Abstract
A competitive-inhibition enzyme-linked immunosorbent assay (cELISA) was evaluated for the detection of serum antibodies to the surface envelope (SU) of caprine arthritis-encephalitis virus (CAEV) in goats. This assay utilized 96-well microtiter plates containing CAEV-63 SU captured by monoclonal antibody (MAb) F7-299 and measured the competitive displacement of horseradish peroxidase-conjugated MAb GPB 74A binding by undiluted goat sera (F. Özyörük, W. P. Cheevers, G. A. Hullinger, T. C. McGuire, M. Hutton, and D. P. Knowles, Clin. Diagn. Lab. Immunol. 8:44-51, 2001). Two hundred serum samples from goats in the United States were used to determine the sensitivity and specificity of cELISA based on the immunoprecipitation (IP) of [35S]methionine-labeled viral antigens as a standard of comparison. A positive cELISA was defined as >33.2% inhibition of MAb 74A binding based on 2 standard deviations above the mean percent inhibition of 140 IP-negative serum samples. At this cutoff value, there were 0 of 60 false-negative sera (100% sensitivity) and 5 of 140 false-positive sera (96.4% specificity). Additional studies utilized IP-monitored cELISA to establish a CAEV-free herd of 1,640 dairy goats.
Caprine arthritis-encephalitis virus (CAEV) is a lentivirus which causes arthritis and mastitis in goats (3). In the United States, the prevalence of CAEV infection has been reported to be as high as 81%, as defined by agar gel immunodiffusion (AGID) with CAEV as the antigen (5). A majority of CAEV-infected goats are lifelong carriers without clinical signs but are potentially capable of transmitting CAEV, primarily through colostrum and milk (1, 14). Therefore, accurate diagnostic tests for CAEV are needed for successful eradication.
Four monoclonal antibodies (MAb) to the conformation-dependent epitopes of the gp135 surface envelope (SU) of the 79-63 isolate of CAEV were previously described (13). Additional studies (13) determined that sera from infected goats could block the binding of MAb to viral SU for possible use in a competitive-inhibition enzyme-linked immunosorbent assay (cELISA). Horseradish peroxidase-conjugated MAb GPB 74A was selected for detailed studies based on binding assays using SU applied directly to or captured on microtiter plates with MAb F7-299. As expected, sera from goats infected with homologous CAEV-63 inhibited the binding of MAb 74A to CAEV SU. Sera from goats infected with heterologous CAEV-Co also inhibited MAb 74A binding, demonstrating the potential utility of this assay for the evaluation of field sera.
In the present study, 200 goat sera from CAEV-positive herds in the United States were used to evaluate the sensitivity and specificity of cELISA. The standard of comparison was the immunoprecipitation (IP) of [35S]methionine-labeled CAEV, which detects antibodies to all viral structural proteins (6, 9). Additional studies utilized cELISA monitored by IP to establish a CAEV-free dairy goat herd maintained by GTC Biotherapeutics.
MATERIALS AND METHODS
Goat sera.
Two hundred serum samples selected from CAEV-positive goat herds in the United States were obtained by VMRD, Inc., Pullman, Wash. Serum samples were also obtained from all of the goats comprising a dairy herd of Saanen, Alpine, and Toggenburg goats maintained by GTC Biotherapeutics. This herd initially included 557 animals and was expanded to 1,640 animals during the course of this study.
Experimentally infected goats.
Some experiments utilized sera from goats experimentally infected with CAEV. For these experiments, eight yearling goats from a CAEV-free Saanen herd maintained at Washington State University were inoculated intravenously with 104 50% tissue culture infective doses (TCID50) of CAEV-Co. Virus was derived from an infectious molecular clone of CAEV-Co provirus (15). Goat synovial membrane (GSM) cells were transfected with proviral DNA, and syncytia were noted 2 weeks posttransfection. GSM cells were inoculated with transfection supernatant and incubated for 12 days to produce a virus stock. The virus stock contained 8.4 × 106 TCID50 of virus/ml determined by infectivity titration in GSM cells (8). For inoculation of goats, the virus stock was diluted in Dulbecco minimal essential medium to contain 104 TCID50/ml.
cELISA.
