Abstract
Background
Although IL-4 and IL-13 share the IL-13 receptor, IL-13 exhibits unique functions. To elicit the cellular basis of these differences, signal transduction processes have been compared. Additionally, the role of the IL-4 receptor alpha (IL-4Rα) variant Q551R was investigated.
Methods
Peripheral blood mononuclear cells from donors were stimulated with IL-4 and IL-13. The phosphorylation status of effector substrates was detected by immunostaining. Binding of SHP-2 to IL-4Rα was investigated by using synthetic peptides.
Results
SHP-2 bound IL-4Rα synthetic peptide; this binding was reduced in the presence of the R551 variant. Stimulation with IL-4 increased SHP-1 phosphorylation, however, stimulation with IL-13 increased SHP-2 phosphorylation. PI3-kinase phosphorylation was elevated following stimulation with IL-13 in all individuals and with IL-4 only in R551 individuals. Jak1, Tyk2 and IRS-2 signals were reduced after IL-13 stimulation in Q551 individuals. STAT3 phosphorylation was markedly increased in R551 individuals, following stimulation with both IL-4 and IL-13. However, STAT3 was only detected immediately in nuclear extracts from variant individuals after stimulation with IL-13; in wildtype individuals STAT3 was only detected after IL-4 treatment.
Conclusion
IL-4 and IL-13 appear to promote distinct signal transduction cascades. SHP-1 seems to be predominately activated by IL-4 and to influence the PI3-kinase, in contrast, SHP-2 seems to be predominately activated by IL-13 and to influence Jak1, Tyk2 and IRS-2. Both phosphatases control STAT3. In the presence of the variant R551, SHP-1/2 activation is reduced and signal transduction is altered. STAT3 signaling appears be further regulated on the level of nuclear translocation.
Keywords: asthma, IL-4, IL-13, SHP, STAT3
Introduction
Asthma and atopy represent a group of complex diseases with a broad variety of clinical phenotypes. The individual risk of developing atopic diseases seems to be influenced by genetic susceptibility and environmental factors. During the past decade, a great number of studies have tried to investigate the genetic basis of atopy. Functional studies of genetic variants contributing to the susceptibility of asthma and atopy become immensely important in an effort to understand the complex immunological processes underlying the development of these diseases. Two important regulators of the human immune system are the pleiotropic cytokines IL-4 and IL-13. They play major roles in stimulating B-cell proliferation and in influencing B-cell differentiation towards IgE production. In addition, IL-4 shifts the Th1/Th2 balance of activated Th cells towards Th2 cells [1-3]. The effect of IL-13 on human T cells, if any, is still unknown. Among others roles, these two cytokines play important roles in the development of inflammatory diseases [4]. B-cell activation and Th2 type immune responses underlie atopic disorders. Animal models have revealed that IL-13 induces the pathophysiological features of asthma independently of IL-4, but is strongly dependent on IL-4Rα [5,6], whereas IL-4 initiates a more general inflammatory response. Furthermore, IL-13 null mice fail to clear helminthic infections, fail to generate goblet cells responsible for mucus overproduction in asthmatics, and fail to recover basic IgE levels even after stimulation with IL-4 [7]. Thus it seems likely, that IL-4 and IL-13 play distinct roles at the cellular level, e.g. in signal transduction.
IL-4 and IL-13 share a functional receptor, named the IL-13 receptor. It is composed of a 140 kDa high affinity binding chain (IL-4Rα) plus the IL-13Rα1 chain (60–70 kDa) [8]. Both chains are members of the hematopoietin receptor superfamily [9]. Association studies with common polymorphisms in the coding part of the human (hu) IL-4Rα gene suggest the involvement of this gene in atopy, systemic lupus erythematosus and transplant rejection [10-14]. Functional studies have revealed that amino acid variants of the huIL-4Rα protein, I50V (in Japanese individuals), S478P and Q551R = Q576R (where Q551R is the mature protein) (in Caucasians), strongly influence the structure and consequently the substrate binding and signaling processes of this chain [12,15,16].
IL-4 and IL-13 promote activation of a number of cell substrates such as kinases of the Janus type (e.g Jak1), insulin receptor-like substrates (IRS-1/2), Phosphatidylinositol 3-kinase (PI3-kinase) and the transcription factor STAT6, the latter being a unique substrate for the IL-4Rα pathway [17]. After binding of IL-4 and IL-13, activation of the receptor-associated kinases (Jak) takes place, followed by the direct activation of IRS-1/-2 (acting as an interface between signaling proteins with Src homology-2 domains [SH2 proteins]) and STAT6 (an IL-4-specific transcription factor) and further signal transduction cascades (e.g. IRS-1/2 initiates PI3-kinase). PI3-kinase is a lipid kinase that phosphorylates the inositol ring of phosphatidylinositol and related compounds at the 3-prime position. The products of these reactions are thought to serve as second messengers (e.g. in growth signaling pathways).
In addition, both IL-4 and IL-13 initiate signal transduction cascades through further effector substrates of the IL-13Rα1 chain like the kinase Tyk2 and STAT3 [18], which are activated via phosphorylation. Activated STAT3 molecules dimerize, translocate to the nucleus and finally serve as transcription factors for various genes (e.g. IRF-1, junB or glycoprotein 130) [19,20]. Activation of effector substrates and translocation in the case of STAT are negatively controlled by phosphatases (SHPs, SHIP), which generally regulate growth and functional responses of hematopoietic cells through tyrosine phosphorylation of proteins [21]. Regulation is further accomplished by specific inhibitors such as SSI-1/SOCS-1 or PIAS3 [22,23].
The fact that some of the defects in IL-13 null mice cannot be overcome even by high IL-4 concentrations [6] points towards similar yet distinct signal transduction cascades. The aim of this study was to prove this hypothesis. Additionally, the role of the functional IL-4Rα variant Q551R has been studied.
Materials and methods
Typing of IL-4Rα polymorphisms
Typing of polymorphisms was performed as described previously [16,24]. Mainly, DNA was extracted from peripheral blood leukocytes following standard protocols and column purified (DNA midi kit; Qiagen, Germany). To amplify the target DNA in the polymorphic regions prior to RFLP analysis, the following oligonucleotide primer pairs, incorporating restriction endonuclease sites, were used (the respective restriction enzyme sites are in brackets): I50V = 5'-GCCTCCGTTGTTCTCAGGTA-3' and 5'-TCTGTCCTCGCATCCGTGAT-3' (BstZ17 I); E375A = 5'-TTAGCCGGGCCACAAAGGCC-3' and 5'-TGGAGATCAGCAAGACAGTC-3' (StuI); S478P = 5'-CTTACCGCAGCTTCAGGTAC-3' and 5'-TTTCTGGCTCAGGTTGGGGC-3' (KpnI); Q551R = 5'-GGCCCCCACCAGTGGCGATC-3' and 5'-GCAAGCAGGCTTGAGAAGGC-3' (PvuI). PCR was carried out in a volume of 10 μl containing 30 ng DNA, 5 pmol of each primer, 0.06 U Taq polymerase (Pharmacia, Uppsala, Sweden); and a 2 mmol dNTP mix. Annealing temperatures were 60°C for I50V, 64°C for E375A, 59°C for S478P and 60°C for Q551R. Restriction digestion was performed in a volume of 10 μl containing 5 μl of the PCR product and the buffer recommended by the supplier for 90 min at 37°C. The fragments were resolved on 10% or 12% polyacrylamide gels. In each reaction individuals with known genotypes were included as positive and negative controls. The genotyping was performed by two investigators unaware of the phenotypes.
