Abstract
The transport of preproteins into or across the mitochondrial inner membrane requires the membrane potential Δψ across this membrane. Two roles of Δψ in the import of cleavable preproteins have been described: an electrophoretic effect on the positively charged matrix-targeting sequences and the activation of the translocase subunit Tim23. We report the unexpected finding that deletion of a segment within the sorting sequence of cytochrome b2, which is located behind the matrix-targeting sequence, strongly influenced the Δψ-dependence of import. The differential Δψ-dependence was independent of the submitochondrial destination of the preprotein and was not attributable to the requirement for mitochondrial Hsp70 or Tim23. With a series of preprotein constructs, the net charge of the sorting sequence was altered, but the Δψ-dependence of import was not affected. These results suggested that the sorting sequence contributed to the import driving mechanism in a manner distinct from the two known roles of Δψ. Indeed, a charge-neutral amino acid exchange in the hydrophobic segment of the sorting sequence generated a preprotein with an even better import, i.e. one with lower Δψ-dependence than the wild-type preprotein. The sorting sequence functioned early in the import pathway since it strongly influenced the efficiency of translocation of the matrix-targeting sequence across the inner membrane. These results suggest a model whereby an electrophoretic effect of Δψ on the matrix-targeting sequence is complemented by an import-stimulating activity of the sorting sequence.
INTRODUCTION
The mitochondrial outer and inner membranes contain protein complexes that are responsible for the import of nuclear-encoded preproteins (Ryan and Jensen, 1995; Schatz and Dobberstein, 1996; Neupert, 1997; Pfanner et al., 1997). Three preprotein translocases have been identified. The transport of preproteins across the outer membrane is mediated by the translocase of the outer membrane (TOM) that contains receptors for preproteins and a general import pore. Two translocases of the inner membrane (TIM) exist. The TIM23 complex is responsible for import of the major class of cleavable mitochondrial preproteins. Each cleavable preprotein carries an amino-terminal extension (presequence) that directs the protein across the outer and inner membranes into the matrix and is termed the matrix-targeting sequence. The TIM23 complex consists of two integral membrane proteins, Tim23 and Tim17, that constitute the import channel and a peripherally attached import motor, which are formed by a dynamic complex between the matrix heat shock protein Hsp70 and Tim44 (Schatz, 1996; Jensen and Johnson, 1999; Voos et al., 1999; Bauer et al., 2000). The second TIM is the TIM22 complex that mediates the insertion of a class of hydrophobic preproteins without presequence into the inner membrane. The metabolite carriers of the inner membrane are typical representatives of these preproteins that contain internal targeting information in the mature protein part (Davis et al., 1998; Koehler et al., 1999; Truscott and Pfanner, 1999; Bauer et al., 2000; Kerscher et al., 2000).
Two import driving forces have been found for the translocation of preproteins into mitochondria (Schleyer et al., 1982; Pfanner and Neupert, 1986; Eilers et al., 1987; Kang et al., 1990; Scherer et al., 1990; Martin et al., 1991; Gambill et al., 1993). The membrane potential Δψ across the inner membrane is required for transport of preproteins via both the TIM23 complex and the TIM22 complex. The ATP-dependent import motor consisting of matrix Hsp70 and Tim44 is needed for preproteins imported by the TIM23 complex. In the case of cleavable preproteins, Δψ promotes the transport of the amino-terminal matrix-targeting sequence (Schleyer and Neupert, 1985; Martin et al., 1991), while Hsp70 is crucial for import of the mature portion of a preprotein by direct binding to the unfolded polypeptide chain (Kang et al., 1990; Ostermann et al., 1990; Scherer et al., 1990; Gambill et al., 1993). Two roles for Δψ in the import of cleavable preproteins have been assigned. 1) An electrophoretic effect of Δψ on the positively charged matrix-targeting sequence has been concluded from several observations: studies with synthetic presequence peptides indicated that the positively charged residues are driven in by the electrical gradient (Roise and Schatz, 1988; Roise, 1992; de Kruijff, 1994); the electrical component of the proton–motive force across the inner membrane is essential for protein import, while the ▵pH is dispensable (Martin et al., 1991); a matrix-targeting sequence with a low positive net charge required a high Δψ for import, while a matrix-targeting sequence with a high positive net charge could be imported at a lower Δψ (Martin et al., 1991). 2) Δψ supports the dimerization of Tim23, a likely prerequisite for the interaction of a matrix-targeting sequence with the TIM23 complex (Bauer et al., 1996).
The TIM23 complex does not only transport preproteins into the matrix. A number of preproteins destined for the intermembrane space or inner membrane are imported via this translocase (Bömer et al., 1997; Kurz et al., 1999). These preproteins contain an additional sorting sequence besides the matrix-targeting sequence. The intermembrane space protein cytochrome b2 represents a typical example. Its preprotein carries a second cleavable segment, the sorting sequence, which is located between the matrix-targeting signal and the mature protein (Hurt and van Loon, 1986; Hartl et al., 1987; Glick et al., 1992; Koll et al., 1992; Gärtner et al., 1995a; Gruhler et al., 1995). While the matrix-targeting signal is directed into the matrix space and cleaved off, the adjacent sorting sequence is arrested in the inner membrane and prevents a complete translocation of the preprotein across the inner membrane. Subsequently, the inner membrane peptidase I (Pratje and Guiard, 1986; Schneider et al., 1991; Kalousek et al., 1993) cleaves off the sorting sequence and releases the mature protein to the intermembrane space. A number of alterations of the sorting sequence, such as partial deletions and amino acid substitutions, have been described that inactivate its sorting function and cause a complete translocation of the mutant cytochrome b2 into the matrix (Koll et al., 1992; Beasley et al., 1993; Schwarz et al., 1993; Voos et al., 1993; Gärtner et al., 1995a; Merlin et al., 1997; Bömer et al., 1997, 1998).
In this report, we studied the import of mutant forms of the precursor of cytochrome b2 and made the surprising finding that a sequence beyond the matrix-targeting signal, i.e. the sorting sequence, strongly influenced the Δψ-dependence of protein import. We analyzed the properties of this Δψ-dependence and found that it cannot be attributed to the two roles of Δψ known so far. The sorting sequence contributes to the import driving mechanism in a novel manner and thus modulates the effectiveness of Δψ action.
MATERIALS AND METHODS
Yeast Strains
Unless stated otherwise, the experiments in this study were performed using mitochondria isolated from the Saccharomyces cerevisiae wild-type strain PK82 (MATα his4–713 lys2 ura3–52 ▵trp1 leu2–3112) (Gambill et al., 1993). For the experiments shown in Figure 6, the strains PK82 and PK83 (MATα ade2–101 lys2 ura3–52 leu2–3112 ▵trp1 ssc1–3(LEU2)) (Gambill et al., 1993), MB3–46 (MATα ade2–101 his3-▵200 leu2-▵1 lys2–801 ura3::LYS2 tim23–2) (Dekker et al., 1993), and the corresponding wild-type strain MB3 (MATα ade2–101 his3-▵200 leu2-▵1 lys2–801 ura3::LYS2) were used.
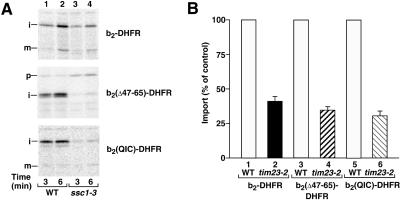
Figure 6.
The requirement of mtHsp70 and Tim23 for the import of b2-DHFR preproteins. (A) Requirement for mtHsp70. pb2-DHFR, pb2(▵47–65)-DHFR, and pb2(QIC)-DHFR were imported into mitochondria isolated from the scc1–3 mutant strain and the corresponding wild-type strain (WT). The mitochondria were incubated at 37°C for 15 min before import (3% BSA in import buffer). After an import for 3 or 6 min at 25°C, all samples were treated with proteinase K (final concentration, 40 μg/ml) for 15 min on ice. After reisolation of the mitochondria, the proteins were separated by SDS-PAGE. (B) Requirement for Tim23. pb2-DHFR, pb2(▵47–65)-DHFR, and pb2(QIC)-DHFR were imported into tim23–2 or the corresponding wild-type mitochondria for 5 min at 25°C. After import, the samples were treated as described above. For quantitation of the amount of protein imported into tim23–2 mitochondria, the import of the respective protein into wild-type mitochondria was set to 100% (control). Bars indicate the SEs of the means (from three to four independent experiments).
Construction of b2-Dihydrofolate Reductase Fusion Proteins
For in vitro transcription of pb2(167)-dihydrofolate reductase (DHFR), pb2(▵47–65)-DHFR, and pb2(K48I,R49C)-DHFR, pGEM4Z plasmids containing the respective open reading frames were used (Rassow et al., 1989; Koll et al., 1992; Bömer et al., 1997). To construct the other fusion proteins, oligonucleotide-directed missense mutagenesis polymerase chain reaction (PCR) was used with the following primers and templates: forward primer 5′-GTCGTTCGAACAAGACT CGCAAAT-ATGCACACAGTCATG-3′ and reverse primer 5′-CATGACTGTG TGCATATTTGCGAGTCTTGTTCGAACGAC-3′ on pb2(K48I,R49C)-DHFR (Bömer et al., 1997) as a template to generate pb2(QIC)-DHFR; forward primer 5′- GTCATGGACTGCCTTGCAGGTCGGTGCAA-TTCTAG-3′ and reverse primer 5′- CTAGAATTGCACCGACCTGCAAGGCAGTCCATGAC-3′ on pb2(QIC)-DHFR as a template to generate pb2(QIC-Q)-DHFR; forward primer 5′-CAAAAT CCAAGTCG-TTCCAACAAAACTCAAGAAAACGCAC-3′ and reverse primer 5′-GTGCGTTTTCTTGAGTTTTGTTGGAACGACTTGGATTTTG-3′ on pb2(167)-DHFR (Rassow et al., 1989; Voos et al., 1993) as a template to generate pb2(E43Q,D45N)-DHFR; forward primer 5′-GTTCGAACAAGACTCAGTCGGTGCAATTCTAG-3‘ and reverse primer 5‘-CTAGAATTGCACCGACTGAGTCTTGTTCGAAC-3‘ on pb2(167)-DHFR to generate pb2(▵47–57)-DHFR. After the PCR reaction, the template DNA was digested with DpnI. To construct pb2(A63P)-DHFR, a PCR was performed on pb2(167)-DHFR using 5′-GAATTGGATTTAGGTGACACTATA-3′ as a forward primer and 5′-GAACTAGRAGCGGGTAGAATTGCACCG-3′ as a reverse primer. The resulting product was used as a forward primer in a subsequent PCR with 5′-CAAGCTCTAATACGACTCACTATA-3′ as a reverse primer, using pb2(167)-DHFR as a template. After digestion with DpnI, the product was cut with EcoRI and MscI and was ligated into EcoRI- and MscI-digested pb2(167)-DHFR. All constructs were transformed into the Escherichia coli strain XL-1 blue (Stratagene, La Jolla, CA). Formation of the correct products was confirmed by DNA sequencing.