Sera were evaluated for anti-CAEV SU antibodies by using a CAEV cELISA antibody test kit (VMRD, Inc.). The CAEV cELISA test kit utilizes 96-well microtiter plates containing CAEV-63 SU captured by MAb F7-299 and measures the displacement of horseradish peroxidase-conjugated MAb GPB 74A binding by undiluted goat sera. Each test kit included positive and negative goat sera verified by the IP of [35S]methionine-labeled CAEV antigens (see below). Results were expressed as the percent inhibition of MAb GPB74A binding calculated by [(1 − OD620 of test sample)/(OD620 of negative plate control)] × 100, where OD620 is the optical density at 620 nm (13). Anti-CAEV SU antibody titers were determined by end point cELISA reactivity with serial twofold or fivefold dilutions of goat sera. End points were extrapolated by linear regression analysis of percent inhibition plotted against serum antibody dilutions.
IP.
Serum antibodies to CAEV structural antigens were detected by IP of the lysates of CAEV labeled with [35S]methionine followed by electrophoresis in polyacrylamide gel electrophoresis (PAGE) gels containing sodium dodecyl sulfate (SDS). Production of labeled CAEV in GSM cells in the presence of [35S]methionine, preparation of viral lysates, IP with protein G, SDS-PAGE, and fluorography have been described previously (6, 9). Each SDS-PAGE gel included CAEV-positive goat serum 8517 and CAEV-negative goat serum 8505 (2). Positive IP results were defined by the detection of CAEV gp135 SU and/or gp90 oligomeric transmembrane (TM) Env proteins (12). Antibodies to CAEV p28, p19, and p16 Gag proteins were also detected in most Env-positive sera. However, only 1 of 757 serum samples immunoprecipitated a Gag protein (p28) in the absence of SU or TM.
Statistical analysis of cELISA sensitivity and specificity.
The 95% confidence intervals (CI) for the sensitivity and specificity of cELISA compared to those for IP were determined as follows: 95% CI = [P(1 − P)/n][1/2] × 1.96, where P is the proportion of cELISA to IP concordant samples and n is the total number of samples (11). The kappa statistic was utilized to assess the agreement between the cELISA and IP (11, 16).
RESULTS
cELISA of goat sera.
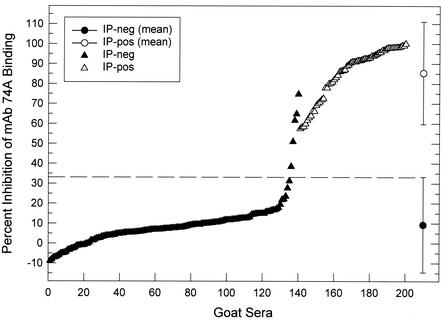
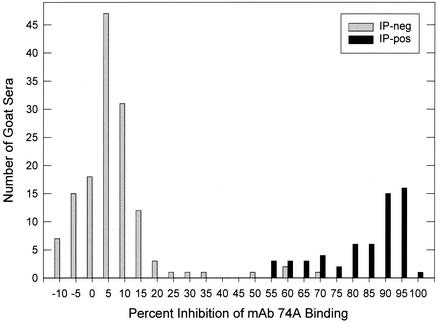
cELISA results in relation to the IP status of 200 goat sera are shown in Fig. 1. There were 140 IP-negative sera, with cELISA values ranging from −8.9 to 74.8% inhibition of MAb 74A binding, and 60 IP-positive sera, with cELISA values ranging from 57.7 to 100% inhibition. The frequency distribution plot of percent inhibition values (Fig. 1) indicated a sharp distinction between IP-negative and IP-positive sera, and histogram analysis also indicated a distinct bimodal distribution of percent inhibition values corresponding to IP-negative and IP-positive sera (Fig. 2). A cELISA cutoff value of 33.2% inhibition was defined to be 2 standard deviations (SD) above the mean percent inhibition of the 140 IP-negative sera. Based on this cutoff, goat sera with cELISA values of >33.2% inhibition were considered positive.
FIG. 1.
Evaluation of 200 goat sera by cELISA and IP. Data indicate the distribution of cELISA values [percent inhibition of MAb 74A binding] as a function of IP status. The mean percent inhibition values ± 2 SD for IP-negative and IP-positive sera are shown. The cELISA cutoff value (33.2% inhibition) is indicated by the dotted line.
FIG. 2.
Frequency distribution of cELISA values in 5% increments plotted as a function of IP status.
