Abstract
Enzyme immunoassays (EIAs) for detection of serum antibodies to simian virus 40 (SV40), BK virus (BKV), and JC virus (JCV) were developed by using virus-like-particles (VLPs) produced in insect cells from recombinant baculoviruses expressing the VP1 protein of the respective virus. Rhesus macaque sera with neutralizing antibodies to SV40 showed a high level of reactivity in the SV40 VLP-based EIA, and these sera also showed lower levels of reactivity in the BKV and JCV VLP-based EIAs. Rhesus macaque sera negative for neutralizing antibodies to SV40 were negative in all three EIAs. Competitive binding assays showed that SV40 VLPs inhibited BKV reactivity. In rhesus macaque sera, high optical density (OD) values for antibodies to SV40 VLPs were correlated with high OD values for antibodies to BKV but not with high OD values for antibodies to JCV VLPs. Human sera with neutralizing antibodies to SV40 were more reactive to SV40 VLPs than human sera without neutralizing antibodies to SV40. The greater SV40 reactivities of human sera were correlated with greater reactivities to BKV VLPs but not JCV VLPs. These data suggest that cross-reactivity with BKV antibodies may account for part of the low-level SV40 reactivity seen in human sera. With their greater versatility and their suitability for large-scale testing, the VLP-based EIAs for SV40, BKV, and JCV are likely to contribute to a better understanding of the biology of these viruses.
In recent years a controversy has been brewing over the role of simian virus 40 (SV40), a polyomavirus of rhesus macaques, in human cancers (8). SV40 is closely related to the human polyomaviruses BK virus (BKV) and JC virus (JCV). The SV40 genome has about 70% nucleotide sequence homology with the BKV and JCV genomes. Primary infections with BKV and JCV occur in childhood and are largely asymptomatic. The viruses remain latent following primary infection and are reactivated in patients with conditions associated with T-cell deficiency. BKV infection is associated with hemorrhagic cystitis in bone marrow transplant recipients and nephropathy in renal transplant recipients. JCV is etiologically linked to the demyelinating disease progressive multifocal encephalopathy, which is a frequent complication of AIDS and other immunocompromising conditions.
Since 1992 several reports have appeared in the scientific literature describing the detection of SV40 DNA sequences by PCR in a variety of human tumors, including ependymomas (1), osteosarcomas (5), pleural mesotheliomas (4, 30), and non-Hodgkin's lymphomas (25, 31). Although SV40 is known to be a natural infection only of Asiatic macaques, human exposure to the virus occurred on a wide scale as a result of inadvertent contamination of the poliovirus vaccine from 1955 to 1963 (22). In addition, the detection of SV40 in tumors in children too young to have received the contaminated poliovirus vaccine has been interpreted by some investigators as evidence that the virus is circulating in human populations and transmitted from person to person (3). SV40-induced carcinogenesis is biologically plausible because the virus encodes an oncoprotein (20), can transform human cells in vitro (24), and induces tumors in rodents (7, 13). However, there are also reasons for skepticism concerning a role for SV40 in human cancers. Not all investigators have detected SV40 DNA in human tissues (15, 17, 27, 28). Epidemiological studies have generally failed to detect an increased risk of cancer in birth cohorts potentially exposed to contaminated poliovirus vaccines (18, 29). Among the studies that have detected SV40 DNA in human tissues, the prevalence of SV40 DNA in different types of tumors and in normal human tissues has varied greatly (23). The levels of virus found in most tumor specimens are very low and suggest that only a fraction of the cancer cells contain SV40 genomic sequences (30). Yet, current paradigms of viral carcinogenesis postulate that tumors arise by clonal expansion of a virus-infected cell and that continued expression of viral oncoproteins is necessary to maintain the cancer phenotype (33).
Another tool that could be brought to bear on this controversy is serological assays. The presence of virus-specific antibodies in serum or other body fluids is a well-established biomarker of viral infection. Antibodies to SV40 can be measured by plaque inhibition neutralization assays. However, the assays take up to 2 weeks to perform and are labor-intensive. Enzyme immunoassay (EIA) technology is the preferred method for measurement of antiviral antibodies because EIA provides greater sensitivity and precision than tissue culture-based assays. In addition, EIAs are economical for large-scale seroepidemiological studies. We have established EIAs to detect antibodies to SV40 and two human polyomaviruses, BKV and JCV. In this report, we describe the development of the three EIAs and an initial characterization of the reactivities of sera from rhesus macaques and humans.
MATERIALS AND METHODS
Human and macaque serum samples.
A total of 56 serum samples from rhesus macaques were kindly provided by Diane Griffin. These animals were previously used in a study of measles and measles virus vaccines. The sera were collected over the past 10 years and stored frozen at −20°C. By SV40 plaque inhibition assay, 39 of these serum samples were positive for neutralizing antibodies and 17 serum samples were negative for neutralizing antibodies. In addition, preinoculation and postinoculation serum specimens from four rhesus macaques experimentally infected with SV40 (7 log10 50% tissue culture infective doses of SV40 via the intravenous route) were also tested.
One hundred thirty serum samples were obtained from two serum banks from healthy donors established in Washington County, Maryland (the Clue I serum bank in 1974 and the Clue II serum bank in 1989), for research into serological markers of future disease. As described previously (19), these sera were part of a nested matched case-control study which aimed to investigate if the presence of antibodies to SV40, BKV, and JCV was associated with incident cases of brain tumors. The study population comprised 44 case patients (who subsequently developed brain tumors) and 88 control subjects (who had not developed cancer). The age range of the donors was 13 to 87 years. An insufficient volume of serum from two donors was available for serological testing.
Recombinant baculoviruses.
