Abstract
To assess the utility of fluorescence in situ hybridization (FISH) for analysis of MYCN gene amplification in neuroblastoma, we compared this assay with Southern blot analysis using tumor specimens collected from 232 patients with presenting characteristics typical of this disease. The FISH technique identified MYCN amplification in 47 cases, compared with 39 by Southern blotting, thus increasing the total number of positive cases by 21%. The major cause of discordancy was a low fraction of tumor cells (≤30% replacement) in clinical specimens, which prevented an accurate estimate of MYCN copy number by Southern blotting. With FISH, by contrast, it was possible to analyze multiple interphase nuclei of tumor cells, regardless of the proportion of normal peripheral blood, bone marrow, or stromal cells in clinical samples. Thus, FISH could be performed accurately with very small numbers of tumor cells from touch preparations of needle biopsies. Moreover, this procedure allowed us to discern the heterogeneous pattern of MYCN amplification that is characteristic of neuroblastoma. We conclude that FISH improves the detection of MYCN gene amplification in childhood neuroblastomas in a clinical setting, thus facilitating therapeutic decisions based on the presence or absence of this prognostically important biologic marker.
Keywords: neuroblastoma, fluorescence in situ hybridization, MYCN gene, gene amplification, double minute chromatin bodies
Introduction
Amplification of the MYCN proto-oncogene is recognized as an independent prognostic factor in neuroblastoma, identifying children with rapidly progressive disease who respond poorly to conventional therapy [1,2]. Increased MYCN copy numbers are found in less than 5% of patients with low-stage neuroblastoma, as well as stage IV-S tumors, compared with 30% to 40% of those with advanced disease [3,4]. When MYCN amplification occurs, it is almost always present at the time of diagnosis, and thus appears to be an intrinsic property of a subset of highly aggressive tumors that are invariably fatal unless effective alternative treatment can be found [5]. MYCN gene amplification may occur as either intrachromosomal homogeneously staining regions (HSRs) or as genetically unstable extrachromosomal double minutes (DMs) [6–8].
Southern blot analysis has been the standard method for detecting amplified MYCN genes in tumor specimens; however, its usefulness is limited by a number of technical factors. One cannot, for example, obtain reliable measurements with small quantities of DNA, and false-negative results are common when the tissue sample contains an admixture of tumor cells and normal peripheral blood leukocytes, stromal elements, or other nonmalignant elements [9]. Thus, the incidence of MYCN gene amplification may have been underestimated in previous studies based on Southern blot analyses. Nor do Southern assays allow one to discern the cytogenetic basis of MYCN amplification, whether DMs or HSRs.
To compare these two approaches, we used both fluorescence in situ hybridization (FISH) and Southern blot analysis to detect MYCN gene amplification in neuroblastoma samples collected from patients enrolled in therapeutic, as well as nontherapeutic, studies of the Pediatric Oncology Group (POG). The intent was to test the concordance of these two methods applied to tumor samples from the same patients. The results reported here indicate that the FISH assay is the method of choice for detecting MYCN amplification, currently the most reliable marker of resistant disease warranting prompt intensification of treatment.
Materials and Methods
Tumor Tissue and Patient Information
Fresh tumor specimens or bone marrow samples taken from 642 neuroblastoma patients enrolled on therapeutic or nontherapeutic protocols of the POG, between January 1991 and April 1994, met all criteria for analysis. The FISH assay was performed at St. Jude Children's Research Hospital (Memphis, TN) and the Southern blot analysis at Washington University (St. Louis, MO). Wright-stained smears of aliquots of each tumor cell suspension were analyzed for the percentage of tumor cells. Specimen accrual was limited to patients whose diagnosis of neuroblastoma was based on histologic examination of tumor tissue or of involved bone marrow. Clinicopathologic tumor staging was based on standard POG criteria [10]. Selected clinical and laboratory data (e.g., age at diagnosis, sex, tumor site, and ploidy) were retrieved from the POG statistical center.
FISH
MYCN gene amplification was investigated by simultaneous interphase and metaphase FISH analysis of single-cell suspensions from tumor specimens, as described previously [9]. Hybridization studies were performed with a cosmid probe (from the MYCN genomic locus on chromosome 2) that had been nick-translated with digoxigenin-dUTP (Boehringer Mannheim). The labeled probe was combined with human Cot1 DNA and allowed to hybridize overnight at 37°C to fixed tumor cells in a solution containing 50% formamide, 10% dextran sulfate, and 2x SSC. Specific hybridization signals were detected by incubating the hybridized slides in a solution containing fluorescein-conjugated antidigoxigenin antibodies. Following signal detection, the slides were counterstained with propidium iodide and mounted with Vectashield (Vector Laboratories, Burlingame, CA). Probe detection for two-color experiments included Texas red avidin and counterstaining with 4′, 6-diamidino-2-phenylindole (DAPI).