Import of Preproteins into Isolated Mitochondria
Mitochondria were isolated from yeast cells grown on YPG (1% yeast extract, 2% bactopeptone, and 3% glycerol) according to published procedures (Daum et al., 1982; Hartl et al., 1987; Kang et al., 1990; Gambill et al., 1993), were resuspended in SEM buffer (250 mM sucrose, 1 mM EDTA, and 10 mM Mops-KOH, pH 7.2) to a concentration of 5 mg/ml, and were stored at −80°C. Radiolabeled mitochondrial preproteins were synthesized by in vitro translation in rabbit reticulocyte lysate (Amersham Pharmacia Biotech, Uppsala, Sweden) in the presence of [35S]methionine/cysteine after in vitro transcription using SP6 polymerase (Stratagene) (Söllner et al., 1991).
For the in vitro import assay, mitochondria (25–50 μg of protein) were diluted with import buffer (1% [wt/vol] fatty acid-free bovine serum albumin [BSA], 250 mM sucrose, 80 mM KCl, 5 mM MgCl2, 2 mM ATP, 2 mM NADH, and 10 mM Mops-KOH, pH 7.2) to a final volume of 100 μl. The samples were preincubated for 5 min at 25°C before the import reaction was started by adding 2–4 μl of reticulocyte lysate containing 35S-labeled preproteins. For the accumulation of import intermediates, the import was performed in the presence of 5 μM of methotrexate (Sigma Chemical, St. Louis, MO) when indicated. After incubation for 2.5–6 min at 25°C, the import reaction was stopped by the addition of 1 μM valinomycin to dissipate the membrane potential ▵ψ. The samples were treated with proteinase K (40 μg/ml) for 15 min, followed by the addition of 1 mM phenylmethylsulfonyl fluoride and incubation for 10 min on ice. The mitochondria were subsequently reisolated, washed with SEM buffer, and subjected to SDS-PAGE.
Carbonyl Cyanide m-Chlorophenylhydrzone Titration
The mitochondria were partially uncoupled by the addition of the protonophore carbonyl cyanide m-chlorophenylhydrzone (CCCP) (Martin et al., 1991; Gärtner et al., 1995b). CCCP was added (from a stock solution concentrated 100-fold in ethanol) before the preincubation at 25°C and before import. All samples were made chemically identical by adding the corresponding amount of solvent to the control samples. To prevent the generation of an electrochemical potential by a reversed action of the FoF1-ATPase, 20 μM oligomycin was added to the import buffer.
Intramitochondrial Localization of Imported Proteins
To determine the localization of the imported proteins, import was performed as described above. After import, the samples were diluted with five volumes of EM buffer (1 mM EDTA, and 10 mM Mops-KOH, pH 7.2) to rupture the outer membrane by swelling. After 15 min of incubation on ice, five volumes of S500EM buffer (500 mM sucrose, 1 mM EDTA, and 10 mM Mops-KOH, pH 7.2), were added to reestablish the original osmotic conditions. For nonswelling conditions, the import mix was diluted twice with SEM buffer. Mitochondria of all samples were reisolated by centrifugation resuspended in 100 μl of SEM buffer, and proteinase K treatment was carried out as described above. After SDS-PAGE, blotting of the proteins to nitrocellulose membrane and subsequent immunodecoration with control antibodies were performed.
Coimmunoprecipitation
The interaction of imported protein with mtHsp70 was analyzed by coimmunoprecipitation (Voisine et al., 1999). pb2(▵47–65)-DHFR was imported into mitchondria in the presence of different concentrations of CCCP for 5 min at 25°C. The mitochondria were reisolated, washed with SEM, and lysed in buffer A (0.1% [vol/vol] Triton X-100, 100 mM NaCl, 10 mM Tris-HCl, pH 7.4, 1 mM PMSF, and 5 mM EDTA). After a clarifying spin (16,000 × g for 5 min), the supernatants were transferred to antibodies directed against mtHsp70 that were bound to protein-A sepharose. The samples were incubated for 1 h at 4°C rotating end-over-end. Subsequently, the protein-A sepharose beads were washed three times in buffer A and once with 10 mM Tris-HCl, pH 7.4. Bound proteins were eluted by the addition of SDS-sample buffer and were analyzed by SDS-PAGE.
Assessment of the Mitochondrial Membrane Potential Δψ
The membrane potential Δψ of isolated yeast mitochondria was assessed by measuring the fluorescence quenching of the potential-sensitive dye 3,3′-dipropylthiadicarbocyanine iodide (DiSC3(5); Molecular Probes, Eugene, OR) as described before (Sims et al., 1974; Gärtner et al., 1995b). The measurements were performed using a Perkin Elmer-Cetus (Norwalk, CT) LS 50B luminescence spectrometer at 25°C, with excitation at 622 nm, emission at 670 nm, and slits at 5 nm. The measurements were carried out using a buffer containing 600 mM sorbitol, 1% (wt/vol) BSA, 10 mM MgCl2, 0.5 mM EDTA, and 20 mM KPi, pH 7.4. The following reagents were successively added to 3 ml of the buffer and the change in fluorescence was recorded: 3 μl of DiSC3(5) (in ethanol; final concentration, 2 μM); 20 μl of mitochondria (in SEM buffer; final concentration, 33 μg mitochondrial protein per milliliter); and, finally, 3 μl of valinomycin (in ethanol; final concentration, 1 μM) to dissipate ▵ψ. The difference in the fluorescence before and after the addition of valinomycin represents a relative assessment of the membrane potential.
Miscellaneous Methods
Standard techniques were used for SDS-PAGE, immunodecoration, and DNA manipulation. For the detection and quantitation of radiolabeled proteins, a storage phosphor imaging system with software (ImageQuant version 1.11, Molecular Dynamics, Sunnyvale, CA) was used.
RESULTS
A Deletion in the Sorting Sequence of Cytochrome b2 Causes a Higher Δψ-Dependence of Transport into Mitochondria
To modulate the magnitude of the mitochondrial membrane potential Δψ under in vitro protein import conditions into isolated mitochondria, we used different concentrations of the protonophore CCCP (Martin et al., 1991; Nicholls and Ferguson, 1982; Gärtner et al., 1995b; Bömer et al., 1998). Oligomycin was included to inhibit the FoF1-ATPase to prevent the generation of a membrane potential and the depletion of matrix ATP by a reverse action of the ATPase. The membrane potential was assessed by use of the fluorescent dye DiSC3(5) that is taken up by mitochondria and thus quenched in a Δψ-dependent manner (Sims et al., 1974; Gärtner et al., 1995b). The decrease in fluorescence (which is reversed by a complete dissipation of Δψ by the potassium ionophore valinomycin in the presence of potassium in the medium) is used as an assessment for the magnitude of Δψ (Figure 1A, top). By the addition of increasing concentrations of CCCP, this fluorescence quenching was gradually reduced until a complete dissipation was achieved (Figure 1A [middle and bottom] and B).
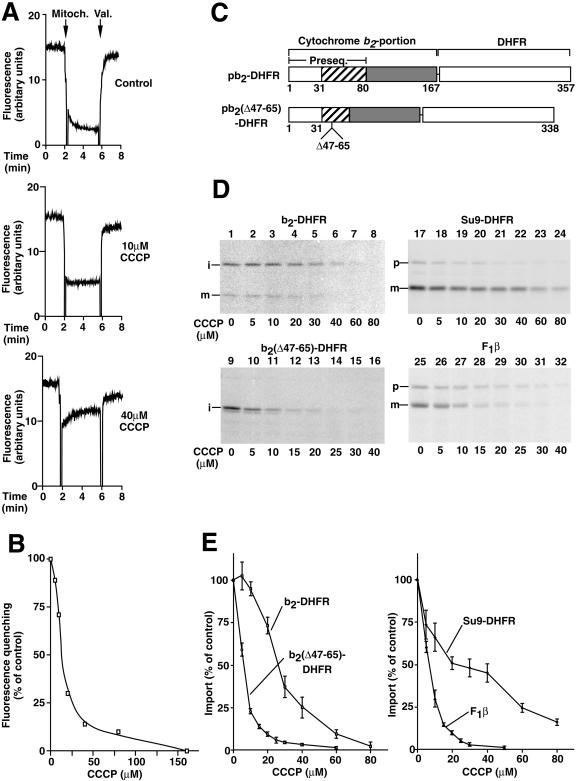
Figure 1.
