Abstract
The existence of specific immunoglobulin E (IgE) allows us to determine the allergens that cause the allergic disease. For the purposes of allergen avoidance and immunotherapy, the measurement of specific IgE is widely applied in clinical laboratories. However, if IgE from the serum of an allergic patient exhibits reactivity to multiple allergens, it would cause a problem. The present study analyzes whether the serum IgE with multiple reactivity is due to the presence of unique IgE against the common epitope shared by different allergens or the presence of multiple IgEs against different epitopes on different allergens. The quantitative-competitive inhibition tests and the immunoblotting were applied to analyze the immunosimilarity among examined allergens. The result shows that the competitive inhibition of IgE binding between shrimp and crab allergens is higher than those between either shrimp and cockroach or between crab and cockroach. Furthermore, the results of immunoblotting are consistent with those of quantitative-competitive inhibition tests. These results allow us to detect the cross-reactivity for atopic IgE against multiple allergens.
Atopic allergy, the most widespread immunologic disorder in human, is characterized by the increased and persistent level of immunoglobulin E (IgE) in serum (4, 11, 18). Family and twin studies have shown that both genetic and environmental factors are involved in the atopic reaction (14, 20). To avoid the allergen from environment exactly, the allergen for an atopic individual should be identified by the measurement of the specific IgE in serum with suspicious allergens. In particular, the cross-reactivity among allergens has been documented (1). Many species of grass pollen, latex, and fruit allergens share their immunological similarities with glycoprotein (2, 7-9, 16, 21). In addition, the tropomyosin in cockroach was identified as a major allergen with potential cross-reactivity with mite and shrimp allergens (12, 13, 19). Calcium-involved carbohydrate-containing IgE epitopes also play a role in the cross-reactivity among several species of fish (3, 10). Furthermore, the investigation of atopic infants revealed that human milk whey protein and cow milk β-lactoglobulin not only share the epitope for IgE binding in vitro but also cause the atopic reaction in vivo (5). Since the serum IgE with the reactivity to multiple allergens is commonly seen in clinical patients (6), we wanted to characterize the immunosimilarity among allergens. First, the atopic sera with the multiple IgE reactivity to cockroach, crab, and shrimp were chosen for the analysis of cross-reactivity with quantitative-competitive inhibition tests. As a control, other atopic sera with multiple IgE reactivities to dust mites, house dust, and dog dander were also examined for the immunosimilarity by quantitative-competitive inhibition tests. Then, the sera with cross-reactivity were further analyzed by immunoblotting. The results allow us to understand the prevalence of immunosimilarity among examined allergens.
MATERIALS AND METHODS
Study populations.
The serum samples of atopic individuals with elevated specific IgE reactive to multiple allergens were collected from the Department of Pediatrics, Kaohsiung Veterans General Hospital. The results of specific IgE, examined by using a MAST CLA allergen-specific IgE assay (Hitachi Chemical Diagnostics, Inc.), were used for screening and selection of patients. From 36 allergens examined, sera with reactivity to multiple allergens, i.e., two or more allergen items, were collected. There were 67 sera collected for this study. After the specific IgE levels were measured, the sera were stored as 1-ml aliquots at −20°C for further analysis.
Quantitative examination of serum IgE.
Based on the results of MAST system, the positive allergen items were chosen for the quantitative examination of atopic sera. The quantitative examinations were performed in Pharmacia CAP-FEIA system (Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden). Generally, 50 μl of the serum was used for each measurement. If the atopic serum appeared to have a titer higher than class 6 (>100 kU/liter), a 10-fold dilution was performed.
Quantitative-competitive inhibition test.
The quantitative-competitive inhibition tests were performed by using Pharmacia CAP-FEIA system. Sera were prebound with ImmunoCAP: this allergen is the candidate with cross-reactivity to the following allergens examined. After 2 h of prebinding, the level of serum IgE was determined according to the procedure of the Pharmacia CAP-FEIA system. The percentage of the quantitative-competitive inhibition test was calculated by using the IgE reactivity with or without the prebinding of allergens as follows: percent inhibition = [(IgEO − IgEA)/IgEO] × 100, where IgEO is the IgE reactivity without pre-binding and IgEA is the IgE reactivity with prebinding with allergens.
IgE immunoblotting.
