Abstract
Heat-killed pathogenic Leptospira interrogans serovar rachmati induced the production of gamma interferon (IFN-γ) and the IFN-γ-inducing cytokines interleukin-12p40 (IL-12p40) and tumor necrosis factor alpha in human whole blood in vitro. The production of IFN-γ was largely dependent on IL-12. These data establish that pathogenic leptospires can stimulate the production of type I cytokines involved in cellular immunity.
Leptospirosis, caused by pathogenic spirochetes of the genus Leptospira, is probably the world's most widespread zoonosis and has been identified as an emerging (or reemerging) infectious disease. The spectrum of symptoms caused by leptospirosis is extremely broad, ranging from flu-like illness to the classical syndrome of Weil (see reference 9).
Gamma interferon (IFN-γ) is a pluripotent proinflammatory cytokine that is produced mainly by activated T cells and natural killer (NK) cells (1). IFN-γ plays an important role in host defense against intracellular pathogens (13). Although leptospires are considered extracellular organisms, recent observations have suggested that IFN-γ may also contribute to immunity against leptospirosis. Indeed, Naiman and coworkers vaccinated cattle with killed Leptospira borgpetersenii serovar hardjo, a major causative agent of leptospirosis in cattle and of zoonotic infection in humans, and found a strong antigen-specific proliferative response of peripheral blood mononuclear cells and enhanced production of IFN-γ by CD4+ T cells, which suggests the induction of a cellular immune response (10).
The production of IFN-γ is tightly controlled by a number of macrophage-derived cytokines. Of these, interleukin-12 (IL-12) is the most potent inducer of IFN-γ release. Other cytokines involved in IFN-γ synthesis include IL-18, IL-15, and tumor necrosis factor alpha (TNF-α) (2, 3, 16, 18). Knowledge of the regulation of IFN-γ production induced by leptospires is not available. Therefore, in the present study we sought to determine whether serovar rachmati, belonging to pathogenic Leptospira interrogans sensu lato, can induce IFN-γ release in vitro and, if so, which of the cytokines previously mentioned play a role therein.
Leptospira serovar rachmati strain Rachmat, allocated to both L. interrogans sensu lato and L. interrogans sensu stricto according to the conventional and the novel DNA-based classification systems, respectively, was obtained from the Leptospirosis Reference Center, Amsterdam, The Netherlands. Cells were inoculated into 500 ml of EMJH medium (Difco, Detroit, Mich.) (5) and grown at 30°C until log phase was achieved. The concentration of bacteria was determined by measuring the optical density of a culture with a spectrophotometer at 420 nm. Cells were harvested by centrifugation, washed twice in MilliQ (Millipore, Etten-Leur, The Netherlands), and resuspended in MilliQ at a concentration of 1010 bacteria/ml. The suspension was subsequently heat inactivated for 10 min at 100°C and stored at −20°C.
Whole-blood stimulations were performed as described previously (7, 8). Heparinized whole blood, collected aseptically from six healthy male individuals, was diluted 1:2 in pyrogen-free RPMI 1640 (Life Technologies, Paisley, Scotland) and stimulated for 48 h at 37°C in the presence or absence of heat-killed L. interrogans cells of serovar rachmati at a concentration of 108 bacteria/ml. In incubations done to examine the regulation of IFN-γ production, anti-IL-12, anti-IL-18, anti-IL-15, or anti-TNF-α (all monoclonal mouse anti-human immunoglobulin G [IgG]; all final concentrations, 10 μg/ml; R&D Systems, Abingdon, United Kingdom) were added. In these experiments, mouse IgG was used as a control antibody (R&D Systems). The concentration at which the anticytokine antibodies were used was sufficient to neutralize the levels of cytokines produced in our whole-blood system (7, 8). Supernatant was obtained after centrifugation and stored at −20°C until assays were performed.
