Abstract
The endodermal cells of the shoot are thought to be the gravity-sensing cells in Arabidopsis. The amyloplasts in the endodermis that sediment in the direction of gravity may act as statoliths. Endodermis-specific expression of SGR2 and ZIG using the SCR promoter could complement the abnormal shoot gravitropism of the sgr2 and zig mutants, respectively. The abnormalities in amyloplast sedimentation observed in both mutants recovered simultaneously. These results indicate that both genes in the endodermal cell layer are crucial for shoot gravitropism. ZIG encodes AtVTI11, which is a SNARE involved in vesicle transport to the vacuole. The fusion protein of SGR2 and green fluorescent protein localized to the vacuole and small organelles. These observations indicate that ZIG and SGR2 are involved in the formation and function of the vacuole, a notion supported by the results of subcellular analysis of the sgr2 and zig mutants with electron microscopy. These results strongly suggest that the vacuole participates in the early events of gravitropism and that SGR2 and ZIG functions are involved.
INTRODUCTION
Gravitropism is an important environmental response in plants. In higher plants, shoots and roots show negative and positive gravitropism, respectively. The gravitropic response is composed of four sequential steps: gravity sensing, signal formation, signal transduction, and differential growth of the upper and lower tissues of the responding organ (Tasaka et al., 1999, 2001). The perception of gravity and the conversion of the physical stimulus (i.e., the gravity vector) to chemical signals in the cell are particularly interesting, but they are the least characterized events in gravitropism. Physiological and cytological analyses with various plants have shown that amyloplasts, which are plastids containing starch granules, sediment in the direction of gravity and are able to move downward in response to the change of gravity stimulation (Konings, 1995; Blancaflor et al., 1998). Thus, amyloplasts are believed to act as statoliths for gravity. The columella cells of the root cap and the endodermal cells or starch sheath of the shoot, which contain sedimented amy-loplasts, are thought to act as gravity-sensing cells (Sack, 1991, 1997).
In the shoot, the statolith hypothesis is supported by genetic studies. Some of the genes involved in the development or differentiation of gravity-sensing cells or amyloplasts have been cloned. In the Arabidopsis shoot, the epidermis, the cortex, the endodermis, and the stele containing vascular tissues are arranged in a radially symmetrical manner progressing from the outside to the inside. The sgr1 (shoot gravitropism 1) and sgr7 mutants, whose inflorescence stems and hypocotyls both lack gravitropic ability, also lack the normal endodermal cell layer (Fukaki et al., 1996, 1998). These two mutants are allelic to scr (scarecrow) and shr (short-root), respectively, both of which were isolated as radial pattern formation mutants in the root and encode putative transcription factors (Di Laurenzio et al., 1996; Fukaki et al., 1998; Helariutta et al., 2000). Moreover, starch-less or starch-reduced mutants that contain immature amyloplasts show reduced gravitropism in both the shoot and the root (Kiss et al., 1989, 1996, 1997; Weise and Kiss, 1999). In addition, the eal1 (endodermal-amyloplastless 1) mutant, which lacks amyloplasts in its hypocotyl endodermal cells, shows abnormal shoot gravitropism, whereas its roots, which contain amyloplasts in the root cap, show normal gravitropism (Fujihira et al., 2000). These studies indicate that endodermal cells containing amyloplasts are essential for shoot gravitropism.
In contrast, there is little information on the genes that are involved directly in the gravity perception and signal formation process. Mutations of ARG (ALTERED RESPONSE TO GRAVITY) cause abnormal gravitropism in both the root and the hypocotyl, and ARG is believed to be involved in gravity perception (Fukaki et al., 1997; Sedbrook et al., 1999). Although it has been suggested that the endoplasmic reticulum (ER) localized at peripheral regions in columella cells might be the site of gravity perception in roots (Hensel and Sievers, 1981; Zheng and Staehelin, 2001), the precise mechanism involved remains unknown. In this work, we report the molecular functions of two additional shoot gravitropism genes, SGR2 and ZIG (ZIGZAG), and suggest that these two genes are involved in the early gravity signaling process in the shoot.
RESULTS
Incomplete Sedimentation of Amyloplasts in the Shoot Endodermis of sgr2 and zig
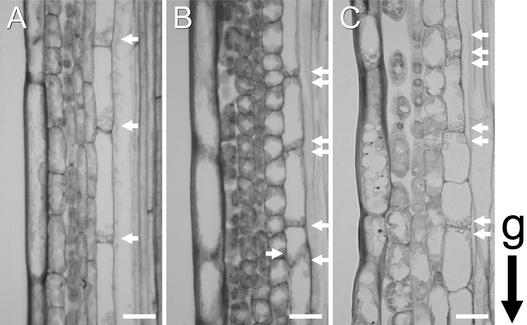
In the wild-type shoot, one epidermal cell layer, usually three cortex layers, and one endodermal cell layer are arranged in a concentric manner from the outer side of the stem inward to its core. The endodermal cells are almost uniform in size and shape and contain amyloplasts that are sedimented in the direction of gravity (Figure 1A). In the sgr2-1 and zig-1 mutants, the patterning of whole tissues was basically normal, although the endodermal cells frequently were irregular in size and shape. In sgr2-1, however, a relevant proportion of amyloplasts accumulated in the upper region of the cells, in the opposite direction with respect to gravity (Figure 1B). The inflorescence stem of zig-1 showed a similar but slightly different pattern, in that the amyloplasts were located both at the bottom and at the top of the cells but also occasionally dispersed peripherally (Figure 1C). The patterns of amyloplast distribution in wild type and mutants are shown quantitatively in Table 1. The pattern of amyloplast distribution varied slightly among alleles in zig (data not shown). In contrast, in the columella cells in the root cap of both sgr2-1 and zig-1, amyloplasts sedimented normally (data not shown). Thus, the defective shoot gravitropism of these two mutants correlates with an impairment of amyloplast sedimentation in their shoot endodermis.
