Abstract
A new immunochromatographic rapid test (Rapid Check HIV 1&2; Núcleo de Doenças Infecciosas) for the detection of antibodies to human immunodeficiency virus type 1 and type 2 in human samples (whole blood, serum, and plasma) was evaluated and compared to the commercially available Determine (Abbott Laboratories). When whole-blood samples were evaluated, the specificity and sensitivity of both tests were 100%. However, when plasma samples were used, sensitivity for the Rapid Check HIV 1&2 and the Determine tests were 100 and 98.58%, respectively. The observed specificity for plasma samples was 98.94% for the Rapid Check HIV 1&2 and 96.97% for the Determine test. The results presented here are encouraging and support the adoption of both tests as an alternative to enzyme-lined immunosorbent assay and/or Western blots in regions where laboratorial infrastructure is not available or for use in the management of occupational accidents for healthcare workers.
The human immunodeficiency virus (HIV) pandemic has become one of the greatest infectious disease threats to human health and social stability that the world has ever faced. Although effective antiretroviral therapy has slowed the epidemic in some industrialized countries, worldwide there are still an estimated 15,000 new HIV infections occurring daily. Nearly 40 million people are living with HIV type 1 (HIV-1) infection, and more than 21 million have already died with AIDS (1, 15). Diagnosis and counseling are the cornerstones of prevention, and both care strategies for HIV-infected individuals and the presence of an effective public health surveillance system are essential to track the spread of the HIV pandemic, guide research needs, and provide a focus for prevention activities (4). In developing countries, the lack of diagnostic capabilities presents a major challenge for diagnosis of HIV infection and for surveillance activities (2). In addition to poorly equipped laboratories, individuals to be tested must often travel long distances to reach a health care facility, and the chances that the person will travel back to the clinic to receive results decrease due to transportation difficulties. Since rapid tests can be performed anywhere, its utilization would help these individuals. Other important contributions of rapid screening are the following: (i) the diagnosis before delivery of HIV-infected mothers who didn't have access to appropriate prenatal care, which could reduce the risk of vertical transmission by the initiation of antiretroviral therapy prior to labor, and (ii) the evaluation of patient-derived samples during accidental exposure of healthcare workers.
The present study compares the performance (sensitivity and specificity), on both whole-blood and plasma samples, of two rapid diagnostic tests: the Determine test (Abbott Laboratories, Abbott Park, Ill.), currently the only rapid test available in Brazil, and a new diagnostic test, Rapid Check HIV 1&2 (Núcleo de Doenças Infecciosas [NDI], Vitória, Espírito Santo, Brazil).
MATERIALS AND METHODS
In order to evaluate the performance of two rapid tests (Rapid Check HIV 1&2 and Determine) on different samples (whole blood and plasma), the present study was divided into two parts. First, an evaluation of 137 HIV-positive and 99 HIV-negative plasma samples was performed. Then, 111 HIV-positive and 50 HIV-negative whole-blood samples from a different patient population were used to determine both the sensitivities and the specificities of the referred tests.
Plasma samples.
One hundred thirty-seven consecutive HIV-positive patients, with known CD4 counts and viral loads, attending to the HIV outpatient clinic at the Hospital da Santa Casa de Misericórdia de Vitória were enrolled. Samples from 137 HIV-positive consecutive patients were then grouped according to their CD4 counts and renumbered from 1 to 137. Blood samples were collected by venipuncture in K3-EDTA-treated tubes (Becton Dickinson, Franklin Lakes, N.J.), and plasma was separated by centrifugation and stored at −20°C until further use. Samples from 99 HIV-negative patients were also tested. The HIV-negative group included 49 HIV-negative healthy individuals and 50 HIV-negative patients who were positive for another infectious disease, such as Chagas' disease (n = 10), dengue (n = 10), tuberculosis (n = 10), hepatitis C (n = 10), and syphilis (n = 10). All samples from both HIV-negative patients and HIV-positive patients were retested serologically using the AXSYM system (Abbott Laboratories) and a confirmatory enzyme-linked immunosorbent assay (ELISA) test.
Whole-blood samples.
Both the Rapid Check HIV 1&2 and the Determine tests were also evaluated using whole-blood samples from 111 consecutive patients, referred to the NDI, at the Biomedical Center, Universidade Federal do Espírito Santo, for HIV viral load evaluation. Fifty healthy HIV-negative individuals, confirmed by ELISA (AXSYM; Abbott Laboratories) were also enrolled as HIV-negative controls. Informed consent was obtained from all patients prior to their enrollment.