Recombinant baculoviruses expressing the VP1 protein of BKV or JCV were a generous gift from Stephen Frye and Peter Jensen (16). An SV40 VP1 recombinant baculovirus was constructed by using the Bac-to-Bac baculovirus expression system (Gibco-BRL, Inc.). The VP1 coding sequence of SV40 was amplified by PCR with purified SV40 genomic DNA as a template. Primers were designed to introduce unique XhoI and KpnI restriction enzyme sites at the 5′ and 3′ ends, respectively, of the coding sequence. In addition, the 5′ primer was constructed to introduce a CTT-to-ACC mutation in the triplet preceding the ATG start codon to create a more efficient translation initiation signal, and the 3′ primer was constructed to introduce an A-to-T mutation at nucleotide 2594 (nucleotide position based on GenBank accession number NC001669) to add a second stop codon downstream of the native STOP codon. The primer sequences were 5′-CATCTCGAGACCATGAAGATGGCCCCAACAAAAAG (sense) and 5′-CATGGTACCTTATCACTGCATTCTAGTTGTGGTTTG (antisense). Restriction sites are underlined, and the putative start and stop codons (antisense) are in boldface. Amplification was performed with 200 μM each deoxynucleotide triphosphate, 0.5 μM each primer, 1.75 U of Expand High Fidelity enzyme mixture, and the buffer provided by the manufacturer with 1.5 mM MgCl2. Cycle conditions were set at 30 cycles of 94°C for 15 s, 55°C for 15 s, and 72°C for 30 s, with a final extension reaction for 10 min at 72°C. After amplification, the PCR products were purified by using a Gene Clean Turbo kit according to the instructions of the manufacturer. The purified PCR product was digested with XhoI and KpnI. The digestion product was purified with the Gene Clean Turbo kit (Bio 101, Inc.) and cloned into the corresponding restriction sites of the baculovirus transfer vector pFastBac downstream of the polyhedrin promoter. Recombinant baculovirus genomic DNA was prepared by transposition of the resulting construct, pFastBacSV40VP1, into a baculovirus shuttle vector (bacmid) propagated in Escherichia coli strain DH10Bac+. A recombinant baculovirus stock was generated by transfection of Spodoptera frugiperda sf9 insect cells with recombinant bacmid DNA by using Cellfectin. Sequencing of the full-length SV40 VP1 open reading frame was carried out. The sequence of the cloned SV40 VP1 gene differed from that of the reference strain (GenBank accession number NC001669) by 7 bases. Six substitutions were silent, and one resulted in an amino acid change: Glu-86 to Asp.
Production of VLPs. (i) Infection of insect cells.
For large-scale production of virus-like particles (VLPs), approximately 2 × 109 Trichoplusia ni (High Five) cells (Invitrogen, Carlsbad, Calif.) were infected with 20 ml of a high-titer recombinant baculovirus stock in 80 ml of Ex-Cell 400 medium (JRH Biosciences, Lenexa, Kans.) for 60 min at room temperature with periodic inversion. Aliquots of infected cells (2 × 108) were grown as adherent cultures in tissue culture plates (245 by 245 mm; Nunc, Naperville, Ill.) in a volume of 100 ml of Ex-Cell 400 medium supplemented with gentamicin (10 μg/ml). After 96 h of incubation at 27°C, the cells were harvested from the plates by scraping and were collected by centrifugation at 2,000 rpm (Sorvall FH18/250 rotor) for 5 min. The cell pellet was frozen at −70°C.
(ii) Isolation of VLPs by sonication and centrifugation.
The cell paste was thawed at 4°C, resuspended in phosphate-buffered saline (PBS; pH 7.0) containing a cocktail of protease inhibitors (Complete, Mini; Roche, Mannheim, Germany), and sonicated at a setting of 5 (550 Sonic Dismembrator with a microtip; Fisher Scientific, Suwanee, Ga.) on ice twice for 60 s each time. The lysate was loaded onto a cushion of 40% sucrose-50 mm MOPSO (3-[N-morpholino]-2-hydroxypropane sulfonic acid) (pH 5.0) containing 0.5 M NaCl and centrifuged in an SW-28 rotor at 28,000 rpm for 2.5 h at 4°C. The pellet was resuspended in 27% (wt/wt) CsCl-50 mM MOPSO (pH 5.0) by short-pulse sonication, and the mixture was centrifuged at 28,000 rpm in an SW-28 rotor overnight at 4°C. The contaminating buoyant layer was removed, and the clarified lysate was then centrifuged at 45,000 rpm in a Vti50 vertical rotor for 48 h at 4°C. The remaining steps of the purification procedure were performed at room temperature. The centrifuge tube was opened from the top, and particulate material was gently removed without disturbing the bands. A spinal needle was placed in the tube, and 2-ml fractions were withdrawn from the bottom of the tube with a peristaltic pump and a fraction collector (model 2110; Bio-Rad Laboratories, Hercules, Calif.). For each fraction, total protein was measured and an aliquot (5 μg) was analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Fractions containing VP1 protein (approximately 40 kDa) were pooled and stored at 4°C.
(iii) Size-exclusion chromatography.
The pooled fractions obtained by centrifugation in the Vti50 vertical rotor were filtered through a Millex AP prefilter (Millipore Corp., Bedford, Mass.) to remove particulate material and loaded at 1.8 ml/min onto a Bio-Gel A15m column (2.5 by 75 cm) preequilibrated with 50 mM MOPSO (pH 5.0) containing 0.66 M NaCl. Elution was carried out in the same buffer at 1.8 ml/min, and 3-ml fractions were collected. VLPs, which eluted in the void volume, were identified by the presence of the VP1 protein on SDS-PAGE.
(iv) Anion-exchange chromatography.
