Abstract
Changes in the number of alveolar macrophages were correlated with organism burden during Pneumocystis carinii infection. The lungs of healthy, dexamethasone-treated, and dexamethasone-treated and P. carinii-infected rats were lavaged with phosphate-buffered saline. Counting of alveolar macrophages in the lavage fluids revealed that P. carinii infection caused a 58% decrease in the number of alveolar macrophages and that higher P. carinii organism burdens caused a more rapid decrease in alveolar macrophage number. As a control, healthy rats were challenged with the same number of organisms as that normally used to generate P. carinii infections in dexamethasone-treated rats. Thirteen days after challenge, these rats had a profound (54%) increase in alveolar macrophage number in response to the challenge, while the number of alveolar macrophages in immunosuppressed and P. carinii-infected rats had decreased significantly by this time point. These experiments created the first animal model to mimic human pneumocystis pneumonia in alveolar macrophage number alterations. Reduction of P. carinii organism numbers by treatment of rats with trimethoprim and sulfamethoxazole brought a slow rebound in alveolar macrophage number, while recovery from P. carinii infection by cessation of immunosuppression brought a rapid rebound in alveolar macrophage number. These results suggest that both the immune state of the host and P. carinii burden affect alveolar macrophage number.
Immunosuppressed populations are at risk for developing a pneumonia caused by the opportunistic organism Pneumocystis carinii (renamed recently as Pneumocystis jiroveci for those strains that infect humans) (31). P. carinii now refers to those organisms that infect rats (31). Infection with pneumocystis organisms brings many changes in the lung environment, including decrease of phosphatidylcholine (27, 29) and increase of sphingomyelin (T. H. Su, V. Natarajan, and W. J. Martin II, abstract from the 1992 Int. Conf. Am. Lung Assoc./Am. Thoracic Soc., Am. Rev. Resp. Dis. 145:A246, 1992), surfactant proteins A and D (22, 26) and the adhesion proteins vitronectin and fibronectin (19). Pneumocystis infection also causes increases in the production of the cytokines tumor necrosis factor alpha (11, 24, 37), interleukin-8 (IL-8), IL-10, IL-12 (2, 39), and gamma interferon (39); the growth factor granulocyte-macrophage colony-stimulating factor (24); and the chemokine monocyte chemotactic protein 1 (2) but down-regulates the expression of the host transcription factor GATA-2 (33). An inflammatory response (41, 43) that changes alveolar macrophage and polymorphonuclear cell populations (7, 42, 44) is also observed during pneumocystis infection.
In immunocompetent humans and animals, alveolar macrophages protect against pneumocystis infection by actively removing the organism from the alveolus. In contrast, it has been shown that alveolar macrophages from pneumocystis-infected animals do not phagocytose pneumocystis organisms to any significant degree (3, 13). In addition to the defect in phagocytosis, the number of alveolar macrophages in humans with P. carinii pneumonia (PCP) has also been found to be reduced (7, 8, 13, 42, 44). Young et al. (42) found that the numbers of both leukocytes and alveolar macrophages are low in the lungs of immunocompromised patients with PCP. This is unusual because alveolar macrophages are increased in other types of infections in immunocompromised patients (7). Furthermore, the major surface glycoprotein (MSG or gpA) of pneumocystis organisms is chemotactic for normal blood monocytes (H. Koziel, J. Baik, M. Y. K. Armstrong, F. F. Richards, and R. M. Rose, abstract from the 1993 Int. Conf. Am. Lung Assoc./Am. Thoracic Soc., Am. Rev. Resp. Dis. 147:A33, 1993); it should have attracted blood monocytes to the lung and increased the alveolar macrophage number. Fleury et al. (7) further showed that alveolar macrophages are 69.5% of the total cell population in bronchoalveolar lavage (BAL) fluids from immunocompromised patients without PCP but are only 38.8% of the total cell population in BAL fluids from PCP patients with AIDS. The reduction in alveolar macrophage number was found in both human immunodeficiency virus-positive and -negative patients with PCP but was not observed in either human immunodeficiency virus-positive or -negative patients without PCP (7, 11). A similar observation was reported by Sadaghdar et al. (28), i.e., that alveolar macrophages account for 64% of total cells in BAL fluids from AIDS patients without PCP and only 45% in BAL fluids from AIDS patients with PCP.
Reduction in alveolar macrophages has not been shown in an experimental animal model of PCP, and the changes in alveolar macrophage number described to this point have been relative to other cell types. To investigate the problem, we determined whether the reduction in alveolar macrophage number also occurs in P. carinii-infected rats. Rats were inoculated with different numbers of P. carinii organisms, and the number of alveolar macrophages at different time points was determined. The effects of treatment for PCP and restoration of immune functions in P. carinii-infected rats on changes in alveolar macrophage number were also investigated. The results of these experiments demonstrated that the rat model of PCP mimics the disease in humans as to the decrease in the number of alveolar macrophages. Results also showed that the absolute number, not just the relative number, of alveolar macrophages was reduced in these animals and that the organisms present in the lung play a role in the regulation of alveolar macrophage number.
MATERIALS AND METHODS
Immunosuppressed rat model of PCP.
Female Sprague Dawley rats (colony 202) were obtained from Harlan (Indianapolis, Ind.). The rats used in this study were housed in microisolator cages and divided into six groups: (i) healthy (immunocompetent) rats—rats supplied with regular drinking water and not inoculated with P. carinii; (ii) healthy challenged rats—rats supplied with regular drinking water and transtracheally inoculated with P. carinii; (iii) Dex rats—rats immunosuppressed with dexamethasone (0.36 mg/kg/day) in their drinking water; (iv) Dex-Pc rats—rats immunosuppressed with dexamethasone for 7 days, transtracheally inoculated with P. carinii, and then maintained on an immunosuppressive regimen by giving the same dose of dexamethasone (0.36 mg/kg/day) in their drinking water; (v) treatment rats—those which were immunosuppressed, transtracheally inoculated with P. carinii, allowed to develop an infection for 3 weeks, and then treated with trimethoprim-sulfamethoxazole (50 and 250 mg/kg of body weight/day, respectively) in dexamethasone-containing drinking water for 3 weeks; and (vi) recovery rats—those which were immunosuppressed, transtracheally inoculated with P. carinii, allowed to develop an infection for 3 weeks, and then switched to drinking water without dexamethasone for the rest of the study. P. carinii organisms were introduced into rat lungs by instillation of organisms directly into the bronchi using a 27-gauge needle inserted between cartilaginous rings of the trachea as previously described (1); rats were inoculated with 7.8 × 106, 3.9 × 106, 1.95 × 106, 9.8 × 105, or 7.8 × 104 P. carinii trophozoites, termed the load or initial load. Additionally, some rats were inoculated with inoculum made from healthy rats not previously exposed to P. carinii as a control for the effects of host lung tissue on alveolar macrophage number.
Lavage of rat alveolar macrophages.
