Abstract
Circular DNA molecules known as T-cell receptor rearrangement excision circles (TREC) arise during T-cell development and are present in cells that have recently emigrated from the thymus. In cross-sectional studies, the number of peripheral blood lymphocytes bearing TREC decreases with age, consistent with an anatomically demonstrated loss of thymic epithelial tissue. TREC numbers increase following hematopoietic stem cell transplantation and during therapy for human immunodeficiency virus (HIV) infection. Quantitation of TREC has therefore been proposed as a parameter of thymic activity. In this study, we used real-time PCR to quantify TREC in peripheral blood samples obtained longitudinally from HIV-seronegative adolescents. TREC values in peripheral blood T cells were very stable throughout adolescence, once thought to be a time of rapid involution of the thymus. In addition, in a cross-sectional analysis, we examined TREC values in a cohort of HIV-positive adolescents and found evidence of ongoing thymopoiesis in perinatally infected individuals, despite lifelong infection. These data demonstrate the utility of TREC assessment in adolescents and that HIV infection does not uniformly result in accelerated thymic involution in childhood.
The thymus is the site of differentiation of bone marrow derived precursors into naive T cells with a diverse T-cell repertoire. Studies utilizing autopsy specimens have shown that the thymus gradually involutes with age. As observed morphologically, this involution consists of enlargement of the perivascular spaces, accompanied by a decrease in the thymic epithelial space, the site of lymphocyte differentiation (17, 34). Until recently, there were no adequate methods to assess functional activity of the thymus. In 1998, measurement of T-cell receptor rearrangement excision circles (TREC) was shown to be a useful marker of thymic activity. TREC are found exclusively in naive T cells and are readily quantified by PCR assay. The number of TREC decreases with increasing age and following either thymectomy or human immunodeficiency virus (HIV) infection. Ex vivo functional studies of lymphocytes from specimens of thymus removed during cardiothoracic surgery or at autopsy confirm that the presence of TREC in peripheral blood specimens correlates with the presence of active thymopoiesis in the epithelial tissue in the thymus, which continues into the sixth and seventh decades of life (11, 19, 22). At one time the thymus was thought to undergo accelerated involution during adolescence due to hormonal changes, resulting in replacement of thymic epithelial tissue by fat (17, 34). Perhaps in agreement, TREC values have been reported to diminish gradually prior to puberty, with a significant decline in the second decade of life (40). However, histological and endocrinological data show no correlation between the size of the thymic epithelial space and hormonal changes during puberty (34). Moreover, several studies have shown a gradual and steady decline in the TREC content of peripheral blood lymphocytes throughout life (3, 11, 32, 40). All of these studies, however, were cross-sectional analyses using samples collected from subjects of different ages, and they examined TREC measured either in peripheral blood mononuclear cells (PBMC) or in T lymphocytes. The correlation between TREC numbers in PBMC and in T lymphocyte subsets has not been carefully examined. In this study, we examined the utility of using the TREC assay to measure changes in thymic activity in adolescents and carefully examined the correlation between the number of TREC in PBMC and in purified CD4+ and CD8+ T cells.
HIV infection has been associated with accelerated involution of the thymus. Both HIV-infected adults and children demonstrate decreases in TREC numbers compared to age-matched seronegative controls (11, 40). Treatment with highly active antiretroviral therapy (HAART) has been associated with improvements in CD4+-T-cell parameters and enlargement of thymic volume, correlating with increases in TREC values (15, 21, 33, 36). No studies have specifically examined the impact of HIV infection on thymic activity during adolescence, a time when accelerated thymic involution may take place. We therefore studied this issue in perinatally and sexually infected HIV-positive adolescents and two groups of high-risk seronegative youths.
MATERIALS AND METHODS
Subjects.
Samples of PBMC were obtained from adolescents enrolled in the REACH Project (Reaching for Excellence in Adolescent Care and Health) and patients monitored at the Adolescent Medicine Clinic at Children's Hospital Los Angeles (CHLA), the Clinical Immunology Service at CHLA, and the Maternal Child Immunology Clinic at UCLA (MCIC). The REACH Project of the Adolescent Medicine HIV/AIDS Research Network was an observational study of HIV-infected and high-risk non-HIV-infected adolescents age 13 to 18 years at enrollment. Biomedical and behavioral data were collected from these youths at 15 different clinical sites in 13 U.S. cities. Characteristics of the cohort, recruitment and eligibility criteria, and study design are reported in detail elsewhere (28, 39). The youngest HIV-seronegative subjects who had a minimum of five specimens collected during study visits conducted at 6-month intervals were selected for this study.
