Abstract
Insulin treatment of fat cells results in the translocation of the insulin-responsive glucose transporter type 4, GLUT4, from intracellular compartments to the plasma membrane. However, the precise nature of these intracellular GLUT4-carrying compartments is debated. To resolve the nature of these compartments, we have performed an extensive morphological analysis of GLUT4-containing compartments, using a novel immunocytochemical technique enabling high labeling efficiency and 3-d resolution of cytoplasmic rims isolated from rat epididymal adipocytes. In basal cells, GLUT4 was localized to three morphologically distinct intracellular structures: small vesicles, tubules, and vacuoles. In response to insulin the increase of GLUT4 at the cell surface was compensated by a decrease in small vesicles, whereas the amount in tubules and vacuoles was unchanged. Under basal conditions, many small GLUT4 positive vesicles also contained IRAP (88%) and the v-SNARE, VAMP2 (57%) but not markers of sorting endosomes (EEA1), late endosomes, or lysosomes (lgp120). A largely distinct population of GLUT4 vesicles (56%) contained the cation-dependent mannose 6-phosphate receptor (CD-MPR), a marker protein that shuttles between endosomes and the trans-Golgi network (TGN). In response to insulin, GLUT4 was recruited both from VAMP2 and CD-MPR positive vesicles. However, while the concentration of GLUT4 in the remaining VAMP2-positive vesicles was unchanged, the concentration of GLUT4 in CD-MPR-positive vesicles decreased. Taken together, we provide morphological evidence indicating that, in response to insulin, GLUT4 is recruited to the plasma membrane by fusion of preexisting VAMP2-carrying vesicles as well as by sorting from the dynamic endosomal-TGN system.
INTRODUCTION
Insulin stimulates glucose transport in adipocytes and myocytes by triggering the translocation of the glucose transporter GLUT4 (Birnbaum, 1989; Charron et al., 1989; James et al., 1989) from intracellular locations to the cell surface (Cushman and Wardzala, 1980; Suzuki and Kono, 1980). Establishing the precise nature of the intracellular compartment(s) from which GLUT4 is recruited to the cell surface will provide major insight into the molecular basis for this effect. Insulin regulates both endosomal and trans-Golgi network (TGN) trafficking in a variety of cell types. For example, in adipocytes, insulin triggers translocation of transmembrane proteins other than GLUT4 to the cell surface, such as the ubiquitously expressed glucose transporter GLUT1 (Piper et al., 1991), the cation-independent mannose-6-phosphate receptor (MPR; Appell et al., 1988) and the transferrin-receptor (TfR; Tanner and Lienhard, 1987). However, several observations suggest that the effects of insulin on GLUT4 trafficking may be unique compared with its effects on endosomal and/or TGN trafficking. First, insulin effects on the endosomal/TGN system are modest compared with GLUT4 trafficking. The surface expression of molecules that are thought to transit the TGN/endosomes, such as GLUT1, MPR, and TfR is increased by only two- to threefold upon insulin stimulation, in contrast to a 10- to 40-fold increase for GLUT4. Secondly, it has been possible to dissociate the effects of insulin on endosomal/TGN trafficking from its effects on GLUT4 trafficking. For instance, phospholinase D inhibitors block insulin-stimulated adipsin secretion but have no detectable effect on insulin-stimulated GLUT4 translocation (Millar et al., 2000). Similarly, a constitutively active PKB construct stimulated GLUT4 translocation to the plasma membrane in a SNAP-23 and synaptobrevin-2/cellubrevin-dependent manner but had little effect on the distribution of either GLUT1 or the transferrin receptor (Foran et at., 1999). Third, there are biochemical (Holman et al., 1994; Livingstone et al., 1996; Martin et al., 1996; Wei et al., 1998; Bogan and Lodish, 1999; Lee et al., 1999; Millar et al., 1999; Hashiramoto and James, 2000) and morphological (Slot et al., 1991a; Slot et al., 1991b; Martin et al., 1996; Malide et al., 1997a; Malide et al., 1997b; Martin et al., 1997) experiments indicating that GLUT4 localizes to specialized compartments. Although, most studies agree on the presence of a major GLUT4 compartment distinct from endosomes and the TGN, the degree of overlap is variable dependent on the experimental method or cell type used, e.g., isolated rat adipocytes versus 3T3-L1 adipocytes (Livingstone et al., 1996; Martin et al. 1996; Malide et al., 1997a; Bogan and Lodish, 1999; Lee et al., 1999; Millar et al., 1999; Hashiramoto and James, 2000).