The mitochondrial import of two b2-DHFR preproteins shows a differential dependence on the membrane potential Δψ. (A) The membrane potential ▵ψ of isolated yeast mitochondria was assessed at 25°C using the potential-sensitive dye DiSC3(5). CCCP was added as indicated. ▵ψ was assessed by the difference in fluorescence before and after the addition of valinomycin (Val.). (B) The fluorescence quenching at different concentrations of CCCP was quantified. The quenching in the absence of CCCP was set to 100%. (C) Cytochrome b2 hybrid proteins used in this experiment. pb2-DHFR consists of the first 167 amino acids of the cytochrome b2 precursor fused to the entire mouse DHFR by a linker of two amino acids. The bipartite cytochrome b2 presequence consists of an amino-terminal matrix-targeting sequence (residues 1–31) and a sorting sequence (residues 32–80). In pb2(▵47–65)-DHFR, residues 47–65 of pb2-DHFR have been deleted. (D and E) Isolated mitochondria were incubated with reticulocyte lysate containing 35S-labeled mitochondrial preproteins in the presence of the indicated concentrations of CCCP. After incubation for 5 min at 25°C, the import was stopped by the addition of 1 μM valinomycin. To remove nonimported preproteins, all samples were treated with proteinase K (final concentration, 40 μg/ml) for 15 min on ice. After reisolation and separation by SDS-PAGE, the amounts of imported proteins were quantified by phosphorimage analysis. The amount of protein imported in the absence of CCCP was set to 100% (control). Bars indicate the SEs of the means (from four to six independent experiments). p, precursor form; i, intermediate-sized form; m, mature form.
b2-DHFR fusion proteins are widely used to study mitochondrial protein import since they are efficiently synthesized and radiolabeled in rabbit reticulocyte lysate and their intramitochondrial processing and localization can be unambiguously determined (Hartl et al., 1987; Rassow et al., 1989, 1990; Koll et al., 1992; Beasley et al., 1993; Glick et al., 1993; Schwarz et al., 1993; Voos et al., 1993; Stuart et al., 1994; Voisine et al., 1999). The 167 amino-terminal amino acid residues of cytochrome b2 that were used as the basis for the preproteins of this study contain the complete targeting and sorting information of the preprotein as follows: the matrix-targeting sequence (residues 1–31); the sorting sequence (residues 32–80); and 87 residues of the mature protein. Fused to the entire DHFR, the resulting chimeric preprotein b2-DHFR (Figure 1C) has been shown to be sorted to the intermembrane space like authentic cytochrome b2 (Koll et al., 1992; Voos et al., 1993) and will be referred to as the wild-type preprotein in this study. b2-DHFR was synthesized in rabbit reticulocyte lysates in the presence of [35S]methionine/cysteine and was incubated with isolated yeast mitochondria. Import was determined by monitoring the processing of the preprotein to the intermediate- and mature-sized forms and by protection against externally added protease (Figure 1D, lane 1). On addition of increasing concentrations of CCCP, the import of b2-DHFR was gradually inhibited (Figure 1D, lanes 2–8). In the short import time that is required to be in the kinetically linear import range (Söllner et al., 1991; Alconada et al., 1995), the slow second processing step generated only small amounts of the mature form. Since the Δψ-dependent step of import takes place before the generation of the intermediate-sized form by the matrix-processing peptidase (Schatz, 1996; Neupert, 1997; Pfanner et al., 1997), the sum of protease-protected intermediate- and mature-sized forms was used for the quantitation of import (Figure 1E).
The deletion of a 19-residue segment in the sorting sequence of b2-DHFR generates the preprotein b2(▵47–65)-DHFR, which is sorted into the matrix space and cleaved to the intermediate-sized form (Koll et al., 1992; Voos et al., 1993; Voisine et al., 1999). b2(▵47–65)-DHFR was efficiently imported into yeast mitochondria in the absence of CCCP (Figure 1D, lane 9) yet was inhibited strongly by the addition of CCCP (Figure 1D, lanes 10–16). A quantitative analysis of the inhibitory effect of CCCP on the import of b2-DHFR and b2(▵47–65)-DHFR revealed a striking difference (Figure 1E, left panel), indicating that the import of b2(▵47–65)-DHFR was significantly more sensitive to a reduction of the mitochondrial membrane potential than that of b2-DHFR.
For comparison, we imported the following two preproteins that were reported to depend differentially on Δψ: the β-subunit of the F1-ATPase (F1β) and a fusion protein between the presequence of Fo-ATPase subunit 9 and DHFR (Su9-DHFR) (Martin et al., 1991). The import of F1β was strongly inhibited by the addition of CCCP (Figure 1D, lanes 25–32), while the import of Su9-DHFR showed a higher resistance to CCCP (Figure 1D, lanes 17–24). The quantitation indicated that the CCCP sensitivity of the import of b2(▵47–65)-DHFR was comparable to that of F1β, while that of b2-DHFR was more related to that of Su9-DHFR (Figure 1E). We conclude that the import of b2-DHFR and of b2(▵47–65)-DHFR show a differential Δψ-dependence. The difference is roughly related to that observed for the import of Su9-DHFR and F1β. However, Su9-DHFR and F1β possess quite different matrix-targeting sequences, whereas b2-DHFR and b2(▵47–65)-DHFR possess the identical matrix-targeting sequence. In the following chapters, we thus asked if characteristics of the sorting sequence of the b2-fusion proteins were responsible for the differential Δψ-dependence.
A Differential Δψ-Dependence of b2-DHFR Preproteins with the Same Intramitochondrial Destination
b2-DHFR is transported to the intermembrane space, whereas b2(▵47–65)-DHFR is completely imported into the matrix (Koll et al., 1992; Voos et al., 1993) as demonstrated here by the differential protease accessibility after opening of the mitochondrial outer membrane by swelling (i.e., formation of mitoplasts). A major fraction of imported b2-DHFR was sensitive to proteinase K like the intermembrane space-exposed portions of the marker protein ADP/ATP carrier (Figure 2A, lane 2, columns 3 and 5), while b2(▵47–65)-DHFR was mainly protected against the protease, which is comparable to the matrix marker mitochondrial GrpE (Mge1) (Figure 2A, lane 2, columns 4 and 6). After lysis of the mitochondrial membranes with detergent, both b2-DHFR fusion proteins as well as the marker proteins were fully accessible to and degraded by proteinase K (Gärtner et al., 1995a; data not shown), excluding an endogenous protease resistance of the proteins as explanation for the different protease protection.
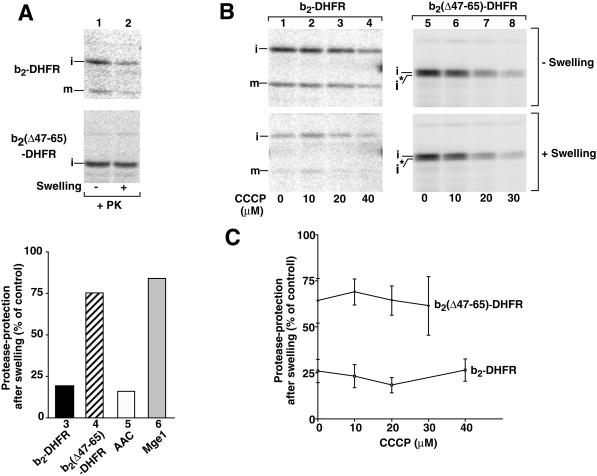
Figure 2.
Lowering of the membrane potential does not change the intramitochondrial sorting of the b2-DHFR preproteins. (A) pb2(▵47–65)-DHFR is transported into the matrix. Radiolabeled pb2-DHFR and pb2(▵47–65)-DHFR were synthesized in reticulocyte lysate and were imported into isolated yeast mitochondria for 5 min at 25°C. After the import, the samples were divided, and one half was diluted with hypotonic EM buffer to generate mitoplasts by swelling (sample 2). The other half was mock-treated with isotonic SEM buffer (sample 1). The samples were left on ice for 15 min. After reisolation and resuspension of the mitochondria in SEM buffer, all samples were treated with proteinase K (PK; 40 μg/ml final concentration) for 15 min on ice. Subsequently, the mitochondria were reisolated and subjected to SDS-PAGE, and the proteins were transferred to nitrocellulose. Radiolabeled proteins were quantified by phosphorimage analysis. To confirm the opening of the intermembrane space by swelling, immunodecoration was performed with antibodies directed against the ATP/ADP carrier (AAC) as an intermembrane space-exposed marker protein and the matrix protein Mge1. For quantitation, the amount of protease-protected protein in nonswollen mitochondria was set to 100% (control). (B and C) Sorting of imported proteins is independent of the membrane potential. Radiolabeled pb2-DHFR (samples 1–4) and pb2(▵47–65)-DHFR (samples 5–8) were imported into mitochondria for 5 min at 25°C in the presence of the indicated concentrations of CCCP. After the import reaction, the samples were split and one half was swollen to open the intermembrane space. Subsequently, the mitochondria were reisolated and resuspended, and all samples were treated with proteinase K (40 μg/ml final concentration). After SDS-PAGE, the amounts of radiolabeled proteins were determined by phosphorimage analysis. The amount of each protein in nonswollen mitochondria at the respective CCCP concentration was set to 100% (control). Bars indicate the SEs of the means (from four independent experiments each). i, i*, intermediate-sized forms; m, mature form.
We asked whether a lowering of the membrane potential altered the intramitochondrial sorting of the b2-fusion proteins. The preproteins were imported at different concentrations of CCCP, and half of each sample was subjected to swelling. Both the i- and m-form of b2-DHFR were largely degraded by added proteinase K (Figure 2B, lower panel, lanes 1–4), whereas i-b2(▵47–65)-DHFR was mainly protected against the protease (Figure 2B, lower panel, lanes 5–8). A quantitative analysis demonstrated that the intramitochondrial locations of b2-DHFR and b2(▵47–65)-DHFR were not affected by the addition of CCCP to the import reaction (Figure 2C). (A second processing to i* that was observed for a small amount of b2(▵47–65)-DHFR [Figure 2B] has been reported for a number of matrix-targeted preproteins and is apparently mediated by the matrix-localized mitochondrial intermediate peptidase [Isaya et al., 1991; Kalousek et al., 1993; Schwarz et al., 1993]. In all experiments, the Δψ-dependence of formation of the i*-form correlated with that of formation of the i-form, and thus our quantitations included both forms.)
