Abstract
The role of pneumococcal (Pnc) surface adhesin A (PsaA) in the adherence of Streptococcus pneumoniae (pneumococcus) to host cells is not well defined. We examined the effect of anti-PsaA antibodies in an inhibition of adherence assay using Detroit 562 nasopharyngeal human epithelial cells. Rabbit polyclonal (Pab) anti-recombinant PsaA (rPsaA) sera, a purified mouse monoclonal antibody (MAb) (MAb 6F62G8E12), and 22 healthy adult sera with known anti-PsaA IgG levels (obtained by enzyme-linked immunosorbent assay) were evaluated for their abilities to inhibit Pnc adherence to confluent monolayers (measured as percent reduction in CFU counts compared to those of uninhibited controls). Pnc adherence was dependent on capsular phenotype (no or low adherence for opaque strains). With an inoculum of 104 to 105 bacteria/well, the mean ± standard deviation count in controls was 163 ± 32 CFU/well for transparent strains. Low adherence was observed for a PsaA-minus mutant even at higher inoculum doses. Mean percent inhibitions of adherence with Pab and MAb were 54 and 50%, respectively. Adult sera showed inhibition in a dose-response fashion with a range of 98 to 8%, depending on the serum anti-PsaA antibody concentration. Absorption of Pab with rPsaA restored Pnc adherence to control levels. Absorption of sera with a PsaA-minus mutant did not result in a significant decrease (P >0.05) of inhibition of adherence activity. Additionally, nearly 100% of Pnc adherence was inhibited by lipidated rPsaA at 2.5 μg/ml. Our data support the argument that PsaA is an adhesin that mediates Pnc adherence to human nasopharyngeal cells. This functional assay may be useful in evaluating antibodies elicited in response to PsaA vaccination.
Streptococcus pneumoniae (pneumococcus) is one of the leading causes of infant mortality worldwide. The high rates of disease observed after infections with this bacterium are largely due to the fact that humans of all ages can be colonized by pneumococcus. Some persons develop disease after colonization, whereas others remain asymptomatic carriers. In an effort to reduce the burden of pneumococcal (Pnc) disease, multiple vaccine formulations have been developed based upon the immunogenicity that is generated by the type-specific capsular polysaccharides. Currently, two types of formulations are licensed in the United States, a 23-valent polysaccharide vaccine and a 7-valent protein conjugated-polysaccharide vaccine (5, 8, 9). The conjugated-polysaccharide vaccines have been shown to reduce Pnc colonization in some populations (14). However, there is still the risk of replacement (infection with other serotypes not included in the vaccine) (S. K. Obaro, R. A. Adegbola, W. A. S. Banya, and B. M. Greenwood, Letter, Lancet 348:271-272, 1996) and serotype switching (natural genetic transformation from one serotype to another) (10). There is a possibility for unmasking of nonvaccine serotypes present at lower levels than the vaccine serotype (19). In addition, the serotype coverage of the conjugated-polysaccharide vaccines is limited depending on the geographic area.
A third generation of Pnc vaccines is under development. These vaccines are based on common proteins (present in all 90 known Pnc serotypes) that are immunogenic in humans after infection and in vaccinated animals (6). The candidate proteins for these vaccines are primarily Pnc surface adhesin A (PsaA), Pnc surface protein A (PspA), pneumolysin (Ply), and PspC, although other common proteins are currently under investigation (3, 6, 22). PsaA is a putative Pnc adhesin and an ABC transporter for manganese (16). The role of naturally developed antibodies to PsaA in prevention of colonization in humans has been previously demonstrated (24). Anti-PsaA antibodies can reduce Pnc colonization and carriage in mice and protect chinchillas from otitis media (6; S. I. Pelton, M. Figueira, R. Albut, and J. Reino, Program Abstr. 2nd Int. Symp. Pneumococci Pneumococcal Dis. 2000, abstr. O38, 2000). Other studies of mice have indicated that antibodies to PsaA can prevent colonization, whereas antibodies to PspA, for example, can reduce bacteremia and pneumonia. When both proteins are combined, a much higher level of protection was observed in mice (6, 22). A recent report demonstrated protection in mice against Pnc lung colonization and septicemia after oral immunization with PsaA (29).
Although the immune response to PsaA antibodies can be measured by enzyme-linked immunosorbent assay (ELISA), there is the need for the development of functional assays that measure the in vivo biological activity of the antibodies formed in response to vaccination. This study demonstrates that anti-PsaA antibodies naturally developed in humans or elicited by recombinant PsaA (rPsaA) in animals can prevent the adherence of pneumococci to nasopharyngeal epithelial cells. This inhibition of adherence assay can be used for the measurement of the functional activity of anti-PsaA antibodies.