Fluorescence microscopy was performed with a Zeiss microscope equipped with either fluorescein filter sets or a three-color filter set for FITC, Texas red, and DAPI; observers had no knowledge of the Southern blot results. Each sample was analyzed to determine the origin of the amplification unit (extrachromosomal DMs or intrachromosomal HSRs) and the proportion of cells with amplified MYCN genes. The MYCN copy number was scored as either amplified or unamplified. Two-color FISH was performed with a biotin-labeled chromosome 2 centromere-specific probe, in addition to the MYCN probe, whenever a modal copy number of 5 to 12 was detected to distinguish gene amplification from chromosome 2 aneusomy.
Southern Blot Analysis for MYCN Gene Amplification
High-molecular-weight DNA was isolated from tumor samples, digested with restriction endonuclease EcoRI, fractionated on agarose gels, transferred to nylon filters, and hybridized to a 32P-labeled MYCN probe, as previously described [1]. Washed filters were exposed for 12 to 48 hours to Kodak XAR-5 film at -80°C with an intensifying screen. Signal intensity was quantitated directly and by serial dilution from autoradiograms with a laser densitometer (LKB Model 2222), as previously described [1]. The gene was considered to be amplified if the copy number was >3 per haploid genome and unamplified if it was <3.
Results
Heterogeneity of MYCN Gene Amplification
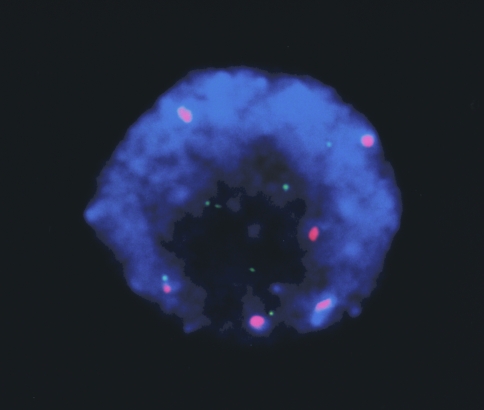
MYCN gene amplification was detected in 105 of 642 neuroblastoma samples examined by FISH analysis. Figure 1 illustrates the typical findings in tumors with single-copy (panel A) or amplified (panels B and C) MYCN genes. Different forms of MYCN amplification within tumor cells from a single clinical sample are shown in Figure 2A, where amplification is present as either DMs or HSRs in interphase cells. Some of the cells have the normal number of MYCN copies. One of the advantages of using the FISH assay is depicted in Figure 3, which illustrates multiple copies of MYCN in a tumor with chromosome 2 aneusomy (tumor cell DNA index, 2.22). In this instance, the extra copies of MYCN did not arise from the formation of DMs or HSRs, but from increased copies of the entire chromosome [11].
Figure 1.
Typical findings obtained by FISH analysis of MYCN genes in neuroblastoma: (A) unamplified; (B) amplified as DMs; and (C) amplified as HSRs.
Figure 2.
Heterogeneity of MYCN copy number among tumor cells in a single diagnostic sample, including amplification as DMs (A); HSRs (B); or single no amplification (C).
Figure 3.
Multiple copies of MYCN in a tumor with chromosome 2 aneusomy.
Comparison of FISH and Southern Analysis
From January 1991 to June 1993, 232 neuroblastoma samples were successfully examined by both FISH and Southern blotting procedures. With the former assay, MYCN amplification was present in 47 of the samples, compared with 39 by Southern blotting, representing a 21% increase in the total number of positive tumors detected by FISH (Table 1). A single tumor with MYCN amplification by Southern blotting was scored as unamplified by FISH (patient 9).
Table 1.
Clinical Features of Discordant Cases.
| Patient Number | Age (year) | Stage* | Percent Tumor Cells | Type of Sample | MYCN Status | ||
| FISH | Southern | FISH | Southern | ||||
| 1 | 2.3 | D | 30 | Marrow | Marrow | Amplified | UA |
| 2 | 3.7 | D | 66 | Tumor | Marrow | Amplified | UA |
| 3 | 1.0 | C | 88 | Tumor | Tumor | Amplified | UA |
| 4 | 4.5 | D | 9 | Marrow | Marrow | Amplified | UA |
| 5 | 1.1 | D | 80 | Tumor | Tumor | Amplified | UA |
| 6 | 1.2 | A | 50 | Tumor | Tumor | Amplified | UA |
| 7 | 2.0 | D | 10 | Marrow | Marrow | Amplified | UA |
| 8 | 2.2 | D | 10 | Marrow | Marrow | Amplified | UA |
| 9 | 1.1 | D | 81 | Marrow | Tumor | NA† | Amplified |
By POG criteria [10].