Cell culture and stimulation
Peripheral blood mononuclear cells (PBMCs) derived from two different groups of probands (homozygous Q551 = wildtype and homozygous R551 = variant) were cultured for 48 h at 37°C with 5% CO2 in RPMI-1640 medium containing 2 mM L-glutamine, 20 mM HEPES, 100 U/ml penicillin, 50 mg/ml streptomycin and 10% fetal calf serum (FCS) (PAN Systems GmbH, Aidenbach, Germany). Samples from at least two different individuals in each group were investigated in all the following experiments.
Cells were made quiescent for 5–16 h in RPMI-1640/glutamine/HEPES/penicillin/streptomycin and 1% FCS. After that all cultured samples were pooled (separately for each proband) and then divided into equal amounts prior to stimulation. Cells (1–2 × 107) were stimulated with 100 nM human IL-4 (PAN Systems GmbH) or IL-13 (Sigma, Deisenhofen, Germany) for 5–15 min at 37°C. Cell pellets were then stored at -80°C. 293 fibroblasts were cultured for 48 h in Dulbeccos'MEM/Glutamax-1/sodiumpyruvate/4,500 mg sodium pyruvate/l-glucose/pyridoxine/penicillin/streptomycin and 10% FCS. Cells were then stimulated with IL-4 and IL-13.
Immunoprecipitation and immunoblot
After thawing, cells were suspended in lysis buffer (10 mM Tris [pH 7.8], 5 mM EDTA, 50 mM NaCl, 30 mM pyrophosphate, 50 mM sodium fluoride, 20 μM sodium orthovanadate, 1%Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 1 μg/ml pepstatin A and 10 μg/ml leupeptin; 108cells/ml buffer) and incubated for 60 min at 4°C. Insoluble material was removed by centrifugation and equal amounts of cell lysates (BioRad protein assay, BioRad Laboratories, Munich, Germany) were incubated with 1 μg/ml of the appropriate antibodies (PI3-kinase and p85α [BIOMOL, Hamburg, Germany]; Tyk2 and IRS-2 [Santa Cruz Biotechnology, Heidelberg, Germany]; STAT3, SHP-1 and SHP-2 [Transduction Laboratories, Lexington, USA]; and Jak1 [Upstate Biotechnology, Lake Placid, USA]) for 16 h at 4°C. Antibodies were precipitated with protein A sepharose beads (CL-4B; Pharmacia, Germany). After four washes with lysis buffer, proteins were analyzed on SDS-PAGE and transferred onto polyvinylidene difluoride filters (Millipore, Bedford, UK). The residual binding sites on the filters were blocked with TPBS (150 mM sodium chloride, 3 mM potassium chloride, 1 mM potassium dihydrogen phosphate, 7 mM disodium hydrogen phosphate, 0.05% Tween 20) and 5% non-fat dried milk overnight. The filters were incubated with anti-phosphotyrosine antibodies (Santa Cruz Biotechnology), the appropriate horseradish-peroxidase (HRP)-coupled secondary antibodies (DAKO GmbH, Germany) and developed using a chemiluminescence kit (ECL; Amersham, Germany). Filters were stripped, washed in TPBS and immunostained with the respective antibody used for precipitation to control for protein concentrations.
Preparation of nuclear extracts
Nuclear extracts were prepared as described for electrophoretic mobility shift assays (EMSA) [25]. Immunoprecipitation and immunoblots were performed as described above. STAT3 in the extract was detected with anti-tyrosine (Santa Cruz) or anti-STAT3 (Transduction Laboratories) antibodies. STAT6 was detected with anti-STAT6 antibody (Dianova). Experiments were repeated several times using at least two different individuals in each group. For concentration curves, 0, 5, 10, 50 and 100 nM of IL-4 or IL-13 were used for stimulation. In time-course experiments cells were stimulated with 100 nM IL-4 or IL-13 for 0, 1, 5, 15 or 20 min. To test for purity of the extracts, immunoprecipitation was performed with anti-Tyk2 antibody. Tyk2 was not detectable in all cases (data not shown in Fig. 4C, 4D).
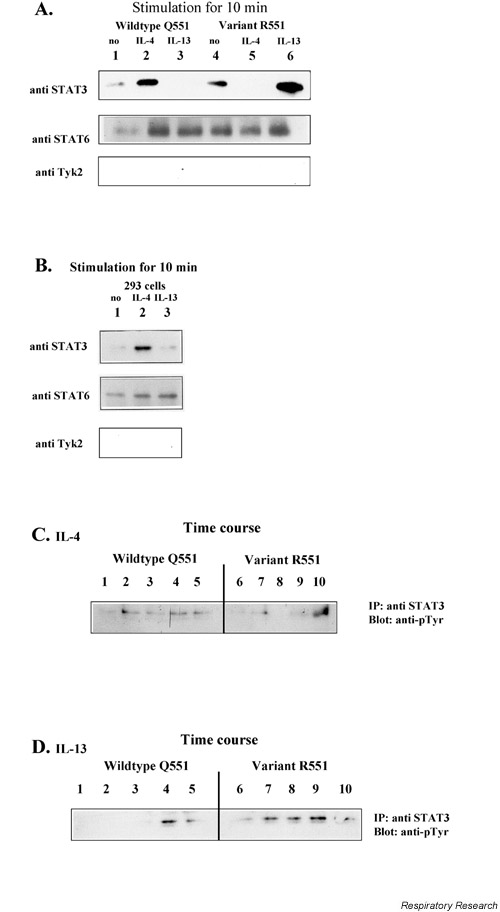
Figure 4.
