Abstract
Recessive mutations of the early phase change (epc) gene in maize affect several aspects of plant development. These mutations were identified initially because of their striking effect on vegetative phase change. In certain genetic backgrounds, epc mutations reduce the duration of the juvenile vegetative phase of development and cause early flowering, but they have little or no effect on the number of adult leaves. Except for a transient delay in leaf production during germination, mutant plants initiate leaves at a normal rate both during and after embryogenesis. Thus, the early flowering phenotype of epc mutations is explained completely by their effect on the expression of the juvenile phase. The observation that epc mutations block the rejuvenation of leaf primordia in excised shoot apices supports the conclusion that epc is required for the expression of juvenile traits. This phenotype suggests that epc functions normally to promote the expression of the juvenile phase of shoot development and to suppress the expression of the adult phase and that floral induction is initiated by the transition to the adult phase. epc mutations are epistatic to the gibberellin-deficient mutation dwarf1 and interact additively with the dominant gain-of-function mutations Teopod1, Teopod2, and Teopod3. Genetic backgrounds that enhance the mutant phenotype of epc demonstrate that, in addition to its role in phase change, epc is required for the maintenance of the shoot apical meristem, leaf initiation, and root initiation.
INTRODUCTION
Growth of the plant shoot can be divided into several discrete phases based on the character of the organs produced during these phases and the capacity of the shoot for reproductive development (Hackett, 1985; Zimmerman et al., 1985; Poethig, 1990). Genetic analyses of phase change in herbaceous plants have focused primarily on floral induction—that is, on the transition from an adult vegetative phase of shoot development to a reproductive phase in which the shoot produces flowers and flowering branches (Simpson et al., 1999). The regulation of earlier developmental transitions is poorly understood. We are particularly interested in the regulation of the transition from the juvenile to the adult phase of vegetative growth. Traditionally, this transition has been defined as a change in the reproductive competence of the shoot, but it also is marked by species-specific changes in a variety of vegetative traits, including leaf shape, leaf anatomy, root production, disease resistance, and the synthesis of a number of secondary compounds (Hackett, 1985; Kerstetter and Poethig, 1998). Mutations affecting this vegetative transition have been identified in several species, including pea (Wiltshire et al., 1994), Arabidopsis (Telfer and Poethig, 1998; Clarke et al., 1999; Hamada et al., 2000; Berardini et al., 2001; Prigge and Wagner, 2001), and maize (Freeling et al., 1992; Lawson and Poethig, 1995), and are beginning to provide some insight into the mechanism of this important but neglected aspect of plant development.
In maize, the juvenile and adult phases of vegetative development are distinguished primarily by features of the epidermis of the leaf blade, the most obvious of which are the presence of epicuticular wax (a juvenile trait) and epidermal hairs (an adult trait) (Freeling and Lane, 1994; Lawson and Poethig, 1995; Bongard-Pierce et al., 1996). Genes that regulate vegetative maturation in maize have been identified by screening for mutations that affect one or more of these phase-specific traits. Genes that promote the juvenile-to-adult transition are defined by recessive loss-of-function mutations that prolong the expression of juvenile traits and delay the appearance of adult traits. This class includes the gibberellin (GA)-deficient dwarf (an1, d1, d3, and d5) (Evans and Poethig, 1995) and vivaparous8 (vp8) (Evans and Poethig, 1997) genes. Genes that promote juvenile development have been defined primarily by dominant gain-of-function mutations that prolong the expression of juvenile traits. This class of genes includes Teopod1 (Tp1), Teopod2 (Tp2), Teopod3 (Tp3), and Corngrass (Cg), all of which are thought to act in a developmental pathway that operates independently of the pathway that regulates adult traits (Poethig, 1988).
Two other dominant mutations, Hairy sheath frayed-O (Bertrand-Garcia and Freeling, 1991) and Lax midrib-O (Schichnes and Freeling, 1998), affect the expression of phase-specific traits and many other aspects of leaf morphogenesis and are believed to play a general role in the regulation of developmental timing rather than being involved specifically in vegetative phase change. The only gene known to be required for the expression of juvenile vegetative traits in maize is glossy15 (gl15). Recessive, loss-of-function gl15 mutations partially transform the epidermis of juvenile leaves into an adult epidermis (Evans et al., 1994; Moose and Sisco, 1994) but have no other obvious effects on leaf morphology or shoot development. This phenotype, and genetic evidence indicating that gl15 acts downstream of the Tp genes, Cg (Evans et al., 1994; Moose and Sisco, 1994), vp8 (Evans and Poethig, 1997), and the gibberellins (Evans and Poethig, 1995), indicate that gl15 functions late in the pathway that promotes juvenile development. gl15 is predicted to encode a protein with a domain that is nearly identical to the putative DNA binding domain of APETALA2 in Arabidopsis (Moose and Sisco, 1996).
Although genetic analyses of vegetative phase change in maize have revealed some of the major features of this process, one of the major limitations of these studies is that they have largely used dominant gain-of-function mutations. Dosage studies have provided some information about the nature of these mutations, but their effect on gene function still is unknown. As noted above, gene function can be inferred accurately only from loss-of-function mutations, and only a few such mutations have been identified in maize. Here, we describe a new gene, early phase change (epc), that is required for the expression of the juvenile phase and that plays a critical role in several other aspects of shoot and root development.
RESULTS
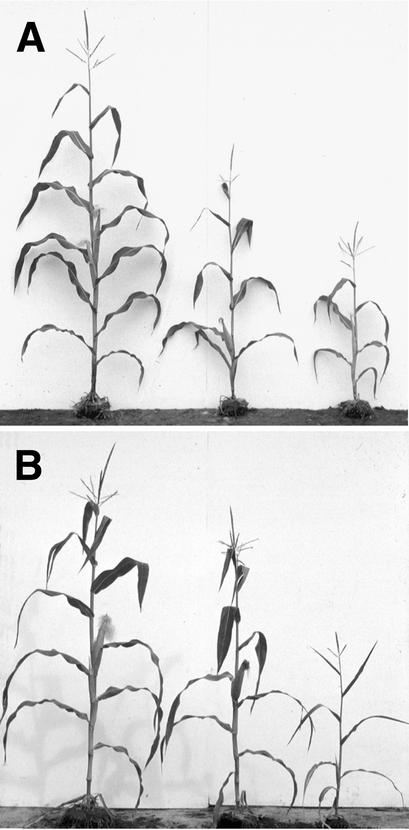
Most of the work presented here was conducted with two alleles of epc, one that arose spontaneously in a W23 genetic background (epc-W23) and an allele (epc-1s2p) present in the inbred background 1s2p. Several other alleles have been identified in our laboratory and by others (see Methods), but these were not characterized in detail in this study. The phenotype of all of these alleles is influenced strongly by genetic background and undefined environmental and/or developmental conditions. In a W23 background, epc-W23 and epc-1s2p primarily affect the number of juvenile and transition leaves, although sometimes this phenotype is poorly penetrant and variably expressed in this inbred background. This is illustrated for epc-W23 (W23) in Figure 1A and Table 1, rows 3 to 9, where each row represents the progeny of a single self-pollinated plant from the row above it. Even in this highly inbred background, some families have only a few affected progeny with an extremely mild phenotype, whereas others have a large number of affected plants with a wide range of phenotypes. This phenomenon also is characteristic of epc-1s2p in a W23 background (Table 2) as well as epc-W23/epc-1s2p (W23/1s2p) hybrids (Figure 1B, Table 1, rows 11 and 12). In other genetic backgrounds, the phenotype of epc-W23, epc-1s2p, and other epc alleles is much more severe and is fully penetrant (Figure 2B, Table 2). In the Oh43 and A632 backgrounds, for example, mutant shoots either fail to emerge above soil level or produce only one or two rudimentary leaves. epc mutations have no obvious effect on endosperm morphology.
Figure 1.
Wild-Type and epc Mutant Plants.
(A) Left, W23-Rnj; right, two epc-W23 (W23) plants.
(B) Left, +/epc-1s2p (W23/1s2p); right, two epc-W23/epc-1s2p (W23-Rnj/1s2p) plants.
Table 1.
