Abstract
Among the toxins that Staphylococcus aureus is able to secrete, bi-component toxins named leukotoxins target specifically leukocytes, mainly phagocytic cells. Isolates from cows, goats and ewes with mastitis were selected on the basis of the presence or not of the genes encoding the recently described LukM/LukF′-PV leukotoxin. Of the 128 isolates tested, 126 had moderate to high leukotoxic activity to bovine polymorphonuclear cells (PMN). The supernatants of lukM-positive isolates were much more leukotoxic than the supernatants of lukM-negative isolates: mean leukotoxic titers were 122 versus 20 and 581 versus 26 for isolates of bovine and caprine origin, respectively. Among lukM/lukF′-PV positive isolates, those of caprine and ovine origins were more leukotoxic than were isolates of bovine origin (P < 0.01). The two most abundant proteins in the culture supernatant of a highly toxic isolate were purified and identified as the two components of LukM (LukM and LukF′-PV) on the basis of their molecular mass, N-terminal amino acid sequence, and high synergistic activity. LukM/LukF′-PV induced the flattening of bovine PMN at a concentration as low as 3.6 ng/ml (0.1 nM). A higher concentration (18 ng/ml) was necessary to produce the same effect on caprine or ovine PMN. Affinity-purified antibodies to LukM or to LukF′-PV neutralized the leukotoxic effect of all the culture supernatants. They neutralized with the same efficiency the toxic activity of supernatants from lukM/lukF′-PV positive or negative isolates. These results establish that LukM/LukF′-PV is very active on PMN of ruminants and suggest that this leukotoxin could be the most active leukotoxin produced by mastitis isolates. They prompt further studies to delineate the contribution of LukM/LukF′-PV to the pathogenesis of mastitis in ruminants and the protective effect of antibodies to this leukotoxin.
Staphylococcus aureus is one of the pathogens most frequently isolated from the milk of infected mammary glands of cows, and it is isolated occasionally from the milk of ewes or goats (2, 11). This pathogen, which causes contagious mastitis, is mainly transmitted from gland to gland during the milking process. It is generally associated with long-lasting chronic infections of subclinical or moderate clinical forms in the cow, whereas severe clinical mastitis is more frequent in goats and ewes (1, 23). S. aureus can secrete several toxins which are supposed to contribute to the pathogenesis of mastitis (18). Among these exotoxins, some have the capacity to selectively kill phagocytic cells such as polymorphonuclear cells (PMN) and monocytes. These leukotoxins belong to the family of bi-component leukotoxins, composed of two distinct proteins, the S-related (slow-eluted) and the F-related (fast-eluted) components, which act synergistically to form holes in the membrane of phagocytes. The staphylococcal leukotoxin family comprises the long-known Panton-Valentine leukocidin (LukS-PV + LukF-PV), γ-hemolysin (HlgA + HlgB and HlgC + HlgB), and the more recently described LukM (LukM + LukF′-PV) and LukE/D (LukE + LukD) (4, 8, 9, 24).
Because phagocytosis by PMN is regarded as one of the most important defense mechanisms of the mammary gland (5), toxins produced by staphylococci that can interfere with this defense are of potential importance in the pathogenesis of staphylococcal mastitis, in which case neutralizing antibodies (Ab) could contribute to the protection of the mammary gland. In vivo production of leukotoxins is likely, since cows with chronic S. aureus mastitis have higher antileukocidin Ab titers than uninfected cows (12). It was shown that Ab to staphylococcal leukotoxin protect bovine PMN from cytotoxicity (13). Also, vaccination of ewes with partially purified leukotoxin (probably the Panton-Valentine leukocidin), contaminated with α-hemolysin, conferred partial protection against an intramammary challenge with a mastitis-causing strain of S. aureus (22). Although the precise nature of the leukotoxin studied was unknown and the purification of the toxins was incomplete, these earlier reports suggest that antileukotoxin Ab could have an important role in protection against mammary infection of ruminants.
Recently, it was shown by PCR that S. aureus isolated from ruminants with mastitis possess the genes for several leukotoxins (B. Poutrel et al., unpublished data). In particular, all the strains had genes for γ-hemolysin and LukE/D, but lukMlukF′-PV genes were harbored by only part of the strains. None of the strains possessed the genes for the Panton-Valentine leukocidin. This finding prompted us to investigate whether the possession of genes for leukotoxins is linked to leukotoxic activity of culture supernatants and, in particular, whether the intensity of toxicity correlated with the genetic equipment of the mastitis isolates. As it appeared that the presence of genes encoding LukM coincided with strong toxicity of culture supernatants and that the activity of LukM, reported to be low on human PMN (15), was unknown on bovine PMN, we decided to measure the toxicity of this newly described leukotoxin on the PMN of ruminants. In addition, we evaluated the capacity of Ab to LukM to inhibit the leukotoxic activity of culture supernatants of S. aureus mastitis isolates.
MATERIALS AND METHODS
Strains (Table 1).
TABLE 1.