STAT3 and STAT6 in nuclear extracts. PBMC (1–2 × 107) from different probands (wildtype Q551 or variant R551) were stimulated with IL-4 or IL-13. Nuclear extracts were obtained according to the EMSA protocol. Immunoprecipitation was performed using anti-STAT3, followed by western blotting and immunostaining with either p-Tyr or ant-STAT3. Staining with Tyk2 served as a control. In (A) and (B) cells were stimulated for 10 min. In (C) and (D) a time course has been performed. (A). PBMCs 1= WT unstimulated.; 2= WT/ IL-4, 3= WT/ IL-13; 4= R551 unstimulated.; 5= R551/ IL-4; 6= R551/ IL-13; (B). 293 cells 1= unstimulated.; 2= IL-4; 3= IL-13; (C). Time-course experiment IL-4. 1= WT 0 min, 2= WT 1 min, 3= WT 5 min, 4= WT 15 min, 5= WT 20 min, 6= R551 0 min, 7= R551 1 min, 8= R551 5 min, 9= R551 15 min, 10= R551 20 min; (D). Time-course experiment IL-13. 1= WT 0 min, 2= WT 1 min, 3= WT 5 min, 4= WT 15 min, 5= WT 20 min, 6= R551 0 min, 7= R551 1 min, 8= R551 5 min, 9= R551 15 min, 10= R551 20 min. PBMC = Peripheral blood mononuclear cell; WT = wildtype.
Immunofluorescence
PBMCs were grown and stimulated as described above, and then transferred to culture chamber slides (Falcon; Becton Dickinson, Franklin Lakes, USA) and spun down at 800 × g (Megafuge 3.0R; Heraeus Instruments, Hanau, Germany). This procedure was repeated after each incubation step. Cells were stained as described for the immunoblots (see above). A FITC-conjugated goat anti-rabbit antibody (DAKO) was used as a secondary antibody. The B cells were examined under a Zeiss Axioplan2 microscope (C Zeiss GmbH, Jena, Germany).
In vitro binding assays
This assay was performed as previously described [12,16]. The following synthetic peptides, corresponding to the amino acids 545–558 of the mature IL-4Rα, were used: wildtype (Q551) phosphorylated Y550 (NH2-SAPTSG(PY)QEFVHAVE-COOH) and mutant (R551) phosphorylated Y550 (NH2-SAPTSG(PY)REFVHAVE-COOH) (INTERACTIVA Biotechnology GmbH, Ulm, Germany). Amino acids 726–784 of IL-4Rα, expressed in Escherichia coli, were available as a control peptide (the corresponding DNA was amplified by PCR at 55°C using primers 5'-GGGGGGATCCAGGTCCTCGCCCCCTACAAC-3' and 5'-GGGGGGATCCGGGGGTCTGGCTTGAGCTCT-3', cloned into pQE-30 [Qiagen, Hilden, Germany] and in E. coli BL21pLysS, and affinity purified by Ni-NTA agarose [Qiagen] according to standard protocols). Further control peptides from the amino acids of the I4R-motif of the IL4Rα : wildtype unphosphorylated Y497 (NH2-LVIAGNPAYRSFSNSLSQSP-COOH), wildtype phosphorylated Y497 (NH2-LVIAGNPA(pY)RSFSN SLSQSP-COOH) and mutant phosphorylated Y497 (NH2-LVIAGNPA(pY)RSFSN PLSQSP-COOH) were also used. The peptides were coupled to Affigel 10 beads (BioRad Laboratories, München, Germany) at a ratio of 3 mg peptide per ml of beads. Afterwards, sufficient binding was confirmed by testing for proteins in the supernatant (BioRad protein assay).
To assess the binding of cellular proteins to the peptides, 20 μl of peptide-conjugated beads were incubated with lysates from IL-13-activated cells (3 × 107 cells). The peptide-associated SHP-2 were analyzed by immunoblotting with specific antibodies (monoclonal anti- SHP-2; Transduction Laboratories) and developed using a chemiluminescence kit (ECL; Amersham, Germany).
Results
First, all blood donors were typed for the common IL-4Rα variants I50V, E375A, S478P and Q551R [15,16]. Those individuals bearing the intracellular R551 variant of the IL-4α (homozygous R551 = variant) and no other intracellular variant, and those bearing no intracellular variant at all (homozygous Q551 = wildtype) were selected for the experiments. All probands showed the extracellular I50V variant. Two or three probands in each group were examined and all experiments were repeated at least twice. PBMCs were stimulated either with IL-4 or IL-13. Unstimulated cells served as controls. Effector substrates of the IL-13 receptor were investigated in cytoplasmatic extracts, and in the case of STAT3 also in nuclear extracts. SHP-2 binding was assayed using synthetic peptides of IL-4Rα. Examples of the experiments are shown in all figures. There was no obvious variation between the different groups of probands.
SHP-2 binding to synthetic peptides
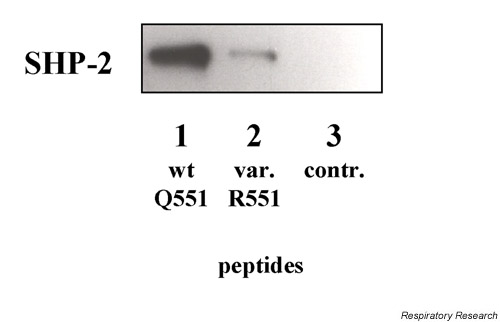
In vitro experiments using synthetic peptides revealed strong binding of SHP-2 to the IL-4Rα in the region of the amino acids 445–558. However, reduced binding was seen in the presence of the R551 variant. No binding was seen with the control peptides (Fig. 1 and data not shown).
Figure 1.
In vitro binding assay using synthetic IL-4Rα peptides (amino acids 545–558 = Q551/R551; control amino acids = 726–784). To assess the binding of cellular proteins to the peptides, 20 μl of peptide-conjugated Affigel beads were incubated with lysates from IL-13-activated cells (3 × 107 cells). The peptide-associated SHP-2 were analyzed by immunoblotting with specific antibodies (monoclonal anti SHP-2; Transduction Laboratories) and developed using a chemiluminescence kit (ECL; Amersham, Germany). Cells were (1): wildtype Q551; (2): variant R551; (3): control. SHP =SH2 containing phosphatase.
SHP-1/2
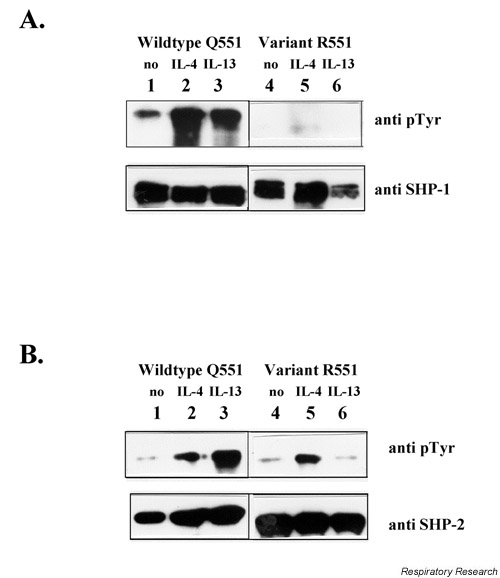
Investigating cytoplasmatic extracts after IL-4 or IL-13 stimulation, SHP-1 phosphorylation was generally reduced in individuals bearing the variant R551 compared to wildtype Q551 individuals. Furthermore, in wildtype individuals SHP-1 phosphorylation was markedly increased after stimulation with IL-4. IL-13 also induced SHP-1 phosphorylation to a slightly lesser extent (Fig. 2A).
