Abstract
Integrins play an important role in tumour progression by influencing cellular responses and matrix-dependent adhesion. However, the regulation of matrix-dependent adhesion assembly in epithelial cells is poorly understood. We have investigated the integrin and signalling requirements of cell-matrix adhesion assembly in colon carcinoma cells after plating on fibronectin. Adhesion assembly in these, and in the adenoma cells from which they were derived, was largely dependent on αvβ6 integrin and required phosphorylation of FAK on tyrosine-397. The rate of fibronectin-induced adhesion assembly and the expression of both αvβ6 integrin and FAK were increased during the adenoma-to-carcinoma transition. The matrix-dependent adhesion assembly process, particularly the final stages of complex protrusion that is required for optimal cell spreading, required the activity of extracellular signal-regulated kinase (ERK). Furthermore, phosphorylated ERK was targeted to newly forming cell-matrix adhesions in the carcinoma cells but not the adenoma cells, and inhibition of FAK-tyrosine-397 phosphorylation or MEK suppressed the appearance of phosphorylated ERK at peripheral sites. In addition, inhibition of MEK-ERK activation blocked the formation of peripheral actin microspikes that were necessary for the protrusive phase of cell-matrix adhesion assembly. Thus, MEK-ERK-dependent peripheral actin re-organization is required for the full development of integrin-induced adhesions and this pathway is stimulated in an in vitro model of colon cancer progression.
Keywords: integrin-mediated adhesion assembly, colon cancer, focal adhesion kinase, extracellular signal-regulated kinase, actin cytoskeleton
Introduction
Focal adhesions are specialized structures found in fibroblasts where cells contact their surrounding extracellular matrix (ECM). They consist of clustered integrin heterodimers, heterodimers, structural- or cytoskeletal-associated proteins that link the ECM through the integrins to the actin cytoskeleton, and proteins involved in intracellular signal transduction (reviewed in Ref. [1]). The dynamic regulation of the assembly and disassembly of these structures governs the ability of cells to respond to their extracellular environment, thus regulating processes such as adhesion and motility. In epithelial cells, similar matrix-dependent adhesion sites exist although little is known about their regulation. In tumour cells in vivo, the balance between adhesiveness and migration is likely to be determined by the underlying matrix as well as the integrins present on the tumour cell surface. While a reduction in tumour cell matrix should aid migration and tumorigenicity, the cells also require matrix adhesion to derive traction for migration away from other cells. Thus, deregulation of matrix-dependent adhesion assembly in tumour cells alters tumour cell behaviour and may result in more aggressive and invasive tumours.
In this study, we have examined matrix-dependent adhesion assembly in an in vitro model of colon cancer progression. In the model, a cell line was derived from a familial adenoma (PC/AA), from which a non-tumorigenic clonogenic variant was established (AA/C1). This adenoma cell line was transformed in vitro to produce an anchorage-independent adenocarcinoma line, which was tumorigenic when injected into nude mice (AA/C1/SB10) [2]. Analysis of the genetic and cellular changes that occur during this conversion mimics those seen in vivo, indicating that this is a relevant model for the study of cellular changes that occur during the acquisition of a malignant phenotype [2,3]. Using this model, we have identified changes in the regulation of integrin-dependent adhesion assembly during the adenoma-to-carcinoma transition. In addition, examination of the matrix-dependent adhesion assembly process, which is poorly understood in epithelial cells, identified specific signalling requirements for the cytoskeletal re-arrangements at the cell periphery that were required for the protrusive final phase of the adhesion assembly process.
Integrins function not only as adhesion receptors, but also play a role in activating intracellular signalling pathways. Mediators of integrin-induced signalling include focal adhesion kinase (FAK) and the Src family of tyrosine kinases, which we have identified as possible mediators of epidermal growth factor-induced invasion of AA/C1/SB10 colon carcinoma cells [4]. Specifically, FAK is a key regulator of cell spreading [5–7], focal adhesion turnover [8], and cell motility [9,10]. It is rapidly phosphorylated on a number of tyrosine residues after plating cells on matrix proteins [11–14], including autophosphorylation of tyrosine-397 [15], which plays a key role in integrin-mediated events such as cell spreading and migration [5–7].
Extracellular signal-regulated kinase (ERK) activation is also associated with numerous biological events including integrin engagement [16–19], where it has been shown to be downstream of FAK although this appears to be cell type- and context-dependent. Recently, activated ERK has been observed at newly forming focal adhesions in fibroblasts [20], which is consistent with a role for ERK signalling in cell migration via phosphorylation of myosin light chain kinase (MLCK) [21,22].
We set out to analyze the process of fibronectin-induced adhesion assembly in the in vitro model of colon cancer progression described, and to study the role of signalling intermediates downstream of integrins in this process. Fibronectin-induced adhesion assembly in the colon cancer cells was mediated, at least in part, by αvβ6 integrin that was upregulated in the carcinoma cells. Furthermore, cell-matrix adhesion assembly required phosphorylation of FAK on tyrosine-397. In addition, activation of MEK-ERK was required for the final protrusion of adhesion complexes that had formed at the cell periphery. Specifically, ERK activation was required for the generation of actin microspikes that emanated from the peripheral cortical actin and mediated the protrusion of adhesion complexes at the cell periphery and optimal cell spreading.
Materials and Methods
Cell Culture and Transfections
The AA/C1 series of cell lines has been previously described in detail [2]. Chicken FAK and FAK (Y397F) both with a Myc tag at the carboxy terminus [23] were removed from RCAS-FAK or RCAS-397F as ClaI fragments, blunt-ended, and cloned into the EcoRV site of pcDNA3.1 (Invitrogen, Groningen, Netherlands). HA-ERK was a kind gift from J. Pouyssegur [24]. Transient transfections were carried out using Superfect according to the manufacturer's protocol (Qiagen, Crawley, UK).