Figure 1.
Amyloplast Localization in Endodermal Cells.
Longitudinal sections through inflorescence stems (3 to 4 cm below the apex) of wild-type (A), sgr2-1 (B), and zig-1 (C) plants. The growth orientation of stems was maintained during fixation. Arrows indicate amyloplasts. g indicates the orientation of gravity. Bars = 20 μm.
Table 1.
Positioning of Amyloplasts in the Endodermal Cells
| Position | Wild Type | sgr2-1 | zig-1 |
|---|---|---|---|
| Top | 1 (0.5%) | 65 (32.2%) | 44 (20.1%) |
| Middle | 14 (7.3%) | 15 (7.4%) | 80 (36.5%) |
| Bottom | 176 (92.1%) | 122 (60.4%) | 95 (43.4%) |
| Total | 191 | 202 | 219 |
Amyloplasts that were observed in the longitudinal sections as in Figure 1 were counted. Twenty endodermal cells of each plant were used to count amyloplasts. Amyloplasts that touched the top or bottom side of the wall and those that sat closely together were counted as top or bottom. The others were counted as middle.
Endodermal Expression of SGR2 and ZIG Is Essential for Gravitropism
It is unclear whether the aberrant endodermal cells of the mutants are responsible directly for their abnormal gravitropic response, because SGR2 and ZIG are expressed in all of the wild-type plant organs examined (see Figure 5 of the accompanying paper for SGR2; data not shown for ZIG). Furthermore, SGR2 expression has been detected in the sgr7/shr mutant that lacks an endodermis (data not shown). To elucidate the function of SGR2 and ZIG in endodermal cells, we used the SCR promoter to drive these genes in the corresponding mutants. It had been shown that the SCR gene is expressed specifically in the endodermal cell layer of the root, the hypocotyl, and the inflorescence stem (Di Laurenzio et al., 1996; Wysocka-Diller et al., 2000).
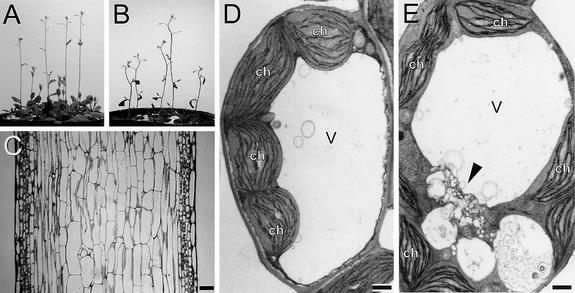
Figure 5.
Morphological and Physiological Characteristics of the zig Mutant at the Tissue Level.
(A) and (B) Four-week-old inflorescence stems of wild-type (A) and zig-1/pSCR::ZIG (B) plants.
(C) Longitudinal section through an inflorescence stem of zig-1/pSCR::ZIG. Bar = 100 μm.
(D) and (E) Ultrastructure of the cortex cell in wild-type (D) and zig-1 (E). Aberrant vacuolar/vesicular structures (arrowhead) are shown in (E). ch, chloroplast; v, central vacuole. Bars = 1 μm.
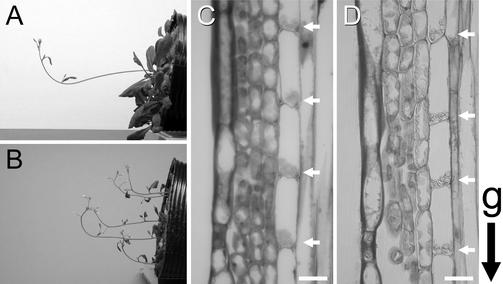
sgr2-1 plants that were transformed with pSCR::SGR2-GFP, which generates green fluorescent protein (GFP) fused to the C terminus of SGR2, showed almost normal gravitropic responses in their inflorescence stems (Figure 2A) and hypocotyls (data not shown). In the wild-type plant, the stem segments began to bend upward within 30 min, and the curvature reached 90° in ∼90 min (see Figure 3 of the accompanying paper). sgr2-1/pSCR::SGR2-GFP plants responded to gravity with similar kinetics, whereas the parental sgr2-1 showed little response even after 24 hr. As described in detail in the accompanying paper, sgr2-1 is characterized by slightly winding primary stems and lateral stems that roll downward. In the transgenic plants, however, the primary and lateral stems grew straight and upward, respectively. Thus, this fusion protein is functional. The zig-1 mutant plants that were transformed with pSCR::ZIG also responded to gravity with similar kinetics to wild-type plants, although the parental zig-1 plants showed little response even after 24 hr. In addition, their lateral stems, which in the parental zig-1 mutant curl downward, grew upward (Figure 2B). Interestingly, however, pSCR::ZIG could not complement the morphological phenotype of zig-1 completely, because the transgenic mutant still had the characteristic zigzag-shaped inflorescence stems and wrinkled leaves of the parent.