The AlaBLOT specific IgE allergen strips (Diagnostic Products Co., Los Angeles, Calif.) were used to identify the serum IgE-reactive allergens by immunochemical means. According to the procedures of the AlaBLOT specific IgE allergen strips test, the diluted serum (50 μl in 500 μl) was added onto the strip, followed by incubation for 2 h at room temperature. The strip was then washed three times and incubated with enzyme-labeled anti-IgE for 30 min. After three washes, the color of the protein bands with reaction to serum IgE was developed by adding substrate reagent. The molecular weights of the proteins were estimated according to the description in each lot of strips by using relative mobility of molecular weight markers. For the inhibition of immunoblotting, the serum was also prebound with ImmunoCAP; these allergens are cross-reactive to the examined allergen. The serum was then used for the IgE immunoblotting to determine the extent of the inhibition of immunoblotting.
RESULTS AND DISCUSSION
Immunosimilarity among allergens.
The sera examined here contained IgE antibodies to multiple allergens. After the examination with the CAP-FEIA system, 63 sera of the 67 collected samples showed reactivity to multiple allergens (Table 1).
TABLE 1.
Atopic sera with IgE reactivity to multiple allergensa
| No. | Positive reaction |
|---|---|
| 1 | i6 e1 e5 d1 d2 h1 m2 m3 m5 |
| 2 | i6 e1 e5 d1 d2 h1 |
| 3 | i6 e5 d1 d2 h1 |
| 4 | i6 d1 d2 h1 |
| 5 | i6 e5 d1 d2 h1 |
| 6 | i6 d1 d2 h1 |
| 7 | i6 e5 d1 d2 h1 |
| 8 | i6 d1 d2 h1 |
| 9 | i6 d1 d2 h1 |
| 10 | i6 d1 d2 h1 |
| 11 | i6 e1 e5 d1 d2 h1 m1 m2 m3 m5 m6 |
| 12 | d1 d2 f23 |
| 13 | i6 d1 d2 h1 f23 |
| 14 | i6 d1 d2 h1 |
| 15 | i6 e1 e5 d1 d2 h1 |
| 16 | f24 |
| 17 | i6 d1 d2 f23 f24 |
| 18 | i6 d1 d2 h1 |
| 19 | i6 d1 d2 h1 |
| 20 | i6 d1 d2 h1 e5 |
| 21 | i6 d1 d2 h1 |
| 22 | i6 d1 d2 h1 e5 |
| 23 | i6 d1 d2 h1 |
| 24 | i6 d1 d2 h1 f23 |
| 25 | i6 d1 d2 h1 f23 f24 |
| 26 | i6 d1 d2 h1 f23 f24 |
| 27 | i6 |
| 28 | i6 |
| 29 | d1 d2 f23 |
| 30 | i6 d1 d2 h1 |
| 31 | i6 d1 d2 h1 |
| 32 | i6 d1 d2 h1 f23 f24 |
| 33 | i6 d1 d2 h1 f24 |
| 34 | i6 d1 d2 h1 e5 f23 f24 |
| 35 | d1 d2 h1 f24 |
| 36 | i6 d1 d2 h1 |
| 37 | i6 d1 d2 h1 |
| 38 | i6 d1 d2 h1 f23 f24 |
| 39 | i6 d1 d2 f23 f24 |
| 40 | i6 e5 d1 d2 f23 f24 |
| 41 | i6 f24 |
| 42 | i6 d1 d2 f23 f24 |
| 43 | e1 e5 d1 d2 f23 |
| 44 | d1 d2 f23 f24 |
| 45 | i6 e5 d1 d2 |
| 46 | i6 d1 d2 |
| 47 | i6 d1 d2 f24 |
| 48 | i6 d1 d2 |
| 49 | i6 d1 d2 f23 |
| 50 | i6 d1 d2 f23 f24 |
| 51 | i6 d1 d2 |
| 52 | i6 e5 d1 d2 f24 |
| 53 | i6 e5 d1 d2 |
| 54 | i6 d1 d2 f23 f24 |
| 55 | d1 |
| 56 | i6 d1 d2 |
| 57 | i6 d1 d2 f23 f24 |
| 58 | i6 e5 d1 d2 f24 |
| 59 | i6 e5 d2 f23 f24 |
| 60 | e1 e5 d2 f23 f24 |
| 61 | i6 d1 d2 f23 f24 |
| 62 | i6 d1 d2 f23 f24 |
| 63 | i6 d1 f24 |
| 64 | i6 d1 d2 |
| 65 | d1 d2 f23 f24 |
| 66 | i6 d1 d2 f23 f24 |
| 67 | i6 d1 d2 f23 f24 |
Reaction types: i6, cockroach; d1, dust mite (Dermatophagoides pteronyssinus); d2, dust mite (Dermatophagoides farinae); e1, cat dander; e5, dog dander; h1, house dust; f23, crab; f24, shrimp; m1, Penicillium spp.; m2, Cladosporium spp.; m3, Aspergillus spp.; m5, Candida albicans; m6, Alternaria spp.