The sensitivity and specificity of cELISA compared to those of the IP are shown in Table 1. The sensitivity of cELISA was 100%, and the specificity of cELISA was 96.4%. There were 0 of 60 false-negative and 5 of 140 false-positive cELISAs. The cELISA values for the five false-positive sera ranged from 38.5 to 74.8% inhibition (Fig. 1). The kappa statistic was 0.94, indicating excellent agreement between cELISA and IP (11, 16).
TABLE 1.
Sensitivity and specificity of cELISA compared to those of IP for detection of CAEV antibodies in goat sera
| cELISA result | No. with IP result:
|
|
|---|---|---|
| Positive | Negative | |
| Positivea | 60 | 5 |
| Negativeb | 0 | 135 |
| Sensitivity | 60/60 (100%) | |
| Specificity | 135/140 (96.4 ± 3.1%c) | |
cELISA values of >33.2 percent inhibition.
cELISA values of ≤33.2 percent inhibition.
95% CI.
Relative sensitivity of cELISA and IP.
One possible explanation for the false-positive results is that cELISA is more sensitive than IP for the detection of antibodies to CAEV gp135 SU. One way to evaluate this possibility is to test sequential sera from false-positive goats by cELISA and IP. However, as sequential sera were unavailable, the relative sensitivity of cELISA and IP for the detection of anti-SU antibodies was evaluated by using experimentally infected goats. In this experiment, serum drawn biweekly from eight goats infected intravenously with CAEV was tested by cELISA and IP. Results are shown in Table 2. Preinfection sera from these goats were IP negative, with cELISA values ranging from 6.4 to 17.1% inhibition. A cELISA cutoff was defined as 2 SD above the mean percent inhibition of the preinfection sera. Based on this cutoff (18.5% inhibition), cELISA detected anti-SU antibodies prior to IP in seven of eight experimentally infected goats (Table 2, data in bold type). We also noted that, by applying the 33.2% inhibition cutoff from the IP-negative field sera (Fig. 1), cELISA detected anti-SU antibodies prior to IP in five of eight experimentally infected goats (Table 2, data in underlined bold type). These results indicate that the status of the five cELISA-positive, IP-negative (false-positive) sera in Table 1 is uncertain and that 96.4% may be a low estimate of cELISA specificity.
TABLE 2.
Detection of CAEV SU antibodies in experimentally infected goats by cELISA and IPa
| Goat serum | Wks postinfection
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
2
|
4
|
6
|
8
|
||||||
| IPb | % Ic | IP | % I | IP | % I | IP | % I | IP | % I | |
| 9910 | Neg | 6.4 | Neg | 11.1 | Neg | 32.6 | Pos | 77.0 | Pos | 87.9 |
| 9912 | Neg | 7.6 | Neg | 14.1 | Neg | 13.6 | Neg | 48.6 | Pos | 62.8 |
| 9913 | Neg | 7.7 | Neg | 30.7 | Pos | 83.6 | Pos | 88.6 | Pos | 81.7 |
| 9914 | Neg | 7.7 | Neg | 14.6 | Neg | 17.0 | Pos | 69.7 | Pos | 77.3 |
| 9938 | Neg | 13.4 | Neg | 17.4 | Neg | 35.4 | Pos | 69.6 | Pos | 79.8 |
| 9939 | Neg | 13.7 | Neg | 27.1 | Neg | 22.1 | Neg | 53.5 | Pos | 90.3 |
| 9940 | Neg | 17.1 | Neg | 30.1 | Neg | 39.7 | Neg | 40.7 | Pos | 92.6 |
| 9941 | Neg | 12.0 | Neg | 25.6 | Neg | 34.3 | Neg | 55.0 | Pos | 79.2 |
| Total no. positive | 0/8 | 4/8 | 1/8 | 6/8 | 4/8 | 8/8 | 8/8 | 8/8 | ||
Goats were infected intravenously with 104 TCID50 of CAEV-Co. The mean ± SD cELISA value for IP-negative preinfection sera was 10.7 ± 3.9% inhibition. IP-negative postinfection sera with cELISA values of >18.5% inhibition are in bold type. IP-negative postinfection sera with cELISA values of >33.2% inhibition are in bold type and underlined.
Neg, IP negative; Pos, IP positive.
% I, percent inhibition.
Relationship between percent inhibition and SU antibody titer by cELISA.