Figure 2.
Endodermis-Specific Expression of SGR2 and ZIG and Their Rescue of Gravitropism in Each Mutant.
(A) and (B) Complementation of sgr2-1 (A) and zig-1 (B) gravitropism with pSCR::SGR2-GFP and pSCR::ZIG, respectively. The transgenic plants were placed horizontally at 23°C for 3 hr in the dark.
(C) and (D) Longitudinal sections of inflorescence stems of sgr2-1/pSCR::SGR2 (C) and zig-1/pSCR::ZIG (D) transgenic plants. Arrows indicate amyloplasts. g indicates the orientation of gravity. Bars = 20 μm.
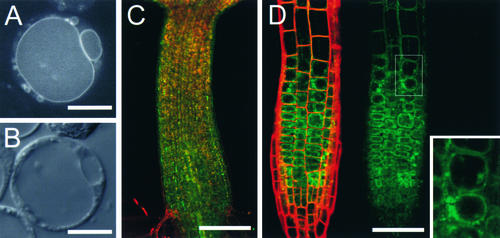
Figure 3.
Subcellular Localization of SGR2.
(A) and (B) Arabidopsis cultured cells expressing the functional SGR2-GFP fusion gene using the 35S promoter of Cauliflower mosaic virus.
(A) Confocal image.
(B) Nomarski image. GFP fluorescence was detected on the vacuolar membrane and small organelles.
(C) and (D) GFP images of sgr2-1/pSGR2::SGR2i-GFP.
SGR2-GFP fusion proteins were expressed under the native SGR2 gene promoter.
(C) The hypocotyl just after germination.
(D) The root tip.
Data from the fluorescein isothiocyanate channel (SGR2-GFP, green image) and the rhodamine channel (cell wall, red image) are merged and shown at left, and the fluorescein isothiocyanate channel data alone are shown at right. GFP fluorescence appeared to be present on tonoplasts in the plant as well as in a protoplast in (A). A magnified image of the boxed area is shown in the inset.
Bars in (A) and (B) = 10 μm; bars in (C) and (D) = 200 μm.
Most importantly, in both transgenic plants all amyloplasts in the endodermis sedimented in the direction of gravity, similar to the wild-type plant. In addition, the shape of the endodermal cells also recovered (Figures 2C and 2D). These results indicate that the expression of SGR2 and ZIG in the endodermis is essential for the shoot gravitropic response, probably for early processes such as gravity sensing and signal formation.
SGR2 Is Localized to Vacuolar Membranes
We know little about the SGR2 protein except that it has sequence similarity to bovine phosphatidic acid–preferring phospholipase A1. The presence of a possible transmembrane domain in SGR2 and the fact that ZIG encodes a SNARE prompted us to examine the subcellular localization of the SGR2 protein. When the SGR2-GFP construct, which generates a functional fusion protein (Figure 2A), was driven in Arabidopsis protoplasts by the 35S promoter of Cauliflower mosaic virus (Figures 3A and 3B), the GFP signal was detected on the membranes of vacuoles and small organelles in the transformed cells. In addition, bright dots were observed on these membranes (Figure 3A).
To examine the localization of SGR2 in planta, the SGR2-GFP fusion gene was linked to the putative SGR2 promoter, which also includes the first intron (pSGR2::SGR2i-GFP). The resulting construct was introduced into the sgr2-1 mutant plant. The construct complemented both the morphology and the gravitropic response of the mutant, indicating that the fusion protein also is functional in planta (data not shown). The fluorescence was detected in the whole seedling just after germination (Figures 3C [hypocotyl] and 3D [root]), but its intensity decreased markedly during seedling growth, except for a region just below the elongation zone of the root.
The subcellular localization of SGR2i-GFP in the roots of transgenic seedlings was examined next. Fluorescence was detected mainly on membranes that are likely to be vacuoles and small organelles and unlikely to be plasma membranes (Figure 3D, magnified image). The localization and the presence of bright dots observed previously in the protoplasts also were noted in the transgenic plant. These results strongly suggest that SGR2 localizes on the membranes of vacuoles and small organelles in various tissues.