Since most sera with reactivity to shrimp allergens were also reactive to crab and cockroach allergens, 15 sera were then chosen for the analysis of cross-reactivity among shrimp, crab, and cockroach serum samples by the quantitative-competitive inhibition tests. As shown in Table 2, the inhibition of the serum IgE is an average of 34% between shrimp and cockroach, 65% between shrimp and crab, and 35% between crab and cockroach. In particular, among the 15 examined sera, 3 showed an inhibition of >50% between shrimp and cockroach sera, 11 showed a inhibition of >50% between shrimp and crab sera, and 4 showed a inhibition of >50% between crab and cockroach sera. Furthermore, the inhibition between shrimp and crab sera was shown to be significant by the chi-square test (P < 0.01). Therefore, this result indicated that the immunosimilarity between shrimp and crab sera is indeed present in the serum IgE of atopic individuals.
TABLE 2.
Quantitative-competitive inhibition tests among cockroach, shrimp, and crab seraa
| No. | f24 (kU/liter)
|
f24 (kU/liter)
|
f23 (kU/liter)
|
|||
|---|---|---|---|---|---|---|
| − | + i6 (% inhibition) | − | + f23 (% inhibition) | − | +i6 (% inhibition) | |
| 17 | 3.63 | 0.94 (74) | 3.63 | 0.78 (79) | 5.09 | 1.39 (73) |
| 25 | 0.51 | <0.35 (31) | 0.51 | 0.45 (12) | 0.57 | 0.36 (37) |
| 34 | 0.50 | <0.35 (30) | 0.50 | <0.35 (30) | 1.01 | 0.63 (38) |
| 38 | 0.90 | 0.74 (18) | 0.90 | 0.58 (36) | 0.83 | 0.69 (17) |
| 39 | 0.83 | 0.73 (12) | 0.83 | <0.35 (58) | 1.25 | 0.99 (21) |
| 40 | 6.44 | 5.34 (17) | 6.44 | 1.85 (71) | 7.03 | 5.37 (24) |
| 42 | 29.9 | 20.1 (33) | 29.9 | 4.05 (86) | 38.2 | 19.3 (49) |
| 44 | 5.46 | 3.30 (40) | 5.46 | <0.35 (94) | 5.40 | 3.83 (29) |
| 50 | 0.75 | 0.55 (27) | 0.75 | <0.35 (53) | 5.60 | 4.09 (27) |
| 54 | 3.68 | 2.86 (22) | 3.68 | 0.7 (81) | 5.1 | 2.28 (55) |
| 57 | 1.85 | 0.72 (61) | 1.85 | <0.35 (81) | 2.27 | 1.01 (56) |
| 61 | 6.56 | 2.96 (55) | 6.56 | 0.39 (94) | 6.97 | 3.33 (52) |
| 62 | 1.34 | 1.16 (13) | 1.34 | 0.7 (48) | 2.2 | 2.1 (5) |
| 66 | 6.32 | 4.10 (35) | 6.32 | 2.14 (66) | 10.5 | 8.65 (18) |
| 67 | 3.42 | 2.00 (42) | 3.42 | 0.37 (89) | 3.48 | 2.49 (28) |
i6, cockroach; f23, crab; f24, shrimp. −, untreated serum; +, serum prebound with the allergen in ImmunoCAP. The mean percent inhibition values for f24 + i6, f24 + f23, and f23 + i6 are 34, 65, and 35%, respectively, and the associated P values are 0.139, 0.007, and 0.022, respectively (as determined by chi-square analysis).
In addition, five sera were chosen for the analysis of cross-reactivity among dust mites, house dust, and dog dander allergens by the quantitative-competitive inhibition tests. As shown in Table 3, small inhibitions of the serum IgE against these allergens were observed. The result indicated that these sera appear to have the distinct IgEs reactive to dust mites, house dust, and dog dander separately.