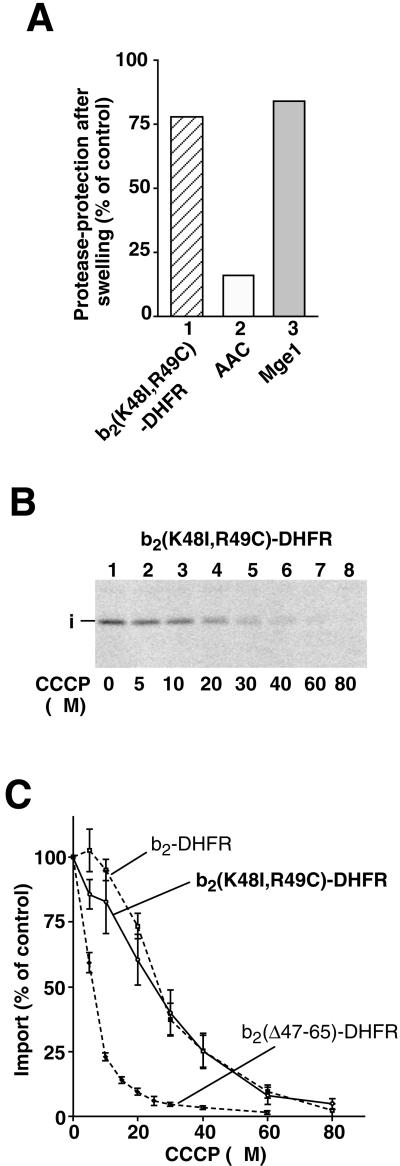
Does the transport of b2-fusion proteins into the matrix require a higher membrane potential than the transport into the intermembrane space? An exchange of two basic amino acid residues (lysine 48 and arginine 49) of the sorting sequence by neutral amino acids impaired the sorting function and caused transport of the mutant preprotein into the matrix (Schwarz et al., 1993; Bömer et al., 1997). We synthesized b2(K48I,R49C)-DHFR and imported it into mitochondria. Figure 3A demonstrates that the imported fusion protein was protected against protease added to mitoplasts like the matrix marker Mge1 (columns 1 and 3) and thus transported into the matrix space. Then the CCCP sensitivity for the import of b2(K48I,R49C)-DHFR was determined (Figure 3B). The import of b2(K48I,R49C)-DHFR was significantly more resistant to CCCP than that of b2(▵47–65)-DHFR yet was similar to that of b2-DHFR (Figure 3C). Thus, b2(K48I,R49C)-DHFR and b2-DHFR show a similar Δψ-dependence, although their sorting pathways are different. In contrast, b2(▵47–65)-DHFR and b2(K48I,R49C)-DHFR are both translocated into the matrix but have a strikingly different Δψ-dependence.
Figure 3.
A matrix-targeted b2-DHFR construct with a similar Δψ-dependence as an intermembrane space-targeted construct. (A) The fusion protein pb2(K48I, R49C)-DHFR was imported into mitochondria, and the intramitochondrial localization was determined as described in the legend of Figure 2A. (B and C) The import of pb2(K48I, R49C)-DHFR shows a dependence on ▵ψ comparable to that of pb2-DHFR. pb2(K48I, R49C)-DHFR was imported into mitochondria in the presence of increasing concentrations of CCCP, as described in the legend of Figure 1, D and E. The amount of protein imported in the absence of CCCP was set to 100% (control). For comparison, the titration curves for the import of pb2-DHFR and pb2(▵47–65)-DHFR also are shown (dashed lines). Bars indicate the SEs of the means (from four to six independent experiments).
These results lead to two related conclusions. First, lowering of Δψ during import does not alter the intramitochondrial sorting of b2-fusion proteins. Second, a differential Δψ-dependence of import of b2-fusion proteins cannot be explained by a different intramitochondrial destination (matrix or intermembrane space). Taken together, these results indicate that the sorting pathway of b2-fusion proteins is not a critical determinant for the Δψ-dependence of import.
The Content of Charged Residues in the Sorting Sequence Is Not Critical for the Δψ-Dependence of Protein Import
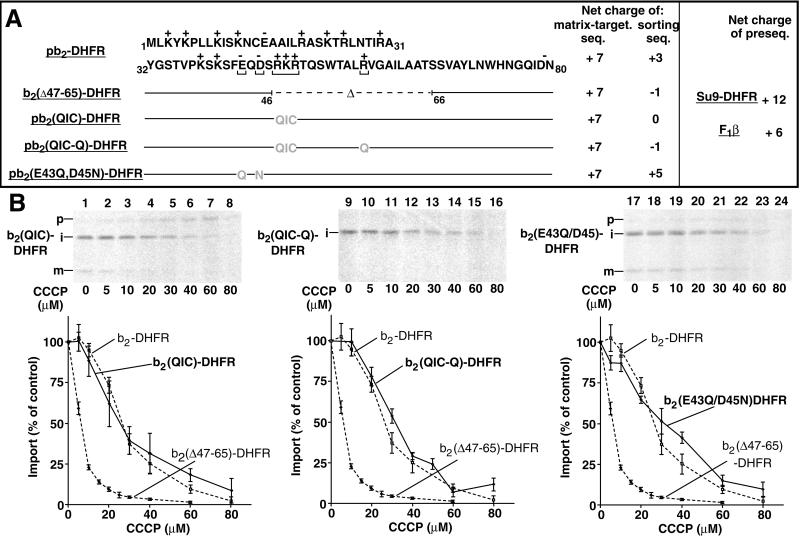
The entire presequence of b2-DHFR contains a net positive charge of 10 (i.e., +7 for the matrix-targeting sequence and +3 for the sorting sequence) (Guiard, 1985). In b2(▵47–65)-DHFR, a segment containing four positively charged residues has been deleted, leading to a net charge of the sorting sequence of −1 (Figure 4A) (Koll et al., 1992). The two classic preproteins that have been shown to differentially depend on a Δψ for import, Su9-DHFR and F1β (see Figure 1, D and E) (Martin et al., 1991), differ in the net charge of their presequences from +12 to +6 (Figure 4A, right panel). It was thus conceivable that a difference in the net positive charge of b2-fusion proteins was responsible for the differential Δψ-dependence.
Figure 4.
Substitution of charged residues in the sorting sequence does not alter the ▵ψ dependence of import. (A) b2-fusion proteins employed. The net charges (amino acid side chains) of the matrix-targeting sequence and the sorting sequence are shown. For comparison, the net charge of the presequences of Su9-DHFR and yeast F1β is also shown. (B) pb2(QIC)-DHFR (samples 1–8), pb2(QIC-Q)-DHFR (samples 9–16), and pb2(E43Q, D45N)-DHFR (samples 17–24) were imported into mitochondria in the presence of 0–80 μM CCCP, as described in the legend of Figure 1, D and E. The amount of protein imported in the absence of CCCP was set to 100% (control). Bars indicate the SEs of the means (from four to six independent experiments). For comparison, the curves for the import of pb2-DHFR and pb2(▵47–65)-DHFR are included (dashed lines).
To test this, three or four positively charged residues in the sorting sequence were replaced by uncharged amino acid residues, leading to the fusion proteins b2(QIC)-DHFR and b2(QIC-Q)-DHFR with net charges of the sorting sequence of 0 or −1, respectively (Figure 4A). These preproteins were imported into mitochondria at different concentrations of CCCP (Figure 4B, lanes 1–16). Surprisingly, the import of the preproteins revealed a similar sensitivity to a decrease of the membrane potential as that of the wild-type presequence of b2-DHFR but was clearly different from that of b2(▵47–65)-DHFR (Figure 4B, left panel and middle panel). In particular, b2(QIC-Q)-DHFR contains the identical charged residues as b2(▵47–65)-DHFR throughout the entire preprotein but shows a much lower Δψ-dependence (Figure 4B, middle panel). Moreover, the replacement of two negatively charged residues of the sorting sequence by uncharged ones generated the preprotein b2(E43Q,D45N)-DHFR with a higher net charge than the wild-type sorting sequence (i.e., +5) (Figure 4A). The import of b2(E43Q,D45N)-DHFR again revealed a Δψ-dependence that was similar to b2-DHFR (Figure 4B, lanes 17–24, right panel). These results demonstrate that the Δψ-dependence of import is independent of the net charge of the b2 sorting sequence.
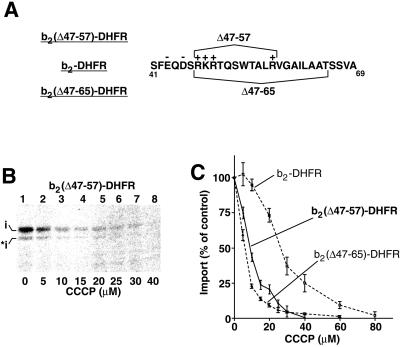
The wild-type b2-DHFR and the constructs b2(K48I,R49C)-DHFR, b2(QIC)-DHFR, and b2(QIC-Q)-DHFR, which share a similar Δψ-dependence (Figures 3C and 4B), all harbor an uncharged segment (residues 58–65) that is lacking in b2(▵47–65)-DHFR. We asked whether a lack of this segment was responsible for the high Δψ-dependence of b2(▵47–65)-DHFR. Thus, we constructed the fusion protein b2(▵47–57)-DHFR that only lacked the segment containing the four positively charged residues (Figure 5A). The preprotein was imported into mitochondria in the presence of different concentrations of CCCP (Figure 5B). The import of b2(▵47–57)-DHFR showed a high sensitivity toward the lowering of the membrane potential that was close to that of b2(▵47–65)-DHFR (Figure 5C). (Although the difference of the Δψ-dependence of b2(▵47–57)-DHFR compared with b2(▵47–65)-DHFR was statistically significant, the difference was rather small when compared with the Δψ-dependence of b2-DHFR [Figure 5C].) This result suggests that a lack of this uncharged segment is not the main determinant for the strong difference in Δψ-dependence of import observed between the wild-type preprotein and b2(▵47–65)-DHFR.
Figure 5.
Effect of the uncharged stretch within the sorting sequence. (A) The fusion protein b2(▵47–57)-DHFR lacks the positive charges of the sorting sequence like b2(▵47–65)-DHFR but maintains most of the uncharged stretch of wild-type b2-DHFR. (B and C) The Δψ-dependence of import of pb2(▵47–57)-DHFR shows only a minor difference with that of pb2(▵47–65)-DHFR. pb2(▵47–57)-DHFR was imported into the mitochondria in the presence of increasing concentrations of CCCP, as described in the legend of Figure 1, D and E. The amount of protein imported in the absence of CCCP was set to 100% (control). For comparison, the titration curves for the import of pb2-DHFR and pb2(▵47–65)-DHFR also are shown (dashed lines). Bars indicate the SEs of the means (from four to six independent experiments).
