Abstract
Several novel, differentially transcribed genes were identified in one centroblastic and one immunoblastic HIV-associated B-cell non-Hodgkin's lymphoma (B-NHL) by subtractive cloning. In both lymphomas, we detected an upregulated transcription of several mitochondrial genes. In the centroblastic B-NHL, we found a high level transcription of nuclear genes including the interferon-inducible gene (INF-ind), the immunoglobulin light chain gene (IgL), the set oncogene, and several unknown genes. The data obtained on upregulated expression of the genes in human B-NHL of HIV-infected patients considerably overlap with those obtained earlier for the B-NHL of simian immunodeficiency virus-infected monkeys. In the centroblastic lymphoma, one transcript revealed a fusion of the 3′-untranslated region of the set gene and the C-terminal region of the IgL gene. This chimeric sequence was confirmed by a site-directed polymerase chain reaction performed with total cDNA and genomic DNA. The expected amplification product was obtained in both cases pointing to a genomic rearrangement. The IgL-set fusion sequence was not found in cDNA preparations and genomic DNA of the immunoblastic HIV-associated B-NHL. Further studies are necessary to determine whether these genes contribute to lymphoma development or can be used as therapeutic targets.
Keywords: HIV, B-cell lymphoma, transcription, genomic rearrangement, subtractive cloning
Introduction
Humans infected with human immunodeficiency virus (HIV) are predisposed to develop B-cell non-Hodgkin's lymphomas (B-NHL) with distinct histomorphological characteristics [1,2]. Studies on the mechanisms of lymphomagenesis have shown that several molecular alterations seem to be associated with tumor development. Different groups of genes may contribute to the chain of events that eventually lead to lymphomagenesis. These groups are comprised of oncogenes, tumor suppressor genes, genes encoding intratumoral cytokines, growth factors, growth factor receptors, transcription factors, and cell adhesion proteins [2–4]. Cellular genes playing a role in growth regulation, cell senescence, and proliferation such as c-myc, bcl-1, bcl-2, bcl-6 and p53 are essential for growth transformation of lymphoma cells. Malignization of cells during lymphomagenesis is also related to genetic lesions in tumor cell chromosomes, e.g., rearrangements and mutations of genes. Some of the alterations cause the formation of novel fused genes [5–8]. In addition, overexpression of some housekeeping genes takes place [9–11].
Viral cofactors of lymphomagenesis have also been postulated. Epstein-Barr virus (EBV) infection has long been associated with Burkitt's lymphoma. It is present in almost 100% of endemic cases and up to 30% in sporadic cases [12]. The prevalence of EBV genomes in tumor cells is about 30% in acquired immunodeficiency syndrome (AIDS)-associated NHLs [1–3]. The incidence of B-NHL is about 10% in HIV-infected patients. However, the role of this herpes virus as well as the immunodeficiency virus as cofactor or etiological agent in the lymphomagenesis is not clear. HIV-associated B-NHL shares some histological and molecular characteristics with spontaneous lymphomas. Basic differences with respect to gene expression were not detected. However, AIDS-associated B-NHL exhibits unique features that distinguish them significantly from NHL arising in individuals with iatrogenic, congenital, or non-HIV immunodeficiencies [13,14]. These findings strongly suggest the presence of unique mechanisms leading to AIDS-associated NHL.
Multiple factors presumably contribute to the development of the AIDS-associated NHL including chronic antigenic stimulation — a tendency towards chromosomal translocations and gene products of HIV itself [2,3,15,16]. In particular, the tat gene of HIV-1 is reported to have oncogenic potential [15,16] and tat can enhance the migration of lymphoma cells and their adhesion to endothelial cells [17].
In order to clarify the mechanisms of lymphomagenesis, several new approaches have been recently proposed [18–21]. The methods allowed to get spectra of genes differently expressed in malignant cells, to more correctly characterize different types of lymphomas, and to reveal new diagnostic markers for them.
Our study aimed to identify genes that are differentially expressed or overexpressed in HIV-associated lymphoma by polymerase chain reaction (PCR)-based subtractive cloning. This kind of expression profiling extends our previous studies describing cytokine gene transcription patterns in HIV-associated human and simian immunodeficiency virus (SIV)-associated monkey lymphomas [4]. Besides, recently, we detected an upregulation of several nuclear and mitochondrial genes in SIV-associated B-cell monkey lymphomas [21]. Our experimental approach allowed us to detect genes which have not yet been thought to be upregulated in human AIDS-associated lymphomas. In addition, we detected for the first time a gene fusion between the IgL gene and the rarely described set gene.
Materials and Methods
Tumor Tissue
Biopsy specimens from lymphomas A and B both from HIV-1-infected AIDS patients (males, ages 43 and 36) were kindly provided by Prof. Dr. I. Schedel (Medical School, Hanover, Germany). Histological and virological characteristics of these tumors are summarized in Table 1. Material from lymphoma A was taken from the left tonsil. Specimens from lymphoma B were taken from the liver hilus. The latter patient was classified as WR-6 stage of AIDS. Both tumors were B-NHLs either of the centroblastic type (lymphoma A) or the immunoblastic type (lymphoma B). Cells from both tumors harbored EBV genomes, and EBER-1 as well as EBNA-2 mRNAs were present [22].
Table 1.
Characteristics of Two AIDS-Associated B-NHLs.
| Histological and Virological Characteristics | Lymphoma A | Lymphoma B |
| REAL classification | Diffuse large B cell | Diffuse large B cell |
| KIEL classification | Centroblastic | Immunoblastic |
| Ki 1 | Positive | Positive |
| Tumor site | Extranodal tonsils | Extranodal lymph node |
| Stage of immunodeficiency | - | WR 6 |
| EBV serology | Positive | Positive |
| EBNA-2 | Positive* | Positive* |
| HIV-1 serology | Positive | Positive |
| p24 | Positive | - |
Immunohistochemistry and/or mRNA in situ.
(-) No data available.
DNA and RNA Isolation
Cellular DNA was isolated from tissue biopsy specimens stored in liquid nitrogen. Tissues were dispersed and lysed in eight volumes of a buffer containing 0.5 M EDTA, pH 8.0, 0.4% sarcosyl [23]. DNA was extracted twice with phenol and precipitated with 96% ethanol. The DNA was stored in Tris-EDTA buffer at a concentration of 100 ng/µl.
Total cellular RNA was isolated from tissues dispersed in liquid nitrogen. It was isolated in the presence of 4 M guanidine isothiocyanate [23]. RNA was extracted twice with phenol and the RNA concentration was determined spectrophotometrically. The quality of the isolated RNA was confirmed by a horizontal agarose gel electrophoresis.