At various time points, rats from each group were anesthetized with an intramuscular injection of 0.15 ml of ketamine cocktail (ketamine hydrochloride, 80 mg/ml; acepromazine, 1.76 mg/ml; atropine, 0.38 μg/ml). The thoracic cavity and trachea of anesthetized rats were exposed by dissection and cutting away of the ribs. Five milliliters of pyrogen-free, sterile phosphate-buffered saline (PBS) (pH 7.4, warmed to 37°C) was injected into the lungs with a 10-ml syringe via a 14-gauge angiocatheter (Becton Dickinson, Franklin Lakes, N.J.) and recovered to a sterile 50-ml centrifuge tube. Lavage was continued with 7- to 10-ml aliquots of PBS until a total of 100 ml of lavage fluid was recovered from each rat. The lavage fluid was centrifuged at 300 × g for 5 min at 25°C, and the pelleted cells were resuspended to 1.5 × 106/ml in media containing RPMI 1640 (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% fetal bovine serum, 1 mM pyruvate, 1% nonessential amino acids, 14 mM glucose, 17.9 mM NaHCO3, 10 mM HEPES, penicillin (100 U/ml), and streptomycin (0.1 mg/ml). Lavaged cells were identified by morphology or by cell-type-specific staining (anti-rat macrophage activator [anti-RMA] antigen for alveolar macrophages) and counted on hemocytometer.
Identification of alveolar macrophages by anti-RMA immunocytochemistry.
To confirm the identity of lavaged cells, the cells were reacted with a monoclonal antibody against the RMA (BD Pharmingen, San Diego, Calif.). Anti-RMA antibody reacts with a 120-kDa cell surface antigen found on rat alveolar macrophages and a small subset of pulmonary dendritic cells (40). An aliquot of the lavage sample containing 30,000 to 50,000 cells was placed on a Superfrost+ slide (Fisher, Pittsburgh, Pa.) by the cytospin method (Cytospin II; Shandon Instruments, Pittsburgh, Pa.) at 750 rpm (65 × g) for 5 min. The slides were air dried, fixed in methanol for 1 min, and incubated for 5 min with 1 part 3% H2O2 and 4 parts methanol to inactivate the endogenous peroxidase activity, and this was followed by a wash in PBS. The slide was covered with blocking buffer (10% fetal bovine serum and bovine serum albumin [0.5 mg/ml] in PBS) for 1 h at 25°C in a humidified chamber and then drained, but not rinsed. The anti-RMA antibody was diluted 1:10,000 in PBS and placed on the slides for 1 h at 25°C in the same humidified chamber. The slides were washed in blocking buffer four times, and the secondary antibody (rabbit anti-mouse immunoglobulin G, peroxidase conjugated) (Sigma Chemical Co.) was diluted 1:500 in PBS and placed on the slides for 45 min at 25°C in the humidified chamber. The slides were washed four times in PBS and once in 50 mM Tris-HCl (pH 7.4) for a total of 30 min. The bound antibody was visualized by incubating the cells with diaminobenzidine (DAB) solution (DAB liquid substrate dropper system; Sigma Chemical Co.) for 2 min. The reaction was enhanced by addition of cobalt chloride to a final concentration of 0.03% (wt/vol) in the DAB solution. Cells that reacted with the anti-RMA antibody showed a punctate blue or black precipitate on the cell membrane and were 20 to 25 μm in diameter with a large U-shaped nucleus and a distinct nucleolus, irregular and shaggy cell margins, and visible granules within the cytoplasm. These morphological properties are characteristic of macrophages. Large cells with segmented nuclei not stained by the anti-RMA antibody were identified as neutrophils, and small, anti-RMA unreactive cells with large nuclei were identified as lymphocytes.
Enumeration of alveolar macrophages in situ.
Lungs of healthy, Dex, or Dex-Pc rats were removed after anesthetization and cardiac exsanguination. Some rats were subjected to BAL with 100 ml of PBS prior to cardiac exsanguination. The lungs were fixed in 4% paraformaldehyde, paraffin embedded, and sectioned by microtome. Five-micrometer-thick lung sections were stained with Grocott methenamine-silver nitrate stain to visualize the granules of alveolar macrophages. Alveolar macrophages were identified by size, morphology, location inside the alveolus and the presence of dark granules. At least 50 oil immersion microscopic fields (magnification, ×1,000) were analyzed from three different lung sections separated by more than 200 μm and from at least three different rats of each experimental condition. Results were expressed as the number of alveolar macrophages per thousandfold-magnified oil immersion field.
Analysis of infection in rats transtracheally inoculated with P. carinii.
At various time points after inoculation with P. carinii, animals were anesthetized as previously described (1) and exsanguinated by cardiac puncture, and their lungs were removed. Severity of P. carinii infection was determined by scoring numbers of organisms on histochemically stained impression smears of lung tissue. The smears were stained with Wright's-Giemsa stain for both trophozoite and cyst forms and with Grocott methenamine-silver nitrate stain for cyst forms (1). The slides were examined as unknowns by two experienced microscopists. An infection score for each slide was determined by using the following roughly logarithmic scale: 5+ was more than 100 organisms per thousandfold-magnified field, 4+ was 11 to 100 organisms per thousandfold-magnified field, 3+ was 1 to 10 organisms per thousandfold-magnified field, 2+ was 2 to 9 organisms in 10 thousandfold-magnified fields, 1+ was one or more organisms in 50 thousandfold-magnified fields, and 0 was no organisms in 50 thousandfold-magnified fields (1). These scores are termed the burden or organism burden, representing P. carinii organism number in infected lungs.
Statistics.
Determinations of significant statistical difference are made using a one-way analysis of variance method for multiple samples or Student's t test for comparison of two samples where appropriate. The SigmaStat (Jandel Scientific, San Rafael, Calif.) software package was used to make these calculations.
RESULTS
Percentages of various immune cells in BAL fluid.
In three trials where 300 cells were counted on each cytospin-treated slide after reaction with anti-RMA antibody, an average (mean ± standard deviation unless otherwise specified) of 98% ± 1.30% of nucleated cells in BAL fluid obtained from healthy rats 56 days after arriving at our facility (49 days postinoculation for Dex-Pc rats) were alveolar macrophages. A similar percentage (97.4% ± 1.76%) of alveolar macrophages was seen in BAL fluid from Dex rats, while only 42.9% ± 1.12% of lavaged cells from Dex-Pc rats were alveolar macrophages. A small percentage of BAL cells from healthy (3.38% ± 0.51%) and Dex (0.39% ± 0.21%) rats were lymphocytes. A slightly higher percentage (5.62% ± 0.77%) of lymphocytes were seen in BAL fluid from Dex-Pc rats. This small increase in lymphocyte number in Dex-Pc rats has also been reported previously (7, 8, 13, 42, 44) but was not statistically significant compared to those observed in healthy rats in this study. Neutrophils represent only 0.42% ± 0.09% and 0.56% ± 0.41% of total BAL cells from healthy and Dex rats, respectively, but are 50.6% ± 1.11% of those from Dex-Pc rats as described previously (14, 44).
Number of lavaged alveolar macrophages.
Rats were maintained as immunocompetent or immunosuppressed for 7 days with dexamethasone and then transtracheally inoculated with P. carinii. The largest inoculation dose was the normal load (7.8 × 106 trophozoites) used in the originally described model (1). Other doses were equal to 0.5 times (3.9 × 106 trophozoites), 0.25 times (1.95 × 106 trophozoites), 0.125 times (9.8 × 105 trophozoites), or 0.01 times (7.8 × 104 trophozoites) this load.
