Abstract
The purpose of this study was to determine whether systemic administration of interferon-alpha (IFN-α) can inhibit liver metastasis produced in nude mice by human colon cancer cells. KM12L4 (IFN-α-sensitive) or KM12L4 IFNR (IFN-α-resistant) cells were injected into the spleen of nude mice. Seven days later, the mice were treated with subcutaneous (s.c.) injections of IFN-α (70,000 units/week) at different dosing schedules (1, 2, or 7 times/week). Significant inhibition of tumor growth, vascularization and expression of basic fibroblast growth factor (bFGF) or matrix metalloproteinase-9 (MMP-9) mRNA and protein occurred in mice given daily injections of IFN-α. Kinetic analysis of therapy showed that daily s.c. administrations of 10,000 units of IFN-α induced apoptosis in liver metastasis-associated endothelial cells, followed by inhibition of tumor cell division and apoptosis of tumor cells. These data suggest that the antiangiogenic activity of IFN-α-2a depends on frequent administration of the optimal biologic dose.
Keywords: angiogenesis, apoptosis, interferon
Introduction
Colorectal cancer is the fourth most common malignancy in the United States and the second leading cause of death from cancer [1]. The major cause of death from colon cancer is hepatic metastases that are resistant to conventional therapies [2,3]. The process of metastasis consists of multiple sequential and selective steps. To produce metastasis, tumor cells must invade into the vasculature, disseminate to distant organs, arrest in capillary beds, extravasate into the organ parenchyma, and proliferate [3]. The growth of neoplasms and production of metastasis depend on induction of adequate and new blood supply, i.e., angiogenesis. The extent of angiogenesis is determined by the balance between proangiogenic molecules, including basic fibroblast growth factor (bFGF) [4–6], interleukin-8 (IL-8) [7], type IV collagenase [6–8], and vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) [9,10], and by antiangiogenic molecules, such as interferon-alpha (IFN-α) and -beta (-β), which are released by both tumor cells and host cells [11,12]. After being discovered in the 1950s on the basis of their antiviral activities [13], studies of the IFNs revealed that they also control cell growth and differentiation [14,15]. Recently, IFN-α and -β have also been shown to down-regulate the expression of several proangiogenic molecules, such as bFGF [12,16,17], matrix metalloproteases (MMP-2 and MMP-9) [16–19], and IL-8 [20,21], and to activate host immune cells [22–24].
Pharmacokinetic studies have demonstrated that in the circulation of patients, the half-life of IFNs is on the order of minutes [25]; the resulting lack of sustained therapeutic levels [25,26] may be responsible, therefore, for the disappointing results of using IFNs to treat solid neoplasms in humans [14,15,22,23]. We recently reported that daily administration of an optimal biologic dose of human IFN-α to nude mice bearing human bladder cancer cells (implanted into the bladder wall) produced a significant downregulation of bFGF MMP-2/-9 and IL-8 that was associated with significant regression of the disease, whereas intermittent administration of high doses of IFN-α was ineffective [16,17]. Because the expression levels of bFGF, IL-8, and MMPs correlate with the malignant potential of human colon carcinomas [27,28], we wished to determine whether downregulation of these genes by systemic administration of IFN-α could inhibit production of liver metastasis. We developed a reliable preclinical in vivo model to study the biology and therapy of human colon carcinoma [29] subsequent to implantation of KM12L4 human colon carcinoma cells into the spleen of nude mice; this highly metastatic human colon cancer produces numerous experimental hepatic metastases that express high levels of bFGF, MMP-9, and IL-8 [28]. We used this model to demonstrate that systemic administration of human IFN-α can inhibit angiogenesis — hence the metastatic potential of colon cancer — by downregulating proangiogenic molecules and inducing apoptosis in liver endothelial cells.
Materials and Methods
Cell Culture
The highly metastatic human KM12L4 colon carcinoma cell line was isolated from a rare liver metastasis produced by the KM12C low metastatic cell line [29,30]. The cell line was maintained as a monolayer in Eagle's minimum essential medium (MEM) with 10% fetal bovine serum (FBS), vitamins, sodium pyruvate, l-glutamine, and nonessential amino acids. The KM12L4 IFNR cell line was isolated from the KM12L4 cell line by prolonged culture with increasing concentrations of IFN-α-2a. The adherent monolayer cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. The cultures were free of mycoplasma, reovirus type 3, pneumonia virus of mice, K virus, encephalitis virus, lymphocyte choriomeningitis virus, and lactate dehydrogenase virus.
In Vitro Cytostasis Assay
Tumor cells (1x103) were seeded into 38-mm2 wells of 96-well flat-bottomed plates in quadruplicate and allowed to adhere overnight. The cultures were washed and refed with medium (control) or medium containing different concentrations of IFN-α. After 6 days, the antiproliferative activity was determined by the 3- [4,5-dimethylthiazol-2-yl]-2.5-diphenyltetrazolium bromide (MTT) assay [31] using an MR-5000 96-well microtiter plate reader at 570 nm. Percentage of cytostasis was calculated by the formula: (1-[A/B]) x 100 where A is the absorbance of cells incubated with IFN-α and B is the absorbance of cells incubated in control medium.
Elisa for bFGF
The level of bFGF protein in cells and serum was analyzed by the Quantikine ELISA kit (R&D Systems, Minneapolis, MN). The concentration of bFGF in the samples was determined by comparing their absorbance with a standard curve. The minimum detectable level of bFGF by this assay is 1 pg/ml [12,32].
Mice
Male athymic BALB/c nude mice were obtained from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research Center (Frederick, MD). The mice were maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and the National Institutes of Health. The mice were used according to institutional guidelines when they were 8–10 weeks of age.
Experimental Liver Metastasis
To produce hepatic metastases, cultures (50% confluence) of KM12L4R cells were given fresh medium 24 hours before harvest by brief treatment with 0.25% trypsin-0.02% EDTA. Only single-cell suspensions with a viability exceeding 90% (trypan blue) were used for the in vivo studies. Nude mice anesthetized with pentobarbital sodium were placed in a supine position. A midline abdominal incision was made and the spleen exteriorized. The incision was closed in one layer with wound clips [29]. Tumor cells (1x106/mouse) were injected into the spleen, and at the end of the study, the mice were euthanized. Livers were removed and placed in Bouin's solution. The number of experimental liver metastases was determined with the aid of a dissecting microscope. The number of liver lesions smaller than 1 mm in diameter was determined in representative sections stained with H and E using a light microscope.