Subtractive Cloning
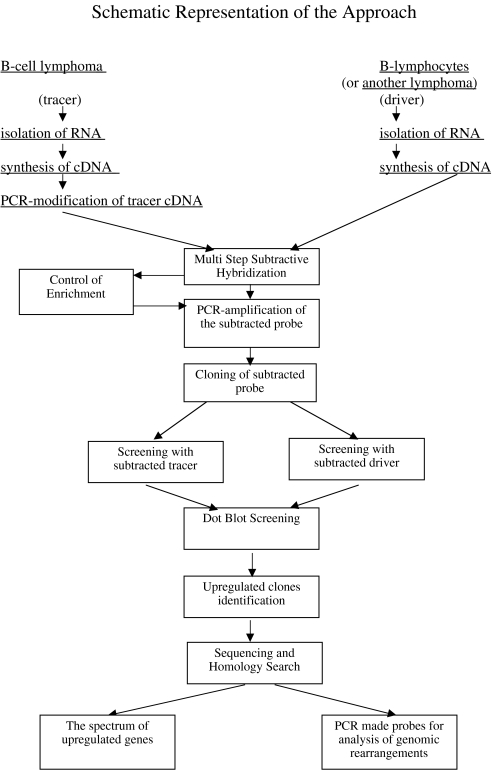
A PCR-based technique was performed. As tracer, we used RNA from lymphoma cells and as driver RNA from B lymphocytes of an uninfected human or from other types of lymphoma. A detailed protocol on how to generate cDNA libraries, isolation of lymphoma-specific cDNA by subtractive hybridization, and the differential screening was published [21,24,25]. In general, schematic representation of the approach is presented in Figure 1.
Figure 1.
Schematic representation of the approach.
PCR Analyses of Total cDNA and Genomic DNA to Detect Fused Ig-set Sequences
The PCR analyses were performed with 500 ng of genomic DNA and 30 ng of cDNA. The reaction was performed in a 20-µl volume containing 10x PCR buffer (Promega), 0.3 mM of each dNTP (Fermentas), 10 pmol of each primer, and 0.2 µ of TaqI polymerase. The following primers were used: set1: 5′-AAACAAGAGAAAGTAGACAG-3′; set2: 5′-CCTGTGTAGTAGTGTATAG-3′; set3: 5′-TGTTGGTAAATGCTAACTGTCCA-3′; set4: 5′-CTTGGCATTAGAGCACCAGG-3′; set5: 5′-GGAGCTCAACTCCAACCACGACGGG-3′; set6: 5′-CCTCTCCTAACTCATCAGCACCTGC-3′; Ig1: 5′-AATGAGGATATTTATTGGGG-3′; Ig2: 5′-TTTCATGAGTGCGGTGAGAG-3′; Ig3: 5′-GCATGTTTCCTTTCCCAATG-3′; X14: 5′-CATTCGTATTTTTTGACCCAGACC-3′. The PCR consisted of 35 cycles for the DNA analyses and 22 cycles for the cDNA.
Southern Blot Analysis
Hybridization was performed with DNA isolated from different tissues as described previously [23]. Total DNA was digested with HindIII and run on 0.8% agarose gels, transferred onto nylon filters (Hybond N, Amersham), and fixed by UV light. The 32P-labeled PCR fragment containing a part of the coding region of the set gene (primers set 5 and 6) was used as hybridization probe.
Northern Blot Analyses
About 10 µg of total cellular RNA was separated on a 1% agarose formaldehyde gel and transferred to a Hybond-N + membrane (Amersham) in 25 mM potassium phosphate (pH 6.5). Membranes with RNA were UV-irradiated and hybridized with 32P-labeled SalI fragments of cDNA clones generated by subtraction coupled with differential screening. As a control, we used a 32P-labeled β-actin PCR amplification product which is 658 bp in size [4].
Sequencing of DNA
Plasmid-cloned cDNA was sequenced as described [26] using Amersham Quick-Denature Plasmid-Sequencing Kit. Search for similarity of the subtracted sequences to known sequences was performed with the BLAST DataBase.
Results
Subtracted Lymphoma-Specific cDNA Libraries of AIDS-Associated B-Cell NHL
To perform the subtractive hybridization experiments, total RNA from tumor cells of two AIDS associated B-NHLs was isolated. According to their histomorphology and immunophenotype, over 90% of cells in these tumors was composed of B cells contaminated with a small percentage of infiltrating macrophages and T cells. Lymphoma A was classified as centroblastic and lymphoma B as immunoblastic (Table 1). RNA from peripheral blood B cells of a healthy donor was taken as control RNA. The cDNA synthesized from mRNA of lymphoma cells was used as tracer, cDNA synthesized from mRNA of normal B cells was used as driver.
Lymphoma-specific sequences were 40x enriched by the subtractive hybridization. After subtractive hybridization and subsequent PCR amplification of the subtracted material, the two libraries were constructed by cloning the enriched and amplified cDNA sequences into pBlue-ScriptSK vector.
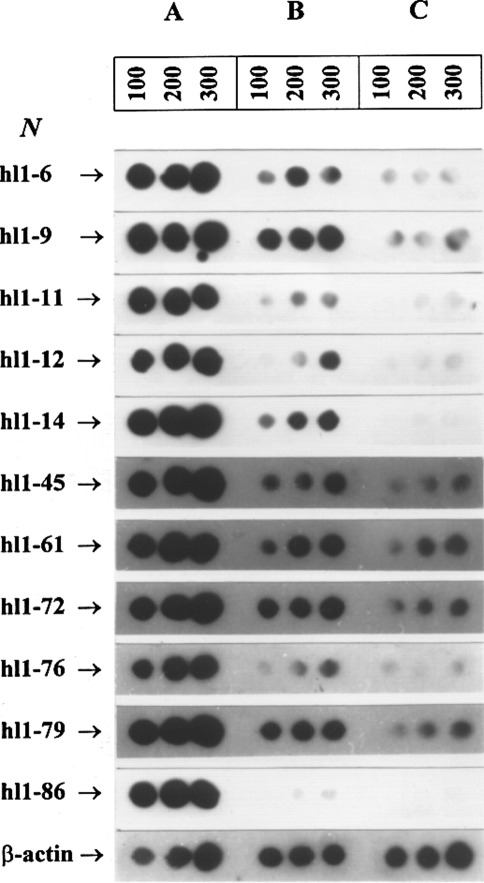
To eliminate contaminating “nonlymphoma-specific” clones in the subtracted libraries, a two-step differential screening was introduced. The first step of the screening of the subtracted cDNA library was based on the screening of colonies of transformed bacteria by hybridization [23]. At this step of the differential screening procedure, we analysed 200 and 500 clones of the subtracted cDNA libraries from lymphomas A and B, respectively. The cDNA clones selected at the first step were then used for the second step differential screening (Figure 2). Strong hybridization signals to graded amounts of subtracted cDNA indicate a high specificity of the respective clones (Figure 2A). The difference in the strength of the hybridization signals between subtracted cDNA sequences and total tracer cDNA (Figure 2A and B) corresponds to the enrichment of labeled “lymphoma-specific” cDNA sequences in the subtracted cDNA. The observed differences in the strength of hybridization signals between tracer (total cDNA from the lymphoma) and driver (total cDNA from normal B cells) indicated the differential expression level of the respective genes (Figure 2B and C). Scanning of the autoradiographic images obtained after reduced exposure times (when compared to that on Figure 2) allowed to determine semiquantitatively the upregulation of genes with this selection procedure. After the second step differential screening, we identified 21 positive clones out of 200 clones from the subtracted cDNA library of lymphoma A and 16 clones out of 500 clones from the subtracted cDNA library of lymphoma B. Thus, about 4–10% of the clones of these libraries contained cDNAs sequences representing differentially expressed genes. Most of the genes selected by this approach were upregulated by a factor of 3–14.