Immunofluorescence
Permanox chamber slides were coated with fibronectin (10 µg/ml; Becton Dickinson, Oxford, UK) by overnight incubation at 4°C. All wells were incubated with 0.1% BSA for 2 hours at 37°C to block non-specific binding and then washed twice with phosphate-buffered saline (PBS). Cells were then plated onto the coated chambers and allowed to adhere for the indicated times. The medium was removed and the cells fixed in 3.7% formaldehyde in PBS for 10 minutes at room temperature, permeabilized for 15 minutes with 0.5% Triton X-100/1% BSA in PBS, and blocked for 30 minutes with 10% fetal bovine serum in PBS. The cells were incubated with an anti-vinculin antibody (10 µg/ ml; Sigma, Poole, UK) for 1 hour, washed in PBS, and then incubated with anti-mouse FITC-labeled secondary antibody (Stratech Scientific, Luton, UK). For visualization of Myc-tagged FAK proteins, fixed cells were incubated with rabbit anti-Myc antibody (10 µg/ ml; TCS Biologicals, Botolph, Claydon, UK) and for detection of HA-tagged ERK, with mouse anti-HA (2 µg/ ml; Roche Diagnostics, Lewes, UK), then the appropriate Texas Red labeled secondary antibody (Stratech Scientific, Luton, UK). Detection of phospho-ERK was carried out as described previously [20].
Cell-Matrix Adhesion Assembly
Permanox chamber slides were coated with fibronectin (10 µg/ml) by overnight incubation at 4°C. All wells were incubated with 0.1% BSA for 2 hours at 37°C to block non-specific binding and then washed twice with PBS. Cells were plated onto the coated chambers and allowed to attach for the indicated times. The cells were then fixed and stained with anti-vinculin antibody as described above. Experiments with antibodies and inhibitors were carried out in two ways. Either the cells were allowed to attach for 30 minutes before their addition or the cells were pre-incubated with them for 15 minutes prior to attachment. Similar results were obtained in each case and results are shown for the second procedure. Antibodies used were anti-β1 (mAb13, 5 µg/ ml; Becton Dickinson, Oxford, UK), anti-αv antibody (L230, ATCC hybridoma, 10 µg/ml), and anti-αvβ6 (10D5, a kind gift from Dean Sheppard, UCSF, 10 µg/ml). Inhibitor concentrations were PD098059 (25 µM) (Calbiochem, Nottingham, UK) and UO126 (20 µM; Promega, Southampton, UK). To monitor adhesion assembly on immobilised αvβ6 antisera, chambers were coated overnight with rabbit anti-mouse IgG (10 µg/ml), blocked in 0.1% BSA, then incubated with anti-αvβ6 antibody (E7P6, 10 µg/ml) for 2 hours at 37°C before plating the cells.
Cell Attachment
Fibronectin (Becton Dickinson, Oxford, UK) and collagen type IV (Sigma, Poole, UK) were used at a concentration of 10 µg/ml to coat 96-well plates by overnight incubation at 4°C. All wells were incubated with 0.1% BSA for 2 hours at 37°C to block non-specific binding and then washed twice with PBS. Cells were trypsinized and labeled with 51Cr by incubation with 90 µCi sodium chromate (Amersham, Little Chalfont, UK) in serum containing medium for 1 hour at 37°C. Serum proteins and unincorporated 51Cr were removed by washing three times in serum-free medium. The labeled cells were then resuspended in serum-free medium and 1x104 cells added to each well of the pre-treated plate. After 1 hour, the non-attached cells were removed by washing twice in PBS and the number of remaining attached cells determined by counting the radioactivity on the bottom of each well using a gamma counter. Non-specific attachment was determined on wells coated with 0.1% BSA, which was typically around 1% to 5% in both cell lines. This was then subtracted from each value. The results were either expressed as percentage attachment, where 100% attachment corresponds to the total radioactivity added to each well, or as percentage of control attachment in untreated cells. Wells were set up in quadruplicate and the values are the mean±SD taken from a representative experiment in a series of at least three.
Immunoprecipitation and Immunoblotting
For FAK immunoprecipitation, cell extracts were prepared in RIPA buffer (50 mM Tris pH 7.4, 150 mM sodium chloride, 5 mM EGTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 10 mM sodium pyrophosphate, 0.5 mM sodium fluoride, 1 mM PMSF, 10 µg/ml aprotinin, and 100 µM sodium orthovanadate) and immunoprecipitated with either saturating concentrations of anti-FAK antisera raised against a C-terminal peptide [25] or phospho-FAK-Y397 (4 µg [23]). The immune complexes were collected on protein A-Sepharose and washed five times with RIPA. Proteins were separated by 7% SDS-PAGE and visualized after blotting with either anti-FAK antibody (0.5 µg/ ml; Becton Dickinson, Oxford, UK) or anti-phosphotyrosine antibody (PY20, 1 µg/ml; Becton Dickinson, Oxford, UK).
Fluorocytometric Analysis
Cells were trypsinized and resuspended in ice-cold wash buffer (PBS supplemented with 0.1% BSA). Cells (2x105) were incubated with the appropriate integrin antibodies for 1 hour at 4°C and then washed three times with wash buffer before incubation with FITC-labeled secondary antibody for 30 minutes at 4°C. After three washes in wash buffer, the cells were fixed with 3.7% formaldehyde and stored at 4°C prior to analysis. Analysis was performed using a FACSCAN analyzer (Becton Dickinson). Background fluorescence was determined by omitting the primary antibody in one set of samples. Antibodies used were anti-αvβ5 (P1F6, 10 µg/ml; Chemicon, Harrow, UK) and anti-αvβ6 (E7P6, a kind gift from Dean Sheppard, UCSF, 10 µg/ml).
Results
Cell-Matrix Adhesion Assembly on Fibronectin is Enhanced during the Adenoma-to-Carcinoma Transition
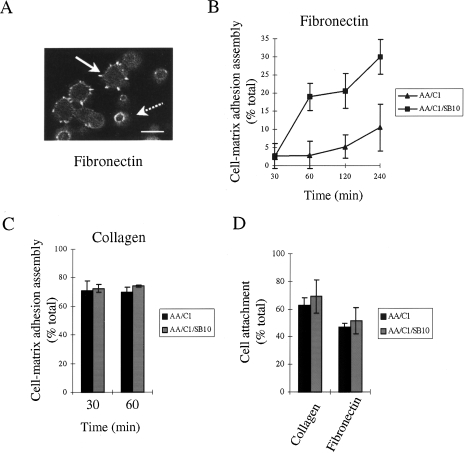
We have examined the assembly of matrix-dependent cellular adhesions in colorectal cancer cells (AA/C1/SB10) and their adenoma progenitors (AA/C1). When these cells spread after attaching to fibronectin, prominent vinculin-containing protrusions (referred to from here as cell-matrix adhesions) are assembled at the cell periphery (Figure 1A, solid arrow). In contrast, cells that have adhered to fibronectin, but which have not spread, remain rounded and are apparently tethered by a ring of vinculin-containing smaller structures on the basal cell surface (Figure 1A, broken arrow). Using these criteria, we have quantitated cell-matrix adhesion assembly on fibronectin by monitoring the number of cells in the adherent population that have peripheral vinculin-containing protrusions. We found that the malignant colorectal cells, AA/C1/SB10, assembled cell-matrix adhesions and spread more rapidly following adhesion to fibronectin than the non-malignant cells, AA/C1, from which they were derived (Figure 1B). However, the adhesion assembly process was unaltered between the two cell lines following adhesion of the cells to collagen (Figure 1C), indicating that the enhanced assembly of protruding adhesions was matrix-specific. The difference in cell-matrix adhesion assembly on fibronectin between the adenoma and carcinoma cells was therefore not due to an intrinsic inability of the adenoma cells to assemble such structures. The difference in the adhesion assembly process between the two cell lines was also not a result of differences in the ability of the cells to attach, as there was no difference in attachment to fibronectin between the two cell lines (Figure 1D). In this experiment, we measured the number of cells that attached to the matrix proteins after 1 hour regardless of whether they had spread and assembled peripheral adhesions. Thus, cell-matrix adhesion assembly can be monitored independently of attachment to fibronectin and the enhanced assembly of cell-fibronectin adhesion protrusions accompanies the adenoma-to-carcinoma transition that occurs in the in vitro model of colon cancer progression.