The VP1 protein containing eluant from the Bio-Gel column was diluted with 1 volume of 50 mM MOPSO (pH 5.0) to reduce the salt concentration of the solution to ∼0.33 M NaCl and fractionated by column chromatography by using POROS 50HS strong cation-exchange resin (PerSeptive Biosystems, Framingham, Mass.). The column (1.0 by 2.5 cm) was preequilibrated with 50 mM MOPSO (pH 5) containing 0.33 M NaCl. After the VLP-containing solution was loaded at 1.0 ml/min, the column was washed with 10 column volumes and proteins were eluted with a linear salt gradient (0.33 to 1.5 M NaCl) in the same buffer over 50 ml at 1.0 ml/min. Fractions (1.5 ml) were collected and analyzed by SDS-PAGE. The peak VP1 protein-containing fractions were pooled, filter sterilized through a 0.45-μm-pore-size Durapore (polyvinylidene difluoride) filter (Millipore), and stored at 4°C.
(v) Electrophoresis and protein estimation.
Aliquots of VLP-containing samples were concentrated by precipitation with 20% trichloroacetic acid at 4°C for 30 min. The pellets were resuspended at a concentration of 1.3 mg/ml in 1× Laemmli sample buffer containing 200 mM dithiothreitol and heated at 70°C for 5 min. A sufficient volume to give 2 to 5 μg of total protein was loaded on precast 4 to 20% polyacrylamide gels (NOVEX, San Diego, Calif.) by using a NuPage morpholineethanesulfonic acid-SDS buffer system (Invitrogen). The gels were electrophoresed at a constant current of 70 to 80 mA/gel for 30 min. Protein bands were fixed with 50% methanol and 10% acetic acid in distilled water and visualized with a commercial colloidal Coomassie blue reagent (Invitrogen) according to the recommendations of the manufacturer. Total protein was measured by using the Bio-Rad protein assay kit and immunoglobulin G as a standard.
Electron microscopy.
Electron microscopy was performed in the facilities of the Johns Hopkins Department of Cell Biology, Johns Hopkins School of Medicine. Briefly, an aliquot of diluted VLPs was placed on a 300-mesh carbon-coated copper grid and air dried. A drop (20 μl) of 2% phosphotungstic acid (pH 7.0) was placed on the grid for 30 s. The grid was allowed to air dry prior to examination by transmission electron microscopy. The microscopy was performed with an Hitachi HU-12A transmission electron microscope, with micrographs of random sections taken at various magnifications.
VLP-based EIA.
The optimum coating concentration of VLP protein, serum dilution, conjugate dilution, incubation times and temperature, and blocking reagent were determined in preliminary checkerboard titration experiments. For the optimized EIAs, SV40, BKV, and JCV VLP proteins were diluted to 0.3, 0.2, and 0.2 μg per ml, respectively, in PBS (pH 7.2); 100 μl was added to each well of 96-well polystyrene flat-bottom PolySorp plates (Nunc); and the plates were incubated overnight at 4°C. After the antigen solution was removed, each well of the plates was blocked for 3 h at room temperature with 300 μl of 10% (vol/vol) Superblock (Pierce, Rockford, Ill.)-0.05% (vol/vol) Tween 20 (Sigma, St. Louis, Mo.) in PBS. The blocking solution was removed, 300 μl of PBS was added to each well, and the plates were covered with a plastic sealer and stored at −20°C. Before use, the plates were thawed at room temperature and washed three times with wash solution (PBS, 0.05% Tween 20) in an automatic plate washer (Skanwasher 300; Skatron, Lier, Norway), and 90 μl of 10% Superblock-0.05% Tween 20 was immediately added to each well. By using a MultiPROBE II robotic liquid handling system (Packard Instruments, Meriden, Conn.), serum samples were diluted 1:40 in 10% Superblock-0.05% Tween 20, and 10 μl of the diluted serum sample was added to the antigen-coated plates in which each well contained 90 μl of 10% Superblock-0.05% Tween 20. The plates were incubated at 37°C for 1 h on a microplate shaker and then washed twice, rotated 180°, and washed two more times. Goat anti-human immunoglobulin G (gamma chain specific) conjugated with horseradish peroxidase (Zymed, San Francisco, Calif.) was diluted 1:4,000 in 10% Superblock-2.5% (wt/vol) polyethylene glycol (molecular weight, 20,000; Sigma)-0.5% (vol/vol) Igepal CA-630 (Sigma) in PBS, and 100 μl was added to each well. The plates were incubated at 37°C for 30 min on a microplate shaker and then washed as described above. Freshly prepared 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) hydrogen peroxide solution (Kirkegaard & Perry, Gaithersburg, Md.) prewarmed to 50°C was added at 100 μl per well. The plates were incubated at room temperature in the dark for 20 min. The enzyme reaction was stopped by the addition of 100 μl of 1% sodium dodecyl sulfate to each well of all plates. The plates were read at 405 nm in an automated microtiter plate reader (Molecular Devices, Menlo Park, Calif.) with a reference wavelength of 490 nm.
VLP-based EIA with disrupted VLPs.
VLPs were disrupted by incubation for 1 h at room temperature in 0.2 M carbonate buffer (pH 9.6) containing 50 mM dithiothreitol. Disrupted SV40 VLP protein was diluted to 0.3 μg per ml in PBS (pH 7.2), and 100 μl was added to each well of a 96-well polystyrene flat-bottom PolySorp plate (Nunc). The plates were processed and the assay was performed as described above.
Competitive VLP binding assay.
Microtiter plates were coated with SV40 or BKV VLPs, treated with blocking solution, and stored as described above. Before use the plates were prepared as described above. Serum samples were diluted 1:40 in 10% Superblock-0.05% Tween 20 containing 3 μg of SV40 or BKV VLPs per ml, and the plates were incubated for 30 min at 37°C. Then, 10 μl of the diluted serum sample was added to antigen-coated plates with 90 μl of 10% Superblock-0.05% Tween 20 per well. The assay was completed as described above. The percent competition was defined as 1 minus the mean optical density (OD) value for wells containing diluent with added VLP divided by the mean OD value for wells with buffer alone.
