Abstract
Cytogenetically unrelated clones have been detected by chromosome banding analysis in many breast carcinomas. Because these karyotypic studies were performed on short-term cultured samples, it may be argued that in vitro selection occurred or that small clones may have arisen during culturing. To address this issue, we analyzed 37 breast carcinomas by G-banding and comparative genomic hybridization (CGH), a fluorescent in situ hybridization-based screening technique that does not require culturing or tumor metaphases. All but two of the 37 karyotypically abnormal cases presented copy number changes by CGH. The picture of genomic alterations revealed by the two techniques overlapped only partly. Sometimes the CGH analysis revealed genomic imbalances that belonged to cell populations not picked up by the cytogenetic analysis and in other cases, especially when the karyotypes had many markers and chromosomes with additional material of unknown origin, CGH gave a more reliable overall picture of the copy number gains and losses. However, besides sometimes revealing cell populations with balanced chromosome aberrations or unbalanced changes that nevertheless remained undetected by CGH, G-banding analysis was essential to understand how the genomic imbalances arose in the many cases in which both techniques detected the same clonal abnormalities. Furthermore, because CGH pictures only imbalances present in a significant proportion of the test sample, the very detection by this technique of imbalances belonging to apparently small, cytogenetically unrelated clones of cells proves that these clones must have been present in vivo. This constitutes compelling evidence that the cytogenetic polyclonality observed after short-term culturing of breast carcinomas is not an artifact.
Keywords: breast cancer, clonality, cytogenetics, CGH, chromosome aberrations
Introduction
Chromosome banding analysis of breast carcinomas has demonstrated extensive clonal cell-to-cell variation in the tumors [1,2]. Intriguingly, this intratumor cytogenetic heterogeneity takes the form of karyotypically unrelated clones in a significant proportion of cases, approaching 70% in series subjected to particularly detailed analysis [3,4]. Because the karyotypic studies demonstrating cytogenetic polyclonality were all performed after short-term culture of the tumor cells and because different cell populations may have different growth rates in vitro, the relative clonal sizes revealed by the cytogenetic analysis does not necessarily correspond to the in vivo situation. It can furthermore be argued that some of the observed clonal changes, especially the ones detected in only a small number of metaphases, may have arisen during culturing.
Comparative genomic hybridization (CGH) is a fluorescent in situ hybridization-based screening technique that, in a quantitative manner, detects gains and losses of genomic material [5,6]. Because CGH does not require tumor metaphases and hence is independent of the mitotic activity of clonal subpopulations, the technique is free from selection biases. However, only imbalances present in a significant proportion of the test sample are revealed by CGH and hence intratumor heterogeneity manifesting itself as minor clones will go unnoticed. The simultaneous detection by CGH of genomic imbalances pertaining to cytogenetically unrelated clones would therefore, regardless of how many metaphase cells displayed the characteristic aberrations after in vitro culturing, provide compelling evidence that each clone was large in vivo and, indirectly, that both or all constituted significant parts of the tumor parenchyma. Taking advantage of these disparate qualities of the two techniques, we performed a combined G-banding and CGH analysis of a series of breast carcinomas to obtain more information on the nature of genetic polyclonality in breast cancer.
Materials and Methods
Tumor Specimens
Thirty-seven breast carcinomas from patients admitted to Odense University Hospital from 1992 to 1994 were selected because they had been shown to carry clonal chromosome abnormalities by chromosome banding analysis and because frozen material for DNA extraction was available. The samples for G-banding and CGH analyses were obtained before any chemo- or radiotherapy.
Histopathology
The histopathologic classification (Table 1), which included examination of slides from tissue immediately adjacent to that processed for genetic analysis, was made in accordance with WHO recommendations [7]. The carcinomas were ductal not otherwise specified (NOS, 17 cases), comedo (11 cases), papillary, cribriform (two cases each), mucinous, lobular, mixed ductal and lobular, ductal carcinoma in situ (DCIS), and ductal carcinoma with extensive DCIS component (one case each). Histopathologic grading was based on the three components tubular formation, mitotic index, and nuclear pleomorphism, and quantified to allow a correlation study with the complexity of the genetic changes. Each component was scored from 1 to 3 (Table 1) with their sum corresponding to grade I (3 to 5), grade II (6 or 7), or grade III (8 or 9). The scores 1 and 2 for each of the three components, as well as grades I and II, were grouped together for the purpose of statistical analysis (Table 2).