Figure 1.
Cell-matrix adhesion assembly on fibronectin is enhanced during the progression of colon cancer. (A) Visualization of AA/C1/SB10 carcinoma cell-matrix adhesion assembly 1 hour after plating on fibronectin. Cells were fixed and stained for vinculin. The solid arrow represents a spread cell with prominent vinculin-containing protrusions, while the broken arrow represents an adherent, but non-spread cell which is tethered to the surface by a ring of small vinculin-containing structures. Scale bar, 25 µm. (B) Cell-matrix adhesion assembly on fibronectin in AA/C1 and AA/C1/SB10 cells was quantitated by counting the percentage of cells with prominent adhesions in >500 adherent cells for the indicated times. Values are mean±SD from triplicate wells. (C) Cell-matrix adhesion assembly on collagen was measured by counting the percentage of cells with prominent focal adhesions in >500 cells. Values are mean±SD from triplicate wells. (D) Cell attachment to fibronectin and collagen was measured after 60 minutes. Values are mean±SD from quadruplicate wells.
Cell-Matrix Adhesion Assembly on Fibronectin is Integrin-Mediated
That the enhanced assembly of protruding adhesions was matrix-dependent was confirmed by the observation that the colorectal carcinoma cells contained no discernible vinculin-containing structures after attachment to poly-l-lysine (not shown). To ascertain which integrin heterodimer(s) was responsible, we used previously characterized antibodies that inhibit the ability of particular integrins to act as receptors for their matrix ligands [26,27].
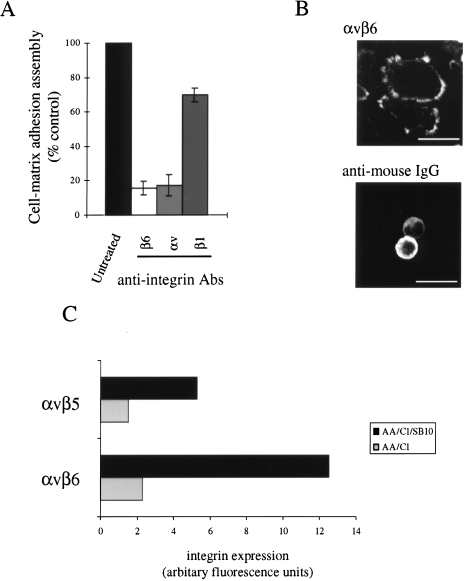
Using these antibodies, we previously demonstrated that the attachment of both the AA/C1 and AA/C1/SB10 cells to fibronectin was mediated by the α5β1 and αvβ6 integrins (results not shown). We therefore examined the involvement of these integrins in the fibronectin-mediated adhesion assembly process. The β6 inhibitory antibody suppressed adhesion assembly by around 85% (similar results were also seen with an αv subunit inhibitory antibody), while there was only around a 30% inhibition with the β1 inhibitory antibody (Figure 2A). Furthermore, clustering of the αvβ6 integrin by plating cells on immobilised αvβ6 antibody clustered with anti-mouse IgG was sufficient to induce adhesion assembly (Figure 2B, top panel). In contrast, very few cells adhered to the chambers coated only with anti-mouse IgG and those that did attach had no peripheral vinculin staining (Figure 2B, bottom panel). Thus, we concluded that fibronectin-induced adhesion assembly in the AA/C1/SB10 colon carcinoma cells (and also in the AA/C1 adenoma progenitors, not shown) was largely dependent on the function of αvβ6 heterodimers, while the β1 integrin may play a lesser role. αvβ6 is also a receptor for vitronectin; however, for reasons that are unexplained, neither of the cell lines was able to adhere to vitronectin (results not shown). Interestingly, we found that αvβ6 integrin expression was four-fold higher in the carcinoma cells when compared to the adenoma cells from which they were derived, with small changes in other integrins known to act as receptors for fibronectin such as αvβ5, also seen between the two cell lines (Figure 2C). The increased cell surface expression of αvβ6 may contribute to the enhanced cell-matrix adhesion assembly that is displayed by the AA/C1/SB10 cells on fibronectin (Figure 1B), which we have shown to be largely inhibited by αvβ6 blocking antibodies (Figure 2A).
Figure 2.
Cell-matrix adhesion assembly on fibronectin is integrin-mediated. (A) Cell-matrix adhesion assembly in AA/C1/SB10 cells on fibronectin was measured by counting the percentage of cells with prominent adhesions in >500 cells in the presence of inhibitory integrin antibodies as indicated. Values are mean±SD from triplicate wells. (B) Cell-matrix adhesion assembly on αvβ6 antibody and anti-mouse IgG coated chambers (top panel) or anti-mouse IgG alone-coated chambers (bottom panel). (C) Cell surface integrin expression was determined in the AA/C1 and AA/C1/SB10 cells following FACS analysis using integrin antibodies as described in Materials and Methods section. Relative fluorescence values were determined using the arbitrary geometric mean values calculated for the individual histograms, from which the non-specific fluorescence was deducted. Non-specific fluorescence was measured in the absence of antibody. Values are taken from a representative experiment in a series of three.