An insulin-responsive aminopeptidase, IRAP (Ross et al., 1996; Malide et al. 1997b; Martin et al., 1997; Ross et al., 1997; Elmendorf et al., 1999; Garza and Birnbaum, 2000), and a V-SNARE, VAMP2, are colocalized with GLUT4 in this discrete intracellular compartment. Although the trafficking of IRAP in response to insulin is very similar to that of GLUT4 (Keller et al., 1995), its function is not known. Because VAMP2 is involved in the docking and fusion of synaptic vesicles with the presynaptic plasma membrane in neurons, it has been speculated that it may play a similar role for GLUT4 vesicles in adipocytes (reviewed in Rea and James, 1997), and indeed, studies have implicated a role for VAMP2 in insulin-dependent trafficking in adipocytes (Olson et al., 1997; Martin et al., 1998).
Immunofluorescence microscopy has revealed some overlap between GLUT4 and endosomal/TGN markers as well as discrete labeling of an additional compartment (Malide et al., 1997a). Intriguingly both IRAP and VAMP2 localized to this discrete compartment as well (Martin et al., 1996; Malide et al., 1997a; Malide et al., 1997b; Martin et al., 1997). GLUT4 has also been localized by immuno-electron microscopy (IEM) in white and brown adipocytes and in cardiac and skeletal muscle (Slot et al., 1991a; Slot et al., 1991b; Smith et al., 1991; Rodnick et al., 1992; Ralston and Ploug, 1996; Slot et al., 1997; Ploug et al., 1998; Malide et al., 2000). In all cases, under basal conditions, the majority of GLUT4 resides in tubulo-vesicular elements. However, the precise morphological and biochemical nature of this mature GLUT4 compartment remains uncertain. While these studies point to the presence of a GLUT4 compartment in adipocytes that is distinct from endosomes and the TGN, the precise morphological and biochemical relationship between all of these compartments remains uncertain. For example, one possibility is that this separate GLUT4 compartment is a tubular extension or subdomain of endosomes or the TGN. This possibility is difficult to exclude using either immunofluorescence microscopy or even IEM in sectioned material. Such limitations in resolution combined with relatively poor labeling efficiencies on sections compromises quantitative assignment of GLUT4 carrying membranes. In an effort to circumvent some of these problems in the present study we have developed a novel morphological approach for studying the distribution of molecules in isolated rat adipocytes, which preserves 3-D information of GLUT4 compartments and at the same time results in efficient immuno-gold labeling. Using this approach we provide morphological evidence that GLUT4 is recruited from specialized insulin-responsive VAMP2-carrying vesicles, as well as from the endosome/TGN.
MATERIALS AND METHODS
Antibodies
Affinity-purified rabbit antibodies to the C-terminus of GLUT4 (Calderhead et al., 1990) and the cytoplasmic tail of IRAP (Keller et al., 1995) were generously provided by Dr Lienhard (Dartmouth Medical School, Hanover, NH). Affinity purified rabbit antibodies against the cytoplasmic tails of Lgp-120 and CD-MPR (Klumperman et al., 1993) were kindly provided by Dr Hopkins (University College, London, United Kingdom) and Dr Hille-Rehfeld (Georg-August-Universität, Göttingen, Germany), respectively. Affinity-purified autoimmune antibodies against EEA1 were kindly provided by Dr Toh (Monash Medical School, Melbourne, Australia) (Mu et al., 1995). Rabbit polyclonal anti-actin was from Dr Chaponnier (Geneva University, Geneva, Switzerland). The monoclonal antibodies directed against VAMP2 (Edelmann et al., 1995), vimentin (clone V9), and β-tubulin were obtained from Synaptic Systems (Göttingen, Germany), Sigma Chemical Co. (St. Louis, MO), and Dr Shermin (EMBL, Heidelberg, Germany), respectively. Rabbit anti-mouse Ig was from DAKO A/S (Glostrup, Denmark).
Preparation of Isolated Rat Adipocytes
Male Ola rats (170–200 g, SD strain, Harlan CPB, Zeist, The Netherlands) were killed by decapitation. Isolated rat adipocytes were obtained by collagenase digestion (collagenase, type I, Worthington, Lakewood, NJ) of epididymal fat pads (Weber et al., 1988) at 37°C in Krebs-Ringer-bicarbonate-HEPES buffer, pH 7.4 (130 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 4.7 mM KH2PO4, 10 mM NaHCO3, 30 mM HEPES, 200 nM adenosine) supplemented with 1% BSA (fraction V, Intergen, Purchase, NY). When indicated, isolated adipose cells were incubated in the presence of 700 nM insulin (Sigma Chemical Co., St. Louis, MO). The integrity of each adipocyte preparation was monitored by measuring [U-14]C-glucose uptake as described previously (Foley et al., 1983).