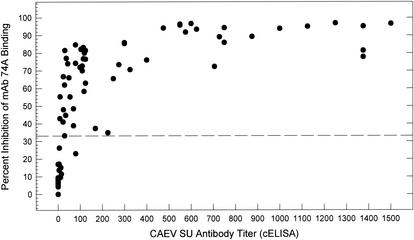
The absence of false-negative sera in Fig. 1 suggested that the use of undiluted serum enabled the cELISA to detect positive sera with low anti-CAEV SU antibody titers. We addressed this question by comparing the percent inhibition values for undiluted sera to the cELISA antibody titers for the same sera. The results demonstrated that the cELISA with undiluted serum readily detected anti-SU antibody titers of <100 (Fig. 3). In addition, the percent inhibition for most undiluted sera reached a maximum at antibody titers of >100, after which the addition of more competing antibody did not significantly affect the percent inhibition (Fig. 3).
FIG. 3.
Relationship between cELISA percent inhibition of undiluted serum and anti-CAEV SU antibody titers determined by cELISA. Serum samples from CAEV-infected goats 9910, 9912, 9913, and 9914 drawn periodically between 1 and 52 weeks following CAEV infection were evaluated. Percent inhibition values obtained by cELISA analysis of undiluted serum samples are plotted against anti-CAEV SU antibody titers determined by end point dilution of the same sera.
Establishment of a CAEV-free dairy goat herd.
cELISA and IP were used to monitor a dairy goat herd maintained by GTC Biotherapeutics. This herd was initially comprised of 557 goats in 1997 and was subsequently expanded by importation and breeding to include 1,640 goats in 2002.
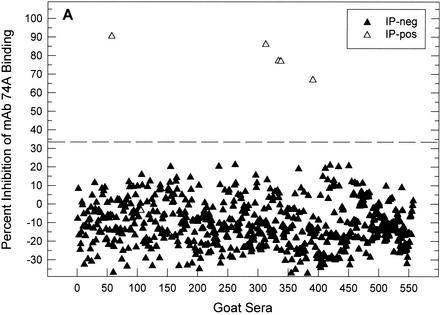
Figure 4 shows cELISA results in relation to the IP status of the 557 goats in the initial herd. Based on the cutoff cELISA value of 33.2% inhibition determined in Fig. 1, 5 of 557 serum samples were positive by IP and cELISA. One serum sample (goat 106) was cELISA negative and IP positive. This serum immunoprecipitated CAEV p28 Gag exclusively and was unique among the 757 field samples evaluated by both cELISA and IP in this study. The six IP-positive goats whose data are shown in Fig. 4 were culled. Of the remaining 551 goats, 158, 66, 47, and 32 were retained in the herd for 2, 3, 4, and 5 years, respectively. All of these goats remained negative by cELISA.
FIG. 4.
cELISA and IP evaluation of 557 goats from GTC Biotherapeutics. Data indicate the distribution of cELISA values (percent inhibition) as a function of IP status. The cELISA cutoff value of 33.2% inhibition determined in Fig. 1 is shown by the dotted line.
In 1999, 1,203 goats were added to this dairy herd by importation and breeding. In 2000, 2001, and 2002, the herd was expanded by breeding to include 1,527, 1,579, and 1,640 goats, respectively. All of these goats were monitored by cELISA. In the 1999 screen, one goat with replicate cELISA values of 48.4 and 52.9% inhibition was culled. There were no cELISA-positive goats in the 2000 screen. In 2001, one goat that was cELISA negative in 1999 and 2000 had triplicate cELISA values of 36.7, 41.9, and 40.0% inhibition in 2001. This goat was culled, and there were no cELISA-positive goats among the 1,640 animals in the 2002 screen. The only goats that tested cELISA positive and were culled in the GTC Biotherapeutics herd from years 1997 through 2002 were imported goats.
DISCUSSION
This study describes a cELISA for the detection of antibodies to CAEV based on serum inhibition of MAb binding to CAEV gp135 SU glycoprotein. Tested against 200 field sera from CAEV-positive goat herds in the United States, cELISA exhibited excellent sensitivity and specificity, utilizing IP as a standard of comparison. cELISA values of IP-positive sera ranged from 57.7 to 100% inhibition, with a mean ± SD of 85.4 ± 12.9% inhibition. Thus, there were no false-negative sera by cELISA based on the cutoff value established at 33.2% inhibition. In contrast, there were five false-positive sera, defined as IP-negative sera with cELISA values of >33.2% inhibition. A study utilizing cELISA and IP to measure the initial seroconversion of experimentally infected goats demonstrated that cELISA is more sensitive than IP for the detection of serum antibodies to CAEV gp135 SU. These results indicate that the high sensitivity of cELISA may contribute to the prevalence of apparently false-positive samples among field sera.