TABLE 3.
Quantitative-competitive inhibition tests among dust mites, house dust, and dog dander
| Examined allergena | Quantitation of IgE for the atopic sera (kU/liter) for sample:
|
||||
|---|---|---|---|---|---|
| No. 1 | No. 5 | No. 7 | No. 11 | No. 22 | |
| d2 without treatment | >100 | 99.8 | >100 | >100 | >100 |
| Prebinding with d1 | 82.1 | 52.1 | 92.2 | >100 | >100 |
| Prebinding with h1 | >100 | 84.3 | >100 | >100 | >100 |
| Prebinding with e5 | >100 | 91.0 | >100 | >100 | >100 |
| d1 without treatment | >100 | 80.5 | >100 | >100 | >100 |
| Prebinding with h1 | >100 | 68.0 | 98.9 | >100 | >100 |
| h1 without treatment | 71.1 | 14.0 | 37.3 | 70.8 | 46.6 |
| Prebinding with e5 | 65.6 | 13.3 | 40.6 | 63.7 | 47.1 |
| e5 without treatment | 0.59 | 0.91 | 0.63 | 0.51 | 0.48 |
| Prebinding with d1 | 0.45 | 0.77 | 0.57 | 0.44 | 0.37 |
d1, dust mite (Dermatophagoides pteronyssinus); d2, dust mite (Dermatophagoides farinae); h1, house dust; e5, dog dander.
The quantitative-competitive inhibition test has been applied to examine the level of immunosimilarity between allergens. For example, it was used by Petersen et al. for the examination of IgE cross-reactivity between tomato fruit and grass pollen allergens (17) and by Hansen et al. for the examination of IgE cross-reactivity among different species of fish (10). The results of quantitative-competitive inhibition tests in our study indicate that the extent of immunosimilarity was low among dust mites, house dust, and dog dander and higher among shrimp, crab, and cockroach sera.
The quantitative-competitive inhibition tests in this study were performed with ImmunoCAP for prebinding of serum to allergen. The limitation of this method is that only one dose of allergen could be applied. However, we used this method to examine the cross-reactivity between two allergens in the clinical laboratory and showed that it is appropriate to analyze the cross-reactivity between two allergens.
Immunoblotting of serum IgE against shrimp, crab, and cockroach.
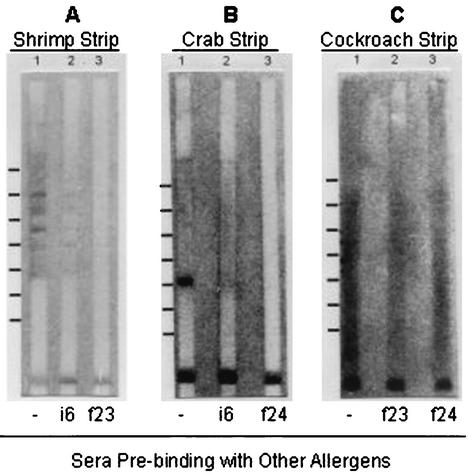
To examine the molecular features of cross-reactivity among shrimp, crab, and cockroach, the immunoblotting and inhibition of immunoblotting were performed. Since the result from Table 2 showed that a high degree of immunosimilarity is present between shrimp and crab and partial immunosimilarity is evident between either shrimp and cockroach or between crab and cockroach, the serum of sample 61 was chosen for this experiment. We demonstrated (Fig. 1A) that this serum exhibited reactivity to several shrimp proteins. Moreover, reactivity to shrimp proteins was dramatically inhibited if the serum was prebound with crab allergens and slightly inhibited if the serum was prebound with cockroach allergens. Figure 1B shows the immunoblotting of crab with a major reactivity to a protein of 31 kDa. In addition, the inhibition of immunoblotting is higher by shrimp allergens (lane 3) than that by cockroach allergens (lane 2). Figure 1C shows the immunoblotting of cockroach sera, and a smear of protein bands seems to indicate that many cockroach proteins were recognized by the serum IgE. In addition, the inhibition of immunoblotting with the serum prebound by either shrimp or crab allergens was consistent with the results of the quantitative-competitive inhibition tests given in Table 2. Therefore, the results of immunoblotting appear to be consistent with those of quantitative-competitive inhibition tests.