Distribution of 128 strains of S. aureus isolated from ruminants with mastitisa
| Origin of isolates | No. of isolates
|
|
|---|---|---|
| Lacking lukM | lukM positive | |
| Cow | 31 | 17 |
| Goat | 15 | 32 |
| Ewe | 1 | 32 |
Strains are listed as a function of the animal species of origin and of the presence of the genes of the LukM/LukF′-PV leukotoxin. All the isolates have the genes for γ-hemolysin and for the leukotoxin LukE/D.
A total of 128 strains of S. aureus isolated from the milk of infected mammary glands of ruminants were selected on the basis of the animal species of origin and the presence of the lukM/lukF′-PV genes. All the isolates, which belong to the strain collection of our laboratory, have been typed by PCR (Poutrel et al., unpublished data) and shown to possess the genes for the leukotoxins γ-hemolysin (hlgv) and LukE/LukD (lukE and lukD). On the other hand, only part of the isolates have the lukM/lukF′-PV genes (Table 1). Only one lukM-negative isolate of ovine origin was available. All the available lukM-positive isolates of bovine origin and lukM-negative isolates of caprine origin were included in the study. The isolates selected for the study originate from different herds, or had different PCR gene profiles when originating from the same herd. Bacteria were cultivated for 24 h at 37°C in either brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) or yeast extract-Casamino acids-sodium pyruvate (YCP) growth medium (8). After one subculture under the same conditions, bacteria were sedimented by centrifugation (2,500 × g for 30 min) and supernatants were aliquoted and stored frozen at −20°C.
Purification of leukotoxins (LukM/LukF′-PV).
A strain of S. aureus (Ch122) isolated from the milk of an infected goat was cultivated overnight at 37°C with vigorous shaking (150 rpm) in 2-liter Erlenmeyer flasks containing 0.5 liter of BHI culture medium. Bacteria were centrifuged (9,000 × g, 30 min) and the culture supernatant was filtered (0.45-μm-pore-size filter; Millipore) before passage over a carboxy-methyl-Sephadex C-25 column equilibrated in 0.03 M phosphate buffer, pH 6.5. The adsorbed material was eluted with a gradient of NaCl (0 to 0.5 M) in phosphate buffer. Fractions obtained at different elution concentrations were assayed by the PMN spreading assay (see below). The leukotoxic fractions were pooled and dialysed against sodium 0.03 M phosphate buffer, pH 6.5. The dialysed pool was loaded onto a cation-exchange chromatography column (MonoS HR 10/10; Pharmacia LKB Biotechnology AB, Uppsala, Sweden) equilibrated with the same buffer. Elution of bound proteins was achieved with a gradient of NaCl (0 to 0.6 M over 200 ml) in equilibrating buffer, applied with a system for fast protein liquid chromatography (BioLogic chromatography system; Bio-Rad Laboratories Inc., Hercules, Calif.). Absorbance (at 280 nm) peaks augmenting the leukotoxic activity of suboptimal concentration of crude supernatant were further purified by hydrophobic chromatography on a t-butyl HIC Econo-Pac cartridge (Bio-Rad Laboratories Inc.) equilibrated in 0.1 M phosphate buffer (pH 6.5) plus 1.5 M ammonium sulfate. Elution was performed with a declining gradient of ammonium sulfate from 1.5 to 0 M in the phosphate buffer. The purified components were then dialyzed against 0.05 M phosphate buffer (pH 7.0) plus 0.15 M NaCl. Protein analysis was performed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% polyacrylamide, wt/vol) under reducing conditions (10). Protein bands were visualized by staining with silver nitrate (14). Characterization of the purified proteins was performed by determining their N-terminal peptide sequences after blotting on polyvinylidene difluoride membranes.
Assay of leukotoxic activity on PMN.
The previously described shape change of PMN induced by staphylococcal leukotoxins (6, 7), which is well suited for a screening assay, was adapted for use in microtiter plates. Bovine PMN were isolated by centrifugation of peripheral blood anticoagulated with EDTA and hypotonic lysis of erythrocytes (3). The cells obtained by this procedure were essentially granulocytes (95%). PMN were resuspended (2 × 106/ml) in RPMI 1640 medium without sodium carbonate, supplemented with 0.1% gelatin (Difco) and 20 mM HEPES (RPMI-GH). PMN from goats and sheep were isolated by the same procedure. Cell preparations with a viability greater than 95% (trypan blue exclusion) were used. Serial twofold dilutions in RPMI-GH of culture supernatants or purified toxins (80 μl) were distributed in the wells of a 96-well flat-bottom microtiter plate previously coated with gelatin (0.5% in water for 15 min at 37°C). To every well was added 20 μl of PMN suspension, and the plate was incubated for 20 min at 37°C. Then, the shape of PMN was examined with an inverted microscope. Under the influence of leukotoxins, the PMN flattened and spread on the gelatin-coated plastic surface, whereas in control wells without toxin (containing RPMI-GH or unused broth culture medium) the cells remained rounded. The last dilution inducing the spreading of more than 95% of PMN (rated +++) was taken as the last active dilution, and the titer of the culture supernatant was the inverse of this dilution. When the titration of a given culture supernatant was repeated on different days with PMN from different cows, the titers were usually the same, or differed only by one dilution. Initially, two different investigators scored the plates, but this procedure was stopped when it appeared that their scores were identical. Culture supernatants could be frozen and stored for at least several months at −20°C without loss in activity.