The Differential Δψ-Dependence Is Not Attributable to the Dependence on mtHsp70 or Tim23
We asked whether the differential Δψ-dependence of b2-fusion proteins could be explained by the requirement for the second import driving force, mtHsp70, or the dependence on the function of Tim23. We used the preproteins b2-DHFR, b2(▵47–65)-DHFR, and b2(QIC)-DHFR to address this problem. In the yeast mutant ssc1–3, mtHsp70 carries an amino acid exchange in the ATPase domain that strongly inhibits its function (Gambill et al., 1993; Voos et al., 1993, 1996). The isolated mitochondria were preincubated at 37°C to induce the mutant phenotype. The import of b2(▵47–65)-DHFR and b2(QIC)-DHFR was strongly inhibited in the mutant mitochondria (Figure 6A, middle panel and lower panel, lanes 3 and 4), while the import of b2-DHFR was only partially reduced (Figure 6A, upper panel, lanes 3 and 4). Therefore, the requirement for mtHps70 does not correlate with the Δψ-dependence, since b2-DHFR and b2(QIC)-DHFR have a similar low Δψ-dependence while b2(▵47–65)-DHFR has a strong Δψ-dependence. The dependence on Tim23 function was analyzed with tim23–2 mutant mitochondria in which the oligomerization of the TIM23 translocase is destabilized and, thus, the transport efficiency of preproteins is reduced (Dekker et al., 1997; Bömer et al., 1997). All three b2-fusion proteins were inhibited in import into tim23–2 mitochondria by ∼60–70% compared with wild-type mitochondria (Figure 6B). In particular, no significant difference of b2(▵47–65)-DHFR compared with b2-DHFR or b2(QIC)-DHFR was recognizable, indicating that the differential Δψ-dependence cannot be attributed to a different requirement for Tim23.
A b2-DHFR Construct That Is Less Dependent on Δψ Than the Wild-Type Version
The results obtained so far have suggested a novel characteristic of the membrane potential-driven import of preproteins, which depends on properties of the sorting sequence in a charge-independent manner. We wondered whether further independent evidence for this novel role of Δψ could be obtained. Beasley et al. (1993) had selected a number of mutations in the preprotein of cytochrome b2. We asked whether the mutation of an uncharged residue to another uncharged residue in the sorting sequence of cytochrome b2 would influence the Δψ-dependence of import. The residues in the segment deleted in b2(▵47–65)-DHFR seemed to be of particular importance to us.
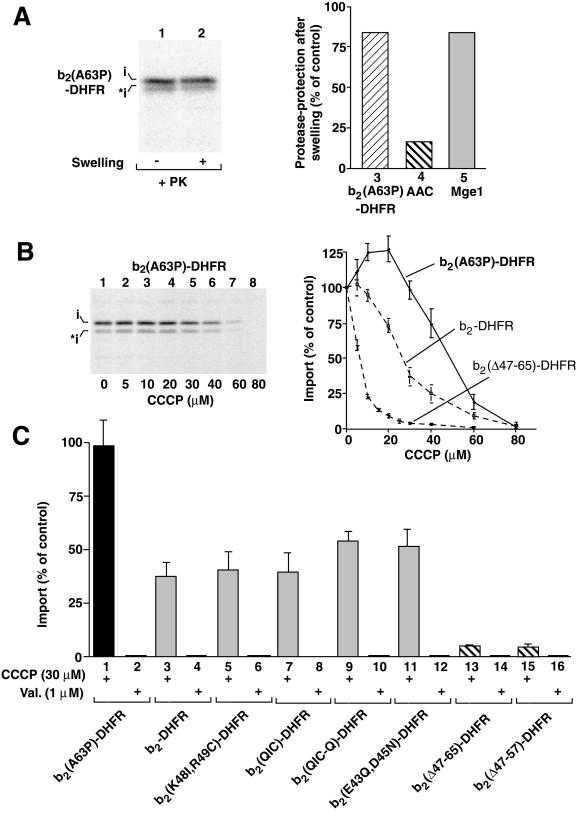
Finally, we found a single mutation, a substitution of alanine 63 by proline, that generated a preprotein with a remarkable Δψ-dependence. b2(A63P)-DHFR was targeted to the matrix space since it remained protease-protected in mitoplasts like the matrix marker Mge1 (Figure 7A, lane 2, columns 3 and 5) (Beasley et al., 1993). When the sensitivity of import to CCCP was analyzed, b2(A63P)-DHFR showed a higher resistance than any preprotein tested before (Figure 7B, lanes 1–8, quantitation). CCCP concentrations up to 30 μM that lead to a clear reduction of Δψ (see Figure 1B) did not inhibit the import of b2(A63P)-DHFR but actually led to a slight stimulation (Figure 7B, panel on the right). The import of b2(A63P)-DHFR was only inhibited at higher concentrations of CCCP. Therefore, b2(A63P)-DHFR requires less Δψ for import than the wild-type presequence of b2-DHFR, proving that uncharged residues in the b2 sorting sequence can play a critical role in the membrane potential-dependence of import.
Figure 7.
An alteration in the hydrophobic segment of the sorting sequence lowers the Δψ-dependence. (A) The hybrid protein b2(A63P)-DHFR was imported into isolated yeast wild-type mitochondria, and the intramitochondrial localization was determined as described in the legend of Figure 2A. (B) pb2(A63P)-DHFR was imported into mitochondria in the presence of increasing concentrations of CCCP, as described in the legend of Figure 1, D and E. The amount of protein imported in the absence of CCCP was set to 100% (control). For comparison, the curves for the import of pb2-DHFR and pb2(▵47–65)-DHFR are included (dashed lines). (C) b2-DHFR fusion proteins can be divided into three groups according to the ▵ψ dependence of their import. The preproteins were imported into mitochondria for 5 min at 25°C in the presence or absence of 30 μM CCCP (for a partial reduction of Δψ) and in the presence or absence of 1 μM valinomycin (Val.; to completely dissipate Δψ). After import, the samples were subjected to treatment with proteinase K and SDS-PAGE, as described in the legend of Figure 1, D and E. The import of the respective protein in the absence of CCCP/valinomycin was set to 100% (control). Bars indicate the SEs of the means (from four to six independent experiments).
In Figure 7C, we compared the b2-fusion proteins used in this study. First, all preproteins required a membrane potential for import since a complete dissipation of Δψ by the addition of valinomycin (in the presence of potassium in the import buffer) blocked the import of each protein (Figure 7C, even-numbered columns). Second, roughly three classes of preproteins can be distinguished when an intermediate level of Δψ is generated by the addition of CCCP (shown are the import results at 30 μM CCCP) (Figure 7C, odd-numbered columns): (1) A series of constructs with a different number of charged residues in the sorting sequence (Figure 7C, columns 5, 7, 9, and 11) show a Δψ-dependence that is roughly similar to that of b2-DHFR with the wild-type presequence (Figure 7C, column 3); (2) b2(▵47–65)-DHFR and b2(▵47–57)-DHFR reveal a much stronger Δψ-dependence (Figure 7C, columns 13 and 15); while (3) b2(A63P)-DHFR still can be efficiently imported at a low Δψ (Figure 7C, column 1).
Early Function of the Sorting Sequence for Translocation of the Matrix-targeting Sequence
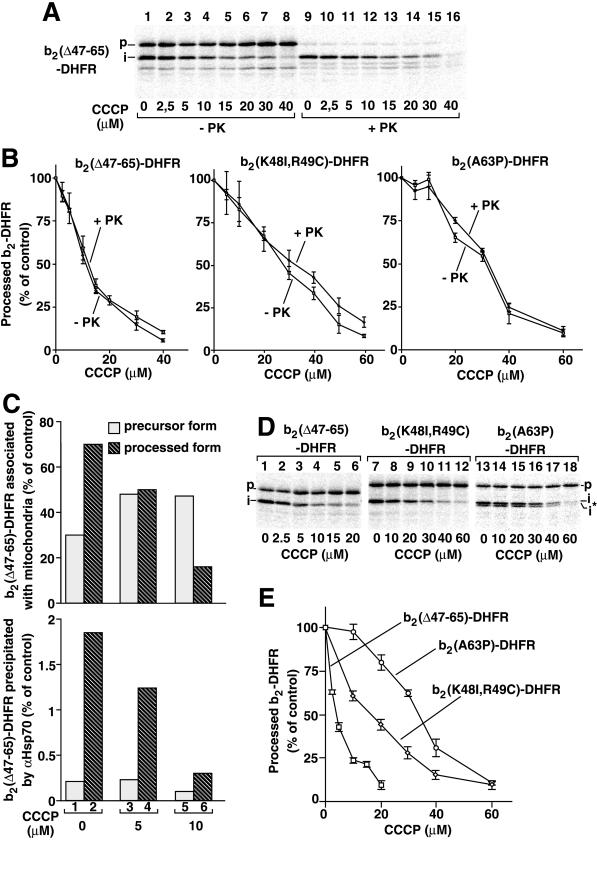
The experiments described so far have analyzed the influence of the sorting sequence on the entire import process, since the processed forms of the fusion proteins protected against externally added proteinase K were quantified. We asked whether the sorting sequence already had affected the early import stage of translocation of the matrix-targeting sequence or whether the sorting sequence functioned only in a later stage by promoting the translocation of carboxy-terminal parts of the presequence and the mature protein parts. In the latter case, the translocation of the matrix-targeting sequence of b2(▵47–65)-DHFR should have a lower Δψ-dependence than the translocation of the entire fusion protein. Therefore, we analyzed the efficiency of processing of b2(▵47–65)-DHFR, i.e., the removal of the matrix-targeting sequence by the matrix-processing peptidase, without treating the mitochondria with proteinase K (Figure 8A, lanes 1–8). A direct comparison with the protease protection of the processed protein, however, did not reveal a difference (Figure 8, A, lanes 9–16, and B, left panel). The Δψ-dependence of the formation of i-b2(▵47–65)-DHFR was indistinguishable from that of translocation of the entire protein to a protease-protected location (Figure 8B, left panel). With both b2(K48I,R49C)-DHFR and b2(A63P)-DHFR, which are imported into the matrix like b2(▵47–65)-DHFR, the translocation of the matrix-targeting sequence to the matrix-processing peptidase showed a lower Δψ-dependence than that of b2(▵47–65)-DHFR (Figure 8B); however, the Δψ-dependence was not lower than that of the complete import of the respective protein (Figure 8B, middle panel and right panel). These results suggested that the sorting sequence influenced the Δψ-dependence at a very early import stage.