FIG. 1.
Immunoblotting with strips of allergens. (A, B, and C) Immunoblots of shrimp, crab, and cockroach sera with atopic sera, respectively. Lane 1 in each panel represents immunoblotting with untreated serum. Lanes 2 and 3 in each panel represent the immunoblotting with the prebound sera. The molecular masses of protein bands were estimated as described in Materials and Methods. Markers are indicated on the left side of each panel, and the molecular masses from top to bottom are 117, 83, 59, 42, 30, 21, and 15 kDa for panel A; 135, 94, 66, 46, 32, 23, and 16 kDa for panel B; and 123, 86, 60, 42, 30, 21, and 14 kDa for panel C.
Pascual et al. have demonstrated the cross-reactivity between IgE binding proteins from Anisakis simplex larvae (parasite of seafoods), Blattella germanica (German cockroach), and Chironomidae (red mosquito larvae) (15). Also, Santos et al. have identified tropomyosin as a major allergen of cockroach (19). However, our results show that the inhibitions between either cockroach and shrimp or between cockroach and crab were only partial. In particular, our examinations, including quantitative-competitive inhibition tests and the inhibition of immunoblotting, showed that the inhibition between shrimp and crab seems to be higher. These results indicate that similar epitopes of shrimp and crab allergens are involved in IgE binding. Interestingly, Cantisani et al. also showed that the common structure of epitopes indeed existed in human milk whey proteins and β-lactoglobulin in cow's milk proteins (5). Although cross-reactivity between epitopes in vivo may not have correlation to cross-reactivity in a clinical setting, further studies on the peptide sequence and tertiary structure of common epitopes should still shed light on the molecular mechanism of IgE cross-reactivity.
In conclusion, the immunosimilarity among several allergens was quantitated with quantitative-competitive inhibition tests and analyzed based on the inhibition of immunoblotting. A high degree of immunosimilarity between shrimp and crab allergens was determined. The result indicates that the cross-reactivity between shrimp and crab sera was induced by a similar, if not identical, allergen epitope. In addition, the result of immunoblotting indicates that the 31-kDa crab protein is involved in the binding of allergen to IgE.
Acknowledgments
We thank Chi-Shan Chen for the collection of serum samples.
This study was supported by grants from National Science Council, Republic of China (NSC 89-2314-B-242-005), and the Fooyin Institute of Technology (FIT-88-Med-022).
REFERENCES
- 1.Aalberse, R. C. 1998. Allergens from mites: implications of cross-reactivity between invertebrate antigens. Allergy 53:47-48. [DOI] [PubMed] [Google Scholar]
- 2.Batanero, E., M. Villalba, R. I. Monsalve, and R. Rodriguez. 1996. Cross-reactivity between the major allergen from olive pollen and unrelated glycoproteins: evidence of an epitope in the glycan moiety of the allergen. J. Allergy Clin. Immunol. 97:1264-1271. [DOI] [PubMed] [Google Scholar]
- 3.Bugajska-Schretter, A., L. Elfman, T. Fuchs, S. Kapiotis, H. Rumpold, R. Valenta, and S. Spitzauer. 1998. Parvalbumin, a cross-reactive fish allergen, contains IgE-binding epitopes sensitive to periodate treatment and Ca2+ depletion. J. Allergy Clin. Immunol. 101:67-74. [DOI] [PubMed] [Google Scholar]
- 4.Burrows, B., F. D. Martinez, M. Holonen, R. A. Barbee, and M. G. Clin. 1989. Association of asthma with serum IgE levels and skin test reactivity to allergens. N. Engl. J. Med. 320:271-277. [DOI] [PubMed] [Google Scholar]
- 5.Cantisani, A., M. G. Giuffrida, C. Fabris, E. Bertino, A. Coscia, R. Oggero, G. Monti, P. Stroppiana, and A. Conti. 1997. Detection of specific IgE to human milk proteins in sera of atopic infants. FEBS Lett. 412:515-517. [DOI] [PubMed] [Google Scholar]