The lungs of healthy rats were lavaged to establish a baseline number of alveolar macrophages. In 14 healthy rats examined on day −7, the average number (mean ± standard deviation) of alveolar macrophages obtained from 100 ml of lavage fluid was (4.73 ± 0.76) × 106. Some healthy rats were housed for 48 days (the entire length of the study) before their alveolar macrophages were counted to control for possible changes due to increased weight, size, and age of these rats. These rats had an average of (4.47 ± 0.36) × 106 alveolar macrophages, statistically no difference from those lavaged at the beginning of the study (Table 1). Healthy rats transtracheally inoculated with inoculum made from uninfected rats showed no change in alveolar macrophage number over the course of the study, indicating that syngeneic host tissue had no effect on host response (data not shown). Dexamethasone treatment alone did not significantly reduce the number of alveolar macrophages lavaged from rats. Seven days after initiation of immunosuppression, designated day 0, the number was reduced by only 6%, from (4.73 ± 0.76) × 106 to (4.43 ± 0.26) × 106 (P = 0.2805 versus the value for healthy rats). At subsequent time points (days 6, 13, 20, 27, 34, and 41), the number of alveolar macrophages lavaged was very close to that of day 0 (Table 1).
TABLE 1.
Alveolar macrophage number in normal and immunosuppressed rats with or without P. carinii infection over time
| Rat grouph | Mean alveolar macrophage no. ± SD (106) at indicated day after P. carinii inoculation
|
P vs. Dexg | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 13 | 20 | 27 | 34 | 41 | ||
| Healthya | 4.61 ± 0.42 | 4.56 ± 0.46 | NAi | NA | NA | NA | 4.47 ± 0.36 | |
| Dexb | 4.43 ± 0.26 | 4.23 ± 0.02 | 4.30 ± 0.27 | 4.36 ± 0.17 | 4.32 ± 0.11 | 4.25 ± 0.17 | 4.35 ± 0.10 | |
| Healthy challengedc (1×) | NA | 4.78 ± 0.10 | 6.85 ± 0.26 | 6.72 ± 0.40 | 7.12 ± 0.65 | 3.54 ± 0.27 | 4.27 ± 0.33 | 0.0005 |
| Healthy challenged (0.5×) | NA | 4.65 ± 0.11 | 6.39 ± 0.25 | 6.61 ± 0.45 | 6.83 ± 0.69 | 4.20 ± 0.26 | 4.51 ± 0.16 | 0.0053 |
| Healthy challenged (0.25×) | NA | 4.55 ± 0.14 | 6.88 ± 0.73 | 6.21 ± 0.48 | 5.39 ± 0.33 | 4.67 ± 0.42 | 4.45 ± 0.32 | 0.0058 |
| Healthy challenged (0.125×) | NA | 4.42 ± 0.12 | 4.38 ± 0.15 | 4.90 ± 0.50 | 4.29 ± 0.16 | NA | NA | >0.05 |
| Dex-Pcd (1×) | NA | 1.88 ± 0.14 | 1.77 ± 0.21 | 1.75 ± 0.15 | 2.03 ± 0.08 | 1.87 ± 0.74 | 2.19 ± 0.23 | 0.0007 |
| Dex-Pc (0.5×) | NA | 2.06 ± 0.16 | 1.97 ± 0.09 | 1.63 ± 0.23 | 2.11 ± 0.23 | 1.85 ± 0.23 | 1.83 ± 0.22 | <0.0001 |
| Dex-Pc (0.25×) | NA | 4.51 ± 0.13 | 2.21 ± 0.36 | 1.84 ± 0.48 | 1.69 ± 0.22 | 1.83 ± 0.09 | 1.83 ± 0.32 | 0.0003 |
| Dex-Pc (0.125×) | NA | 4.45 ± 0.39 | 1.86 ± 0.08 | 1.85 ± 0.15 | 1.92 ± 0.28 | 1.82 ± 0.24 | 1.53 ± 0.20 | 0.0001 |
| Dex-Pc (0.01×) | NA | NA | NA | 3.49 ± 0.29 | 1.86 ± 0.32 | 1.84 ± 0.09 | NA | 0.0002 |
| Treatmente (1×) | 1.75 ± 0.16 | 3.08 ± 0.33 | 3.28 ± 0.25 | 3.91 ± 0.11 | 0.0033 | |||
| Treatment (0.5×) | 1.63 ± 0.23 | 2.96 ± 0.20 | 3.55 ± 0.07 | 4.07 ± 0.20 | 0.041 | |||
| Treatment (0.25×) | 1.84 ± 0.48 | 2.38 ± 0.38 | 3.98 ± 0.21 | 3.96 ± 0.19 | 0.048 | |||
| Treatment (0.125×) | 1.85 ± 0.15 | 1.71 ± 0.24 | 2.87 ± 0.24 | 3.23 ± 0.21 | 0.0044 | |||
| Recoveryf (1×) | 1.75 ± 0.14 | 6.16 ± 0.10 | 5.18 ± 0.18 | 4.45 ± 0.15 | 0.0011 | |||
| Recovery (0.5×) | 1.63 ± 0.23 | 4.12 ± 0.11 | 6.89 ± 0.40 | 4.68 ± 0.24 | 0.0045 | |||
| Recovery (0.25×) | 1.84 ± 0.48 | 3.45 ± 0.10 | 5.80 ± 0.30 | 5.14 ± 0.14 | 0.0035 | |||
| Recovery (0.125×) | 1.85 ± 0.15 | 4.09 ± 0.13 | 4.42 ± 0.17 | 4.50 ± 0.15 | >0.05 | |||
Rats that were not immunosuppressed (immunocompetent rats).
Rats that were immunosuppressed with dexamethasone.
Immunocompetent rats inoculated with different loads of P. carinii.
Immunosuppressed rats inoculated with different loads of P. carinii.
Immunosuppressed, P. carinii-infected rats treated with trimethoprim-sulfamethoxazole starting on day 21.
Immunosuppressed, P. carinii-infected rats recovered from immunosuppression by cessation of dexamethasone treatment starting on day 21.
P for the smallest significant difference compared to the dexamethasone-treated group at the same time point. P values were calculated using Student's t-test.
Loads of P. carinii are indicated in parentheses as follows: 1×, 7.8 × 106; 0.5, 3.9 × 106; 0.25, 1.95 × 106; 0.125, 9.8 × 105; 0.01, 7.8 × 104.
NA, not assessed.
Immunocompetent rats inoculated with P. carinii (healthy challenged rats) responded by increasing the number of alveolar macrophages. Six days (day 6) after inoculation with 7.8 × 106 trophozoites, these rats showed a slight increase in alveolar macrophage number [from (4.56 ± 0.46) × 106 to (4.78 ± 0.10) × 106, P = 0.3902 versus the value for healthy rats at day 6]. Thirteen days (day 13) after inoculation, the number of lavaged alveolar macrophages increased significantly to (6.85 ± 0.26) × 106 (P = 0.0002 versus Dex rats at day 13). The number of alveolar macrophages remained high at day 20 [(6.72 ± 0.40) × 106] and peaked at day 27 [(7.12 ± 0.65) × 106] but was reduced slightly at day 34 [(3.54 ± 0.27) × 106]. At day 41, the number of alveolar macrophages returned to the normal level [(4.27 ± 0.33) × 106] (Table 1).