Preparation of affinity-purified bovine Ab to LukM.
A healthy lactating cow was immunized by two subcutaneous injections of the two components F (LukF′-PV) and S (LukM) of purified leukotoxin. Each component (40 μg in 500 μl of phosphate-buffered saline) was emulsified with 500 μl of incomplete Freund adjuvant and injected twice separately in different subcutaneous locations 1 month apart. Two weeks after the second injection, blood was drawn and serum was prepared.
Two immunoabsorbents were prepared by coupling either component S or component F to an affinity coupling gel. Three milliliters of EAH Sepharose 4B (Pharmacia LKB Biotechnology AB), equilibrated in sodium 0.1 M phosphate buffer, pH 8.0, were activated with 1.25% glutaraldehyde (Merck) and mixed with 1 mg of either component F or component S of the purified leukotoxin. After a 30-min incubation at 37°C under end-over-end agitation, the gels were washed with phosphate buffer, and the potential remaining coupling sites were blocked with Tris-HCl buffer (0.1 M), pH 7.5. Gel slurries were poured into empty PD-10 columns (Pharmacia LKB Biotechnology AB) and washed with 20 mM HEPES buffer, pH 7.0, plus 0.15 M NaCl (HBS). Bovine antiserum to purified leukotoxin (F+S) was passed sequentially over columns of LukF′-PV-Sepharose and LukM-Sepharose. The columns were washed with 1.0 M NaCl until the absorbance at 280 nm returned to baseline, and the bound Ab were eluted with 1.0 M NH4OH (21). The eluted fractions were neutralized with 4 N HCl, and their specificities were assayed by indirect enzyme-linked immunosorbent assay. Microtiter plates (Immunoplate; Nunc, Roskilde, Denmark) were coated with either purified LukM or LukF′-PV diluted at 2.5 μg/ml in 0.1 M carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. The plates were blocked with 0.5% (wt/vol) gelatin for 30 min at 37°C, and dilutions of immunosorbent eluates were incubated for 1 h at 37°C. After five washes with phosphate-buffered saline (PBS) supplemented with 0.05% Tween 20, peroxidase-conjugated rabbit anti-bovine immunoglobulin G (IgG) (heavy and light chain) (Jackson Immunoresearch Laboratory, West Grove, Pa.) diluted 10,000-fold was incubated for 30 min at 37°C. After five washes, peroxidase substrate [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) + H2O2] was added and the absorbance was read at 415 nm. The purified Ab were also tested by Western blotting. Culture supernatant of strain Ch122 was subjected to SDS-PAGE, and the proteins were transferred to a nitrocellulose membrane (Sartorius AG, Göttingen, Germany) (20) using a semidry method. Nonspecific sites were blocked by incubating the blot for 30 min at room temperature with PBS supplemented with 0.5% (wt/vol) gelatin. The sequence of incubation steps, separated by three washes with PBS supplemented with 0.1% (vol/vol) Tween 20 (PBS-T), was as follows: (i) bovine specific Ig anti-LukM or anti-LukF (2 μg/ml) in PBS plus 0.1% gelatin during 2 h at room temperature, (ii) a 1/1,500 dilution of a peroxidase-conjugated mouse IgG anti-bovine IgG (heavy- and light-chain specific; Jackson Immunoresearch Laboratory) during 1 h at room temperature, and (iii) detection of peroxidase-conjugated Ab using a 1/10 dilution of a 3,3′,5,5′-tetramethylbenzidine solution in stable substrate peroxide buffer (1-Step TMB-blotting; Pierce, Rockford, Ill.).
Assay of leukotoxin neutralization.
The capacity of affinity-purified Ab to LukM or LukF′-PV to neutralize the leukotoxic activity of staphylococcal culture supernatants was measured to test whether the leukotoxic activity observed with the PMN spreading assay was due to LukM/LukF′-PV leukotoxin or cross-reacting leukotoxins. One toxin limit concentration (TLC) was defined as the concentration of toxin (F + S) just sufficient to cause the characteristic morphological change (+++ intensity) of 4 × 104 bovine PMN, in 100 μl of reagent mixture at the end of a 30-min incubation at 37°C. The two components of leukotoxin (M + F′-PV) were used in equal amounts. The affinity-purified bovine Ab to LukM or to LukF′-PV were titrated against purified leukotoxin (F + S) by determining the amount of Ab necessary to inhibit the activity of the purified components F and S of the leukotoxin (ratio, 1/1). One neutralizing limit concentration (NLC) of Ab was defined as the minimum amount of Ab of either component S or component F specificity able to neutralize 1 TLC of LukM.