FAK Phosphorylation is Required for Cell-Matrix Adhesion Assembly
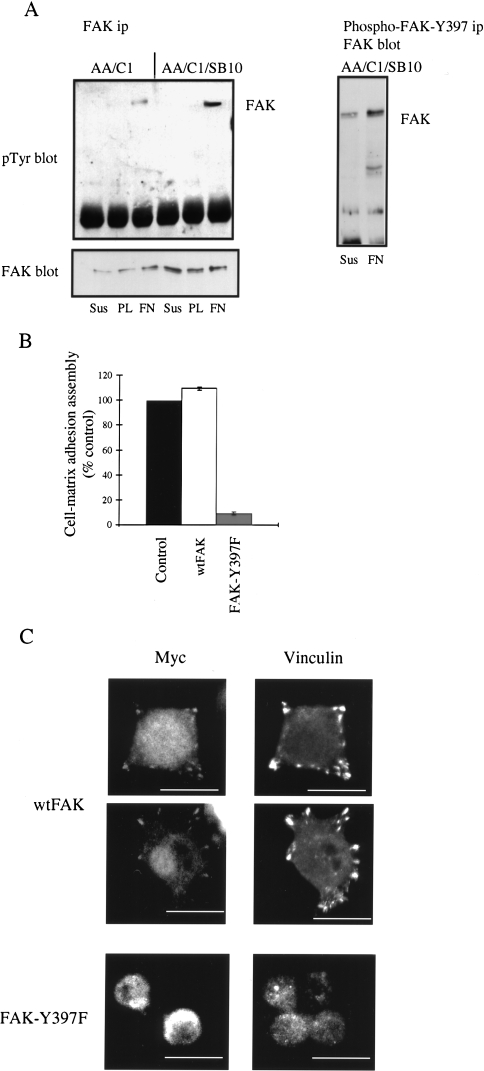
We next investigated the signalling pathways that regulate integrin-dependent assembly of cell-matrix adhesions. One potential regulator of integrin-dependent adhesion assembly is FAK, which is phosphorylated on tyrosine-397 after plating cells on matrix proteins and is required for FAK-mediated functions [5–7,28]. As we had previously reported that protein levels of FAK were increased in the AA/C1/SB10 carcinoma cells as compared to the AA/C1 adenoma cells [4], we addressed whether FAK tyrosine phosphorylation was involved in integrin-mediated adhesion assembly in the colon epithelial cells. We found that FAK was tyrosine-phosphorylated after plating both the AA/C1 and AA/C1/SB10 cells on fibronectin but not on poly-l-lysine (Figure 3A, left hand, top panel), although the level of phosphorylation was greater in the AA/C1/SB10 cells. This most likely reflects the increased expression of FAK in the carcinoma cells (Figure 3A, left hand, bottom panel [4]). Furthermore, using an antibody, which specifically recognises FAK phosphorylated on tyrosine-397 [23], we demonstrated that this residue was phosphorylated in the AA/C1/SB10 cells (Figure 3A, right hand panel). Since these cells cannot readily be stably transfected to express exogenous proteins, we have used a combination of transient transfection and drug treatment to further monitor the cell-matrix adhesion assembly process on a single cell basis. We therefore transiently transfected AA/C1/SB10 colon carcinoma cells with Myc-tagged FAK or a FAK-Y397F mutant, and monitored the assembly of cell-matrix adhesions. In cells expressing exogenous FAK-Y397F, the number of cells that were able to assemble adhesions was reduced to around 10% of that seen in the untransfected control cells, while expression of the wild-type protein had no effect (Figure 3B). Using immunofluorescence, we demonstrated that expression of the wild-type protein resulted in its localization to cell-matrix adhesions co-incident with the localization of vinculin at these sites (Figure 3C). In contrast, Nature Publishing Groupcells expressing the FAK-Y397F remained rounded with diffuse staining for both vinculin and the myc-tagged mutant FAK protein (Figure 3C). These data indicate that integrin-dependent cell spreading in AA/C1/SB10 colon carcinoma cells is accompanied by FAK phosphorylation on tyrosine-397, and that this is also required for fibronectin-induced adhesion assembly.
Figure 3.
FAK phosphorylation is required for integrin-mediated adhesion assembly. (A) FAK phosphorylation in cells either held in suspension (Sus), plated on fibronectin (FN), or poly-l-lysine (PL) for 1 hour. FAK was immunoprecipitated from cell lysates and tyrosine-phosphorylated FAK visualized by blotting and probing with PY20, a general phosphotyrosine antibody (left hand, top panel). The same filters were reprobed with anti-FAK antibody (left hand, bottom panel). FAK phosphorylated on tyrosine-397 was immunoprecipitated from AA/C1/SB10 cell lysates using a phospho-specific antibody and immunoprecipitated proteins visualized by probing with an anti-FAK antibody (right panel). (B) AA/C1/SB10 cells were transfected with Myc-tagged wtFAK or FAK-Y397F and trypsinized after 24 hours before plating on fibronectin. After 1 hour, the cells were fixed and stained with an antibody to vinculin and the Myc epitope tag. Cell-matrix adhesion assembly was quantitated in the non-transfected and transfected cells. Values are mean±SD from triplicate wells with >500 transfected cells. (C) Visualization of Myc-tagged proteins following double staining with anti-Myc and anti-vinculin antibodies. Scale bars, 25 µm.