Vacuole Defects in zig-1 and sgr2-1
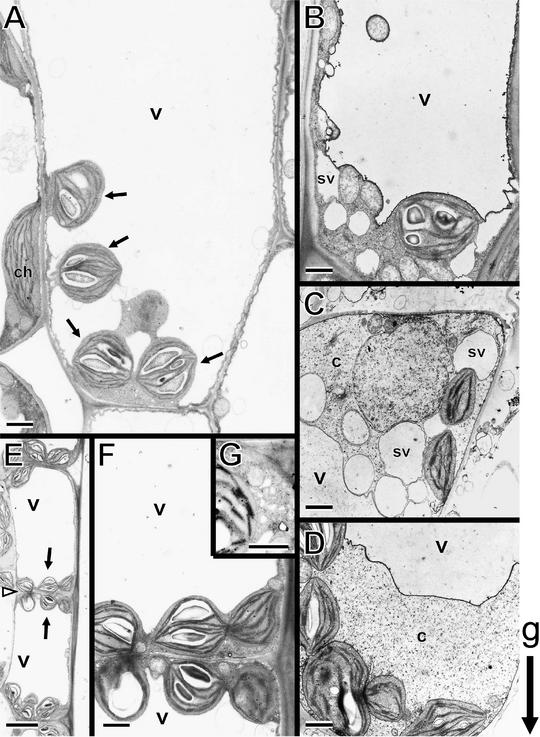
To investigate the effect of the sgr2 and zig mutations in more detail at the subcellular level, inflorescence stem tissues were processed for electron microscopy (Figure 4). In wild-type stems, the sedimented amyloplasts in the endodermis often appeared to be suspended by falling into a “hollow” of the vacuolar membrane (Figure 4A). They were surrounded by cytoplasm and the vacuolar membrane and sometimes were connected via transvacuolar strands. In contrast, in sgr2-1 mutants, amyloplasts were located on both the top and bottom sides of the endodermal cell, and few amyloplasts that got into transvacuolar strands were observed. Many spherical compartments that looked like small-vacuole membrane structures (Figures 4B and 4C) and accumulations of cytoplasm occasionally were observed on both the top and bottom sides of the cells (Figure 4D). With regard to the zig-1 mutant, although the amyloplasts also were located on both the top and bottom sides of the endodermal cell and could not get into transvacuolar strands, like those of the sgr2 mutants, there was a distinct difference (Figures 4E and 4F). The amyloplasts appeared to be pressed against the cell periphery by a large central vacuole in zig-1. Moreover, the cytoplasm between the amyloplasts and the plasma membrane did not contain the small-vacuole-like organelles that often were observed in sgr2-1 endodermal cells. Instead, aberrant vacuolar/vesicular structures occasionally were found in the cytoplasm (Figure 4G). Their shape was distorted, and they were smaller than the small-vacuole-like structures observed in sgr2-1. A few amyloplasts in both mutants seemed to contain less starch, especially in sgr2-1. However, starch granules stained with potassium iodide were observed in both the sgr2-1 and zig-1 mutants as well as in the wild type (data not shown).
Figure 4.
Ultrastructure of the Endodermal Cell in sgr2-1 and zig-1 Stems.
The growth orientation of stems was maintained during fixation.
(A) Wild type (Columbia). The lower side of an endodermal cell is shown. The arrows indicate amyloplasts sedimented in the direction of gravity.
(B) to (D) sgr2-1. Amyloplasts are observed in the lower side ([B] and [D]) and the upper side (C) of an endodermal cell. The small-vacuole-like membrane structures and the accumulation of cytoplasm are shown.
(E) to (G) zig-1.
(E) Amyloplasts (arrows) are observed at the top and bottom of an endodermal cell. The open arrowhead indicates the borders of neighboring cells.
(F) Higher magnification of the area around the borders of neighboring endodermal cells in (E).
(G) Occasionally, aberrant vacuolar/vesicular structures were observed in zig-1.
c, cytoplasm; ch, chloroplast; sv, small-vacuole-like membrane structures; v, central vacuole. g indicates the orientation of gravity.
Bars in (A) to (D), (F), and (G) = 1 μm; bars in (E) = 5 μm.
Tissue-Specific Function of ZIG
As shown in Figure 5B, the endodermis-specific expression of ZIG could complement the gravitropic response of zig-1 but not its morphological phenotype, because the inflorescence stems still elongated in the same characteristic zigzag fashion and the relatively small leaves shrank. At the tissue level, certain anomalies in the endodermis, such as the amyloplast localization and the cell shape and size, were restored (Figure 2D). However, the shape and arrangement of the cortex and pith cells remained abnormal (Figure 5C; see Figure 7D of the accompanying paper). These observations suggest that the aberrant endodermis of zig-1 is not responsible directly for the stem shape.
In zig-1 mutants, vacuole anomalies such as fragmentation and vesiculation were observed more frequently in the cortex cells of the inflorescence stem than in the endodermal cells (Figures 4G and 5E, arrowhead). In contrast, wild-type cortex cells are filled with large central vacuoles (Figure 5D). The lesions in zig-1 would be consistent with the putative role for ZIG as a SNARE that is involved in vacuolar membrane biogenesis and dynamics. Together, these results suggest that the expression of ZIG in the endodermis is essential for the shoot gravitropic response, whereas ZIG expressed in other tissues may be responsible for the correct stem and leaf shape. Both functions probably are implemented by ZIG, mediating appropriate vacuole biogenesis and vacuolar functions.