Position of the First Transition Leaf in Wild-Type and epc Mutant Plants
| First Transition Leaf (% of Plants)
|
|||||||
|---|---|---|---|---|---|---|---|
| Rowa | Genotype | 2 | 3 | 4 | 5 | 6 | n |
| 1 | W23-Rnj | 67 | 33 | 24 | |||
| 2 | epc-W23 (W23-Rnj) | 73 | 17 | 25 | |||
| 3 | epc-W23 (W23) | 20 | 80 | 25 | |||
| 4 | epc-W23 | 11 | 11 | 44 | 33 | 9 | |
| 5 | epc-W23 | 50 | 20 | 30 | 10 | ||
| 6 | epc-W23 | 78 | 17 | 5 | 18 | ||
| 7 | epc-W23 | 14 | 86 | 22 | |||
| 8 | epc-W23 | 5 | 58 | 37 | 19 | ||
| 9 | epc-W23 | 4 | 7 | 64 | 25 | 28 | |
| 10 | +/epc-1s2p (W23-Rnj/1s2p) |
17 | 83 | 24 | |||
| 11 |
epc-W23/epc-1s2p (W23/1s2p) |
58 | 34 | 8 | 12 | ||
| 12 |
epc-W23/epc-1s2p (W23/1s2p) |
27 | 33 | 7 | 33 | 15 | |
Row 1, W23, ACR-nj; row 2, epc-W23 crossed six times to W23-Rnj; rows 3 to 9, epc-W23 in its original W23 background (each row represents the progeny of a single, self-pollinated plant from the row above it); row 10, progeny of a cross between W23-Rnj and the 1s2p inbred line; rows 11 and 12, progeny of a cross between epc-W23 in its original W23 background and the 1s2p inbred line.
Table 2.
Phenotypes of epc Mutant Alleles in Different Genetic Backgrounds
| Mutant Phenotypes
|
||||||
|---|---|---|---|---|---|---|
| Genotypea | Early Phase Change | Shoot Abortion | Nongerminating | Wild Type | Mutant Frequency | Expected Frequency |
| epc-W23/+ × epc-W23 (W23-Rnj) | 54 | 0 | 3 | 44 | 0.56 | 0.50 |
| epc-W23/+ Oh43 (7) × self | 0 | 33 | 58 | 230 | 0.28 | 0.25 |
| epc-W23/+ A632 (5) × self | 6 | 45 | 18 | 169 | 0.29 | 0.25 |
| epc-1s2p/+ W23 (3) × self | 15 | 0 | 0 | 104 | 0.14b | 0.25 |
| epc-1s2p/+ Oh43 (4) × self | 38 | 12 | 16 | 171 | 0.28 | 0.25 |
| epc-1s2p/+ A632 (5) × self | 0 | 21 | 47 | 231 | 0.23 | 0.25 |
| epc-Mo/+ Oh43 (3) × self | 13 | 18 | 25 | 183 | 0.23 | 0.25 |
| epc-mu/+ Oh43 (2) × self | 0 | 17 | 26 | 157 | 0.22 | 0.25 |
Numbers in parentheses indicate the number of times the allele was crossed to the inbred line.
Significantly different from 0.25 (P < 0.01).
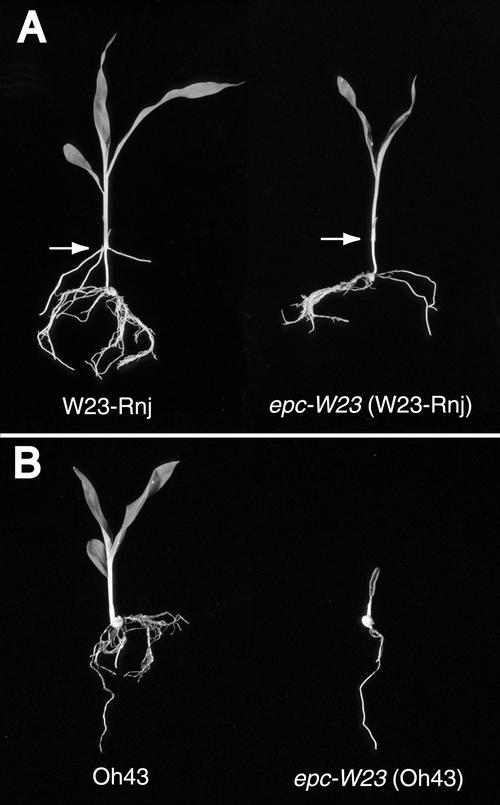
Figure 2.
Phenotype of epc-W23 in Different Genetic Backgrounds.
(A) epc-W23 (W23-Rnj).
(B) epc-W23 (Oh43).
Seedlings are 12 days old. Arrows indicate the coleoptile node.
epc was mapped by testing linkage to simple sequence repeat (SSR) markers using F2 families from self-pollinated epc-W23/+ (W23/Oh43) plants. Only mutant progeny were genotyped to avoid misscoring as a result of the poor penetrance of this mutation. Data from 133 mutant progeny place epc on the long arm of chromosome 8 in the following position: epc—0.4 centimorgan (cM)—bnlg 2082, bnlg 1067—10.9 cM—bnlg 162—1.5 cM—bnlg 666.
Effect of epc on Vegetative Identity
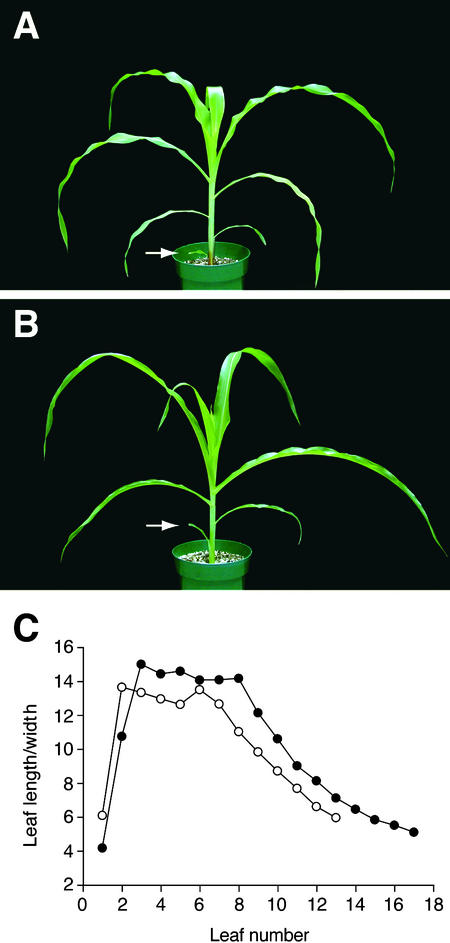
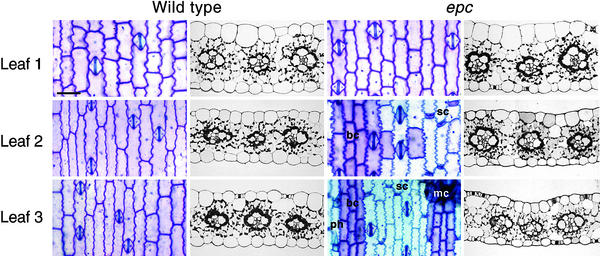
The effects of epc mutations on vegetative identity are apparent in a W23 genetic background, in which the deleterious effects of these mutations on shoot viability are suppressed. The effect of epc on leaf identity is particularly striking in the case of the first leaf. In wild-type plants, this leaf differs from leaves at higher nodes in that it is small and has a blunt or indented tip rather than a pointed tip. In contrast, the first leaf of epc-W23 (W23) plants is unusually long and narrow and often has a pointed tip (Figures 2A and 3); in these respects, it resembles leaves produced at higher nodes on wild-type plants. epc leaves formed later also are similar in shape to leaves at higher nodes on wild-type plants (Figure 3). This effect on leaf shape is accompanied by the precocious expression of all known phase-specific epidermal traits (Figure 4), including the production of epidermal hairs, bulliform cells and short cells, highly crenelated lateral cell walls, a flat outer cell wall, a thick cuticle, and aqua cell wall staining with toluidine blue. Although these traits are first observed on leaf 6 in wild-type plants, in epc mutants they usually are first apparent on leaf 2. epc mutations also cause the leaf blade to be uprolled slightly, with the result that mutant leaves often are held in a more upright position than wild-type leaves (Figures 3A and 3B).
Figure 3.
Effect of epc-W23 on Leaf Shape.
(A) W23-Rnj.
(B) epc-W23 (W23-Rnj). Leaves are slightly uprolled and are more upright than normal. The first few leaves also are longer than the corresponding wild-type leaves.
(C) The length/width ratio of successive leaves of W23-Rnj (closed circles) and epc-W23 (W23-Rnj) (open circles).
Arrows in (A) and (B) indicate leaf 1.
Figure 4.
Leaf Anatomy in Wild-Type and epc Mutant Plants.
The first and second columns illustrate the anatomy of leaves 1, 2, and 3 (numbered in the order in which they were initiated during shoot development) from a wild-type plant; the third and fourth columns illustrate the anatomy of leaves 1, 2, and 3 from an epc mutant plant. The first and third columns show adaxial epidermal peels stained with toluidine blue; the second and fourth columns show cross-sections of expanded leaves. bc, bulliform cell; mc, macrohair; ph, prickle hair; sc, short cell. Bar = 100 μm.