Table 1.
Histopathologic Data, G-Banding Karyotype, and CGH Copy Number Changes of the 37 Breast Carcinomas.
| Case no. | Histology* | Grade† | LNM‡ | G-banding karyotype§,¶ | CGH copy number changes¶ |
| 317/92 | Com | 3,3,2 | No | 47–49,XX,add(1)(p31),der(1)add(1)(p13)add(1)(q43),+der(1)t(1;11)(q10;q10)add(1)(q42),-3,-4,-7,der(9)t(9;13)(p13;q14),del(10)(p12),-11,-13,-13,-14,-15,add(19)(p13),+4–6mar[32]/91–98,idemx2[11] | rev ish enh(1q21q22,3q24q28,6p,6q21q22,8q,16q23qter) |
| 363/92 | D | 3,2,2 | No | 44–46,XX,+7[4]/46,XX[47] | rev ish dim(1p13p31,4,9p13pter,13q14qter) |
| 386/92 | D | nd | No | 46,XX,+1,der(1;16)(q10;p10)[3]/46,XX[75] | rev ish enh(4q22qter,8p12qter,17q12q21, 20q12qter),dim(3p12p14,5q15q33,6q13q25,7q31qter,9p21pter,9q21,13q14qter,16q),amp(8q) |
| 394/92 | D | 2,2,2 | 18/18 | 48,XX,+5,+10[5]/46,XX[45] | rev ish enh(3q21q24,3q27qter,5pterq13,5q34qter,7q33qter,8p21qter,16p,17q24qter),dim(Xpterq22,11q23qter,13q14qter,16q,22q13) |
| 431/92 | Com | 3,3,3 | No | 47,XX+7[8]/46,XX[45] | rev ish enh(3p21p24,3q,4p16,8p11qter,11p14q14,14q,16p11p13,16q23qter,17q21qter,19q,20q,22q11q12),dim(1p31,4q23qter,5q11q22,6q16qter,8p22pter,9pterq33,11q23qter,20p12pter,21q21),amp(8q24,11q13) |
| 439/92 | Com | 3,1,3 | 9/11 | 66–80,XXX,+X,t(1;14)(p13;q12)x2,del(2)(q31)x2,-4,add(?6)(q14),+7,+7,+8,-9,+10,add(11)(p14),add(12)(p13),-14,-15,-17,-17,-17,-18,-19,+20,-21,+4mar[5]/46,XX[55] | rev ish enh(10q21q22) |
| 501/92 | Crib | 2,1,2 | No | 46,XX,del(4)(p12-3p15)[4]/46,XX[45] | rev ish enh(1q21q41),dim(6q16q22),amp(1q32) |
| 503/92 | D | 3,1,3 | 4/20 | 47–49,XX,+i(1)(q10),der(8)del(8)(p21)ins(8;10)(q22;q22q24),der(8)add(8)(p11)ins(8;10)(q22;q22q24),+der(8)add(8)(p11)ins(8;10)(q22;q22q24)x2,inv(9)(q12q34),-10,add(11)(q23),add(16)(q22)[cp19]/46,XX,t(1;19)(p13;q13)[4]/46,XX[10] | rev ish enh(1q21qter,8q,10q21q22),dim(8p22pter,10q25qter,16q) |
| 509/92 | D | 3,2,3 | No | 47–48,XX,+X[3]/46,XX[19] | rev ish enh(8p12qter,16p,19p,20q13),dim(6q12q21,13q21q22) |
| 512/92 | D | 3,3,3 | 19/19 | 66–67,XXX,del(3)(p13),+del(3)(p13),-4,+5,-7,-8,-8,-9,-10,-10,-13,-14,add(14)?(p11),-15,-15,-16,-17,-17,-17,-18,add(20)(q13),+add(20)(q13),-21,-21,-21,inc[2]/46,XX[55] | rev ish enh(1q21q32,7p,8q,15q,17q11q21,17q25,19,20q13,22q11q12),dim(1p21pter,1q42qter,3p21pter,4q24q28, 4q34qter,7q21q31,8p12pter,10q24qter,11q22qter,13q,18q12qter),amp(1q25q31,17q11q21) |
| 515/92 | Papil | nd | 1/8 | 47,XX,+X[2]/47,XX,+7[2]/46,XX[46] | rev ish enh(1q,4p16,4p14q24,8p12qter,12q24, 16p,17q,19,20q),dim(3p12p13,6p24pter,6q,8p22pter,10q,11q14qter,13q,14q,16q,20p12pter),amp(8q21qter,20q12qter) |
| 46/93 | Crib | 2,1,2 | No | 47,XX,+der(1;16)(q10;p10)[2]/47–48,XX,idem,+der(1;16)(q10;p10)x2,-16[cp5]/46,XX[56] | rev ish enh(1q,16p) |
| 80/93 | Com | 3,3,3 | No | 68–75,XX,-X,add(1)(p22),+add(1)(p22),-2,inv(2)(p13q37)x2,+3,del(3)(p12)x2,-4,-4,-5,-5,-5,+6,add(6)(q23)x2,add(7)(p21),+add(7)(p21),der(8)t(1;8)(p22;q24),der(8)t(3;8)(p24;p22),-10,-11,-12,dup(12)(q13q22)x2,-13,-13,-14,-15,+add(16)(p12),der(16)del(16)(p11)add(16)(q23),-17,-18,-19,-21,-22,add(22)(q13),+8mar[cp114]/46,XX[11] | rev ish enh(1p36qter,2p11p13,3p21pter,3p14qter,5q32qter,6pterq22,7,8p12qter,9p21pter,10p14p15,11p15q12,12p13,12q14q21,16p12,17p13,18pterq21,18q22qter,19p13q13.1,21q21,22q11q12),dim(X,2p21pter,2q21q32,2q35qter,4p15qter,5p14q23,6q24qter,8p23pter,13q,14q21q22),amp(6p23pter) |
| 92/93 | D+DCIS | 3,1,2 | No | 46,XX,del(6)(q21q23)[2]/46,XX[120] | rev ish enh(12q23qter) |
| 100/93 | Com | 3,3,3 | No | 46–47,XX,+7[4]/46,XX,+1,der(1;15)(q10;q10),ins(1;?)(q11;?)[2]/46,XX[64] | rev ish enh(Xq26qter,1q31q32,3p24pter,8p12qter,12q14q22,17q21q25,20q11q13),dim(Xp21pter,2p24pter,3p13p21,4q33qter,11p15,11q21qter,13q12q21,14q,16q,20p12pter,21q21q22) |
| 113/93 | D | 2,1,1 | No | 46,XX,der(3)inv(3)(p12p26)del(3)(p13p21)[4]/46,XX,t(7;9)(p14;p24)[2]/46,XX[64] | rev ish enh(1q21q42,16p),dim(12p,13q21q31) |
| 136/93 | D | 3,1,2 | No | 40–44,XX,add(1)(p34),-3,der(8)hsr(8)(p?21)hsr(8)(q24),add(11)(q21),-13,-16,+1–2mar[cp4]/46,XX,+der(1;16)(q10;p10),-10[3]/46,XX,t(5;6)(p14;q15)[2]/46,XX[85] | rev ish enh(1q22qter,6p24,9p13),dim(11q21qter,13q13qter,16q,21q) |
| 138/93 | D | 3,2,3 | No | 46,XX,t(1;20)(p13;q13)[14]/48,XX,+3mar[2]/46,XX[37] | None |
| 145/93 | D | 2,2,3 | 5/5 | 67–74,XXX,der(1)(1qter→1p36::6qter→q15::?),i(1)(q10),add(2)(q36),t(3;6)(p12;p22),-4,dic(4;16)(q31;q12),add(6)(p23),del(6)(q15),del(7)(p12),der(7)(7pter→7q11::?::3p12→3pter),i(7)(p10),-8,der(8)(1pter→1p12::?::hsr::8p22→8qter),-9,add(9)(p23),der(10)t(3;10)(p14;p13),add(11)(q14),-13,-14,-14,add(14)(p11),-15,del(16)(p11),del(16)(q12),-17,add(17)(q25),add(19)(q13),add(20)(q13),i(21)(q10),-22,+9–16mar[6]/46,XX[71] | rev ish enh(3q25qter,6p21,8q21q22,8q24,11p11p14,12p13,17q24qter,18p,22q13) |
| 167/93 | Com | 3,2,3 | 9/14 | 37–42,XX,add(1)(p11),del(2)(q23),add(3)(p12),-5,-8,-9,der(10)t(3;10)(p23;q22),der(11)t(8;11)(q12;p14),-14,-15,del(17)(p11p12),-18,-19,-21,add(22)(q12),+1–4mar[cp13]/74–80,idemx2,-add(1)(p11),+add(1)(p13)x2,-3,+der(3)t(3;8)(p12;q21),-8,+i(8)(q10)[cp15]/149–150,idemx4[2] | rev ish enh(8q21qter),dim(11p13pter,17p13) |