The adolescents from CHLA and MCIC consisted of three groups: adolescents infected perinatally (n = 12) or via neonatal (n = 2) transfusion (PI group), adolescents infected by high-risk “adult” behavior (AB group), and adolescents who are HIV seronegative (SN group). The adolescents recruited from the UCLA MCIC and clinics at CHLA were recruited sequentially, without regard to the use of antiretroviral therapy, age, or gender.
Informed consent for study participation was obtained in accordance with approvals from the Institutional Review Board at each institution.
Cell isolation.
Peripheral blood samples from CHLA and MCIC were collected into acid citrate dextrose tubes, and separation of PBMC was performed by Ficoll-Hypaque density gradient centrifugation. Each sample was processed within 24 h of collection. Samples from the adolescents in the REACH project were obtained as cryopreserved mononuclear cells, prepared as described previously (13); they were immediately stored in liquid nitrogen until use.
Cell staining and flow cytometry.
Whole-blood cell staining was done to determine the percentages of CD3+ CD4+, CD3+ CD8+, and CD4+ CD45RA+ CD62L+ T cells in samples obtained from CHLA and MCIC. Staining was done with CD8-conjugated fluorescein isothiocyanate, CD3-conjugated phycoerythrin, CD4-conjugated allophycocyanin, and CD45RA/CD62L-conjugated fluorescein isothiocyanate and phycoerythrin antibodies according to the instructions of the manufacturer (BD Biosciences, San Jose, Calif.). Flow cytometric analysis was performed with a FACSCalibur flow cytometer, and the results were analyzed with CellQuest Software (BD Biosciences). The methodologies for the REACH laboratory-based measures used in this study have been previously described in detail (13, 14, 29). Quantitative immunophenotyping of CD4+- and CD8+-T-cell lymphocytes was done at the individual clinical sites in certified laboratories using AIDS Clinical Trials Group standardized flow cytometry protocols. Samples for quantitative immunophenotyping of an expanded panel of flow cytometric markers were analyzed centrally at the Immunology Core Laboratory at The Children's Hospital of Philadelphia after overnight shipping as previously reported (14). The following cell types from the expanded panel were used in this analysis: CD4+/CD45RO−/CD45RA+ (naive helper T cell), CD4+/CD45RO+/CD45RA− (memory helper T cell), CD8+/CD45RO−/CD45RA+ (naive suppressor T cell), CD8+/CD45RO+/CD45RA− (memory suppressor T cell), and CD8+/CD38+/HLA-DR+ and CD8+/CD38+ (activated cytotoxic T-cell immunophenotypes). For all of the above-mentioned T-cell subsets, associations were analyzed for both percentages of each cell type and absolute cell counts; both were used as continuous variables.
Isolation of CD4+ and CD8+ cells.
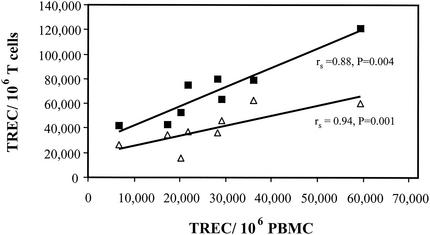
CD4+ and CD8+ T cells were isolated from PBMC of HIV-negative adolescents by using the MACS magnetic bead separation system (Miltenyi Biotec, Auburn, Calif.) according to the manufacturer's directions. The purity of the sorted cells was typically greater than 80% as evaluated by flow cytometry (FACSCaliber; BD Biosciences) with CD3, CD4, and CD8 fluorochrome-conjugated antibodies (BD Biosciences). The TREC contents of the PBMC and purified T cells were analyzed as described below (see Fig. 1). DNA from additional aliquots of PBMC from these HIV-seronegative adolescents was used for TREC analysis (see Fig. 3 and 4).
FIG. 1.
Correlation between TREC in PBMC and magnetic-bead-purified CD4+ (triangles) and CD8+ (squares) T cells from eight HIV-seronegative subjects. Spearman's correlation coefficients and regression lines are shown.
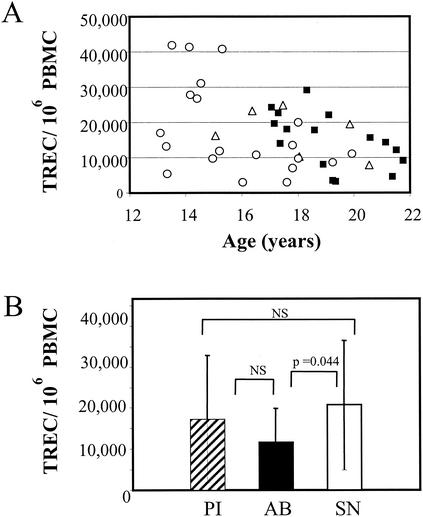
FIG. 3.
Comparisons of TREC values from the PI, AB, and SN groups. (A) TREC values for each individual. Circles, PI group; squares, AB group; triangles, SN group. (B) Mean TREC values in each cohort. Error bars indicate standard deviations. NS, not significant.