All cytokines were measured by specific enzyme-linked immunosorbent assays according to the instructions of the manufacturers (TNF-α and IFN-γ, Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands; IL-12p40, IL-12p70, and IL-15, R&D Systems; IL-18, Biosource, Camarillo, Calif.). The detection limits of TNF-α, IFN-γ, IL-12p40, IL-12p70, IL-15, and IL-18 were 2.1, 3.1, 5.5, 1.7, 4.1, and 78.1 pg/ml, respectively. Data are expressed as means ± standard errors of the means of results for six donors. Statistical analysis was performed by paired t test. A P of <0.05 was considered to represent a statistical difference.
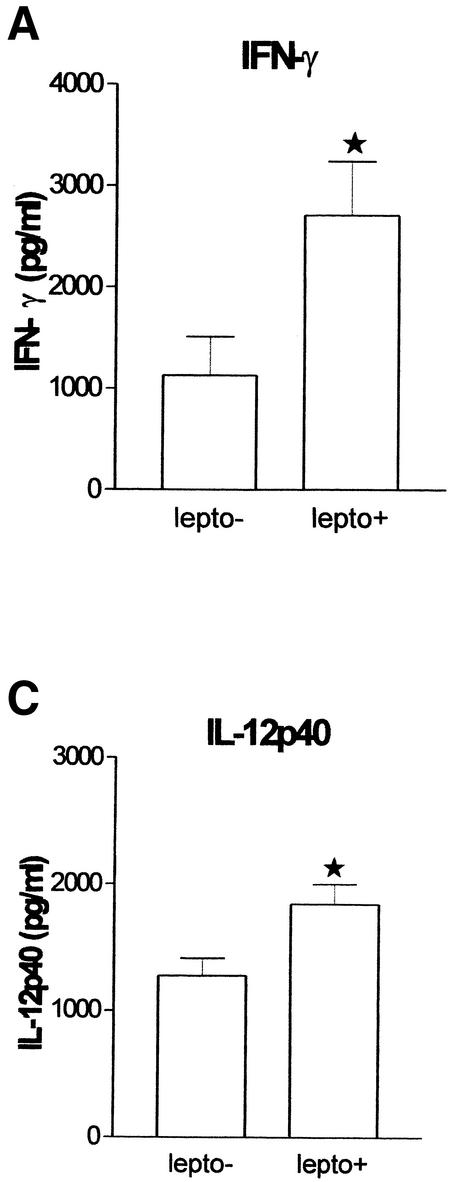
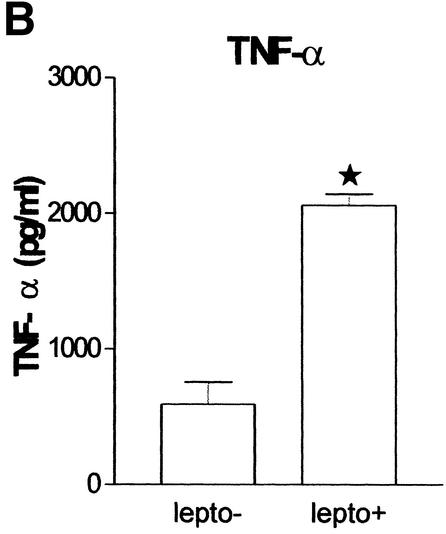
In preliminary experiments, we established that IFN-γ production could be detected after incubations with Leptospira for 24 h and that IFN-γ reached the highest concentrations after incubations of 48 h (data not shown). Incubation of whole blood with heat-killed L. interrogans for 48 h resulted in a significant increase in IFN-γ (P = 0.0005), TNF-α (P = 0.0005), and IL-12p40 (P = 0.0109) and in a nonsignificant increase in IL-18 in culture supernatants (Fig. 1). IL-12p70 and IL-15 concentrations were low or undetectable and remained so after stimulation with leptospires (data not shown).
FIG. 1.
L. interrogans induces IFN-γ (A), TNF-α (B), and IL-12p40 (C) release. lepto−, L. interrogans not added; lepto+, L. interrogans added. A star indicates a significant difference.