| 199/93 | Com | nd | No | 46,XX,del(3)(p14p22)[10]/46,X,t(X;3)(q22;p14)[5]/60–61,X,-X,-X,add(1)(p12),-2,-2,der(3)add(3)(p14)add(3)(q26),-4,-5,-5,-6,-7,-8,-8,-9,-10,-11,i(12)(p10),-13,-14,-14,-15,dic(16;?) (q23;?),-17,-17,der(20)t(5;20)(q13;p13),-21,-21, add(22)(p11),+3mar,inc[3] | rev ish enh(1q24q31,7p,13q,14q12q21),dim(3p13p22,6p22pter,8p12pter,10q25qter,11p15,11q,14q24qter,15q21qter,17p12pter,18p11.3) |
| 208/93 | Com | 3,3,3 | No | 38–41,X,-X,add(1)(p36),-2,add(3)(q2?4),del(3)(p12),add(4)(p16),der(4)t(3;4) (q2?4;q25),add(7)(q32),-8,-9,-10,der(10)t(4;10)(q21;p15),-11,der(12)inv dup(12)(q13q24)add(12)(q13),-13,-14,der(8;14)(q10;q10),-15,-15,der(17)t(3;17)(p13;p12),-18, add(19)(p13),add(21)(p11),+2mar[10]/38–41,idem,-der(12)inv dup(12)(q13q24)add(12)(q13),+der(12)inv dup(12)(q13q24)t(1;12)(q21;q13)[6]/38-41,idem,-add(1)(p36),+del(1)(q41q43),-5,+del(5)(q14q23),-6,+add(6)(q21),-add(7)(q32),+der(7)add(7)(q32)t(7;13)(p15;q12),-8,+del(12)(p11),-der(12)inv dup(12)(q13q24)add(12)(q13),-13,-14,+15,-der(17)t(3;17)(p13;p12), +der(17)t(3;17)(p13;p12)add(17)(q25),+18,+2mar[24]/76–82,idemx2,-add(1)(p36)x2,+add(1)(p12)x2[8] | rev ish enh(Xp22,1q32,2q32q33,4q12q31,6q22q24,7p14pter,8q,10p,11p14p15,12q13q22,20q12qter),dim(3p14p21,7q32qter,9p23pter,10q21qter,11q23qter,13q,14q,15q26,17p12pter,18p11.3,19q13),amp(7p21p22,8q23,10p12p14) |
| 236/93 | D+Lob | 1,1,1 | 4/14 | 46,XX,i(1)(q10)[2]/46,XX[158] | rev ish enh(16p13),dim(16q,22q13) |
| 244/93 | Muc | nd | No | 46,XX,t(3;19)(p21;q13),t(11;20)(q14;q13)[7]/46,X,der(X)t(X;3)(q22;p21),del(2)(q14),add(3)(p21),del(4)(p14p15),del(4)(q21),add(5)(p15),del(5)(q23),del(7)(q22q32),add(10)(p15),add(12)(p13),der(16)t(5;16)(q31;p13)add(16)(q23)[5]/46,XX,t(1;11)(p36;p14),-4,der(6)t(6;7)(q13;q22),add(7)(q22),der(18)t(4;18)(q13;q22),+r[3]/47,XX,t(7;19)(p15;q13)[2]/46–47,XX,t(16;17)(p11;q21)[2]/46,XX[41] | None |
| 248/93 | Com | 3,3,2 | No | 56–59,X,add(1)(p35),add(1)(p12),t(1;6)(p34;p24),add(3)(p24),dic(7;7)(q22;q22),add(10)(q22),add(11)(q22),add(16)(p13),+r,+3mar,inc[14]/46,XX,t(1;16)(p34;q23)[2]/46,XX[170] | rev ish enh(1p31p34,1p13p21,1q21q31,2p24pter,2p12p13,2q22q32,3q13qter,5p13p15,6p21qter,7p15qter,8q21qter,10p,10q11q21,11q13q22,12p,13q12q31,16p13,20q),dim(Xp11pter,1p36,3p24pter,4p11p15,4q21qter,5q11q34,8p21pter,10q23q25,11p11pter,15q22qter,18q22qter),amp(7p11p14,7q11q21,8q24) |
| 335/93 | D | 3,3,2 | 7/18 | 62–68,XX,-X,del(1)(p22),der(1)(1qter→cen→1p31::1p36→1p31::?),-2,-4,-5,add(6)(q23),+del(7)(q32),-8,-8,-9,-9,+add(12)(p13),-13,-15,-15,-16,add(16)(q23),del(16)(q22),-17,-18,-21,-22,inc[16] | rev ish enh(1p21qter,5p12p15,7pterq21,8q21qter,12,13q,17q,20) |
| 361/93 | Com | 3,2,2 | 4/24 | 59–63,XX,-X,der(1;16)(q10;p10)x2,i(1)(q10),add(13)(p11),der(19)t(7;19)(q11;q13),+der(?)t(?;5)(?;q11),inc[2] | rev ish enh(Xq27qter,1q31q32,2q23q32,4q32q34,6q21q22,6q23q24,8q11q24.1,11q13q14,12q14q22,14q12q22,20q11q13),dim(6p21.3,9p12,17p11pter,17q25,19p13) |
| 392/93 | Com | 3,1,2 | No | 79–?84,del(7)(q21),inc[2]/46,XX[67] | rev ish enh(1q24q41,3p24pter,5p13q34,6q16q22,8p11q24.1,9q13q21,9q31q34,18q12q21),dim(2q36qter,13q,17p) |
| 467/93 | D | 3,1,2 | No | 47,XX,+1,der(1;16)(q10;p10),r(?11),+20[cp19]/46,XX[21] | rev ish enh(1q,11p14p15,11q12q14),dim(11q22qter,16q) |
| 495/93 | DCIS | nd | No | 46,XX,t(8;19)(q22;q13)[2]/46,XX[12] | rev ish enh(1q23q32,1q43qter) |
| 507/93 | D | 2,3,3 | No | 46,XX,add(1)(q21)[2] | rev ish enh(1q24qter,3p24pter,3q25qter,6q15q22,7q31q32,8q22qter,9q31q33,10p13pter,11p11p15,17q23,18q12),dim(1p36,5q34qter,10q25qter,16pterq21,17p12pter) |
| 535/93 | Lob | nd | No | 62–80,XXX,+7,+10,+12,+14,+15,+mar[cp5]/46,XX[110] | rev ish enh(1q31,5q23q31,12q15q21) |
| 557/93 | D | 3,3,3 | No | 46,XX,+1,der(1;16)(q10;p10)[2]/46,XX[168] | rev ish enh(Xq25q27,1q31q32,2p24pter,4q13q21,5p,6q15q16,6q22,8q22qter,9p13pter,10p13pter,10q22,12p11p12,20q11qter),dim(8p21pter,14q24qter,15q26) |
| 566/93 | D | 2,2,1 | No | 46,XX,+1,der(1;10)(q10;p10)[2]/46,XX[81] | rev ish enh(1p13p31,1q23q32,3p22p24,3p12p14,3q24q26,4q12q33,5p13,8p12qter,12p11p12,12q21,16p11pter),dim(6p21pter,16q22qter,17p11p12),amp(8q) |
| 574/93 | D | 2,1,2 | No | 46–47,XX,t(3;6)(q21;q25)[2]/46,XX[78] | rev ish enh(1q24q32),dim(6p12p21.1,6q23qter,16q,17pterq21) |
| 2/94 | D | 3,2,3 | No | 74–80,XX,i(1)(q10)x2,+3r,inc[2]/46,XX[47] | rev ish enh(1q,5p,7p21p22,10q21q23,15q21qter,16p13,17q21qter),dim(8p,10q25qter,11q22qter,13q,16q22qter,17p12pter,18,22q),amp(1q32qter,17q22q24) |
| 7/94 | Papil | nd | No | 73–76,XXX,del(1)(q21),i(1)(?::q44→q10::q10→q44::?),del(7)(q21),add(16)(q22),del(16)(q13),inc[6]/46,XX[53] | rev ish enh(1p32qter,2p16pter,3q,5p,6p22q16,6q22q25,7p14pter,7q21,8q13qter,10p,11pterq14,12q22,13q13q32,18),dim(1p34pter,2q11q35,3p12pter,5q13q14,7q31qter,10q21q22,11q23qter,12q14q15,14q,15q11q25,17pterq12,17q24qter,20q),amp(7p21) |
Com, comedocarcinoma; Crib, cribriform carcinoma; D, ductal carcinoma; DCIS, ductal carcinoma in situ; D+DCIS, ductal carcinoma with extensive in situ component; Lob, lobular carcinoma; Muc, mucinous carcinoma; Papil, papillary carcinoma.