Systemic In Vivo Therapy with Human IFN-α-2a
A fixed daily dose of 1000 or 10,000 units of IFN-α-2a was given to nude mice bearing experimental liver metastases. Therapy started on day 7 after tumor cell implantation. Groups of mice (n=10) were injected subcutaneously (s.c.) with saline (control) or IFN-α-2a (specific activity, 6x106 IU/mg of protein) according to the schedule shown in Table 1. The treatment continued for 5 weeks. On day 42, the mice were euthanized. To evaluate the presence of tumor, the livers were removed, weighed, and processed for histologic, immunohistochemical, and in situ hybridization (ISH) analyses. In one experiment, mice (n=10) were treated with daily s.c. injections of saline or IFN-α at 10,000 U/dose beginning 7 days later. On days 14, 21, and 28, three mice from each group were euthanized and their livers processed for immunohistochemical assay.
Table 1.
Inhibition of Angiogenesis and Human KM12L4 Colon Cancer in Nude Mice by IFN-α.
| IFN-α Treatment* | Liver Metastasis | Serum bFGF (pg/ml)† | Microvessel Density‡ | ||
| Dose (Units) | Schedule | Incidence§ | Liver wt (g) Mean±SD | ||
| Saline | Daily | 9/10 | 1.74±0.2 | 206 | 59±4 |
| 70,000 | 1xweek | 9/10 | 1.45±0.4 | 232 | 51±6 |
| 35,000 | 2xweek | 8/10 | 1.37±0.2 | 175 | 53±3 |
| 10,000 | Daily | 1/10¶ | 1.2±0.2¶ | 141 | 35±3¶ |
KM12L4 cells (1x106) were injected into the spleen of nude mice. Therapy began on day 7. Mice were euthanized on day 42.
Determined by ELISA.
Microvessel density was determined by identifying tumor areas showing the most intense CD-31 staining. Mean number of vessels±SD per 20 100xfields.
Number of tumor-positive mice/number of mice injected.
P<.01 versus control.
Histologic and Immunohistochemical Analysis
Livers that contained colon cancer metastases were divided into fragments and placed in either 10% (vol/vol) neutral formalin or OCT compound (Miles Laboratories, Elkhart, IN) to be snap-frozen in liquid nitrogen. For histologic studies, consecutive 5-µm sections were stained with H and E. For immunohistochemical analysis, frozen sections (10 µm) were fixed with cold acetone. Tissue sections (5 µm) of formalin-fixed, paraffin-embedded specimens were deparaffinized in xylene, rehydrated in graded alcohol, and transferred to PBS. The slides were rinsed twice with PBS, and endogenous peroxidase was blocked by the use of 3% hydrogen peroxidase in PBS for 12 minutes. Nonspecific reactions were blocked by incubating the sections for 20 minutes at room temperature with a solution containing 5% normal horse serum and 1% normal goat serum. Excess blocking solution was drained and the slides were incubated overnight at 4°C with the appropriate dilution (1:400) of monoclonal mouse anti-CD-31 antibody (Pharmingen, San Diego, CA), a 1:25 dilution of a rabbit polyclonal anti-IL-8 antibody (Biosource International, Camarillo, CA), a 1:500 dilution of rabbit polyclonal anti-bFGF antibody (Sigma Chemical, St. Louis, MO), a 1:1000 dilution of rabbit polyclonal anti-VEGF/VPF antibody (Santa Cruz Biotech, Santa Cruz, CA), a 1:50 dilution of rabbit polyclonal anti-MMP-9 antibody (Calbiochem, La Jolla, CA), or a 1:50 dilution of a mouse monoclonal antiproliferative cell nuclear antigen (PCNA)-PC10 antibody (Dako, Carpinteria, CA).
The samples were then rinsed three times with PBS and incubated for 60 minutes at room temperature with the appropriate dilution of peroxidase-conjugated anti-rabbit IgG or anti-rat IgG [33]. The sections were rinsed with PBS and incubated for 5 minutes with diaminobenzidine (Research Genetics, Huntsville, AL). Then the sections were washed three times with distilled water and counterstained with Gill's aqueous hematoxylin (Sigma), washed once with distilled water and once with PBS, and rinsed again with distilled water. The slides were mounted with a Universal mount (Research Genetics) and examined under a bright-field microscope. A positive reaction was indicated by a reddish-brown precipitate in the cytoplasm. The intensity of staining was quantified in three different areas of each sample by an image analyzer using the Optimas software program (Bioscan, Edmonds, WA) to yield an average measurement [34].
In Situ Hybridization (ISH)
Specific antisense oligonucleotide cDNA probes complementary to the mRNA transcripts of four metastasis-related genes, bFGF, VEGF/VPF, IL-8, and MMP-9, identified on the basis of published reports of the cDNA sequences, were designed as previously described [35]. All DNA probes were synthesized with 6 biotin molecules (hyperbiotinylated) at the 3′ end through direct coupling using standard phosphormidine chemistry (Research Genetics) [36,37]. The lyophilized probes were reconstituted to a 1-µg/µl stock solution in 10 mM Tris-HCI (pH 7.6) and 1 mM EDTA. The stock solution was diluted with probe diluent (Research Genetics) immediately before use.
ISH was performed as described previously [27,28,38,39]. The Microprobe manual staining system (Fisher Scientific, Pittsburgh, PA) was used to stain tissue sections (4 µm) of formalin-fixed, paraffin-embedded livers. Two from each treatment group were mounted on Silane-coated ProbeOn slides (Fisher Scientific) [40]. The slides were placed into the Microprobe slide holder, dewaxed, and dehydrated with Autodewaxer and Autoalcohol (Research Genetics), followed by enzymatic digestion with pepsin. Hybridization of the probe was carried out for 80 minutes at 45°C, then the samples were washed three times with 2x SSC for 2 minutes at 45°C. The samples were incubated with alkaline phosphatase-linked enhancer (Biomeda, Foster City, CA) for 1 minute and finally incubated at 45°C for 40 minutes with chromogen substrate FastRed (Research Genetics). A positive reaction in this assay stained red. Control for endogenous alkaline phosphatase included treatment of the samples in the absence of biotinylated probe and use of chromagen in the absence of any oligonucleotide probes. The integrity of the mRNA in each sample was verified by using a poly d(T)20 probe. All specimens analyzed produced an intense histochemical reaction, indicating that the mRNA was well preserved. To avoid variabilities in probe concentration, all specimens were stained in a single session for each probe.
Stained sections were examined in a Zeiss photomicroscope (Carl Zeiss, Thornwood, NY) equipped with a threechip charged coupled device (CCD) color camera (model DXC-960 MD, Sony, Tokyo, Japan). The images were analyzed using Optimas image analysis software, version 5.2 (Bothell, WA). Prescreening was done to determine the range in staining intensity of the slides to be analyzed. Images covering the range of staining intensities were captured electronically, a color bar (montage) was created, and a threshold value was set in the red, green, and blue (RGB) mode of the color camera. All subsequent images were quantified based on this threshold. The integrated OD of the selected fields was determined based on its equivalence to the mean log inverse gray scale value multiplied by the area of the field. The samples were not counterstained, so the OD was solely the product of the ISH reaction. Measured areas of 1 mm2 were located at the center or edge of the tumor. In each area, cytoplasmic staining of 20 to 30 tumor cells was measured, and the level of cytoplasmic staining/area was quantified to derive an average value [41]. The intensity of staining was compared by integrated OD of poly d(T)20 and standardized by comparison with the integrated OD of nonpathologic tissue, which was set at 100.
