Abstract
Mixed connective tissue disease is an overlap syndrome characterized by features of different systemic autoimmune diseases and a high titer of U1-snRNP antibodies. We examine here the autoantibodies to nucleoporin p62 in a severe case of mixed connective tissue disease in a young male patient. Thus far, p62 antibodies have mainly been described in cases of primary biliary cirrhosis. We speculate that the presence of p62 antibodies is an indication of a poor prognosis in connective tissue disorders.
U1-RNP is a component of the spliceosome complex. Human sera in cases of mixed connective tissue disease (MCTD) recognize the U1-RNA and the U1-specific polypeptides. High anti-U1-RNA titers have been shown to correlate with disease activity (3). Several other antibodies, in addition to the anti-U1-RNP antibodies, have been associated with MCTD (5).
During the last 10 years new autoantigens in the nuclear envelope have been characterized. Examples include gp210, lamins, lamin-associated polypeptides, the lamin B receptor, and nucleoporin p62 (7). Nucleoporin p62 has been cloned and sequenced in 1991 (1) and is not identical with p62 characterized by Zhang et al. (11).
In the present study we show for the first time nucleoporin p62 antibodies in a young male patient with severe MCTD. The amino acid sequence of nucleoporin p62 contains phenylalanine and glycine (FG)-rich peptide motives, which are binding sites for nuclear transport factors (9). Nucleoporin p62 antibodies seem to be characteristic of patients with primary biliary cirrhosis (PBC) (4, 10). In addition, four cases of Sjögren syndrome (6) and two cases of systemic lupus erythematosus (4) with p62 antibodies have been described.
Case report.
A 32-year-old male patient was diagnosed with MCTD 7 years previously. His primary manifestations were extremely painful arthralgia and myalgia, Raynaud symptoms, and muscle weakness (accompanied by a pathological pattern in electromyography). Myositis was verified by elevated lactate dehydrogenase, creatinine kinase, and myoglobin levels. Intermittently, pleural effusions and leukopenia were observed. In December 2001 the patient was hospitalized due to very painful myalgia, muscle weakness, and arthralgia. He was nearly unable to walk or move. He reported fatigue and chest pain at deep inspiration. Auscultation revealed percussion dullness and decreased breath sounds over the left lung base. Laboratory parameters showed elevated levels of C-reactive protein (up to 12 mg/dl [0 to 0.5 mg/dl]), fibrinogen (4.7 g/liter [1.8 to 3.5 g/liter]), and immunoglobulin G (IgG; 1,550 mg/dl [690 to 1,400 mg/dl]). Immunologic parameters revealed positive antinuclear antibodies (1:5,120), highly positive anti-RNP, and slightly positive anti-Ro (SS-A). Anti-ds DNS antibodies were occasionally positive. Pulmonary function studies showed restrictive changes of the lung, with a forced vital capacity that was 44% of the predicted value and a diffusing capacity that was 58% of the predicted value. A computed tomographic scan of the chest revealed diaphragmatic fibrosis on the left side of the lung and a thickening of the pericardium; this was probably due to former pericarditis. Abdominal sonography indicated that the spleen and liver were enlarged. Because of the severity of MCTD and to avoid high steroid doses, the patient was treated with different immunosuppressive drugs. His painful arthralgias, body stiffness, and fatigue responded well to immune suppression.
Expression and purification of p62 fusion proteins.
p62 was expressed in three fragments (p62I [amino acid residues 1 to 329], p62II [amino acid residues 330 to 456], and p62III [amino acid residues 457 to 522]) with three sets of nondegenerate oligonucleotide primers (5′-ATGAGCGGCTTTAATTTTGGAGG-3′ and 5′-GGTCATGGCGGAGCTGGCAG-3′; 5′-TACGCGCAGCTGGAGAGCCT-3′ and 5′-CTCGATGATGTCCTTGAGATCCT-3′; and 5′-CACCTGAACACGTCCGGGGC-3′ and 5′-GTCAAAGGTGATCCGGAAGCTG-3′), with additional restriction sites for HindIII (5′) and XhoI (3′). The corresponding PCR products were inserted between these restriction sites in pET21-b vector (Novagen, Madison, Wis.) containing a six-histidine tag. The constructs were transformed into Escherichia coli BL21(DE3). p62-His6 fusion proteins were purified under denaturing conditions as described by the manufacturer.
Gel electrophoresis and immunoblotting.
Samples of nuclear envelopes, prepared as described previously (8), and p62 fusion proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10 μg/lane) and transferred to Hybond ECL membranes (Amersham, Braunschweig, Germany) at 50 V for 3 h.