Others have reported ELISAs that rely on the detection of goat antibodies bound to whole virus lysate or recombinant CAEV antigens by using anti-goat immunoglobulin G reagents. Two of these indirect ELISAs using whole CAEV lysate and compared to IP or Western blot analysis had sensitivity and specificity values comparable to those of the cELISA reported here (7, 17). In addition, one other indirect ELISA utilizing recombinant CAEV Gag and TM antigens and compared to Western blot analysis also had sensitivity and specificity values similar to those for cELISA (4).
The ability to detect positive sera with low anti-SU antibody titers is a major advantage of the cELISA over indirect ELISA formats requiring the dilution of test sera. By utilizing recently infected goats, the high sensitivity of cELISA for sera diluted <1:100 is clearly evident (Fig. 3). Therefore, the cELISA has high sensitivity for the detection of recently exposed goats. However, it is also important to note that maximal percent inhibition values are reached at low serum dilutions (Fig. 3). Thus, the use of diluted sera in the cELISA will increase the prevalence of false-negative tests, as was the case for indirect ELISA formats (4, 7, 17).
AGID is another widely used diagnostic test for small, ruminant lentiviruses. Unambiguous AGID tests were recorded for 193 of the 200 goat serum samples evaluated here. AGID sensitivity and specificity values for these sera compared to those for IP were 92.6 and 98.6%, respectively. These results support a previous study indicating that AGID with CAEV antigen performs well (10). However, subjective interpretation of AGID results is a major limitation of this test, and seven ambiguous AGID results were recorded in the present study. Six of these ambiguous AGID results were resolved by cELISA and IP. Based on these data, we conclude that cELISA is a more reliable diagnostic test than AGID.
Our effort to establish a CAEV-free goat herd by cELISA supports the following conclusions and recommendations. (i) Culling of cELISA-positive goats from a herd followed by annual testing will reliably ensure CAEV-free status. (ii) cELISA prior to the importation of goats is a reliable method to prevent the introduction of CAEV-infected goats into a herd. One qualification to these recommendations involves goats with cELISA values higher than the 33.2% inhibition cutoff but less than ∼60% inhibition. Our results indicate that the status of goats with percent inhibition values in this range is uncertain but can be resolved by annual testing. Therefore, a particularly valuable goat with a cELISA value in this range could be separated and retested the following year.
In summary, the present study describes a new diagnostic test for the detection of serum antibodies to CAEV. The test was validated against IP by using sera from CAEV-positive herds from throughout the United States. A major advantage of the cELISA is the use of undiluted serum, which allows the reliable detection of recently exposed animals with comparatively low serum antibody titers to CAEV. cELISA also detects antibodies in colostrum and milk samples (data not shown). Another advantage of cELISA is that the test does not rely on the subjective assessment of results. We also propose recommendations for the use of cELISA based on the annual testing of a dairy herd maintained by GTC Biotherapeutics.
Acknowledgments
We thank Massaro Ueti for performing IP and Kathy Pretty-On-Top for performing IP and cELISA titrations with sera from experimentally infected goats. Isidro Hötzel constructed the infectious molecular clone of the CAEV provirus, and Kevin Snekvik and Jessie Trujillo assisted with studies by using experimentally infected goats.
This work was supported by USDA-ARS CWU 5348-32000-019-00D and NIH grant R01 AR 43718.