Vascular Density
Blood vessels in liver metastases of human colon cancer were counted under a light microscope after immune staining of sections with anti-CD31 antibodies [42]. Areas containing the highest number of capillaries and small venules were identified by scanning the tumor sections at low power. After the areas of high vascular density were identified, individual vessels were counted in x100 fields (x10 objective and x10 ocular [0.739 mm2/field]). We classified structures as vessels on the basis of criteria described by Weidner et al. [43], so that observation of a vessel lumen was not required.
Immunofluorescence Double Staining for CD31/PECAM-1 (Endothelial Cells) and TUNEL (Apoptotic Cells)
Frozen tissues were sectioned (8 to 10 µm), mounted on positively charged slides, air-dried for 30 minutes, and fixed for 5 minutes each in cold acetone, acetone+chloroform (1:1), and acetone. Samples were washed three times with PBS, incubated with protein-blocking solution containing 5% normal horse serum and 1% normal goat serum in PBS for 20 minutes at room temperature, and incubated with the appropriate dilution (1:400) of rat monoclonal anti-mouse CD31 antibody (human cross-reactive) over 18 hours at 4°C. After the samples were rinsed four times for 3 minutes each with PBS, the slides were incubated with the appropriate dilution (1:200) of secondary goat anti-rat conjugated to Texas Red for 1 hour at room temperature in the dark. Samples were washed twice with PBS containing 0.1% Brij and washed with PBS for 5 minutes [33].
TdT-mediated dUTP-biotin nick-end labeling (TUNEL) was performed using a commercially available apoptosis detection kit with the following modifications: samples were fixed with 4% paraformaldehyde (methanol-free) for 10 minutes at room temperature, washed with PBS twice for 5 minutes, then incubated with 0.2% Triton X-100 for 15 minutes at room temperature. After two washes of 5 minutes each with PBS, the samples were incubated with equilibration buffer (from kit) for 10 minutes at room temperature. The equilibration buffer was drained, and reaction buffer containing equilibration buffer, nucleotide mix, and TdT enzyme was added to the tissue sections and incubated in a humid atmosphere at 37°C for 1 hour in the dark. The reaction was terminated by immersing the samples in 2x SSC for 15 minutes. Samples were washed three times for 5 minutes to remove unincorporated fluorescein-dUTP. For quantification of endothelial cells, the samples were incubated with 300 µg/ml of Hoechst stain for 10 minutes at room temperature. Fluorescent bleaching was minimized by treating the slides with an enhancing reagent (Prolong solution). Immunofluorescence microscopy was performed using a 40x objective (Zeiss Plan-Neofluar) on an epifluorescence microscope equipped with narrow bandpass excitation filters mounted in a filter wheel (Ludl Electronic Products, Hawthorne, NY) to individually select for green, red, and blue fluorescence. Images were captured using a cooled CCD camera (Photometries, Tucson, AZ) and SmartCapture software (Digital Scientific, Cambridge, England) on a Macintosh computer. Images were further processed using Adobe Photoshop software (Adobe Systems, Mountain View, CA). Endothelial cells were identified by red fluorescence, and DNA fragmentation was detected by localized green and yellow fluorescence within the nucleus of apoptotic cells. Quantification of apoptotic endothelial cells was expressed as an average of the ratio of apoptotic endothelial cells to total number of endothelial cells in 5 to 10 random 0.011-mm2 fields at 400x magnification. For the quantification of total TUNEL expression, the number of apoptotic events was counted in 10 random 0.159-mm2 fields at 100x magnification [33].
Statistical Analysis
The significance of the differences in the in vitro data was analyzed by the unpaired Student's t test. The significance of the differences in the in vivo data was analyzed by the Mann-Whitney U test [44].
Results
Selection of Human Colon Cancer Cells for Resistance to Antiproliferative Effects of Human IFN-α-2a
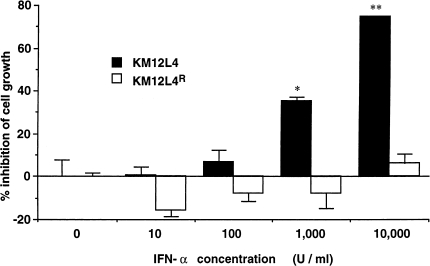
KM12L4 human colon cancer cells were incubated with increasing concentrations of IFN-α-2a (specific activity, 6x106 IU/mg protein) (Hoffman-LaRoche, Nutley, NJ). After 6 weeks, we isolated the KM12L4 IFNR line. The KM12L4 IFNR cells resisted the antiproliferative effects of IFN-α-2a at concentrations exceeding 10,000 U/ml (Figure 1). Regardless of antiproliferative activity, IFN-α-2a (at 100 U/ml)] inhibited production of bFGF by both KM12L4 (IFN-α-sensitive) and KM12L4 IFNR (IFN-α-resistant) cells (44±6% and 39±4%, respectively; P<.01).
Figure 1.
Antiproliferative effects of IFN-α-2a against KM12L4 and KM12L4 IFNR cells. Tumor cells (1x103) plated into 38-mm2 wells were incubated in medium containing different concentrations of IFN-α-2a. Cell proliferation was determined by the MTT assay after 6 days of culture. The values are mean±SEM of six wells. This graph represents one experiment of four. *P<.05; **P<.001.
Systemic Administration of IFN-α-2a as Therapy for Liver Metastasis of Human Colon Cancer: Effect of Schedule
In the first set of in vivo experiments, we evaluated the therapeutic efficacy of systemic administration of IFN-α-2a against experimental liver metastasis established in nude mice by intrasplenic injection of human KM12L4 colon cancer cells [29]. We also determined whether the IFN-α-2a treatment was associated with downregulation of proangiogenic molecules and, therefore, decreased vascularization.
In the first experiment, 1x106 KM12L4 (IFN-sensitive) cells were injected into the spleen of nude mice. Daily IFN-α-2a therapy started on day 7 after tumor implantation. Control mice received daily s.c. injections of saline. IFN-α-2a was administered at a total weekly dose of 70,000 units, as shown in Table 1: Groups of mice (n=10) received s.c. injections of IFN-α-2a once weekly, twice weekly, or once daily for 5 weeks. The mice were bled and serum was collected for ELISA, and the animals were euthanized and necropsied one day after the last daily s.c. injection (day 42 of the study). The livers with tumors were removed and weighed. The data summarized in Table 1 demonstrated that once weekly or twice weekly IFN-α treatment (70,000 or 35,000 units/dose, respectively) did not decrease the incidence or weight of liver metastasis. However, daily injections of IFN-α-2a (10,000 units/dose) significantly decreased the incidence of liver metastasis as well as the weight of the liver (P<.01). The inhibition of liver metastasis correlated with a decrease in the level of bFGF in the serum (P<.01) and a decrease in microvessel density (MVD) in the metastatic lesions (P<.001).