Involvement of ERK in Cell-Matrix Adhesion Assembly on Fibronectin
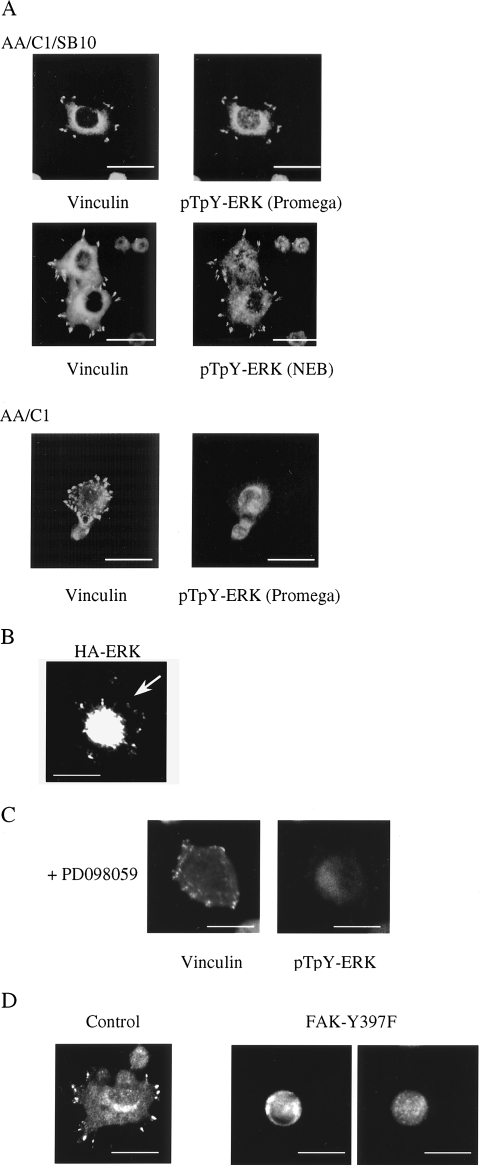
The ERK-MAP kinases have been implicated in the regulation of cell migration [21,22] and are rapidly activated following integrin engagement [16–19]. Furthermore, there is a wide body of evidence implicating FAK in this process, although the details remain to be elucidated (reviewed in Ref. [29]). We therefore examined whether the MEK-ERK signalling cascade was involved in integrin-mediated adhesion assembly downstream of FAK in the colon epithelial cells. Specifically, we monitored ERK activity using dual phospho-specific ERK antibodies raised against a peptide encompassing Thr183 and Tyr185 in their phosphorylated forms. Using mild fixation and biotin-streptavidin amplification in immunofluorescence procedures as described in Fincham et al. [20], we detected phospho-ERK-specific staining at newly formed cell-matrix adhesions as AA/C1/SB10 cells spread on fibronectin (Figure 4A). In particular, phospho-ERK staining with three different antisera was coincident with anti-vinculin staining at cell-matrix adhesions. Data are shown for polyclonal anti-pT183EpY185 ERK (Promega, Southampton, UK) and polyclonal anti-pT183EpY185 (NEB, Hitchin, UK; Figure 4A), and similar results were obtained with polyclonal anti-pY185 ERK (NEB, Hitchin, UK; not shown). Phospho-ERK was not present at the cell periphery in the AA/C1 adenoma cells following plating on fibronectin, and in these cells, it appeared to be nuclear (Figure 4A, bottom panels). We were also able to visualize ERK localized at cell-matrix adhesion structures following transient expression of epitope-tagged ERK in the cells (Figure 4B). There was a very high level of HA-ERK expression in the cells, much of which was in the nucleus, with some also being located at the protruding cell-matrix adhesions. Treatment with PD098059 or UO126 — two chemically distinct inhibitors of ERK kinase (MEK) [30,31], the upstream ERK-activating kinase — both blocked the targeting of phospho-ERK to the cell periphery (Figure 4C, only treatment with PD098059 shown). In repeated experiments, between 35% and 40% of the untreated cells had phospho-ERK staining at the cell periphery and this was inhibited by around 80% in the inhibitor-treated cells.
Figure 4.
Localization of activated ERK in cell-matrix adhesions. (A) Cells were fixed after 1 hour on fibronectin and co-stained with anti-phospho-ERK antibodies and an anti-vinculin antibody. Scale bars, 25 µm. (B) AA/C1/SB10 cells were transfected with HA-tagged ERK and trypsinized after 24 hours before plating on fibronectin. After 1 hour, the cells were fixed and stained with an antibody to HA. Scale bars, 25 µm. (C) AA/C1/SB10 cells treated with PD098059 were fixed after 1 hour on fibronectin and co-stained with anti-phospho-ERK antibodies and an anti-vinculin antibody. (D) AA/C1/SB10 cells were transfected with Myc-tagged FAK (Y397F) and, after 24 hours, trypsinized before plating on fibronectin. After 1 hour, the cells were fixed and stained with an antibody to phospho-ERK and the Myc epitope tag. Control cells were untransfected cells in the same culture. Scale bars, 25 µm.
Since we had also observed that expression of FAK-Y397F inhibited matrix-dependent adhesion assembly of AA/C1/SB10 cells (Figure 3B), we used the peripheral targeting of phosphorylated ERK in individual cells to determine whether there was a link between phosphorylation of FAK on tyrosine-397 and ERK activity at the cell periphery during the assembly process. We found that transient expression of FAK-Y397F also inhibited the targeting of phospho-ERK to the cell periphery (Figure 4D), indicating that FAK phosphorylation on tyrosine-397 was required for this process. In repeated experiments, between 35% and 40% of the untreated cells had phospho-ERK staining at the cell periphery and this was inhibited by around 95% in the cells expressing FAK-Y397F. However, since the transient transfection efficiency is relatively low in AA/C1/SB10 cells, we were unable to determine whether FAK-Y397F inhibited the phosphorylation and activation of ERK, or only its peripheral targeting.
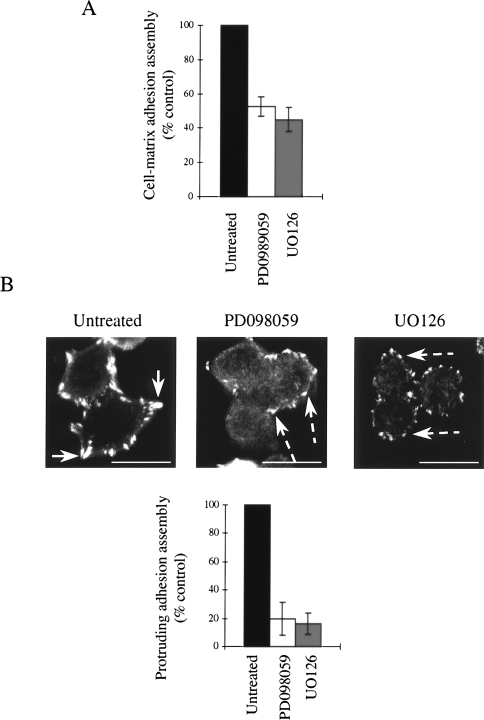
To address the role of the FAK-ERK pathway in the cell-matrix adhesion assembly process, which is altered between the adenoma and carcinoma cells, we treated the cells with PD098059 or UO126. Both inhibitors of ERK activation suppressed the assembly of cell-matrix adhesions in the AA/C1/SB10 cells (Figure 5A). Although treatment with PD098059 or UO126 clearly caused a reduction in the number of cells that formed cell-matrix adhesions (defined as cells which had spread and contained vinculin-containing structures at the cell periphery) at concentrations which inhibited its peripheral targeting, we also found that these vinculin-containing complexes were smaller and less protrusive (Figure 5B, broken arrows) than those seen in untreated cells (Figure 5B, solid arrows). Quantitation of this showed that between 80% and 85% of the inhibitor treated cells was unable to assemble protruding adhesions (Figure 5B, bottom panel). Thus, inhibition of the MEK-ERK pathway had a pronounced effect on the quality of cell-matrix adhesions in AA/C1/SB10 colon carcinoma cells. This suggests that although vinculin can still be incorporated into small adhesion structures at the cell periphery in the presence of selective inhibitors of MEK-ERK activity, these are not able to convert to protrusive adhesions found in untreated cells.