Quantification of CD4+ lymphocytes in blood samples.
Blood was collected from HIV-positive patients by venipuncture in Vacuntainer tubes using K3-EDTA as an anticoagulant and processed within 4 h of collection. Peripheral blood CD4 and CD8 cell counts were determined by flow cytometry (FACScount; Becton & Dickinson, Mountain View, Calif.) using standardized protocol recommended by the manufacturer.
Quantification of HIV-1 RNA in plasma samples.
Plasma was obtained from K3-EDTA-treated whole-blood samples from HIV-positive patients, centrifuged at 600 × g for 7 min, and stored in aliquots at −70°C within 4 h of collection. The HIV-1 viral load in blood plasma was quantified by the NASBA method (Nuclisens; Organon Teknika, Durham, N.C.) according to the protocol recommended by the manufacturer (3, 14). The detection limit of the assay was 40 HIV-1 RNA copies/ml.
Rapid Check HIV 1&2 assay.
The Rapid Check HIV 1&2 assay (NDI) is a rapid immunoassay based on the immunochromatographic sandwich principle. Recombinant proteins representing immunodominant regions of the envelope proteins of HIV-1 (gp41 and gp120) and HIV-2 (gp36) are immobilized at the test region of the nitrocellulose strip. The Rapid Check HIV 1&2 assay incorporates the new generation of HIV antigens, synthetic peptides, for the detection of antibodies to HIV-1 and HIV-2. The test method employs colloidal gold-labeled conjugates. The multiple epitopes (gp41, gp120, and gp36) of both HIV-1 and HIV-2 proteins are produced synthetically according to the viral genome, and the synthetic peptide antigens for both HIV-1 and HIV-2 are bound to the membrane (solid phase matrix). An internal control region is present to validate the result obtained: in order for a test to be considered valid, the presence of a positive control red line is mandatory, regardless of the status of the tested sample.
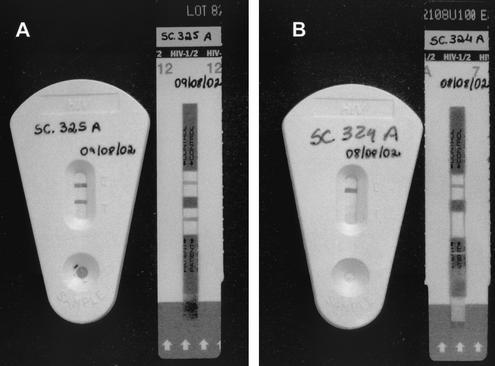
The test is performed by applying 60 μl of whole blood, serum, or plasma to the sample port at the bottom of the test cassette. Two drops (approximately 60 μl) of the wash buffer is then added to the sample port and incubated at room temperature for 10 min to allow the reaction to occur. Results are interpreted visually by the presence of a narrow red band at the test region of the device. A red band must be present at the control region even in the absence of a band at the test region in order to validate the test. The test strip is encased in a protective plastic cassette with a slot, which allows the interpretation of the results without exposing the operator to potentially hazardous fluids (Fig. 1) and also facilitates sample identification due to a large area for writing.
FIG. 1.
Immunochromatographic tests (Rapid Check HIV 1&2 and Determine) used for the detection of HIV-specific antibodies: left pair, positive samples; right pair, negative samples.
Determine assay.
The Determine test is an immunochromatographic test for the qualitative detection of HIV-1 and HIV-2. The test is performed by applying 50 μl of plasma, serum, or whole blood to the test pad at the bottom of the strip. For whole-blood testing, one drop of the chase buffer is added to the strip and the assay is incubated at room temperature. As the sample migrates, it reconstitutes and mixes with a selenium colloid-antigen conjugate. This mixture continues to migrate through a solid phase until it reaches immobilized recombinant antigens and synthetic peptides in the window where the patient's results are displayed. If antibodies to HIV-1 or HIV-2 are present, a red line forms in the window where the patient's results are displayed. As described for the Rapid Check HIV 1&2 test, an internal control line is included on each strip where a red line forms to ensure quality control of individual strips. Therefore, the absence of a positive control line, with or without a positive test line, invalidates the test, and the sample needs to be retested. Results from the strips are interpreted visually (Fig. 1).