In all subsequent studies, to distinguish between the direct and indirect antiproliferative effects of IFN-α, we used KM12L4 IFNR cells that are resistant to IFN-α or IFN-β antiproliferative effects. Therapy began 7 days after tumor cell implantation into the spleen. Control mice (n=10) received daily s.c. injections of saline. IFN-α was administered s.c. once per day at doses of 1000 or 10,000 U. The mice were euthanized on day 42 of the study and necropsied. The data summarized in Table 2 demonstrated that, in mice injected daily with 10,000 units of IFN-α-2a, the incidence of experimental liver metastasis was significantly reduced from 10/10 to 2/10. The median (range) number of experimental metastases in control mice, 38 (3–100), was reduced to 0 (0–15) (P<.001). The inhibition of metastasis was associated with a significant reduction in the level of bFGF in the serum (P<.01).
Table 2.
Systemic Administration of IFN-α-2a to Treat KM12L4 IFNR Liver Metastasis in Nude Mice.
| IFN-α Treatment* | Liver Metastasis | Liver wt (g) Mean±SD | Serum bFGF† (pg/ml) | Microvessel Density‡ | |
| Median | Range | ||||
| Saline | 38 | 3–100 | 2.4±0.4 | 482 | 73±4 |
| 1000 U | 12 | 0–50 | 1.7±0.2 | 303 | 58±3 |
| 10,000 U | 0 | 0–15§ | 1.4±0.1¶ | 156¶ | 52±6¶ |
KM12L4 IFNR cells (1x106) were injected into the spleen of nude mice. Daily s.c. injections of saline or IFN-α-2a began on day 7. Mice were euthanized on day 42.
Determined by ELISA.
Microvessel density was determined by identifying tumor areas showing the most intense CD-31 staining. Mean number of vessels±SD
P<.001 versus control.
P<.01 versus control.
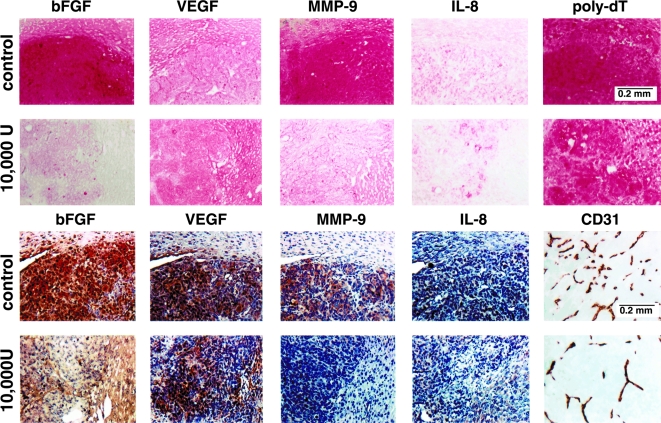
The growth inhibition of experimental liver metastases in mice treated with daily injections of 10,000 U of IFN-α-2a correlated with expression of bFGF and MMP-9 mRNA (Figure 2 and Table 3). Specifically, the mRNA level (as measured by intensity of ISH staining) for bFGF was reduced from a mean of 113±7 in the control mice to 36±5 in mice receiving daily injections of 10,000 U of IFN-α-2a (P<.001). For MMP-9, the mRNA intensity level was reduced from a mean of 111±12 in control tumors to 52±6 in mice given daily injections of IFN-α-2a (P<.01). No discernible differences in expression of IL-8 or VEGF mRNA were found between the groups (Figure 2).
Figure 2.
ISH and immunohistochemical (IHC) analyses of KM12L4 human colon cancer cells growing in the liver of control and IFN-α-2a-treated nude mice. KM12L4 cells (1x106) were injected into the spleen of nude mice. Seven days later, groups of mice (n=10) received daily s.c. injections of saline (control) or 10,000 U of IFN-α-2a. Treatment continued for 5 weeks. The mice were euthanized on day 42 and necropsied The livers were resected, weighed, and processed for ISH (top 2 rows) and IHC (bottom 2 rows). Note a significant decrease in reaction intensity for bFGF and MMP-9 in lesions of mice treated with daily s.c. injections of IFN-α-2a. Microvessel density directly correlated with expression of bFGF and MMP-9.
Table 3.
In Situ Hybridization and Immunohistochemical Analyses of Control and IFN-α-2a-Treated KM12L4 IFNR-Induced Liver Metastases.
| IFN-α Treatment* | In Situ Hybridization† | Immunohistochemical Assay | Tumor Cells‡ | Endothelial Cells§ | |||||||
| bFGF | MMP-9 | IL-8 | VEGF | bFGF | MMP-9 | IL-8 | VEGF | PCNA+ (%) | TUNEL+ (%) | CD-31/TUNEL (%) | |
| Saline | 113±7 | 111±12 | 27±2 | 64±2 | 100±6 | 100±8 | 100±20 | 100±6 | 74±3 | 3.4±1 | 1.6±0.6 |
| 1000 U | 89±13 | 109±7 | 31±3 | 74±6 | 83±12 | 72±6 | 111±30 | 90±3 | 47±4 | 4.1±08 | 4.9±1.8 |
| 10,000 U | 36±5¶ | 52±6# | 26±1 | 75±3 | 53±4# | 47±4# | 109±16 | 83±7 | 35±5# | 9.9±1.5# | 23.2±7.7¶ |
KM12L4 IFNR cells (1x106) were injected into the spleen of nude mice. Daily s.c. injections of saline or IFN-α-2a began on day 7. Mice were euthanized on day 42.
ISH and immunohistochemical analysis were performed on three livers from each treatment group. Image analysis was carried out on five areas of each section. For image analysis of ISH, expression of each factor was normalized to expression of poly d(T) in the same area. For image analysis of immunohistochemical characteristics, expression of each factor was compared to that of control sections assigned the value of 100.
Percentage of dividing cells (PNCA+) and apoptotic cells (TUNEL+) was calculated by examining at least 100 nuclei/sample.
CD-31 (Texas Red) and TUNEL (FITC green).
P<.001 versus control.
P<.01 versus control.
Immunohistochemical analysis agreed with the results of the ISH analyses (Figure 2). The intensities of bFGF and MMP-9 immunostaining in liver metastases was significantly reduced in mice given daily s.c. injections of 10,000 U of IFN-α-2a. Specifically, MMP-9 intensity was reduced from a mean of 100±8 in control mice to 47±4 in treated mice (Figure 2; Table 3, P<.001), and bFGF intensity was reduced from a mean of 100±6 in control mice to 53±4 in treated mice (P<.01).