After a blocking step with 5% skimmed milk in phosphate-buffered saline (PBS; pH 7.4) for 1 h at room temperature, the Hybond sheets were incubated overnight at 4°C with the patient (serum from June 2001) and control sera (diluted 1:500 in 5% milk). Bound antibodies were visualized with horseradish peroxidase-labeled goat anti-human IgG (Jackson Immunoresearch Laboratories, West Grove, Pa.) diluted 1:4,000 in PBS, including 5% milk by using enhanced chemiluminescence (Amersham, Braunschweig, Germany). In addition to the patient serum from June 2001, we confirmed our data with patient serum from December 2001 (not shown). Furthermore, we used sera of PBC patients and the serum of a healthy donor. Monoclonal antibody (MAb) 414 to the FG-rich domain (2) was a gift of M. Rout (Rockefeller University, New York, N.Y.).
Cell culture and immunofluorescence.
HeLa cells were grown on coverslips until they reached subconfluency. After a brief wash with ice-cold PBS, the cells were fixed and permeabilized for 10 min in ice-cold methanol and then blocked for 1 h with 2% bovine serum albumin (BSA)-0.05% Tween 20 in PBS. The fixed and permeabilized cells were incubated overnight with patient and control sera diluted 1:100 in 0.1% BSA-0.5% Tween 20 in PBS. The coverslips were washed five times for 5 min with 0.1% BSA-0.5% Tween 20 in PBS and then incubated with rabbit anti-human IgA plus IgG plus IgM (diluted 1:50; ICN Biomedicals, Aurora, Ohio). Antibody binding was detected by incubation with cyanin 3-labeled polyclonal donkey anti-rabbit IgG (Jackson ImmunoResearch). Coverslips were mounted and examined as described previously (8).
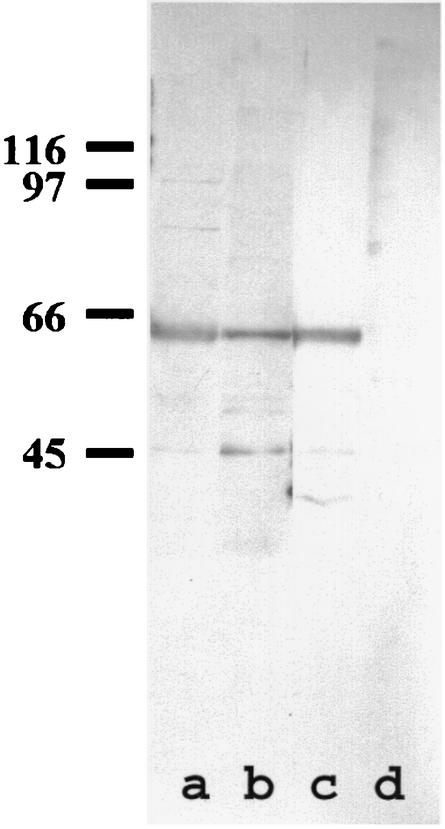
The serum of our patient was screened for p62 antibodies. Proteins of purified nuclear envelopes were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunostained with MCTD patient serum, the sera of PBC patients (as a positive control), and the serum of a healthy donor (as a negative control) (Fig. 1). The serum of our patient, as the positive control, strongly labeled a 62-kDa protein in nuclear envelopes.
FIG. 1.
p62 antibodies. Western blot of nuclear envelopes (SDS-10% PAGE, 10 μg/lane) probed with the serum of a healthy donor (lane d), with the sera of two patients with PBC (a and b), and with the serum of a patient with MCTD (c). The positions of the marker proteins are shown on the left.
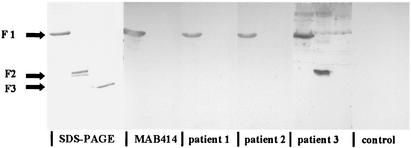
To investigate whether this 62-kDa protein is indeed nucleoporin p62, we expressed the entire nucleoporin in three fragments in E. coli. The first fragment contains the FG-rich domain. The sera of two patients with PBC, i.e., the sera of our patient and an MAb against the FG-motives, labeled the first fragment, indicating that the autoantibodies might bind to the FG-motives in p62. The serum of our MCTD patient also recognized the second fragment. (Fig. 2).
FIG. 2.
SDS-15% PAGE of p62 fusion proteins stained with Coomassie blue (SDS-PAGE) or transferred to nitrocellulose membrane. Blots are incubated with MAb 414 (MAB414), the serum of a healthy donor (control), sera of two patients with PBC (patients 1 and 2), and the serum of a patient with MCTD (patient 3).