Unamplified.
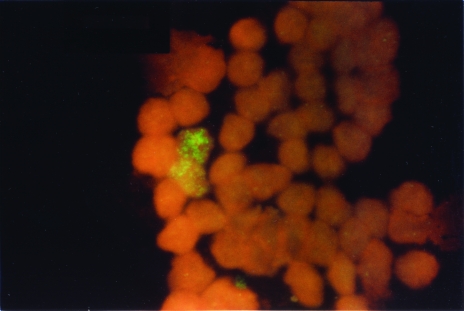
Reasons for the discordant findings are suggested by the specimen characteristics reported in the table. Four of nine samples (from patients 1, 4, 7, and 8) consisted of tumor or bone marrow with a low percentage of malignant cells (9% to 30%), a situation conducive to false-negative measurements by the Southern method. With FISH, by contrast, it was possible to analyze multiple interphase nuclei rapidly, permitting the detection of clumps of tumor cells with amplified MYCN genes, regardless of their abundance in the sample (Figure 4). In two additional cases (2 and 3), the interphase tumor cells contained low levels of DMs. Apparently, the low MYCN copy levels in these tumor cell populations were below the limits of detection by Southern blotting. The same pattern of amplification was observed in case 4, which probably contributed (with the low tumor cell fraction) to a false-negative Southern result. The Southern blot finding in case 5 can be attributed to a degraded DNA sample that yielded a falsely low MYCN hybridization signal. Finally, the false-negative FISH classification of case 9 was due to a clerical error resulting in the analysis of a tumor-free marrow specimen. Thus, the FISH technique not only shows a high level of agreement with Southern blotting, but detects authentic gene amplification in a significant number of additional cases. A review of the 223 specimens with concordant FISH and Southern results revealed that all had more than 30% tumor cells, indicating that Southern blotting should be reserved for samples with intermediate to high fractions of tumor cells.
Figure 4.
Low percentage of amplified neuroblastoma cells in a discordant diagnostic bone marrow sample.
Discussion
Amplification of the MYCN oncogene identifies a high-risk subset of neuroblastoma patients who respond poorly to conventional therapy [2,12,14]. Hence, the ability to detect this biologic marker promptly and reliably is a prerequisite for sound clinical management. The data we have presented indicate that FISH offers distinct advantages over Southern blotting for the detection of MYCN amplification, in agreement with the recently reported study of Taylor et al. [15]. It can be readily performed on small tissue samples that may be only partially involved by tumor. Moreover, in contrast to Southern blotting, interphase FISH distinguishes a spectrum of MYCN changes that may have prognostic significance. Single-copy genes appear as two discrete fluorescence signals corresponding to each copy of the MYCN gene. Amplified sequences, whether HSRs or DMs, are clearly distinguished as multiple punctate or coalesced signals in interphase nuclei. In cases with chromosome 2 aneusomy, simultaneous cohybridization of interphase nuclei with a chromosome 2-specific alpha-satellite probe allows direct enumeration of the number of copies of chromosome 2 relative to MYCN [9]. Finally, the FISH procedure allows one to visualize cell-to-cell differences in the MYCN copy number due to the unequal segregation of DMs between daughter cells during mitosis, resulting in a heterogenous distribution of amplified sequences within a defined cell population [13].
Consistent with previous reports [7,14], we found that the MYCN oncogene was most frequently amplified as DMs. We did not observe HSRs in any of the discordant cases, although such regions were noted in two cases found to have amplified MYCN by both FISH and Southern blot analysis. The basis for this differential pattern of amplification is not known; however, experimental models of gene amplification suggest that it may reflect primary differences in the stage of tumor progression, with DMs representing an earlier event than the intrachromosomal integration of amplified sequences [9,13].