Figure 2.
Dot blot hybridization of the second step differential screening of the subtracted cDNA libraries: The 32P-labeled cloned cDNA sequences were hybridized to graded amounts (in ng) of total subtracted cDNA (lane A), tracer cDNA (lane B), and driver cDNA (lane C).
The size of the cloned cDNA ranged from 200 to 800 bp. These cDNA inserts were partially sequenced and the sequences were analyzed with the BLAST DataBase.
The Subtracted cDNA Library from the Centroblastic Lymphoma
Sequences lacking homology to known genes. Out of 21 sequenced lymphoma A-specific cDNA sequences, nine of them could not be easily assigned to sequences deposited in gene databases (Table 2). These cloned cDNA sequences were not unique, although some of them showed nucleotide sequence similarity to each other of up to 78%. We could not decide whether these sequence differences are caused by the error-prone PCR or are indicative of a family of closely related genes.
Table 2.
Homology Search with Differentially Expressed Subtracted cDNA of a Human Centroblastic Lymphoma (Lymphoma A).
| Clone Number | Accession Number | Size of the Sequenced Fragment (bp) | Related Gene or cDNA | Accession Number | Similarity | |
| bp | % | |||||
| hL1–1 | Y16696 | 139 | Unknown | - | - | - |
| hL1–2 | Y16715 | 161 | EST | AIO50976 | 104 | 95 |
| hL1–5 | Y16713 | 255 | Unknown | - | - | - |
| hL1–6 | Y16702 | 194 | λ2(3)IgL | X51755 | 71 | 84 |
| hL1–8 | Y16712 | 183 | Unknown | - | - | - |
| hL1–9 | Y16700 | 203 | λ1IgL-set | X51755/M93651 | 48/154 | 96/97 |
| hL1–10 | Y16708 | 272 | EST | AI377400 | 229 | 95 |
| hL1–11 | Y16697 | 108 | Unknown | - | - | - |
| hL1–12 | Y16714 | 110 | IFN-ind | U22970 | 36 | 97 |
| hL1–14 | Y16709 | 152 | set | M93651 | 152 | 97 |
| hL1–15 | Y16706 | 143 | Unknown | - | - | - |
| hL1–35 | Y16711 | 109 | Unknown | - | - | - |
| hL1–45 | Y16703 | 108 | Unknown | - | - | - |
| hL1–55 | Y16710 | 100 | Unknown | - | - | - |
| hL1–61 | Y16704 | 245 | ND-I | V00662 | 117 | 95 |
| hL1–72 | Y16701 | 199 | Unknown | - | - | - |
| hL1–76 | Y16699 | 131 | ND-IV | V00662 | 51 | 94 |
| hL1–79 | Y16707 | 129 | ND-IV | V00662 | 128 | 99 |
| hL1–86 | Y16705 | 278 | IFN-ind | U22970 | 45 | 97 |
| hL1–98 | Y16698 | 203 | λ1Ig-set | X51755/M93651 | 48/154 | 96/97 |
| hL1–101 | Y17174 | 250 | 16S rRNA | V00662 | 247 | 99 |
Clones hL1–2 and hL1–10 with lymphoma A-specific cDNAs were homologous to previously described rat and human cDNA sequences (EST), respectively.
cDNA with known sequences. Sequence similarities of lymphoma A-specific subtracted cDNA to already known genes or cDNAs are shown in Table 2. Homologous sequences were up to 250 bp in size and similarities ranged from 84% to 99%. Among these were clones homologous to the human IgL lambda 1 and 2 chain genes, to a human and monkey interferon-inducible gene (INF-ind), to the human mitochondrial NADH dehydrogenase subunits I and IV (ND-I and ND-IV) genes, and 16S rRNA genes.
Three most abundantly represented clones (hL1–9, hL1–14, and hL1–98) from the subtracted cDNA library generated from the lymphoma A gave strong differential signals. They appeared to be homologous to the 3′-untranslated region of the set oncogene [27]. The sequences of set-specific cDNAs from lymphoma A comprise a sequence of the 3′-untranslated region adjacent to the open reading frame of the set gene. A more detailed sequence analysis revealed that such a transcript could emerge in case an internal poly (A) stretch (within the 3′-untranslated region of the set mRNA) anneals with the oligo (dT) primers that initiate the cDNA synthesis.
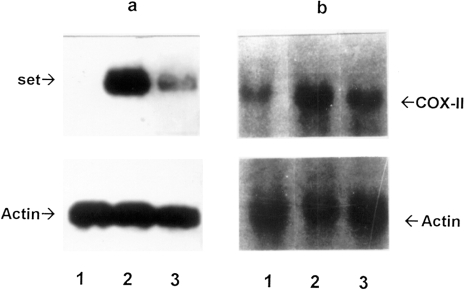
Multiple set-specific cDNA clones in the lymphoma-specific cDNA library are additional evidence for a transcriptional upregulation of this gene. To determine the relative levels of set sequence containing transcripts in lymphoma A and of those found in normal B lymphocytes and in lymph nodes, we performed Northern blot analyses with several RNA preparations (Figure 3A). The results indicated that the 3′ noncoding region of set gene is about 10-fold more abundantly transcribed in lymphoma cells than in the lymph nodes of a naive monkey; it was not detected in B lymphocytes of a naive animal under our experimental conditions.
Figure 3.
Northern blot analysis to confirm differential transcription of lymphoma-specific genes. A 32P-labeled pL1–14 (plasmid containing cDNA homologous to the 3′-untranslated region of the set gene) (a) and a PCR fragment of the COX-II gene (b) were hybridized to RNA isolated from naive normal B lymphocytes (lane 1), lymphoma A (lane 2), and lymph node (lane 3). As a control, rehybridization with a 32P-labeled PCR fragment of β-actin gene was used (on the bottom).
The Subtracted cDNA Library from the Immunoblastic Lymphoma
Each lymphoma B-specific cDNA identified by subtractive cloning is homologous to already known genes or cDNA. Sequence similarities are shown in Table 3. The homologous sequences were up to 349 bp long and have sequence similarities of 92–99%. Each of the 16 cDNA clones analyzed so far represents sequences of mitochondrial genes. They code for 16S rRNA, cytochrome c oxidase II (COX-II), ATP synthase VI (AS-VI), ND-IV, and cytochrome b (CYTb). The clones from the subtracted lymphoma B cDNA library with sequence homologies to the ND-IV and 16S rRNA genes were overrepresented (five and seven cDNA clones, respectively) and both the 16S rRNA and ND-IV cDNA were also present in the subtracted, lymphoma A-specific cDNA library (Table 2).