Similar to those inoculated with 7.8 × 106 trophozoites, immunocompetent rats inoculated with lesser doses (0.5 or 0.25 times the normal load) of P. carinii also showed increases in alveolar macrophage numbers (Table 1). For both of these two inoculation loads, the increase in alveolar macrophage number occurred 6 to 13 days after transtracheal inoculation of organisms. By day 34, the number of alveolar macrophages in these two groups of rats had returned to normal levels. Interestingly, immunocompetent rats inoculated with 0.125 times the normal load (9.8 × 105 trophozoites) showed no increase in alveolar macrophage number over the 34-day period studied (Table 1).
In direct contrast to the response in immunocompetent rats challenged with P. carinii, immunosuppressed rats inoculated with P. carinii trophozoites (Dex-Pc rats) displayed decreased numbers of alveolar macrophages. Dex-Pc rats inoculated with a full load of trophozoites showed a 56% decrease in alveolar macrophage number 6 days after inoculation [from (4.23 ± 0.06) × 106 to (1.88 ± 0.14) × 106] compared to Dex rats. Alveolar macrophage numbers in the Dex-Pc rats stayed low throughout the remaining study period (Table 1). Dex-Pc rats inoculated with 0.5 times the normal load showed similar patterns of decrease in alveolar macrophage number (Table 1).
Lower initial inoculation loads brought similar decreases in alveolar macrophage number, but at later time points (Table 1). A load of 0.25 times the normal did not affect alveolar macrophage number until day 13. At day 6, alveolar macrophage number was normal [(4.51 ± 0.13) × 106], but at day 13 it was reduced to (2.21 ± 0.36) × 106 (P = 0.0004 versus Dex rats at day 13). As with larger initial loads, once the macrophage number decreased, it remained low throughout the course of disease (Table 1). Dex-Pc rats inoculated with 0.125 times the normal load displayed patterns similar to those of rats inoculated with 0.25 times the normal load. Alveolar macrophage numbers in those inoculated with 0.01 times the normal load did not significantly decrease until day 27 (Table 1).
Correlation of alveolar macrophage number with P. carinii burden.
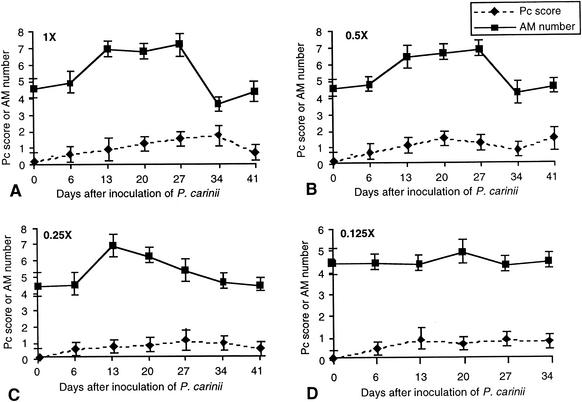
At each time point when alveolar macrophage numbers were assessed, the P. carinii organism burden was also determined. Impression smears of P. carinii-infected rat lung tissue were stained to count organisms. Both trophozoites and cysts were counted. In most instances, there were 95 to 99% trophozoites and 1 to 5% cysts. Healthy challenged rats did not develop organism burdens with a score greater than 1.5, regardless of the initial inoculation load. Healthy rats inoculated with the largest load of P. carinii first showed small increases in organism burdens 6 days after inoculation (Fig. 1A). These rats began to clear the organisms between day 34 and day 41, as evidenced by reduced organism burden (Fig. 1A). Lower initial loads translated to lower organism burden over time, but the increases and decreases in organism number showed a pattern similar to the highest inoculation load. The increase in alveolar macrophage number that was noted by day 13 in rats inoculated with the three largest loads, was preceded by an increase in organism burden by day 6 in each case (Fig. 1). The return of alveolar macrophages to normal levels toward the end of the study period preceded a decrease in organism number by at least 6 days. Interestingly, a measurable organism burden remained even when the alveolar macrophage number returned to the normal level. In the case of 0.125 times the normal load, there was no increase in alveolar macrophage number throughout the entire study period (Fig. 1D), even though the organism burdens were comparable to those inoculated with 0.25 times the normal load at various time points.
FIG. 1.
Correlation of P. carinii (Pc) burden with alveolar macrophage (AM) number in healthy challenged rats with different loads of P. carinii. The y axis is the scale of both P. carinii burden and macrophage numbers. The scale for the organism burden is the actual number shown, whereas that for alveolar macrophage numbers is the number shown on the scale multiplied by 106. Both alveolar macrophage and P. carinii organism numbers shown are averages derived from results for at least three rats of each experimental condition (error bars, standard deviations). The rats were inoculated at day 0 with different loads of P. carinii: 7.8 × 106 trophozoites (1 times the normal load) (A), 3.9 × 106 trophozoites (0.5 times the normal load) (B), 1.95 × 106 trophozoites (0.25 times the normal load) (C), and 9.8 × 105 trophozoites (0.125 times the normal load) (D).
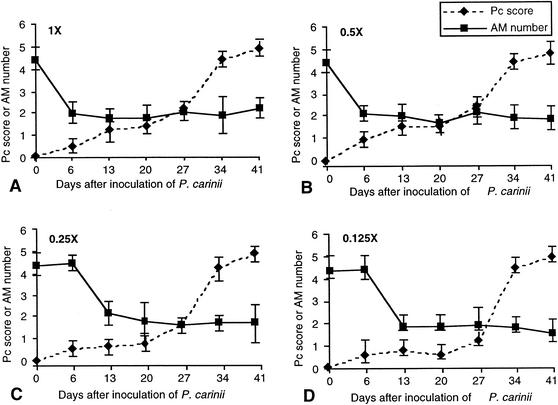
Dex-Pc rats, as with healthy challenged rats, had low organism burdens soon (day 6) after inoculation (Fig. 2), but they developed progressive, severe infections over the next 34 days, regardless of the initial inoculation load. Significant differences in organism burden between healthy challenged rats and Dex-Pc rats were evident by day 20 or day 27 (P < 0.05, at day 20 for the two largest initial loads and at day 27 for 0.25 and 0.125 times the normal load).
FIG. 2.
Correlation of P. carinii (Pc) burden with alveolar macrophage (AM) number in Dex-Pc rats. The symbols and scales are the same as those of Fig. 1. All numbers shown are the averages of results for three rats in the same experimental group (error bars, standard deviations). Rats were inoculated at day 0 with different loads of P. carinii: 7.8 × 106 trophozoites (1 times the normal load) (A), 3.9 × 106 trophozoites (0.5 times the normal load) (B), 1.95 × 106 trophozoites (0.25 times the normal load) (C), and 9.8 × 105 trophozoites (0.125 times the normal load) (D).