(This work was presented in part at the 101st General Meeting of the American Society for Microbiology [poster E-80].)
MATERIALS AND METHODS
Bacterial strains.
Pnc strains were isolated from nasopharyngeal swabs as part of a Pnc colonization vaccine trial conducted in the Navajo nation (K. L. O'Brien, M. A. Bronsdon, G. M. Carlone, R. R. Facklam, B. Schwartz, R. R. Reid, and M. Santosham, Abstr. 70th Annu. Meet. Soc. Pediatr. Res. 2001, abstr. 1463, 2001). Other Pnc strains were obtained from invasive isolates sent to the Centers for Disease Control and Prevention (CDC) for bacterial identification and serotyping (strains provided by Richard Facklam, Streptococcal Reference Laboratory, CDC). All strains were screened for their phenotypic expression of opacity factors, following the methodology described by Weiser et al. (31). Only strains that were transparent in phenotype were used for adherence studies (12). Transparent strains have been reported to have a lower capsular polysaccharide content than opaque strains (17). We selected both well-characterized transparent strains which are nearly 100% transparent as previously described (17) and recent nasopharyngeal isolates (O'Brien et al., Abstr. 70th Annu. Meet. Soc. Pediatr. Res. 2001) for which ≥75% of the colonies were of the transparent phenotype. A complete list of strains used in this study is given in Table 1. Pnc strains were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract to an optical density at 420 nm of 0.4 to 0.6 (mid-logarithmic growth phase for the majority of strains tested) and stored frozen in the same medium after addition of 15% sterile glycerol. Viability of these frozen Pnc stocks for adherence assays was determined to be as long as 8 months.
TABLE 1.
Adherence of S. pneumoniae to Detroit 562 cells
| S. pneumoniae serotype | Strain | Inoculum size (CFU/well) | Mean adherence [CFU/well (CV)]c | Phenotype | Source and/or reference |
|---|---|---|---|---|---|
| 2 | D39 | 105 | 155 (3)* | Mixed | 4 |
| 2 | PsaA− | 106 | 29 (72) | Intermediate | 4 |
| 4 | 0215-95 | 104 | 6 (72) | Opaque | Blood (26) |
| 6A | 3666 | 104 | 189 (25)* | Transparent | NPd |
| 6B | DS2212-94 | 104 | 13 (66) | Opaque | Blood (26) |
| 9V | P64 | 105 | 217 (21)* | Transparent | 17 |
| 18C | SP116 | 104 | 4 (70) | Opaque | SSIa |
| 18C | P73 | 105 | 114 (18)* | Transparent | 17 |
| 19A | 91/6571 | 104 | 276 (8)* | Mixed | CDCb |
| 19F | 6133 | 104 | 169 (19)* | Transparent | NPd |
| 23F | SP264 | 104 | 475 (11)* | Intermediate | 10 |
| 23F | 3476 | 103 | 133 (19)* | Mixed | NPd |
SSI, Statens Seruminstitut, Denmark (source unknown).
Multidrug-resistant strain originally reported by Steve McDougal and currently available at the Streptococcal Reference Laboratory, CDC, Atlanta, Ga.
An asterisk designates the percent coefficient of variance (CV) for adherent serotypes (mean CV = 16).
O’Brien et al., Abstr. 70th Annu. Meet. Soc. Pediatr. Res. 2001.
Tissue culture cells.
Nasopharyngeal human carcinoma epithelial cells (Detroit 562 cells) were purchased from the American Type Culture Collection, Manassas, Va. (CCL138). Cells were thawed and reconstituted in minimal essential medium with Earle's salts (EMEM) and without l-glutamine (Life Technologies, Grand Island, N.Y.) supplemented with 10% fetal calf serum (HyClone, Logan, Utah). Cells were grown in 75-cm2 tissue culture bottles (Corning Costar Co., Cambridge, Mass.) at 37°C and in a 5% CO2 atmosphere. After a period of 6 days cells reached confluency and were passed to a new culture flask and/or seeded to 96-well tissue culture treated plates (Corning Costar Co., Corning, N.Y.) for use in the adherence assays. Only confluent monolayers were used for the adherence assays. When monolayers that were only ∼90% confluent were used, there was a loss of integrity of the monolayer, resulting in lower numbers of adherent CFU. Monolayers that were 1 to 2 days older than the maximum confluence period maintained integrity during the performance of the assay; however, there was higher variability in the number of adherent CFU. Cell monolayers were released by incubation with 0.05% trypsin solution and 0.53 mM EDTA (Life Technologies) for 10 to 15 min at 37°C. To enhance the effect of the trypsin-EDTA solution, all of the culture medium was removed and the monolayer was initially rinsed with 2 ml of the trypsin-EDTA solution, which was immediately discarded, and an additional 2 ml of trypsin-EDTA was added. A 70-ml volume of fresh tissue culture medium was added to the trypsin-treated monolayers, and the cells were gently resuspended in a single-cell suspension before counting of the viability by trypan blue (0.4%) exclusion. Cell suspensions were adjusted by addition of EMEM to yield either 1 × 105 or 2 × 105 cells/ml. Stock cultures were seeded at 1 × 105cells/ml, and tissue culture microtiter plates were seeded with 200 μl of a 2 × 105 cells/ml suspension per well, equivalent to 4 × 104 cells/well. All cultures were grown in the absence of antibiotics. The final cell yield was 1.2 × 105 cells/well after 6 days of incubation.