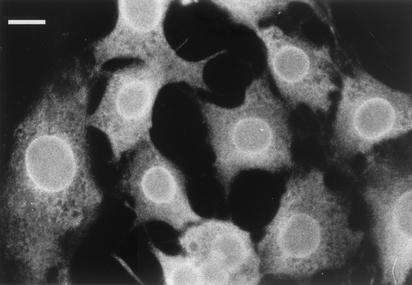
To further confirm our data, we examined the patient serum by immunofluorescence in HeLa cells. The patient serum showed clear rim staining in the nuclear plane (Fig. 3), as well as punctate staining, when it was focused on the top of the nucleus (not shown). This is the typical staining pattern of antibodies against nuclear pore proteins (8).
FIG. 3.
Immunofluorescence microscopy of HeLa cells. Cells grown on coverslips were fixed and permeabilized in methanol and then probed with patient serum. Focusing on the nuclear periphery yielded nuclear rim staining. Bar, 10 μm.
In the present study we discuss the case of a young male patient who was diagnosed with MCTD. On account of the intensive course of the disease, requiring repeatedly high doses of steroids, the patient was treated with various immunosuppressive regimens.
Because of the unusual seriousness of the disease and since various additional antibodies to the U1-RNP antibodies have been described in association with MCTD syndrome, we checked the serum of the patient for nucleoporin p62 antibodies. We used the anti-p62-positive sera of patients with PBC as controls. In a Western blot of nuclear envelopes, the serum of the patient with MCTD showed a strong signal at 62 kDa. Furthermore, the patient's serum recognized the first and second fragments of p62 fusion proteins. In addition, the patient serum showed nuclear pore labeling in immunofluorescence studies in HeLa cells.
In summary, our data suggest that p62 autoantibodies are not only specific for PBC (4) but that they can be found in connective tissue diseases. To our knowledge, this is the first case of a patient with both MCTD syndrome and p62 antibodies that has thus far been described. We speculate that p62 antibodies are not only a sign of poor prognosis in PBC (4) but also in connective tissue disorders. This hypothesis needs to be further evaluated by studying a panel of MCTD patients.
Acknowledgments
We thank I. Davydenko for excellent technical assistance.
D.M.K. was supported by grants (Bayerischer Habilitationsförderpreis 2000, Wilhelm Sander Stiftung 1999.119.2).
REFERENCES
- 1.Carmo-Fonseca, M., H. Kern, H., and E. C. Hurt. 1991. Human nucleoporin p62 and the essential yeast nuclear pore protein NSP1 show sequence homology and a similar domain organization. Eur. J. Cell Biol. 55:17-30. [PubMed] [Google Scholar]
- 2.Davis, L. I., and G. Blobel. 1987. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc. Natl. Acad. Sci. USA 84:7552-7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoet, R. M., I. Koornneef, D. J. de Rooij, L. B. van de Putte, and W. J. van Venrooij. 1992. Changes in anti-U1 RNA antibody levels correlate with disease activity in patients with systemic lupus erythematosus overlap syndrome. Arthritis Rheum. 35:1202-1210. [DOI] [PubMed] [Google Scholar]
- 4.Invernizzi, P., M. Podda, P. M. Battezzati, A. Crosignani, M. Zuin, E. Hitchman, M. Maggioni, P. L. Meroni, E. Penner, and J. Wesierska-Gadek. 2001. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J. Hepatol. 34:366-372. [DOI] [PubMed] [Google Scholar]
- 5.Kasukawa, R. 1999. Mixed connective tissue disease. Intern. Med. 38:386-393. [DOI] [PubMed] [Google Scholar]
- 6.Miyachi, K., M. Shibata, Y. Onozuka, F. Kikuchi, N. Imai, and T. Horigome. 1996. Primary biliary cirrhosis sera recognize not only gp210 but also proteins of the p62 complex bearing N-acetylglucosamine residues from rat liver nuclear envelope: anti-p62 complex antibody in PBC. Mol. Biol. Rep. 23:227-234. [DOI] [PubMed] [Google Scholar]
- 7.Nesher, G., R. Margalit, and Y. J. Ashkenazi. 2001. Anti-nuclear envelope antibodies: clinical associations. Semin. Arthritis Rheum. 30:313-320. [DOI] [PubMed] [Google Scholar]
- 8.Radu, A., G. Blobel, and R. W. Wozniak. 1993. Nup155 is a novel nuclear pore complex protein that contains neither repetitive sequence motifs nor reacts with WGA. J. Cell Biol. 121:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart, M., and W. D. Clarkson. 1996. Nuclear pores and macromolecular assemblies involved in nucleocytoplasmic transport. Curr. Opin. Struct. Biol. 6:162-165. [DOI] [PubMed] [Google Scholar]
- 10.Wesierska-Gadek, J., H. Hohenuer, E. Hitchman, and E. Penner. 1996. Autoantibodies against nucleoporin p62 constitute a novel marker of primary biliary cirrhosis. Gastroenterology 110:840-847. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, J. Y., E. K. L. Chan, X. X. Peng, and E. M. Tan. 1999. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J. Exp. Med. 189:1101-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]