Figure 2.
Phosphorylation of SHP-1 and SHP-2. PBMCs (1–2 × 107) from different probands (wildtype Q551 or variant R551) were stimulated with IL-4 or IL-13 for 10 min. Cells were suspended in lysis buffer [10 mM Tris (pH 7.8), 5 mM EDTA, 50 mM NaCl, 30 mM pyrophosphate, 50 mM sodium fluoride, 20 μM sodium orthovanadate, 1%Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 1 μg/ml pepstatin A and 10 μg/ml leupeptin; 108cells/ml buffer] and incubated for 60 mins at 4°C. Insoluble material was removed by centrifugation and equal amounts of cell lysates (BioRad protein assay, BioRad Laboratories) were incubated with 1 μg/ml of the appropriate antibodies, anti-SHP1 or anti-SHP-2, followed by western blotting and immunostaining with either p-Tyr or the respective control antibody. (A). SHP-1, 3= WT/IL-13; 2= WT/IL-4; 1= WT unstimulated. (stain 2 min); 6= R551/ IL-13; 5= R551/ IL-4; 4= R551 unstimulated. (stain 5 min); (B). SHP-2, 3= WT/ IL-13; 2= WT/ IL-4; 1= WT unstimulated; 6= R551/ IL-13; 5= R551/ IL-4; 4= R551 unstimulated. PBMC = Peripheral blood mononuclear cell; SHP =SH2 containing phosphatase; WT = wildtype.
SHP-2 phosphorylation was generally reduced in individuals bearing the variant R551 compared to the wildtype Q551, where SHP-2 phosphorylation was markedly increased after stimulation with IL-13. IL-4 also induced SHP-2 phosphorylation in the variant (Fig. 2B).
PI3-Kinase, Jak1, Tyk2 and IRS-2
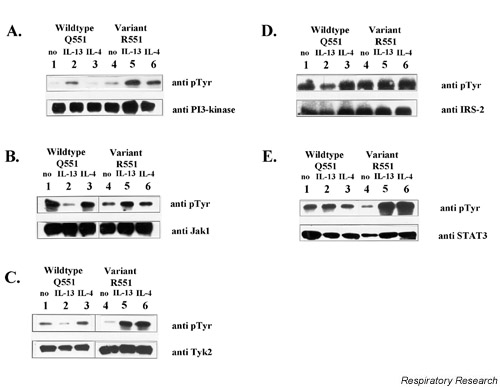
In cytoplasmic extracts, phosphorylation of the p85α subunit of PI3-kinase was markedly increased after stimulation with IL-13 in both groups of probands. After IL-4 stimulation, enhanced phosphorylation was seen in the presence of the R551 variant, but not in wildtype Q551 individuals and unstimulated controls (Fig. 3A).
Figure 3.
Effector substrates. Phosphorylation of PI3-kinase, Jak-1, Tyk2, IRS-2 and-STAT3. PBMCs (1–2 × 107) from different probands (wildtype Q551 or variant R551) were stimulated with IL-4 or IL-13 for 10 mins. Cells were suspended in lysis buffer [10 mM Tris (pH 7.8), 5 mM EDTA, 50 mM NaCl, 30 mM pyrophosphate, 50 mM sodium fluoride, 20 μM sodium orthovanadate, 1%Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 1 μg/ml pepstatin A and 10 μg/ml leupeptin; 108cells/ml buffer] and incubated for 60 mins at 4°C. Insoluble material was removed by centrifugation and equal amounts of cell lysates (BioRad protein assay, BioRad Laboratories) were incubated with 1 μg/ml of the appropriate antibodies. Immunoprecipitation was performed using anti-PI3-kinase (p85α), anti-Jak-1, anti-Tyk2, anti IRS-2 or anti-STAT3, followed by western blotting and immunostaining with either p-Tyr or the respective control antibody. (A). PI3-kinase (p85α), 6= R551/ IL-4; 5= R551/ IL-13; 4= R551 unstimulated.; 3= WT and IL-13; 2= WT and IL-4; 1= WT unstimulated. (B). Jak-1, 6= R551/ IL-4; 5= R551/ IL-13; 4= R551 unstimulated.; 3= WT/ IL-4; 2= WT/ IL-13; 1= WT unstimulated. (C). Tyk2, 6= R551/ IL-4; 5= R551/ IL-13; 4= R551 unstimulated.; 3= WT/ IL-4; 2= WT/ IL-13; 1= WT unstimulated. (D). IRS-2, 6= R551/ IL-4; 5= R551/ IL-13; 4= R551 unstimulated.; 3= WT/ IL-4; 2= WT/ IL-13; 1= WT unstimulated. (E). STAT3, 6= R551/ IL-4; 5= R551/ IL-13; 4= R551 unstimulated.; 3= WT/ IL-4; 2= WT/ IL-13; 1= WT unstimulated. JAK =Janus kinase; PBMC = Peripheral blood mononuclear cell; SHP =SH2 containing phosphatase; STAT =signal transducer and activator of transcription;WT = wildtype.
In the case of Jak1, elevated phosphorylation was seen in individuals bearing the variant R551 after stimulating with IL-13, compared to the wildtype Q551 (Fig. 3B).
Tyk2 phosphorylation appeared to be down-regulated after stimulation with IL-13 in wildtype cells, whereas no effect was seen for the R551 variant. Indeed, in variant cells both IL-4 and IL-13 seemed to activate Tyk 2 to a great extent (Fig. 3C). The same situation described for Tyk2 – that is, down-regulation after IL13 stimulation in case of the wildtype situation – applies to IRS-2 (Fig. 3D).
STAT3
In cytoplasmic extracts, STAT3 phosphorylation was markedly increased after stimulation with IL-4 as well as IL-13 in individuals bearing the R551 variant compared to wildtype individuals and unstimulated controls (Fig. 3E).