Evaluation of reproducibility.
In order to evaluate the reproducibility of the Rapid Check HIV 1&2 assay, 30 HIV-positive and 20 HIV-negative plasma samples were randomly selected among the samples tested earlier. Randomization was accomplished by grouping samples by their CD4 counts, assigning each HIV-positive sample a number from 1 to 137 and assigning a number from 1 to 99 for the HIV-negative samples, and drawing 30 random numbers for the HIV-positive samples and 20 numbers for the HIV-negative samples. Each sample was then retested six times for each test, and the results were evaluated by two independent operators.
RESULTS
Studied samples.
All the samples used throughout this study were also screened by ELISA for anti-HIV antibodies (AXSYM system). All 137 HIV-positive plasma samples had titers of antibody (delta values) at least 17 times higher than the ELISA cutoff value (Table 1). Samples from all the 99 HIV-negative individuals (both healthy and disease control individuals) were negative by ELISA (data not shown).
TABLE 1.
Evaluation of CD4+-T-cell counts, viral load, and ELISA and HIV rapid test results from 137 HIV-positive plasma samplesa
| No. of CD4+ (T cells/μl) | Sample no. | Result for:
|
|||
|---|---|---|---|---|---|
| ELISA | LOG CV | RC | DT | ||
| <200 | 1 | 26.90 | 4.75 | + | + |
| 2 | 28.77 | 2.20 | + | + | |
| 3 | 24.95 | <LDL | + | + | |
| 4 | 22.57 | 4.18 | + | + | |
| 5 | 20.57 | 4.46 | + | + | |
| 6 | 21.86 | 5.20 | + | + | |
| 7 | 42.97 | 2.59 | + | + | |
| 8 | 29.07 | <LDL | + | + | |
| 9 | 33.61 | 3.49 | + | NR | |
| 10 | 34.04 | 3.51 | + | + | |
| 11 | 23.36 | <LDL | + | + | |
| 12 | 32.06 | 2.08 | + | + | |
| 13 | 32.53 | 2.15 | + | + | |
| 14 | 28.51 | 1.90 | + | + | |
| 15 | 30.04 | 3.85 | + | + | |
| 16 | 34.09 | 3.90 | + | + | |
| 17 | 34.78 | 2.56 | + | + | |
| 18 | 35.80 | <LDL | + | + | |
| 19 | 38.81 | 2.74 | + | + | |
| 20 | 39.93 | <LDL | + | + | |
| 21 | 25.25 | 2.08 | + | + | |
| 22 | 31.65 | 2.49 | + | + | |
| 23 | 39.63 | 3.67 | + | + | |
| 24 | 30.30 | 2.77 | + | + | |
| 25 | 19.79 | 3.41 | + | + | |
| 26 | 25.20 | 3.82 | + | + | |
| 27 | 35.78 | 3.81 | + | + | |
| 28 | 24.44 | 4.04 | + | + | |
| 29 | 38.37 | 3.71 | + | + | |
| 30 | 24.36 | 3.18 | + | + | |
| 31 | 30.71 | <LDL | + | + | |
| 32 | 17.75 | 4.74 | + | + | |
| 33 | 40.19 | 1.90 | + | + | |
| 34 | 39.52 | 3.40 | + | + | |
| 35 | 17.33 | 5.04 | + | + | |
| 36 | 26.24 | 3.00 | + | + | |
| 37 | 31.53 | 5.04 | + | + | |
| 38 | 22.41 | 4.08 | + | + | |
| 39 | 30.18 | 4.88 | + | + | |
| 40 | 19.33 | 5.08 | + | + | |
| >200 and <499 | 41 | 29.49 | 4.26 | + | + |
| 42 | 29.36 | 2.32 | + | + | |
| 43 | 27.42 | 3.79 | + | + | |
| 44 | 33.28 | 2.94 | + | + | |
| 45 | 33.16 | 3.63 | + | + | |
| 46 | 34.92 | 3.64 | + | + | |
| 47 | 33.62 | 3.45 | + | + | |
| 48 | 28.22 | 3.11 | + | + | |
| 49 | 40.28 | 3.11 | + | + | |
| 50 | 29.36 | 5.34 | + | + | |
| 51 | 28.08 | 5.04 | + | + | |