Histologic grade as subdivided in three components, i.e., tubule formation, mitotic activity, and nuclear pleomorphism; nd, not determined (histologic grading is done routinely only for ductal carcinoma not otherwise specified).
Lymph node metastasis.
The G-banding karyotypes of cases 386/92, 503/92, 46/93, 136/93, 145/93, 361/93, and 467/93 were published in Tsarouha et al. [10].
In bold are shown the G-banding and CGH findings that are likely to belong to the same clonal cell populations in the individual cases.
Table 2.
Relationship Between Histopathologic Grade and the Number of Chromosome Abnormalities (G-banding) and Copy Number Changes (CGH).
| Grade* | G-banding† | CGH‡ | G-banding/CGH§ | ||||||
| Tubular formation | 0–10 | >10 | P=.200 | 0–10 | >10 | P=.690 | 0–10 | >10 | P=.230 |
| 1–2 | 8 | 1 | 6 | 3 | 5 | 4 | |||
| 3 | 12 | 9 | 11 | 10 | 6 | 15 | |||
| Mitotic index | 0–10 | >10 | P=.045¶ | 0–10 | >10 | P=.007¶ | 0–10 | >10 | P=.004¶ |
| 1–2 | 16 | 4 | 15 | 5 | 11 | 9 | |||
| 3 | 4 | 6 | 2 | 8 | 0 | 10 | |||
| Nuclear pleomorphism | 0–10 | >10 | P=.440 | 0–10 | >10 | P=.270 | 0–10 | >10 | P=.140 |
| 1–2 | 12 | 4 | 11 | 5 | 8 | 8 | |||
| 3 | 8 | 6 | 6 | 8 | 3 | 11 | |||
| Combined grade | 0–10 | >10 | P=.120 | 0–10 | >10 | P=.063 | 0–10 | >10 | P=.026¶ |
| I–II | 13 | 3 | 12 | 4 | 9 | 7 | |||
| III | 7 | 7 | 5 | 9 | 2 | 12 | |||
The combined grade is arrived at by adding the three individual scores for tubular formation, mitotic index, and nuclear pleomorphism (each with a score of 1 to 3). Grade I: 3 to 5; grade II: 6 or 7; grade III: 8 or 9. The individual scores 1 and 2 and the grades I and II were grouped together for the purpose of statistical analysis.
The number of cytogenetic abnormalities in each case was arrived at by counting the abnormalities described in the karyotype. Multiple copies of structural or numerical abnormalities were counted only once; in derivative chromosomes, each structural change was counted; each individual marker was counted once.
The score was arrived at by adding the number of copy number gains and losses indicated in Table 1; amplifications were not counted because the regions involved are always part of usually wider regions registered as copy number gains.
The highest number of changes detected by G-banding or CGH was used for a combined score.
Statistically significant finding.
Chromosome Banding Analysis
Cells were short-term cultured and analyzed cytogenetically as previously described [8]. Briefly, all samples were mechanically and enzymatically disaggregated, and the resulting cells were plated out in 25-cm2 Primaria flasks or Vitrogen-coated slide-flasks. The cultures were fed an appropriate medium that facilitates epithelial growth and harvested after 5 to 8 days. The cells were exposed to demecolcine, dislodged by trypsinization, subjected to hypotonic shock in 0.05 M KCl, and fixed in methanol:acetic acid (3:1). G-banding was obtained with Wright stain. The clonality criteria and the description of karyotypes followed the recommendations of the ISCN (1995) [9]. The G-banding karyotypes of 7 of the 37 cases have been published before [10] (cases 386/92, 503/92, 46/93, 467/93, 136/93, 361/93, and 145/93); the remainder are described here for the first time.
Comparative Genomic Hybridization
The CGH procedure of Kallioniemi et al. [6] was performed with the modifications previously described in detail by Kraggerud et al. [11]. Briefly, test (tumor) and reference (peripheral blood lymphocytes from a healthy female) DNA was extracted using standard methods and labeled in nick-translation reactions using two fluorochrome-conjugated nucleotides in each (New England Nuclear, Boston, MA; FITC-12-dCTP and FITC-12-dUTP for tumor DNA and Texas Red-6-dCTP and Texas Red-6-dUTP for normal DNA), after which DNA fragment lengths between 300 and 2000 bp were obtained. The same amounts of labeled tumor and reference DNA (800 ng each) were mixed with 20 µg unlabeled Cot-1 DNA (Life Technologies, Rockville, MD), ethanol-precipitated, dried and dissolved in hybridization buffer (Vysis, Downers Grove, IL). Normal metaphases were obtained by lymphocyte culture from healthy donors or, in six cases, from commercially available slides (Vysis). After denaturing the chromosomes and the DNA probe, hybridization was allowed to occur for 2 to 3 days in a humidified chamber at 37°C. After a series of washes, the slides were mounted in an antifade solution with DAPI (Vectashield; Vector Laboratories, Burlingame, CA).