To determine if epc regulates mesophyll identity, we examined its effect on the expression of Ragged leaves (Rg), a dominant mutation that causes adult leaves to develop necrotic lesions as a result of cytolysis of mesophyll cells (Mericle, 1950; Evans et al., 1994; Bongard-Pierce et al., 1996), and on Rolled leaf (Rld), a dominant mutation that affects leaf polarity and causes leaf curling (Hay et al., 2000). We found that leaf necrosis occurred two leaves earlier in epc Rg/+ double mutants than in Rg/+ plants and that this effect was correlated with the first leaf expressing adult-specific epidermal traits (i.e., the first transition leaf). Rg/+ plants produced their first transition leaf at node 5.7 ± 0.2 and their first ragged leaf at node 5.8 ± 0.2, whereas epc Rg/+ double mutants produced their first transition leaf at node 3.2 ± 0.1 and their first ragged leaf at node 3.5 ± 0.3. A similar result was obtained with Rld. Rld/+ plants produced their first transition leaf and their first rolled leaf at node 5.6 ± 0.2, whereas epc Rld/+ plants produced their first transition leaf and their first rolled leaf at nodes 3.3 ± 0.2 and 3.9 ± 0.1, respectively. This result is consistent with the effects of epc on leaf morphology and leaf anatomy and suggests that epc affects all aspects of phase-specific leaf identity.
To quantify the effect of epc mutations on the duration of juvenile and adult development, we examined their effects on the two most obvious phase-specific traits, epicuticular wax (a juvenile trait) and epidermal hairs (an adult trait) (Table 3). The data demonstrate that epc reduces the number of juvenile leaves but has only a minor effect on adult leaf number. In a W23-Rnj background, epc-W23 had approximately one juvenile leaf, one transition leaf, and 10 adult leaves, compared with wild-type plants, which usually had five juvenile leaves, two transition leaves, and nine adult leaves. epc-W23/epc-1s2p (W23/1s2p) plants had one or two juvenile leaves, one or two transition leaves, and 10 adult leaves, whereas +/epc-1s2p (W23/1s2p) plants produced five juvenile leaves, three transition leaves, and 10 or 11 adult leaves. The reduced leaf number of mutant plants therefore is accounted for entirely by a reduction in the number of juvenile and transition leaves.
Table 3.
Number of Juvenile, Transition, and Adult Leaves in epc and Wild-Type Plants
| Genotype | Juvenile Leavesa |
Transition Leavesb |
Adult Leavesc |
n |
|---|---|---|---|---|
| W23-Rnj | 4.7 ± 0.1 | 2.1 ± 0.1 | 9.4 ± 0.1 | 12 |
|
epc-W23 (W23-Rnj) |
1.3 ± 0.1 | 0.9 ± 0.1 | 10.1 ± 0.1 | 15 |
| +/epc-1s2p (W23-Rnj/1s2p) |
4.8 ± 0.1 | 2.8 ± 0.1 | 10.6 ± 0.1 | 17 |
|
epc-W23/epc-1s2p (W23-Rnj/1s2p) |
1.5 ± 0.2 | 1.3 ± 0.25 | 10.2 ± 0.4 | 12 |
All values are n ±sem.
Completely covered with epicuticular wax, no epidermal hairs. b Partially covered with epicuticular wax and epidermal hairs.
No epicuticular wax, completely covered with epidermal hairs.
Effect of epc on Shoot Growth
epc mutants often germinated slightly later than wild-type seedlings and usually had fewer expanded leaves than wild-type plants throughout shoot growth. The severity of this effect usually was correlated with the severity of the phase change phenotype of mutant seedlings, raising the possibility that the primary function of epc may be to regulate leaf production and shoot growth rather than the developmental identity of the shoot. If epc mutations delayed leaf production without affecting the timing of vegetative maturation, mutant plants would produce fewer juvenile leaves. To test this hypothesis, we studied the effect of epc mutations on the rate of leaf initiation before and after germination.
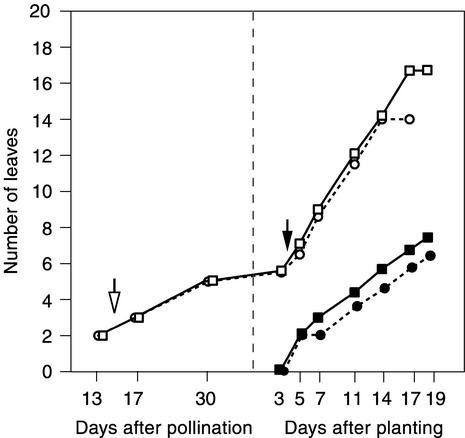
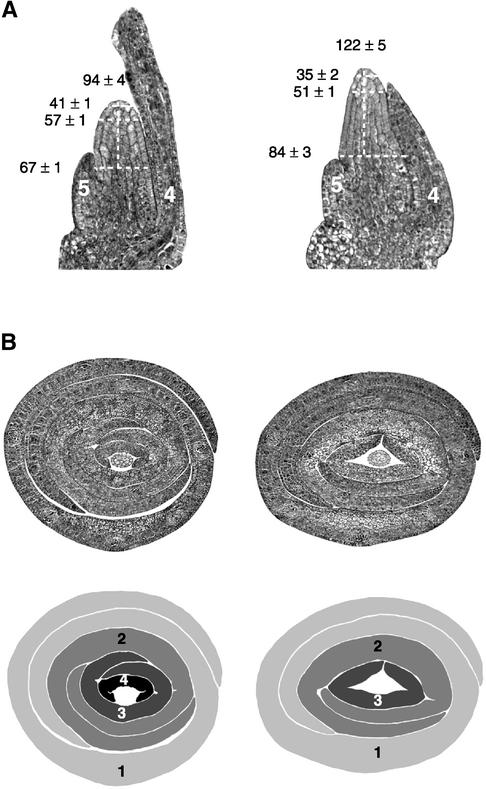
epc had no effect on the rate of leaf initiation early in embryogenesis (Figure 5), but it had a significant effect on leaf and meristem growth late in embryogenesis. By 30 days after pollination, leaves 3, 4, and 5 were significantly smaller in mutant embryos than in wild-type embryos (Table 4, Figure 6), and mutant shoot apical meristems (SAMs) were morphologically aberrant (Figure 6A). The dimensions of the SAM in 30-day-old mutant and wild-type embryos revealed that the epc SAM is significantly (P < 0.01 for each dimension) broader at the base, narrower at the tip, and taller than the wild-type SAM (Figure 6A).
Figure 5.
Rate of Leaf Initiation and Leaf Emergence in Wild-Type and epc Mutant Plants.
The data at left represent leaves initiated during seed development and were collected from specimens grown under field conditions; the data at right represent leaves initiated after germination and were collected from specimens grown in a growth chamber (see Methods). The open arrow indicates the time at which the first transition leaf in epc was initiated; the closed arrow indicates the initiation time of the first transition leaf in wild-type plants. Open squares, total number of leaves and leaf primordia in wild-type plants; open circles, total number of leaves and leaf primordia in epc mutant plants; closed squares, visible leaves in wild-type plants; closed circles, visible leaves in epc mutant plants. sem bars are obscured by the symbols. Five to 10 samples were analyzed at each time point.
Table 4.
Lengths (μm) of Leaf Primordia in 30-Day-Old Wild-Type and epc Mutant Embryos
| Leaf No.
|
|||||
|---|---|---|---|---|---|
| Genotype | 1 | 2 | 3 | 4 | 5 |
| +/epc-1s2p (W23-Rnj/1s2p) | 1610 ± 29 | 1166 ± 31 | 835 ± 36 | 423 ± 16 | 74 ± 7 |
| epc-W23/epc-1s2p (W23/1s2p) | 1560 ± 30 | 1107 ± 25 | 654 ± 40a | 196 ± 20a | 16 ± 2a |
n = 9 for each genotype.
Significantly different from wild type (P < 0.01).
Figure 6.
Morphology of Wild-Type (Left) and epc Mutant (Right) Shoot Apices in 30-Day-Old Embryos.
(A) Median longitudinal sections. The height (μm ±sem) of the SAM, its width 20 and 40 μm below the tip, and its width just above the last visible leaf primordium are indicated. Leaves 4 and 5 are indicated. These data represent an average of 10 wild-type and 10 epc samples.
(B) Cross-sections taken at the tip of the SAM (top) and a scheme of the leaf primordia in these sections (bottom). Note that leaves 2 and 3 do not extend as far around the shoot apex in mutant plants as in wild-type plants and that leaves 4 and 5 of mutant embryos are too short to be included in this plane. Leaves are numbered in the order in which they were initiated during shoot development, with leaf 1 being the first initiated leaf.
Mutant seedlings exhibited a transient delay in leaf initiation during germination, but they initiated leaves at the same rate as wild-type plants throughout the remainder of shoot growth and produced a tassel 3 days earlier than normal (Figure 5). This effect on tassel initiation was reflected in the timing of anthesis, which also occurred 3 days earlier in mutant than in wild-type plants (49.7 versus 53.3 days after planting). The reduced leaf number of mutant plants therefore is attributable to both an early pause in leaf production and early flowering.