DISCUSSION
SGR2 and ZIG Expression in the Endodermis Is Essential for the Gravitropic Response
The gravitropic response can be divided into four consecutive processes: the perception of gravity, signal formation, signal transduction, and differential growth that probably is mediated by asymmetric auxin distribution (Tasaka et al., 1999). It is accepted generally that starch-containing amyloplasts function as statoliths in gravity perception because they sediment in the direction of gravity in specialized cells (Sack, 1991, 1997). In Arabidopsis shoots, sedimenting amyloplasts are found in the starch sheath or the endodermis. The starch statolith hypothesis is strongly supported by genetic evidence (Kiss et al., 1989, 1996, 1997; Fukaki et al., 1998; Weise and Kiss, 1999; Fujihira et al., 2000).
It is thought that the transport and distribution of auxin is involved in both gravitropism and phototropism (Kaufman et al., 1995). Because the sgr2 and zig mutants both can perform the phototropic response, it is more likely that SGR2 and ZIG are involved in the early gravitropic processes, that is, either the perception of gravity or the initial signaling step (Fukaki et al., 1996; Yamauchi et al., 1997). This assumption is consistent with anomalies observed in the endodermis of both sgr2-1 and zig-1 (Figure 1). Although the endodermal cell layer containing amyloplasts clearly exists in these mutants, not all amyloplasts sediment in the direction of gravity. When the mutants were transformed with SGR2 and ZIG driven by the endodermis-specific SCR promoter, both the defect in amyloplast sedimentation and the abnormal gravitropic response recovered. These results indicate that the expression of these genes in the endodermis is essential for the gravitropic response.
The Vacuole Is Involved in the Early Step of Gravity Sensing
Subcellular observations by electron microscopy showed that the endodermal cells of wild-type inflorescence stems are filled with large vacuoles and that sedimented amyloplasts are surrounded by cytoplasm and vacuolar membranes (Figure 4A). Both SGR2 and ZIG appear to be vacuole-related genes. ZIG is a SNARE that probably is involved in the vesicle transport pathway to vacuoles (Zheng et al., 1999). And we have shown in this report that SGR2 localizes to the membranes of vacuoles and small organelles in wild-type plants (Figure 3). The expression of these genes in the endodermis was found to be essential for proper gravitropism (Figure 2). In the endodermal cells of the zig-1 mutant, amyloplasts were located outside of the vacuole on both the top and bottom sides of the endodermal cells and appeared to be pressed against the cell periphery (Figures 4E through 4G). A similar abnormal localization of amyloplasts as well as anomalies in the distribution of cytoplasm and in the vacuolar structure also were observed in the endodermal cells of sgr2-1 (Figures 4B through 4D). These observations suggest that a certain impairment in vacuolar function may affect amyloplast localization.
What kind of defect could cause the abnormal gravitropic response in the sgr2-1 and zig-1 mutants? The most plausible explanation is that the mutants are unable to respond to gravity as a result of their failure to sense the direction of gravity because the instruments for determining the direction of gravity, the amyloplasts, are distributed abnormally. Alternatively, the abnormal localization of amyloplasts may not be the primary result of the aberrant gravitropic response; rather, the mutants may have lost the molecular mechanism(s) involved in constructing the primary signal and transducing it. The latter possibility cannot be excluded completely because the inflorescence stems of zig-3 still respond to gravity, albeit very slowly, despite the fact that their amyloplasts are relatively dispersed (data not shown). Thus, our study has brought a new insight into the study of gravitropism, namely, that vacuole functions may be involved in the early step of the gravitropic response occurring in shoot statocytes.
Possible Molecular Function(s) of SGR2 and ZIG in the Gravitropic Response
Our results strongly suggest that both SGR2 and ZIG are involved in the vacuolar functions of the endodermal cells in the shoot. It is likely that these two proteins participate in vacuolar function in distinct ways, because the sgr2-1 and zig-1 mutations resulted in slightly different defects in the endodermis (Figure 4). Because ZIG encodes a SNARE, it is possible that at least some of the proteins of the prevacuolar compartment/vacuoles may be transported by a ZIG-dependent pathway. Thus, missorting of cargo proteins may cause multiple downstream effects that result in the vacuole abnormalities observed in the zig-1 mutant.
Possible interaction between SGR2 and ZIG in the endodermal cells can be hypothesized as follows. First, SGR2 may be one of the proteins transported by a ZIG-dependent traffic pathway and may play a role in the vacuole for the gravitropic response. Second, ZIG may transport another unidentified protein(s) that regulates SGR2 function directly or that constructs the appropriate vacuolar environment wherein SGR2 can be activated or regulated properly. Third, SGR2 and ZIG may be involved independently in the gravitropic response via vacuolar function. These possibilities should be evaluated in the near future.