Ten good-quality metaphase spreads were selected for analysis in each case. Three images, corresponding to FITC (green) and Texas Red (red) hybridization signals and DAPI counterstain, were sequentially captured with a Cohu 4900 CCD (12-bit gray scale) camera, using an automated filter wheel coupled to a Zeiss Axioplan fluorescence microscope (Zeiss, Oberkochen, Germany), and a CytoVision system (Applied Imaging, Santa Clara, CA). Chromosomes were identified based on their inverted DAPI appearance and the relative hybridization signal intensity determined along each chromosome. Data obtained from the 10 cells were combined to generate average ratio profiles with 95% confidence intervals for each chromosome. The threshold values 1.25 and 0.75 were used to score gain and loss of DNA sequences, respectively, corresponding to the ability of detecting one copy number change in at least 50% of the cells in a diploid tumor. Scoring was performed independently by two of the authors (M.R.T. and R.A.L.) with few interobserver differences; these were resolved after joint reevaluation. A negative (normal versus normal) and a positive (the cell line LOVO with known copy number changes) control were included in every set of experiments. Furthermore, the findings were controlled by repeated, independent analyses of 16 tumors, including the use of reverse labeling in four tumors. The description of the CGH copy number changes followed the guidelines suggested in the ISCN (1995) [9].
Evaluation of Genetic Complexity
The karyotypic complexity, i.e., the number of cytogenetic abnormalities detected by chromosome banding analysis, was determined by counting the aberrations described in the karyotype in each case according to the following criteria: Multiple copies of structural or numerical abnormalities were counted only once; in derivative chromosomes, each structural change was counted, and each individual marker was counted once. The number of changes detected by CGH was arrived at by adding the number of copy number gains and losses indicated in Table 1. Because the average number of changes per case detected by G-banding and CGH was, respectively, 9.6 and 10.5, cases with 0 to 10 and more than 10 genetic changes detected by either technique were tabulated for statistical analysis (Table 2).
Statistical Analysis
Fisher's exact test was used to determine the relationship between the number of genetic changes and grade (including each of its components individually) and between genetic complexity and histological type. The correlation coefficient (r) was determined to study the correlation between the number of changes detected with G-banding and CGH, as well as the correlation between the copy number gains and losses detected by CGH. Two-tailed P values ≤.05 were considered statistically significant.
Results
The G-banding analysis revealed 24 cases with a single karyotypically abnormal clone and 13 cases (35%) with multiple clones. Nine of the 13 cases showed polyclonality in the form of two to five cytogenetically unrelated clones per case. Twelve cases presented a neartriploid clone and three other cases with stemlines in the neardiploid range showed additional, related clones that were neartriploid, neartetraploid, and/or nearhexaploid. The remaining 22 cases had only neardiploid clones with variable degrees of karyotypic complexity. The most common structural chromosome abnormalities were der(1;16)(q10;p10) (detected in six cases, in three as the sole change and in three as part of complex karyotypes) and i(1)(q10) (five cases, in one of them as the sole change), whereas +7 was the most frequent numerical change (six cases, in four of them as the sole change). A detailed description of all clonal chromosome abnormalities is presented in Table 1.
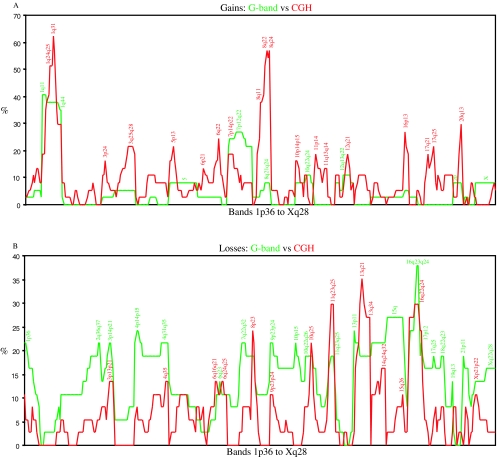
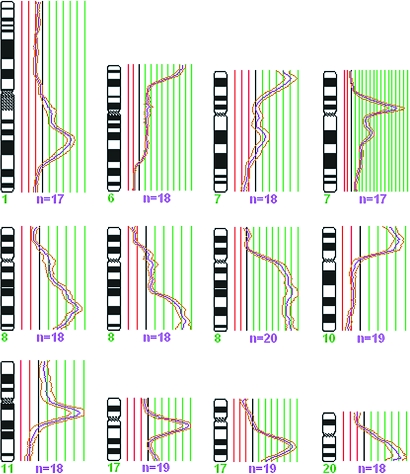
All but two cases presented copy number changes by CGH (Table 1). The number of imbalances per case ranged from 0 to 30 with an average of 10.5 per case. The number of copy number gains varied from to 0 to 20 (average: 6.2) and the losses from 0 to 13 (average: 4.4); gains and losses were significantly correlated with one another (r=0.63; P<.001). The chromosome arms from which material was most frequently gained were 1q (the band most commonly gained was 1q31 with 62.2%), 8q (56.8% at 8q22 and 8q24), 20q (29.7% at 20q13), and 16p (27.0% at 16p13), whereas the ones most often displaying losses were 13q (35.1% at 13q21), 11q (29.7% at 11q23q25), 16q (29.7% at 16q22q24), 8p (24.3% at 8p23), and 17p (24.3% at 17p12p13; Figure 1). Eleven tumors showed amplifications (here defined as ratios above 2.0; Table 1) in one to three discrete chromosomal regions, most frequently at 8q (six cases), 1q (three cases), 7p (three cases), and 17q (two cases).
Figure 1.
Graphic comparison of the genomic gains (A) and losses (B) detected by G-banding (green) and CGH (red) from 1p36 to Xq28 (all cases pooled). For every case, the presence or absence of imbalance in every chromosome band was computed in a spreadsheet. The total number of imbalances detected by each technique in every band was then used to prepare the graphic comparison. Imbalances in some areas of the genome are equally often detected by both techniques (e.g., gain of 1q and losses of 3p, 6q, 8p, 11q, and 16q). However, CGH seems to detect more often gains of 3q, 6q, 8q, 11, 16p, 17q and 20q, whereas G-banding more often detects losses of 1p, 2q, 4, 9q, 15q, and 17q. These differences might be explained by the preferential detection of disparate clones by each technique or by the frequent presence of the said chromosomal segments in marker chromosomes or in chromosomes that by G-banding are seen to have addition of unknown material.
The comparison between genetic complexity and the individual components of the histopathologic grade revealed a statistically significant association between high mitotic index and the presence of many genetic changes detected both by G-banding and CGH (P=.045 and P=.007, respectively; Table 2). No such association was discernible with the other two components or with the overall grade when the findings of each technique were considered in isolation. However, when the findings of both techniques were combined so that the one yielding the highest value was used, a statistically significant association emerged between grade III and genetically complex tumors (i.e., >10 genetic changes; P=.026). Genetic complexity varied also with histologic tumor type. The carcinomas with a cribriform, lobular or DCIS histology presented relatively few genetic changes by G-banding/CGH (average 2.7 changes), whereas the two papillary carcinomas and the single mucinous carcinoma presented 19, 27, and 22 genetic changes, respectively (Table 1). Differences were also seen in the two more common groups of tumors: All 11 comedocarcinomas (range: 11 to 36; average: 23.4), but only 10 of 17 ductal carcinomas NOS (range: 4 to 44; average: 12.9) had more than 10 genetic changes per case by G-banding/CGH (P=.023). Amplifications were more often seen in tumors with many genetic changes (P=.057). No association was detected between genetic complexity and the presence of lymph node metastases. The correlation between the number of changes detected by G-banding and CGH was not statistically significant (r=0.20; P=.212).