In the families used for this study, the majority of mutant plants produced their first transition leaf at node 2 or 3 and their first completely adult leaf at node 3 or 4 (Table 1, row 11, and Table 3). Most wild-type plants produced their first transition leaf at node 6 and their first completely adult leaf at node 8 (Table 1, row 10, and Table 3). Thus, mutant plants produce their first adult leaves during embryogenesis, whereas wild-type plants do not make this transition until after germination. We conclude that the effect of epc mutations on leaf identity does not result from a delay in the timing of leaf initiation because mutant plants actually initiate adult leaves earlier than wild-type plants.
epc Prevents Rejuvenation in Vitro
Previous studies have shown that adult shoot meristems (Irish and Karlen, 1998) and immature adult leaf primordia (Orkwiszewski and Poethig, 2000) revert to a juvenile state when adult shoot apices are excised and cultured in vitro. To determine if epc normally is required for the production of juvenile traits, we tested the effect of this treatment on mutant and wild-type shoots. Consistent with previous results, we found that adult leaf primordia on wild-type shoots (n = 12) were rejuvenated completely or partially if these primordia were 3 mm or less in length at the time the shoot apex was placed in culture (Figure 7A). In contrast, none of the 11 mutant shoots produced rejuvenated leaves (Figure 7B), even though the leaf primordia on these mutant apices were the same size as wild-type leaf primordia at the time of culture. This result demonstrates that epc is required for the expression of juvenile vegetative traits and provides additional evidence that the effect of epc mutations on leaf identity is not an indirect effect of a delay in leaf initiation.
Figure 7.
Toluidine Blue–Stained Adaxial Epidermal Peels from the Base of Leaf 9 of Wild-Type and epc Mutant Shoots Cultured in Vitro.
(A) +/epc-1s2p (W23-Rnj/1s2p).
(B) epc-W23/epc-1s2p (W23/1s2p).
Effect of epc on Shoot Viability and Root Growth
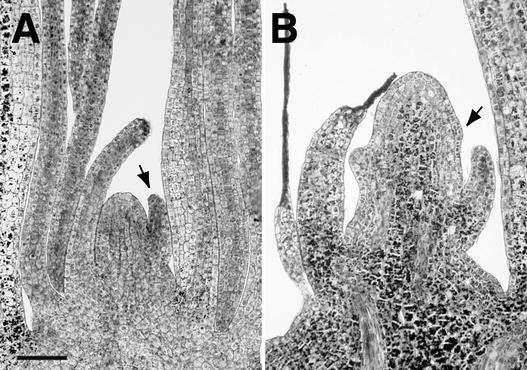
As noted above, the phenotypes of epc-W23 and epc-1s2p are particularly strong in the Oh43 and A632 inbred backgrounds. In both of these backgrounds, the shoots of most mutant plants either fail to emerge above soil level or produce only one or two rudimentary expanded leaves (Figure 2B). To determine the basis of this phenotype, five mutant and five wild-type F2 seedlings from a self-pollinated epc-W23/Oh43 plant were harvested 5 days after planting and analyzed histologically. At this stage, leaf 1 had just emerged from the coleoptile. Mutant seedlings could be distinguished from wild-type seedlings by their unfused coleoptiles and short mesocotyls; confirmation that these abnormal seedlings were homozygous for epc-W23 was obtained by genotyping these seedlings for the SSR marker bnlg 2082, located 0.4 cM from epc. The shoot apices of mutant seedlings had six leaf primordia, the youngest of which (leaves 4, 5, and 6) were significantly smaller than the corresponding wild-type leaves (Table 4). There was no obvious sign of cell death in leaf 1, but other leaves in the shoot were either partially or completely necrotic (Figure 8). Mutant seedlings also had an unusually large SAM compared with wild-type SAMs and had a lower rate of cell division. Wild-type SAMs had 56 ± 6 cells in a median longitudinal section, whereas mutant plants had 139 ± 18. Wild-type plants had a mitotic index of 0.015 ± 0.003 in the SAM, whereas the mitotic index in epc was 0.004 ± 0.003. The cells in mutant SAMs also had an unusually large amount of starch or, in the case of the most severely affected SAMs, were highly vacuolated. This phenotype is consistent with the morphology of mutant SAMs in viable seedlings and suggests that shoot abortion in mutant seedlings occurs either late in seed development or during germination—that is, at approximately the same time as the transient delay in leaf initiation observed in less severely affected plants.
Figure 8.
Median Longitudinal Sections of Wild-Type and Aborted epc Mutant SAMs 5 Days after Planting.
(A) Wild type (Oh43).
(B) epc-1s2p (Oh43).
Arrows indicate leaf 6. Bar = 100 μm.
epc mutations also affect root development. As shown in Figure 2A, epc-W23 plants produce few lateral roots and produce no roots at the coleoptile node by 12 days after planting. In contrast, wild-type plants produce an extensively branched root system and usually have three or four roots at the coleoptile node by this stage. Adventitious roots usually develop some time later at the coleoptile node and other basal nodes of mutant plants, but the number and extent of these roots usually is reduced significantly compared with wild-type plants. The most severely affected plants have such a rudimentary root system that they have dif-ficulty remaining upright. The effect of epc mutations on lateral root development is more severe in genetic backgrounds in which these mutations cause shoot abortion (Figure 2B). Although this phenotype may be an indirect result of the effect of these mutations on the growth of the shoot, it is true that adventitious root production is a juvenile trait in maize (Poethig, 1988), as it is in many other species (Hackett, 1985). Therefore, the reduction in root production in mutant plants may either result from the effect of this mutation on phase change or reflect a more direct role for this gene in root initiation.
Genetic Interactions
epc and id
To study the basis for the early flowering phenotype of epc mutants, we examined the interaction of mutant alleles with the late flowering mutation indeterminate (id). id encodes a zinc finger protein that regulates the production of a leaf-derived diffusible factor required for flowering (Colasanti et al., 1998). epc id double mutants underwent vegetative phase change at exactly the same node as epc mutants and had the vegetative phenotype of epc plants (Figure 9A). However, like their id siblings, epc id double mutants did not flower before the end of the summer growing season. This result demonstrates either that id is epistatic to epc with respect to flowering time or that these mutations interact additively in controlling this trait. We were unable to distinguish between these alternatives because none of the id plants in this experiment flowered before the end of the growing season. However, the observation that epc id plants are not early flowering (i.e., that the epc mutation is not epistatic to id with respect to flowering time) implies that wild-type epc function is not required for the late flowering phenotype of id mutants.
Figure 9.
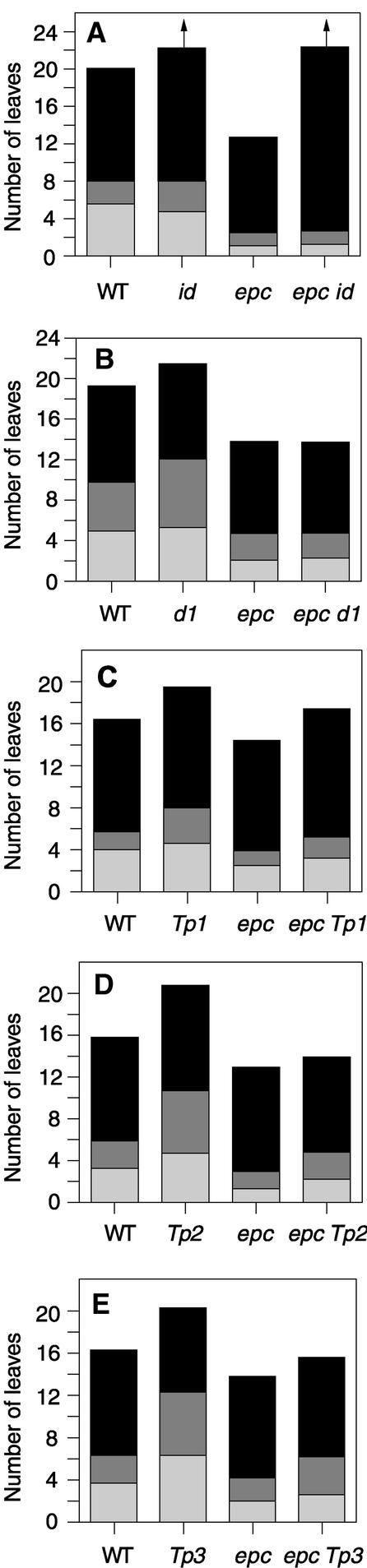
Effect of Mutations Affecting Flowering Time and Vegetative Phase Change on Leaf Identity in Single Mutants and Double Mutants with epc.
(A) epc and id. The arrows indicate that flowering had not occurred by the time the experiment was terminated.
(B) epc and d1.
(C) epc and Tp1/+.
(D) epc and Tp2/+.