Figure 5.
ERK is required for integrin-mediated adhesion assembly. (A) Cell-matrix adhesion assembly on fibronectin in the presence of PD098059 (25 µM) or UO126 (20 µM) was measured by counting the percentage of cells with prominent adhesions in >500 adherent cells. Values are mean±SD from triplicate wells. (B) Visualization of cell-matrix adhesions in untreated and inhibitor-treated cells stained with anti-vinculin antibody. Scale bars, 25 µm. Quantitation of cells with protruding cell-matrix adhesions from >500 cells (lower panel). Values are mean±SD from triplicate wells.
Actin Cytoskeletal Rearrangements during Cell-Matrix Adhesion Assembly
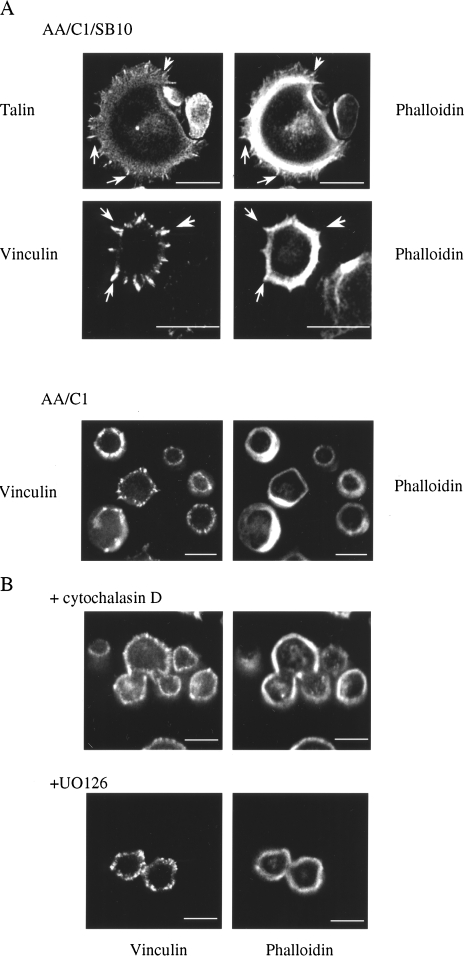
As ERK was known to influence the actin cytoskeleton via phosphorylation of MLCK [21] and as peripheral targeting of active ERK was required for the full development of protrusive cell-matrix adhesions in the colon carcinoma cells, we examined the actin cytoskeleton in the colon epithelial cells as they assembled adhesions on fibronectin. Use of labeled phalloidin revealed a tight ring of cortical actin in all cells; however, in AA/C1/SB10 cells that had prominent cell-matrix adhesion protrusions, it was possible to detect filamentous actin in microspike-like structures, which emanated from the ring of cortical actin that colocalized with the focal adhesion proteins vinculin and talin (Figure 6A, arrow heads). These actin-containing microspikes were seen in around 35% of the AA/C1/SB10 cells, but were not seen in the AA/C1 adenoma cells and vinculin-containing structures rarely protruded into the extracellular space (Figure 6A, bottom panels). Thus, the protrusive stage of assembly of cell-matrix adhesions was associated with the presence of filamentous actin spikes in the cell protrusions. In accordance with a role for the actin cytoskeleton in the protrusive stage of cell-matrix adhesion assembly, treatment with the actin filament-disrupting drug cytochalasin D prevented the assembly of actin microspikes associated with vinculin-containing protrusions at the cell periphery, but had no effect on the cortical ring of actin at the cell periphery (Figure 6B) or on the ability of the cells to attach to fibronectin (results not shown). As with the MEK inhibitors, in cytochalasin D-treated epithelial cells, the vinculin-containing complexes were confined to a ring of non-protruding structures at the cell periphery, which were reminiscent of the vinculin-containing structures in the adenoma cells. Inhibition of MEK-ERK activation by UO126 also blocked the formation of actin microspikes (Figure 6B), indicating that the activation of MEK-ERK, and ERK activity at the cell periphery, is required for the actin cytoskeletal rearrangements that accompany the final protrusive stages of the cell-matrix adhesion assembly process.
Figure 6.
Actin rearrangements during cell-matrix adhesion assembly. (A) Cells were fixed after 1 hour on fibronectin and co-stained with phalloidin-FITC and either antibodies to talin or vinculin. (B) As above, except AA/C1/SB10 cells were pre-treated with cytochalasin D (0.25 µg/ ml) or UO126 (20 µM) for 15 minutes prior to plating. Scale bars, 25 µm.
Discussion
Focal adhesions are points of cell-ECM contact, which are found at the end of stress fibers in fibroblasts. The assembly of stress fibers and focal adhesions is under the control of the small GTPase Rho, now believed to be via stimulation of myosin-based contractility that contributes to the bundling of actin filaments into stress fibers after the clustering of integrins at focal adhesions sites (reviewed in Ref. [32]). In epithelial cells, the situation is complex as there are few stress fibers, with the major actin structure being a peripheral ring of cortical actin. For this reason, the control of cell-matrix adhesion assembly in epithelial cells is not well understood. In the AA/C1/SB10 colon carcinoma cells, we found that upon initial integrin engagement, small structures, which contained vinculin, formed on the basal surface of the cell. We propose that these ring-like structures may represent early “focal contacts” that comprise complexes of focal adhesion proteins, which progress to form mature cell-matrix adhesions seen as prominent vinculin-containing protrusions at the cell periphery. In this context, we have demonstrated here for the first time a major role for ERK activation in the latter stages of this process, as known inhibitors of ERKs upstream-activating kinase MEK blocked the full maturation of small focal contacts into the prominent protrusive cell-matrix adhesions in the epithelial cells.