Discussion
The overall picture of the karyotypic findings in the 37 breast carcinomas we present does not differ significantly from that of a larger, unselected series previously analyzed with the same technique [1], neither with regard to which clonal chromosome aberrations were the most common nor with regard to the clonal composition of the tumors. Similarly, in spite of the fact that cases with an abnormal G-banding karyotype had been selected for, the genomic imbalances detected by CGH in our study were similar to those found in a consecutive series of 55 breast carcinomas [12]. Therefore, the findings we arrived at by combined G-banding and CGH analysis of the chosen set of 37 tumors seem to be representative of breast carcinomas in general.
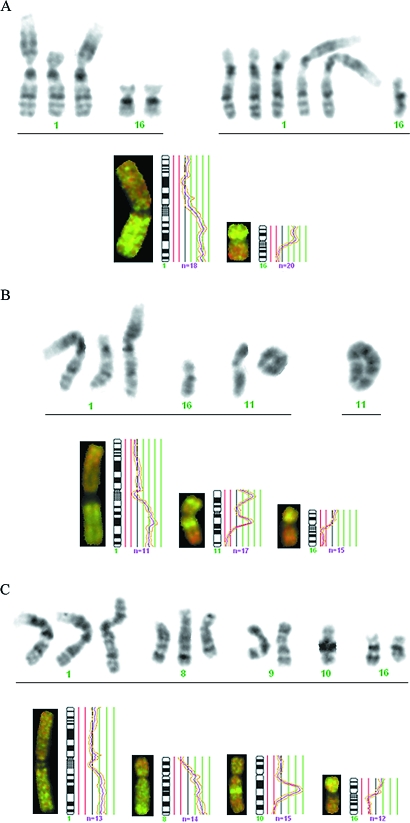
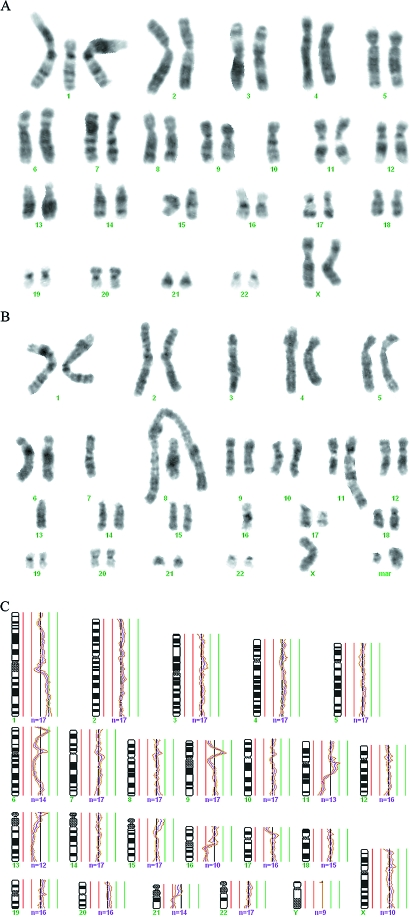
To be able to compare the G-banding and CGH findings, the chromosomal imbalances in each case were deduced from the karyotypic data (Figure 1). Similar imbalances detected by both techniques were discernible in 14 of the 37 breast carcinomas, making it overwhelmingly likely that the same clonal cell populations were being looked at in these cases. In the two cases in which no imbalance was detected by CGH, the chromosome banding analysis had revealed only balanced rearrangements in the mainline, and so the findings here, too, may be considered to be in agreement. Some of the matching cases (e.g., 512/92, 80/93, and 335/93; Table 1 and Figure 2) presented neartriploid clones with numerous chromosomal alterations, whereas others had neardiploid clones with many (e.g., cases 503/92, 136/93, 167/93, and 208/93; Figures 3 and 4) or only a few changes (e.g., case 46/93 presented only rev ish enh(1q,16p) due to a supernumerary der(1q;16p)). Because the imbalances corresponding to the banding karyotypes were detected by CGH, both the clones with simple chromosomal abnormalities and the more complex ones must have made up a significant fraction of the test sample, even when the G-banding analysis after culturing revealed only a few representative metaphases (e.g., cases 512/92, 46/93, 136/93, and 2/94). The results therefore provide further evidence that breast carcinomas are clonally heterogeneous and that cytogenetic complexity is an insufficient criterion to judge the pathogenetic relevance of these clones by.
Figure 2.
G-banding karyogram (A) and CGH profile (B) showing the same clonal cell population in a breast carcinoma (case 80/93) with complex genomic changes. See Table 1 for a full description of the acquired clonal aberrations. The changes present in this metaphase that are not indicated in the karyotype are nonclonal.
Figure 3.
Partial karyograms and CGH profiles showing the increased understanding of the acquired genetic changes obtained by the combined analysis. (A) The genomic imbalances detected by CGH (rev ish enh(1q,16p)) in case 46/93 are due to one (left) and three (right) supernumerary der(1q;16p) present in two related subclones. The loss of one chromosome 16 occurred only in one subclone, which explains why the ratio profile did not reach 0.75 at 16q. (B) The presence of a der(1q;16p) instead of a normal chromosome 16 explains the gain of 1q and loss of 16q seen by CGH in case 467/93. The imbalances detected by CGH in chromosome 11 showed that the ring chromosome contained multiple copies of 11p14p15 and 11q12q14 but no 11q22qter material. The ring was unstable and was larger in some cells (right). (C) Chromosome banding analysis showed that the rev ish enh(8q,10q21q22) and rev ish dim(8p) detected by CGH in case 503/92 derived from the presence of multiple copies of an abnormal chromosome 8 having an insertion of a segment of 10q in its long arm as well as loss of 8p material. In this case, the gain of 1q and loss of 16q are independent events, because the gain of 1q resulted from an i(1q) and not from a der(1q;16p). The inv(9) is not detectable by CGH.
Figure 4.
Imbalances brought about by two cytogenetically unrelated clones (A and B) were simultaneously detected by CGH (C) in case 136/93, indicating that both clones were large in vivo and, by inference, were part of the tumor parenchyma. See Table 1 for a full description of the acquired aberrations. The changes not indicated in the karyotype are nonclonal.