FIG. 4.
Correlation between TREC in PBMC and naive CD4+ T cells in the PI, AB, and SN groups. (A) TREC in PBMC versus percentage of naive CD4+ T cells/μl of blood. (B) TREC in PBMC versus absolute number of naive CD4+ T cells. Circles, PI group; squares, AB group; triangles, SN group. Spearman's correlation coefficients and regression lines are shown. Solid line, PI group; dotted line, AB group; dashed line, SN group.
Quantitation of TREC and cellular DNA.
DNAs from PBMC and purified CD4+ and CD8+ cells were extracted by using 100 μg of proteinase K (Boehringer Ingelheim, Petersburg, Va.) per ml. Samples were incubated for at least 1 h at 56°C and then for 10 min at 95°C. TREC were quantified by real-time PCR analysis, using the 5′ nuclease (TaqMan) assay and the ABI Prism 7700 sequence detector system (PE Biosystems) as described by others (12). A 25-μl PCR mixture consisted of 5 μl of genomic DNA solution, 2.5 μl of 10× PCR buffer, 1.75 μl of 50 mM MgCl2, 1 μl of 5 mM deoxynucleoside triphosphates, 1 μl of 12.5 pM forward primer (CACATCCCTTTCAACCATGCT), 1 μl of 12.5 pM reverse primer (GCCAGCTGCAGGGTTTAGG), 1 μl of 5 μM probe (FAM-5′-ACACCTCTGGTTTTTGTAAAGGTGCCACT-3′-TAMRA) (MegaBases), 0.25 μl of 10 μM Blue 636 reference dye (MegaBases), and 0.125 μl of platinum Taq polymerase. The PCR conditions were set at 95°C for 5 min, 95°C for 30 s, and 60°C for 1 min for 40 cycles. A standard curve was established with 25 copies up to 1,000,000 copies of plasmids containing signal joint TREC fragment (provided by D. Douek). TREC values for the unknown samples were determined by using the software provided with the ABI Prism 7700 system. All samples were analyzed in triplicates. The means of the triplicate TREC values were used for data analysis; the coefficient of variation was less than 20% for nearly all samples.
The number of cells in each test sample was confirmed by using real-time PCR to amplify CCR5 DNA sequences with a primer-probe combination supplied by D. Douek. A 25-μl PCR mixture consisted of 5 μl of genomic DNA solution, 2.5 μl of 10× PCR buffer, 1.75 μl of 50 mM MgCl2, 1 μl of 5 mM deoxynucleoside triphosphates, 1 μl of 12.5 pM forward primer (TACCTGCTCAACCTGGCCAT), 1 μl of 12.5 pM reverse primer (TTCCAAAGTCCCACTGGGC), 1 μl of 5 μM probe (5′-FAM-TTTCCTTCTTACTGTCCCCTTCTGGGCTC-TAMRA-3′), 0.25 μl of 10 μM Blue 636 reference dye, and 0.125 μl of platinum Taq polymerase. The PCR conditions were set at 95°C for 5 min, 95°C for 30 s, and 60°C for 1 min for 40 cycles. A standard curve was established with cellular DNA at 1.2 μg/5 μl to 750 ng/5 μl. The DNA amount in each test sample was determined by using the software provided with the ABI Prism 7700 system. Test samples were analyzed in duplicates. The mean DNA quantity was used for data analysis. We used an estimate of 8 μg of DNA per million PBMC.
HIV viral loads.
HIV plasma RNA concentrations were measured at UCLA and CHLA by using Amplicor HIV-1 Monitor testing, with a limit of sensitivity of 50 copies/ml (Roche Diagnostics, Indianapolis, Ind.).
Statistical analysis.
Analyses were conducted with SAS 8.2 (SAS, Inc., Cary, N.C.) software to examine data from the TREC assays on cell lysates from PBMC and CD4+ and CD8+ sorted cells. T-lymphocyte counts were compared by using a two-sided Wilcoxon rank sum test. Patients with undetectable HIV plasma RNA were assigned a value of 50 copies/ml for statistical analysis. Univariate analyses utilized SAS PROC CORR to calculate simple descriptive statistics and Pearson correlation coefficients for selected laboratory marker results. The longitudinal data were analyzed by repeated-measures mixed-effects regression modeling with SAS PROC Mixed.
RESULTS
Correlation between TREC in PBMC and purified CD4+ and CD8+ T cells.