Table 3.
Homology Search with Differentially Expressed Subtracted cDNA of a Human Immunoblastic Lymphoma (Lymphoma B).
| Clone Number | Accession Number | Size of the Sequenced Fragment (bp) | Related Gene or cDNA | Accession Number | Similarity | |
| bp | % | |||||
| hL2–201 | Y17170 | 422 | 16S rRNA | V00662 | 349 | 99 |
| hL2–222 | Y17172 | 230 | ND-IV | V00662 | 175 | 98 |
| hL2–229 | Y17171 | 364 | AS-VI | V00662 | 328 | 99 |
| hL2–253 | Y17173 | 329 | ND-IV | V00662 | 194 | 99 |
| hL2–254 | Y17174 | 243 | 16S rRNA | V00662 | 242 | 98 |
| hL2–264 | Y17176 | 259 | ND-IV | V00662 | 246 | 98 |
| hL2–274 | Y17175 | 196 | COX-II | V00662 | 180 | 97 |
| hL2–378 | Y17177 | 95 | CYTb | V00662 | 78 | 92 |
| hL2–383 | Y17178 | 298 | ND-IV | V00662 | 296 | 98 |
| hL2–494 | Y17179 | 264 | AS VI | V00662 | 264 | 95 |
| hL2–498 | Y17180 | 265 | ND-IV | V00662 | 208 | 98 |
| hL2–500 | Y17170 | 227 | 16S rRNA | V00662 | 225 | 99 |
| hL2–501 | Y17170 | 302 | 16S rRNA | V00662 | 299 | 99 |
| hL2–502 | Y17170 | 205 | 16S rRNA | V00662 | 203 | 99 |
| hL2–503 | Y17170 | 274 | 16S rRNA | V00662 | 271 | 99 |
| hL2–504 | Y17170 | 255 | 16S rRNA | V00662 | 252 | 99 |
Abundantly Transcribed Genes Common to Both Lymphomas
Bearing in mind that subtractive cloning reveals only that limited set of genes changes significantly its transcription levels, we performed dot blot hybridization to look for transcriptional upregulation of mitochondrial genes in both lymphomas. To determine the abundant transcription of several genes in lymphomas A and B, we took as probes the cDNA clones hL2-274 (COX-II), hL2-229 (AS-VI), hL2–378 (CYTb) and hL1–98 (set). As a control, we used a PCR fragment from the mitochondrial ND-II gene — an apparently not overexpressed gene in both lymphomas gene (has not been detected by subtractive cloning). Each probe was hybridized to graded cDNA amounts in the same way as it was done during the second step differential screening (see Figure 2). The results of these experiments are shown in Table 4. It became evident that the mitochondrial 16S rRNA, COX-II, and ND-IV genes are abundantly transcribed in both lymphomas and thus may be essential for malignization. In contrast, the upregulation of the AS-VI and CYTb genes seemed to be specific for lymphoma B only. There was no detectable upregulation of the ND-II gene in both lymphomas.
Table 4.
Upregulated Mitochondrial Gene Transcription in Two AIDS-Associated Human NHL Detected by Dot Blot Hybridization*.
| Genes/Probes | Centroblastic (Lymphoma A) | Immunoblastic (Lymphoma B) |
| 16S rRNA | + | + |
| Cytochrome c oxidase II | + | + |
| ATP synthase VI | - | + |
| Cytochrome b | - | + |
| NADH dehydrogenase I | + | + |
| NADH dehydrogenase IV | + | + |
| NADH dehydrogenase II | - | - |
As in Figure 1.
(+) Upregulation; (-) no changes.
The results of the dot blot hybridizations were confirmed by Northern blot analyses with subtracted cDNA clones as probes; results for the COX-II gene transcription in lymphoma A are shown in Figure 3B.
Abundantly Transcribed Genes Different in Two Lymphomas
Apart from the genes overexpressed in both lymphomas, we have found a number of genes overexpressed only in the centroblastic lymphoma (see Tables 2 and 3). In order to demonstrate the real difference between two types of lymphomas and to estimate the adequacy and potentials of the subtractive hybridization method applied, we have carried out an additional subtraction between cDNA from the centroblastic lymphoma as tracer and cDNA from the immunoblastic lymphoma as driver. The data obtained are presented in Table 5. It can be seen that the spectrum of genes overexpressed in lymphoma A as compared to lymphoma B is similar to that revealed earlier on closer examination of the overexpressed genes spectra in these lymphomas obtained by subtractive hybridization with cDNA of B lymphocytes as driver (Tables 2 and 3). The subtractions revealed that the INF-ind gene, the set gene, the IgL genes, and the ND-I and ND-IV genes are upregulated in the centroblastic lymphoma as compared to the immunoblastic one. In addition, two new genes [the interleukin 4 receptor (ILR4) gene and the ABC transporter protein 2 located in human MHC class II (TAP2) gene] and some unknown genes were revealed.
Table 5.
Homology Search with Differentially Expressed Subtracted cDNA of a Human Centroblastic Lymphoma (Lymphoma A) in Comparison with Immunoblastic Lymphoma (Lymphoma B).
| Clone Number | Size of the Sequenced Fragment (bp) | Related Gene or cDNA | Accession Number | Similarity | |
| bp | % | ||||
| hss1–204 | 137 | ND-I | V00662 | 84 | 83 |
| hss1–206 | 107 | Unknown | - | - | - |
| hss1–209 | 88 | Unknown | - | - | - |
| hss1–219 | 144 | set | M93651 | 128 | 96 |
| hss1–221 | 98 | ND-IV | V00662 | 47 | 100 |
| hss1–225 | 105 | λIgL | X51755 | 54 | 100 |
| hss1–250 | 143 | set | M93651 | 139 | 97 |
| hss1–254 | 161 | ND-IV | V00662 | 161 | 95 |
| hss1–255 | 171 | set | M93651 | 147 | 97 |
| hss1–257 | 65 | set | M93651 | 65 | 95 |
| hss1–301 | 33 | IFN-ind | U22970 | 26 | 100 |
| hss1–316 | 77 | IL4R | X52425 | 55 | 92 |
| hss1–328 | 128 | TAP2 | U07844 | 94 | 95 |
| hss1–332 | 256 | Unknown | - | - | - |
Analysis of Fused Transcripts Containing Partial Sequences of the Set Gene
Sequencing of the cloned set-like cDNAs (lymphoma A) revealed that apart from 154 bp of the 3′ region of the set gene, some clones contained adjacent sequences not related to the set gene (Figure 4). In two cDNA clones hL1–9 and hL1–98, the set-like sequence was fused to a sequence approximately 50 bp in size having high similarity to the 3′ terminal part of the IgL lambda gene.