(E) epc and Tp3/+.
WT, wild type. Light gray bars, juvenile leaves; dark gray bars, transition leaves; black bars, adult leaves.
epc and d1
In maize, GA promotes vegetative and reproductive maturation (Evans and Poethig, 1995). In addition to reducing stem and leaf expansion, this mutation has been shown to prolong the expression of juvenile traits and to increase total leaf number (Evans and Poethig, 1995). To test the hypothesis that epc promotes adult development by increasing the endogenous level of GA or the sensitivity of cells to this hormone, we examined the interaction between epc-1s2p and the GA-deficient d1 mutation. The phenotypes of single and double mutant plants were compared in F2 families segregating both mutations to minimize the effects of genetic background. Wild-type, d1, epc, and d1 epc-1s2p double mutant plants could be identified readily in these families by their unique phenotypes. Double mutant plants were identical to epc single mutants in terms of leaf identity and leaf number, but they had the dwarf phenotype of d1 (Figures 9B and 10A). Thus, the epc mutation is completely epistatic to d1 with respect to phase-specific traits but does not correct the leaf and stem expansion phenotype of d1. We conclude that the wild-type allele of epc is required for the effect of the d1 mutation on both vegetative and reproductive phase change and that epc acts downstream of GA.
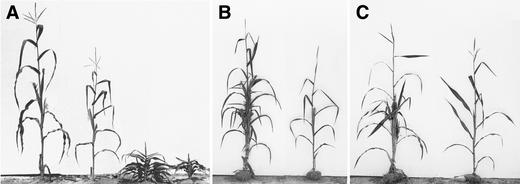
Figure 10.
Phenotypes of d1, Tp1, and Tp2 and Their Interaction with epc.
(A) Left to right: wild type, epc, d1, epc d1.
(B) Left, Tp1/+; right, Tp1/+ epc-W23.
(C) Left, Tp2/+; right, Tp2/+ epc-W23.
epc and the Teopod Mutations
Tp1, Tp2, and Tp3 are dominant gain-of-function mutations that prolong the expression of juvenile vegetative traits (Poethig, 1988). In contrast to the epistasis displayed by epc in combination with d1 and id, epc mutations interacted with all three of these dominant mutations in an additive fashion. In families segregating epc and any one of the Tp1, Tp2, or Tp3 mutations, plants with a wild-type and a Tp mutant phenotype occurred at the expected ratio of 1:1 (data not shown). However, approximately half of these Tp plants produced adult traits at the same, or earlier, leaf position than wild-type plants and had the same, or fewer, total leaves than wild-type plants. Because Tp1, Tp2, and Tp3 always produce more juvenile leaves and more total leaves than wild-type plants (Poethig, 1988), these plants were assumed to be epc Tp double mutants. In all three cases, the number of juvenile and transition leaves in putative epc Tp double mutants was, on average, intermediate between epc and Tp/+ plants (Figures 9C to 9E). Furthermore, although the overall morphology of these putative double mutants was “Tp-like,” this phenotype clearly was less severe than that of Tp single mutants (Figures 10B and 10C). In particular, putative double mutants had broader leaves, fewer tillers, and, in the case of epc Tp2, a more highly branched tassel than single mutant plants. Thus, epc mutations partially suppress the dominant gain-of-function phenotype of these mutations, suggesting that wild-type epc function normally is required for this phenotype.
DISCUSSION
Recessive mutant alleles of epc affect many aspects of the vegetative phase identity of the shoot and, in some genetic backgrounds, also affect root production, seedling morphology, and the viability of the shoot apex. We were particularly interested in the effect of these mutations on phase change because they have a more dramatic effect on this process than any mutations described previously in maize. Recessive mutations of gl15 are phenotypically similar to epc mutations in accelerating the expression of adult traits, but gl15 mutations affect only a subset of phase-specific epidermal traits and have no obvious effect on the development of the first two juvenile leaves or on flowering time (Evans et al., 1994; Moose and Sisco, 1994). The dominant gain-of-function mutations Tp1, Tp2, Tp3, and Cg have a phenotype opposite to that of epc mutations in that they prolong the expression of many juvenile traits. However, these mutations do not significantly affect the onset of the adult vegetative phase (Poethig, 1988) and, at least in the case of Tp1 and Tp2, do not affect the duration of shoot growth or reproductive competence (Bassiri et al., 1992). vp8 (Evans and Poethig, 1997) and GA-deficient mutations (Evans and Poethig, 1995) delay both vegetative and reproductive phase change, but they have relatively subtle effects on these transitions compared with epc mutations. Therefore, the dramatic effect of epc mutations on vegetative and reproductive phase change is unusual, and suggests that this gene plays a crucial role in the regulation of shoot maturation.
Role of epc in Phase Change
The pleiotropic phenotype of epc mutants suggests two possible explanations for their effects on the expression of juvenile traits. One possibility is that the reduction in the number of juvenile leaves in mutant plants is a consequence of a delay in leaf production early in shoot development or the necrosis of early formed leaves. In this case, the primary function of epc would be to regulate processes important for shoot and root growth, not phase change per se. An alternative possibility is that epc regulates several aspects of plant development, one of which is the transition from the juvenile to the adult phase. Our results support the latter conclusion. Specifically, we found that epc mutations do not affect the rate of leaf initiation in mutant and wild-type plants during the first four to five plastochrons of shoot growth and produce only a transient delay in leaf production after germination. In a W23 genetic background, all of the primordia produced during this early period of shoot development survive and develop features typical of adult leaves. Although epc mutations produce leaf necrosis in some genetic backgrounds, this effect was observed only in association with shoot abortion and does not account for the phase change phenotype of epc mutations in these backgrounds, because leaves produced before the shoot abortion express adult traits. The observations that epc mutations prevent leaf rejuvenation in adult shoot apices cultured in vitro and partially suppress the juvenile phenotype of Tp1, Tp2, and Tp3 clearly demonstrate that epc is required for the expression of juvenile traits and suggest that it functions throughout shoot development. Because this effect on leaf identity is associated with a corresponding change in leaf number, we conclude that epc regulates the transition from juvenile to adult development rather than being involved solely in the regulation of juvenile organ identity, as is the case with gl15 (Evans et al., 1994; Moose and Sisco, 1994).
The relationship between vegetative maturation and reproductive maturation is a major unsolved problem in plant biology. Although the vegetative morphology of a shoot generally is correlated with its reproductive competence, it is unknown if these processes are regulated independently or by a single developmental program. This question has been addressed by investigators studying woody plants (Greenwood and Hutchinson, 1993; Hackett and Murray, 1993, 1997), but with a few exceptions investigators studying reproductive development in herbaceous plants have ignored the relationship between vegetative and reproductive maturation. In Arabidopsis, loss-of-function mutations in EMBRYONIC FLOWER1 (EMF1) and EMBRYONIC FLOWER2 (EMF2) completely eliminate the development of the rosette (Yang et al., 1995). Based on this phenotype and the interaction between emf1 and emf2 and mutations that affect flowering time and floral morphogenesis, it has been proposed (Yang et al., 1995) that these genes regulate all phase transitions during shoot growth. Whether EMF1 and EMF2 play a role in the vegetative phase is unknown, however, because severe mutant alleles never produce a rosette and the effect of antisense suppression of EMF1 (which has a less severe phenotype than mutant alleles) on vegetative phase change has not been examined (Aubert et al., 2001).
Other genes involved in phase change fall into three classes with respect to their effect on vegetative and reproductive development. Genes that play a role in both vegetative phase change and floral induction are defined by mutations/transgenes that simultaneously affect the duration of both the juvenile and adult phases of vegetative development. We are not aware of any mutations with this phenotype in maize, but several such mutations and transgenes have been described in Arabidopsis (Martinez-Zapater et al., 1995; Telfer et al., 1997; Melzer et al., 1999; Scott et al., 1999). Interestingly, most of these genes are involved either in the synthesis of or the response to GA or in the phytochrome response pathway. A second class consists of genes that regulate floral induction after the shoot is in the adult phase. This class is represented by mutations (Wiltshire et al., 1994; Telfer et al., 1997; Soppe et al., 1999; Vladutu et al., 1999; Gomez-Mena et al., 2001) and transgenes (Weigel and Nilsson, 1995) that affect the duration of the adult phase but have little or no effect on the duration of the juvenile vegetative phase. The vegetative phenotype of the id mutation and its interaction with epc suggest that it is a member of this class of genes. Mutations in the third class affect the duration of the juvenile phase but have only a minor effect on the duration of the adult phase. In maize, this class includes epc and mutations in genes involved in GA biosynthesis (Evans and Poethig, 1995); in Arabidopsis, it is represented by hasty (Telfer and Poethig, 1998) and squint (Berardini et al., 2001).