We examined the relationship between TREC in PBMC and magnetic-bead-purified CD4+ T and CD8+ T cells from eight HIV-seronegative adolescents monitored at the Adolescent Medicine Clinic at CHLA. As a group, the mean number of TREC was higher in the CD8+ T-cell subset (69,400 TREC/106 cells) than in the CD4+ the T-cell subset (39,800 TREC/106 cells). PBMC had the lowest number of TREC (27,400 TREC/106 cells). The number of TREC in PBMC correlated highly with the number of TREC in purified CD4+ T cells (rs= 0.94; P = 0.001) and purified CD8+ T cells (rs= 0.88; P = 0.004) (Fig. 1).
Stability of TREC.
In order to better understand thymic activity during adolescence, we measured TREC in PBMC obtained every 6 months for up to 4 years from 11 female and 5 male HIV-seronegative individuals from the REACH Cohort (13, 14, 28, 39) (Fig. 2). These 16 subjects were 14 to 17 years of age at the time of study entry. The median TREC values at each time point ranged from 29,900 to 73,500. Applying mixed-effects modeling, which allows each individual to have a different time trend in their TREC values as well as taking into account the correlation of the TREC values over time, there was no significant change in TREC values over the period of follow-up (P > 0.20). The number of TREC per million PBMC correlated positively with the percentage of CD4+ CD45RA+ T cells (P = 0.004).
FIG. 2.
Stability of TREC number during adolescence. Longitudinal TREC values from 16 HIV-seronegative adolescents (14 to 17 years old at entry) monitored for up to 48 months are shown.
Comparison of TREC values in HIV-positive and -seronegative adolescents.
In order to examine the impact of HIV-1 perinatal infection on the magnitude of thymic activity during adolescence, we compared TREC concentrations in PBMC from perinatally infected individuals (PI group) and youths who were recently infected via adult behavior, generally sexual contact (AB group). Seronegative adolescents recruited from the same clinics (SN group) served as an additional comparison group.
The AB subjects were slightly older than the other two groups and were predominantly (85%) male (Table 1). There was a more balanced distribution of the sexes in the PI and SN groups (57 and 55% female, respectively). The PI group had more advanced HIV-related disease than the AB group; most had had an AIDS-defining illness or were Centers for Disease Control and Prevention class B or C (5, 6). More patients in the PI group than the AB group were receiving multidrug antiretroviral therapy (13 out of 14 versus 8 out of 14). Although the mean plasma viral load in the PI group was higher than that in the AB group, the difference was not significant (17,300 versus 12,300 copies/ml; P = 0.24). Two out of 14 PI subjects (14%) and 3 out of 14 AB subjects (21%) had undetectable plasma HIV-1 RNA (<50 copies/ml). The HIV-infected groups had lower mean CD4+ and higher mean CD8+ T-cell numbers and percentages than the HIV-seronegative group. Importantly, the AB subjects had a lower mean naive CD4+ T-cell percentage than the SN youths. However, the PI and SN youths had similar fractions of naive CD4+ T cells (45 versus 48%) (Table 1).
TABLE 1.
Characteristics of subjects
| Characteristic | Value for groupa
|
||
|---|---|---|---|
| PI | AB | SN | |
| No. of subjects | 14 | 14 | 9 |
| Age (yr) | 16.7 (2.2) | 20.3 (1.8) | 17.5 (2.2) |
| Gender (male/female) | 6/8 | 12/2 | 4/5 |
| CDC classification | |||
| N | 1 | 0 | |
| A | 0 | 12 | |
| B | 4 | 1 | |
| C | 9 | 1 | |
| No. on antiretroviral therapy | 13 | 8 | |
| Viral load (copies/ml) | 17,500 | 12,300 | |
| No. with plasma HIV RNA level of ≤50 copies/ml | 2 | 3 | |
| CD4+ T cells | |||
| % of T cells | 21 (12) | 34 (4) | 50 (14) |
| Count (cells/μl) | 412 (332) | 534 (239) | 1,158 (501) |
| CD8+ T cells | |||
| % of T cells | 50 (10) | 40 (14) | 25 (9) |
| Count (cells/μL) | 852 (336) | 650 (341) | 540 (117) |
| CD4+ naive T cells | |||
| % of CD4+ T cells | 45 (16) | 36 (10) | 48 (10) |
| Count (cells/μl) | 215 (166) | 201 (120) | 575 (318) |
Values are expressed as means; standard deviations are in parentheses.
The numbers of TREC in PBMC from the PI, AB, and SN groups were evaluated by real-time PCR. TREC values were significantly lower in the AB youths than in the seronegative subjects (11,700 versus 20,700) (P = 0.047). In contrast, the TREC values were similar in the PI (17,300) and SN groups (Fig. 3). Due to the small number of cells available for this study, we did not attempt to purify subsets of T cells. However, one would predict that TREC values in the peripheral blood would correlate with the number of naive T cells present in peripheral blood (11). Indeed, in the three cohorts, we found a significant positive correlation between the number of TREC in PBMC and either the number or percentage of naive CD4+ T cells. The correlation was the strongest in the AB group (P < 0.0001 for both comparisons) (Fig. 4).