Plaque inhibition assay.
Sera were examined for neutralizing antibodies that can inhibit plaque formation (26). Briefly, serum dilutions of 1:10 and 1:40 were tested in duplicate plates for their ability to inhibit SV40 plaque formation. Sera that reduced the number of plaques by more than 50% at both dilutions were scored as positive, and sera that reduced the number of plaques by more than 50% at a 1:10 dilution, but not at a 1:40 dilution, were scored as weakly positive. The categories of positive and weakly positive were combined for this analysis.
Statistical analysis.
The distributions of OD values between groups were compared by the Mann-Whitney U test (and by the Kruskal-Wallis test for more than two groups). Nonparametric tests were used because the distributions of OD values were skewed. Spearman rank correlation coefficients were calculated to assess immunological cross-reactivity between polyomavirus antibodies.
RESULTS
Characterization of SV40, BKV, and JCV VLPs.
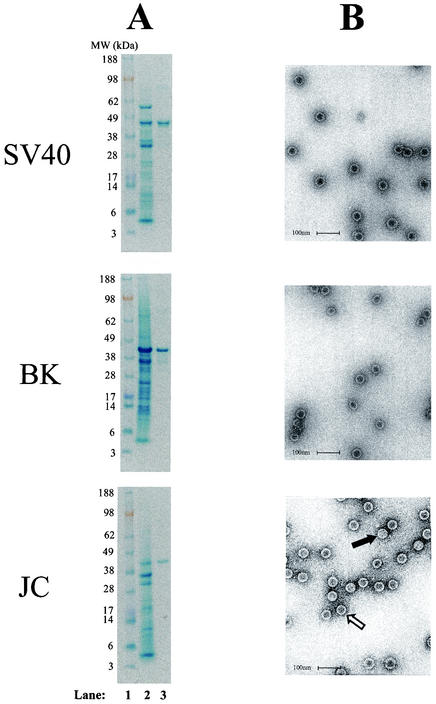
Insect cells were infected with recombinant baculoviruses expressing the SV40, BKV, or JCV VP1 protein; and VLPs were purified as described in the Materials and Methods section. The yields of total SV40, BKV, and JCV VLP proteins from 2 × 109 infected insect cells were 3.1, 10.2, and 0.1 mg, respectively. The low yield of JCV VLPs was due to a low level of expression of JCV VP1 and not to the poor recovery of VLPs during the purification process. SDS-PAGE analysis of purified JCV, SV40, and BKV VLPs showed major protein bands migrating at ∼40, ∼43, and ∼40 kDa, respectively (Fig. 1A). Electron micrographs of purified SV40, BKV, and JCV VLPs showed that most of the VLPs exhibited the typical morphology of empty polyomavirus capsids, having a diameter of 45 to 50 nm, and were present as individual, well-defined particles with minimal aggregation (Fig. 1B). A few VLPs had the appearance of full particles.
FIG. 1.
SDS-PAGE of crude and purified SV40, BKV, and JCV VLPs (A) and transmission electron micrographs of SV40, BKV, and JCV VLPs (B). A lysate of insect cells infected with the respective VP1-expressing recombinant baculovirus (lane 2) or 5 μg of purified VLP protein (lane 3) was subjected to SDS-PAGE. Molecular weight markers (103) are shown in lane 1. Electron micrographs of purified VLPs are shown at ×105,000 magnification. The bars correspond to 100 nm. The open arrow points to an empty particle, and the solid arrow points to a full particle.
Reactivities of rhesus macaque sera in polyomavirus VLP-based EIAs.
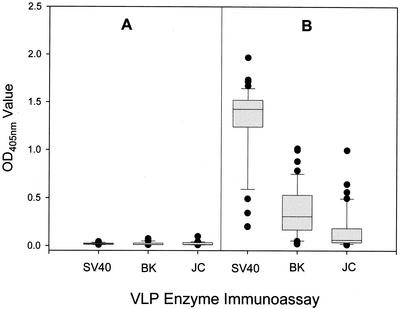
Thirty-nine serum samples from rhesus macaques positive for SV40-neutralizing antibodies and 17 serum samples from rhesus macaques negative for SV40-neutralizing antibodies were tested in each of the three VLP-based polyomavirus EIAs (Fig. 2). The antibody-negative sera gave uniformly low reactivities in all three EIAs, with mean OD values of 0.020, 0.021, and 0.024 in the SV40, BKV, and JCV VLP-based EIAs, respectively. In contrast, high levels of reactivity were observed among the antibody-positive sera in the SV40 VLP-based EIA, with a median OD value of 1.43 (interquartile range, 1.24 to 1.52). The difference in seroreactivities between antibody-positive and -negative sera was statistically significant (P < 0.0001). The median OD values for the antibody-positive sera in the BKV and JCV VLP-based EIAs were 0.307 (interquartile range, 0.167 to 0.530) and 0.061 (interquartile range, 0.035 to 0.183), respectively. The reactivities of the antibody-positive sera were significantly greater that those of antibody-negative sera in the BKV and JCV VLP-based EIAs (P < 0.0001 for both comparisons).
FIG. 2.
Reactivities of SV40-neutralizing antibody-positive and -negative rhesus macaque sera in the SV40, BKV, and JCV VLP-based EIAs. Serum samples were tested for neutralizing antibodies in the SV40 plaque inhibition assay. The distributions of the 17 antibody-negative serum samples (A) and 39 antibody-positive serum samples (B) are shown. The length of each box corresponds to the interquartile range, with the upper boundary of the box representing the 75th percentile and lower boundary of the box representing the 25th percentile. The horizontal line in the box indicates the median value. The lines extending upward and downward from the box mark the 10th to 90th percentile range. Outlier values are shown as closed circles.