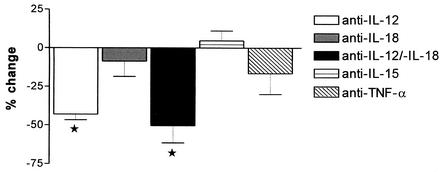
Having established that heat-killed L. interrogans can induce the release of IFN-γ in whole blood, we next determined the roles of IL-12, IL-18, IL-15, and TNF-α herein (Fig. 2). For this purpose, we added neutralizing antibodies to each of these cytokines into whole-blood cultures stimulated with leptospires. In addition, we investigated the effect of concurrent inhibition of IL-12 and IL-18, considering that these two cytokines exert strong synergistic effects on IFN-γ production (18). Anti-IL-12 markedly reduced IFN-γ concentrations in whole blood stimulated with L. interrogans (P = 0.002 compared with values for the control IgG). Neither anti-IL-18, anti-IL-15, nor anti-TNF-α influenced IFN-γ concentrations. The addition of anti-IL-18 to anti-IL-12 modestly but not significantly increased the inhibiting effect of the latter antibody (P = 0.021 compared with values for the control IgG and differences were nonsignificant compared with values for anti-IL-12 only).
FIG. 2.
IFN-γ release induced by L. interrogans is largely dependent on IL-12.
Leptospirosis is caused by spirochetes of the pathogenic species L. interrogans sensu lato, which live extracellularly. Recent investigations have indicated that IFN-γ may play a role in the protective immune response to pathogenic leptospires (10). We therefore considered it of interest to evaluate the regulation of IFN-γ production induced by L. interrogans in vitro. We demonstrate here for the first time that L. interrogans can stimulate IFN-γ release in vitro. Moreover, IL-12 was found to be the main mediator of L. interrogans-induced IFN-γ secretion in whole blood.
The main producers of IFN-γ are activated NK cells, T helper 1 cells, and cytotoxic T cells (1). In the present and earlier investigations by members of our laboratory, whole blood was used as an in vitro system to investigate IFN-γ release in order to minimize potential artifacts due to the procedures necessary to isolate NK cells and/or T cells (6, 8). In addition, whole-blood cultures provide a more natural environment for cells in which their biological function can be studied in the presence of other cell types and humoral factors. Using this system, we demonstrated that heat-killed L. interrogans is capable of stimulating the release of IFN-γ in vitro. This finding is in line with a recently published study in which vaccination of cattle with killed bacteria induced IFN-γ production by CD4+ T cells and the proliferation of peripheral blood mononuclear cells (10). It remains to be established whether L. interrogans can activate NK cells and T cells directly. In whole blood, the production of IFN-γ elicited by L. interrogans appeared to be largely dependent on the release of IL-12, a cytokine mainly derived from monocytes/macrophages, which is in line with the results of earlier investigations that examined the production of IFN-γ induced by lipopolysaccharide or heat-killed Burkholderia pseudomallei, the organism that causes melioidosis (7, 8). In this context, it should be noted that both lipopolysaccharide and B. pseudomallei are more-potent inducers of IFN-γ release in whole blood, yielding concentrations that are 5- to 10-fold higher than those produced by L. interrogans (7, 8). L. interrogans has been reported to activate macrophages through CD14, a pattern recognition receptor that recognizes various bacterial antigens, and Toll-like receptor 2; this interaction with the Toll-like receptor 2 signaling pathway is predominantly mediated by the lipopolysaccharide component of intact L. interrogans (17).
Biologically active IL-12p70 is a heterodimer consisting of a p35 subunit and a p40 subunit, each encoded by a separate gene (13, 14, 15). The p40 subunit mediates binding to the IL-12 receptor (but does not induce signal transduction), while the p35 subunit is critical for signal transduction. The p40 subunit is able to form homodimers, which bind to the IL-12 receptor with affinities similar to those of the IL-12 heterodimer without eliciting a cellular effect. Therefore, p40 homodimers act as inhibitors of IL-12 activity by blocking IL-12 receptor-binding sites, although they may also have some proinflammatory activities (4, 12). The production of p35 and that of p40 are differentially regulated, and to a given stimulus, cells secrete 10- to 100-fold more free p40 than the biologically active p35-p40 heterodimers. Although the levels of IL-12p70 were marginally and not significantly elevated in supernatants of L. interrogans-stimulated whole-blood cultures, the addition of anti-IL-12 had a profound inhibitory effect on IFN-γ release. Our laboratory made a similar observation with whole blood stimulated with lipopolysaccharide or heat-killed B. pseudomallei (7, 8), suggesting that very low concentrations of IL-12p70 can stimulate the production of IFN-γ.