DISCUSSION
We observed a strong correlation between the number of TREC in PBMC and TREC in CD4+ and CD8+ T lymphocytes and that TREC values did not change substantially during adolescence. In addition, TREC quantitation also provided evidence of ongoing thymopoiesis in perinatally infected adolescents, despite prolonged and poorly controlled HIV infection. The number of TREC found in PBMC correlated with the number and percentage of naive (CD45RA+ CD62L+) CD4+ T cells, as expected (11).
A correlation between the number of TREC in PBMC and TREC in purified CD4+ and CD8+ T cells has been previously noted by Chavan et al. in a study involving HIV-infected children who were 2 months to 16 years of age (median, 9 years) (7). However, TREC were more abundant in the CD4+ T cells than in the CD8+ T cells of these infected children. In contrast, we found that that TREC were more abundant in CD8+ T cells of uninfected adolescents (Fig. 1). These differences are probably a consequence of immune activation and T-cell proliferation triggered by HIV infection (23). Using quantification of Ki67, a nuclear antigen indicative of T-cell proliferation, Starr et al. recently found evidence in support of increased T-cell proliferation in HIV-infected adolescents monitored in the REACH study (31). Ki67 levels were higher in the CD8+ T-cell population, which would be expected to lead to dilutional reduction in the concentration of TREC compared to CD4+ T cells (11, 18).
In a similar analysis, Steffens et al. found that coding joint TREC were more numerous in CD4+ T cells than in either PBMC or CD8+ T cells from HIV-infected and uninfected adults (33). However, comparisons of these data to those presented by Chavan et al. (7) and in Fig. 1 are complicated by the fact that cell proliferation occurs between the points of signal joint and coding joint TREC formation.
It was once commonly accepted that thymic involution begins at sexual maturity and is completed by midlife (17, 28, 34). In agreement with this notion, one recent report described a decline of 1 to 1.5 log10 units in TREC number in the third decade of life compared to that in childhood and adolescence (40). However, this conclusion was based on an analysis of TREC content within PBMC obtained from a cross-section of infants, children, and adults. Despite the use of a very similar method for quantifying TREC, our data do not support the concept of accelerated thymic involution during adolescence, as TREC changed little in adolescents monitored for up to 4 years. Moreover, other cross-sectional analyses have indicated a constant rate of decline in TREC number with increasing age (3, 11, 15, 32). In addition, anatomical data correlating changes in gonadal hormones that occur during puberty with thymic involution show that only the volume of the perivascular space, connective tissue, and fat are affected. Atrophy of the thymic epithelial space, the site of thymopoiesis, is independent of hormonal changes seen in puberty and continues at a constant rate from infancy into adulthood (34). Thus, our unprecedented longitudinal analysis corroborates other evidence that thymopoiesis changes little during human adolescence.
It is vital that we gain a clearer understanding of the regenerative capacity of the immune system in adolescents, in view of the changing epidemiology of pediatric HIV infection in the United States and other resource-rich nations. Due to changes in obstetric care and the use of antiretroviral therapy, fewer children are infected perinatally (37). Moreover, the use of HAART, especially regimens that contain HIV protease inhibitors, has markedly diminished the annual mortality of infected children (16), leading to a burgeoning population of perinatally infected adolescents. In one recent study, 25% of the HIV-infected children monitored in 1995 to 1998 were in their second decade of life, compared to 5% just a few years earlier (1). This creates an impetus for understanding the long-term consequences of HIV infection on the thymuses of perinatally infected children. HIV is capable of directly infecting and killing thymocytes (2, 4, 26), with disruption of the thymic architecture (17). Despite these effects, TREC values and lymphocyte parameters indicated a level of thymopoiesis that was similar to that in the HIV-seronegative control group. The majority of the PI group were receiving antiretroviral therapy, which may in part account for the high level of TREC observed. Several short-term studies with children have shown an increase in TREC following the institution of antiretroviral therapy (7, 10, 20). Additional evidence for increased thymic activity following HAART includes a rise in naive CD4+ T cells that correlates with the increase in TREC number (20, 32). Most of the PI group still had detectable plasma HIV RNA, despite antiretroviral therapy, but this does not exclude continued beneficial effects of treatment. The benefits of antiretroviral therapy persist in at least some individuals with persistent viremia (8), as protease inhibitors appear to select for mutations that also lower the replicative capacity of HIV in thymic tissue (35).
As noted previously, HIV infection is associated with increased cellular activation and destruction that may complicate the interpretation of TREC assay data. The number of TREC present within a population of T cells depends not only on thymic output but also on the rate of cell proliferation and death (11, 18, 27). Thus, TREC values are an imperfect indicator of changes in thymopoiesis, as antiretroviral therapy may alter cell proliferation and survival (9, 24, 25, 27, 32). In the future, we plan to use longitudinal evaluation of markers of cellular activation, such as Ki67 quantification (30), in order to better quantify T-cell production and turnover in HIV-infected adolescents.