Assays of the neutralization of the toxic activity of culture supernatants were carried out in microtiter plates with a mixture of anti-M and anti-F′-PV Ab in 40 μl (equal concentrations) and 40 μl of culture supernatant. The mixture was incubated for 1 h at 20 to 25°C under agitation, and 20 μl of PMN suspension was added. Visual assessment of the wells was done after 1 h of incubation at rest at 37°C. The reaction mixture contained 2 NLCs, and the culture supernatants were tested at twice the limit concentration causing the spreading of PMN. All dilutions were in RPMI-HG. When required, higher concentrations of antitoxin were incubated with the 2 TLCs of toxin.
Statistical analysis.
Results were analyzed for significant differences by use of analysis of variance and the Wilcoxon test, using a computer software program (StatView; Abacus Concepts, Berkeley, Calif.).
RESULTS
Titration of toxic activity of culture supernatants of isolates from animals with mastitis.
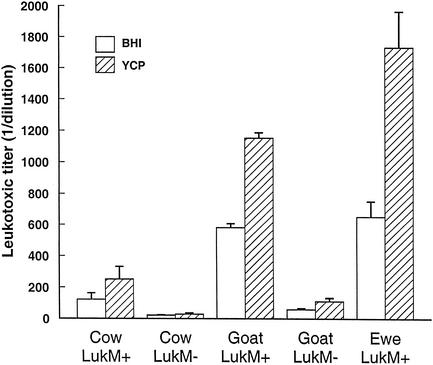
All the culture supernatants were toxic for bovine PMN, with the exception of the supernatants of two lukM-negative isolates from goats, which were active only undiluted. The two media, BHI and YCP, were used in parallel because variable production of leukotoxins (LukE/LukD and γ-hemolysin) in these media has been reported (8). In our study, leukotoxic titers of cultures in YCP medium were systematically higher than the titers of BHI cultures, but the growth of staphylococci was also better in YCP medium than in BHI broth (results not shown). Overall, the leukotoxic titers by optical density (OD) unit (turbidity) were comparable in YCP and in BHI media. Marked differences in leukotoxic titers were seen as a function of the origin of the isolates and of the presence of the lukM/lukF′-PV genes (Fig. 1). The supernatants of isolates possessing the genes encoding the LukM/LukF′-PV toxin were much more leukotoxic than the supernatants of isolates devoid of these genes. This was true of isolates of bovine origin (mean titer of 122 versus 20 in BHI; P < 0.01) and of caprine origin (mean titer of 581 versus 26 in BHI; P < 0.01). The only isolate of ovine origin devoid of the lukM gene produced a supernatant weakly leukotoxic (titer = 10) compared to the mean leukotoxic titer of lukM-positive isolates (titer = 651). Among isolates equipped with lukM/lukF′-PV genes, those of caprine and ovine origins produced supernatants more leukotoxic than did the isolates of bovine origin (P < 0.01) (Fig. 1).
FIG. 1.
Mean leukotoxic titers + standard errors of the means (error bars) of culture supernatants of lukM-positive and lukM-negative S. aureus isolated from cows (n = 48), goats (n = 47), or ewes (n = 32) with mastitis. Bacteria were cultivated in BHI or YCP broths. Titration of supernatants was performed with bovine PMN. Titers are the inverse of the last dilution of supernatants causing the spreading of more than 80% of PMN. The only lukM-negative strain of ovine origin yielded a leukotoxic titer of 10.
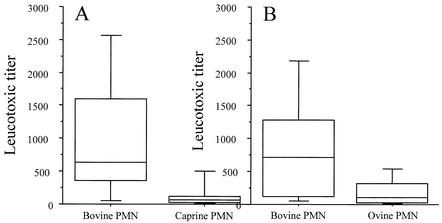
The supernatants of eight isolates of caprine and eight isolates of ovine origins (all lukM positive) were titrated with PMN of the respective species, simultaneously with PMN of bovine origin. It appeared that the leukotoxic titers obtained with PMN from ewes and goats were much lower than the titers obtained with PMN from cows (Fig. 2). The differences were statistically significant for bovine versus caprine PMN (P = 0.012) and for bovine versus ovine PMN (P = 0.012). The median values of these ratios (titer on bovine PMN/titer on small-ruminant PMN) were 10.6 for caprine PMN and 7.2 for ovine PMN.
FIG. 2.
Comparison of the leukotoxic activity of S. aureus on bovine and small-ruminant PMN. (A) Supernatants of isolates of caprine origin (n = 8) were tested on bovine and caprine PMN. (B) Supernatants of isolates of ovine origin (n = 8) were tested on bovine and ovine PMN. Supernatants are from cultures in BHI broth. The data are presented as box-and-whiskers plots. Horizontal box lines denote the 25th and 75th percentile values. The median is indicated by the line within each box. The lower and upper error bars denote the 5th and 95th percentile values.
The observation that all culture supernatants were leukotoxic to PMN prompted us to investigate the contribution of leukotoxins to the toxic activity measured in the culture supernatants. Because lukM-positive isolates had much higher leukotoxic activities than had lukM-negative isolates, we gave a particular emphasis to the LukM/LukF′-PV toxin.