The observation that epc mutations affect the duration of the juvenile phase but not the adult phase is significant for several reasons. First, this phenotype demonstrates that the vegetative development of the shoot is divided into at least two discrete developmental phases. Although this has been accepted widely by investigators studying woody plants, investigators studying herbaceous plants tend to think of vegetative shoot maturation as a gradual, quantitative process (Schultz and Haughn, 1993; Yang et al., 1995) rather than as a progression between discrete developmental states. Furthermore, the fact that epc mutations have little effect on the number of adult leaves suggests that the timing of floral induction during the adult phase (which is what determines the number of adult leaves in determinate plants such as maize) does not depend on the length of the juvenile phase. Thus, in maize, the timing of floral induction appears to depend on the timing of the juvenile-to-adult transition, not on total leaf number. The effect of epc mutations on juvenile and adult leaf number also suggests that transition to the adult phase is not only permissive for floral induction but actually initiates this process. If floral induction and vegetative phase change were completely independent, a truncation of the juvenile phase would be associated with an extension of the adult phase because a larger fraction of the vegetative growth of the shoot would be spent in this phase. It should be emphasized that the juvenile-to-adult transition clearly is not the only factor that regulates the timing of floral induction. Many environmental conditions, such as photoperiod and nutrition, have significant effects on this process. However, these factors appear to affect the mechanism of floral induction primarily during the adult phase, not during the juvenile phase. In our experience, the leaf position of this transition does not vary dramatically in inbred plants grown under a wide range of field conditions or in the greenhouse, despite the significant effects these conditions often have on total leaf number. We propose that in maize, environmental conditions that affect flowering time act primarily to regulate the duration of the adult phase of shoot development rather than the duration of the juvenile phase.
Genetic Interactions
In maize, epc and GA specifically affect vegetative phase change: epc delays this transition and GA promotes it. To determine if GA and epc act in the same regulatory pathway, we examined the interaction between epc mutations and the GA-deficient mutation d1. We found that epc d1 double mutants have the dwarf stature of d1 but the vegetative identity of epc single mutants; that is, epc mutations are completely epistatic to the effect of the d1 mutation on phase change. This result demonstrates that the prolonged expression of juvenile traits in d1 mutant plants requires wild-type epc function, whereas the precocious expression of adult traits in epc mutant plants either does not require GA or requires very low levels of this hormone. This observation is consistent with the hypothesis that these factors act in the same pathway, with epc functioning downstream of GA.
The functional relationship between epc and the tp genes is less clear, largely because the tp genes are defined by gain-of-function mutations. Thus, although the additive interaction between epc and the Tp mutations could mean that epc and the tp genes operate in parallel pathways, it is conceivable that these genes act in the same pathway. For example, if epc is an upstream activator of tp2, epc-W23 could partially correct the Tp2 mutant phenotype by reducing the expression level of this neomorphic mutation.
Function of epc
The effect of epc mutations on phase change does not represent the full range of this gene's function. In the Oh43 and A632 inbred backgrounds, the phenotype of epc-W23 is fully penetrant, uniform, and quite severe. In these inbred backgrounds, epc-W23 produces distorted seedlings and arrests shoot development at approximately the fourth or fifth plastochron, if not before. Aborted seedlings have abnormally enlarged shoot meristems and unusually small leaf primordia, suggesting that shoot abortion may be a consequence of a defect in leaf initiation or leaf expansion. The abnormal morphology of mutant meristems is consistent with this hypothesis. The SAM of viable mutant shoots at 30 days after pollination is unusually tall and is broader at the base and narrower at the tip than normal. This morphology could result from a defect in leaf initiation or leaf expansion because such a defect would cause cells that otherwise would become part of a leaf primordium to be retained within the SAM, producing an increase in the number of cells at the base of the SAM. If developing leaf primordia promote cell division in more apical regions of the SAM, this defect also would account for the reduced size of the meristem tip. The subsequent expansion of cells in suppressed leaf primordia and in the more apical region of the SAM would account for the unusually large size of aborted SAMs. An alternative possibility is that epc mutations disrupt leaf initiation and meristem growth independently, in which case the overall size of the SAM at any particular time in development would depend on the sum of these effects.
This severe phenotype and the pleiotropic effects of epc mutations on shoot and root development in backgrounds in which these mutations are partially suppressed indicate that epc is involved in a number of different processes in maize. In addition to regulating vegetative phase change and leaf initiation, epc plays a role in leaf expansion (mutant leaves are slightly uprolled) and root development. This does not, of course, make its role in phase change any less interesting. Most major regulatory factors have diverse roles in growth and development; obvious examples include all major phytohormones and photoreceptors. Defining the functions of such molecules by mutational analysis can be difficult because loss-of-function mutations often have pleiotropic phenotypes that obscure the role of these factors in specific developmental or physiological processes. In such cases, weak alleles or conditions that suppress some aspects of a mutant phenotype provide critical information about gene function. Thus, the phenotype of epc mutations in W23 reveals a role for this gene in the regulation of phase change that is not obvious from its more severe phenotype in other genetic backgrounds. The genes responsible for this difference in expressivity clearly are of interest and could be identified by analyzing crosses between mutant lines that display suppressed and more severe phenotypes.
Penetrance and Expressivity of epc
The penetrance and expressivity of epc-W23 in a W23 genetic background are quite variable. This could be because of the variable activity of epc-W23 or the variable activity of suppressors present in this genetic background. Preliminary evidence (M. Sauer and R.S. Poethig, unpublished observations) indicates that the phenotype of other mutant alleles also is suppressed in this genetic background, but we have not yet characterized the penetrance or expressivity of this phenotype. We assume that these alleles will display the same variability as epc-W23 if this variability is a function of the variable activity of the suppressors present in this background. The poor penetrance of epc-W23 in W23 has implications for investigators who use this inbred background and related genetic stocks in their research. We have found that W23-converged stocks obtained from several investigators contain an epc mutation. In addition, the inbred line 1s2p is fixed for an allele of epc that is phenotypically indistinguishable from epc-W23 and has the same polymorphisms as epc-W23 at several SSR loci (phi 119, umc 1034, bnlg 669, bnlg 1067, bnlg 2082, phi 014, bnlg 119) in a 20-cM region surrounding this gene. These observations sug-gest that epc-W23 and epc-1s2p represent a mutation that arose some time ago that may be present in many maize stocks, particularly those that have these inbred lines in their pedigree.
The paucity of mutations in maize that have the precocious adult phenotype of epc mutations is surprising because mutations with this phenotype block epicuticular wax production. Many mutations that reduce or eliminate epicuticular wax have been identified in maize because the mutants have an obvious “glossy” appearance. However, we identified only one phase change gene, gl15, in the existing collection of glossy mutations at the Maize Stock Center (Urbana, IL). It is possible that genes involved in promoting juvenile development have an aborted shoot phenotype like that of epc, in which case they would not exist in collections of glossy mutations. The other obvious possibility is that many genes that regulate juvenile development in maize are functionally redundant and therefore have no obvious loss-of-function phenotype. This conclusion is supported by the observation that an ethyl methanesulfonate–induced revertant of the dominant Tp1 mutation and a Mutator-induced revertant of the dominant Tp2 mutation both have homozygous wild-type phenotypes (R.S. Poethig, unpublished results). At present, epc is the only gene known to be essential for the expression of the juvenile phase in maize. Therefore, it is an important entry point into the mechanism of phase change in this species.
METHODS
Genetic Stocks and Alleles
epc-W23 was identified in an inbred maize (Zea mays) W23 line obtained from E.H. Coe, Jr. (University of Missouri, Columbia). The epc-1s2p allele was identified in a 1s2p stock obtained from M. Freeling (University of California, Berkeley); the 1s2p inbred line was found subsequently to be homozygous for this mutation. For the sake of clarity, the 1s2p line is referred to as epc-1s2p (1s2p) in this article. Two other mutant alleles of epc have been identified in our laboratory. epc-mu was identified in a targeted screen for Mutator-induced alleles of epc; it was the only allele identified in a screen of ∼100,000 F1 plants produced by crossing epc-W23 to lines containing Robertson's Mu. epc-Mo was preexisting in a Mu stock obtained from Guri Johal (University of Missouri, Columbia). epc-mu and epc-Mo mutations are unlikely to represent reisolates of epc-W23 and epc-1s2p because the stocks in which they arose possess unique alleles of one or more SSR markers (bnlg 2082, bnlg 1067, bnlg 667) located 0.4 centimorgan (cM) from epc. The aborted shoot 3 gene, which was identified by D. Laudencia-Chingcuanco (Plant Gene Expression Center, Albany, CA), and the narrow leaf 4 gene, which was identified by R.F. Baker and M. Freeling (University of California, Berkeley), also have been shown to be allelic to epc.