Acknowledgments
P.A.K. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility, which is supported by NIH awards CA-16042 and AI 28697, the Jonsson Cancer Center, the UCLA AIDS Institute, and the David Geffen School of Medicine. This study was conducted with samples from the REACH Project (investigators listed below), which was supported by the National Institute of Child Health and Human Development with supplemental funding from the National Institutes of Drug Abuse, Allergy and Infectious Diseases, and Mental Health and the Health Resources and Services Administration.
The following investigators of The Adolescent Medicine HIV/AIDS Research Network, listed by institution (with individual names in parentheses) in order of the numbers of subjects enrolled, participated in the REACH study: University of Miami (L. Friedman, L. Pall, D. Maturo, and A. Pasquale); Montefiore Medical Center (D. Futterman, D. Monte, M. Alovera-DeBellis, N. Hoffman, and S. Jackson); University of Pennsylvania and the Children’s Hospital of Philadelphia (D. Schwarz and B. Rudy); Children’s Hospital of Philadelphia (M. Tanney and A. Feldman); Children’s Hospital of Los Angeles (M. Belzer, D. Tucker, C. Hosmer, and K. Chung); Tulane Medical Center (S. E. Abdalian, L. Green, C. McKendall, and L. Wenthold); Children’s National Medical Center (L. J. D’Angelo, C. Trexler, C. Townsend-Akpan, R. Hagler, and J. A. Morrissy); University of Maryland (L. Peralta, C. Ryder, S. Miller, and S. Calianno); Cook County Hospital/University of Chicago (L. Henry-Reid and R. Camacho); Children’s Hospital, Birmingham (M. Sturdevant, A. Howell, and J. E. Johnson); Children’s Diagnostic and Treatment Center (A. Puga, D. Cruz, and P. McLendon); Emory University (M. Sawyer, J. Tigner, and A. Simmonds); St. Jude Children’s Research Hospital (P. Flynn, K. Lett, J. Dewey, and S. Discenza); Mt. Sinai Medical Center (L. Levin and M. Geiger); University of Medicine and Dentistry of New Jersey (P. Stanford and F. Briggs); SUNY Health Science Center at Brooklyn (J. Birnbaum, M. Ramnarine, and V. Guarino). The following investigators have been responsible for the basic science agenda: C. Holland (Center for Virology, Immunology, and Infectious Disease, Children’s Research Institute, Children’s National Medical Center); A. B. Moscicki (University of California at San Francisco), D. A. Murphy (University of California at Los Angeles), S. H. Vermund (University of Alabama at Birmingham), P. Crowley-Nowick (The Fearing Laboratory, Brigham and Women’s Hospital, Harvard Medical School, Boston, Mass.), and S. D. Douglas (University of Pennsylvania and the Children’s Hospital of Philadelphia). Network operations and analytic support are provided by C. M. Wilson and C. Partlow at the University of Alabama at Birmingham and by S. J. Durako, J. H. Ellenberg, B. Hobbs, A. Bennett, M. Camarca, K. Clingan, J. Houser, V. Junankar, O. Leytush, L. Muenz, Y. Ma, R. Mitchell, T. Myint, P. Ohan, L. Paolinelli, M. Rakheja, M. Sarr, and A. Soloviov at Westat, Inc.
We thank D. Douek, R. Koup, and S. Wolinsky for their help in establishing the real-time PCR assay for TREC and CCR5 sequences.