Figure 8.
The sorting sequence influences the Δψ-dependence of translocation of the matrix-targeting sequence. (A and B) The preproteins of b2(▵47–65)-DHFR, b2(K48I, R49C)-DHFR, and b2(A63P)-DHFR were imported into mitochondria for 2.5 min at 25°C in the presence of the indicated concentrations of CCCP. After import, the samples were split, and one half was treated with proteinase K (+PK; 40 μg/ml) for 15 min on ice, while the other half was left untreated (−PK). After reisolation and separation by SDS-PAGE, the amounts of processed proteins were quantified by phosphorimage analysis. The amount of protein processed in the absence of CCCP was set to 100% (control). Bars indicate the SEs of the means (from four independent experiments). p, precursor; i, intermediate-sized form. (C) Coimmunoprecipitation with antimtHsp70. pb2(▵47–65)-DHFR was imported into mitochondria for 5 min at 25°C in the presence of the indicated concentrations of CCCP. The mitochondria were reisolated. A portion of each sample was directly analyzed by SDS-PAGE and digital autoradiography (upper panel). The rest of each sample was lysed by nonionic detergent in the presence of EDTA and was subjected to immunoprecipitation with antibodies against mtHsp70 (αHsp70). The bound material was analyzed by SDS-PAGE and digital autoradiography (lower panel). The precursor and processed forms were quantified. The total amount (precursor plus processed form) associated with the mitochondria in the absence of CCCP was set to 100% (control). (D and E) Δψ-dependence of processing of membrane-spanning translocation intermediates. The preproteins of b2(▵47–65)-DHFR, b2(K48I, R49C)-DHFR, and b2(A63P)-DHFR were incubated with isolated mitochondria in the presence of 5 μM MTX and different concentrations of CCCP for 15 min at 25°C. After reisolation and separation by SDS-PAGE, the amounts of processed proteins were quantified by phosphorimage analysis. The amount of each protein processed by the mitochondria in the absence of CCCP was set to 100% (control). Bars indicate the SEs of the means (from four independent experiments).
To obtain independent evidence, we probed the accessibility of the preprotein to matrix Hsp70. mtHsp70 can bind to the matrix-targeting sequence as soon as it emerges on the matrix side of the inner membrane import channel, even when the processing site has not yet been exposed to the matrix (Ungermann et al., 1994). Thereby, the very first stage of translocation of the matrix-targeting signal into the matrix can be analyzed. b2(▵47–65)-DHFR was imported at different concentrations of CCCP. Mitochondria were lysed with nonionic detergent, and the association of the fusion protein with mtHsp70 was determined by coimmunoprecipitation. In the absence of CCCP, ∼2.5% of processed b2(▵47–65)-DHFR was recovered together with mtHsp70 (Figure 8C, column 2); this represents a typical yield for the coimmunoprecipitation (including several washing steps) of accumulated substrate with mtHsp70 (Ungermann et al., 1994; Voisine et al., 1999). In the presence of CCCP, the processing of b2(▵47–65)-DHFR (Figure 8C, upper panel, columns 4 and 6) as well as the coprecipitation of i-b2(▵47–65)-DHFR decreased (Figure 8C, lower panel, columns 4 and 6). The amount of the precursor form of b2(▵47–65)-DHFR associated with mitochondria increased in the presence of CCCP (Figure 8C, upper panel, columns 3 and 5 versus column 1); however, coprecipitation of the precursor form with antimtHsp70 remained at a very low level under all conditions and was not increased at low Δψ (Figure 8C, lower panel, columns 1, 3 and 5), indicating that the precursor form was not accessible for binding to mtHsp70. Together with the protease sensitivity of the precursor form (Figure 8A), this result shows that the precursor form is located on the mitochondrial surface and has not yet entered the matrix space. The coprecipitation of b2(▵47–65)-DHFR with anti-mtHsp70 thus confirms the result obtained with the processing assay that the initial translocation of the amino-terminal portion of the preprotein across the inner membrane shows a strong sensitivity to CCCP like the translocation of the complete protein. We conclude that the deletion in the sorting sequence of b2(▵47–65)-DHFR already affects the Δψ-dependence of translocation of the matrix-targeting sequence across the inner membrane.
The specific ligand methotrexate (MTX) stabilizes the DHFR moiety of the cytochrome b2 fusion proteins and, thus, arrests the importing protein after the first step of translocation in a processed state, with the folded DHFR still outside the mitochondria (Eilers and Schatz, 1986; Rassow et al., 1989). The preproteins of b2(▵47–65)-DHFR, b2(K48I,R49C)-DHFR, and b2(A63P)-DHFR were imported in the presence of MTX and different concentrations of CCCP (Figure 8D). The efficiency of translocation arrest was demonstrated by the accessibility of the intermediates to proteinase K (not shown). A quantification of the processing efficiency revealed that the Δψ-dependence of the constructs significantly differed (Figure 8E), as was observed for the translocation of the entire proteins; i.e., b2(A63P)-DHFR showed the lowest Δψ-dependence, and b2(▵47–65)-DHFR showed the highest Δψ-dependence (compare Figure 8E to 7C). We conclude that the sorting sequence of cytochrome b2 contributes to the Δψ-dependence of import at an early stage when the major portion of the mature protein is still outside the mitochondrion.
DISCUSSION
The membrane potential Δψ is essential for the transport of preproteins into or across the mitochondrial inner membrane. We report that the sorting sequence of a cleavable preprotein strongly influences the requirement for Δψ. All cytochrome b2 fusion proteins used here contain the identical matrix-targeting sequence and the identical mature protein part, and differences were only introduced in the sorting sequence in the form of deletions or of amino acid substitutions. All b2-fusion proteins were efficiently imported into fully energized mitochondria (i.e., at a high Δψ) and were blocked fully in transport across the inner membrane upon a complete dissipation of Δψ. However, significant differences in import efficiency became apparent when the magnitude of the membrane potential was gradually lowered by the protonophore CCCP. Since the sorting sequence determines the intramitochondrial sorting of b2-fusion proteins to the intermembrane space or matrix, an obvious assumption was that a differential Δψ-dependence would be related to the sorting pathway of the preproteins. However, we found that the Δψ-dependence was independent of the intramitochondrial destination, and, in particular, matrix-targeted b2-fusion proteins with both a high and a low Δψ-dependence were found.
It has to be emphasized that CCCP selectively inhibits the Δψ-dependent step of protein import and does not unspecifically impair the import competence of preproteins or mitochondrial function for the following reasons. In the presence of high concentrations of CCCP, preproteins still specifically interact with the TOM machinery of the outer membrane (Hines and Schatz, 1993; Haucke et al., 1995; Ryan et al., 1999). The import block by CCCP can be reversed by the removal of CCCP, and, thus, arrested preproteins can be imported completely (Hines and Schatz, 1993; Haucke et al., 1995; Ryan et al., 1999). The induction of a potassium diffusion potential (by valinomycin in the presence of low potassium in the medium) abolishes the dissipation of Δψ by CCCP and allows import of preproteins, even in the presence of high concentrations of CCCP (Pfanner and Neupert, 1985; Martin et al., 1991).
The differential Δψ-dependence of the b2-fusion proteins was not attributable to a differential dependence on the function of mtHsp70. The intramitochondrial sorting pathway of b2-fusion proteins is critical for the requirement for mtHsp70, since matrix-targeted preproteins, but not intermembrane space-targeted preproteins, strongly depend on the chaperone (Voos et al., 1993; Stuart et al., 1994; Gärtner et al., 1995a), while the Δψ-dependence is independent of the sorting pathway. Moreover, preproteins with a low and a high Δψ-dependence showed the same requirement for Tim23 function. In fact, the modulatory effect of the sorting sequence on the Δψ-dependence of protein import was much stronger than the effect of Δψ on Tim23 dimerization (Figure 7C) (Bauer et al., 1996), excluding the fact that the effect of the sorting sequence on the Δψ-dependence was mediated by Tim23.
b2(▵47–65)-DHFR that strongly depends on Δψ lacks four positively charged residues compared with the wild-type sorting sequence of b2-DHFR with a lower Δψ-dependence, raising the possibility that the membrane potential exerted an electrophoretic effect not only on the matrix-targeting sequence, but also on the sorting sequence. Thus, we constructed a series of b2-fusion proteins in which positively or negatively charged residues of the sorting sequence were replaced by neutral residues. Surprisingly, however, no differences in the responses to the membrane potential were observed, although the difference in net charge of the sorting sequence was up to 6 among different fusion proteins. These results indicate that the sorting sequence of cytochrome b2 influences the requirement for a Δψ in a novel manner that is independent of the net charge of the sorting sequence.
As the deleted segment of b2(▵47–65)-DHFR not only contained charged residues but also an uncharged stretch, we reinserted this segment in a further construct, but the Δψ-dependence did not change substantially. Since neither the charge nor the length of the hydrophobic segment of the sorting sequence seems to be crucial, it is unlikely that the simple physicochemical properties of this segment are critical for the differential Δψ-dependence, raising the possibility that more complex structural properties of the sorting sequence are important. Evidence for this hypothesis was obtained by constructing a b2-fusion protein that showed a lower Δψ-dependence than the wild-type presequence. The only modification was the replacement of an alanine (residue 63) by a proline, thereby breaking a predicted α-helix in the hydrophobic segment of the sorting sequence. This observation is puzzling in view of the typical model that the precursor polypeptide is translocated as an extended chain across both mitochondrial membranes, since this residue then would not even be in contact with the inner membrane. As an extended chain, 50 residues are sufficient to span both mitochondrial membranes (Rassow et al., 1990; Ungermann et al., 1994; Matouschek et al., 1997; Bömer et al., 1998); the first processing step occurs after residue 31, while residues of the mature part (beyond 80) are still on the outside of the outer membrane. Residue 63, therefore, would have to be positioned at the inner side of the outer membrane, making it difficult to explain the profound effect on the Δψ-dependence of the first processing event because the translocation of preproteins across the outer membrane does not require a Δψ (Schatz, 1996; Neupert, 1997; Pfanner et al., 1997). The observation, however, fits well with studies on the mechanism of insertion and sorting of cytochrome b2 at the inner membrane that indicated the formation of a loop in the inner membrane that was formed mainly by the sorting sequence (Gruhler et al., 1995; Gärtner et al., 1995a; Kanamori et al., 1997). It is tempting to speculate that insertion of a helix-breaking residue increases the conformational flexibility of the sorting sequence, thereby facilitating insertion of the sorting sequence into the inner membrane and substituting in part for Δψ as the driving force.