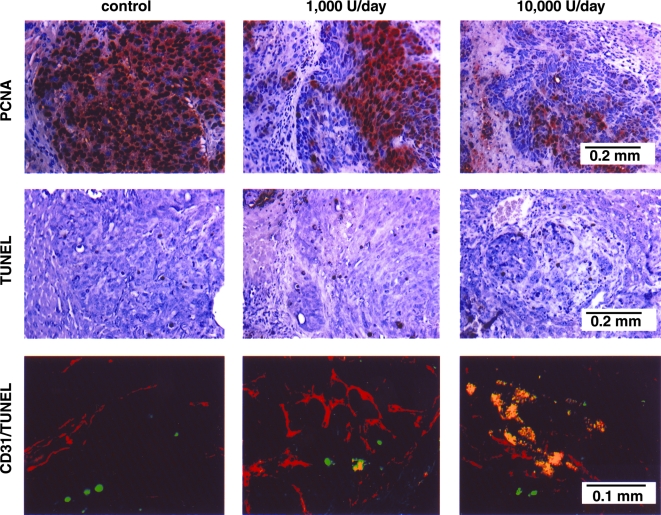
The inhibition of liver metastasis directly correlated with microvessel density measured by staining with antibodies against CD-31 (Figure 2, Table 2). The significant decrease in MVD was associated with a significant decrease in PCNA-positive tumor cells (Figure 3, Table 4) and a significant increase in the number of TUNEL-positive apoptotic cells (Figure 3, Table 4). The CD-31/TUNEL fluorescent double-labeling technique revealed that many endothelial cells in liver lesions treated with IFN-α-2a (10,000 U/day) were undergoing apoptosis (yellow reaction). The increase from 1.6±0.6% of apoptotic endothelial cells in control mice to 23.2±7.7% in IFN-α-2a-treated mice was highly significant (P<.001) (Table 4).
Figure 3.
Immunohistochemical analyses of livers with tumors harvested from control and IFN-α-2a-treated mice. The sections were immunostained for expression of PCNA (to show cell proliferation) and TUNEL (to show cell death). The sections were also stained with imunofluorescent anti-CD-31 antibody and TUNEL. A representative sample (x400) of this CD-31/TUNEL (fluorescent double-staining) is shown. Fluorescent red, CD-31-positive endothelial cells; fluorescent green, TUNEL-positive cells; fluorescent yellow, TUNEL-positive endothelial cells.
Table 4.
Induction of Apoptosis in Liver Endothelial Cells by IFN-α-2a.
| Treatment* | Liver Metastasis† | Tumor Cells‡ | Endothelial Cells§ | |||
| Median | Range | PCNA+ (%) | TUNEL+ (%) | CD-31/TUNEL (%) | Microvessel Density¶ | |
| Saline | ||||||
| 7 | 0 | 0–3 | 59±4 | 4.6±0.8 | 2.0±0.9 | 68±6 |
| 14 | 15 | 4–25 | 67±5 | 5.4±1.1 | 2.7±1.5 | 79±11 |
| 21 | 17 | 2–50 | 66±3 | 3.8±0.6 | 5.4±2.6 | 81±4 |
| IFN-α-2a | ||||||
| 7 | 0 | 0–2 | 56±6 | 5.3±1.1 | 2.9±2.1 | 70±9 |
| 14 | 3 | 0–30# | 57±3 | 4.1±1.2 | 12.1±3.9# | 37±4# |
| 21 | 2 | 1–22# | 39±2** | 1.4±2.1** | 20.2±4.5# | 29±6# |
KM12L4 IFNR cells (1x106) were injected into the spleen of nude mice. Daily s.c. injections of saline or 10,000 U of IFN-α-2a began on day 7. Groups of mice were euthanized after 7, 14, or 21 days of treatment. The livers were harvested for immunohistochemical assay.
The number of tumor foci was determined with the aid of a dissecting microscope.
Percentages of dividing cells (PCNA+) and apoptotic cells (TUNEL+) were calculated by examining at least 100 nuclei/sample.
Immunohistochemical double-staining for CD-31 (red) and TUNEL (green).
Microvessel density was determined by identifying tumor areas with the most intense CD-31 staining. Mean number of vessels±SD.
P<.001 versus control.
P<.01 versus control.
Induction of Apoptosis in Hepatic Endothelial Cells Inhibits Formation of Metastasis
The experiments described above demonstrated that IFN-α-2a administered at the dose of 10,000 U/day inhibited angiogenesis and growth of human colon cancer metastases in the liver of nude mice. In the next set of experiments, we determined whether liver metastases were inhibited by apoptosis of endothelial cells that were induced to divide by proangiogenic molecules released by the tumor cells. Spleens of nude mice were injected with 1x106 viable KM12L4 IFNR cells. Therapy with daily s.c. injections of 10,000 U of IFN-α-2a began 7 days later. Control mice received daily injections of Hank's balanced salt solution (HBSS). Groups of mice were euthanized after 7,14, and 21 days of therapy (days 14, 21, and 28 of the study). The livers were removed, the number of experimental metastases was recorded, and the livers were prepared for immunohistochemical evaluation of dividing cells (PCNA), apoptotic cells (TUNEL), and apoptotic endothelial cells (CD-31/TUNEL). The immunohistochemical data is shown in Figure 4 and summarized in Table 4.
Figure 4.
Induction of apoptosis in liver endothelial cells. KM123L4 IFNR cells (1x106) were injected into the spleen of nude mice. Seven days later, groups of mice (n=10) received daily s.c. injections of saline (control) or 10,000 U of IFN-α-2a. Mice were euthanized after 7, 14, or 21 days of treatment. The livers were processed for immunohistochemical evaluation. The sections were immunostained for expression of PCNA (to show cell proliferation) and TUNEL (to show cell death). The sections were also stained with immunofluorescent anti-CD-31 antibody and TUNEL A representative sample (x400) of this CD-31/TUNEL (fluorescent double-staining) is shown. Fluorescent red, CD-31-positive endothelial cells; fluorescent green, TUNEL-positive cells; fluorescent yellow, TUNEL-positive endothelial cells.
In control mice, microvessel density and percentage of dividing tumor cells, apoptotic tumor cells, and apoptotic endothelial cells were similar throughout the study. In contrast, in mice treated daily with 10,000 U of IFN-α-2a, microvessel density within experimental metastases was significantly reduced after 14 days of treatment (70±9 reduced to 29±4, P<.001). This decrease in microvessel density directly correlated with an increase in percentage of apoptotic endothelial cells (2.9±2.1 as compared with 20.2±4.5, P<.001). A significant decrease in dividing tumor cells (PCNA-positive) and an increase in apoptotic tumor cells (TUNEL-positive) occurred one week after the induction of apoptosis in the tumor-associated endothelial cells, suggesting that the induction of apoptosis in the endothelial cells was an earlier event.