Generally, decreases in alveolar macrophage number preceded significant increases in organism number (Fig. 2). In the three largest initial loads, the alveolar macrophage number was significantly reduced by day 6 to 13, while the organism burden was significantly increased by day 13 to 27. In addition, once the alveolar macrophage number decreased to approximately 1.85 × 106, increases in organism number did not have any additional effect on macrophage number.
Number of alveolar macrophages in rat lung sections before and after lavage.
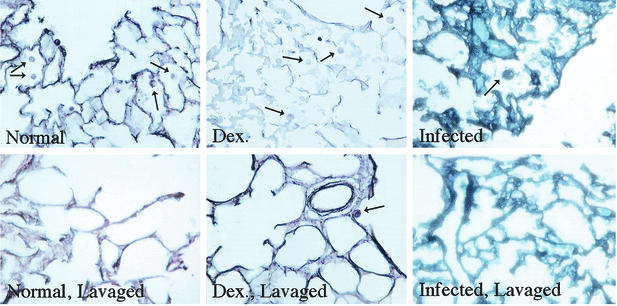
To confirm that alveolar macrophage number is reduced in the lungs of Dex-Pc rats, alveolar macrophage numbers in lung sections were determined. Five-micrometer-thick sections were cut from paraformaldehyde-fixed lung tissues of healthy, Dex, and Dex-Pc rats. Some of these rats were subjected to BAL prior to removal of the lungs. The sections were stained with silver stain to visualize P. carinii cysts and the cytoplasmic granules of alveolar macrophages. Alveolar macrophages in at least 100 alveoli from more than three rats of each condition were counted, and the number was expressed as the average number of alveolar macrophages per thousandfold-magnified oil immersion field. Sections of lungs from rats before and after lavage were assessed.
Lung sections of Dex-Pc rats were from those inoculated with the full load (7.8 × 106 trophozoites) at the day 6 time point. This time point was chosen because the alveoli had not completely filled with P. carinii organisms and exudates so that alveolar macrophages could still be identified and counted. Dex rats had an average of 2.96 ± 0.23 alveolar macrophages per thousandfold-magnified field before lavage and 0.249 ± 0.04 alveolar macrophages after lavage. Healthy rats had similar patterns: 3.05 ± 0.26 and 0.234 ± 0.04 alveolar macrophages before and after lavage, respectively (P = 0.2532). Lung sections from Dex-Pc rats had many fewer macrophages (1.06 ± 0.07) before lavage, but a similar number of macrophages remained in the rat lung sections after lavage (0.22 ± 0.03; P = 0.318), agreeing well with our results derived from BAL fluids.
The significant reduction in alveolar macrophage number in Dex-Pc rats before lavage compared to both Dex and healthy rats (P = 0.0002) indicates that fewer alveolar macrophages are indeed present in the lungs of Dex-Pc rats. The observation that similar numbers of alveolar macrophages were present in lung sections of all three groups of rats after lavage indicates that the decrease in macrophage counts in lavage samples are not due to increased retention of macrophages in the lung during P. carinii infection.
Representative lung sections of each condition are shown in Fig. 3. Lung sections from healthy and Dex rats had numerous alveolar macrophages before lavage. The lavage process removed nearly all of those cells. Sections of Dex-Pc rats had few alveolar macrophages before lavage and very few alveolar macrophages after lavage, indicating that the lavage process also removed them all.
FIG. 3.
Alveolar macrophages in lung tissue before and after BAL. Lungs sections from healthy (Normal), dexamethasone-treated (Dex.), and P. carinii-infected (Infected) rats before and after lavage were assessed for alveolar macrophage number. Upper panels are sections of lungs before lavage, and lower panels are those after lavage. Arrows indicate alveolar macrophages.
Alveolar macrophage number in treatment and recovery.
Experiments were then performed to determine whether the alveolar macrophage number would return to normal levels in Dex-Pc rats when P. carinii organisms were eradicated by drugs or by restoration of host immune functions. Dex-Pc rats were treated with trimethoprim-sulfamethoxazole (50 and 250 mg/kg/day, respectively) starting on day 21 after inoculation with various loads (1, 0.5, 0.25, and 0.125 times) of P. carinii trophozoites. After 6 days of treatment (day 27), alveolar macrophage number rebounded in animals that received 1, 0.5, or 0.25 times the normal load, but in each case this increase did not return to the level observed in Dex rats (Table 1). In those inoculated with 0.125 times the normal load, a significant increase in alveolar macrophage number was not noted until 13 days (day 34) after initiation of treatment. Further increase in alveolar macrophage number was observed after 20 days (day 41) of treatment (Table 1).
In treatment rats, the degree of increase in alveolar macrophage number correlated with the initial inoculation load. After 6 days (day 27) of treatment, alveolar macrophage numbers were increased the most in animals that received full initial loads (76.0% increase), while those inoculated with 0.5 and 0.25 times the normal load had smaller increases (71.6 and 29.3% increases, respectively). Thirteen days (day 34) after initiation of treatment, rats that had received the three larger loads (1, 0.5, and 0.25 times) showed further increases in alveolar macrophage numbers, but these numbers remained below the level observed in the Dex rats. A further increase in alveolar macrophage number was observed after 20 days (day 41) of treatment in all rats (Table 1). As observed at other time points, in no treatment group did this increase equal the number noted in the Dex rats at this time point (P < 0.05).
Recovery rats, those which were allowed to recover their immune systems by removing dexamethasone from their drinking water after 21 days of infection, also showed a large rebound in alveolar macrophage number at 6 days of recovery (day 27) (Table 1). For the full load condition, 6 days after initiation of recovery, the alveolar macrophage number increased to (6.16 ± 0.10) × 106. This is above that of the healthy or Dex rats (Table 1; P < 0.05 versus healthy or Dex rats at day 27). Those that received the lower three doses of P. carinii also increased their alveolar macrophages to or above the control level after 6 days of recovery. After 6 days of recovery (day 27), the alveolar macrophage numbers in the animals that received the lower three doses of P. carinii also increased to the control level or more (Table 1).
Thirteen days (day 34) after cessation of immunosuppression, the animals that had received a full load of organisms had a significant reduction in alveolar macrophage numbers [(5.18± 0.18) × 106] compared to the day 27 levels [(6.16± 0.1) × 106] (P = 0.0011), but these numbers remained above control levels (Table 1). In contrast, animals that had received the three lower loads showed a further increase in alveolar macrophage number from day 27 to day 34. At day 41 (20 days of recovery), the alveolar macrophage number in rats inoculated with the three larger loads was reduced (Table 1). Only in the group that received 0.25 times the normal load was the alveolar macrophage number greater than those observed in healthy or Dex controls after 20 days of recovery. Compared to drug-treated rats, recovery rats showed significantly more alveolar macrophages for each condition tested at each time point (P < 0.05).
Correlation of alveolar macrophage number with P. carinii burden during treatment and recovery.