Adherence assays.
Confluent monolayers of Detroit 562 cells grown in 96-well tissue culture plates were washed twice with EMEM (200 μl/well). A 130-μl volume of EMEM was added to each well, and bacterial suspensions of various densities (103 to105 bacteria/well) were added in a 20-μl volume of Hanks buffer with Ca2+ and Mg2+ (Life Technologies) and supplemented with 0.2% bovine serum albumin. Bacteria were allowed to adhere to the monolayer by incubation at 37°C for 2 h without shaking in a 5% CO2 atmosphere. Time course experiments for optimal adherence were conducted with 30-min intervals ranging from 0.5 to 3 h by incubation of the infected monolayers with 104 bacteria/well. After the 2-h incubation period, an average of 150 to 200 CFU adhered to each well. Nonadherent bacteria were removed by five washes with phosphate-buffered saline (200 μl/well; 100 mM, pH 7.2) supplemented with 0.2% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.). Extended incubation periods (>2 h) resulted in higher adherence rates and lower percentages of inhibition by the test sera (data not shown). Monolayers were maintained moist at all times, so a thin film of buffer was left after each wash, and plate covers were replaced in between washes. A 65-μl volume of bacterial growth medium cooled to 42°C was added to each microtiter well. The bacterial growth medium was composed of Todd-Hewitt broth supplemented with 0.5% yeast extract, 0.5% agar, and 0.1% 2,3,5-triphenyl tetrazolium chloride (Difco Laboratories Inc., Detroit, Mich.) for improved visualization of CFU. Microtiter plates were allowed to solidify before incubation overnight at 37°C and in a 5% CO2 atmosphere. Adherent CFU were quantitated by manual count under a stereoscope or by use of an automated colony counter (AlphaImager; Alpha Innotech, San Leandro, Calif.). Higher coefficients of variance were found in the outer wells of the microtiter plate, rows A and H and columns 1 and 12; therefore, these wells were not used for adherence assays, even if confluent cell growth was observed. These wells were washed and treated similarly and served as sterility and cross-contamination controls for each plate. Wells without inoculum helped in determining if the monolayers maintained integrity during the entire assay.
Inhibition of adherence.
Six 10-fold dilutions of test serum were performed in a 100-μl final volume in sterile microtiter plates. A 40-μl volume of bacterial suspension usually containing 2 × 104 bacteria per well was added to the diluted sera and allowed to incubate for 15 min at 37°C and in a 5% CO2 atmosphere. The initial bacterial inoculum was adjusted for some Pnc serotypes (103 bacteria/well for serotype 23F and 105 bacteria/well for serotype 18C) to yield an average of 150 CFU/well. However, for most serotypes 104 bacteria/well was a sufficient inoculum to yield enough adherent CFU per well. Seventy microliters of this mixture was transferred to the washed tissue culture monolayers containing 80 μl of EMEM. Assays were run in duplicate for each serum sample. The remainder of the assay was performed as described above for adherence assays. Colony counts for each well were compared to the mean of the adherence control wells (12 wells, columns 2 and 11 in the microtiter plate). The percent inhibition of adherence was calculated for each serum sample as well as for reaction mixtures containing various concentrations of lipidated rPsaA (15). Inhibitions of adherence were only calculated if the mean adherence in the control wells was between 2 standard deviations (SD) of the calculated mean adherence for a given target strain, as shown in Table 1.
The following parameters were found to be optimal for assay performance: (i) confluent cell monolayers grown for 6 days in 96-well microtiter plates, (ii) use of EMEM for initial washing of the monolayers, (iii) addition of EMEM (80 μl) to monolayers immediately after the initial washes, (iv) use of a bacterial dilution that yields 120 to 150 adherent CFU per well, (v) serum dilutions starting at 10−1, (vi) a 2-h incubation period to allow for bacterial adherence, and (vii) a minimum of five washes to remove nonadherent bacteria. Although we primarily used a 10-fold serum dilution scheme, other dilution schemes (two-, three-, or five-fold) have been tested successfully. This parameter can be varied according to the serum antibody levels.
Serum samples.