Whole Mount Preparation
To obtain grid-attached cytoplasmic rims, basal and insulin-treated rat adipocytes were rapidly cooled in 1 μl aliquots on ice-cold Petri dishes. Cold poly-L-lysine treated formvar-coated grids were placed on top of these droplets for 10 s to allow attachment of floating adipocytes. Attached cells were rapidly washed twice in ice-cold cytoskeleton-stabilizing PHEM buffer (105 mM PIPES, 20 mM HEPES, 10 mM EGTA, 1 mM MgCl2, set at pH 6.9 with KOH), and the grid, with the cells facing downwards, was placed on top of a presoaked cold nitrocellulose membrane. The grid was pressed gently against the membrane, removed, and immediately placed into cold fixative (2% glutaraldehyde in 0.1 M phosphate buffer) and incubated overnight at 4°C. Grids were then washed with PBS, and aldehydes were quenched for 10 min in PBS containing 20 mM glycine. Immunolabeling was performed in PBS containing 0.1% cold water fish gelatin (Sigma), 0.1% saponin, and 20 mM glycine (blocking buffer). Protein A coupled 5-nm, 10-nm, and 15-nm-colloidal gold particles were prepared as described (Slot and Geuze, 1985). Monoclonal antibodies were detected using rabbit anti-mouse Ig (DAKO A/S, Glostrup, Denmark) as an intermediate step. Grids were preincubated for 1 h in blocking buffer before incubation with antibodies. Grids were then washed in PBS and fixed in 1% glutaraldehyde/PBS to immobilize antibody/proteinA gold complexes and to prevent antibody cross reactivity during a second labeling step (Slot et al., 1991a). Cross reactivity was checked by omitting the primary antibodies during the second labeling. No significant labeling of the C-terminal GLUT4 antibody was found when this antibody was tested for nonspecific labeling in whole mount preparations of A431 cells (Stoorvogel et al., 1996). After labeling, grids were washed extensively in water and stained for 20 s with 1% OsO4/1.5% K4Fe(CN)6. Preparations were dehydrated in ethanol, critical point dried, coated with a carbon-layer, and examined with a JOEL JEM 1010 transmission electron microscope. For quantitative IEM, representative rims were first selected at low magnification. At high magnification the sample was then moved in a straight line from one edge of the rim to the other, allowing examination of a random part of the rim. Vesicles (between 60 and 100 nm in diameter), vacuoles (larger spherical structures between 120 and 500 nm), tubules, a nondefined pool, and the plasma membrane were defined by morphology. For counting the intracellular distribution of GLUT4, all gold particles encountered were assigned to one of the morphologically defined structures. Examination of double or triple labeling was done in the same way, but now only gold particles assigned to a specific structure were counted. Only structures with a minimal amount of gold particles in total were counted in double or triple-labeled adipocyte rims.
RESULTS
Whole Mount View of GLUT4-Positive Structures
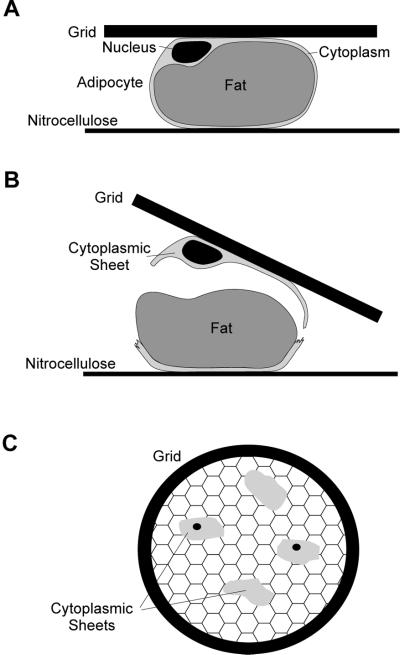