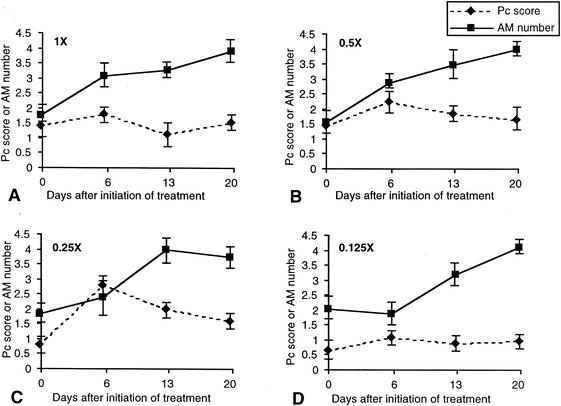
An increase in alveolar macrophage number was seen in Dex-Pc rats infected with 1, 0.5, and 0.25 times the normal loads after only 6 days (day 27) of treatment with trimethoprim-sulfamethoxazole despite increases in P. carinii organism burden over this same time period (Table 1; Fig. 4). The alveolar macrophage number continued to increase as the organism burden began to decrease (Fig. 4). Rats inoculated with 0.125 times the normal load and treated starting on day 21 showed no immediate increase in alveolar macrophages but a slight increase in organism burden 6 days after initiation of treatment, and this was followed by a slow increase in macrophages and a slight decrease in organism burden. It is important to note that over this same time period, and in all initial loads tested, the organism number decreased over time, but the infection was never completely cleared (Fig. 4).
FIG. 4.
Correlation of P. carinii (Pc) burden with alveolar macrophage (AM) number during treatment of PCP. The symbols and scales are the same as those of Fig. 1. All numbers shown are the averages of at results for least three rats in the same experimental group (error bars, standard deviations). Immunosuppressed rats were inoculated at day 0 with different loads of P. carinii: 7.8 × 106 trophozoites (1 times the normal load) (A), 3.9 × 106 trophozoites (0.5 times the normal load) (B), 1.95 × 106 trophozoites (0.25 times the normal load) (C), and 9.8 × 104 trophozoites (0.125 times the normal load) (D). These rats were treated with trimethoprim-sulfamethoxazole (50 and 250 mg/kg/day, respectively) starting on day 21 and were on an immunosuppression regimen throughout the entire 41-day study period.
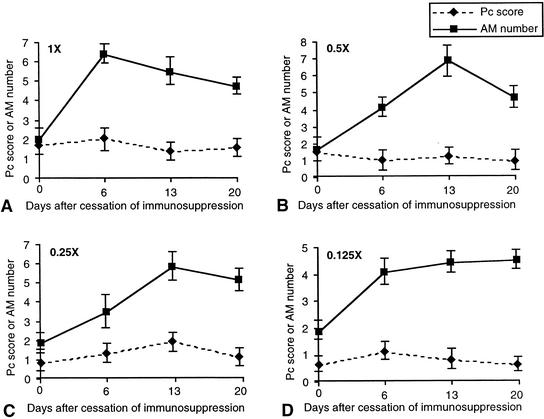
Compared to treatment rats, recovery rats showed faster, more-dramatic increases in alveolar macrophage number, but this did not result in a faster resolution of infection (Fig. 5). In each inoculation group, recovery rats had organism burdens lower than those observed in treatment rats at 6 (day 27) and 13 (day 34) days after cessation of immunosuppression, suggesting that recovery of the immune system is more effective than drug treatment at controlling P. carinii number. However, both recovery and treatment rats had similar organism burdens in all inoculation groups at day 41 (20 days of treatment or recovery).
FIG. 5.
Correlation of P. carinii (Pc) burden with alveolar macrophage (AM) number during recovery from P. carinii pneumonia. The symbols and scales are the same as those of Fig. 1. All numbers shown are the averages of results for three rats in the same experimental group (error bars, standard deviations). Immunosuppressed rats were inoculated at day 0 with different loads of P. carinii: 7.8 × 106 trophozoites (1 times the normal load) (A), 3.9 × 106 trophozoites (0.5 times the normal load) (B), 1.95 × 106 trophozoites (0.25 times the normal load) (C), and 9.8 × 104 trophozoites (0.125 times the normal load) (D). These rats were allowed to recover their immunity by cessation of immunosuppression starting on day 21.
During the first 6 days of recovery, the organism burden increased in all inoculation groups except in those inoculated with 0.5 times the normal loads, in which there was a slight decrease in organism number. At the point when alveolar macrophage number was the highest (6 days for 1 times the normal and 13 days for 0.5, 0.25, and 0.125 times the normal load after cessation of immunosuppression), the organism burden was also the greatest. The organism burdens in all inoculation groups were not reduced to zero even though the macrophage number was increased to normal or above-normal levels (Fig. 5).
DISCUSSION
Several studies in the 1980s and 1990s showed that the number of alveolar macrophages in BAL fluid from AIDS patients with PCP was reduced relative to other cell types (7, 8, 11, 42, 44). It was unknown whether this change is due to a decrease in the number of alveolar macrophages or an increase in other cell types. In this study, we determined the absolute number of alveolar macrophages in BAL fluid and found that there is a 58% decrease in the absolute number of alveolar macrophages in Dex-Pc rats compared to immunosuppressed, noninfected rats. Several methods were used to confirm that the alveolar macrophage number in Dex-Pc rats was reduced. Absolute numbers of alveolar macrophages in BAL fluid from Dex-Pc rats were counted and compared to those for uninfected controls. Alveolar macrophages were also counted in sections of infected and control lungs to confirm that in situ counts correlate with counts in BAL fluid (Fig. 3). Finally, the percentages of alveolar macrophages, relative to other immune cell types, in BAL fluids were determined. In all cases studied, alveolar macrophages were decreased only in immunosuppressed, Dex-Pc rats; immunosuppression alone did not lower the number or percentage of these cells. Late in the infection, the number of neutrophils increased while the number of alveolar macrophages remained decreased. The role of neutrophils in the host response and inflammation associated with PCP is discounted because the increase occurs only very late in infection in AIDS patients (15), but neutrophil number does correlate inversely with prognosis in these patients (5, 18).
Previous investigations of alveolar macrophage numbers in PCP had been conducted with samples from human patients. Although the information is useful, it is not possible to manipulate the parameters of infection, treatment, or host response in humans to further investigate this phenomenon. An animal model of PCP in which the alveolar macrophage number decreases during P. carinii infection in a fashion similar to that in human PCP is needed. The present study of rats shows that alveolar macrophages are 42.9% of the total cells in BAL fluid during PCP. These results agree well with previous studies of humans with PCP, which showed that percentages of alveolar macrophages in PCP are between 38.7% (7) and 45% (28). Therefore, this study represents the first evidence that the alveolar macrophage number in a rodent model of PCP is reduced just as it is in the human form of the disease. This discovery will enable further studies to investigate the possible mechanisms for the decrease in alveolar macrophage number during P. carinii infection.
The decrease in alveolar macrophage number occurred very early in this model of P. carinii infection. Six days after inoculation with 7.8 × 106 P. carinii trophozoites, Dex-Pc rats lost 58% of their alveolar macrophages (Fig. 2). The decrease in alveolar macrophage number noted in these rats is even more profound when compared to the response of healthy rats challenged with the same number of P. carinii trophozoites. These healthy challenged rats responded to P. carinii with alveolar macrophage numbers increased by 54% (Table 1). Therefore, the total decrease in alveolar macrophage number in Dex-Pc rats is the decrease in these rats plus the increase in healthy challenged rats.