Discussion
Our results demonstrated that the systemic administration of human IFN-α-2a to nude mice with liver metastasis of human colon cancer inhibits angiogenesis associated with apoptosis of endothelial cells, thereby inhibiting tumor growth. Altering the time schedule and dose of administration influenced angiogenesis and the therapeutic outcome, i.e., daily s.c. injections of IFN-α-2a at far below maximum tolerated doses [16,17] produced maximal antiangiogenic and antimetastatic effects; they achieved these by decreasing the expression level of the proangiogenic molecules bFGF and MMP-9 and by inducing apoptosis in endothelial cells that supply the metastatic lesions. The therapy mediated by IFN-α was independent of direct antiproliferative effects.
The progressive growth of primary neoplasms and metastases depends on angiogenesis [5–7], whereas the extent of angiogenesis is determined by the balance between proangiogenic and antiangiogenic molecules [45–47]. The angiogenic properties of tumor cells can be modulated by cytokines released by host cells into the tumor microenvironment [48]. For example, orthotopic implantation of human renal cell carcinomas (HRCC) (into the kidney of nude mice) allows the growth of hypervascular lesions, whereas ectopic implantation (into the subcutis of nude mice) does not; expression of bFGF by the tumor cells and of IFN-β by the surrounding host stroma account for the contrast [4].
This inverse correlation between expression of bFGF and IFN-β was also found in human colon cancer cells growing in the cecal wall or subcutis of nude mice [28,49]. IFN-α and IFN-β have been shown to down regulate the steady-state mRNA expression and protein production of bFGF by a mechanism that is independent of antiproliferative activity [12–17], and our data showing that IFN-α-2a can inhibit production of bFGF in KM12L4 IFNR cells (resistant to the antiproliferative effects of IFN-α) are in good agreement with these findings.
Immunohistochemical analyses revealed that IFN-α-2a administration produced a significant decrease in the number of tumor-associated blood vessels in the tumor specimens of mice. This decrease could be attributed to the well-known effect of IFN-α on expression of bFGF, MMP-9, and IL-8 by tumor cells, and to the fact that angiogenesis induced by colon carcinomas is associated with production of bFGF, VEGF, IL-8, and MMP-9 [8,9,19,28,50]. These angiogenic molecules regulate endothelial cell migration, division, degradation of the extracellular matrix, tube formation, and survival [51,52]. Our immunohistochemical analyses of tumor specimens clearly showed that daily treatment with IFN-α-2a decreased expression of bFGF and MMP-9 in the liver metastases. Recent findings by others suggested that bFGF can act as a survival factor for immature blood vessel endothelial cells and that VEGF can protect endothelial cells from apoptosis induced by cytostasis [53–56]. Because bFGF is expressed at higher levels on the leading edge of a tumor (as compared with the center), its downregulation may well be responsible for the inability of dividing endothelial cells to survive.
IFN-α has been widely used alone and in combination with other agents to treat a variety of neoplasms, including colon cancer. The response rate has been modest, and the optimal treatment schedule has yet to be determined [57–63]. In most trials, IFN-α was used as an antiproliferative agent and therefore was used at maximum tolerated doses (5–50 million units/m2), given two to three times per week, with response rates ranging from 5% to 20% [64]. Pharmacokinetic studies concluded that 1 hour after an IV injection of 6 million units of IFN-α, serum levels drop to <8 units/ml [64,65]. These concentrations are too low to inhibit expression of MMPs [16–19] or bFGF [12,16,17]: To produce antiangiogenic effects, IFN-α or IFN-β must be administered chronically [16,17]. For example, chronic administration of IFN-α has produced regression of life-threatening hemangiomas of infancy, especially when given at a low dose (3 MU/m2 s.c. daily) over a period of 9 to 12 months [66–68]. Moreover, daily administration of IFN-α-2a to a child with a rapidly growing giant-cell tumor inhibited the expression of bFGF, suppressed angiogenesis, and induced involution of the large lesion [69].
Our current findings agree with those of our previous report [16] that the antiangiogenic activity of IFN-α requires the maintenance of low levels, which can be achieved by daily administration. Kinetic analysis of liver metastases in mice given daily s.c. injections of IFN-α-2a revealed that apoptosis of tumor-associated endothelial cells was detected 7 days before apoptosis of tumor cells, suggesting that the inhibition of tumor cell division and growth of liver metastases was primarily due to apoptosis of tumor-associated endothelial cells. The fact that the metastases were produced by KM12L4 IFNR cells also supports this conclusion.
In summary, daily subcutaneous administration of 10,000 units of IFN-α-2a downregulated the expression of bFGF and MMP-9 in KM12L4 IFNR cells growing in the liver of nude mice. The inhibition of proangiogenic molecules correlated with apoptosis of metastasis-associated blood vessels. These data suggest that optimization of IFN-α dose and schedule may be beneficial in therapy for colon cancer metastasis to the liver.
Acknowledgements
The authors thank Lore Feldman for her critical editorial review and Lola López for expert assistance in the preparation of this manuscript.