Phosphorylation and activation of FAK are associated with attachment of cells to matrix proteins and we have shown that phosphorylation of FAK on tyrosine-397 is also required for the assembly of cell-matrix adhesions in the colon epithelial cells (Figure 3B). This is consistent with other reports in CHO cells and fibroblasts where tyrosine-397 is important for cell spreading [5,6,28]. Furthermore, use of phospho-specific antibodies has shown that FAK phosphorylated on tyrosine-397 is present in early focal contacts, which are apparent during the initial spreading of fibroblasts on fibronectin [33]. However, fibroblasts derived from FAK-null embryos are not defective in focal adhesion assembly and indeed have an increased number of focal adhesions and impaired motility, suggesting a role for FAK in focal adhesion turnover during cell motility in these cells [9]. Furthermore, phosphorylation of FAK on tyrosine residues other than tyrosine-397 has been linked to focal adhesion disassembly [23]. Taken together, these apparently disparate observations imply that phosphorylation of FAK on tyrosine-397 is required for integrin-dependent adhesion assembly in some cells such as the AA/C1/SB10 colon carcinoma cells, in which endogenous FAK is present. Although there was detectable FAK phosphorylation in the AA/C1 cells (Figure 3A), it was not sufficient to sustain the elevated level of cell-matrix adhesion assembly seen in the carcinoma cells. It may be that FAK phosphorylation is limiting in this process, resulting in the inability of the AA/C1 cells to rapidly form adhesions on fibronectin (Figure 1B).
There is a wide body of evidence implicating FAK in the integrin-mediated activation of ERK. However, this area remains controversial, with several studies favouring a model in which integrin-mediated activation of ERK occurs independently of FAK [34,35]. In this study, we have shown that phosphorylation of FAK on tyrosine-397 is required for the peripheral targeting of activated ERK to cell-matrix adhesions, although we were unable to demonstrate whether activation of ERK was inhibited or only its peripheral targeting. However, expression of FAK-Y397F in human 293 cells does inhibit fibronectin-induced ERK activation [36], which is consistent with a model whereby phosphorylation of FAK on tyrosine-397 results in association of the non-receptor tyrosine kinase Src with FAK, which in turn leads to phosphorylation of FAK by Src on other residues including tyrosine-925. This would in turn result in binding of the adaptor protein Grb2 to FAK at tyrosine-925 and the subsequent activation of the Ras-ERK cascade. Furthermore, the interaction of FAK with the non-receptor tyrosine kinase Src, through interactions with tyrosine-397 of FAK, plays a role in cell spreading and migration [5,28] and may also play a role in the adhesion assembly process in the colon epithelial cells.
The assembly of cell-matrix adhesions requires actin cytoskeletal rearrangements and ERK may regulate adhesion assembly through its effects on the actin cytoskeleton [21,22]. In this regard, we observed in AA/C1/SB10 cells with robust cell-matrix adhesions that filamentous actin was found in microspikes present in these peripheral protrusions. Treatment with cytochalasin D prevented the latter stages of the adhesion assembly process, but did not disrupt cell attachment and the formation of the smaller peripheral adhesion complexes. Thus, the block in the cell-matrix adhesion assembly process with cytochalasin D is analogous to that seen when ERK was inhibited (Figure 5B and 6B). Taken together with the observation that inhibition of ERK activation also blocked the formation of the actin-containing microspikes (Figure 6B), this indicates that ERK-mediated actin cytoskeletal rearrangements are required for the protrusive stages of the cell-matrix adhesion assembly maturation process.
We have recently shown that activated ERK locates to newly forming adhesions in primary fibroblasts in response to integrin engagement or v-Src activation [20], consistent with earlier reports demonstrating accumulation of components of the Ras-ERK signalling pathway to integrin-coated beads [37]. In the colon epithelial cells used in this study, we also found activated ERK in cell-matrix adhesions following plating on fibronectin (Figure 4A), suggestive of a role for activated ERK at these adhesion sites. Several other reports have also provided clues to the possible function of integrin-mediated activation of ERK. In particular, MLCK has been identified as an ERK substrate [21,38], and is required for cell migration under some circumstances [21,22]. Thus, since phosphorylation of myosin light chains by MLCK is a critical step in generating contractile force, and actomyosin contractility has been implicated in the process of integrin clustering and focal adhesion assembly (reviewed in Ref. [32]), it seems likely that ERK-mediated phosphorylation and activation of MLCK may be involved in generating contractile strength that results in actin microspike formation required for the maturation of cell-matrix adhesions in AA/C1/SB10 colon carcinoma cells. In the AA/C1 adenoma cells, activated ERK was not present at the periphery in the smaller number of cells that had assembled peripheral complexes of adhesion proteins, although ERK was activated in the adenoma cells to a similar level as the carcinoma cells (results not shown). Thus, the inability of these cells to efficiently assemble cell-matrix adhesions following integrin engagement may be associated with the inability of activated ERK to localize to newly forming adhesions at the cell periphery. The mechanism of translocation of phosphorylated ERK to cell-matrix adhesions remains unclear. In this context, it is interesting to note that activated MEK has also been shown to locate at the plasma membrane under some circumstances [39], and MEKK1, an upstream regulator of c-Jun N-terminal kinase, can also localize to focal adhesions [40]. Thus, there is evidence that a number of components of the pathways leading to the activation of MAPKs can locate at the cell periphery.
The observation, that αvβ6 integrin function is required downstream of attachment to fibronectin to induce matrix-dependent adhesion assembly that is enhanced during colorectal carcinogenesis in vitro, is particularly interesting. αvβ6 has been detected in a number of tumour cell lines and also in material from tumour tissues of different origins, including colon, being absent from normal adjacent tissue [41,42]. Furthermore, in squamous cell carcinomas, β6 expression is restricted to the basal layer adjacent to stroma and is, in many cases, concentrated at the invading edge of tumour islands, suggesting a role for αvβ6 in tumour cell migration and invasion [41]. In addition, migration of keratinocytes from β6 -/- mice on fibronectin is reduced [27], indicating a role for the β6 integrin subunit in epithelial cell migration. We observed a four-fold increase in αvβ6 expression in the AA/C1/SB10 carcinoma cells compared to the adenoma cells from which they were derived. Although this represents a modest increase in cell surface integrin expression, low levels of αvβ6 have been shown previously to mediate biological effects [43]. In tumour cells, upregulation of FAK expression has also been associated with enhanced motility [44], while enhanced FAK expression is found in invasive tumours, including those of the colon [45,46]. It is interesting that FAK expression is also increased in AA/C1/SB10 carcinoma cells when compared to the non-malignant AA/C1 progenitor cells [4], indicating that a number of components associated with cell-matrix adhesion assembly, such as αvβ6 integrin and FAK, are upregulated during the acquisition of the malignant phenotype in the AA/C1/SB10 cells [2,4]. Furthermore, EGF-induced invasion of the AA/C1/SB10 carcinoma cells is associated with phosphorylation of FAK, and also the activation and localization of Src tyrosine kinase to cell-matrix adhesions [4]. In contrast, the adenoma AA/C1 adenoma cells are non-invasive, suggesting that signalling complexes at cell-matrix adhesions may regulate cell behaviour associated with increased tumorigenicity. Although we have shown an increase in αvβ6 expression associated with colon cancer progression, other integrins which bind to fibronectin such as α5β1 are known to be lost during the acquisition of a malignant phenotype. The balance between adhesiveness and the ability of cells to move, which is mediated by distinct integrin heterodimers, will therefore govern the behaviour of tumour cells.