Leukotoxin purification.
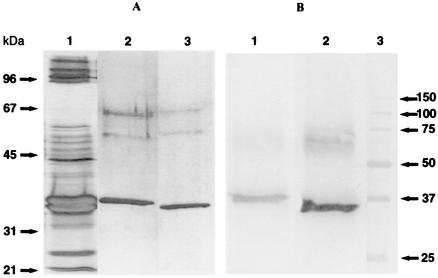
A strain of S. aureus isolated from the milk of a mastitic goat was selected on the basis of the potent leukotoxicity of its culture supernatant. This strain (Ch122) is equipped with the genes encoding Hlgv, LukE/D, and LukM/LukF′-PV toxins. The separation and purification of the toxins was undertaken to identify the main leukotoxin produced. The sequence of cation-exchange and hydrophobic chromatography which has previously been used to purify leukotoxins of S. aureus (16) was efficient for the purification of the main toxins secreted by the mastitis isolate Ch122. The chromatogram (absorbance at 280 nm) obtained by the gradient elution from the MonoS column of the anion-exchange fraction of culture supernatant showed only two major peaks, the first eluting at 200 mM NaCl and the second eluting at 340 mM NaCl. The apparent molecular mass was found to be about 35 kDa for the slow-eluting component (lane 3) and about 37 kDa for the fast-eluting component by SDS-PAGE (Fig. 3, lane 2). This is in agreement with the reported molecular mass of 31 to 32 kDa for S components and 35 to 37 kDa for F components of staphylococcal leukotoxins (15). After transfer onto a polyvinylidene difluoride membrane, the bands corresponding to the two proteins were subjected to N-terminal sequencing. The band corresponding to the first peak eluted from ion-exchange chromatography (component F) had the N-terminal sequence AQHITPVSEK, which had 100% identity with LukF′-PV (9). The other band (component S) yielded the N-terminal sequence TTNAEDIGDD, which is identical with the N-terminal sequence of LukM (4). About 10 mg of LukM and 7 mg of LukF′-PV could be obtained from 1 liter of culture medium.
FIG. 3.
(A) SDS-polyacrylamide gel (12% polyacrylamide) under reducing conditions of the chromatographic fractions containing the leukotoxic components after staining with silver nitrate. Lane 1, culture supernatant (BHI broth) of strain 122.25; lane 2, LukM; lane 3, LukF′-PV. The two bands around 55 and 65 kDa are due to impurities in the sample buffer brought along with β-mercaptoethanol. (B) Immunoblot of culture supernatant of strain Ch122 and affinity-purified Ab to LukM (lane 1) or affinity-purified Ab to LukF′-PV (lane 2). Lane 3, molecular mass markers in kilodaltons.
Titration of toxins and neutralizing Ab.
The purified toxin was titrated against bovine PMN by incubation with 4 × 104 PMN in a final volume of 100 μl in wells of microtiter plates. When the purified components were tested alone, they displayed toxic activity at high concentrations. After a 20-min incubation at 37°C, the lowest concentration causing the spreading of bovine PMN (+++ intensity) was 13.8 μg/ml with LukM and 7.2 μg/ml with LukF′-PV. The synergy between the components was assessed by incubating mixtures of equal concentrations of the two components with bovine PMN. After a 20 min-incubation at 37°C, the lower concentration causing a +++ intensity spreading of PMN was 7.2 ng/ml, and the equivalent concentration was 3.6 ng/ml after a 1-h incubation. The titration was repeated three times with PMN of different cows, yielding the same result. Consequently, 1 TLC corresponds to 3.6 ng/ml (0.1 nM) of toxin (1/1 ratio of the two components) according to the definition given in the Materials and Methods section. The synergy between the two components was approximately 2,000 (3.6 ng/ml versus 7.2 μg/ml). Indeed, minor contamination (1/2,000 would be enough to account for the activity of separated components) of either of the purified components by the other component of the couple cannot be excluded.
The specificity of the affinity-purified Ab was assessed by Western blotting with a culture supernatant of strain Ch122. With Ab eluted from the LukF′-PV immunoabsorbent, one band major band at 37 kDa was revealed, and with Ab eluted from the LukM immunoabsorbent, one major band at about 34 kDa was revealed (Fig. 3). A diffuse band also appeared near 70 kDa, which could correspond to heterodimers of the two components of the leukotoxin. The enzyme-linked immunosorbent assay was also used to test the cross-reactivity of the affinity-purified Ab. The eluate from the LukM-Sepharose column gave an OD of 0.54 in LukM-coated wells (dilution, 1/10,000) versus an OD of 0.001 (dilution, 1/1000) in LukF′-PV-coated wells. Conversely, the eluate from the LukF′-PV-Sepharose column yielded an OD of 0.45 in LukF′-PV-coated wells (dilution, 1/10,000) compared with an OD of 0.002 in LukM-coated wells (dilution, 1/1,000). As the eluates did not show cross-reactivity, they were considered specific bovine Ab to LukM and bovine Ab to LukF′-PV, respectively.