A comparison of these findings to protein export into and across the bacterial plasma membrane reveals both interesting differences and similarities. Several distinct effects of the electrochemical potential were described for bacterial export, including an electrophoretic effect (Driessen and Wickner, 1991; Geller et al., 1993; Andersson and von Heijne, 1994; Cao et al., 1995; Duong et al., 1997; Kiefer et al., 1997; Kiefer and Kuhn, 1999; Schuenemann et al., 1999). Some bacterial preprotein constructs could be transported into the plasma membrane in the absence of any electrochemical gradient, apparently driven by an increase of hydrophobic force (upon removal of charged amino acid residues and the extension of a hydrophobic segment) (Zimmermann et al., 1982; Geller and Wickner, 1985; Lee et al., 1992; Cao et al., 1995; Kiefer and Kuhn, 1999; Schuenemann et al., 1999). In contrast, protein transport at the mitochondrial inner membrane is blocked when the membrane potential is dissipated completely, but, as shown here, it can occur at a low membrane potential when a sequence beyond the matrix-targeting sequence supports transport. Moreover, the model preprotein b2(A63P)-DHFR, which shows the lowest Δψ-dependence of any preprotein transported at the mitochondrial inner membrane, was generated by lowering the hydrophobic moment in the sorting sequence. Interestingly, the insertion of a proline residue directly after the signal peptide of OmpF or OmpA fusion proteins strongly decreased the requirement for a membrane potential during bacterial export (Lu et al., 1991). It was concluded that the conformational flexibility caused by the inserted proline (helix break) facilitated a loop formation under conditions of low proton motive force (Lu et al., 1991). Although mitochondrial import and bacterial export occur in opposite directions across the evolutionary conserved membrane, the proline-effect suggests that in both cases secondary structure properties such as conformational flexibility facilitate membrane insertion and lower the requirement for a membrane potential.
Finally, we found that the cytochrome b2 sorting sequence functioned at a very early import stage since it modulated the efficiency of translocation of the matrix-targeting sequence across the inner membrane under conditions of low Δψ. Since the first processing step (i.e., the removal of the matrix-targeting sequence) represents an early event in the import of cytochrome b2 proteins independently of their final destination (Glick et al., 1992; Koll et al., 1992; Gärtner et al., 1995a; Gruhler et al., 1995; Stuart and Neupert, 1996), this provides further evidence that the influence of the sorting sequence on the Δψ-dependence of translocation of the matrix-targeting sequence is independent of the sorting pathway of the preproteins. In summary, we report the unexpected observation that a preprotein region outside the matrix-targeting sequence strongly influences the dependence of mitochondrial protein import on the membrane potential. This modulatory effect of the sorting sequence is independent of the charge, hydrophobicity, and actual sorting function of the sorting sequence but is related to a conformational flexibility of this segment. We propose that an electrophoretic effect of Δψ on the matrix-targeting sequence is complemented by an additional import-driving activity of the sorting sequence. The sorting sequence thus can modulate the effectiveness of Δψ action.
ACKNOWLEDGMENTS
We thank Drs. Elizabeth Craig and Michiel Meijer for yeast strains and Dr. Wolfgang Voos for helpful discussion. This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 388 Freiburg, and the Fonds der Chemischen Industrie.
REFERENCES
- Alconada A, Gärtner F, Hönlinger A, Kübrich M, Pfanner N. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 1995;260:263–286. doi: 10.1016/0076-6879(95)60144-9. [DOI] [PubMed] [Google Scholar]
- Andersson H, von Heijne G. Membrane protein topology: effects of ▵μH+ on the translocation of charged residues explain the “positive inside” rule. EMBO J. 1994;13:2267–2272. doi: 10.1002/j.1460-2075.1994.tb06508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MF, Sirrenberg C, Neupert W, Brunner M. Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell. 1996;87:33–41. doi: 10.1016/s0092-8674(00)81320-3. [DOI] [PubMed] [Google Scholar]
- Bauer MF, Hofmann S, Neupert W, Brunner M. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10:25–31. doi: 10.1016/s0962-8924(99)01684-0. [DOI] [PubMed] [Google Scholar]
- Beasley EM, Müller S, Schatz G. The signal that sorts yeast cytochrome b2 to the mitochondrial intermembrane space contains three distinct functional regions. EMBO J. 1993;12:2303–2311. doi: 10.1002/j.1460-2075.1993.tb05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bömer U, Meijer M, Guiard B, Dietmeier K, Pfanner N, Rassow J. The sorting route of cytochrome b2 branches from the general mitochondrial import pathway at the preprotein translocase of the inner membrane. J Biol Chem. 1997;272:30439–30446. doi: 10.1074/jbc.272.48.30439. [DOI] [PubMed] [Google Scholar]
- Bömer U, Maarse AC, Martin F, Geissler A, Merlin A, Schönfisch B, Meijer M, Pfanner N, Rassow J. Separation of structural and dynamic functions of the mitochondrial translocase: Tim44 is crucial for the inner membrane import sites in translocation of tightly folded domains, but not of loosely folded preproteins. EMBO J. 1998;17:4226–4237. doi: 10.1093/emboj/17.15.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Kuhn A, Dalbey RE. The translocation of negatively charged residues across the membrane is driven by the electrochemical potential: evidence for an electrophoresis-like membrane transfer mechanism. EMBO J. 1995;14:866–875. doi: 10.1002/j.1460-2075.1995.tb07068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G, Böhni PC, Schatz G. Import of proteins into mitochondria: cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982;257:13028–13033. [PubMed] [Google Scholar]
- Davis AJ, Ryan KR, Jensen RE. Tim23p contains separate and distinct signals for targeting to mitochondria and insertion into the inner membrane. Mol Biol Cell. 1998;9:2577–2593. doi: 10.1091/mbc.9.9.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B. Anionic phospholipids and protein translocation. FEBS Lett. 1994;346:78–82. doi: 10.1016/0014-5793(94)00404-8. [DOI] [PubMed] [Google Scholar]
- Dekker PJT, Keil P, Rassow J, Maarse AC, Pfanner N, Meijer M. Identification of MIM23, a putative component of the protein import machinery of the mitochondrial inner membrane. FEBS Lett. 1993;330:66–70. doi: 10.1016/0014-5793(93)80921-g. [DOI] [PubMed] [Google Scholar]
- Dekker PJ, Martin F, Maarse AC, Bömer U, Müller H, Guiard B, Meijer M, Rassow J, Pfanner N. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 1997;16:5408–5419. doi: 10.1093/emboj/16.17.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen AJ, Wickner W. Proton transfer is rate-limiting for translocation of precursor proteins by the Escherichia coli translocase. Proc Natl Acad Sci USA. 1991;88:2471–2475. doi: 10.1073/pnas.88.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong F, Eichler J, Price A, Leonard MR, Wickner W. Biogenesis of the Gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- Eilers M, Oppliger W, Schatz G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria. EMBO J. 1987;6:1073–1077. doi: 10.1002/j.1460-2075.1987.tb04860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M, Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986;322:228–32. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Gambill BD, Voos W, Kang PJ, Miao B, Langer T, Craig EA, Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993;123:109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F, Bömer U, Guiard B, Pfanner N. The sorting signal of cytochrome b2 promotes early divergence from the general mitochondrial import pathway and restricts the unfoldase activity of matrix Hsp70. EMBO J. 1995a;14:6043–6057. doi: 10.1002/j.1460-2075.1995.tb00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F, Voos W, Querol A, Miller BR, Craig EA, Cumsky MG, Pfanner N. Mitochondrial import of subunit Va of cytochrome c oxidase characterized with yeast mutants. J Biol Chem. 1995b;270:3788–3795. doi: 10.1074/jbc.270.8.3788. [DOI] [PubMed] [Google Scholar]
- Geller B, Zhu HY, Cheng S, Kuhn A, Dalbey RE. Charged residues render pro-OmpA potential dependent for initiation of membrane translocation. J Biol Chem. 1993;268:9442–9447. [PubMed] [Google Scholar]
- Geller BL, Wickner W. M13 procoat inserts into liposomes in the absence of other membrane proteins. J Biol Chem. 1985;260:13281–13285. [PubMed] [Google Scholar]
- Glick BS, Brandt A, Cunningham K, Müller S, Hallberg RL, Schatz G. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell. 1992;69:809–822. doi: 10.1016/0092-8674(92)90292-k. [DOI] [PubMed] [Google Scholar]
- Glick BS, Wachter C, Reid GA, Schatz G. Import of cytochrome b2 to the mitochondrial intermembrane space: the tightly folded heme-binding domain makes import dependent upon matrix ATP. Protein Sci. 1993;2:1901–1917. doi: 10.1002/pro.5560021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhler A, Ono H, Guiard B, Neupert W, Stuart RA. A novel intermediate on the import pathway of cytochrome b2 into mitochondria: evidence for conservative sorting. EMBO J. 1995;14:1349–1359. doi: 10.1002/j.1460-2075.1995.tb07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard B. Structure, expression and regulation of a nuclear gene encoding a mitochondrial protein: the yeast L(+)-lactate cytochrome c oxidoreductase (cytochrome. b2) EMBO J. 1985;4:3265–3272. doi: 10.1002/j.1460-2075.1985.tb04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Ostermann J, Guiard B, Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987;51:1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- Haucke V, Lithgow T, Rospert S, Hahne K, Schatz G. The yeast mitochondrial protein import receptor Mas20p binds precursor proteins through electrostatic interaction with the positively charged presequence. J Biol Chem. 1995;270:5565–5570. doi: 10.1074/jbc.270.10.5565. [DOI] [PubMed] [Google Scholar]