STAT in nuclear extracts
In nuclear extracts from R551 positive individuals, STAT3 was predominately found when stimulating with IL-13 for 10 min, while almost no STAT3 was detectable when stimulating with IL-4. Interestingly, the opposite was seen in Q551 homozygeous individuals. STAT3 was only found after stimulation with IL-4 and almost no STAT3 proteins were detectable after stimulation with IL-13 for 10 min (Fig. 4A). Exactly the same results were achieved when staining with anti-STAT3 or anti-phosphotyrosine antibodies (results not shown).
The results were further confirmed by a direct immunofluorescence-staining with anti-STAT3 antibodies in stimulated and unstimulated B cells (data not shown). The 293 fibroblasts (lacking the common γ chain) showed the same pattern as wildtype individuals (Fig. 4B). Typing these cells for the IL-4Rα polymorphisms indeed revealed the Q551 situation.
As a control STAT6 was analyzed in all nuclear extracts, and no differences could be detected 10 mins after IL-4 or IL-13 stimulation.
Different concentrations of IL-4 and IL-13 did not have an detectable effect on the phosphorylation status in the experiments (data not shown). However, looking at different time points revealed that the response is delayed in the respective case. That means, for example, in the case of IL-4 stimulation, STAT3 appears after 15–20 min in nuclei of variant, whereas STAT3 appears immediately in nuclei of wildtype individuals. In the case of IL-13 stimulation, STAT3 appears immediately in nuclei of variants, whereas its appearance is delayed in nuclei of wildtype individuals (Fig. 4C, 4D).
Discussion
Our knowledge concerning the importance of IL-4 and IL-13 in the context of IgE regulation, as well as in the development of inflammatory and atopic diseases, increases. However, it is not yet known if these cytokines play distinct roles in signal transduction processes. Only mouse models suggest these unique roles of both cytokines, especially in the context of asthma.
IL-4 and IL-13 share the IL-13 receptor (IL-4Rα and IL-13Rα1), which is prominent on human B cells. However, in this study PBMCs were chosen for investigations, as the role of IL-13 on other cells (T cells, eosinophils etc.) is not completely understood. By using a mixture of cells, stimulation with IL-4 would, of course, also lead to activation via the functional IL-4 receptor (IL-4Rα and the common γ chain). We chose this model system to reflect the in vivo and complex medically relevant situation as best as possible. Therefore, we used freshly isolated PBMCs and cultured them for only 2 days. Impairments due to different cell and receptor numbers were limited by pooling the cultured samples before stimulation experiments (for further details see Materials and methods) and by comparing phosphorylation statuses only in samples within each proband.
Previous studies have shown that polymorphisms in the gene encoding the IL-4Rα chain exhibit strong influences on the structure and signal transduction through this receptor chain [12,15,16].
Hershey et al. showed impaired binding of the phosphatase SHP-1 in the presence of the R551 variant of the IL-4Rα chain. Possible influences on the transcription factor STAT6 and elevated CD23 expression were discussed [12]. However, another study could not repeat these results [26] and a further study revealed even slightly reduced phosphorylation of STAT6, following IL-4 stimulation of PBMCs derived from individuals bearing the R551 variant [16]. Moreover, the variant was associated with lowered total serum IgE levels [16], in direct contrast to Hershey's findings. Nevertheless, the impaired SHP-1 binding might well influence intracellular substrates other than STAT6, which could explain the controversy.
In order to study possible the influence of the Q551R variant on signal transduction processes, and here especially on the IL-13 receptor, two groups of probands were selected: exclusively homozygous wildtype Q551, or variant R551 in the intracellular part of the IL-4Rα chain. Influences from the other intracellular variants such as E375A and S478P on signal transduction were excluded for clarity. Their effect will, of course, need to be considered in the future, especially because S478P and Q551R are in strong linkage disequilibrium [16]. Due to the high allelic frequency of the variant V50, all probands in this study bore the extracellular variant I50V. The frequency of individuals showing only the R551 variant and no other polymorphism in IL-4Rα is less than 1% in the German population (unpublished data).
As the transcription factor STAT3 is an effector substrate of the IL-13 receptor α1 chain, our initial interest was to test whether the variant R551 would influence STAT3 signaling through an impaired SHP binding and activation capacity (in accordance with the work of Hershey et al.[12]).
SHP-1/2
STAT3 signaling is controlled by the phosphatase SHP-2 [27]. SHP-2 possesses a structure very similar to SHP-1 [21], which has previously been shown to bind to the Y550 region of IL-4Rα [12]. Therefore, this region was also considered a potential docking site for SHP-2. By using synthetic peptides it was indeed confirmed that SHP-2 binds to the Y550 region of IL-4Rα. Furthermore, binding to the variant R551 (Fig. 1) was impaired as expected [12]. Impaired SHP-1 binding with R551 could be repeated [data not shown].
Interestingly, SHP-1 and SHP-2 seem to be activated differentially by IL-4 and IL-13. In wildtype individuals SHP-1 phosphorylation is predominately induced by IL-4, while SHP-2 phosphorylation is predominately induced by IL-13. As expected, this phosphorylation was in each case markedly reduced in individuals bearing the variant R551 (Fig. 2A, 2B). The phosphorylation of SHP-2 was also slightly increased in the presence of the variant R551 after stimulation, for example, with IL-4, which was comparable to the wildtype (Fig. 2B). This observation hints at docking sites for SHP-2 on the IL-4Rα protein other than Y550, as has been described earlier [28]. We might even have seen a multiplied effect due to the presence of the conventional IL-4 receptor as we worked with cell mixtures (PBMCs, see above).
In conclusion, depending on the stimulating agent (IL-4 or IL-13) SHP-1 and SHP-2 evidently regulate different effector substrates and therefore activate distinct signal transduction cascades.
The two phosphatases have a wide variety of intracellular substrates [29-32]. We went on to test several substrates of the IL-13 receptor for phosphorylation (activation status) after IL-4 or IL-13 treatment.
PI3-kinase, Jak1, Tyk-2 and IRS-2
Stimulation with IL-4 reduced phosphorylation of PI3-kinase compared to stimulation with IL-13 (Fig. 3A). Imani et al. also found decreased PI3-kinase phosphorylation after IL-4 treatment and proposed that SHP-1 mediates this effect [30]. IL-13 does not seem to induce SHP-1 and, as expected, markedly increased phosphorylation of PI3-kinase was seen in both wildtype and variant cells (Fig. 3A). Possibly due to the impaired SHP-1 activation, IL-4 stimulation slightly increased PI3-kinase phosphorylation in the variant compared to the wildtype situation.
Jak1 phosphorylation was markedly reduced in wildtype individuals after IL-13 as compared with IL-4 stimulation (Fig. 3B). This would imply that SHP-2 is responsible for this effect, as it is specifically activated by IL-13 (stated above). Jak1 has indeed been shown to interact with SHP-2 [33]. Impaired binding of SHP-2 in the case of the variant R551 consequently leads to increased phosphorylation of Jak1 (Fig. 3B). As this increase in phosphorylation is also higher than after stimulation with IL-4 in both groups of probands, one can even speculate that Jak1 is specifically induced by IL-13.