| 52 | 26.65 | 5.43 | + | + | |
| 53 | 39.89 | 5.72 | + | + | |
| 54 | 41.56 | 2.84 | + | + | |
| 55 | 35.25 | 4.08 | + | + | |
| 56 | 39.30 | 2.91 | + | + | |
| 57 | 39.43 | <LDL | + | + | |
| 58 | 23.44 | 4.18 | + | + | |
| 59 | 32.08 | 3.32 | + | + | |
| 60 | 30.56 | 3.45 | + | + | |
| 61 | 30.69 | 2.28 | + | + | |
| 62 | 32.56 | 3.67 | + | + | |
| 63 | 33.28 | 3.62 | + | + | |
| 64 | 30.79 | 3.04 | + | + | |
| 65 | 26.97 | 2.86 | + | + | |
| 66 | 28.13 | 3.63 | + | + | |
| 67 | 28.53 | 4.00 | + | ? | |
| 68 | 27.73 | <LDL | + | + | |
| 69 | 34.32 | <LDL | + | + | |
| 70 | 42.18 | 3.87 | + | + | |
| 71 | 43.47 | 2.96 | + | + | |
| 72 | 43.58 | <LDL | + | + | |
| 73 | 29.28 | 5.20 | + | NR | |
| 74 | 30.44 | <LDL | + | + | |
| 75 | 29.54 | 2.40 | + | + | |
| 76 | 26.04 | 3.96 | + | + | |
| 77 | 17.49 | 4.11 | + | + | |
| 78 | 32.06 | 4.81 | + | + | |
| 79 | 32.37 | 4.57 | + | + | |
| 80 | 47.51 | 2.94 | + | + | |
| 81 | 48.32 | <LDL | + | + | |
| 82 | 30.53 | 3.82 | + | + | |
| 83 | 31.95 | 1.93 | + | + | |
| 84 | 33.52 | 4.62 | + | + | |
| 85 | 34.07 | 4.76 | + | + | |
| 86 | 34.40 | 3.82 | + | + | |
| 87 | 32.71 | 3.58 | + | + | |
| 88 | 33.14 | 4.52 | + | + | |
| 89 | 27.66 | 4.84 | + | + | |
| 90 | 30.70 | 3.11 | + | + | |
| >500 | 91 | 31.37 | 5.11 | + | + |
| 92 | 32.27 | 5.08 | + | + | |
| 93 | 27.22 | 5.15 | + | + | |
| 94 | 38.00 | 2.15 | + | + | |
| 95 | 37.93 | 1.90 | + | + | |
| 96 | 32.20 | 3.63 | + | + | |
| 97 | 28.97 | 2.91 | + | + | |
| 98 | 30.16 | 3.00 | + | + | |
| 99 | 40.16 | 4.92 | + | + | |
| 100 | 41.38 | 5.43 | + | + | |
| 101 | 33.92 | 5.32 | + | + | |
| 102 | 26.56 | 4.60 | + | + | |
| 103 | 24.67 | 2.88 | + | + | |
| 104 | 24.19 | 5.11 | + | + | |
| 105 | 35.63 | 2.36 | + | + | |
| 106 | 32.75 | 3.59 | + | + | |
| 107 | 31.30 | 3.26 | + | + | |
| 108 | 31.06 | 5.18 | + | + | |
| 109 | 29.64 | 4.49 | + | + | |
| 110 | 28.11 | 4.77 | + | + | |
| 111 | 28.48 | 3.46 | + | + | |
| 112 | 35.07 | 1.74 | + | + | |
| 113 | 35.73 | 4.04 | + | + | |
| 114 | 26.50 | 4.32 | + | + | |
| 115 | 28.43 | 4.04 | + | + | |
| 116 | 20.01 | 4.40 | + | + | |
| 117 | 27.63 | 4.89 | + | + | |
| 118 | 19.63 | 4.40 | + | + | |
| 119 | 17.62 | 5.18 | + | + | |
| 120 | 37.38 | 3.92 | + | + | |
| 121 | 38.39 | 2.66 | + | + | |
| 122 | 44.84 | 2.59 | + | + | |
| 123 | 41.35 | 3.97 | + | + | |
| 124 | 44.06 | 3.68 | + | + | |
| 125 | 34.46 | 4.95 | + | + | |
| 126 | 35.58 | 6.08 | + | + | |
| 127 | 32.09 | 4.04 | + | + | |
| 128 | 36.78 | 4.30 | + | + | |
| 129 | 38.25 | 5.76 | + | + | |
| 130 | 30.67 | 6.36 | + | + | |
| 131 | 25.32 | 5.28 | + | + | |
| 132 | 45.74 | 5.30 | + | + | |
| 133 | 48.64 | 5.15 | + | + | |
| 134 | 32.60 | 6.18 | + | + | |
| 135 | 33.72 | 5.15 | + | + | |
| 136 | 31.95 | 3.65 | + | + | |
| 137 | 28.66 | 5.11 | + | + | |
RC, Rapid Check HIV 1&2 Test; DT, Determine Test; Log CV, Log10 HIV RNA copies/ml; NR, negative results; ?, inconclusive results. LDL, lower detection limit.