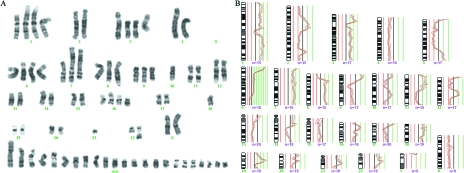
In the remaining 21 tumors, the comparison of the G-banding and CGH findings revealed no clear concordance. This has two main explanations: First, the extreme complexity of the karyotypic abnormalities in some cases, with numerous chromosomes with additional material of unknown origin and markers (many of these corresponding to amplifications detected by CGH; Figure 5), made detailed and complete description of the chromosomal anatomy based on G-banding appearance impossible (e.g., cases 145/93, 248/93, and 392/93). A reliable assessment of karyotypic imbalances based on chromosome banding analysis alone is difficult in such instances, and so it is nevertheless likely that both techniques did detect the same clones also in these cases. Second, the chromosomal changes detected by the G-banding and CGH analyses might be present in different tumor cell subpopulations. Because CGH requires that one copy loss or gain be present in at least 50% (or more in a hyperdiploid tumor) of the cells to be scored using thresholds of 0.75 and 1.25, respectively, anything but major clones will remain undetected. This probably explains why der(1q;16p) was detected as the single chromosomal abnormality by both techniques in case 46/93, but was not seen by CGH in case 557/93. However, although the cytological nature of Gbanding analysis enables it to pick up also small cell subpopulations and clones presenting only balanced chromosome rearrangements (e.g., cases 495/93 and 574/93), selection bias may occur during culturing if some clones thrive better than others in vitro. This explains the CGH copy number changes that the G-banding karyotypes of some cases (e.g., 394/92, 431/92, and 507/93) cannot account for. The study by Persson et al. [13] permitted a similar conclusion. Thus, both G-banding and CGH analyses underestimate the cytogenetic heterogeneity of breast carcinomas and, in principle, tumor cell populations in general, although their combined use goes some way toward providing a more realistic picture. This conclusion is also supported by the lack of a statistically significant correlation between tumor grade and the number of genetic changes detected by either G-banding or CGH, whereas grade III carcinomas were significantly correlated with high genetic complexity as ascertained by the combined G-banding/CGH approach (Table 2).
Figure 5.
Amplifications (ratio >2.0) were mostly seen in cases with complex abnormalities detected by either G-banding or CGH. Top row: amp(1q25q31), amp(6p23p25), amp(7p21p22), and amp(7p11p14,7q11q21). Middle row: amp(8q23), amp(8q24), amp(8q), and amp(10p12p14). Bottom row: amp(11q13), amp(17q11q21), amp(17q22q24), and amp(20q12q13).
Chromosome banding analysis revealed cytogenetically unrelated clones in eight cases (cases 503/92, 515/92, 100/93, 113/93, 136/93, 199/93, 244/93, and 248/93). Although CGH reveals the genomic imbalances of an idealized, average cell, the copy number changes detected in two of these cases (503/92 and 199/93) could be ascribed to only one clone, in all likelihood the quantitatively dominant one in vivo. This is in keeping with the commonly held view that, at any one time, the selection pressure will work in favor of only one neoplastic cell subpopulation [14]. The apparently disparate findings seen in other cases are explained by the methodological limitations indicated above. Interestingly, however, the copy number changes detected by CGH in case 136/93 corresponded to the genomic imbalances seen in two of the three cytogenetically unrelated clones revealed by G-banding (the third clone carried a balanced translocation only, and could therefore not be detected by CGH; Figure 4). The rev ish enh(1q22qter) is attributable to the der(1;16)(q10;p10) of one clone, the rev ish dim(11q21qter,13q13qter) is attributable to the add(11)(q21) and monosomy 13 of the other clone with more complex chromosomal abnormalities, whereas the ish rev dim(16q) is explained by the average of the two aforementioned clones, because one presents monosomy 16 and the other gain of 16p due to the presence of a der(1q;16p) in addition to two normal copies of chromosomes 1 and 16. The additional, apparently nonmatching imbalances are accounted for by the markers, the homogeneously staining regions, and the additional material of unknown origin detected by chromosome banding analysis. In this case, therefore, both clones must have made up a considerable fraction of the tumor providing strong, albeit indirect, evidence that the cytogenetically unrelated clones often detected in breast carcinomas are part of the neoplastic parenchyma. Polyclonal tumorigenesis in some breast carcinomas is the simplest explanation for these findings.
Because the picture of genomic alterations revealed by the two techniques overlapped only partly, we were able to combine them to extract more information than is possible with each of them alone. CGH, besides sometimes revealing genomic imbalances that belonged to cell populations not picked up by the cytogenetic analysis, gave a more reliable overall picture of the copy number gains and losses in cases where the G-banding karyotypes had many markers and chromosomes with additional material of unknown origin. Furthermore, when both techniques detected relatively few changes that could more easily be seen to be the same, the CGH findings helped determine the origin of chromosomal segments and to refine breakpoints. For instance, in case 503/92 the CGH analysis helped us to understand that the segment of 10q material inserted in 8q was 10q21q22 instead of the more distal 10q22q24, whereas in case 467/93 CGH identified the segments of chromosome 11 that were lost (11q22qter) and gained (11p14p15 and 11q12q14) in the ring chromosome (Figure 3). No gain of chromosome 20 was found by CGH in spite of the +20 detected by G-banding in case 467/93, however, which was compatible with the finding of this trisomy by the latter in only four of the 19 cells with r(11).
However, besides sometimes revealing cell populations with balanced chromosome aberrations or unbalanced changes that nevertheless remained undetected by CGH, chromosome banding analysis was essential to understand how the genomic imbalances arose in the many cases in which both techniques detected the same clonal abnormalities. For instance, in case 46/93 the G-banding data tell us that the rev ish enh(1q,16p) arose through the presence in the stemline of a der(1;16)(q10;p10) in addition to two normal chromosomes 1 and 16, whereas a subclone had acquired two additional der(1q;16p). Simple though it was, the nature of this chromosome abnormality could not be deduced from the CGH data alone, especially against the background that the der(1q;16p) in most breast carcinomas is seen instead of a normal chromosome 16, resulting in gain of 1q but loss of 16q. Loss of one normal chromosome 16 did indeed occur in a subclone, but the ratio profile at 16q did not reach the 0.75 level as this chromosomal arm was lost only in a subpopulation of neoplastic cells. Even more strikingly, in case 503/92 the chromosome banding analysis clearly showed that the rev ish enh(8q,10q21q22) and rev ish dim(8p) detected by CGH were not independent events, but instead derived from the presence of multiple copies of an abnormal chromosome 8 having an insertion of a segment of 10q in its long arm as well as loss of 8p material (Figure 3). Knowledge of the chromosomal organization of karyotypic changes, not only of the resulting imbalances, is important in order to study possible position effects, including the occurrence of fusion genes, resulting from them.