REFERENCES
- 1.Abrams, E. J., J. Weedon, J. Bertolli, K. Bornschlegel, J. Cervia, H. Mendez, G. Lambert, T. Singh, P. Thomas, et al. 2001. Aging cohort of perinatally human immunodeficiency virus-infected children in New York City. Pediatr. Infect. Dis. J. 20:511-517. [DOI] [PubMed] [Google Scholar]
- 2.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 3.Al-Harthi, L., G. Marchetti, C. M. Steffens, J. Poulin, R. Sekaly, and A. Landay. 2000. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction-enzyme linked immunosorbent assay (PCR-ELISA). J. Immunol. Methods 237:187-197. [DOI] [PubMed] [Google Scholar]
- 4.Bonyhadi, M. L., L. Rabin, S. Salimi, D. A. Brown, J. Kosek, J. M. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1992. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb. Mortal. Wkly. Rep. 41:1-19. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1994. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morb. Mortal. Wkly. Rep. 43:1-10. [Google Scholar]
- 7.Chavan, S., B. Bennuri, M. Kharbanda, A. Chandrasekaran, S. Bakshi, and S. Pahwa. 2001. Evaluation of T cell receptor gene rearrangement excision circles after antiretroviral therapy in children infected with human immunodeficiency virus. J. Infect. Dis. 183:1445-1454. [DOI] [PubMed] [Google Scholar]
- 8.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D. C., M. R. Betts, B. J. Hill, S. J. Little, R. Lempicki, J. A. Metcalf, J. Casazza, C. Yoder, J. W. Adelsberger, R. A. Stevens, M. W. Baseler, P. Keiser, D. D. Richman, R. T. Davey, and R. A. Koup. 2001. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 167:6663-6668. [DOI] [PubMed] [Google Scholar]
- 10.Douek, D. C., R. A. Koup, R. D. McFarland, J. L. Sullivan, and K. Luzuriaga. 2000. Effect of HIV on thymic function before and after antiretroviral therapy in children. J. Infect. Dis. 181:1479-1482. [DOI] [PubMed] [Google Scholar]
- 11.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690-695. [DOI] [PubMed] [Google Scholar]
- 12.Douek, D. C., R. A. Vescio, M. R. Betts, J. M. Brenchley, B. J. Hill, L. Zhang, J. R. Berenson, R. H. Collins, and R. A. Koup. 2000. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 355:1875-1881. [DOI] [PubMed] [Google Scholar]
- 13.Douglas, S. D., B. Rudy, L. Muenz, A. B. Moscicki, C. M. Wilson, C. Holland, P. Crowley-Nowick, S. H. Vermund, et al. 1999. Peripheral blood mononuclear cell markers in antiretroviral therapy-naive HIV-infected and high risk seronegative adolescents. AIDS 13:1629-1635. [DOI] [PubMed] [Google Scholar]
- 14.Douglas, S. D., B. Rudy, L. Muenz, S. E. Starr, D. E. Campbell, C. Wilson, C. Holland, P. Crowley-Nowick, S. H. Vermund, et al. 2000. T-lymphocyte subsets in HIV-infected and high-risk HIV-uninfected adolescents: retention of naive T lymphocytes in HIV-infected adolescents. Arch. Pediatr. Adolesc. Med. 154:375-380. [DOI] [PubMed] [Google Scholar]
- 15.Franco, J. M., A. Rubio, M. Martinez-Moya, M. Leal, E. Merchante, A. Sanchez-Quijano, and E. Lissen. 2002. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood 99:3702-3706. [DOI] [PubMed] [Google Scholar]
- 16.Gortmaker, S. L., M. Hughes, J. Cervia, M. Brady, G. M. Johnson, G. R. Seage III, L. Y. Song, W. M. Dankner, and J. M. Oleske. 2001. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N. Engl. J. Med. 345:1522-1528. [DOI] [PubMed] [Google Scholar]
- 17.Haynes, B. F., M. L. Markert, G. D. Sempowski, D. D. Patel, and L. P. Hale. 2000. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 18:529-560. [DOI] [PubMed] [Google Scholar]
- 18.Hazenberg, M. D., S. A. Otto, J. W. Cohen Stuart, M. C. Verschuren, J. C. Borleffs, C. A. Boucher, R. A. Coutinho, J. M. Lange, T. F. Rinke de Wit, A. Tsegaye, J. J. van Dongen, D. Hamann, R. J. de Boer, and F. Miedema. 2000. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat. Med. 6:1036-1042. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson, B. D., D. C. Douek, S. Killian, L. E. Hultin, D. D. Scripture-Adams, J. V. Giorgi, D. Marelli, R. A. Koup, and J. A. Zack. 1999. Generation of functional thymocytes in the human adult. Immunity 10:569-575. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, A. M., M. E. Valentine, J. Ottinger, R. Baydo, V. Gryszowka, C. Vavro, K. Weinhold, M. St. Clair, and R. E. McKinney. 2001. Immune reconstitution in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy: a cohort study. Pediatr. Infect. Dis. J. 20:941-946. [DOI] [PubMed] [Google Scholar]
- 21.Kolte, L., A. M. Dreves, A. K. Ersboll, C. Strandberg, D. L. Jeppesen, J. O. Nielsen, L. P. Ryder, and A. S. Nielsen. 2002. Association between larger thymic size and higher thymic output in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J. Infect. Dis. 185:1578-1585. [DOI] [PubMed] [Google Scholar]
- 22.Krogstad, P. 2002. Quantification of recent thymic emigrants: T-cell receptor excision circles, p. 291-295. In N. R. Rose, R. G. Hamilton, and B. Detricks (ed.), Manual of clinical laboratory immunology, 6th ed. ASM Press, Washington, D.C.