Characteristics of HIV-positive samples.
In addition to ELISA results confirming HIV infection, CD4 cell counts and viral load were determined for all 137 plasma samples from HIV-positive patients; 40 samples showed CD4 cell counts of >500 cells/μl, 50 samples showed cell counts between 200 and 499 cells/μl, and 47 showed cell counts below 200 cells/μl. Viral loads ranged from less than 40 copies/ml (1.59 log10 copies/ml) to 2.300.000 copies/ml (6.36 log10 copies/ml) (Table 1).
Performance of both Determine and Rapid Check HIV 1&2 rapid tests using plasma samples.
In our evaluation, Rapid Check HIV 1&2 and Determine immunoassay tests presented specificities of 100 and 98.54%, respectively. The observed sensitivity for the Rapid Check HIV 1&2 was 98.99%, and that for the Determine assay was 96.97% (Table 2). A false-positive result was reported for one of the HIV-negative samples when it was tested with the Rapid Check HIV 1&2, whereas three false-positive results for the HIV-negative samples were reported when the Determine test was used. Since the Determine test was not able to detect 2 out of the 137 HIV-positive samples, we decided to analyze anti-HIV specific ELISA results to rule out low immunoglobulin levels among the tested samples as the cause of test failure. We found neither discrepancies nor low delta values among the ELISA results for the analyzed plasma samples. In addition to that, in order to exclude the possibility that negative results were due to operational problems, these two samples were retested in triplicate with both the Determine and the Rapid Check HIV 1&2 assays. The results obtained on the second round did not differ from the previous results (data not shown). Even patients with CD4 counts below 200 cells/μl were positive by both the Determine and the Rapid Check HIV 1&2 tests, demonstrating that both tests are extremely sensitive and specific even on samples from patients at advanced stages of the disease, when the immune system is severely compromised.
TABLE 2.
Sensitivity and specificity for Rapid Check HIV 1&2 and Determine tests for both plasma and whole-blood samples
| Assay | Result | Plasma samples
|
Whole-blood samples
|
||||
|---|---|---|---|---|---|---|---|
| No. HIV positive (n = 137) | No. HIV negative (n = 99) | % Sensitivity (% Specificity) | No. HIV positive (n = 111) | No. HIV negative (n = 50) | % Sensitivity (% Specificity) | ||
| Rapid Check HIV 1&2 | Positive | 137 | 1 | 100 (98.99) | 111 | 0 | 100 (100) |
| Negative | 0 | 98 | 0 | 50 | |||
| Determine | Positive | 135 | 3 | 98.54 (96.97) | 111 | 0 | 100 (100) |
| Negative | 2 | 96 | 0 | 50 | |||
Performance of both Determine and Rapid Check HIV 1&2 rapid tests on whole-blood samples.
When HIV-positive and HIV-negative whole-blood samples were tested, the observed specificity and sensitivity when samples were tested with either Rapid Check HIV 1&2 or Determine tests were both 100% (Table 2).
Evaluation of reproducibility.
The Rapid Check HIV 1&2 assay presented a reproducibility index of 100% for both the 30 HIV-positive samples and the 20 HIV-negative samples. Results were evaluated separately by two technicians, and their results compared. No discordant results were observed either when a given sample was tested six times by each of the tests or when the tests results from the two technicians were compared.