A nonrandom association between genetic complexity and histologic tumor type was apparent in the series we present. With regard to the cases with special histology, all carcinomas with a cribriform, lobular or DCIS histology presented relatively few genetic changes by the combined G-banding/CGH analysis, whereas the two papillary carcinomas and the single mucinous carcinoma presented numerous genetic changes (Table 1). Furthermore, comedocarcinomas are more often genetically complex than ductal carcinomas NOS. That different histological subtypes of breast carcinoma may be associated with differen degrees of genetic complexity has also been found in other studies, both with chromosome banding analysis [15–17] and CGH [12,18–20]. Further analysis of larger series is nevertheless necessary to test whether genetic subtyping of breast carcinomas really leads to new information reliable enough to have an impact on how these patients should be treated.
Acknowledgements
The technical assistance of Mette Eknæs and Lill Andersen is gratefully acknowledged.
Footnotes
This work was supported by grants from the Norwegian Cancer Society (M.R.T., R.A.L., and S.H.) and the Hellenic Anticancer Institute (N.P.). S.M.K. is a research fellow of the Norwegian Cancer Society.
References
- 1.Pandis N, Jin Y, Gorunova L, Petersson C, Bardi G, Idvall I, Johansson B, Ingvar C, Mandahl N, Mitelman F, Heim S. Chromosome analysis of 97 primary breast carcinomas: identification of eight karyotypic subgroups. Genes Chromosomes Cancer. 1995;12:173–185. doi: 10.1002/gcc.2870120304. [DOI] [PubMed] [Google Scholar]
- 2.Adeyinka A, Mertens F, Idvall I, Bondeson L, Ingvar C, Mitelman F, Pandis N. Different patterns of chromosomal imbalances in metastasising and non-metastasising primary breast carcinomas. Int J Cancer. 1999;84:370–375. doi: 10.1002/(sici)1097-0215(19990820)84:4<370::aid-ijc7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira MR, Pandis N, Bardi G, Andersen JA, Mitelman F, Heim S. Clonal heterogeneity in breast cancer: karyotypic comparisons of multiple intra- and extra-tumorous samples from 3 patients. Int J Cancer. 1995;63:63–68. doi: 10.1002/ijc.2910630113. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira MR, Pandis N, Bardi G, Andersen JA, Heim S. Karyotypic comparisons of multiple tumorous and macroscopically normal surrounding tissue samples from patients with breast cancer. Cancer Res. 1996;56:855–859. [PubMed] [Google Scholar]
- 5.Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258:818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- 6.Kallioniemi OP, Kallioniemi A, Piper J, Isola J, Waldman FM, Gray JW, Pinkel D. Optimizing comparative genomic hybridization for analysis of DNA sequence copy number changes in solid tumors. Genes Chromosomes Cancer. 1994;10:231–243. doi: 10.1002/gcc.2870100403. [DOI] [PubMed] [Google Scholar]
- 7.Sobin LH. Histological Typing of Breast Tumors. 2nd edn. Geneva, Switzerland: World Health Organization; 1981. [Google Scholar]
- 8.Pandis N, Heim S, Bardi G, Limon J, Mandahl N, Mitelman F. Improved technique for short-term culture and cytogenetic analysis of human breast cancer. Genes Chromosomes Cancer. 1992;5:14–20. doi: 10.1002/gcc.2870050103. [DOI] [PubMed] [Google Scholar]
- 9.Mitelman F. ISCN (1995): An International System for Human Cytogenetic Nomenclature. Basel, Switzerland: S. Karger; 1995. [Google Scholar]
- 10.Tsarouha H, Pandis N, Bardi G, Teixeira MR, Andersen JA, Heim S. Karyotypic evolution in breast carcinomas with i(1)(q10) and der(1;16)(q10;p10) as the primary chromosome abnormality. Cancer Genet Cytogenet. 1999;113:156–161. doi: 10.1016/s0165-4608(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 11.Kraggerud SM, Szymanska J, Abeler V, Kærn J, Eknæs M, Heim S, Teixeira MR, Tropé CG, Peltomäki P, Lothe RA. DNA copy number changes in malignant ovarian germ cell tumors. Cancer Res. 2000;60:3025–3030. [PubMed] [Google Scholar]
- 12.Tirkkonen M, Tanner M, Karhu R, Kallioniemi A, Isola J, Kallioniemi OP. Molecular cytogenetics of primary breast cancer by CGH. Genes Chromosomes Cancer. 1998;21:177–184. [PubMed] [Google Scholar]
- 13.Persson K, Pandis N, Mertens F, Borg Å, Baldetorp B, Killander D, Isola J. Chromosomal aberrations in breast cancer: a comparison between cytogenetics and comparative genomic hybridization. Genes Chromosomes Cancer. 1999;25:115–122. [PubMed] [Google Scholar]
- 14.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 15.Pandis N, Idvall I, Bardi G, Jin Y, Gorunova L, Mertens F, Olsson H, Ingvar C, Beroukas K, Mitelman F, Heim S. Correlation between karyotypic pattern and clinicopathologic features in 125 breast cancer cases. Int J Cancer. 1996;66:191–196. doi: 10.1002/(SICI)1097-0215(19960410)66:2<191::AID-IJC9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Adeyinka A, Mertens F, Idvall I, Bondeson L, Ingvar C, Heim S, Mitelman F, Pandis N. Cytogenetic findings in invasive breast carcinomas with prognostically favourable histology: a less complex karyotypic pattern? Int J Cancer. 1998;79:361–364. doi: 10.1002/(sici)1097-0215(19980821)79:4<361::aid-ijc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Flagiello D, Gerbault-Seureau M, Sastre-Garau X, Padoy E, Vielh P, Dutrillaux B. Highly recurrent der(1;16)(q10;p10) and other 16q arm alterations in lobular breast cancer. Genes Chromosomes Cancer. 1998;23:300–306. [PubMed] [Google Scholar]
- 18.Nishizaki T, Chew K, Chu L, Isola J, Kallioniemi A, Weidner N, Waldman FM. Genetic alterations in lobular breast cancer by comparative genomic hybridization. Int J Cancer. 1997;74:513–517. doi: 10.1002/(sici)1097-0215(19971021)74:5<513::aid-ijc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Roylance R, Gorman P, Harris W, Liebmann R, Barnes D, Hanby A, Sheer D. Comparative genomic hybridization of breast tumors stratified by histological grade reveals new insights into the biological progression of breast cancer. Cancer Res. 1999;59:1433–1436. [PubMed] [Google Scholar]
- 20.Vos CB, ter Haar NT, Rosenberg C, Peterse JL, Cleton-Jansen AM, Cornelisse CJ, van de Vijver MJ. Genetic alterations on chromosome 16 and 17 are important features of ductal carcinoma in situ of the breast and are associated with histologic type. Br J Cancer. 1999;81:1410–1418. doi: 10.1038/sj.bjc.6693372. [DOI] [PMC free article] [PubMed] [Google Scholar]