Rat adipocytes isolated from epididymal fat pads are large round cells comprised of a large central lipid droplet that is surrounded by a thin (200–500 nm thick) cytoplasmic rim, thickened only in the perinuclear area. In the present study we have taken advantage of the unusual architecture of this cell to develop a novel whole mount IEM approach to visualize and characterize intracellular GLUT4 compartments. Cytoplasmic rims were isolated by sandwiching isolated adipocytes between a poly-L-lysine-coated grid and a nitrocellulose membrane (Figure 1). Cells were then mechanically ruptured in a cytoskeleton-stabilizing buffer by pulling the grids and nitrocellulose apart. The grids to which the cytoplasmic rims were attached were immediately fixed, labeled with antibodies, stained with OsO4, and processed for IEM as described previously (Stoorvogel et al., 1996). Due to the thickness of the cytoplasmic rim, OsO4-stained samples could be visualized by transmission electron microscopy (EM) (Figure 2A). Distinct structures, such as the nucleus, large vesicles (endosomes, lysosomes, or mitochondria), tubules and small vesicles, and cytoskeletal elements could be discerned morphologically. Most of the vesicles observed had a diameter of 50–100 nm. In addition, larger spherical membranes of diameter 120–500 nm, which often contained tubular protrusions, were identified as endosomal vacuoles (see Figure 3). Since the whole mount rims are attached to the grid via their exoplasmic surface, the plasma membrane is viewed from the inside. To further characterize these structures, we labeled with antibodies specific for a variety of marker proteins followed by protein A/gold. The major cytoskeletal elements, microtubules, intermediate filaments, and actin filaments were well preserved (Figure 2, B-D). In cryosections it is difficult to distinguish between GLUT4-carrying tubules and vesicles, and these compartments therefore have collectively been referred to as tubular-vesicular elements (Slot et al., 1991a). When we labeled for GLUT4 in whole mount preparations of isolated rat adipocytes, we were able to clearly distinguish tubular and vesicular GLUT4-carrying membranes (Figure 3). Quantification revealed that the majority of GLUT4 is associated with small vesicles (Figure 3 and 5). However, we also detected significant labeling of tubules and vacuoles as well as the plasma membrane.
Figure 1.
Isolation of whole mount rims from isolated rat adipocytes. (A) Basal or insulin stimulated adipocytes were sandwiched between a poly-L-lysine-coated grid and nitrocellulose membrane. (B) Cells were mechanical disrupted by pulling the grid from the nitrocellulose membrane in a cytoskeleton stabilizing buffer in the cold and fixed immediately. (C) This resulted in patches of the thin cytoplasmic rims on the grid, which were processed further for IEM.
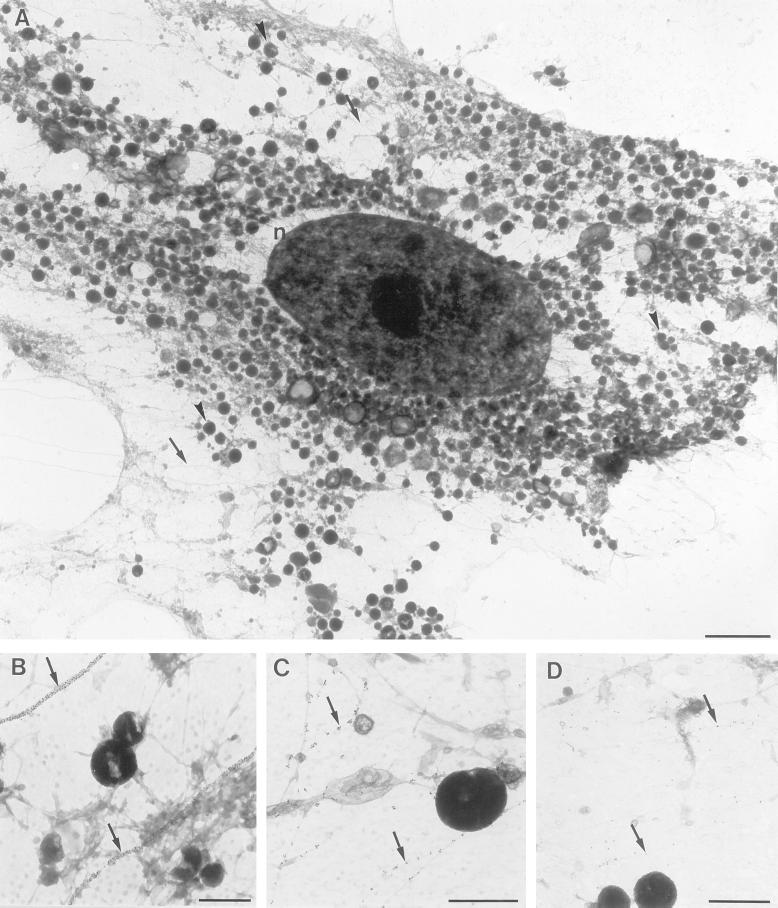
Figure 2.
A) Overview of a whole mount adipocyte rim obtained as described in Figure 1. The nucleus (n), small round structures (vacuoles or mitochondria, for example, see arrowheads), and cytoskeletal elements (for example see arrows) were readily discerned by morphology. Bar, 2 μm. Microtubules (B), intermediate filaments (C) or actin-filaments (D) were labeled with anti-β-tubulin, anti-vimentin, or anti-β-actin antibodies, respectively followed by 10 nm colloidal gold coupled to protein A. In B-D the arrows point to single or clusters of gold particles labeling the distinct cytoskeletal elements, the microtubules in (B) are completely covered by gold particles. Bars, 500 nm.