The epc-W23 (W23) stocks used in this study were generated by crossing epc-W23 six times to an ACR-nj W23 stock (W23-Rnj) obtained from J. Beckett (University of Missouri, Columbia) that does not contain this mutation. epc-W23 (OH43) was generated by crossing epc-W23 six times to Oh43. F1 progeny produced by crossing epc-1s2p (1s2p) to the original epc-W23 (W23) stock are vigorous and display a strong phase change phenotype; they were used for many of the experiments described in this article. The wild-type controls for these mutants were the progeny of a cross between epc-1s2p (1s2p) and W23-Rnj.
Families segregating epc-W23 and the dominant Ragged leaves1 (Rg1) were constructed by crossing epc-W23 as males onto Rg1/+ and then crossing F1 Rg1 plants as females by epc. The first leaves with any small chlorotic regions on the blade were scored as the first leaves to express Rg1. Families segregating epc-1s2p and Rld were constructed in a similar fashion. Families segregating indeterminate growth (id) and epc were generated by crossing +/id females by epc-1s2p and self-crossing the F1. Mutant id plants were identified as those that had not flowered by the end of the summer field season. Families segregating epc and Tp1, Tp2, or Tp3 were generated by crossing Tp/+ (W23-Rnj) females by epc-W23 (W23) and then backcrossing F1 Tp plants by epc-W23 (W23). Families segregating d1 and epc were generated by crossing epc-1s2p females by d1 and self-pollinating the F1.
In all of these crosses, double mutants were identified as plants that expressed a combination of traits not found in either single mutant. Double mutants between epc and mutations that prolong the expression of juvenile traits (d1, Tp1, Tp2, Tp3) were identified as plants that had the distinctive morphology of these mutants and produced glossy leaves with epidermal hairs (adult traits) earlier than the wild-type plants segregating in the same family. We assumed that these plants were homozygous for epc because d1, Tp1, Tp2, and Tp3 single mutants always produce these adult traits later than wild-type plants (Poethig, 1988; Evans and Poethig, 1995). epc id plants were identified as late flowering plants that produced adult leaves at the same position as the epc single mutants segregating in the same family.
Phenotypic Analysis
Leaves were numbered according to their position in relation to the base of the plant, so that the first formed, most basal leaves had the lowest numbers. Plants were scored visually for the presence of wax and hairs on the leaf blade after the leaf had expanded fully. A leaf was scored as having wax if wax was present anywhere on the leaf blade. A leaf was scored as having hairs even if only one macrohair was present on the leaf blade, excluding the hairs along the margin of the leaf blade and prickle hairs and microhairs. The effect of epc mutations on other epidermal traits was determined using epidermal peels and histological sections of embedded leaves.
Leaf dimensions were measured on plants growing in the greenhouse in 6-inch pots in Metromix 200 (Scotts, Hope, AR). Leaf width was measured at the widest part of the leaf blade, and leaf length was measured from the tip of the leaf blade to the ligule.
To study the basis for the effect of epc mutations on shoot viability, seed from a self-pollinated plant heterozygous for epc-W23 (OH43) were planted on filter paper in a growth chamber (25°C, 16-hr-light/8-hr-dark) and harvested 5 days later. The shoots of germinated seedlings were fixed and processed for histological examination as described below. To distinguish mutant from wild-type seedlings, each specimen was genotyped for the SSR marker bngl 2082 before embedding using root tissue harvested at the time of fixation. The size of the shoot apical meristem (SAM) was determined by counting the number of cells in the region of the shoot apex above the last leaf primordium in a median longitudinal section of five mutant and five wild-type specimens. The mitotic index was calculated as the ratio of the number of mitotic figures (metaphase through anaphase) to the total number of cells in the SAM in this longitudinal section.
Leaf Initiation
Timed pollination experiments were conducted during the summer using field-grown plants. Mutant embryos were produced by pollinating epc-W23 (W23) ears with epc-1s2p (1s2p); wild-type control embryos were produced by crossing W23-Rnj to epc-1s2p (1s2p). Pollinations were performed over several days, but only plants that were pollinated on the same day were harvested at each time point. Kernels were excised from each ear without removing the ear from the plant and fixed in 4% formaldehyde or CRAFIII (Jensen, 1962). Because the penetrance and expressivity of epc-W23/epc-1s2p vary in different ears from the same cross, mature kernels from each sampled ear were grown in the greenhouse to determine the cross in which epc was best expressed. Specimens from these ears then were processed for histological analysis.
The rate of leaf initiation after germination was determined using plants from these same crosses. Plants were grown in a growth chamber (16-hr-light/8-hr-dark, 25°C), six to 10 plants of each genotype were dissected every few days with the aid of a stereomicroscope, and the total number of leaves and visible leaf primordia was counted. Some of these plants were transferred to the greenhouse after 3 weeks to measure the effect of epc on flowering time.
Shoot Tip Culture
Shoot tips were cultured from +/epc-1s2p (W23-Rnj/1s2p) and epc-W23/epc-1s2p (W23/1s2p) plants grown in a growth chamber (16-hr-light/8-hr-dark, 25°C). Plants were harvested 10 days after planting, when they had initiated 11 to 12 leaf primordia. A section containing the mesocotyl and ∼1 cm of shoot tissue was excised and sterilized by transferring it three times between 70% ethanol, 5% Clorox, and sterile distilled water (30 sec each step). After removing the first six leaves, shoot tips were sterilized a second time (10 sec each step). Leaf 7 was removed, and the outermost leaf (leaf 8) was measured with an ocular micrometer. The numbers and lengths of the other leaf primordia on the shoot were determined from an examination of cleared specimens (Herr, 1982) harvested at the same time. Shoot tips were cultured as described previously (Orkwiszewski and Poethig, 2000) and harvested 4 weeks later, when they had two fully expanded leaves.
Histological Analysis
Analysis of the effect of epc mutations on leaf anatomy was conducted using leaf samples taken at the midpoint of fully expanded leaves. Samples used for epidermal peels were vacuum-infiltrated with 1% solution of cellulase (Sigma) and 0.5% pectinase (Sigma) in 0.1 M acetate buffer, pH 5, and incubated at room temperature for 2 to 3 hr. The adaxial epidermis was removed, cleaned gently with a paintbrush to remove adhering mesophyll cells, and then stained for 30 sec at room temperature in 1 part 0.05% toluidine blue (0.01 M sodium acetate, pH 4.4) to 15 parts water. Other samples were fixed in 3% glutaraldehyde (PO4 buffer, pH 6.8) overnight at 4°C, postfixed in 1% OsO4 at 4°C for 2 hr, dehydrated in ethanol, and then embedded in Spurr's resin (Electron Microscopy Sciences, Fort Washington, PA). Sections (1 to 2 μm) were stained with 0.2% toluidine blue in 2.5% sodium carbonate.
The effect of epc-W23/epc-1s2p on the anatomy of the shoot apex was studied using shoot apices fixed in formalin–acetic acid–ethanol or CRAFIII and embedded in paraffin. Sections (10 μm) were deparaf-finized, stained with periodic acid–Schiff's reagent–Weigert's hematoxylin, mounted in Permount (Fisher Scientific), and photographed with Kodak Ektachrome 160 film or with a digital camera. Images were adjusted and composed subsequently using Photoshop 5.5 (Adobe Systems, Mountain View, CA).
SSR Analysis
epc was mapped relative to simple sequence repeat (SSR) markers (primers obtained from Research Genetics, Huntsville, AL) using F2 families from a cross of epc-1s2p to the inbred line Oh43. DNA was extracted according to Dellaporta (1994). Polymerase chain reaction was performed using the conditions described by Kozumplik et al. (1996), and the products were separated on a 4% Metaphor gel (FMC Bioproducts, Rockland, ME).
Acknowledgments
We are grateful to Doris Wagner and Christine Hunter for helpful comments on the manuscript and to Jennifer Schumacher for technical assistance. This research was supported by a National Institutes of Health training grant to S.H.V. and by grants from the National Science Foundation and the National Institutes of Health to R.S.P.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010406.
References
- Aubert, D., Chen, L., Moon, Y.-H., Martin, D., Castle, L.A., Yang, C.-H., and Sung, Z.R. (2001). EMF1, a novel protein involved in the control of shoot architecture and flowering in Arabidopsis. Plant Cell 13, 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiri, A., Irish, E.E., and Poethig, R.S. (1992). Heterochronic effects of Teopod2 on the growth and photosensitivity of the maize shoot. Plant Cell 4, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardini, T.Z., Bollman, K., Sun, H., and Poethig, R.S. (2001). Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291, 2405–2407. [DOI] [PubMed] [Google Scholar]
- Bertrand-Garcia, R., and Freeling, M. (1991). Hairy-sheath frayed1-O: A systemic, heterochronic mutant of maize that specifies slow developmental stage transitions. Am. J. Bot. 78, 747–765. [Google Scholar]
- Bongard-Pierce, D.K., Evans, M.M.S., and Poethig, R.S. (1996). Heteroblastic features of leaf anatomy in maize and their genetic regulation. Int. J. Plant Sci. 157, 331–340. [Google Scholar]
- Clarke, J.H., Tack, D., Findlay, K., Van Montagu, M., and Van Lijsebettens, M. (1999). The SERRATE locus controls the formation of the early juvenile leaves and phase length in Arabidopsis. Plant J. 20, 493–501. [DOI] [PubMed] [Google Scholar]
- Colasanti, J., Yuan, Z., and Sundaresan, V. (1998). The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93, 593–603. [DOI] [PubMed] [Google Scholar]
- Dellaporta, S. (1994). Plant DNA miniprep and microprep: Versions 2.1–2.3. In The Maize Handbook, M. Freeling and V. Walbot, eds (New York: Springer-Verlag), pp. 522–525.