How, then, could SGR2 operate in the vacuole? As discussed in the accompanying paper, SGR2 is a possible phospholipase A1. It may regulate vacuolar membrane structure, fluidity, or function by degrading specific phospholipids through its phospholipase activity. This, in turn, may affect amyloplast distribution via vacuole membranes. It has been reported that in the endodermis, amyloplasts move through a narrow cytoplasmic space between the plasma membrane and the vacuole. Occasionally, amyloplasts appear to travel through the vacuole either enclosed by the tonoplast membrane or by passing through transvacuolar strands (Clifford et al., 1989). Thus, the flexible membrane structure of the vacuole might be required to permit the amyloplasts to fall freely. Another possibility is that SGR2 may contribute to the formation of the gravity-sensing or downstream signal transduction. The fatty acids or lysophospholipids produced by phospholipase A1 could act as signaling molecules. Therefore, the SGR2 that is activated by gravistimulation subsequently may produce such signaling molecules. Vacuoles store various ions such as Ca2+ and H+, whose concentrations in the vacuoles are higher than in the cytosol. Ca2+ and H+ ions are thought to be involved in the gravitropic response (Sinclair and Trewavas, 1997; Scott and Allen, 1999; Fasano et al., 2001). Possibly, SGR2 may directly or indirectly regulate the transport of these ions in or out of the vacuole. The first step required to understand the role that SGR2 plays in the molecular mechanism of gravitropic signaling will be to investigate its molecular function(s), such as its enzyme activity.
Root and Shoot Gravitropism Are Mediated by Different Mechanisms
Roots exhibit positive gravitropism, and it is accepted generally that the columella cells in the root cap that contain the amyloplasts are the gravity-sensing cells (Sack, 1991). Although amyloplasts probably play a common role as statoliths in both the shoot and the root, there are many different features between these two organs. In the root, the sensing tissues are arranged conically at the root tip, whereas those in the shoot form a radial pattern, with tissues being distributed rather widely through the elongation region (Fukaki et al., 1998; Tasaka et al., 1999). The subcellular structure of columella cells has been observed in detail. The cells have a polarity, with the nucleus and ER being localized at the proximal side and the periphery of the cell, respectively (Sack and Kiss, 1989; Sack, 1991). It has been reported that the columella cells in tobacco contain ER of a specialized form that has been termed nodal ER (Zheng and Staehelin, 2001). It has been postulated that amyloplasts that sediment or move on the ER stimulate the ER membrane to produce signal molecules in the columella cells (Hensel and Sievers, 1981). In the shoot, in contrast, although little information on cell polarity is available, the gravity-sensing endodermal cells are filled with a large central vacuole and amyloplasts appear to be surrounded by the vacuolar membrane. Such a large vacuole should affect the sedimentation of amyloplasts and/or subsequent signaling. Consequently, based on the subcellular structures of gravity-sensing cells in the root and the shoot, it is likely that vacuolar function affects gravitropism more profoundly in the shoot than in the root. This is consistent with the observation that sgr2 and zig/sgr4 have normal root gravitropism but impaired shoot gravitropism (Fukaki et al., 1996; Yamauchi et al., 1997). This observation strongly supports the notion that the gravitropic mechanisms in these two organs differ at the genetic level despite their common use of amyloplasts as statoliths.
Tissue-Specific Function of ZIG
Interestingly, the morphological and physiological functions of ZIG could be determined at the tissue level (Figures 5B and 5C), because the endodermis-specific expression of ZIG was able to rescue gravitropism but not the abnormal morphology seen in the zig-1 mutants. This finding suggests that ZIG plays tissue-specific roles, participating in gravitropism in the endodermis and in morphogenesis in other tissues.
As observed with electron microscopy (Figure 5E), the vacuole structure and probably its function in inflorescence stem tissues were severely affected by the zig mutation. The vacuoles in other tissues that have not been investigated yet probably also are affected. It is possible, therefore, that the abnormal cell shapes or sizes of leaf pavement cells and trichomes observed in the zig mutant also are caused by the impairment of vacuole structure or function. As discussed above, the zig mutation probably affects vacuolar membrane dynamics by impairing membrane transport to the vacuole.
The plant vacuole has long been recognized as a multifunctional compartment with roles in solute storage, digestion, signaling, and defense (Leigh and Sanders, 1999). However, more recent research has shown that physiologically distinct vacuoles may coexist in the same cell (Robinson and Rogers, 2000). Nevertheless, one of its unique features remains an osmotic one: it is responsible for the generation of turgor, and in some cells it may occupy up to 90% of the total cell volume. This means that, together with the cell wall, the vacuole is an important factor in determining cell shape and size. Thus, the aberrant organ shape of the zig mutant may be attributable partly to the accumulation of cells with abnormal shapes and sizes, which implies that a correctly functioning vacuole is imperative for plant morphogenesis.
METHODS
Histological Analysis
Stem segments were cut from primary inflorescence stems of Arabidopsis thaliana that grew upright after bolting and were fixed in 10% (v/v) formaldehyde, 5% (v/v) acetic acid, and 50% (v/v) ethanol in 0.2-mL tubes under vacuum, with the growth orientation of the stems being maintained. Stems that did not grow upright were carefully excluded from the experiments, especially for mutants. After fixation, samples were dehydrated by a series of ethanol washes and embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany) according to the manufacturer's instructions. Sections (3 μm) were stained with toluidine blue.