- 23.Lawn, S. D., S. T. Butera, and T. M. Folks. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14:753-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCune, J. 2001. The dynamics of CD4+ T cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 25.McCune, J. M., M. B. Hanley, D. Cesar, R. Halvorsen, R. Hoh, D. Schmidt, E. Wieder, S. Deeks, S. Siler, R. Neese, and M. Hellerstein. 2000. Factors influencing T-cell turnover in HIV-1-seropositive patients. J. Clin. Investig. 105:R1-R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCune, J. M. 1997. Thymic function in HIV-1 disease. Semin. Immunol. 9:397-404. [DOI] [PubMed] [Google Scholar]
- 27.Mohri, H., A. S. Perelson, K. Tung, R. M. Ribeiro, B. Ramratnam, M. Markowitz, R. Kost, A. Hurley, L. Weinberger, D. Cesar, M. K. Hellerstein, and D. D. Ho. 2001. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 194:1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers, A. S., D. K. Futterman, A. B. Moscicki, C. M. Wilson, J. Ellenberg, and S. H. Vermund. 1998. The REACH Project of the Adolescent Medicine HIV/AIDS Research Network: design, methods, and selected characteristics of participants. J. Adolesc. Health 22:300-311. [DOI] [PubMed] [Google Scholar]
- 29.Rudy, B. J., P. A. Crowley-Nowick, S. D. Douglas, et al. 2001. Immunology and the REACH study: HIV immunology and preliminary findings. J. Adolesc. Health 29:39-48. [DOI] [PubMed] [Google Scholar]
- 30.Sachsenberg, N., A. S. Perelson, S. Yerly, G. A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 187:1295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starr, S. E., M. Sarr, D. E. Campbell, C. M. Wilson, and S. D. Douglas. 2002. Increased proliferation within T lymphocyte subsets of HIV-infected adolescents. AIDS Res. Hum. Retrovir 18:1301-1310. [DOI] [PubMed]
- 32.Steffens, C. M., L. Al-Harthi, S. Shott, R. Yogev, and A. Landay. 2000. Evaluation of thymopoiesis using T cell receptor excision circles (TRECs): differential correlation between adult and pediatric TRECs and naive phenotypes. Clin. Immunol. 97:95-101. [DOI] [PubMed] [Google Scholar]
- 33.Steffens, C. M., K. Y. Smith, A. Landay, S. Shott, A. Truckenbrod, M. Russert, and L. Al-Harthi. 2001. T cell receptor excision circle (TREC) content following maximum HIV suppression is equivalent in HIV-infected and HIV-uninfected individuals. AIDS 15:1757-1764. [DOI] [PubMed] [Google Scholar]
- 34.Steinmann, G. G., B. Klaus, and H. K. Muller-Hermelink. 1985. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand. J. Immunol. 22:563-575. [DOI] [PubMed] [Google Scholar]
- 35.Stoddart, C. A., T. J. Liegler, F. Mammano, V. D. Linquist-Stepps, M. S. Hayden, S. G. Deeks, R. M. Grant, F. Clavel, and J. M. McCune. 2001. Impaired replication of protease inhibitor-resistant HIV-1 in human thymus. Nat. Med. 7:712-718. [DOI] [PubMed] [Google Scholar]
- 36.Vigano, A., S. Vella, M. Saresella, A. Vanzulli, D. Bricalli, S. Di Fabio, P. Ferrante, M. Andreotti, M. Pirillo, L. G. Dally, M. Clerici, and N. Principi. 2000. Early immune reconstitution after potent antiretroviral therapy in HIV-infected children correlates with the increase in thymus volume. AIDS 14:251-261. [DOI] [PubMed] [Google Scholar]
- 37.Watts, D. H. 2002. Management of human immunodeficiency virus infection in pregnancy. N. Engl. J. Med. 346:1879-1891. [DOI] [PubMed] [Google Scholar]
- 38.Weksler, M., R. Schwab, and D. Ai-hao. 1996. Aging and the immune system, p. 789-795. In R. R. Rich, T. Fleisher, B. D. Schwartz, W. T. Shearer, and W. Strober (ed.), Clinical immunology: principles and practice. Mosby-Year Book Inc., St. Louis, Mo.
- 39.Wilson, C. M., J. Houser, C. Partlow, B. J. Rudy, D. C. Futterman, and L. G. Friedman. 2001. The REACH (Reaching for Excellence in Adolescent Care and Health) project: study design, methods, and population profile. J. Adolesc. Health 29:8-18. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, L., S. R. Lewin, M. Markowitz, H. H. Lin, E. Skulsky, R. Karanicolas, Y. He, X. Jin, S. Tuttleton, M. Vesanen, H. Spiegel, R. Kost, J. van Lunzen, H. J. Stellbrink, S. Wolinsky, W. Borkowsky, P. Palumbo, L. G. Kostrikis, and D. D. Ho. 1999. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J. Exp. Med. 190:725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]