In trimethoprim-sulfamethoxazole-treated rats, the alveolar macrophage number was slowly increased, and this was followed by a decrease in organism burden over the 21 days of treatment (Fig. 4). This rebound in alveolar macrophage number takes place in the presence of continued immunosuppression; therefore, the only variable in this system is the change in organism number. A swifter increase in alveolar macrophages was observed in recovery rats (Fig. 5). In addition, larger initial loads resulted in a more rapid decrease in alveolar macrophage number (Table 1), and smaller loads (0.125 times the normal load) of P. carinii caused no increase in alveolar macrophage number in healthy rats (Fig. 1D). Taken together, these results suggest a strong relationship between the organism burden and the alveolar macrophage number in the host. We conclude that the P. carinii organism number is partially responsible for the control of alveolar macrophage number in infected and challenged hosts.
The decrease in alveolar macrophage number is significant in the host response to the organism. The alveolar macrophage is an important cell for the removal of P. carinii from the alveoli. The alveolar macrophage phagocytoses the organism (6, 15, 16) and liberates reactive oxygen and cytotoxic metabolites in response to P. carinii (36). Loss of the alveolar macrophage population may diminish the removal of P. carinii from the lower respiratory tract, permitting proliferation of the organism (15). In addition, the alveolar macrophage plays a role in limiting the inflammatory response in the lung (9, 10, 20). Alveolar macrophage also regulates the antigen processing ability of dendritic cells (12) and limits the proliferation and function of lymphocytes (9, 20). With a loss of alveolar macrophages, the regulation of the inflammatory response is compromised, which may lead to the fulminant inflammation and pulmonary damage seen in PCP.
There may be many causes for the decrease in alveolar macrophage number. P. carinii infection may cause alveolar macrophages to die or to leave the lung, thus reducing the number of alveolar macrophages. Lack of recruitment of macrophages or peripheral blood monocytes to the lung is another possibility. The epithelial lining of alveoli, in particular the type II pneumocyte, releases chemokine RANTES (35), monocyte chemotactic protein 1 (23, 30), and granulocyte-macrophage colony-stimulating factor (4). Removal of any of these factors may decrease alveolar macrophage chemotaxis (21). Alveolar epithelial cells also produce significant amounts of the complement factor C5 (38); its cleavage product, C5a, is a powerful chemoattractant for macrophages and monocytes. If C5 is depleted in the lung, there would be a decrease in alveolar macrophage migration (17). Since there is significant damage to the alveolar epithelial cells during PCP (41), the production of chemoattractants would be diminished. There may also be decreased differentiation of monocytes to alveolar macrophages during P. carinii infection. Our recent discovery of GATA-2 down-regulation in resident lung monocytes during P. carinii infection (32) supports this possibility, since GATA-2 regulates differentiation (25, 32, 34).
Acknowledgments
This study was supported by NIH grant RO1 HL65170.
REFERENCES
- 1.Bartlett, M. S., J. A. Fishman, M. M. Durkin, S. F. Queener, and J. W. Smith. 1988. A new rat model of Pneumocystis carinii infection. J. Clin. Microbiol. 26:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benfield, T. L., B. Lundgren, J. H. Shelhammer, and J. D. Lundgren. 1999. Pneumocystis carinii major surface glycoprotein induces interleukin-8 and monocyte chemoattractant protein-1 release from a human alveolar epithelial cell line. Eur. J. Clin. Investig. 29:717-722. [DOI] [PubMed] [Google Scholar]
- 3.Chen, W., J. W. Mills, and A. G. Harmsen. 1992. Development and resolution of Pneumocystis carinii pneumonia in severe combined immunodeficient mice: a morphological study of host inflammatory responses. Int. J. Exp. Pathol. 73:709-720. [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen, P. J., L. R. Armstrong, J. J. Fak, G. H. Chen, R. A. McDonald, G. B. Toews, and R. Paine III. 1995. Regulation of rat pulmonary dendritic cell immunostimulatory activity by alveolar epithelial cell-derived granulocyte-macrophage colony-stimulating factor. Am. J. Resp. Cell Mol. Biol. 13:426-433. [DOI] [PubMed] [Google Scholar]
- 5.Colangelo, G., R. P. Baughman, M. N. Dohn, and P. T. Frame. 1991. Follow-up bronchoalveolar lavage in AIDS patients with Pneumocystis carinii pneumonia. Pneumocystis carinii burden predicts early relapse. Am. Rev. Resp. Dis. 143:1067-1071. [DOI] [PubMed] [Google Scholar]
- 6.Ezekowitz, R. A. B., D. J. Williams, H. Koziel, M. Y. K. Armstrong, A. Warner, F. F. Richards, and R. M. Rose. 1991. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 351:155-158. [DOI] [PubMed] [Google Scholar]
- 7.Fleury, J., E. Escudier, M-J. Pochelle, C. Carre, and J. F. Bernaudin. 1985. Cell population obtained by bronchoalveolar lavage in Pneumocystis carinii pneumonitis. Acta Cytol. 29:721-728. [PubMed] [Google Scholar]
- 8.Fleury-Feith, J., J. T. V. Nhieu, C. Picard, E. Escudier, and J. F. Bernaudin. 1989. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Chest 95:1198-1203. [DOI] [PubMed] [Google Scholar]
- 9.Holt, P. G. 1986. Down-regulation of the immune responses in the lower respiratory tract: the role of the alveolar macrophages. Clin. Exp. Immunol. 63:261-270. [PMC free article] [PubMed] [Google Scholar]
- 10.Holt, P. G., J. Oliver, N. Bilyk, C. McMenamin, G. Kraal, and T. Thepen. 1993. Down-regulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Med. Exp. 177:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huhn, C., C. Nerl, A. Siebert, L. Hogl, W. Doering, W. Kaboth, and D. Eichenlaub. 1990. The prognostic significance of lymphocyte subpopulations and macrophages in peripheral blood and in bronchoalveolar lavage in AIDS patients with suspected Pneumocystis carinii pneumonia. Klin. Wochenschr. 68:853-856. [DOI] [PubMed] [Google Scholar]
- 12.Kolls, J. K., J. M. Beck, S. Nelson, W. R. Summer, and J. Shellito. 1993. Alveolar macrophage release of tumor necrosis factor during murine Pneumocystis carinii pneumonia. Am. J. Resp. Cell. Mol. Biol. 8:370-376. [DOI] [PubMed] [Google Scholar]
- 13.Lanken, P. N., M. Minda, G. G. Pietra, and A. P. Fishman. 1980. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am. J. Pathol. 99:561-588. [PMC free article] [PubMed] [Google Scholar]