Electron Microscopy
Stem segments (1 to 2 cm below the apex) were fixed with glutaraldehyde, postfixed with osmium tetroxide, dehydrated, embedded in Spurr's resin, sectioned (70 to 90 nm) longitudinally, and stained with uranyl acetate and lead citrate. The growth orientation of stems was maintained during fixation.
cDNA Cloning and Plasmid Constructions
Total RNA was isolated from etiolated Columbia wild-type hypocotyls with the RNeasy Plant Mini Kit (Qiagen, Valencia, CA). cDNAs were synthesized using Superscript II Reverse Transcriptase (Life Technologies, Grand Island, NY). Nested polymerase chain reaction (PCR) for SGR2 cDNA was performed with the following primer sets: mSGR2-F (5′-TTGGATCCTCGTCTAAAGCTTCCTCC-3′) and mSGR2-R (5′-ATGGATCCAATGTGGCAAAACCATGTCG-3′); cSGR2-F1 (5′-CTGGATCCTGATGGAAGATAGAG-3′) and cSGR2-R1 (5′-ACT-GGATCCTACTTCTGCACGG-3′). Nested PCR for ZIG cDNA was performed essentially as described (Zheng et al., 1999). The PCR products were cloned into pGEM-T Easy vector (Promega).
Green fluorescent protein (GFP; S65T) (Niwa et al., 1999) with a three-glycine linker at both terminal ends was synthesized by PCR and cloned into the SmaI site of pUC19 (resulting in pUC19_ gggGFPgggp). Inverse PCR for SGR2 cDNA including the vector was performed using the following primer pairs: SGR2 N′_1U (5′-ATGTGT-CTCTCTATCTTCCAT-3′) and SGR2 N′_1D (5′-TTAGGAACTCGGGAGGTTAAT-3′) for GFP-SGR2; SGR2 C′_1U (5′-AGGCTTCTT-CAAATATTTCTT-3′) and SGR2 C′_1D (5′-TAAGACTTTGACCGTAGG-TTT-3′) for SGR2-GFP. The products were ligated with gggGFPgggp fragments digested by SmaI.
The SGR2 and SGR2-GFP genes were inserted into the BamHI site of pBI101 that lacks the uidA gene. The 2.5-kb HindIII fragment of the SCR promoter (kindly donated by Dr. Philip N. Benfey, New York University) was inserted into the pBI121 plasmid that lacks the uidA gene (pBI121del_pSCR). The SGR2, SGR2-GFP, and ZIG genes were inserted into pBI121del_ pSCR downstream of the SCR promoter.
The 1.2-kb region upstream from the SGR2 start codon, which was sufficient to complement the sgr2 phenotypes, was defined as the SGR2 promoter. The 1.2-kb fragment, which includes a region spanning the first exon and part of the second exon of the SGR2 gene, was conjugated with SGR2 cDNA, resulting in pSGR2::SGR2i. GFP (S65T) with a four-proline linker at its N-terminal end was generated by PCR and fused in frame at the C-terminal end of SGR2 in pSGR2::SGR2i. The resulting plasmid is pSGR2::SGR2i-GFP. All of these constructs were transformed into sgr2-1 or zig-1 plants.
Cell Culture Conditions and Transient Assay
Cultured Arabidopsis cells were maintained in modified Murashige and Skoog (1962) medium at 23°C in the dark. Plasmids and carrier DNA were introduced into protoplasts that had been generated from suspension cultures. The experiments and the observations were performed according to the method described previously (Takeuchi et al., 2000).
Imaging of GFP Expression
To clarify the outlines of root cells, seedlings were soaked in 10 μg/mL propidium iodide (Sigma) for 1 min and mounted with water on a slide as described previously (Helariutta et al., 2000). Fluorescence was imaged by confocal laser scanning microscopy (LSM510; Zeiss, Jena, Germany). GFP fluorescence (green) was detected through the fluorescein isothiocyanate channel; autofluorescence (red) and propidium iodide fluorescence were detected through the rhodamine channel. Images were processed in Adobe Photoshop 5.0 (Mountain View, CA) to improve their tone and contrast.
Acknowledgments
We thank Dr. Philip N. Benfey for providing the plasmid containing the SCR promoter, Dr. Yasuo Niwa for the GFP (S65T) vector, and Dr. Hidehiro Fukaki for critical reading of the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture of Japan (10182214), by a grant from the Research for the Future Program from the Japan Society for the Promotion of Science to M.T., and by Grants-in-aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan to M.T.M.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010216.
References
- Blancaflor, E.B., Fasano, J.M., and Gilroy, S. (1998). Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 116, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford, P.E., Douglas, S., and McCartney, G.W. (1989). Amyloplast sedimentation in shoot statocytes having a large, central vacuole: Further interpretation from electron microscopy. J. Exp. Bot. 40, 1341–1346. [Google Scholar]
- Di Laurenzio, L., Wysocka-Diller, J., Malamy, J.E., Pysh, L., Helariutta, Y., Freshour, G., Hahn, M.G., Feldmann, K.A., and Benfey, P.N. (1996). The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86, 423–433. [DOI] [PubMed] [Google Scholar]
- Fasano, J.M., Swanson, S.J., Blancaflor, E.B., Dowd, P.E., Kao, T., and Gilroy, S. (2001). Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihira, K., Kurata, T., Watahiki, M.K., Karahara, I., and Yamamoto, K.T. (2000). An agravitropic mutant of Arabidopsis, endodermal-amyloplast less 1, that lacks amyloplasts in hypocotyl endodermal cell layer. Plant Cell Physiol. 41, 1193–1199. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Fujisawa, H., and Tasaka, M. (1996). SGR1, SGR2, SGR3: Novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol. 110, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki, H., Fujisawa, H., and Tasaka, M. (1997). The RHG gene is involved in root and hypocotyl gravitropism in Arabidopsis thaliana. Plant Cell Physiol. 38, 804–810. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Wysocka-Diller, J., Kato, T., Fujisawa, H., Benfey, P.N., and Tasaka, M. (1998). Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 14, 425–430. [DOI] [PubMed] [Google Scholar]
- Helariutta, Y., Fukaki, H., Wysocka-Diller, J., Nakajima, K., Jung, J., Sena, G., Hauser, M.T., and Benfey, P.N. (2000). The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101, 555–567. [DOI] [PubMed] [Google Scholar]
- Hensel, W., and Sievers, A. (1981). Induction of gravity-dependent plasmatic responses in root statocytes by short time contact between amyloplasts and the distal endoplasmic reticulum complex. Planta 153, 303–307. [DOI] [PubMed] [Google Scholar]
- Kaufman, P.B., Wu, L., Brock, T.G., and Kim, D. (1995). Hormones and the orientation of growth. In Plant Hormones, P.J. Davies, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 547–571.