Rabbit polyclonal (Pab) sera specific for the lipidated rPsaA protein (15), a purified mouse monoclonal antibody (MAb) (MAb 6F62G8E12) (11), and normal adult sera (n = 22) with known anti-PsaA immunoglobulin G (IgG) concentrations (shown by enzyme-linked immunosorbent assay [ELISA]) were evaluated for their ability to inhibit Pnc adherence to confluent monolayers. Rabbits (5-month-old New Zealand White rabbits; n = 3) received two subcutaneous doses in the dorsal area between the shoulders. Each dose consisted of 10 mg of rPsaA in 100 ml of alum [Al(OH)3]-phosphate-buffered saline. Doses were given 3 weeks apart, and animals were bled 21 days after dose 1 and 25 days after dose 2. Preimmunization serum was collected 5 days prior to dose 1. Normal healthy donor sera (age range, 24 to 62 years) were obtained through the Emory donor services, Atlanta, Ga. Sera were stored frozen at −70°C in 100-μl aliquots until ready to use. The ELISA methodology used was previously reported (24, 30). Anti-PsaA IgG concentrations determined by ELISA were calculated in micrograms per milliliter and compared to the U.S. National Reference Preparation (USNRP no. IS1644; Centers for Disease Control and Prevention, Atlanta, Ga.) used for determinations of specific human serum proteins (25) and after cross-standardization against the Food and Drug Administration (Bethesda, Md.) reference serum lot 1983 standard (anti-polyribosyl-ribitol phosphate IgG antibodies [60.9 μg/ml]) for Haemophilus influenzae type b (20).
Statistical analysis.
Inhibition of Pnc adherence was calculated as percent reduction in CFU counts per well compared to uninhibited adherence controls. Significant differences between two sets of data were calculated by the t test, with significance defined as a P of <0.05. The Mann-Whitney rank sum test was used when the data were not normally distributed. Antibody concentrations determined by ELISA were calculated against a standard curve using the four-parameter logistic log curve-fitting technique (23). The Pearson product moment correlation coefficient was used for comparison among anti-PsaA antibody concentrations and the percent inhibition of adherence. A weighted average of the percent inhibition of adherence observed with a given serum sample was calculated by using the log10 of each serum dilution factor as the weight.
RESULTS
Pnc adherence.
Pnc adherence to nasopharyngeal epithelial cells (Detroit 562) was dependent on capsular phenotype as previously described for other epithelial cells by Cundell et al. (12). There was very low or no adherence for opaque strains regardless of the serotype. The opaque phenotype correlates with high capsular polysaccharide content (17). On the other hand, transparent or intermediate strains demonstrated high adherence to Detroit 562 cells. With an inoculum of 104 bacteria/well, the mean count ± 1 SD for adherence controls was 163 ± 32 CFU/well (coefficient of variation = 20%; n = 420 wells) for transparent strains. In general, the invasive Pnc strain isolates were opaque and the nasopharyngeal Pnc strain isolates were transparent or exhibited a mixture of opaque and transparent colonies. Differential adherence to Detroit 562 cells depending on the strain used is shown in Table 1. Transparent strains of different serotypes appeared to have the same capacity to adhere. Addition of type-specific polysaccharide (500 μg/ml) to the reaction mixture did not result in reduced adherence. However, low adherence (mean = 23 CFU) was observed for a PsaA-minus mutant even at higher inoculum doses of 106 bacteria/well. The absence of the PsaA protein in this mutant was confirmed by negative colony blot staining with the anti-PsaA mouse MAb (MAb 6F62G8E12) and resistance to erythromycin (≤0.2 μg/ml), as previously reported by Berry and Paton (4). Pnc adherence was successfully obtained with tissue cultures of high passage number (passage numbers evaluated were between 40 and 120).
Inhibition of Pnc adherence.