The affinity-purified Ab were then titrated against the purified toxin. Complete inhibition of 2 TLCs of toxin (equal amounts of F + S) was achieved with 160-ng/ml concentrations of either anti-LukF or anti-LukM Ab, and partial inhibition (spreading of PMN rated ++) was achieved with a 40-ng/ml concentration of either Ab. The mixture of anti-LukF and anti-LukM Ab was not more neutralizing than Ab to one component alone, indicating that neutralization of only one of the two components was sufficient. According to the definition given in the Materials and Methods section, 1 NLC corresponds to an 80-ng/ml concentration of Ab.
To ascertain that the toxic activity of culture supernatants was the result of the action of the known staphylococcal leukotoxins, neutralization with affinity-purified Ab of the spreading effect on bovine PMN was tested on a number of culture supernatants. The isolates tested were chosen on the basis of two criteria: the species of origin (bovine, caprine or ovine), and the possession or not of the LukM/LukF′-PV genes. The supernatants were diluted with RPMI-GH to give 2 TLCs and mixed with affinity-purified Ab (anti-LukM + anti-LukF′-PV in equal concentration) diluted to yield 2 or 4 NLCs. The activity of the 36 supernatants tested was completely inhibited with 2 (33 supernatants) or 4 (3 supernatants) NLCs of Ab (Table 2). Supernatants from lukM-negative isolates were neutralized as well as those from lukM-positive isolates were.
TABLE 2.
Neutralization of culture supernatants of S. aureus isolates selected according to the presence of lukM/lukF′-PV genes and species of origina
| Genotype of isolate and origin | No. of supernatants tested | No. completely inhibitedb |
|---|---|---|
| lukM positive | ||
| Cow | 6 | 5 |
| Goat | 6 | 6 |
| Ewe | 6 | 5 |
| lukM negative | ||
| Cow | 12 | 11 |
| Goat | 6 | 6 |
Supernatants were adjusted by dilution with RPMI-GH to yield 2 TLCs, mixed with 2 or 4 NLCs of affinity-purified Ab (anti-LukM + anti-LukF), and incubated for 1 h before addition of bovine PMN. Spreading of PMN was visually (with an inverted microscope) assessed after 20 min at 37°C. Complete inhibition means that <2% PMN had flattened (not different from control PMN incubated with RPMI-GH without culture supernatant). The leukotoxic activity of all supernates (2 TLCs) was completely inhibited with 4 NLCs of affinity-purified Ab.
NLC = 2.
Comparison of sensitivity of bovine, caprine, and ovine PMN.
PMN of ruminants (six cows, six goats, and six ewes) were prepared in parallel (six per day), and their sensitivities to the LukM/LukF′-PV toxin were measured by the spreading method. Some variability of sensitivity among donors occurred when the spreading was assessed after 20 min of incubation: the lowest active concentrations of toxin were 7.4 to 9.2 ng/ml for bovine PMN, 36 to 46 ng/ml for caprine PMN, and 18 to 46 ng/ml for ovine PMN. The lowest concentration of toxin inducing the spreading of PMN was remarkably constant after a 1-h incubation: it was 3.6 ng/ml for bovine PMN and 18 ng/ml (0.5 nM) for caprine and ovine PMN. It can thus be concluded that caprine and ovine PMN were five times more resistant than were bovine PMN to the spreading effect of LukM/LukF′-PV leukocidin.
DISCUSSION
Certain strains of S. aureus produce exotoxins which are cytotoxic to PMN and macrophages (19). These staphylococcal leukotoxins constitute a family of pore-forming binary toxins acting in two steps, first by the binding of the class S component at the surface of the cellular membrane and then by the association of the class F component to the complex membrane-class S component. Bi-component leukotoxins are known to be secreted by some strains of mastitis-causing S. aureus (12), but information on the prevalence of leukotoxins among isolates from ruminants with mastitis is scarce, partly because some of these toxins have been identified only recently. The recent screening of our collection of S. aureus isolates from ruminants with mastitis to detect the genes encoding the known leukotoxins indicated that all isolates possessed at least the genes of two leukotoxins, the γ-hemolysin and LukE/D (Poutrel et al., unpublished data). This prompted us to gain information on the leukotoxic activity of the culture supernatants of isolates from animals with mastitis. Isolates from the three ruminant dairy species were selected on the basis of the presence or not of the genes for LukM, since the previous screening had shown that lukM genes were found in almost all isolates of ovine origin, in about two-thirds of isolates of caprine origin, and in about 10% of isolates of bovine origin. Almost all of the culture supernatants tested in the present study (126 out of 128) were toxic for bovine PMN. This is appreciably more than the 47% of bovine strains (32 out of 68) previously reported (17). The difference may result from the different geographical origin of the isolates (Germany versus France) or from technical variations like the culture medium (peptone versus BHI or YCP medium) or the assessment of toxicity (cytometry versus microscopy). While almost all supernatants were cytotoxic, leukotoxic titers varied considerably according to the origin of isolates and to the presence of LukM genes. Culture supernatants of lukM-positive isolates were markedly more leukotoxic than supernatants of lukM-negative isolates, whatever their origin. The association of the lukM gene with strong leukotoxicity could suggest that LukM is responsible for the higher toxicity. Nevertheless, LukM is weakly toxic for human PMN (15), and its activity on PMN of ruminants was unknown. To clarify the point, we purified the two components of LukM and measured its activity on PMN of ruminants. LukM proved to be highly active on bovine PMN, inducing spreading at a concentration as low as 3.6 ng/ml. This is a much lower concentration than the previously reported activity of the leukotoxin partially purified from a bovine mastitis isolate (strain P83), which produced typical morphological changes on bovine neutrophils at a concentration of 650 ng/ml (13). Another observation in favor of the contribution of LukM to the strong toxicity of lukM-positive isolates is the capacity of affinity-purified Ab to LukM to neutralize completely the leukotoxicity (Table 2). In fact, this demonstrates only that the toxic activity of culture supernatants was due to one or several of the staphylococcal leukotoxins, since antigenic cross-reactivity exists on one hand among class S and on the other hand among class F components of staphylococcal leukotoxins (8).