- Kiss, J.Z., Hertel, R., and Sack, F.D. (1989). Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177, 198–206. [PubMed] [Google Scholar]
- Kiss, J.Z., Wright, J.B., and Caspar, T. (1996). Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol. Plant. 97, 237–244. [DOI] [PubMed] [Google Scholar]
- Kiss, J.Z., Guisinger, M.M., Miller, A.J., and Stackhouse, K.S. (1997). Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 38, 518–525. [DOI] [PubMed] [Google Scholar]
- Konings, H. (1995). Gravitropism of roots: An evaluation of progress during the last three decades. Acta Bot. Neerl. 44, 195–223. [DOI] [PubMed] [Google Scholar]
- Leigh, R.A., and Sanders, D. (1999). The Plant Vacuole. (New York: Academic Press).
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Niwa, Y., Hirano, T., Yoshimoto, K., Shimizu, M., and Kobayashi, H. (1999). Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J. 18, 455–463. [DOI] [PubMed] [Google Scholar]
- Robinson, D.G., and Rogers, J.C. (2000). Vacuolar Compartments. Annual Plant Review, Vol. 5. (Sheffield, UK: Academic Press).
- Sack, F.D. (1991). Plant gravity sensing. Int. Rev. Cytol. 127, 193–252. [DOI] [PubMed] [Google Scholar]
- Sack, F.D. (1997). Plastids and gravitropic sensing. Planta 203, S63–S68. [DOI] [PubMed] [Google Scholar]
- Sack, F.D., and Kiss, J.Z. (1989). Root cap structure in wild type and in starchless mutant of Arabidopsis. Am. J. Bot. 76, 454–464. [PubMed] [Google Scholar]
- Scott, A.C., and Allen, N.S. (1999). Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol. 121, 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook, J.C., Chen, R., and Masson, P.H. (1999). ARG1 (altered response to gravity) encodes a DnaJ-like protein that potentially interacts with the cytoskeleton. Proc. Natl. Acad. Sci. USA 96, 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, W., and Trewavas, A.J. (1997). Calcium in gravitropism: A re-examination. Planta 203, S85–S90. [DOI] [PubMed] [Google Scholar]
- Takeuchi, M., Ueda, T., Sato, K., Abe, H., Nagata, T., and Nakano, A. (2000). A dominant negative mutant of Sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 23, 517–525. [DOI] [PubMed] [Google Scholar]
- Tasaka, M., Kato, T., and Fukaki, H. (1999). The endodermis and shoot gravitropism. Trends Plant Sci. 4, 103–107. [DOI] [PubMed] [Google Scholar]
- Tasaka, M., Kato, T., and Fukaki, H. (2001). Genetic regulation of gravitropism in higher plants. Int. Rev. Cytol. 206, 135–154. [DOI] [PubMed] [Google Scholar]
- Weise, S.E., and Kiss, J.Z. (1999). Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int. J. Plant Sci. 160, 521–527. [DOI] [PubMed] [Google Scholar]
- Wysocka-Diller, J.W., Helariutta, Y., Fukaki, H., Malamy, J.E., and Benfey, P.N. (2000). Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127, 595–603. [DOI] [PubMed] [Google Scholar]
- Yamauchi, Y., Fukaki, H., Fujisawa, H., and Tasaka, M. (1997). Mutations in the SGR4, SGR5 and SGR6 loci of Arabidopsis thal-iana alter the shoot gravitropism. Plant Cell Physiol. 38, 530–535. [DOI] [PubMed] [Google Scholar]
- Zheng, H., von Mollard, G.F., Kovaleva, V., Stevens, T.H., and Raikhel, N.V. (1999). The plant vesicle-associated SNARE AtVTI1a likely mediates vesicle transport from the trans-Golgi network to the prevacuolar compartment. Mol. Biol. Cell 10, 2251–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H.Q., and Staehelin, L.A. (2001). Nodal endoplasmic reticulum, a specialized form of endoplasmic reticulum found in gravity-sensing root tip columella cells. Plant Physiol. 125, 252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]