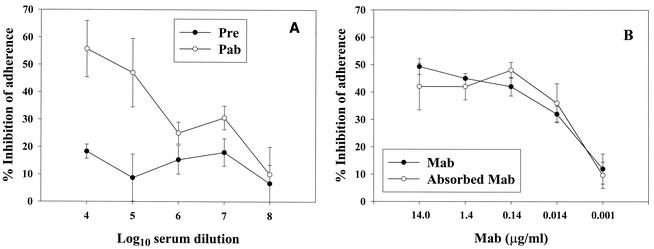
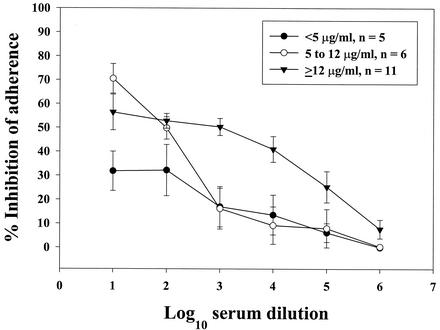
Mean percent inhibition of adherence for five transparent strains with a selected Pab (10−5 dilution) was 54%. Figure 1A gives the mean percent inhibition of adherence obtained with three preimmunization and three Pab rabbit sera for Pnc serotype 19F. No inhibition of adherence was detected in pooled baby rabbit serum or in nonimmunized rabbits. Figure 1B gives the inhibition of adherence (serotype 19F) observed with MAb with and without absorption with the PsaA minus mutant. No significant change (P = 0.95) in the inhibition of adherence was observed after absorption with this mutant. Absorption of a 10−2 dilution of Pab, MAb, and a human serum with a high anti-PsaA concentration with rPsaA (0.5 μg) restored Pnc adherence to control levels for Pab and for the human serum; however, the inhibitory activity of MAb could only be absorbed by 61% (data not shown). Healthy adult donor sera showed inhibition in a dose-response fashion with a range of 98 to 8%, depending on the serum anti PsaA antibody concentration, Fig. 2. The adherence of multidrug-resistant strains, serotype 19A (91/6571) and serotype 23F (SP264), was inhibited 95 and 78% (respectively) by healthy human sera (average inhibition of a 1:10 serum dilution from donors 259 and 7047). Sera with high levels of anti-PsaA antibodies needed to be diluted further to observe the linear portion of the inhibition of adherence. Therefore, it was necessary to use 10-fold-dilution schemes instead of twofold-dilution schemes when testing for serum inhibitory activity.
FIG. 1.
(A) Mean percent inhibition of adherence (± standard error [error bars]) to Detroit 562 cell monolayers by anti-rPsaA antibodies after preabsorption of serum samples with a PsaA-minus mutant (4). Experiments were performed with Pnc serotype 19F (strain 6133). Postimmunization pooled sera were highly reactive with rPsaA by Western blot analysis at a dilution of 1:128,000. Abbreviations: Pre, preimmunization rabbit sera (n = 3, each serum tested in duplicate); Pab, postimmunization rabbit sera (n = 3, each serum tested in duplicate). (B) Mean percent inhibition of adherence (± standard error [error bars]) to Detroit 562 cell monolayers by a purified mouse MAb, 6F62G8E12 (1.4 mg/ml), specific for PsaA (11). Absorption of MAb with a PsaA-minus mutant (4) yielded similar inhibition of adherence (P = 0.95, t test). Three independent experiments with Pnc serotype 19F (strain 6133) were performed in duplicate to calculate the means for absorbed and unabsorbed MAb.
FIG. 2.
Mean percent inhibition of adherence (± standard error [error bars]) to Detroit 562 cell monolayers by normal human donor sera (n = 22). Inhibition assays were performed in duplicate with either Pnc serotype 19F or 18C. Symbols indicate the concentration of anti-PsaA IgG in serum. There was a significant increase (P < 0.05, t test) in the percent inhibition observed with sera containing anti-PsaA IgG antibody anti-PsaA IgG antibody at ≥12 μg/ml when compared to the percent inhibition of sera containing anti-PsaA IgG antibody at <5 μg/ml.
Specificity of the inhibition of Pnc adherence.
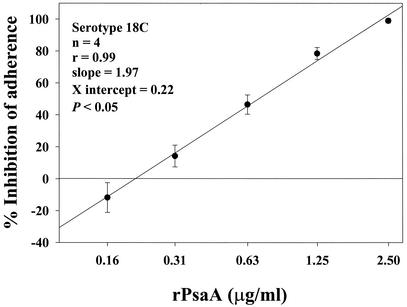
The inhibition of Pnc serotype 19F adherence by selected human sera before or after the absorption with the PsaA-minus strain was determined. Absorption (30 min at room temperature) of human sera (100 μl) with a PsaA-minus mutant pellet containing 108 CFU did not result in a significant decrease (P = 0.11, paired t test) of the maximum percent inhibition of Pnc adherence to Detroit 562 cells. The mean percent inhibition of adherence ± SD for unabsorbed and absorbed sera (n = 18) was 72% ± 18% and 68% ± 16%, respectively. Addition of ≥2.5 μg rPsaA/ml (≥0.25 μg/well) to confluent cell monolayers 15 min prior to the addition of S. pneumoniae can block nearly 100% of Pnc adherence (Fig. 3). No significant effect was seen by direct addition of homologous Ps type 18C (500 μg/well) to the monolayers prior to addition of bacterial inoculum. Effect of cell wall polysaccharide addition (250 μg/well) was not significant if the strains were encapsulated; however, addition of cell wall polysaccharide (preincubation of monolayers with 100 μg/well for 15 min at room temperature) to rough controls (R36A unencapsulated strain) resulted in a significant decrease in adherence of this strain.