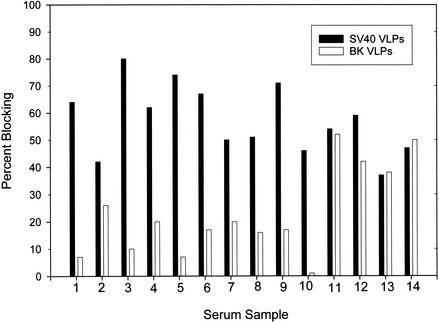
To further explore the reactivities of antibody-positive sera in the BKV and JCV VLP-based EIAs, a correlation analysis was done to test for systematic covariation, which is a characteristic of cross-reactivity. The reactivity in the BKV VLP-based EIA was moderately correlated with the reactivity in the SV40 VLP-based EIA (r = 0.51; P < 0.0001). There was no statistically significant correlation between JCV and SV40 seroreactivities (r = 0.16; P = 0.32). Next, competitive binding assays were performed to determine the antigen specificity of the reactivity. Because the correlation analysis suggested serological cross-reactivity between BKV and SV40, assays were done with these two VLPs. The 14 serum samples that gave the highest levels of reactivity in the BKV VLP-based EIA were tested. Ten samples were obtained from animals naturally infected with SV40, and four samples were from animals experimentally inoculated with SV40. On SV40-coated plates, the median reactivity of the 14 serum samples was reduced by 63% (range, 42 to 80%) after preincubation with 3 μg of SV40 VLP protein per ml (Fig. 3). In contrast, preincubation with the same concentration of the BKV VLP protein only minimally inhibited the SV40 reactivities of the 10 serum samples from naturally infected animals (median inhibition, 16%; range, 0 to 26%). The SV40 reactivities of the four serum samples from experimentally inoculated animals were inhibited less than those of sera from naturally infected animals and were inhibited equivalently by the BKV VLP protein (median inhibition, 46%; range, 38 to 52%) and the SV40 VLP protein (median inhibition, 50%; range, 37 to 59%).
FIG. 3.
Percent blocking of SV40 VLP reactivities of 14 rhesus macaque serum samples by SV40 and BKV VLPs. Ten serum samples from SV40-seropositive rhesus macaques that were naturally infected (samples 1 to 10) and four serum samples from SV40-seropositive macaques that were infected by experimental inoculation (samples 11 to 14) were preincubated with 3 μg of either SV40 or BKV VLP protein per ml or buffer alone and added to an SV40-coated plate. Percent blocking was calculated as 1 − ODbuffer alone/ODVLP.
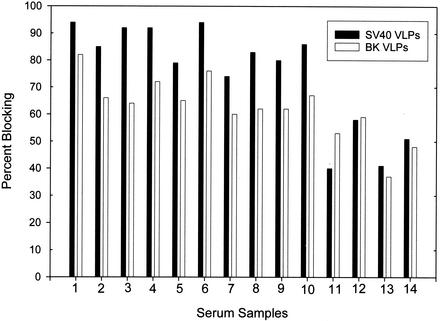
In the tests with sera from naturally infected animals, both BKV and SV40 VLPs inhibited the BKV reactivities of these serum samples (Fig. 4). Preincubation of serum samples with the BKV and SV40 VLP proteins reduced the median reactivities on a BKV-coated plate by 65% (range, 60 to 80%) and 85% (range, 74 to 94%), respectively. At the concentrations of VLP protein used in the competitive binding assay, the BKV reactivities of serum samples from experimentally inoculated animals were not inhibited as strongly as those from naturally infected animals. The median levels of inhibition of BKV reactivity by BKV and SV40 VLP proteins were 50% (range, 37 to 59%) and 46% (range, 40 to 58%), respectively.
FIG. 4.
Percent blocking of BKV VLP reactivities of 14 rhesus macaque serum samples by SV40 and BKV VLPs. Serum samples were tested on BKV-coated plates as described in the legend to Fig. 3.
Reactivities of human sera in the SV40, BKV, and JCV VLP-based EIAs.
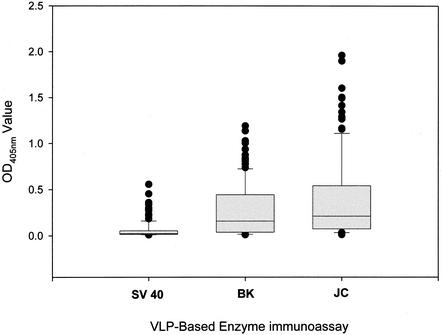
One hundred thirty human serum samples were tested in the BKV and SV40 VLP-based EIAs, and 123 samples were tested in the JCV VLP-based EIA. The distribution of OD values in the three assays is shown in Fig. 5. Seroreactivity in the SV40 VLP-based EIA was tightly clustered at the low end of the OD scale, with an interquartile range of 0.014 to 0.054 OD units. In contrast, the distributions of OD values in the BKV and JCV VLP-based EIAs were much broader, with interquartile ranges of 0.038 to 0.444 and 0.074 to 0.541 OD units in the BKV and JCV VLP-based EIAs, respectively.
FIG. 5.
Reactivities of human sera in the SV40, BKV, and JCV VLP-based EIAs. One hundred thirty serum samples were tested in the SV40 and BKV VLP-based EIAs, and 123 serum samples were tested in the JCV VLP-based EIA. See the legend to Fig. 2 for an explanation of the box plot diagrams.