IL-18 was first described as an IFN-γ-inducing factor. However, by itself IL-18 is not a potent IFN-γ inducer in vitro. IL-18 can synergistically enhance IL-12-induced IFN-γ release, an effect caused by upregulation of IL-18 receptors by IL-12 (18). In accordance, we found no role for IL-18 per se in the IFN-γ release elicited by L. interrogans. Yet, anti-IL-18 tended to further reduce IFN-γ concentrations in cultures incubated with anti-IL-12, indicating that IL-18 and IL-12 may interact in mediating IFN-γ production stimulated by L. interrogans.
Our study did not examine the possible role of IL-23 in IFN-γ production induced by Leptospira. This recently discovered cytokine, a heterodimer composed of p40 and p19, shares a number of biological activities with IL-12, including the induction of IFN-γ release (11). Unfortunately, at present neither reagents for the measurement nor reagents for the neutralization of IL-23 are readily available. Therefore, the role of IL-23 in Leptospira-induced IFN-γ production should be addressed in future investigations.
In conclusion, we showed that heat-killed L. interrogans can induce the production of IFN-γ, IL-12p40, and TNF-α in human whole blood in vitro and that the production of IFN-γ is largely dependent on IL-12. Thus, pathogenic leptospires are capable of stimulating the production of type 1 cytokines involved in the induction of a cellular immune response.
REFERENCES
- 1.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15: 749-795. [DOI] [PubMed] [Google Scholar]
- 2.Carson, W. E., M. E. Ross, R. A. Baiocchi, M. J. Marien, N. Boiani, K. Grabstein, and M. A. Caligiuri. 1995. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 96:2578-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 4.Heinzel, F. P., A. M. Hujer, F. N. Ahmed, and R. M. Rerko. 1997. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 158:4381-4388. [PubMed] [Google Scholar]
- 5.Johnson, R. C., and V. G. Harris. 1967. Differentiation of pathogenic and saprophytic leptospires. I. Growth at low temperatures. J. Bacteriol. 94:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauw, F. N., D. Pajkrt, C. E. Hack, M. Kurimoto, S. J. van Deventer, and T. van der Poll. 2000. Proinflammatory effects of IL-10 during human endotoxemia. J. Immunol. 165:2783-2789. [DOI] [PubMed] [Google Scholar]
- 7.Lauw, F. N., A. J. Simpson, J. M. Prins, M. D. Smith, M. Kurimoto, S. J. van Deventer, P. Speelman, W. Chaowagul, N. J. White, and T. van der Poll. 1999. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J. Infect. Dis. 180:1878-1885. [DOI] [PubMed] [Google Scholar]
- 8.Lauw, F. N., T. ten Hove, P. E. P. Dekkers, E. de Jonge, S. J. H. van Deventer, and T. van der Poll. 2000. Reduced Th1, but not Th2, cytokine production by lymphocytes after in vivo exposure of healthy subjects to endotoxin. Infect. Immun. 68:1014-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naiman, B. M., D. Alt, C. A. Bolin, R. Zuerner, and C. L. Baldwin. 2001. Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γδ T lymphocytes. Infect. Immun. 69:7550-7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 12.Piccotti, J. R., S. Y. Chan, K. Li, E. J. Eichwald, and D. K. Bishop. 1997. Differential effects of IL-12 receptor blockade with IL-12 p40 homodimer on the induction of CD4+ and CD8+ IFN-gamma-producing cells. J. Immunol. 158:643-648. [PubMed] [Google Scholar]
- 13.Shtrichman, R., and C. E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251-259. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri, G. 1998. Immunobiology of interleukin-12. Immunol. Res. 17:269-278. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83-243. [DOI] [PubMed] [Google Scholar]
- 16.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto, T., K. Takeda, T. Tanaka, K. Ohkusu, S. Kashiwamura, H. Okamura, S. Akira, and K. Nakanishi. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161:3400-3407. [PubMed] [Google Scholar]