FIG. 3.
Effect of rPsaA on S. pneumoniae adherence to Detroit 562 nasopharyngeal cells. Addition of rPsaA at ≥2.5 μg/ml (≥0.25 μg/well) to confluent cell monolayers 15 min prior to the addition of S. pneumoniae can prevent nearly 100% of Pnc adherence. Shown in this figure is the rPsaA effect (mean percent inhibition ± standard error [error bars]) on an adherent strain of serogroup 18C; however, similar results were obtained with serotype 19F (94% inhibition of Pnc adherence when rPsaA was added to the cell monolayer at 0.5 μg/well). Pearson's product moment correlation coefficient (r) = 0.99.
Reproducibility among Pnc serotypes.
The maximum inhibitions of adherence (mean ± SD) observed with five human sera (1:10 dilution) for serotypes 2, 6A, 18C, 19F, and 23F were 74% ± 14%, 67% ± 19%, 65% ± 7%, 83% ± 18%, and 73% ± 29%, respectively. There was no significant difference (P > 0.05) in the maximum percentages of inhibition among different serotypes. Within serotype coefficients of variance for adherence were calculated to be 18 to 25% depending on the serotype.
Correlation with ELISA IgG levels.
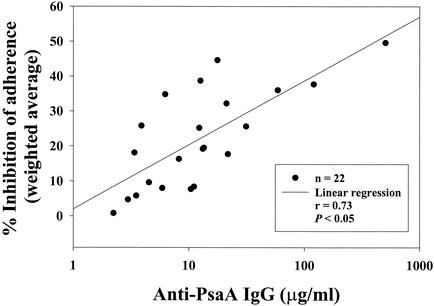
The overall correlation (r = 0.73) of anti-rPSA IgG antibodies (in micrograms per milliliter, as determined by ELISA) and the weighted average of the percent inhibition of adherence were significant (P < 0.05), as shown in Fig. 4. In general, a trend was observed that human serum samples with high inhibiting activity also had high anti-rPsaA IgG concentrations as shown by ELISA.
FIG. 4.
Correlation of functional antibody activity as measured by the weighted average of the percent inhibition of adherence and ELISA concentration of anti-PsaA IgG antibodies naturally found in human donor sera (n = 22). A weighted average of the percent inhibition of adherence observed with a given serum sample was calculated by using the log10 of each serum dilution factor as the weight. Analysis by the Pearson product moment correlation coefficient yielded a significant (P < 0.05) level of correlation (r = 0.73) between the calculated weighted average of the percent inhibition and the measured anti-PsaA IgG concentration of the human serum. Inhibition assays were performed with either Pnc serotype 18C or 19F.
DISCUSSION
PsaA was first discovered in Pnc by Russell et al. (27). This protein is highly immunogenic and is conserved across all 90 serotypes of S. pneumoniae. These characteristics made PsaA a good candidate for a Pnc common protein vaccine to be used alone or in combination with other common protein antigens. Although serum antibody levels can be measured by ELISA (30), this is the first study describing an assay for the measurement of functional activity elicited by PsaA.
The inhibitory effect of rPsaA and both human and animal anti-PsaA antibodies on Pnc adherence to nasopharyngeal epithelial cells was demonstrated with this functional assay. This inhibitory effect was observed across multiple serotypes, and it was not restricted to a particular Pnc strain. In general, a higher CFU count was observed with transparent strains than with opaque strains, in agreement with previous colonization studies by Cundell et al. (12). Preliminary studies indicate that there are no major differences in PsaA protein expression between these two phenotypes (data not shown). Weiser et al. reported that other surface proteins such as the amidase LytA are found at higher levels in the transparent phenotype than in the opaque phenotype (31).
The role of PsaA as a potential Pnc adhesin was first demonstrated by Berry and Paton by the low adherence to type II pneumocytes observed with a PsaA-minus mutant (4). This mutant was also used in this study with similar low adherence for nasopharyngeal cells. However, the inhibitory effect in Pnc adherence by the purified lipidated rPsaA protein is described for the first time in this study. Although we are only presenting information for nasopharyngeal cells, preliminary studies evaluated the capacity of MAb to inhibit approximately 68% of the adherence of Pnc to two types of human endothelial cell lines (human umbilical vein endothelial cells and human microvascular endothelial cells) and to resting type II pneumocytes (A549 lung epithelial cells) (J. S. Sampson, E. W. Ades, S. Romero-Steiner, S. Johnson, H. Daugharty, J. Dykes, A. Stinson, J. Crook, and G. M. Carlone, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. E8, p. 273, 1999). However, the reproducibility of Pnc adherence and inhibition of adherence by anti-PsaA antibodies was better demonstrated with nasopharyngeal Detroit 562 cells than with the other cell lines evaluated.