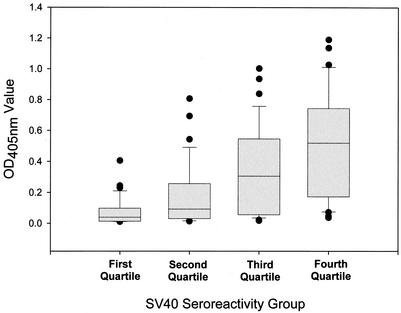
The observation that SV40-reactive monkey sera cross-reacted with BKV prompted us to analyze the human sera for evidence of cross-reactivity. The reactivity of human sera with SV40 was moderately correlated with the BKV reactivity (r = 0.60; P < 0.0001) and to a lesser extent with the reactivity with JCV (r = 0.18; P = 0.06); however, the JCV reactivity did not reach statistical significance. The median OD value in the BKV VLP-based EIA increased linearly with each successively higher quartile of seroreactivity with SV40 (Fig. 6). The median OD value in the BKV VLP-based EIA rose from 0.039 in the lowest quartile for SV40-reactive sera to 0.092, 0.305, and 0.519 in the second, third, and highest quartiles, respectively (by analysis of variance on ranks, P < 0.0001). There was no association of JCV reactivity with quartile for SV40-reactive sera (by analysis of variance on ranks, P = 0.16). In addition, there was no correlation between BKV and JCV seroreactivities (r = −0.10; P = 0.28).
FIG. 6.
Reactivities of human sera in the BKV VLP-based EIA by quartile of SV40 reactivity. The distribution of the BKV reactivities of 130 human serum samples is plotted by quartile of SV40 seroreactivity. See the legend to Fig. 2 for an explanation of the box plot diagrams.
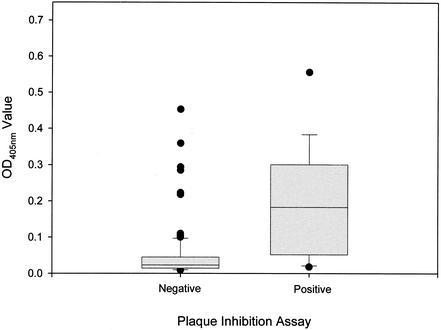
One hundred twenty-eight of the 130 human serum samples were tested for neutralizing antibodies in the SV40 plaque inhibition assay, and 13 scored positive. In the SV40 VLP-based EIA, the OD values of the 13 antibody-positive serum samples were significantly higher than those of the 115 antibody-negative serum samples (median OD values, 0.183 versus 0.023 [P < 0.0001]) (Fig. 7). The OD values of 77% of the antibody-positive serum samples were greater than 0.050 units, while the OD values of 79% of the antibody-negative serum samples were less than 0.050 units. However, a small proportion of the antibody-negative serum samples had high OD values; 6 (5.2%) of the antibody-negative serum samples had OD values exceeding the median OD value for the antibody-positive serum samples. The 20 serum samples that gave the highest reactivities in the SV40 VLP-based EIA and for which an adequate volume of serum was available were tested on plates coated with disrupted SV40 VLPs. The median OD values for the 20 serum samples on plates coated with intact and disrupted VLPs were 0.221 (interquartile range, 0.097 to 0.297) and 0.028 (interquartile range, 0.017 to 0.050), respectively (P < 0.0001).
FIG. 7.
Reactivities of 13 SV40-neutralizing antibody-positive and 115 antibody-negative human serum samples in the SV40 VLP-based EIA. Serum samples were tested for neutralizing antibodies in the SV40 plaque inhibition assay. See the legend to Fig. 2 for an explanation of the box plot diagrams.
DISCUSSION
Expression of the VP1 capsid proteins of SV40, BKV, and JCV in insect cells allowed us to produce highly purified VLPs. VLPs are empty capsids that can be generated by heterologous expression and spontaneous self-assembly of viral capsid proteins. VLPs resemble morphologically native virions and retain many of the immunological properties of native virions. This includes display of surface-exposed B-cell epitopes, which are commonly the viral type-specific and neutralizing epitopes. VLPs have previously been used to establish EIAs to detect antibodies to human papillomaviruses (12, 32). Because polyomaviruses and papillomaviruses share many biological features, we thought that the same technology could be used to develop polyomavirus-specific EIAs. The capsid proteins of SV40, BKV, and JCV have been produced in eukaroytic expression systems and have been shown to form VLPs, but these reagents have not previously been used to develop EIAs (6, 9, 10, 14, 16, 21).
The major finding from our study was the demonstration of reciprocal serological cross-reactivity between SV40 and BKV and to a lesser extent with JCV. In other words, SV40 infection of rhesus macaques appears to elicit antibodies that cross-react with BKV; and, conversely, BKV infection of humans, as evidenced by detection of BKV-reactive antibodies, appears to induce SV40-reactive antibodies. This conclusion was based on the observation that sera from rhesus macaques that contained SV40 VLP-reactive antibodies also reacted with BKV VLPs by EIA. An interpretation of cross-reactivity was supported by the correlation between the BKV and SV40 VLP reactivities and by the blocking of BKV seroreactivity by both BKV and SV40 VLPs. Dual infection due to exposure of monkeys to BKV from human handlers seems unlikely because BKV seroreactivity was not observed in monkeys that were SV40 seronegative. Among the human serum samples tested, SV40 reactivity was correlated with BKV reactivity. Competitive binding assays might have provided additional evidence of cross-reactivity, but we were unable to perform these assays because insufficient volumes of the human sera were available. Cross-reactivity between SV40 and BKV has not previously been demonstrated with sera from SV40-infected monkeys, but it has been proposed as an explanation for the detection of low levels of SV40-reactive antibodies in human serum samples. In tests with 151 serum samples from a primitive human population that did not receive SV40-contaminated vaccines or have contact with SV40-infected primates, Brown et al. (2) detected SV40-neutralizing antibodies in 35% of BKV-seropositive individuals but in only 5% of BKV-seronegative individuals, suggesting that infection with BKV may be responsible for antibodies against SV40 in humans. Another study failed to find serological evidence of cross-reactivity between SV40 and human polyomaviruses. Hamilton et al. (11) found no significant titers of antibodies to SV40 by dot immunoassay in sera from a sample of 97 Japanese adults, many of whom had elevated titers of antibodies to JCV or BKV. The failure to demonstrate cross-reactivity in the latter study may be due to the low prevalence (9%) of high-titer, BKV-reactive antibodies.