DISCUSSION
Results presented here demonstrated that both the Determine and the Rapid Check HIV 1&2 tests yielded rapid, accurate, and easy-to-interpret results on both whole-blood and plasma samples and may be considered valuable tools for prompt screening of patient samples at point-of-care facilities, reference laboratories, prenatal clinics, and delivery and emergency rooms located in regions where laboratorial infrastructure is neither present nor well outfitted. This is of particular importance in HIV testing, if one considers that negative results can be given to the patient at the time of testing, eliminating the need of return visits (11). On the other hand, although a positive, single rapid HIV test result requires confirmation by another serological test or by Western blotting, the patient with a positive result can receive immediate feedback on the likelihood of infection with HIV and counseling encouraging the adoption of risk-reducing behaviors (5, 7, 8). Pregnant women who have not received appropriate prenatal care (which must include screening for HIV) can be quickly tested just before labor and, if HIV positive, start the appropriate antiretroviral drug regimen in order to reduce the risk of vertical transmission during delivery. Nogueira et al. demonstrated that the use of two rapid HIV immunochromatographic tests with female patients at labor was faster than and as efficient as the standard combination of ELISA and Western blot for detection of HIV infection (12). Another important application for rapid tests is the event of an occupational accident, when the sample/index case can be readily tested to determine whether postexposure therapy should begin immediately. This approach has been suggested by several authors as a practical way to reduce costs with laboratory tests and postexposure prophylactic medications as well as with lost work time and psychological counseling (6, 9).
Both the Determine and the Rapid Check HIV 1&2 tests were highly specific and sensitive; therefore, both are suitable for the diagnosis of HIV on both whole-blood and plasma samples. Neither viral load titers nor CD4 counts interfered with the results on either test. Both the Determine and the Rapid Check HIV 1&2 tests were able to give a valid positive result with either whole-blood or plasma samples from patients with CD4 counts below 200 cells/μl or with an undetectable viral load, demonstrating that both tests can be used in the diagnosis of HIV infection regardless of the patient's clinical status. Although not statistically different, when plasma samples were used, the sensitivity of the Rapid Check HIV 1&2 test was slightly higher than that presented by the Determine assay. The Rapid Check HIV 1&2 assay also presented a couple of physical advantages over the Determine. The former test strip is encased in a hard plastic cassette which protects the operator from biological fluids without compromising result interpretation. Due to a large write-on area, the plastic cassette also facilitates the identification of the sample.
The World Health Organization has addressed the issue of increasing in-country capabilities for infectious disease diagnoses, particularly in the case of HIV infection, and incorporating the use of rapid diagnostic tests into standard laboratory formats and point-of-care facilities (15). For example, the strategy for HIV testing recommends sequential testing of samples by either ELISA or rapid assays with different formats. Samples are screened by one method, and reactive samples can then be tested by a second assay. Thus, screening of samples by the rapid assay could serve as first-line diagnostic support, with follow-up by the secondary method done only for samples that appear to be positive upon initial testing. Since these tests are completed within 15 min, the patient benefits from a rapid result. This can allay fears and anxiety when negative results are obtained or can provide rapid treatment options and/or counseling in the case of a positive result. Either way, patients are immediately aware of their potential disease status. Studies performed in West Africa and in Central America demonstrated the positive impact of HIV rapid tests on the diagnosis of HIV infection and their suitability to the limited laboratorial infrastructure found in developing countries (1, 13).
According to the World Health Organization, an ideal test for the rapid diagnosis of HIV infection should be rapid, inexpensive, highly sensitive and specific, and easy to perform and have results that are easy to interpret. In addition to these characteristics, the test should be able to be stored at room temperature with a long shelf life and should require no additional equipment or ancillary supplies to be performed (10). In conclusion, both the Determine test and the Rapid Check HIV 1&2 Assay fulfill these criteria and, as such, would provide a powerful tool for helping in the diagnosis and control of the HIV pandemic in developing countries.