Cundell and Tuomanen have described three families of Pnc permeases that may have a role in Pnc adherence to different eukaryotic cell types (13). One of these families is the PsaA family, which bears homology to the FimA protein of Streptococcus parasanguis (28). Although PsaA is important for Pnc adherence, it is not responsible for all Pnc adherence. At least two other families (PlpA and AmiA) are involved in adherence to endothelial cells and type II pneumocytes (13). Cundell and Tuomanen have established that the endothelial and type II pneumocyte receptors are Gal NAcβ1-3 Gal and Gal NAcβ1-4 Gal. When these cells are activated by cytokines a new receptor (GlcNAc) is formed (13). The nasopharyngeal cell receptor was first characterized by Anderson et al. as a neolactose, Glc NAcβ1-3 Gal (1, 2). A recent report indicates that the human polymeric immunoglobulin receptor is involved in the invasion of Detroit 562 epithelial cells by unencapsulated variants via the choline binding protein A (CbpA). However, encapsulated strains did not invade the epithelial cell monolayers despite the expression of CbpA (7). The cell receptor for PsaA in epithelial cells still remains to be elucidated.
Anti-PsaA antibodies have been previously demonstrated to confer protection in mouse colonization models and protection against otitis media in chinchillas (6; Pelton et al., Program Abstr. 2nd Int. Symp. Pneumococci Pneumococcal Dis. 2000). More recently, protection elicited by oral immunization with PsaA against lung colonization and septicemia was reported in mice (29). This type of protection in mice had not been observed previously. Phase 1 trials with humans have been conducted for PspA and will eventually be conducted for PsaA. A surveillance study in Finland demonstrated that incidence of otitis media was lower for children with higher levels of anti-PsaA antibodies and higher for those children with lower levels of anti-PsaA antibodies (24). While production of antibodies to Pnc proteins is age dependent (18, 24), it is likely that the presence of anti-PsaA antibodies prevents colonization and therefore transmission of pneumococcus. The results of this study suggest that natural exposure to Pnc induces anti-PsaA antibody levels with functional activity (up to 98% inhibition of adherence in healthy adult donor sera). However, the induction of higher antibody levels postvaccination remains to be determined. Since PsaA is present in all Pnc serotypes (11, 21), a vaccine including this protein may provide wider serotype coverage than those formulations that confer protection against a limited number of serotypes. Future vaccine evaluation will require the use of assays that measure not only the quantity of the antibodies elicited by the vaccine antigen but also the functional activity of such antibodies. In the case of Pnc polysaccharide antigens, such assays (ELISA and opsonophagocytosis assay) have been identified and standardized. However, for evaluating responses to Pnc protein antigens, these assays have not been established. In addition, the type of functional assay may vary depending on the protein function. Although Pnc adherence can be mediated by multiple adhesins, our data support the role of PsaA in Pnc adherence to human cells and the argument that this protein is a major Pnc adherence factor regardless of the serotype tested. This functional assay may be useful in evaluating antibodies elicited in response to PsaA vaccination.
Acknowledgments
We thank James Paton, Women's and Children Hospital, Adelaide, Australia, for kindly providing the PsaA mutant strain and Melinda Bronsdon, CDC, for support in immunoblotting and nasopharyngeal Pnc isolates. We also thank Kate O'Brien, Johns Hopkins University, Baltimore, Md., and Jeffrey Weiser, University of Pennsylvania, Philadelphia, for providing Pnc nasopharyngeal isolates and Pnc strains of the transparent phenotype. We also thank Richard Facklam, CDC, for providing reference Pnc strains and materials for antimicrobial assays; Wayne Harris, CDC, for valuable assistance in the cross-standardization of the PsaA ELISA; and Joseph Caba, CDC, for additional inhibition of adherence assays with serotype 18C.
This research was funded in part by an unrestricted cooperative research agreement between Aventis-Pasteur, Toronto, Ontario, Canada, and the CDC.