The evidence for cross-reactivity between SV40 and JCV was less clear. The levels of reactivity to JCV VLPs were higher in SV40 antibody-positive monkeys than in antibody-negative monkeys; however, for both rhesus macaque and human serum samples, the correlation between SV40 seroreactivity and JCV seroreactivity did not reach statistical significance. To further resolve this question, testing of serum samples in competitive binding assays would be useful, but we were unable to perform these assays because we did not have enough JCV VLP protein.
Among the 128 human serum samples tested, SV40 reactivity in the VLP-based EIA was strongly associated with the presence of SV40-neutralizing antibodies, suggesting that the EIA can measure responses to virus exposure. The EIA also provided evidence that the major portion of serum antibodies to the VP1 capsid protein of SV40 is directed against epitopes exposed on the surfaces of virions, and these are thus likely to be neutralizing epitopes, because chemical disruption of the capsid structure significantly reduced the seroreactivity. A number of SV40-neutralizing antibody-negative human serum samples were strongly reactive in the SV40 VLP-based EIA. This reactivity may reflect a greater sensitivity of the EIA compared to that of the plaque assay for measurement of exposure to SV40.
VLP-based EIAs for SV40 antibodies can be a useful tool in studies of the role of SV40 in human tumors; however, our data suggest that serological cross-reactivity between SV40 and human polyomaviruses may complicate the interpretation of assay results. SV40-specific antibodies will have to be distinguished from cross-reactive antibodies by competitive binding assays or other absorption techniques. Alternatively, mapping of the type-specific and cross-reactive epitopes on the SV40 VP1 protein may lead to the development of assays with greater specificities for SV40. Another critical issue is how to set a cutoff point for SV40 seropositivity when human serum samples are tested by EIA. In addition to the problem posed by cross-reactivity with BKV, it is difficult to define epidemiologically a population not exposed to SV40. The use of contaminated poliovirus vaccine was widespread, and it has been suggested that SV40 now circulates in human populations. However, even if highly specific serological cutoff points are difficult to define, present serological assays should be useful in comparing the responses of cases and those of controls.
Acknowledgments
This work was supported by NIH grant RO1-AI42058 (to R.P.V.).
REFERENCES
- 1.Bergsagel, D. J., M. J. Finegold, J. S. Butel, W. J. Kupsky, and R. L. Garcea. 1992. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N. Engl. J. Med. 326:988-993. [DOI] [PubMed] [Google Scholar]
- 2.Brown, P., T. Tsai, and D. C. Gajdusek. 1975. Seroepidemiology of human papovaviruses. Discovery of virgin populations and some unusual patterns of antibody prevalence among remote peoples of the world. Am. J. Epidemiol. 102:331-340. [DOI] [PubMed] [Google Scholar]
- 3.Butel, J. S., S. Jafar, C. Wong, A. S. Arrington, A. R. Opekun, M. J. Finegold, and E. Adam. 1999. Evidence of SV40 infections in hospitalized children. Hum. Pathol. 30:1496-1502. [DOI] [PubMed] [Google Scholar]
- 4.Carbone, M., H. I. Pass, P. Rizzo, M. Marinetti, M. Di Muzio, D. J. Mew, A. S. Levine, and A. Procopio. 1994. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene 9:1781-1790. [PubMed] [Google Scholar]
- 5.Carbone, M., P. Rizzo, A. Procopio, M. Giuliano, H. I. Pass, M. C. Gebhardt, C. Mangham, M. Hansen, D. F. Malkin, G. Bushart, F. Pompetti, P. Picci, A. S. Levine, J. D. Bergsagel, and R. L. Garcea. 1996. SV40-like sequences in human bone tumors. Oncogene 13:527-535. [PubMed] [Google Scholar]
- 6.Chang, D., C. Y. Fung, W. C. Ou, P. C. Chao, S. Y. Li, M. Wang, Y. L. Huang, T. Y. Tzeng, and R. T. Tsai. 1997. Self-assembly of the JC virus major capsid protein, VP1, expressed in insect cells. J. Gen. Virol. 78:1435-1439. [DOI] [PubMed] [Google Scholar]
- 7.Cicala, C., F. Pompetti, and M. Carbone. 1993. SV40 induces mesotheliomas in hamsters. Am. J. Pathol. 142:1524-1533. [PMC free article] [PubMed] [Google Scholar]
- 8.Ferber, D. 2002. Monkey virus link to cancer grows stronger. Science 296:1012-1015. [DOI] [PubMed] [Google Scholar]