Abbreviations
- bFGF
basic fibroblast growth factor
- FBS
fetal bovine saerum
- IFN-α
interferon-alpha
- IHC
immunohistochemistry
- IL
interleukin
- ISH
in situ hybridization
- EMEM
Eagle's minimum essential medium
- MMP
matrix metalloprotease
- MVD
microvessel density
- VEGF/VPF
vascular endothelial growth factor/vascular permeability factor
- MMP
matrix metalloprotease
- PCNA
proliferative cell nuclear antigen
- TUNEL
TdT-mediated dUTP-biotin nick-end labeling
Footnotes
This work was supported in part by Cancer Center Support Core grant CA16672 and grant R35-CA42107 from the National Cancer Institute, National Institutes of Health, and by the SPORE in Ovarian Cancer grant P50-CA83639 from the National Institutes of Health.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PH. Cancer statistics. Ca Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 2.August DA, Ottow RT, Sugarbaker PH. Clinical perspective on colorectal cancer. Cancer Metastasis Rev. 1984;3:303–325. doi: 10.1007/BF00051457. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eight GHA Clowes Memorial Award Lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 4.Kumar SR, Bucana CD, Gutman M, Fan D, Wilson MR, Fidler IJ. The influence of the organ microenvironment on the expression of basic fibroblast growth factor in renal cell carcinoma cells. Am J Pathol. 1994;145:365–374. [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman MJ. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 6.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 7.Kumar SR, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- 8.Ellis LM, Fidler IJ. Angiogenesis and metastasis. Eur J Cancer. 1996;32A:2451–2460. doi: 10.1016/s0959-8049(96)00389-9. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, Ellis LM, Wilson MR, Bucana CD, Kitadai Y, Fidler IJ. Progressive upregulation of metastasis-related genes in human colon cancer cells implanted into the cecum of nude mice. Oncol Res. 1996;8:163–169. [PubMed] [Google Scholar]
- 10.Kayton ML, Rowe DH, O'Toole KM, Thompson RB, Schwarz MA, Stolar CJ, Kandel JJ. Metastasis correlates with production of vascular endothelial growth factor in a murine model of human Wilms' tumor. J Pediatr Surg. 1999;34:743–747. doi: 10.1016/s0022-3468(99)90367-6. [DOI] [PubMed] [Google Scholar]
- 11.Fidler IJ, Ellis LM. The implication of angiogenesis of biology and therapy of metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 12.Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons alpha and beta downregulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci USA. 1995;92:4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc London B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 14.Baron S, Dianzani F. The interferons: a biological system with therapeutic potential in viral infections. Antiviral Res. 1994;24:97–110. doi: 10.1016/0166-3542(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 15.Hertzog PJ, Hwang SY, Kola I. Role of interferons in the regulation of cell proliferation, differentiation, and development. Mol Reprod Dev. 1994;39:226–232. doi: 10.1002/mrd.1080390216. [DOI] [PubMed] [Google Scholar]
- 16.Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-α-mediated downregulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res. 1999;10:2726–2734. [PubMed] [Google Scholar]
- 17.Dinney CP, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, Fidler IJ. Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res. 1998;58:808–814. [PubMed] [Google Scholar]
- 18.Gohji K, Fidler IJ, Tsan R, Radinsky R, von Eschenbach AC, Tsuruo T, Nakajima M. Human recombinant interferons-β and -γ decrease gelatinase production and invasion by human KG-2 renal carcinoma cells. Int J Cancer. 1994;58:380–384. doi: 10.1002/ijc.2910580313. [DOI] [PubMed] [Google Scholar]
- 19.Fabra A, Nakajima M, Bucana CD, Fidler IJ. Modulation of the invasive phenotype of human colon carcinoma cells by organ-specific fibroblasts of nude mice. Differentiation. 1992;52:101–110. doi: 10.1111/j.1432-0436.1992.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira IC, Sciavolino PJ, Lee TH, Vilcek J. Down-regulation of interleukin 8 gene expression in human fibroblasts: unique mechanism of transcriptional inhibition by interferon. Proc Natl Acad Sci USA. 1992;89:9049–9053. doi: 10.1073/pnas.89.19.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar SR, Gutman M, Llansa N, Fidler IJ. Interferon-β prevents the upregulation of interleukin-8 expression in human melanoma cells. J Interferon Cytokine Res. 1996;16:577–584. doi: 10.1089/jir.1996.16.577. [DOI] [PubMed] [Google Scholar]
- 22.Gutterman JU. Cytokine therapeutics: lessons from interferon-α. Proc Natl Acad Sci USA. 1994;91:1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krown SE. Interferons in malignancy: biological products or biological response modifiers? J Natl Cancer Inst. 1988;80:306–309. doi: 10.1093/jnci/80.5.306. [DOI] [PubMed] [Google Scholar]
- 24.Thomas H, Balkwill FR. Effects of interferons and other cytokines on tumors in animals: a review. Pharmacol Ther. 1991;52:307–314. doi: 10.1016/0163-7258(91)90030-p. [DOI] [PubMed] [Google Scholar]
- 25.Salmon P, LeCotonnec JY, Galazka A, Abdul-Ahad A, Darragh A. Pharmacokinetics and pharmacodynamics of recombinant human interferon-β in health male volunteers. J Interferon Cytokine Res. 1996;16:759–764. doi: 10.1089/jir.1996.16.759. [DOI] [PubMed] [Google Scholar]
- 26.Einhorn S, Grander D. Why do so many cancer patients fail to respond to interferon therapy? J Interferon Cytokine Res. 1996;16:275–281. doi: 10.1089/jir.1996.16.275. [DOI] [PubMed] [Google Scholar]
- 27.Kitadai Y, Ellis LM, Tucker SL, Greene GF, Bucana CD, Cleary KR, Takahashi Y, Tahara E, Fidler IJ. Multiparametric in situ mRNA hybridization analysis to predict disease recurrence in patients with colon carcinoma. Am J Pathol. 1996;149:1541–1551. [PMC free article] [PubMed] [Google Scholar]
- 28.Kitadai Y, Bucana CD, Ellis LM, Tahara E, Fidler IJ. In situ mRNA hybridization technique for analysis of metastasis-related gene in human colon carcinoma cells. Am J Pathol. 1996;147:1238–1247. [PMC free article] [PubMed] [Google Scholar]
- 29.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1998;48:1943–1948. [PubMed] [Google Scholar]
- 30.Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1998;48:6863–6871. [PubMed] [Google Scholar]
- 31.Fan D, Chakrabarty S, Seid C, Bell CW, Schackert H, Morikawa K, Fidler IJ. Clonal stimulation or inhibition of human colon carcinomas mediated by transforming growth factor-α1. Cancer Commun. 1989;1:117–125. doi: 10.3727/095535489820875327. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Kuniyasu H, Bucana CD, Wilson MR, Fidler IJ. Spatial and temporal expression of angiogenic molecules during tumor growth and progression. Oncol Res. 1998;10:301–311. [PubMed] [Google Scholar]
- 33.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, Fidler IJ. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 34.Dinney CPN, Fishbeck R, Singh RK, Eve B, Pathak S, Brown N, Xie B, Fan D, Bucana CD, Fidler IJ, Killion JJ. Isolation and characterization of metastatic variants from human transitional cell carcinoma passaged by orthotopic implantation in athymic nude mice. J Urol. 1995;54:1532–1538. [PubMed] [Google Scholar]