Conclusion
We have shown that increased expression of αvβ6 integrin during the adenoma-to-carcinoma transition in an in vitro model of colon cancer progression is associated with increased integrin-mediated assembly of adhesions on fibronectin. In addition, integrin-mediated adhesion assembly requires “inside-out” signalling provided by phosphorylation of FAK tyrosine-397 and peripheral translocation of active ERK to newly assembling adhesions. Inhibition of MEK-ERK activity suppressed both the number of cells that assembled cell-matrix adhesions and the conversion of smaller, perhaps immature, vinculin-containing structures into fully developed, protrusive adhesions, most likely as a result of inhibition of actin microspike formation at the cell periphery.
Acknowledgements
We thank John Wyke and Brad Ozanne for critical reading of the manuscript.
Abbreviations
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- MAPK
mitogen-activated protein kinase
- MEK
ERK kinase
- MLCK
myosin light chain kinase
Footnotes
This work was supported by the Cancer Research Campaign (V.J.F., G.W.M., C.P., M.C.F.), the Medical Research Council (V.G.B.) and The Wellcome Trust (S.J.W.).
References
- 1.Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- 2.Williams AC, Harper SJ, Paraskeva C. Neoplastic transformation of a human colonic epithelial cell line: in vitro evidence for the adenoma to carcinoma sequence. Cancer Res. 1990;50:4724–4730. [PubMed] [Google Scholar]
- 3.Manning AM, Williams AC, Game SM, Paraskeva C. Differential sensitivity of human colonic adenoma and carcinoma cells to transforming growth factor β (TGF-β): conversion of an adenoma cell line to a tumorigenic phenotype is accompanied by a reduced response to the inhibitory effects of TGF-β. Oncogene. 1991;6:1471–1476. [PubMed] [Google Scholar]
- 4.Brunton VG, Ozanne BW, Paraskeva C, Frame MC. A role for epidermal growth factor receptor, c-Src and focal adhesion kinase in an in vitro model for the progression of colon cancer. Oncogene. 1997;14:283–293. doi: 10.1038/sj.onc.1200827. [DOI] [PubMed] [Google Scholar]
- 5.Richardson A, Malik RK, Hildebrand JD, Parsons JT. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen JD, Ruest PJ, Fry DW, Hanks SK. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 8.Fincham VJ, Wyke JA, Frame MC. v-Src-induced degradation of focal adhesion kinase during morphological transformation of chicken embryo fibroblasts. Oncogene. 1995;10:2247–2252. [PubMed] [Google Scholar]
- 9.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomyra S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan J, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 13.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 14.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 17.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 18.Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowwaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fincham VJ, James M, Frame MC, Winder SJ. Active extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAP kinase) is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen DHD, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, Weber MJ, Gonias SL. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean GW, Fincham VJ, Frame MC. v-Src induces tyrosine phosphorylation of focal adhesion kinase independently of tyrosine 397 and formation of a complex with Src. J Biol Chem. 2000;275:23333–23339. doi: 10.1074/jbc.M909322199. [DOI] [PubMed] [Google Scholar]
- 24.Meloche S, Pages G, Pouyssegur J. Functional expression and growth factor activation of an epitope-tagged p44 mitogen-activated protein kinase, p44mapk. Mol Biol Cell. 1992;3:63–71. doi: 10.1091/mbc.3.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112:947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang XZ, Wu JF, Spong S, Sheppard D. The integrin αvβ6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci. 1998;111:2189–2195. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- 28.Cary LA, Chang JF, Guan J. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 29.Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharm Rev. 1998;50:197–262. [PubMed] [Google Scholar]
- 30.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feesner WS, Dyk DEV, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 32.Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;9:86–92. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 33.Ruest PJ, Roy S, Shi E, Mernaugh RL, Hanks SK. Phospho-specific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ. 2000;11:41–48. [PubMed] [Google Scholar]
- 34.Lin HT, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, Aukhil I, Juliano RL. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways inbroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 36.Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances Ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison DL, Sanghera JS, Stewart J, Sutherland C, Walsh MP, Pelech S. Phosphorylation and activation of smooth muscle myosin light chain kinase by MAP kinase and cyclin-dependent kinase-1. Biochem Cell Biol. 1996;74:549–558. doi: 10.1139/o96-459. [DOI] [PubMed] [Google Scholar]
- 39.Kranenburg O, Verlaan I, Moolenaar WH. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J Biol Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 40.Christerson LB, Vanderbilt CA, Cobb MH. MEKK1 interacts with ∝-actinin and localizes to stress fibers and focal adhesions. Cell Motil Cytoskeleton. 1999;43:186–198. doi: 10.1002/(SICI)1097-0169(1999)43:3<186::AID-CM2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillet N, Sheppard D, Matthay MA, Albelda SM, Kramer RH, Pytela R. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 42.Jones J, Watt FM, Speight PM. Changes in the expression of αv integrins in oral squamous cell carcinomas. J Oral Pathol Med. 1997;26:63–68. doi: 10.1111/j.1600-0714.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 43.Kemperman H, Wijnands YM, Roos E. αv integrins on HT-29 colon carcinoma cells: adhesion to fibronectin is mediated solely by small amounts of αvβ6, and αvβ5 is co-distributed with actin fibers. Exp Cell Res. 1997;234:156–164. doi: 10.1006/excr.1997.3599. [DOI] [PubMed] [Google Scholar]
- 44.Akasaka T, van Leeuwen RL, Yoshinga IG, Mihm MC, Byers HR. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J Invest Dermatol. 1995;105:104–108. doi: 10.1111/1523-1747.ep12313396. [DOI] [PubMed] [Google Scholar]
- 45.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342:1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 46.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]