As for Jak1, the phosphorylations of Tyk2 and IRS-2 were reduced after IL-13 stimulation in wildtype cells (Fig. 3C, 3D). SHP-2 seems to be responsible for these effects as well. Though it was suggested that IRS-2 does not associate with SHP-2 after IL-4 treatment [34], which confirms the results with IL-4 (no effect, see Fig. 1D), it obviously does so after IL-13 stimulation. Not much is known yet about Tyk2, but these results suggest that it is regulated by SHP-2 after IL-13 stimulation as well. In contrast to Jak1, no increase in phosphorylation is seen after IL-13 treatment for Tyk2 and IRS-2 in the presence of the variant. So these two substrates do not seem to be particularly activated by IL-13.
In most experiments a rather high level of substrate phosphorylation has been seen in the controls, i.e. without additional stimulation with exogeneously administered IL-4 or IL-13. Two effects might underlie this observation. First, certain amounts of IL-4 and IL-13 might be produced by the cell culture itself, although the incubation has been rather short at 10 min. Second, the signal might represent a lower dephosphorylation due to missing SHP-1 and SHP-2 activation in concordance with our hypothesis.
STAT3
As stated above, STAT3 phosphorylation was believed to be controlled by SHP-2 [27] until recently, when Tenev et al. reported a control by SHP-1 [35].
The results from this study suggest that STAT3 is regulated by both phosphatases, SHP-1/2. We therefore only see a dramatic increase in phosphorylation of STAT3 in the presence of the R551 variant, after both IL-4 and IL-13 stimulation, due to the impaired binding of SHP-1 or SHP-2 (Fig. 3E). No effect at all was seen in the wildtype situation where normal regulation of STAT3 by SHP-1/2 takes place.
Interestingly, the signaling of STAT3 seems to be further regulated on a different level. As a transcription factor it can act only when present in a dimeric form in the nucleus. Recently, it was reported that although STAT3 was activated by IL-4 its nuclear translocation was impaired in several keratinocytic cell lines [36]. We therefore sought similar phenomena. We immediately found STAT3 only in nuclear extract of wildtype cells after IL-4 treatment, whereas in R551 variant nuclear extracts it was present at once predominately after IL-13 stimulation (Fig. 4A), although its phosphorylation, that means activation in the cytoplasm, was markedly increased after treatment with both cytokines (stated above). These results are very specific for the IL-13 receptor. 293 fibroblasts lack the common γ chain and behave the same way as PBMCs (Fig. 4B).
Performing time-course experiments revealed, however, that we are not faced with a "black and white" situation. As expected STAT3 molecules appeared in a delayed fashion (after 20 min) in the nucleus in the respective cases mentioned above (Fig. 4C, 4D). We suggest that specific inhibitors of activated STAT3 are responsible for the observed effects, and that they might interact with STAT3 and consequently prevent or delay it from translocating to the nucleus. A variety of inhibitors of the IL-4/IL-13 pathway have been reported, such as SOCS-1 (also known as SSI-1) [21]; however, this inhibitor was suggested to prevent activation of STAT and, for example, act on Jak proteins [37]. So, SOCS-1 does not seem to be the right candidate in this case, because STAT3 activation was not found to be abolished. A better candidate seems to be the inhibitor PIAS3, which was previously shown to specifically bind to activated STAT3 proteins [23]. In addition, it is possible to imagine splice variants of STAT3 acting as anti-substrates; this has previously been suggested (e.g. for STAT6 [21]).
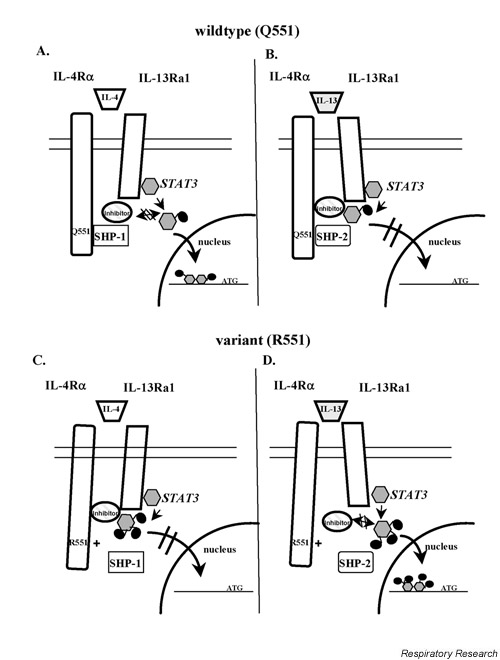
For conformational reasons the inhibitor might not be able to bind to STAT3 in case of the wildtype situation and IL-4 stimulation, so that STAT3 can be translocated to the nucleus. This is suggested in a hypothetical model (Fig. 5A). In the case of IL-13 stimulation, the conformational status of the receptor might allow the inhibitor to interact directly with STAT3 and prevent the molecule from being translocated (Fig. 5B). In the case of the R551 variant the conformation of IL-4Rα would be altered, so that the situation is reversed. The inhibitor interacts with STAT3 in case of IL-4 stimulation (Fig. 5C), however, after IL-13 stimulation this interaction is abolished and consequently STAT3 is translocated to the nucleus (Fig. 5D). It might also be that SHP-1 and SHP-2 are differentially recruited to the receptor depending on the wildtype or variant situation and IL-4 or IL-13 stimulation.
Figure 5.
Hypothetical model of nuclear translocation of STAT3. (A) When stimulating cells from wildtypes (Q551) with IL-4, a potential inhibitor does not bind to STAT3 and nuclear translocation takes place. (B) When stimulating wildtypes (Q551) with IL-13, a potential inhibitor is able to interact with STAT3 and prevents it from being translocated. (C) When stimulating variants R551 with IL-4, the inhibitor is able to interact and prevent the STAT3 translocation. (D) When stimulating the variants with IL-13. STAT3 is translocated STAT = signal transducer and activator of transcription.
On the whole, these results help to understand the complex picture of signal transduction processes in the IL-4/IL-13 pathway. These findings thus provide the first evidence for distinct roles of IL-4 and IL-13 while acting through the same IL-13 receptor. They support the idea of IL-13 being responsible for developing the asthma phenotype by inducing separate intracellular signaling processes independently of IL-4. If this assumption is correct, variants in the IL-13 protein itself might also be able to add to these specific effects. Very recently, the R110Q variant in IL-13 was found to be highly associated with the asthma phenotype, atopic dermatitis and elevated total serum IgE levels in three different populations [38-40]. The variant Gln110 is thought to provide a higher binding affinity to the IL-13 receptor and might therefore influence the IL-13 signaling processes in a specific way.