Assuming that LukM is a major agent of the leukotoxicity of culture supernatants of lukM-positive mastitis isolate is one point, another is the contribution of LukM to the pathogenesis of staphylococcal mastitis. Unfortunately, we do not have information on the clinical severity of the mastitis cases from which the isolates originated. A slight but not significant tendency for leukotoxin-producing isolates to induce clinical rather than subclinical mastitis has been reported (17). It is worth noting that cases of S. aureus mastitis are more often clinical in ewes and goats than in cows, which could relate to the higher leukotoxic titers of culture supernatants from isolates originating from small ruminants than in supernatants from isolates of bovine origin, and to the high frequency of LukM genes among isolates from small ruminants. Moreover, culture supernatants of isolates from goats and ewes were more toxic for PMN, whether or not they possessed the genes for LukM; i.e., lukM-positive strains from small ruminants were far more toxic than lukM-positive strains from cows, and the same applied to lukM-negative strains (Fig. 2). This poses the question of the existence of several subtypes of mastitis strains, adapted to their respective hosts. The higher amount of leukotoxins secreted by isolates from small ruminants may be an adaptation to the higher resistance of ewe and goat PMN to LukM when compared to bovine PMN (Fig. 2). The ratio of activity of culture supernatants of lukM-positive isolates on small ruminants to bovine PMN was comparable to the ratio found with purified LukM (about 5). This could be another observation in favor of the major contribution of LukM to the activity of lukM-positive strains, but we lack information on the relative activity of γ-hemolysin or LukE/D on bovine, caprine, or ovine PMN. Knowledge of the susceptibility of PMN from ruminants to the newly described leukotoxins would help in determining which one would play the major role in the pathogenesis of mastitis.
For leukotoxins to play a part in pathogenesis of mastitis, a prerequisite is that they be produced in vivo. There is an indication that leukotoxins are secreted in vivo during mastitic infections, since Ab titers in milk and serum of infected cows are higher than Ab titers in milk and serum of uninfected animals (12). These infection-induced Ab are able to neutralize the toxic activity of leukotoxin (12). We showed that affinity-purified Ab to LukM can neutralize leukotoxins: they neutralized the leukotoxic activity of lukM-negative isolates as well as the activity of lukM-positive isolates (Table 2). This suggests that the antigenic cross-reactivity of LukM with γ-hemolysin or LukE/D involves the epitopes important for neutralization, and that immunization with LukM, in case it would protect against LukM-producing strains, would also protect against strains producing other leukotoxins.
In conclusion, the great majority of S. aureus isolates from ruminants with mastitis were shown to be toxic for PMN. The isolates originating from small ruminants were more leukotoxic for bovine PMN than were isolates of bovine origin (Fig. 1), but PMN of small ruminants were more resistant to LukM than were bovine PMN. LukM could play a major role in the toxicity of mastitis strains, because lukM-positive isolates were markedly more leukotoxic than were lukM-negative isolates and because LukM proved to be very active on PMN of domestic ruminants. Although information on the relative activities of the different leukotoxins on PMN of ruminants is needed to specify the importance of LukM, the efficient neutralization by Abs to LukM of the activity of culture supernatants of all the tested isolates suggests that an immune response to LukM would protect PMN against all toxin-producing S. aureus strains.
Acknowledgments
We thank Ana-Paula Texeira (Pathologie Infectieuse et Immunologie, Nouzilly, France) for protein sequencing, Jacques Dufrenoy for help with digital art, and Gilles Prévost (Institut de Bactériologie, Strasbourg, France) for fruitful discussions.