- 35.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 36.Park C-S, Manahan LJ, Brigati DJ. Automated molecular pathology: one hour in situ DNA hybridization. J Histotechnol. 1991;14:219–229. [Google Scholar]
- 37.Caruthers MH, Beaucage SL, Efcavitch JW, Fisher EF, Goldman RA, Dehaseth PL, Mandechi W, Matteucci MD, Rosendahl MS, Stabinsky Y. Chemical synthesis and biological studies on mutated gene-control region. Cold Spring Harbor Symp Quant Biol. 1982;47:411–418. doi: 10.1101/sqb.1983.047.01.048. [DOI] [PubMed] [Google Scholar]
- 38.Radinsky R, Bucana CD, Ellis LM, Sanchez R, Cleary KR, Brigati DJ, Fidler IJ. A rapid colorimetric in situ messenger RNA hybridization technique for analysis of epidermal growth factor receptor in paraffin-embedded surgical specimens of human colon carcinomas. Cancer Res. 1993;53:937–943. [PubMed] [Google Scholar]
- 39.Kitadai Y, Ellis LM, Tucker SL, Greene GF, Bucana CD, Cleary KR, Takahashi Y, Tahara E, Fidler IJ. Multiparametric in situ mRNA hybridization analysis to predict disease recurrence in patients with colon carcinoma. Am J Pathol. 1996;149:1541–1551. [PMC free article] [PubMed] [Google Scholar]
- 40.Reed JA, Manahan LJ, Park CS, Brigati DJ. Complete one-hour immunocytochemistry based on capillary action. Biotechniques. 1992;13:434–443. [PubMed] [Google Scholar]
- 41.Kuniyasu H, Ellis L, Evans DB, Abbruzzese JL, Fenoglio CJ, Bucana CD, Cleary KR, Tahara E, Fidler IJ. Relative expression of E-cadherin and type IV collagenase genes predicts disease outcome in patients with resectable pancreatic carcinoma. Clin Cancer Res. 1999;5:25–33. [PubMed] [Google Scholar]
- 42.Vecchi A, Garlanda C, Lampugnami MG, Resnati M, Matteucci C, Stopacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, Dejana E. Monoclonal antibodies specific for endothelial cells of mouse blood vessels: their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
- 43.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 44.Fisher LD, van Belle G. Biostatistics. New York: Wiley; 1993. pp. 786–843. [Google Scholar]
- 45.Folkman J. Seminars in medicine of the Beth Israel Hospital, Boston. Clinical application of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 46.Fidler IJ, Ellis LM. The implications of angiogenesis to the biology and therapy of cancer metastasis (Minireview) Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 47.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 48.Fidler IJ. Modulation of the organ microenvironment for the treatment of cancer metastasis (Commentary) J Natl Cancer Inst. 1995;87:1588–1592. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 49.Nakajima M, Morikawa K, Fabra A, Bucana CD, Fidler IJ. Influence of organ environment on extracellular matrix degradative activity and metastasis of human colon carcinoma cells. J Natl Cancer Inst. 1990;82:1890–1898. doi: 10.1093/jnci/82.24.1890. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 51.Folkman J. Tumor angiogenesis. In: Mendelsohn J, Howley PM, Israel MA, Liotta LA, editors. The Molecular Basis of Cancer. Philadelphia, PA: WB Saunders; 1995. pp. 206–232. [Google Scholar]
- 52.Auerbach W, Auerbach R. Angiogenesis inhibition: a review. Pharmacol Ther. 1994;63:265–311. doi: 10.1016/0163-7258(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 53.Syridopoulos I, Brogi E, Kearney M, Sullivan AB, Cetrulo C, Isner M, Losordo DW. Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-alpha: balance between growth and death signals. J Mol Cell Cardiol. 1997;29:1321–1330. doi: 10.1006/jmcc.1996.0365. [DOI] [PubMed] [Google Scholar]
- 54.Gerber HP, Dixit V, Ferrera N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 55.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe Y, Dvorak HV. Vascular permeability factor/vascular endothelial growth factor inhibits anchorage-disruption-induced apoptosis in microvessel endothelial cells by inducing scaffold formation. Exp Cell Res. 1997;233:340–349. doi: 10.1006/excr.1997.3583. [DOI] [PubMed] [Google Scholar]
- 57.Marshall ME, Wolf M, Crawford ED, Thompson IM, Flanigan R, Balcerzak SP, Meyers FJ. Evaluation of low dose alpha-interferon (Roferon-A) in patients with advanced renal cell carcinoma: a Southwest Oncology Group study. Cancer Biother. 1995;10:205–209. doi: 10.1089/cbr.1995.10.205. [DOI] [PubMed] [Google Scholar]
- 58.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon α-2b adjuvant therapy of high risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 59.Baron S, Tyring SK, Fleischmann WR, Jr, Coppenharer DH, Niesel DW, Klimpel GR, Stanter GJ, Hughes TK. The interferons. mechanisms of action and clinical applications. JAMA. 1991;266:1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 60.Shepherd FA, Beaulieu R, Gelmon K, Thuot CA, Sawka C, Read S, Singer J. Prospective randomized trial of two dose levels of interferon-α with zidovudine for the treatment of Kaposi's sarcoma associated with human immunodeficiency virus infection: a Canadian HIV Clinical Trials Network study. J Clin Oncol. 1998;16:1736–1742. doi: 10.1200/JCO.1998.16.5.1736. [DOI] [PubMed] [Google Scholar]
- 61.Fossa S, Jones M, Johnson P, Joffe J, Holdener E, Elson P, Ritchie A, Selby P. Interferon-alpha and survival in renal cell carcinoma. Br J Urol. 1995;76:286–290. doi: 10.1111/j.1464-410x.1995.tb07702.x. [DOI] [PubMed] [Google Scholar]
- 62.Logothetis CJ, Hossan E, Recondo G, Sella A, Ellerhorst J, Kilbourn R, Zukiwski A, Amato R. 5-Flurouracil and interferon-α in chemotherapy refractory bladder carcinoma: an effective regimen. Anticancer Res. 1994;14:1265–1279. [PubMed] [Google Scholar]
- 63.Negrier S, Escudier B, Lasset C, Douillard J-Y, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T. Recombinant human interleukin-2, recombinant human interferon-α-2a, or both in metastatic renal cell carcinoma. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 64.Dianzani F. Interferon treatments: how to use an endogenous system as a therapeutic agent. J Interferon Res. 1992;(Special Issue):109–118. doi: 10.1089/jir.1992.1992.109. [DOI] [PubMed] [Google Scholar]
- 65.Johns TG, Mackay IR, Callister KA, Hertzog PJ, Devenish RJ, Linnane AW. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon-β. J Natl Cancer Inst. 1992;84:1185–1190. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- 66.Ezekowitz RAB, Mulliken JB, Folkman J. Interferon α-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326:1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 67.Ezekowitz A, Mulliken J, Folkman J. Interferon-α therapy of hemangiomas of infancy (Letter) Br J Haematol. 1991;79:67–68. doi: 10.1111/j.1365-2141.1991.tb08123.x. [DOI] [PubMed] [Google Scholar]
- 68.Ricketts RR, Hatley RM, Corden BJ, Sabio H, Howell CG. Interferon-α-2a for the treatment of complex hemangiomas of infancy and childhood. Ann Surg. 1994;6:605–614. doi: 10.1097/00000658-199406000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaban LB, Mulliken JB, Ezekowitz RA, Ebb D, Smith PS, Folkman J. Antiangiogenic therapy of a recurrent giant cell tumor of the mandible with interferon alfa-2a. Pediatrics. 1999;103:1145–1149. doi: 10.1542/peds.103.6.1145. [DOI] [PubMed] [Google Scholar]