REFERENCES
- 1.Anderson, B., J. Dahmen, F. Torbjörn, H. Leffler, G. Magnusson, G. Noori, and C. Svanborg Eden. 1983. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B., B. Eriksson, E. Falsen, A. Fogh, L. Å. Hanson, O. Nylén, H. Peterson, and C. Svanborg Edén. 1981. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect. Immun. 32:311-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. K. Hollinsghead, and D. E. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. Hansen, L. Elvin, K. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, and The Northern California Kaiser Permanente Vaccine Study Center Group. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 6.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brock, S. C., P. A. McGraw, P. F. Wright, and J. E. Crowe, Jr. 2002. The human polymeric immunoglobulin receptor facilitates invasion of epithelial cells by Streptococcus pneumoniae in a strain-specific and cell type-specific manner. Infect. Immun. 70:5091-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 46(RR-8):1-24. [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2000. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 49(RR-09):1-38. [PubMed] [Google Scholar]
- 10.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinatorial exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 11.Crook, J., J. A. Tharpe, S. E. Johnson, D. B. Williams, A. R. Stinson, R. R. Facklam, E. W. Ades, G. M. Carlone, and J. S. Sampson. 1998. Immunoreactivity of five monoclonal antibodies against the 37-kilodalton common cell wall protein (PsaA) of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 5:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cundell, D. R., J. N. Weiser, J. Shen, A. Young, and E. I. Tuomanen. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cundell, D. R., and E. Tuomanen. 1995. Adherence and interaction of bacteria at respiratory mucosal surfaces, p. 11-16. In J. A. Roth, C. A. Bolin, K. A. Brogden, F. C. Minion, and M. J. Wannemuehler (ed.), Virulence mechanisms of bacterial pathogens, 2nd ed. American Society for Microbiology, Washington, D.C.
- 14.Dagan, R., R. Melamed, M. Muallem, L. Piglansky, D. Greengerg, O. Abramson, P. M. Mendelman, N. Bohidar, and P. Yagupsky. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271-1278. [DOI] [PubMed] [Google Scholar]
- 15.De, B. K., J. S. Sampson, E. W. Ades, R. C. Huebner, D. L. Jue, S. E. Johnson, S. E. Espina, A. R. Stinson, D. E. Briles, and G. M. Carlone. 2000. Purification and characterization of Streptococcus pneumoniae palmitoylated pneumococcal surface adhesin A expressed in Escherichia coli. Vaccine 18:1811-1821. [DOI] [PubMed] [Google Scholar]
- 16.Dintilhac, A., G. Alloing, C. Granadel, and J.-P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J. O., S. Romero-Steiner, U. B. S. Sørensen, J. Blom, M. Carvalho, S. Barnard, G. Carlone, and J. N. Weiser. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lifshitz, S., R. Dagan, M. Shani-Sekler, N. Grossman, G. Fleminger, M. Friger, and Y. M. Nebenzahl. 2002. Age-dependent preference in human antibody responses to Streptococcus pneumoniae polypeptide antigens. Clin. Exp. Immunol. 127:344-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipsitch, M. 1997. Vaccination against colonizing bacteria with multiple serotypes. Proc. Natl. Acad. Sci. USA 94:6571-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madore, D., P. Anderson, B. D. Baxter, G. M. Carlone, K. M. Edwards, R. G. Hamilton, P. Holder, H. Käyhty, D. C. Phipps, C. C. C. Peeters, R. Schneerson, G. R. Siber, J. I. Ward, and C. E. Frasch. 1996. Interlaboratory study evaluating quantitation of antibodies to Haemophilus influenzae type b polysaccharide by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 3:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, K. E., D. Lake, J. Crook, G. M. Carlone, E. Ades, R. Facklam, and J. S. Sampson. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combination of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plikaytis, B. D., P. F. Holder, L. B. Pais, S. E. Maslanka, L. L. Gheesling, and G. M. Carlone. 1994. Determination of parallelism and nonparallelism in bioassay dilution curves. J. Clin. Microbiol. 32:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Käyhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 25.Reimer, C. I., J. S. Smith, T. W. Wells, R. M. Nakamura, P. W. Keitges, R. F. Ritchie; G. W. Williams, D. J. Hanson, and D. B. Dorsey. 1982. Collaborative calibration of the U. S. National College of American Pathologists reference preparations for specific serum proteins. Am. J. Clin. Pathol. 77:12-19. [DOI] [PubMed] [Google Scholar]
- 26.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell, H., J. A. Tharpe, D. E. Wells, E. H. White, and J. E. Johnson. 1990. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J. Clin. Microbiol. 28:2191-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson, J. S., S. P. O'Connor, A. R. Stinson, J. A. Tharpe, and H. Russell. 1994. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect. Immun. 62:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo, J.-Y., S. Y. Seong, B.-Y. Ahn, I. C. Kwon, H. Chung, and S. Y. Jeong. 2002. Cross-protective immunity of mice induced by oral immunization with pneumococcal surface adhesin A encapsulated in microspheres. Infect. Immun. 70:1143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tharpe, J. A., H. Russell, M. Leinonen, B. D. Plikaytis, R. F. Breiman, G. M. Carlone, E. W. Ades, and J. S. Sampson. 1998. Comparison of a pneumococcal common protein (PsaA) antibody ELISA and a PsaA immune complex ELISA for detection of pneumococcal serum antibody. Pathobiology 66:77-83. [DOI] [PubMed] [Google Scholar]
- 31.Weiser, J. N., Z. Markiewicz, E. I. Tuomanen, and J. H. Wani. 1996. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 64:2240-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]