- Evans, M.M., and Poethig, R.S. (1995). Gibberellins promote vegetative phase change and reproductive maturity in maize. Plant Physiol. 108, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, M.M., and Poethig, R.S. (1997). The viviparous8 mutation delays vegetative phase change and accelerates the rate of seedling growth in maize. Plant J. 12, 769–779. [Google Scholar]
- Evans, M.M., Passas, H.J., and Poethig, R.S. (1994). Heterochronic effects of glossy15 mutations on epidermal cell identity in maize. Development 120, 1971–1981. [DOI] [PubMed] [Google Scholar]
- Freeling, M., and Lane, B. (1994). The maize leaf. In The Maize Handbook, M. Freeling and V. Walbot, eds (New York: Springer-Verlag), pp. 17–28.
- Freeling, M., Bertrand-Garcia, R., and Sinha, N. (1992). Maize mutants and variants altering developmental time and their heterochronic interactions. Bioessays 14, 227–236. [DOI] [PubMed] [Google Scholar]
- Gomez-Mena, C., Pineiro, M., Franco-Zorrilla, J.M., Salinas, J., Coupland, G., and Martinez-Zapater, J.M. (2001). early bolting in short days: An Arabidopsis mutation that causes early flowering and partially suppresses the floral phenotype of leafy. Plant Cell 13, 1011–1024. [PMC free article] [PubMed] [Google Scholar]
- Greenwood, M.S., and Hutchinson, K.W. (1993). Maturation as a developmental process. In Clonal Forestry I: Genetics and Biotechnology, M.R. Ahuja and W.J. Libby, eds (New York: Springer-Verlag), pp. 14–33.
- Hackett, W.P. (1985). Juvenility, maturation and rejuvenation in woody plants. Hortic. Rev. 7, 109–155. [Google Scholar]
- Hackett, W.P., and Murray, J.R. (1993). Maturation and rejuvenation in woody species. In Micropropagation of Woody Plants, M.R. Ahuja, ed (Amsterdam: Kluwer Academic Publishers), pp. 93–105.
- Hackett, W.P., and Murray, J.R. (1997). Approaches to understanding maturation or phase change. In Biotechnology of Ornamental Plants, R.L. Geneve, J.E. Preece, and S.A. Merkle, eds (New York: CAB International), pp. 73–86.
- Hamada, S., Onouchi, H., Tanaka, H., Kudo, M., Liu, Y.G., Shibata, D., MacHida, C., and Machida, Y. (2000). Mutations in the WUSCHEL gene of Arabidopsis thaliana result in the development of shoots without juvenile leaves. Plant J. 24, 91–101. [DOI] [PubMed] [Google Scholar]
- Hay, J.O., Moulia, B., Lane, B., Freeling, M., and Silk, W.K. (2000). Biomechanical analysis of the Rolled (RLD) leaf phenotype of maize Am. J. Bot. 87, 625–633. [PubMed] [Google Scholar]
- Herr, J.M., Jr. (1982). An analysis of methods for permanently mounting ovules cleared in four-and-a-half type clearing fluids. Stain Technol. 57, 161–169. [DOI] [PubMed] [Google Scholar]
- Irish, E.E., and Karlen, S. (1998). Restoration of juvenility in maize shoots by meristem culture. Int. J. Plant Sci. 159, 695–701. [Google Scholar]
- Jensen, W.A. (1962). Botanical Histochemistry (San Francisco: W.H. Freeman and Co.).
- Kerstetter, R.A., and Poethig, R.S. (1998). The specification of leaf identity during shoot development. Annu. Rev. Cell Dev. Biol. 14, 373–398. [DOI] [PubMed] [Google Scholar]
- Kozumplik, V., Pejic, I., Senior, L., Pavlina, R., Graham, G., and Stuber, C.W. (1996). Molecular markers for QTL detection in segregating maize populations derived from exotic germplasm. Maydica 41, 211–217. [Google Scholar]
- Lawson, E.J., and Poethig, R.S. (1995). Shoot development in plants: Time for a change. Trends Genet. 11, 263–268. [DOI] [PubMed] [Google Scholar]
- Martinez-Zapater, J.M., Jarillo, J., Cruz-Alvarez, M., Roldan, M., and Salinas, J. (1995). Arabidopsis late-flowering fve mutants are affected in both vegetative and reproductive development. Plant J. 7, 543–551. [Google Scholar]
- Melzer, S., Kampmann, G., Chandler, J., and Apel, K. (1999). FPF1 modulates the competence to flowering in Arabidopsis. Plant J. 18, 395–405. [DOI] [PubMed] [Google Scholar]
- Mericle, L.W. (1950). The developmental genetics of the Rg mutant of maize. Am. J. Bot. 37, 100–116. [Google Scholar]
- Moose, S.P., and Sisco, P.H. (1994). glossy15 controls the epidermal juvenile-to-adult phase transition in maize. Plant Cell 6, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moose, S.P., and Sisco, P.H. (1996). glossy15, an APETALA2-like gene from maize that regulates leaf epidermal cell identity. Genes Dev. 10, 3018–3027. [DOI] [PubMed] [Google Scholar]
- Orkwiszewski, J.A., and Poethig, R.S. (2000). Phase identity of the maize leaf is determined after leaf initiation. Proc. Natl. Acad. Sci. USA 97, 10631–10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig, R.S. (1988). Heterochronic mutations affecting shoot development in maize. Genetics 119, 959–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig, R.S. (1990). Phase change and the regulation of shoot morphogenesis in plants. Science 250, 923–930. [DOI] [PubMed] [Google Scholar]
- Prigge, M.J., and Wagner, D.R. (2001). The Arabidopsis SERRATE gene encodes a zinc-finger protein required for normal shoot development. Plant Cell 13, 1263–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schichnes, D.E., and Freeling, M. (1998). Lax midrib-O, a systemic, heterochronic mutant of maize. Am. J. Bot. 85, 481–491. [PubMed] [Google Scholar]
- Schultz, E.A., and Haughn, G.W. (1993). Genetic analysis of the floral initiation process (FLIP) in Arabidopsis. Development 119, 745–765. [Google Scholar]
- Scott, D.B., Jin, W., Ledford, H.K., Jung, H.S., and Honma, M.A. (1999). EAF1 regulates vegetative-phase change and flowering time in Arabidopsis. Plant Physiol. 120, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, G.G., Gendall, A.R., and Dean, C. (1999). When to switch to flowering. Annu. Rev. Cell Dev. Biol. 15, 519–550. [DOI] [PubMed] [Google Scholar]
- Soppe, W.J., Bentsink, L., and Koornneef, M. (1999). The early-flowering mutant efs is involved in the autonomous promotion pathway of Arabidopsis thaliana. Development 126, 4763–4770. [DOI] [PubMed] [Google Scholar]
- Telfer, A., and Poethig, R.S. (1998). HASTY: A gene that regulates the timing of shoot maturation in Arabidopsis thaliana. Development 125, 1889–1898. [DOI] [PubMed] [Google Scholar]
- Telfer, A., Bollman, K.M., and Poethig, R.S. (1997). Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124, 645–654. [DOI] [PubMed] [Google Scholar]
- Vladutu, C., McLaughlin, J., and Phillips, R.L. (1999). Fine mapping and characterization of linked quantitative trait loci involved in the transition of the maize apical meristem from vegetative to generative structures. Genetics 153, 993–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, D., and Nilsson, O. (1995). A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500. [DOI] [PubMed] [Google Scholar]
- Wiltshire, R.J.E., Murfet, I.C., and Reid, J.B. (1994). The genetic control of heterochrony: Evidence from developmental mutants of Pisum sativum L. J. Evol. Biol. 7, 447–465. [Google Scholar]
- Yang, C.H., Chen, L.J., and Sung, R.Z. (1995). Genetic regulation of shoot development in Arabidopsis: Role of the EMF genes. Dev. Biol. 169, 421–435. [DOI] [PubMed] [Google Scholar]
- Zimmerman, R.H., Hackett, W.P., and Pharis, R.P. (1985). Hormonal aspects of phase change and precocious flowering. Encycl. Plant Physiol. 11, 79–115. [Google Scholar]