- Hines V, Schatz G. Precursor binding to yeast mitochondria: a general role for the outer membrane protein Mas70p. J Biol Chem. 1993;268:449–454. [PubMed] [Google Scholar]
- Hurt EC, van Loon APGM. How proteins find mitochondria and intramitochondrial compartments. Trends Biochem Sci. 1986;11:204–207. [Google Scholar]
- Isaya G, Kalousek F, Fenton WA, Rosenberg LE. Cleavage of precursors by the mitochondrial processing peptidase requires a compatible mature protein or an intermediate octapeptide. J Cell Biol. 1991;113:65–76. doi: 10.1083/jcb.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RE, Johnson AE. Protein translocation: is Hsp70 pulling my chain? Curr Biol. 1999;9:R779–R782. doi: 10.1016/S0960-9822(00)80012-3. [DOI] [PubMed] [Google Scholar]
- Kalousek F, Neupert W, Omura T, Schatz G, Schmitz UK. Uniform nomenclature for the mitochondrial peptidases cleaving precursors of mitochondrial proteins. Trends Biochem Sci. 1993;18:249. doi: 10.1016/0968-0004(93)90174-l. [DOI] [PubMed] [Google Scholar]
- Kanamori T, Nishikawa S, Shin I, Schultz PG, Endo T. Probing the environment along the protein import pathways in yeast mitochondria by site-specific photocrosslinking. Proc Natl Acad Sci USA. 1997;94:485–490. doi: 10.1073/pnas.94.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PJ, Ostermann J, Shilling J, Neupert W, Craig EA, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990;348:137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kerscher O, Sepuri NB, Jensen RE. Tim18p is a new component of the Tim54p-Tim22p translocon in the mitochondrial inner membrane. Mol Biol Cell. 2000;11:103–116. doi: 10.1091/mbc.11.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer D, Hu X, Dalbey R, Kuhn A. Negatively charged amino acid residues play an active role in orienting the Sec-independent Pf3 coat protein in the Escherichia coli inner membrane. EMBO J. 1997;16:2197–2204. doi: 10.1093/emboj/16.9.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer D, Kuhn A. Hydrophobic forces drive spontaneous membrane insertion of the bacteriophage Pf3 coat protein without topological control. EMBO J. 1999;18:6299–6306. doi: 10.1093/emboj/18.22.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler CM, Merchant S, Schatz G. How membrane proteins travel across the mitochondrial intermembrane space. Trends Biochem Sci. 1999;24:428–432. doi: 10.1016/s0968-0004(99)01462-0. [DOI] [PubMed] [Google Scholar]
- Koll H, Guiard B, Rassow J, Ostermann J, Horwich AL, Neupert W, Hartl FU. Antifolding activity of hsp60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell. 1992;68:1163–1175. doi: 10.1016/0092-8674(92)90086-r. [DOI] [PubMed] [Google Scholar]
- Kurz M, Martin H, Rassow J, Pfanner N, Ryan MT. Biogenesis of Tim proteins of the mitochondrial carrier import pathway: differential targeting mechanisms and crossing over with the main import pathway. Mol Biol Cell. 1999;10:2461–2474. doi: 10.1091/mbc.10.7.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Kuhn A, Dalbey RE. Distinct domains of an oligotopic membrane protein are Sec-dependent and Sec-independent for membrane insertion. J Biol Chem. 1992;267:938–943. [PubMed] [Google Scholar]
- Lu HM, Yamada H, Mizushima S. A proline residue near the amino terminus of the mature domain of secretory proteins lowers the level of the proton motive force required for translocation. J Biol Chem. 1991;266:9977–9982. [PubMed] [Google Scholar]
- Martin J, Mahlke K, Pfanner N. Role of an energized inner membrane in mitochondrial protein import: Δψ drives the movement of presequences. J Biol Chem. 1991;266:18051–18057. [PubMed] [Google Scholar]
- Matouschek A, Azem A, Ratliff K, Glick BS, Schmid K, Schatz G. Active unfolding of precursor proteins during mitochondrial protein import. EMBO J. 1997;16:6727–6736. doi: 10.1093/emboj/16.22.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin A, von Ahsen O, Craig EA, Dietmeier K, Pfanner N. A mutant form of mitochondrial GrpE suppresses the sorting defect caused by an alteration in the presequence of cytochrome b2. J Mol Biol. 1997;273:1–6. doi: 10.1006/jmbi.1997.1300. [DOI] [PubMed] [Google Scholar]
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- Nicholls GN, Ferguson SJ. Bioenergetics. London, UK: Academic Press; 1982. [Google Scholar]
- Ostermann J, Voos W, Kang PJ, Craig EA, Neupert W, Pfanner N. Precursor proteins in transit through mitochondrial contact sites interact with hsp70 in the matrix. FEBS Lett. 1990;277:281–284. doi: 10.1016/0014-5793(90)80865-g. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 1985;4:2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N, Neupert W. Transport of F1-ATPase subunit β into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986;209:152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Craig EA, Hönlinger A. Mitochondrial preprotein translocase. Annu Rev Cell Dev Biol. 1997;13:25–51. doi: 10.1146/annurev.cellbio.13.1.25. [DOI] [PubMed] [Google Scholar]
- Pratje E, Guiard B. One nuclear gene controls the removal of transient pre-sequences from two yeast proteins: one encoded by the nuclear the other by the mitochondrial genome. EMBO J. 1986;5:1313–1317. doi: 10.1002/j.1460-2075.1986.tb04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J, Guiard B, Wienhues U, Herzog V, Hartl FU, Neupert W. Translocation arrest by reversible folding of a precursor protein imported into mitochondria: a means to quantitate translocation contact sites. J Cell Biol. 1989;109:1421–1428. doi: 10.1083/jcb.109.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J, Hartl FU, Guiard B, Pfanner N, Neupert W. Polypeptides traverse the mitochondrial envelope in an extended state. FEBS Lett. 1990;275:190–194. doi: 10.1016/0014-5793(90)81469-5. [DOI] [PubMed] [Google Scholar]
- Roise D. Interaction of a synthetic mitochondrial presequence with isolated yeast mitochondria: mechanism of binding and kinetics of import. Proc Natl Acad Sci USA. 1992;89:608–612. doi: 10.1073/pnas.89.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D, Schatz G. Mitochondrial presequences. J Biol Chem. 1988;263:4509–4511. [PubMed] [Google Scholar]
- Ryan KR, Jensen RE. Protein translocation across mitochondrial membranes: what a long, strange trip it is. Cell. 1995;83:517–519. doi: 10.1016/0092-8674(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Ryan MT, Müller H, Pfanner N. Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane. J Biol Chem. 1999;274:20619–20627. doi: 10.1074/jbc.274.29.20619. [DOI] [PubMed] [Google Scholar]
- Schatz G. The protein import system of mitochondria. J Biol Chem. 1996;271:31763–31766. doi: 10.1074/jbc.271.50.31763. [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Krieg UC, Hwang ST, Vestweber D, Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990;9:4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M, Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985;43:339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Schleyer M, Schmidt B, Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982;125:109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Schneider A, Behrens M, Scherer P, Pratje E, Michaelis G, Schatz G. Inner membrane protease I, an enzyme mediating intramitochondrial protein sorting in yeast. EMBO J. 1991;10:247–254. doi: 10.1002/j.1460-2075.1991.tb07944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuenemann TA, Delgado-Nixon VM, Dalbey RE. Direct evidence that the proton motive force inhibits membrane translocation of positively charged residues within membrane proteins. J Biol Chem. 1999;274:6855–6864. doi: 10.1074/jbc.274.11.6855. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Seytter T, Guiard B, Neupert W. Targeting of cytochrome b2 into the mitochondrial intermembrane space: specific recognition of the sorting signal. EMBO J. 1993;12:2295–2302. doi: 10.1002/j.1460-2075.1993.tb05883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims PJ, Waggoner AS, Wang CH, Hoffman JF. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Söllner T, Rassow J, Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Stuart RA, Gruhler A, van der Klei I, Guiard B, Koll H, Neupert W. The requirement of matrix ATP for the import of precursor proteins into the mitochondrial matrix and intermembrane space. Eur J Biochem. 1994;220:9–18. doi: 10.1111/j.1432-1033.1994.tb18593.x. [DOI] [PubMed] [Google Scholar]
- Stuart RA, Neupert W. Topogenesis of inner membrane proteins of mitochondria. Trends Biochem Sci. 1996;21:261–267. [PubMed] [Google Scholar]
- Truscott KN, Pfanner N. Import of carrier proteins into mitochondria. Biol Chem. 1999;380:1151–1156. doi: 10.1515/BC.1999.146. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Neupert W, Cyr DM. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994;266:1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- Voisine C, Craig EA, Zufall N, von Ahsen O, Pfanner N, Voos W. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 1999;97:565–574. doi: 10.1016/s0092-8674(00)80768-0. [DOI] [PubMed] [Google Scholar]
- Voos W, Gambill BD, Guiard B, Pfanner N, Craig EA. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J Cell Biol. 1993;123:119–126. doi: 10.1083/jcb.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W, von Ahsen O, Müller H, Guiard B, Rassow J, Pfanner N. Differential requirement for the mitochondrial Hsp70-Tim44 complex in unfolding and translocation of preproteins. EMBO J. 1996;15:2668–2677. [PMC free article] [PubMed] [Google Scholar]
- Voos W, Martin H, Krimmer T, Pfanner N. Mechanisms of protein translocation into mitochondria. Biochim Biophys Acta. 1999;1422:235–254. doi: 10.1016/s0304-4157(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Watts C, Wickner W. The biosynthesis of membrane-bound M13 coat protein: energetics and assembly intermediates. J Biol Chem. 1982;257:6529–6536. [PubMed] [Google Scholar]