REFERENCES
- 1.Adams, D. S., P. Klevjer-Anderson, J. L. Carlson, T. C. McGuire, and J. R. Gorham. 1983. Transmission and control of caprine arthritis-encephalitis virus. Am. J. Vet. Res. 44:1670-1675. [PubMed] [Google Scholar]
- 2.Cheevers, W. P., D. P. Knowles, and L. K. Norton. 1991. Neutralization-resistant antigenic variants of caprine arthritis-encephalitis lentivirus associated with progressive arthritis. J. Infect. Dis. 164:679-685. [DOI] [PubMed] [Google Scholar]
- 3.Cheevers, W. P., and T. C. McGuire. 1988. The lentiviruses: maedi/visna, caprine arthritis-encephalitis, and equine infectious anemia. Adv. Virus Res. 34:189-215. [DOI] [PubMed] [Google Scholar]
- 4.Clavijo, A., and J. Thorsen. 1995. Bacterial expression of the caprine arthritis-encephalitis virus gag and env proteins and their use in enzyme-linked immunosorbent assay. Am. J. Vet. Res. 56:841-848. [PubMed] [Google Scholar]
- 5.Crawford, T. B., and D. S. Adams. 1981. Caprine arthritis-encephalitis: clinical features and presence of antibody in selected goat populations. J. Am. Vet. Med. Assoc. 178:713-719. [PubMed] [Google Scholar]
- 6.Gogolewski, R. P., D. S. Adams, T. C. McGuire, K. L. Banks, and W. P. Cheevers. 1985. Antigenic cross-reactivity between caprine arthritis-encephalitis, visna and progressive pneumonia viruses involves all virion-associated proteins and glycoproteins. J. Gen. Virol. 66:1233-1240. [DOI] [PubMed] [Google Scholar]
- 7.Heckert, R. A., W. B. McNab, S. M. Richardson, and M. R. Briscoe. 1992. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibodies to caprine arthritis-encephalitis virus in goat serum. Can. J. Vet. Res. 56:237-241. [PMC free article] [PubMed] [Google Scholar]
- 8.Klevjer-Anderson, P., and W. P. Cheevers. 1981. Characterization of the infection of caprine synovial membrane cells by the retrovirus caprine arthritis-encephalitis virus. Virology 110:113-119. [DOI] [PubMed] [Google Scholar]
- 9.Knowles, D., Jr., W. Cheevers, T. McGuire, T. Stem, and J. Gorham. 1990. Severity of arthritis is predicted by antibody response to gp135 in chronic infection with caprine arthritis-encephalitis virus. J. Virol. 64:2396-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles, D. P., Jr., J. F. Evermann, C. Shropshire, J. VanderSchalie, D. Bradway, H. M. Gezon, and W. P. Cheevers. 1994. Evaluation of agar gel immunodiffusion serology using caprine and ovine lentiviral antigens for detection of antibody to caprine arthritis-encephalitis virus. J. Clin. Microbiol. 32:243-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin, S. W., A. H. Meek, and P. Willeberg. 1987. Measurement of disease frequency and production, p. 58-59. In S. W. Martin et al. (ed.), Veterinary epidemiology: principles and methods. Iowa State University Press, Ames, Iowa.
- 12.McGuire, T. C., D. P. Knowles, Jr., W. C. Davis, A. L. Brassfield, T. A. Stem, and W. P. Cheevers. 1992. Transmembrane protein oligomers of caprine arthritis-encephalitis lentivirus are immunodominant in goats with progressive arthritis. J. Virol. 66:3247-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Özyörük, F., W. P. Cheevers, G. A. Hullinger, T. C. McGuire, M. Hutton, and D. P. Knowles. 2001. Monoclonal antibodies to conformational epitopes of the surface glycoprotein of caprine arthritis-encephalitis virus: potential application to competitive-inhibition enzyme-linked immunosorbent assay for detecting antibodies in goat sera. Clin. Diagn. Lab. Immunol. 8:44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe, J. D., N. E. East, C. E. Franti, M. C. Thurmond, N. C. Pedersen, and G. H. Theilen. 1992. Risk factors associated with incidence of seroconversion to caprine arthritis-encephalitis virus on California dairies. Am. J. Vet. Res. 53:2396-2403. [PubMed] [Google Scholar]
- 15.Saltarelli, M., G. Querat, D. A. M. Konings, R. Vigne, and J. E. Clements. 1990. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology 179:347-364. [DOI] [PubMed] [Google Scholar]
- 16.Thrusfield, M. 1995. Veterinary epidemiology, p. 280-282. University Press, Cambridge, Mass.
- 17.VanderSchalie, J., D. S. Bradway, T. E. Besser, and J. F. Evermann. 1994. Evaluation of a kinetic enzyme-linked immunosorbent assay for detection of caprine arthritis-encephalitis virus-specific antibodies. J. Vet. Diagn. Investig. 6:30-33. [DOI] [PubMed] [Google Scholar]