Figure 4.
Nucleotide sequences of lymphoma A-specific cDNA clones consisting of set and IgL gene sequences. Sequences of the cDNA clones hL1–9 and hL1–98 have similarity to the C-region of the IgL gene and to the 3′ untranslated region of the set gene (shaded area). Base pairs, which are different in cDNA clones, and the set and IgL genes are printed in bold. Primer pair set1 and set2 detects only set sequences; primer pair set1 and Ig1 detects fusion products of the IgL gene and the set gene.
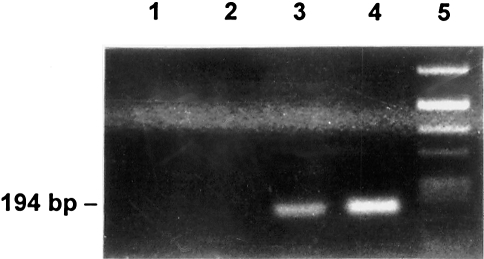
To detect cloning artefacts, we repeatedly prepared total cDNA from lymphomas A and B and from nonmalignant B cells. These preparations were then analyzed by a site-specific PCR designed to detect fusions between the IgL- and set-specific sequences. We obtained the expected 194-bp amplification product only with cDNA from lymphoma A using the set1 and Ig1 primers (Figure 5, lane 3) confirming the presence of this fused mRNA. With the set1 and X14 primers, which should detect the fused sequence present in clone hL1–14, no amplification product was found neither in lymphomas A and B cDNAs nor in normal B cell cDNA (data not shown). Therefore, it is likely that the cDNA of clone hL14 is a cloning artefact. Thus, only lymphoma A contained an mRNA species combining the 3′-untranslated regions of the IgL and set genes.
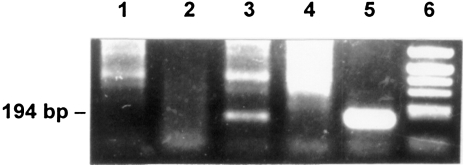
Figure 5.
PCR analyses to detect a fused set-IgL cDNA: A primer pair amplifying a set-IgL fusion was derived from the cDNA clone hL1–9 (set1 and Ig1, see Figure 3). The PCR was performed with total cDNA from normal B lymphocytes (lane 1), from lymphoma B (lane 2), from lymphoma A (lane 3), and with cDNA of clone hL1–98 as a positive control (lane 4). Lane 5, molecular weight marker (Alul digest of pBR322).
A genomic rearrangement in the centroblastic lymphoma is responsible for the fusion of the IgL and set genes. Clones hL1–9 and hL1–98 contained fused sequences. This finding prompted us to obtain evidence for a genomic rearrangement between parts of the set gene and the IgL gene in lymphoma cells. To do this, we performed a site-directed PCR analysis with genomic DNA with the set1 and Ig1 primers located within the set gene and the IgL gene, respectively. The amplification product, 194 bp in size, was synthesized only with genomic DNA of lymphoma A (Figure 6, lane 3). The PCR performed with genomic DNA of lymphoma B, nonmalignant B cells, and spleen tissue did not result in the expected 194-bp amplification product. The detection of such a highly specific amplification product synthesized from genomic DNA of lymphoma A is clear evidence for a chromosomal rearrangement in this lymphoma.
Figure 6.
PCR analyses to detect a fused IgL-set sequence in genomic DNA. To confirm a genomic rearrangement, we used the same primer pair as in the experiments shown in Figure 5. The PCR was performed with genomic DNA from normal B lymphocytes (lane 1), from lymphoma A (lane 3), from lymphoma B (lane 4), and from spleen as a negative control (lane 2). As a positive control, clone hL98 was included (lane 5). Molecular weight marker: Alul digest of pBR322 (lane 6).
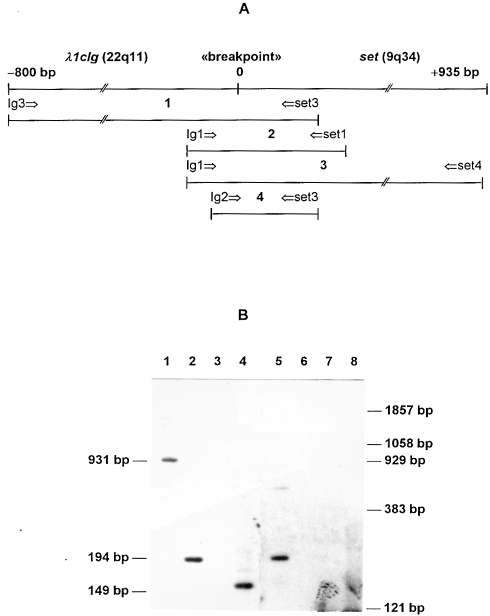
Having the sequences of the C-region of the Ig lambda 1 gene and the set mRNA in mind (the complete set gene sequence is unknown), we constructed several pairs of primers for PCR amplification of sequences spanning the region of the genomic rearrangement in DNA of lymphoma A (Figure 7). The primers were derived from the putative fused DNA sequence, i.e., in each primer pair, one primer was specific for the C-region of the Ig lambda 1 gene whereas the other one was specific for the set mRNA region. After amplification and subsequent gel electrophoresis coupled with a Southern hybridization using labeled 194-bp PCR fragment (a PCR with the Ig1-set1 primer pair), several predictable bands were observed (931-bp PCR fragment with the Ig3-set3 primer pair, lane 1; 194-bp PCR fragment with the Ig1-set1 primer pair, lane 2; 149-bp PCR fragment with the Ig2-set3 primer pair, lane 4). These bands are characteristic for the observed fused chromosomal DNA region and led to a more precise and detailed information about the genome translocation in lymphoma. In case of B lymphocytes, expected bands were absent.
Figure 7.
(A) The putative genomic fusion sequence. The IgL-set sequence, the localization of the breakpoint, and the diagnostic PCR primers. The expected PCR products and corresponding primer pairs are indicated: 1, Ig3-set3; 2, Ig1-set1; 3, Ig1-set4; 4, Ig2-set3. (B) PCR analyses and Southern blot hybridization. PCR was performed on genomic DNA of lymphoma A (lanes 1–4) and normal B lymphocytes (lanes 6–8). The hybridization was performed with the 32P-labeled PCR fragment (primers Ig1 and set1) amplified from the hL1–98 clone. Lanes 1 and 6, Ig3-set3 primer pair; lanes 2 and 7, Ig1-set1 primer pair, lane 3, Ig1-set4 primer pair, lanes 4 and 8, Ig2-set3 primer pair. Lane 5 contains a positive control (PCR fragment Ig1-set1). Molecular weight marker: Alul digest of pBR322.