REFERENCES
- 1.Abu-Samra, M. T., S. M. Elsanousi, M. A. Abdalla, A. A. Gameel, M. Abdel Aziz, B. Abbas, K. E. Ibrahim, and S. O. Idris. 1988. Studies on gangrenous mastitis in goats. Cornell Vet. 78:281-300. [PubMed] [Google Scholar]
- 2.Anderson, J. C. 1983. Veterinary aspects of staphylococci, p. 193-243. In C. S. F. Easmon and C. Adlam (ed.), Staphylococci and staphylococcal infections, vol. 1. Academic Press, London, United Kingdom.
- 3.Carlson, G. P., and J. Kaneko. 1973. Isolation of leukocytes from bovine peripheral blood. Proc. Soc. Exp. Biol. Med. 142:853-856. [DOI] [PubMed] [Google Scholar]
- 4.Choorit, W., J. Kaneko, K. Muramoto, and Y. Kamio. 1995. Existence of a new protein component with the same function as the LukF component of leukocidin or gamma-hemolysin and its gene in Staphylococcus aureus P83. FEBS Lett. 357:260-264. [DOI] [PubMed] [Google Scholar]
- 5.Craven, N., and M. R. Williams. 1985. Defences of the bovine mammary gland against infection and prospects for their enhancement. Vet. Immunol. Immunopathol. 10:71-127. [DOI] [PubMed] [Google Scholar]
- 6.Finck-Barbancon, V., G. Prevost, and Y. Piemont. 1991. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res. Microbiol. 142:75-85. [DOI] [PubMed] [Google Scholar]
- 7.Gladstone, G. P. 1962. The assay of anti-staphylococcal leucocidal components (F and S) in human serum. Br. J. Exp. Pathol. 43:295-310. [PMC free article] [PubMed] [Google Scholar]
- 8.Gravet, A., D. A. Colin, D. Keller, R. Giradot, H. Monteil, and G. Prevost. 1998. Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 436:202-208. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko, J., K. Muramoto, and Y. Kamio. 1997. Gene of LukF-PV-like component of Panton-Valentine leukocidin in Staphylococcus aureus P83 is linked with lukM. Biosci. Biotechnol. Biochem. 61:541-544. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Lerondelle, C., and B. Poutrel. 1984. Characteristics of non-clinical mammary infections of goats. Ann. Rech. Vet. 15:105-112. [PubMed] [Google Scholar]
- 12.Loeffler, D. A., and N. L. Norcross. 1985. Enzyme-linked immunosorbent assay for detection of milk immunoglobulins to leukocidin toxin of Staphylococcus aureus. Am. J. Vet. Res. 46:1728-1732. [PubMed] [Google Scholar]
- 13.Loeffler, D. A., K. A. Schat, and N. L. Norcross. 1986. Use of 51Cr release to measure the cytotoxic effects of staphylococcal leukocidin and toxin neutralization on bovine leukocytes. J. Clin. Microbiol. 23:416-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakley, B. R., D. R. Kirsch, and N. R. Morris. 1980. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal. Biochem. 105:361-363. [DOI] [PubMed] [Google Scholar]
- 15.Prevost, G., D. A. Colin, L. Staali, L. Baba Moussa, A. Gravet, S. Werner, A. Sanni, O. Meunier, and H. Monteil. 1998. Les leucotoxines formant des pores de Staphylococcus aureus: variabilité des cellules-cibles et deux processus pharmacologiques. Pathol. Biol. (Paris) 46:435-441. [PubMed] [Google Scholar]
- 16.Prevost, G., B. Cribier, P. Couppie, P. Petiau, G. Supersac, V. Finck-Barbancon, H. Monteil, and Y. Piemont. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63:4121-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuberth, H. J., C. Krueger, H. Zerbe, E. Bleckmann, and W. Leibold. 2001. Characterization of leukocytotoxic and superantigen-like factors produced by Staphylococcus aureus isolates from milk of cows with mastitis. Vet. Microbiol. 82:187-199. [DOI] [PubMed] [Google Scholar]
- 18.Sutra, L., and B. Poutrel. 1994. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 40:79-89. [DOI] [PubMed] [Google Scholar]
- 19.Szmigielski, S., G. Prevost, H. Monteil, D. A. Colin, and J. Jeljaszewicz. 1999. Leukocidal toxins of staphylococci. Zentbl. Bakteriol. 289:185-201. [DOI] [PubMed] [Google Scholar]
- 20.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang, V. C., and P. P. Wilkins. 1991. Optimum dissociating condition for immunoaffinity and preferential isolation of antibodies with high specific activity. J. Immunol. Methods 138:291-299. [DOI] [PubMed] [Google Scholar]
- 22.Vidal, G. 1968. Vaccination avec la leucocidine dans la mammite staphylococcique de la brebis. Ann. Biol. Anim. Biochem. Biophys. 8:291-299. [Google Scholar]
- 23.Watson, D. L., N. A. Franklin, H. I. Davies, P. Kettlewell, and A. J. Frost. 1990. Survey of intramammary infections in ewes on the New England Tableland of New South Wales. Aust. Vet. J. 67:6-8. [DOI] [PubMed] [Google Scholar]
- 24.Woodin, A. M. 1960. Purification of the two components of leukocidin from Staphylococcus aureus. Biochem. J. 75:158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]