REFERENCES
- 1.Aidoo, S., W. K. Ampofo, J. A. Brandful, S. V. Nuvor, J. K. Ansah, N. Nii-Trebi, J. S. Barnor, F. Apeagyei, D. Ofori-Adjei, and K. Ishikawa. 2001. Suitability of a rapid immunochromatographic test for detection of antibodies to human immunodeficiency virus in Ghana, West Africa. J. Clin. Microbiol. 39:2572-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, H., B. Petchclai, K. Khupulsup, T. Kurimura, and K. Takeda. 1999. Evaluation of a rapid immunochromatographic test for detection of antibodies to human immunodeficiency virus. J. Clin. Microbiol. 37:367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyer, J. R., B. L. Gilliam, J. J. Eron, Jr., L. Grosso, M. S. Cohen, and S. A. Fiscus. 1996. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: comparison of NASBA with Amplicor reverse transcription-PCR amplification and correlation with quantitative culture. J. Virol. Methods 60:161-170. [DOI] [PubMed] [Google Scholar]
- 4.Hu, D. J., T. J. Dondero, M. A. Rayfield, J. R. George, G. Schochetman, H. W. Jaffe, C. Luo, M. L. Kalish, B. G. Weniger, C. Pau, C. A. Schable, and J. W. Curran. 1996. The emerging genetic diversity of HIV. JAMA 275:210-216. [PubMed] [Google Scholar]
- 5.Irwin, K., N. Olivo, C. Schable, J. T. Weber, R. Janssen, J. Ernst, and the CDC Bronx-Lebanon HIV Serosurvey Team. 1995. Performance characteristics of a rapid HIV antibody assay in a hospital with a high prevalence of HIV infection. Ann. Intern. Med. 125:471-475. [DOI] [PubMed] [Google Scholar]
- 6.Kallenborn, J. C., T. G. Price, R. Carrico, and A. B. Davidson. 2001. Emergency department management of occupational exposures: cost analysis of rapid HIV test. Infect. Control Hosp. Epidemiol. 22:289-293. [DOI] [PubMed] [Google Scholar]
- 7.Kassler, W. J. 1997. Advances in HIV testing technology and their potential impact on prevention. AIDS Educ. Prev. 9(Suppl. 3):27-40. [PubMed] [Google Scholar]
- 8.Kassler, W. J., C. Haley, W. K. Jones, A. Gerber, E. J. Kennedy, and J. R. George. 1995. Performance of a rapid, on-site human immunodeficiency virus antibody assay in a public health setting. J. Clin. Microbiol. 33:2899-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado, A. A., R. Martinez, A. A. Haikal, and M. C. Rodrigues da Silva. 2001. Advantages of the rapid HIV-1 test in occupational accidents with potentially contaminated material among health workers. Rev. Inst. Med. Trop. Sao Paulo 43:199-201. [DOI] [PubMed] [Google Scholar]
- 10.Malone, J. D., E. S. Smith, J. Sheffield, D. Bigelow, K. C. Hyams, S. G. Beardsley, R. S. Lewis, and C. R. Roberts. 1993. Comparative evaluation of six rapid serological tests for HIV-1 antibody. J. Acquir. Immune Defic. Syndr. 6:115-119. [PubMed] [Google Scholar]
- 11.Mitchell, S. W., S. Mboup, J. Mingle, D. Sambe, P. Tukei, K. Milenge, J. Nyamongo, O. Mubarak, J. Sankale, D. Hanson, and T. C. Quinn. 1991. Field evaluation of alternative HIV testing strategy with a rapid immunobinding assay and an agglutination assay. Lancet 337:1328-1331. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira, S. A., J. S. Lambert, A. L. Albuquerque, R. Rodrigues, S. Reis, R. Bornia, M. Dias, R. Barbosa, D. Sztanjbock, A. L. Santos, W. Blattner, and N. T. Constantine. 2001. Assessment of rapid HIV test strategy during labor: a pilot study from Rio de Janeiro, Brazil. J. Hum. Virol. 4:278-282. [PubMed] [Google Scholar]
- 13.Palmer, C. J., J. M. Dubon, E. Koenig, E. Perez, A. Ager, D. Jayaweera, R. R. Cuadrado, A. Rivera, A. Rubido, and D. A. Palmer. 1999. Field evaluation of the Determine rapid human immunodeficiency virus diagnostic test in Honduras and the Dominican Republic. J. Clin. Microbiol. 37:3698-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gemen, B., R. van Beuningen, A. Nabbe, D. van Strijp, S. Jurriaans, P. Lens, and T. Kievits. 1994. A one-tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labelled probes. J. Virol. Methods 49:157-168. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. 1992. Recommendation for the selection and use of HIV antibody tests. Wkly. Epidemiol. Rev. 67:145-149. [PubMed] [Google Scholar]