Other factors have, of course, to be considered. The distribution of receptors (IL-4R and IL-13R) varies in mononuclear cells and bronchial tissues [38], and there are further regulation mechanisms through which, for example, the phosphatase SHIP acts on the products of PI3-kinase [41], specific Jak inhibitors [JAB; [37]], soluble IL-4Rα [42] and many others. Also, IL-4 might act differently through the IL-4 receptor than through the IL-13 receptor. Furthermore, more than one docking site for SHP proteins on the IL-4Rα chain (stated above) is responsible for activation, and, very importantly, other functionally relevant polymorphisms exist in the IL-4Rα gene and in the genes of members of the IL-4/IL-13 pathway, apart from the polymorphism encoding the R551 variant, via linkage disequilibrium [43,44]. Further studies will be necessary to understand the complexity of the IL-4/IL-13 signaling pathway and also to deduce its exact implications for the development of asthma and atopy.
Conclusion
Using whole-cell in vivo experiments, we present evidence that IL-4 and IL-13 act through distinct signaling processes by predominately inducing either the phosphatase SHP-1 following binding of IL-4, or SHP-2 following binding of IL-13. Moreover, nuclear translocation of STAT proteins seems to differ following IL-4 versus IL-13 binding. Although some of the effects seen might be due to only IL-4 acting on part of the cells, and IL-4 plus IL-13 on others, this would still not explain some of the observations regarding the unique effects of IL-13 on activated STAT3. The functions of IL-4 and IL-13 are further influenced by some of the IL-4Rα variants. These findings may also have implications for the development of asthma or atopy.
Abbreviation
IL-4 = interleukin 4; IL-4Rα = IL-4 receptor alpha chain; IL-13 = interleukin 13; IL-13Rα1 = IL-13 receptor alpha chain; IRS = insulin receptor-like substrate; JAK = Janus kinase; PBMC = Peripheral blood mononuclear cell; SHP = SH2 containing phosphatase; SH2 = src-homology 2; STAT = signal transducer and activator of transcription.
Acknowledgments
Acknowledgement
This project is supported by a grant from the German Science Foundation (DFG-De386/2-3).
Contributor Information
Susanne Kruse, Email: kruse.susanne@mh-hannover.de.
Sandra Braun, Email: deichman@kkl200.ukl.uni-freiburg.de.
Klaus A Deichmann, Email: deichman@kkl200.ukl.uni-freiburg.de.
References
- Howard M, Farrar J, Hilfiker M, Johnson B, Takatsu K, Hamaoka T, Paul WE. Identification of a T cell-derived β cell growth factor distinct from interleukin 2. J Exp Med. 1982;155:914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- Hu-Li J, Shevach EM, Mizuguchi J, Ohara J, Mosmann T, Paul WE. B cell stimulatory factor-1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J Exp Med. 1987;165:157–172. doi: 10.1084/jem.165.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombacher F. The role of interleukin-13 in infectious diseases and allergy. Bioessays. 2000;22:646–656. doi: 10.1002/1521-1878(200007)22:7<646::AID-BIES7>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann MC, Miyajima A, Puri RK, Paul WE, Leonard WJ. The interleukin-2 receptor γ chain is a functional component of the interleukin-4 receptor. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Cosman D. The hematopoietin receptor superfamily. Cytokine. 1993;5:95–106. doi: 10.1016/1043-4666(93)90047-9. [DOI] [PubMed] [Google Scholar]
- Deichmann KA, Bardutzky J, Forster J, Heinzmann A, Kuehr J. Common polymorphisms in the coding part of the IL-4-receptor gene. Biochem Biophys Res Comm. 1997;231:696–697. doi: 10.1006/bbrc.1997.6115. [DOI] [PubMed] [Google Scholar]
- Deichmann KA, Heinzmann A, Forster J, Dischinger S, Mehl C, Brueggenolte E, Hildebrandt F, Moseler M, Kuehr J. Linkage and allelic association of atopy and markers flanking the IL-4-receptor gene. Clin Exp All. 1998;28:151–155. doi: 10.1046/j.1365-2222.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- Hershey GKK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the α subunit of the interleukin-4 receptor. N Engl J Med. 1997;337:1720–1725. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- Kanemitsu S, Takabayashi A, Sasaki Y, Kuromaru R, Ihara K, Kaku Y, Sakai K, Hara T. Association of interleukin-4 receptor and interleukin-4 promoter gene polymorphisms with systemic lupus erythematosus. Arthritis Rheum. 1999;42:1298–1300. doi: 10.1002/1529-0131(199906)42:6<1298::AID-ANR31>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Hackstein H, Kluter H, Fricke L, Hoyer J, Bein G. The IL-4 receptor alpha-chain variant Q576R is strongly associated with decreased kidney allograft survival. Tissue Antigens. 1999;54:471–477. doi: 10.1034/j.1399-0039.1999.540504.x. [DOI] [PubMed] [Google Scholar]
- Mitsuyasu H, Izuhara K, Mao XQ, Gao PS, Arinobu Y, Enomoto T, Kawai M, Sasaki S, Dake Y, Hamasaki N, Sirakawa T, Hopkin JM. Ile50Val variant of IL-4R alpha upregulates IgE synthesis and associates with atopic asthma. Nat Genet. 1998;19:119–120. doi: 10.1038/472. [DOI] [PubMed] [Google Scholar]
- Kruse S, Japha T, Tedner M, Hauschildt Sparholt S, Forster J, Kuehr J, Deichmann KA. The polymorphisms S503P and Q576R in the interleukin-4 receptor α gene are associated with atopy and influence the signal transduction. Immunology. 1999;96:365–371. doi: 10.1046/j.1365-2567.1999.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A, Roellinghoff M. Biologic functions and signaling of the interleukin-4 receptor complexes. Immunobiology. 2000;201:285–307. doi: 10.1016/S0171-2985(00)80084-4. [DOI] [PubMed] [Google Scholar]
- Orchansky PL, Kwan R, Lee F, Schrader JW. Characterization of the cytoplasmic domain of interleukin-13 receptor-alpha. J Biol Chem. 1999;274:20818–20825. doi: 10.1074/jbc.274.30.20818. [DOI] [PubMed] [Google Scholar]
- Takeda T, Kurachi H, Yamamoto T, Nishio Y, Nakatsuji Y, Morishige K, Miyake A, Murata Y. Crosstalk between the interleukin-6 (IL-6)-JAK-STAT and the glucocorticoid-nuclear receptor pathway: synergistic activation of IL-6 response element by IL-6 and glucocorticoid. J Endocrinol. 1998;159:323–330. doi: 10.1677/joe.0.1590323. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Manolagas SC. Isolation and characterization of the human gp130 promoter. Regulation by STATs. J Biol Chem. 1997;272:15003–15010. doi: 10.1074/jbc.272.23.15003. [DOI] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. ience. 1997;278:Sc1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- Wjst M, Kruse S, Illig T, Deichmann KA. Asthma and IL-4 receptor alpha gene variants. Eur J Immunogenet. 2002;29:263–268. doi: 10.1046/j.1365-2370.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Preparation of nuclear extracts. In Current Protocols in Molecular Biology . Edited by John Wiley & Sons Inc. USA, 1997, 11-1212.