- 9.Goldmann, C., H. Petry, S. Frye, O. Ast, S. Ebitsch, K. D. Jentsch, F. J. Kaup, F. Weber, C. Trebst, T. Nisslein, G. Hunsmann, T. Weber, and W. Luke. 1999. Molecular cloning and expression of major structural protein VP1 of the human polyomavirus JC virus: formation of virus-like particles useful for immunological and therapeutic studies. J. Virol. 73:4465-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hale, A. D., D. Bartkeviciute, A. Dargeviciute, L. Jin, W. Knowles, J. Staniulis, D. W. Brown, and K. Sasnauskas. 2002. Expression and antigenic characterization of the major capsid proteins of human polyomaviruses BK and JC in Saccharomyces cerevisiae. J. Virol. Methods 104:93-98. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton, R. S., M. Gravell, and E. O. Major. 2000. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J. Clin. Microbiol. 38:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirnbauer, R., N. L. Hubbert, C. M. Wheeler, T. M. Becker, D. R. Lowy, and J. T. Schiller. 1994. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J. Natl. Cancer Inst. 86:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirschstein, R., and P. Gerber. 1962. Ependymomas produced after intracerebral inoculation of SV40 into hewborn hamsters. Nature 195:299-300. [DOI] [PubMed] [Google Scholar]
- 14.Kosukegawa, A., F. Arisaka, M. Takayama, H. Yajima, A. Kaidow, and H. Handa. 1996. Purification and characterization of virus-like particles and pentamers produced by the expression of SV40 capsid proteins in insect cells. Biochim. Biophys. Acta 1290:37-45. [PubMed] [Google Scholar]
- 15.Krainer, M., T. Schenk, C. C. Zielinski, and C. Muller. 1995. Failure to confirm presence of SV40 sequences in human tumours. Eur. J. Cancer 31A:1893.. [DOI] [PubMed] [Google Scholar]
- 16.Lenz, P., P. M. Day, Y. Y. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 166:5346-5355. [DOI] [PubMed] [Google Scholar]
- 17.Mulatero, C., T. Surentheran, J. Breuer, and R. M. Rudd. 1999. Simian virus 40 and human pleural mesothelioma. Thorax 54:60-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olin, P., and J. Giesecke. 1998. Potential exposure to SV40 in polio vaccines used in Sweden during 1957: no impact on cancer incidence rates 1960 to 1993. Dev. Biol. Stand. 94:227-233. [PubMed] [Google Scholar]
- 19.Rollison, D. E. M., K. J. Helzlsouer, A. J. Alberg, S. Hoffman, J. Hou, R. Daniel, K. V. Shah, and E. O. Major. Serum antibodies to JC virus, BK virus, simian virus 40 and the risk of incident primary malignant brain tumors in a Maryland cohort. Cancer Epidemiol. Biomarkers Prev., in press. [PubMed]
- 20.Rundell, K., S. Gaillard, and A. Porras. 1998. Small-t and large-T antigens cooperate to drive cell proliferation. Dev. Biol. Stand. 94:289-295. [PubMed] [Google Scholar]
- 21.Sandalon, Z., and A. Oppenheim. 1997. Self-assembly and protein-protein interactions between the SV40 capsid proteins produced in insect cells. Virology 237:414-421. [DOI] [PubMed] [Google Scholar]
- 22.Shah, K., and N. Nathanson. 1976. Human exposure to SV40: review and comment. Am. J. Epidemiol. 103:1-12. [DOI] [PubMed] [Google Scholar]
- 23.Shah, K. V. 2000. Does SV40 infection contribute to the development of human cancers? Rev. Med. Virol. 10:31-43. [DOI] [PubMed] [Google Scholar]
- 24.Shein, H., and J. Enders. 1962. Transformation induced by simian virus 40 in human renal cell cultures. I. Morphology and growth characteristics. Proc. Natl. Acad. Sci. USA 48:1164-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivapurkar, N., K. Harada, J. Reddy, R. H. Scheuermann, Y. Xu, R. W. McKenna, S. Milchgrub, S. H. Kroft, Z. Feng, and A. F. Gazdar. 2002. Presence of simian virus 40 DNA sequences in human lymphomas. Lancet 359:851-852. [DOI] [PubMed] [Google Scholar]
- 26.Steinbaugh, S., and J. L. Melnick. 1962. Plaque formation by vacuolating virus SV40. Virology 16:348-349. [DOI] [PubMed] [Google Scholar]
- 27.Strickler, H. D. 2001. A multicenter evaluation of assays for detection of SV40 DNA and results in masked mesothelioma specimens. Cancer Epidemiol. Biomarkers Prev. 10:523-532. [PubMed] [Google Scholar]
- 28.Strickler, H. D., J. J. Goedert, M. Fleming, W. D. Travis, A. E. Williams, C. S. Rabkin, R. W. Daniel, and K. V. Shah. 1996. Simian virus 40 and pleural mesothelioma in humans. Cancer Epidemiol. Biomarkers Prev. 5:473-475. [PubMed] [Google Scholar]
- 29.Strickler, H. D., P. S. Rosenberg, S. S. Devesa, J. Hertel, J. F. Fraumeni, Jr., and J. J. Goedert. 1998. Contamination of poliovirus vaccines with simian virus 40 (1955-1963) and subsequent cancer rates. JAMA 279:292-295. [DOI] [PubMed] [Google Scholar]
- 30.Testa, J. R., M. Carbone, A. Hirvonen, K. Khalili, B. Krynska, K. Linnainmaa, F. D. Pooley, P. Rizzo, V. Rusch, and G. H. Xiao. 1998. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res. 58:4505-4509. [PubMed] [Google Scholar]
- 31.Vilchez, R. A., C. R. Madden, C. A. Kozinetz, S. J. Halvorson, Z. S. White, J. L. Jorgensen, C. J. Finch, and J. S. Butel. 2002. Association between simian virus 40 and non-Hodgkin lymphoma. Lancet 359:817-823. [DOI] [PubMed] [Google Scholar]
- 32.Viscidi, R. P., K. L. Kotloff, B. Clayman, K. Russ, S. Shapiro, and K. V. Shah. 1997. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among college women. Clin. Diagn. Lab. Immunol. 4:122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.zur Hausen, H. 1999. Viruses in human cancers. Eur. J. Cancer 35:1174-1181. [DOI] [PubMed] [Google Scholar]