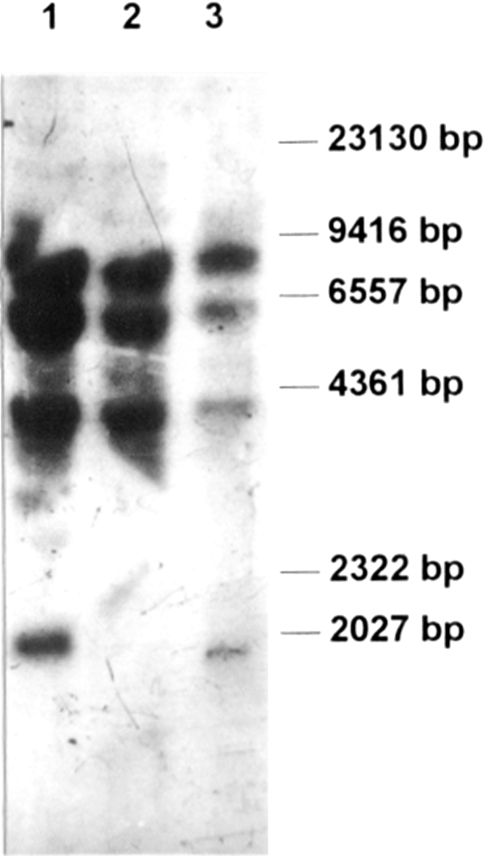
To further characterize this genome rearrangement, a Southern blot analysis of genomic DNA of both lymphomas and B lymphocytes was performed (Figure 8). The 587-bp PCR fragment corresponding to a coding region of the set mRNA (the set4–set5 primer pair and DNA from B lymphocytes) was used as a labeled probe. The Southern blot analysis revealed three major hybridization signals corresponding to common genome fragments of all three DNA preparations. There is also a low molecular weight fragment seen only with DNA of lymphoma B and B lymphocytes, but not with DNA of lymphoma A. To interpret the obtained hybridization patterns, we made a computer search for set-like DNA sequences in the BLAST DataBase. With sequences in the database, we searched for HindIII fragments correlating to the restriction pattern shown in Figure 8. Indeed, we found several genomic sequences whose HindIII fragments were similar in size to the fragments found; i.e., a 8059-bp fragment of chromosome 17 (acc. no. 005666); a 8059-bp DNA fragment of X chromosome (acc. no. Z95126); a 5426-bp DNA fragment of 21 chromosome (acc. no. 004106); and a DNA fragment with an approximate size of 4400 bp of chromosome 3. Although we performed the computer search with updated databases, we did not find any homology to the set-like fragment of approximately 2000 bp, which had not been observed in DNA of lymphoma A. We concluded that this fragment is related to a set sequence, which is not yet available. The absence of the 2000-bp fragment in lymphoma A may also be indicative of a genomic rearrangement leading to the loss of a HindIII restriction site(s).
Figure 8.
Southern blot hybridization of HindIII restricted genomic DNA. DNA isolated from naive B lymphocytes (lane 1), lymphoma A (lane 2), and lymphoma B (lane 3) were hybridized with a 32P-labeled PCR fragment produced by amplification with the set4–set5 primers located inside of the coding sequence of the set gene. Marker, lambda DNA/HindIII (lane 4).
Discussion
Our studies aimed at the detection of specifically expressed or upregulated genes in AIDS-associated B-NHL. We used a full-scale subtractive cloning procedure to obtain a broadened spectrum of genes preferably expressed in lymphomas.
In the centroblastic lymphoma, we detected cDNA clones containing sequences homologous to several known nuclear and mitochondrial genes. Among the lymphoma-specific cDNA clones, eight proved to have no sequence homology to any known sequence in the GenBank database. They may represent novel genes whose transcription was upregulated only in human lymphoma tested. Interestingly, some of these clones share up to 78% sequence identity. However, we cannot yet decide whether these genes are variants of one single gene or are closely related unique genes. Furthermore, we identified two lymphoma-specific cDNA clones (hL1–2 and hL1–10) containing substantial sequence homology with ESTs.
Among the upregulated genes in the AIDS-associated lymphoma A, we detected the INF-ind gene (clones hL1–12 and 86). An enhanced expression of this INF-ind gene had been found to be specifically transcribed in liver tissue of a hepatitis C virus-infected chimpanzee [28,29] and in B-NHL of SIV-infected monkeys [21]. According to recent data, IFN-ind genes have been previously implicated in the regulation of cell growth and malignant transformation [30]. A recently discovered INF-induced factor represses the expression from the HIV-1 long terminal repeat [31].
In both the centroblastic and the immunoblastic AIDS-associated lymphomas, we detected several upregulated mitochondrial genes. The overexpression of the mitochondrial COX-II, ND-IV, and 16S rRNA genes in both lymphomas is remarkable. The detection of cDNA having homology to the 16S rRNA may be due to a fused transcript encoding parts of ND-I and 16S rRNA (these genes are located nearly in mitochondrial genome). Such transcripts have been already detected in cancer cells [9].
Analyses of genes differentially expressed in different types of malignant cells have already revealed altered transcription levels of several mitochondrial genes, including the ND-IV and the COX-II genes [9–11]. Similarly, we also have detected such an abundant transcription of these mitochondrial genes in SIV-associated monkey lymphomas [21]. It is important that ND-II gene used as a control was not overexpressed. It indicates that the observed enhanced expression of some mitochondrial genes' subunits was not due to the increased number of mitochondria in metabolically active cells.
Our experimental findings concerning SIV- and HIV-associated lymphomas, as well as current reports, let us suppose that the lymphomagenesis as well as cancerogenesis in general may be linked to a modulated synthesis of some enzymes belonging to the oxidative phosphorylation pathways or their subunits.
As to EBV- and HIV-specific sequences, we were not able to select any corresponding cDNA by our subtractive technique although EBNA2 and p24 proteins, respectively, were detectable by immunohistochemistry (Table 1). Perhaps, the abundance of these viral mRNA species in lymphoma cells is extremely low and the number of clones analyzed so far is not sufficient to reveal them.
Special experiments revealed that 1) the differences between the spectra of the genes overexpressed in the two lymphomas found using earlier versions of subtraction are reliable; and 2) the method applied here provides a comprehensive spectrum of upregulated genes in the lymphomas.
A high percentage of cDNA clones in the subtracted library of lymphoma A contains an IgL-set gene fusion sequence. According to differential screening and analyses of cDNA, the set gene appeared to be only slightly upregulated in lymphoma B. Quite similar to the set gene expression in HIV-associated lymphomas, we detected an upregulation of the set gene in diffuse large B cell monkey lymphomas associated with SIV [21]. There is no conclusive evidence for any function of the set gene in these lymphomas. Its gene product is a 39-kDa nuclear phosphoprotein playing a role in DNA replication and inhibition of protein phosphatase 2A [32,33].