- 14.Laursen, A. L., N. Obel, J. Rungy, and P. L. Andersen. 1993. Phagocytosis and stimulation of the respiratory burst in neutrophils by Pneumocystis carinii. J. Infect. Dis. 168:1466-1471. [DOI] [PubMed] [Google Scholar]
- 15.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masur, H., and T. C. Jones. 1978. The interaction of Pneumocystis carinii with macrophages and L-cells. J. Exp. Med. 147:157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGavran, P. D., C. J. Butterick, and A. R. Brody. 1990. Tritiated thymidine incorporation and the development of an intestinal lesion in the bronchiolar-alveolar regions of the lungs of normal and complement-deficient mice after inhalation of chrysotile asbestos. J. Environ. Pathol. Toxicol. Oncol. 9:377-392. [PubMed] [Google Scholar]
- 18.Momose, H., and S. Lee. 1991. Pneumocystis carinii as foamy exudate in bone marrow. JAMA 265:1672.. [PubMed] [Google Scholar]
- 19.Neese, L. W., J. E. Standing, E. J. Olson, M. Castro, and A. H. Limper. 1994. Vitronectin, fibronectin, and gp120 antibody enhance macrophage release of TNF-alpha in response to Pneumocystis carinii. J. Immunol. 152:4549-4556. [PubMed] [Google Scholar]
- 20.Nelson, D., D. Strickland, and P. G. Holt. 1990. Selective attrition of non-circulating T cells during normal passage through the lung vascular bed. Immunology 69:476-481. [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien, A., T. J. Standiford, P. J. Christensen, S. E. Wilcoxen, and R. Paine III. 1998. Chemotaxis of alveolar macrophages in response to signals derived from alveolar epithelial cells. J. Lab. Clin. Med. 131:417-424. [DOI] [PubMed] [Google Scholar]
- 22.O'Riordan, D. M., J. E. Standing, K. Y. Kwon, D. Chang, E. C. Crouch, and A. H. Limper. 1995. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J. Clin. Investig. 95:2699-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paine, R., III, M. W. Rolfe, T. J. Standiford, M. D. Burdick, B. J. Rollins, and R. M. Strieter. 1993. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J. Immunol. 150:4561-4570. [PubMed] [Google Scholar]
- 24.Paine, R., III, A. M. Preston, S. Wilcoxen, H. Jin, B. B. Siu, S. B. Morris, J. A. Reed, G. Ross, J. A. Whitsett, and J. M. Beck. 2000. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J. Immunol. 164:2602-2609. [DOI] [PubMed] [Google Scholar]
- 25.Pevny, L., M. C. Simon, E. Robertson, W. H. Klein, S. F. Tsai, V. D'Agati, S. H. Orkin, and F. Costantini. 1991. Erythroid differentiation in chimeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349:257-260. [DOI] [PubMed] [Google Scholar]
- 26.Phelps, D., and R. M. Rose. 1991. Increased recovery of surfactant protein-A in AIDS-related pneumonia. Am. Rev. Resp. Dis. 143:1072-1075. [DOI] [PubMed] [Google Scholar]
- 27.Rice, W. R., F. M. Singleton, M. J. Linke, and P. D. Walzer. 1993. Regulation of surfactant phosphotidylcholine secretion from alveolar type II cells during Pneumocystis carinii pneumonia in the rat. J. Clin. Investig. 92:2778-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadaghdar, H., Z.-B. Huang, and E. Eden. 1992. Correlation of bronchoalveolar lavage findings to severity of Pneumocystis carinii pneumonia in AIDS. Chest 102:63-69. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan, P. M., D. C. Stokes, Y. Yeh, and W. T. Hughes. 1986. Surfactant phospholipids and lavage phospholipase A2 in experimental Pneumocystis carinii pneumonia. Am. Rev. Resp. Dis. 134:526-531. [DOI] [PubMed] [Google Scholar]
- 30.Standiford, T. J., S. L. Kunkel, S. H. Phan, B. J. Rollins, and R. M. Strieter. 1991. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J. Biol. Chem. 266:9912-9918. [PubMed] [Google Scholar]
- 31.Stringer, J. R., C. B. Beard, R. F. Miller, and A. E. Wakefield. 2002. A new name (Pneumocystis jiroveci) for pneumocytsis from humans. Emerg. Infect. Dis. 8:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, X., M. E. Lasbury, D. D. Davidson, M. S. Bartlett, J. W. Smith, and C. H. Lee. 2000. Down-regulation of GATA-2 transcription factor during Pneumocystis carinii infection. Infect. Immun. 68:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai, F.-Y., G. Keller, F. C. Kuo, M. Weiss, J. Chen, M. Rosenblatt, F. W. Alt, and S. H. Orkin. 1994. An early hematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371:221-226. [DOI] [PubMed] [Google Scholar]
- 34.Tsai, F.-Y., and S. H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89:3636-3643. [PubMed] [Google Scholar]
- 35.van oud Albas, A. B., B. van der Linden-Schrever, and R. van Furth. 1983. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intra-alveolar administration of aerosolized heat-killed BCG. Am. Rev. Resp. Dis. 128:276-281. [DOI] [PubMed] [Google Scholar]
- 36.Vassallo, R., C. F. Thomas II, Z. V. Pavlovic, and A. H. Limper. 1999. Alveolar macrophage interactions with Pneumocystis carinii. J. Lab. Clin. Med. 133:535-540. [DOI] [PubMed] [Google Scholar]
- 37.Vassallo, R., J. E. Standing, and A. H. Limper. 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol. 164:3755-3763. [DOI] [PubMed] [Google Scholar]
- 38.Warheit, D. B., G. George, L. H. Hill, R. Synderman, and A. R. Brody. 1985. Inhaled asbestos activates a complement-dependent chemotactic factor for macrophages. Lab. Investig. 52:505-514. [PubMed] [Google Scholar]
- 39.Warschkau, H., H. Yu, and A. F. Kiderlen. 1998. Activation and suppression of natural cellular immune functions by Pneumocystis carinii. Immunobiol. 198:343-360. [DOI] [PubMed] [Google Scholar]
- 40.Yamin, M., D. Lazarus, E. E. Schneeberger, K. McCarthy, W. Xia, and R. Kradin. 1990. Anti-RMA: a murine monoclonal antibody that activates rat macrophages. I. Distribution and characterization of the RMA antigen. Am. J. Resp. Cell Mol. Biol. 2:207-215. [DOI] [PubMed] [Google Scholar]
- 41.Yoneda, K., and P. D. Walzer. 1983. Attachment of Pneumocystis carinii to type I alveolar cells: study by freeze fracture electron microscopy. Infect. Immun. 40:812-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, J. A., J. W. Stone, R. J. S. McGonigle, A. Adu, and J. Michael. 1986. Diagnosing Pneumocystis carinii pneumonia by cytological examination of bronchoalveolar lavage fluid: a report of 15 cases. J. Clin. Pathol. 39:945-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu, M. L., and A. H. Limper. 1997. Pneumocystis carinii induces ICAM-I expression in lung epithelium cells through a TNF-α mediated mechanism. Am. J. Physiol. 273:L1103-L1111. [DOI] [PubMed] [Google Scholar]
- 44.Yung, K. R., Jr., J. A. Rankin, G. P. Naegel, E. S. Paul, and H. Y. Reynolds. 1985. Bronchoalveolar lavage cells and proteins in patients with acquired immunodeficiency syndrome. Ann. Intern. Med. 103:522-553. [DOI] [PubMed] [Google Scholar]