- 6.Chou, M. C., C. Y. Yuo, W. S. Lan, and S. P. Huang. 2001. Seral IgE of atopic individuals exhibit the reactivity to multiple allergens. J. Biomed. Lab. Sci. 13:1-5. [Google Scholar]
- 7.Fischer, S., M. Grote, B. Fahlbusch, W. D. Muller, D. Kraft, and R. Valenta. 1996. Characterization of Phl p4, a major timothy grass (Phleum pratense) pollen allergen. J. Allergy Clin. Immunol. 98:189-198. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs, T., S. Spitzauer, C. Vente, J. Hevier, S. Kspiotis, H. Rumpold, D. Kraft, and R. Valenta. 1997. Natural latex, grass pollen, and weed pollen share IgE epitopes. J. Allergy Clin. Immunol. 100:356-364. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, N., B. M. Martin, D. D. Metcalfe, and P. V. S. Rao. 1996. Identification of a novel hydroxyproline-rich glycoprotein as the major allergen in Parthenium pollen. J. Allergy Clin. Immunol. 98:903-912. [DOI] [PubMed] [Google Scholar]
- 10.Hansen, T. K., C. Bindslev-Jensen, P. S. Skov, and L. K. Poulen. 1997. Codfish allergy in adults: IgE cross-reactivity among fish species. Ann. Allergy Asthma Immunol. 78:187-194. [DOI] [PubMed] [Google Scholar]
- 11.Hayglass, K. T. 1995. Allergy: who, why, and what to do about it? Immunol. Today 16:505-510. [DOI] [PubMed] [Google Scholar]
- 12.Jeoung, B. J., G. Reese, P. Hauck, J. B. Oliver, C. B. Daul, and S. B. Lehrer. 1997. Quantification of the major brown shrimp allergen Pen a1 (tropomyosin) by a monoclonal antibody-based sandwich ELISA. J. Allergy Clin. Immunol. 100:229-234. [DOI] [PubMed] [Google Scholar]
- 13.Leung, P. S., Y. C. Chen, M. E. Gershwin, S. Wong, H. S. Kwan, and K. H. Chu. 1998. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J. Allergy Clin. Immunol. 102:847-852. [DOI] [PubMed] [Google Scholar]
- 14.Newman-Talor, A. 1995. Environmental determinants of asthma. Lancet 345:296-299. [DOI] [PubMed] [Google Scholar]
- 15.Pascual, C. Y., J. F. Crespo, S. San Maetin, N. Ornia, N. Ortega, T. Caballero, M. Munoz-Pereira, and M. Martin-Esteban. 1997. Cross-reactivity between IgE-binding proteins from Anisakis, German cockroach, and chironomids. Allergy 52:514-520. [DOI] [PubMed] [Google Scholar]
- 16.Pastrorello, E. A., V. Pravettoni, M. Ispano, L. Farioli, R. Ansaloni, F. Rotondo, C. Incorvaia, I. Asman, A. Bengtsson, and C. Ortolani. 1996. Identification of the allergenic components of kiwi fruit and evaluation of their cross-reactivity with timothy and birch pollen. J. Allergy Clin. Immunol. 98:601-610. [DOI] [PubMed] [Google Scholar]
- 17.Petersen, A., S. Vieths, H. Aulepp, M. Schlaak, and W. M. Becker. 1996. Ubiquitous structures responsible for IgE cross-reactivity between tomato fruit and grass pollen allergens. J. Allergy Clin. Immunol. 98:805-815. [DOI] [PubMed] [Google Scholar]
- 18.Pollart, S., M. Chapman, G. Fiocco, G. Rose, and A. Platts-Mills. 1989. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J. Allergy Clin. Immunol. 83:875-882. [DOI] [PubMed] [Google Scholar]
- 19.Santos, A. B., M. D. Chapman, R. C. Aalberse, L. D. Vailes, V. P. Ferriani, C. Oliver, M. C. Rizzo, C. K. Naspitz, and L. K. Arruda. 1999. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J. Allergy Clin. Immunol. 104:329-337. [DOI] [PubMed] [Google Scholar]
- 20.Sluyter, R., E. R. Tovey, D. L. Dufy, and W. J. Britton. 1998. Limited genetic control of specific IgE responses to rye grass pollen allergens in Australian twins. Clin. Exp. Allergy 28:322-331. [DOI] [PubMed] [Google Scholar]
- 21.Wahl, R., P. Schmid-Grendelmeier, O. Cromwell, and B. Wuthrich. 1996. In vitro investigation of cross-reactivity between birch and ash pollen allergen extracts. J. Allergy Clin. Immunol. 98:99-106. [DOI] [PubMed] [Google Scholar]