One of the most important findings concerning lymphoma A is that set gene-like sequence appears to be rearranged. The chromosomal breakpoints are supposed to be located 3′ to the Cλ1 region of the IgL gene and in the 3′-untranslated region of the set gene. This primary genetic event may be analogous to the IgL gene sequences translocated into the c-myc gene [7,34]. Previously, part of the set gene was found to be fused to the can oncogene in acute undifferentiated leukemia cells by a chromosomal translocation [27]. Our study demonstrated for the first time the existence of elevated levels of fused transcripts containing a 3′-untranslated element of the set-like gene and the IgL gene in HIV associated B-NHL.
Chromosomal translocations involving different loci are already recognized as common specific cytogenetic abnormalities in lymphoma cells [3]. Studying such translocations allowed to identify several genes involved in lymphomagenesis, e.g., bcl-6 [35], ALK and NPM [5], cycline D [36]. It is known that the gene locus of the lambda-chain of Ig is prone to genetic rearrangements in follicular lymphomas [7,32]. Since the set gene is located on chromosome 9 (q34) and the IgL gene on chromosome 22 (q11), the sequence identified may result from a t (9;22) (q34;q11) translocation event. Unfortunately, about 10 sequences with similarity to the 3′-untranslated region of the set gene are supposed to be present in the human genome [27]. Thus, further studies are needed to confirm the exact site and chromosome where the rearrangement took place. Nevertheless, our subtractive hybridization technique allowed us to detect a genomic rearrangement never observed previously with less sophisticated methods. Recently, molecular studies of the Philadelphia-like translocation t(9;22) (q34;q11) in a follicular lymphoma indicated an IgL-mediated rearrangement of an unknown gene at 9q34 that may be involved in the lymphomagenesis [37]. According to our data, the unknown gene sequence might belong to one of the multiple set gene copies.
The genomic rearrangement of the set and the IgL genes may be a primary genetic event causing the high level of fused transcripts in the centroblastic lymphoma A. In immunoblastic lymphoma B, there was no evidence for an IgL-set translocation, although set gene-containing transcripts were present.
Conclusion
We succeeded in identifying a panel of genes not yet reported to be differentially expressed in AIDS-associated or in other human NHLs. Moreover, we have detected a unique genomic rearrangement between set gene-like sequences and the IgL gene in a centroblastic AIDS-associated NHL. However, further studies are necessary to determine whether these genes contribute to lymphoma development and can be used for lymphoma classification or as therapeutic targets.
Acknowledgements
We thank I. Schedel (Medical School Hanover) for providing biopsy specimens from two AIDS-associated NHL.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- AS
ATP synthase
- B-NHL
B-cell non-Hodgkin's lymphoma
- COX
cytochrome c oxidase
- CYTb
cytochrome b
- IgL
immunoglobulin light chain
- INF-ind
interferon-inducible gene
- ND
NADH dehydrogenase
- IL4R
interleukin 4 receptor
- TAP2
ABC transporter protein 2 located in human MHC class II
- PCR
polymerase chain reaction
- SIV
simian immunodeficiency virus
- EBV
Epstein-Barr virus
- HIV
human immunodeficiency virus
Footnotes
This work was supported by the Volkswagen Foundation (grant I/69398 to W.B.) and the Foundation of Russian Ministry of Sciences.
References
- 1.Levine AM. AIDS-related malignancies: the emerging epidemic. J Natl Cancer Res. 1993;85:1382–1397. doi: 10.1093/jnci/85.17.1382. [DOI] [PubMed] [Google Scholar]
- 2.Herndier BG, Kaplan LD, McGrath MS. Pathogenesis of AIDS lymphomas. AIDS. 1994;8:1025–1049. doi: 10.1097/00002030-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Gaidano G, Pastore C, Gloghini A, Volpe G, Ghia P, Saglio G, Carbone A. AIDS-related non-Hodgkin's lymphomas: molecular genetics, viral infection and cytokine deregulation. Acta Hematol. 1996;95:193–198. doi: 10.1159/000203877. [DOI] [PubMed] [Google Scholar]
- 4.Hannig H, Motz-Rensing K, Kuhn EM, Stahl-Hennig C, Kaup FJ, Hunsmann G, Bodemer W. Cytokine gene transcription in SIV and HIV-associated non-Hodgkin's lymphomas. AIDS Res Hum Retroviruses. 1997;13:1589–1596. doi: 10.1089/aid.1997.13.1589. [DOI] [PubMed] [Google Scholar]
- 5.de Boer CJ, van Krieken JH, Kluin-Nelemans JC, Kluin PM, Schuuring E. Cyclin D messenger RNA overexpression as a marker for mantle cell lymphoma. Oncogene. 1995;10:1833–1840. [PubMed] [Google Scholar]
- 6.Morris SW, Kirstein MN, Valentine MB, Dittmer K, Shapiro DN, Look AT, Saltman DL. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1995;267:316–317. doi: 10.1126/science.267.5196.316-b. [DOI] [PubMed] [Google Scholar]
- 7.Drexler HG, MacLeod RA, Borhardt A, Janssen JW. Recurrent chromosomal translocations and fusion genes in leukemia-lymphoma cell lines. Leukemia. 1995;9:480–500. [PubMed] [Google Scholar]
- 8.Dyer MJS, Lillington DM, Bastard C, Tilly H, Lens D, Heward JM, Stranks G, Morilla R, Monard S, Guglielmi P, Kluin-Nelemans JC, Hagemeijer A, Young BD, Catovsky D. Concurrent activation of MYC and BCL 2 in B cell non-Hodgkin lymphoma cell lines by translocation of both oncogenes to the same immunoglobulin heavy chain locus. Leukemia. 1996;10:1198–1208. [PubMed] [Google Scholar]
- 9.Yamamoto A, Horai S, Yuasa Y. Increased level of mitochondrial gene expression in polyps of familial polyposis coli patients. Biochem Biophys Res Commun. 1989;159:1100–1106. doi: 10.1016/0006-291x(89)92222-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang F-L, Wang Y, Wong W-K, Liu Y, Addivinola J, Liang P, Chen LB, Kantoff PW, Pardee AB. Two differentially expressed genes in normal human prostate tissue and in carcinoma. Cancer Res. 1996;56:3634–3637. [PubMed] [Google Scholar]
- 11.Kornacker M, Merz W, v Kalle C, Ehlers NA, Jox A, Tesch H, Diehl V, Wolf J. Identification of differentially expressed genes in Hodgkin's-derived B-cell lines by differential display RT-PCR. QIAGEN News. 1996;4:12–13. [Google Scholar]
- 12.Gaidano G, Dalla-Favera R. Protooncogenes and Tumor Suppressor genes. In: Knowles DM, editor. Neoplastic Hematopathology. Baltimore: Williams and Wilkins; 1992. pp. 245–261. [Google Scholar]