- Wang HY, Shelburne CP, Zamorano J, Kelly AE, Ryan JJ, Keegan AD. Cutting edge: effects of an allergy-associated mutation in the human IL-4R alpha (Q576R) on human IL-4-induced signal transduction. J Immunol. 1999;162:4385–4389. [PubMed] [Google Scholar]
- Servidei T, Aoki Y, Lewis SE, Symes A, Fink JS, Reeves SA. Coordinate regulation of STAT signaling and c-fos expression by the tyrosine phosphatase SHP-2. J Biol Chem. 1998;273:6233–6241. doi: 10.1074/jbc.273.11.6233. [DOI] [PubMed] [Google Scholar]
- Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J All Clin Immunol. 2000;105:1063–1070. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase SHP-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–33896. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- Imani F, Rager KJ, Catipovic B, Marsh DG. Interleukin-4 (IL-4) induces phosphatidylinositol 3-kinase (p85) dephosphorylation. Implications for the role of SHP-1 in the IL-4-induced signals in human B cells. J Biol Chem. 1997;272:7927–7931. doi: 10.1074/jbc.272.12.7927. [DOI] [PubMed] [Google Scholar]
- Kuhne MR, Pawson T, Lienhard GE, Feng GS. The insulin receptor substrate1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- Gadina M, Stancato LM, Bacon CM, Larner AC, O'Shea JJ. Involvement of SHP-2 in multiple aspects of IL-2 signaling: evidence for a positive regulatory role. J Immunol. 1998;160:4657–4661. [PubMed] [Google Scholar]
- Yin T, Shen R, Feng GS, Yang YC. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J Biol Chem. 1997;272:1032–1037. doi: 10.1074/jbc.272.2.1032. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Pons S, Wang LM, Zhang Y, Yenush L, Burks D, Myers MGJr, Glasheen E, Copeland NG, Jenkins NA, Pierce JH, White MF. The IRS-2 gene on murine chromosome 8 encodes a unique signaling adapter for insulin and cytokine action. Mol Endocrinol. 1997;11:251–262. doi: 10.1210/mend.11.2.9885. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bohmer SA, Kaufmann R, Frese S, Bittorf T, Beckers T, Bohmer FD. Perinuclear localization of the protein-tyrosine phosphatase SHP-1 and inhibition of epidermal growth factor-stimulated STAT1/3 activation in A431 cells. Eur J Cell Biol. 2000;79:261–271. doi: 10.1078/S0171-9335(04)70029-1. [DOI] [PubMed] [Google Scholar]
- Wery-Zennaro S, Letourneur M, David M, Bertoglio J, Pierre J. Binding of IL-4 to the IL-13Ralpha(1)/IL-4Ralpha receptor complex leads to STAT3 phosphorylation but not to its nuclear translocation. FEBS Lett. 1999;464:91–96. doi: 10.1016/s0014-5793(99)01680-4. [DOI] [PubMed] [Google Scholar]
- Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- Heinzmann A, Mao XQ, Akaiwa M, Kroemer RT, Gao PS, Ohshima K, Umeshita R, Abe Y, Braun S, Yamashita T, Roberts MH, Sugimoto R, Arima K, Arinobu Y, Yu B, Kruse S, Enomoto T, Dake Y, Kawai M, Shimazu S, Sasaki S, Adra CN, Kitaichi M, Inoue H, Yamauchi K, Tomichi N, Kurimoto F, Hamasaki N, Hopkin JM, Izuhara K, Shirakawa T, Deichmann KA. Genetic variants of IL-13 signaling and human asthma and atopy. Hum Mol Genet. 2000;9:549–959. doi: 10.1093/hmg/9.4.549. [DOI] [PubMed] [Google Scholar]
- Graves PE, Kabesch M, Halonen M, Holberg CJ, Baldini M, Fritzsch C, Weiland SK, Erickson RP, von Mutius E, Martinez FD. A cluster of seven tightly linked polymorphisms in the IL-13 gene is associated with total serum IgE levels in three populations of white children. J Allergy Clin Immunol. 2000;105:506–513. doi: 10.1067/mai.2000.104940. [DOI] [PubMed] [Google Scholar]
- Liu X, Nickel R, Beyer K, Wahn U, Ehrlich E, Freidhoff LR, Bjorksten B, Beaty TH, Huang SK. An IL13 coding region variant is associated with a high total serum IgE level and atopic dermatitis in the German multicenter atopy study (MAS-90). J All Clin Immunol. 2000;106:167–170. doi: 10.1067/mai.2000.107935. [DOI] [PubMed] [Google Scholar]
- Giallourakis C, Kashiwada M, Pan PY, Danial N, Jiang H, Cambier J, Coggeshall KM, Rothman P. Positive Regulation of IL-4 Mediated Proliferation by the SH2-Containing Inositol 5'-Phosphatase (SHIP). J Biol Chem. 2000;275:29275–29282. doi: 10.1074/jbc.M002853200. [DOI] [PubMed] [Google Scholar]
- Kruse S, Forster J, Kuehr J, Deichmann KA. Characterization of the membrane-bound and a soluble form of human IL-4 receptor alpha produced by alternative splicing. Int Immunol. 1999;11:1965–1970. doi: 10.1093/intimm/11.12.1965. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Deichmann KA, Izuhara K, Mao XQ, Adra CN, Hopkin JM. Atopy and asthma: genetic variants of IL-4 and IL-13 signaling. Immunol Today. 2000;21:60–64. doi: 10.1016/s0167-5699(99)01492-9. [DOI] [PubMed] [Google Scholar]
- Ober C, Leavitt SA, Tsalenko A, Howard TD, Hoki DM, Daniel R, Newman DL, Wu X, Parry R, Lester LA, Solway J, Blumenthal M, King RA, Xu J, Meyers DA, Bleecker ER, Cox NJ. Variation in the interleukin 4-receptor alpha gene confers susceptibility to asthma and atopy in ethnically diverse populations. Am J Hum Genet. 2000;66:517–526. doi: 10.1086/302781. [DOI] [PMC free article] [PubMed] [Google Scholar]