FISH has also shown promise for improving the detection of gene amplification in tumors other than neuroblastoma. Kallioneimi et al. [16] have demonstrated the utility of this method for recognizing both the level and spatial distribution of ERBB2 (HER-2/neu) amplification in breast cancer cell lines and primary breast carcinomas [16]. HER-2/neu amplification in salivary gland carcinomas was characterized by Press et al. [17] using a method similar to ours. They showed that the HER-2/neu gene is amplified and overexpressed in approximately one third of these tumors and is independently associated with a poor prognosis. With increased attention being paid to the oncogenic role of human gene amplification, it will be important to consider FISH for assessment of the amplification pattern in clinical tumor samples and the detection of tumor cell subpopulations with strongly amplified genomic regions.
We conclude that the capacity of FISH to detect small populations of highly amplified neuroblasts within tissue or bone marrow specimens affords a clear advantage when one is evaluating clinical material for MYCN gene status. The improved rate of detection of amplified MYCN copies over Southern blot analysis could be expected to influence the selection of therapy and thus the overall survival of children with neuroblastoma.
Footnotes
Supported, in part, by NIH grants CA 31566, CA 21765 (Cancer Center CORE Grant), and The American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children's Research Hospital.
References
- 1.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 2.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM, Fong CT. Molecular biology and genetics of human neuroblastoma. Cancer Genet Cytogenet. 1989;41:153–174. doi: 10.1016/0165-4608(89)90243-4. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM. Neuroblastoma — clinical applications of molecular parameters. Brain Pathol. 1990;1:47–54. doi: 10.1111/j.1750-3639.1990.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur GM, Hayes FA, Green AA, Casper JT, Wasson J, Wallach S, Seeger RC. Consistent N-myc copy number in simultaneous or consecutive neuroblastoma samples from sixty individual patients. Cancer Res. 1987;47:4248–4253. [PubMed] [Google Scholar]
- 6.Brodeur GM, Sekhon GS, Goldstein MN. Chromosomal aberrations in human neuroblastomas. Cancer. 1977;40:2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Brodeur GM, Green AA, Hayes FA, Williams KJ, Williams DL, Tsiatis AA. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981;41:4678–4686. [PubMed] [Google Scholar]
- 8.Biedler JL, Ross RA, Shanske S, Spengler BA. Human neuroblastoma cytogenetics: search for significance of homogeneously staining regions and double minute chromosomes. Prog Cancer Res Ther. 1980;12:81–96. [Google Scholar]
- 9.Shapiro DN, Valentine MB, Rowe ST, Sinclair AE, Sublett JE, Roberts WM, Look AT. Detection of N-myc gene amplification by fluorescence in situ hybridization: diagnostic utility for neuroblastoma. Am J Pathol. 1993;142:1339–1346. [PMC free article] [PubMed] [Google Scholar]
- 10.Nitschke R, Smith El, Shochat S, Altshuler G, Travers H, Shuster JJ, Hayes FA, Patterson R, McWilliams N. Localized neuroblastoma treated by surgery: a Pediatric Oncology Group Study. J Clin Oncol. 1988;6:1271–1279. doi: 10.1200/JCO.1988.6.8.1271. [DOI] [PubMed] [Google Scholar]
- 11.Trask BJ. Flourescence in situ hybridization: applications in cytogenetics and gene mapping. TIGG. 1991;7:149–154. doi: 10.1016/0168-9525(91)90378-4. [DOI] [PubMed] [Google Scholar]
- 12.Look AT, Hayes FA, Shuster JJ, Douglass EC, Castleberry RP, Bowman LC, Smith El, Brodeur GM. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group Study. J Clin Oncol. 1991;9:581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Wahl GM. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989;49:1333–1340. [PubMed] [Google Scholar]
- 14.Kaneko Y, Kanda N, Maseki N, Sakurai M, Tsuchida Y, Takeda T, Okabe I. Different karyotypic patterns in early and advanced stage neuroblastoma. Cancer Res. 1987;47:311–318. [PubMed] [Google Scholar]
- 15.Taylor CPF, Bown NP, McGuckin AG, Lunec J, Malcolm AJ, Pearson AD, Sheer D. Fluorescence in situ hybridization techniques for the rapid detection of genetic prognostic factors in neuroblastoma. United Kingdom Children's Cancer Study Group. Br°Cancer. 2000;83:40–49. doi: 10.1054/bjoc.2000.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallioneimi OP, Kallioniemi A, Kurisu W, Thor A, Chen LC, Smith HS, Waldman FM, Pinkel D, Gray JW. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci USA. 1992;89:5321–5325. doi: 10.1073/pnas.89.12.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Press MF, Pike MC, Hung G, Zhou JY, Ma Y, George J, Dietz-Band J, James W, Slamon DJ, Batsakis JG. Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: correlation with poor prognosis. Cancer Res. 1994;54:5675–5682. [PubMed] [Google Scholar]