- 13.Delecluse HJ, Hummel M, Marafioti T, Anagnostopoulos I, Stein H. Common and HIV-related diffuse large B-cell lymphomas differ in their immunoglobulin gene mutation pattern. J Pathol. 1999;188:133–138. doi: 10.1002/(SICI)1096-9896(199906)188:2<133::AID-PATH349>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Ioachim HL, Antonescu C, Giancotti F, Dorsett B. EBV-associated primary lymphomas in salivary glands of HIV-infected patients. Pathol Res Pract. 1998;194:87–95. doi: 10.1016/S0344-0338(98)80075-7. [DOI] [PubMed] [Google Scholar]
- 15.Laurence J, Astrin SM. Human immunodeficiency virus induction of malignant transformation in human B lymphocytes. Proc Natl Acad Sci USA. 1991;8:7635–7639. doi: 10.1073/pnas.88.17.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrallini A, Altavilla G, Pozzi L, Bignozzi L, Negrini M, Rimessi P, Gualandi F, Barbanti-Brodano G. Systemic expression of HIV-1 tat gene in transgenic mice induced endothelial proliferation and tumors of different histotypes. Cancer Res. 1993;53:5569–5575. [PubMed] [Google Scholar]
- 17.Chirivi RG, Taraboletti G, Bani MR, Barra L, Piccinini G, Giacca M, Bussolino F, Giavazzi R. Human immunodeficiency virus-1 (HIV-1) Tat protein promotes migration of AIDS-related lymphoma cells and enhances their adhesion to endothelial cells. Blood. 1999;95:1747–1754. [PubMed] [Google Scholar]
- 18.Copie-Bergman C, Gaulard P, Maouche-Chretien L, Briere J, Haioun C, Alonso MA, Romeo PH, Leroy K. The MAL gene is expressed in primary mediastinal large B-cell lymphoma. Blood. 1999;15:3567–3575. [PubMed] [Google Scholar]
- 19.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever Mr, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 20.Wellmann A, Thieblemont C, Pittaluga S, Sakai A, Jaffe ES, Siebert P, Raffeld M. Detection of differentially expressed genes in lymphomas using cDNA arrays: identification of clustering as a new diagnostic marker for anaplastic large cell lymphomas. Blood. 2000;96:398–404. [PubMed] [Google Scholar]
- 21.Tarantul VZ, Nikolaev Al, Martinenko A, Hannig H, Hunsmann G, Bodemer W. Differential gene expression in B-cell non-Hodgkin's lymphoma of SIV-infected monkey. AIDS Res Hum Retro-viruses. 2000;16:173–179. doi: 10.1089/088922200309511. [DOI] [PubMed] [Google Scholar]
- 22.Pingel S, Hannig H, Motz-Rensing K, Kaup FJ, Hunsmann G, Bodemer W. Detection of Epstein-Barr virus small RNAs EBER1 and EBER2 in lymphomas of SIV-infected rhesus monkeys by in situ hybridisation. Int J Cancer. 1997;72:160–164. doi: 10.1002/(sici)1097-0215(19970703)72:1<160::aid-ijc23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. 1989 Cold Spring Harbor Laboratory Press. [Google Scholar]
- 24.Belyavsky A, Vinogradova T, Raevsky K. PCR-based cDNA library construction. General cDNA libraries at the level of a few cells. Nucleic Acids Res. 1989;17:2919–2932. doi: 10.1093/nar/17.8.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisitsyn NA, Lisitsyn N, Wigler M. Cloning the differences between two complex genomes. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Lindern M, van Baal S, Wiegant J, Raap A, Hagemeijer A, Grosveld G. Can a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol Cell Biol. 1992;12:3346–3355. doi: 10.1128/mcb.12.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Esumi M, Yamashita S, Abe K, Shikata T. Interferon-inducible gene expression in chimpanzee liver infected with hepatitis C virus. Virology. 1992;190:856–860. doi: 10.1016/0042-6822(92)90925-f. [DOI] [PubMed] [Google Scholar]
- 29.Kelly JM, Gilbert CS, Stark GR, Kerr IM. Differential regulation of interferon-induced mRNAs by alpha- and gamma-interferons. Eur J Biochem. 1985;153:367–371. doi: 10.1111/j.1432-1033.1985.tb09312.x. [DOI] [PubMed] [Google Scholar]
- 30.Barber GN, Jagus R, Meurs EF, Hovanessian AG, Katze MG. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon induced enzyme RNA-dependent protein kinase. J Biol Chem. 1995;270:17423–17428. doi: 10.1074/jbc.270.29.17423. [DOI] [PubMed] [Google Scholar]
- 31.Tissot C, Mechti N. Molecular cloning of a new interferon-induced factor that represses human immunodeficiency virus type 1 long terminal repeat expression. J Biol Chem. 1995;270:14891–14898. doi: 10.1074/jbc.270.25.14891. [DOI] [PubMed] [Google Scholar]
- 32.Adachi Y, Pavlakis GN, Copeland TD. Identification of in vivo phosphorylation sites of SET, a nuclear phosphoprotein encoded by the translocation breakpoint in acute undifferentiated leukemia. FEBS Lett. 1994;340:231–235. doi: 10.1016/0014-5793(94)80144-4. [DOI] [PubMed] [Google Scholar]
- 33.Nagata K, Kawase H, Handa H, Yano K, Yamasaki M, Ishimi Y, Okuda A, Kikuchi A, Matsumoto K. Replication factor encoded by a putative oncogene SET, associated with myeloid leukemogenesis. Proc Natl Acad Sci USA. 1995;92:4279–4283. doi: 10.1073/pnas.92.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Showe LC, Moore RCA, Erikson J, Croce CM. MYC oncogene involved in a t(8;22) chromosome translocation is not altered in its putative regulatory regions. Proc Natl Acad Sci USA. 1987;94:2824–2828. doi: 10.1073/pnas.84.9.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muramatsu M, Akasaka T, Kadowaki N, Ohno H, Yamabe H, Edamura S, Dor S, Mori T, Okuma M, Fukuhara S. Rearrangement of the BCL6 gene in B-cell lymphoid neoplasms: comparison with lymphomas associated with BCL2 rearrangement. Br J Hematol. 1996;93:911–920. doi: 10.1046/j.1365-2141.1996.d01-1728.x. [DOI] [PubMed] [Google Scholar]
- 36.Chesi M, Bergsagel PL, Brents LA, Smith CM, Gerhard DS, Kuehl WM. Disregulation of cyclin D1 by translocation into an IgH gamma switch region in two multiple myeloma cell lines. Blood. 1996;88:674–681. [PubMed] [Google Scholar]
- 37.Wlodarska I, Pittaluga S, Stul M, Martiat P, Dierlamm J, Michaux L, De Wolf-Peeters C, Cassiman JJ, Mecucci C, Van der Berghe H. Philadelphia-like translocation t(9;22) (q34;q11) found in a follicular lymphoma involving not BCR and ABL but IgL-mediated rearrangement of an unknown gene on 9q34. Genes, Chromosomes Cancer. 1997;20:113–119. doi: 10.1002/(sici)1098-2264(199710)20:2<113::aid-gcc2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]