Figure 3.
GLUT4 localization in whole mount preparations of isolated adipocytes. Cells were incubated for 20 min without (A-C) or with (D, E) 700 nM insulin and processed for whole mount electron microscopy. Whole mount rims were labeled for GLUT4 (10 nm gold particles). In basal cells GLUT4 is found in vesicles (A, large arrowheads), tubules (A and B, arrows), and vacuoles (A and C, v). After stimulation with insulin (D and E), GLUT4 appears at the plasma membrane (for example, see small arrowheads). GLUT4 is also shown in vesicles (large arrowheads) and tubules (arrows). Bars, 100 nm.
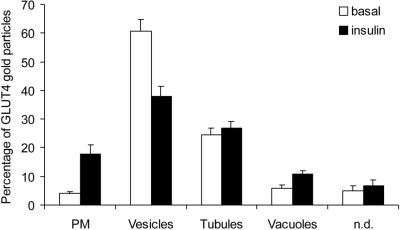
Figure 5.
Insulin recruits GLUT4 from small vesicles. Whole mount adipocyte rims were labeled for GLUT4 as shown in Figure 3. Random parts of the rims were analyzed for the distribution of GLUT4. Three different intracellular structures were defined by morphology: vesicles (between 60 and 100 nm in diameter), vacuoles (larger spherical structures), and tubules. Plasma membrane-associated GLUT4 was represented on the sheet by gold particles that were not associated with any obvious structure. Gold particles associated with structures that could not clearly be assigned to one of the other structures were counted as nondefined. For each condition, in total 1000 gold particles were counted 9 times on samples from three independent experiments (± SEM). In basal cells, GLUT4 is present in small vesicles (61%), tubules (25%), and to a minor extent, in vacuoles (6%). Insulin caused a 4.4-fold increase of GLUT4 at the plasma membrane (p < 0.001) and to a minor extent in vacuoles (1.9-fold, p < 0.01), parallel to a 37% decrease of GLUT4-label in vesicles (p < 0.001).
Characterization of Intracellular GLUT4 Compartments in Basal Rat Adipocytes
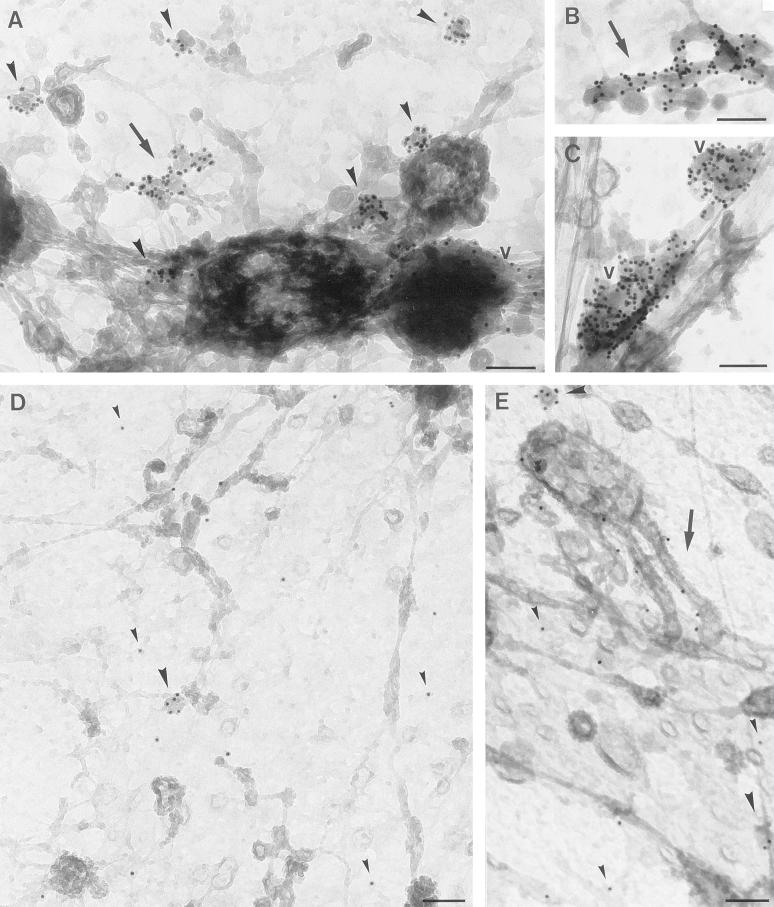
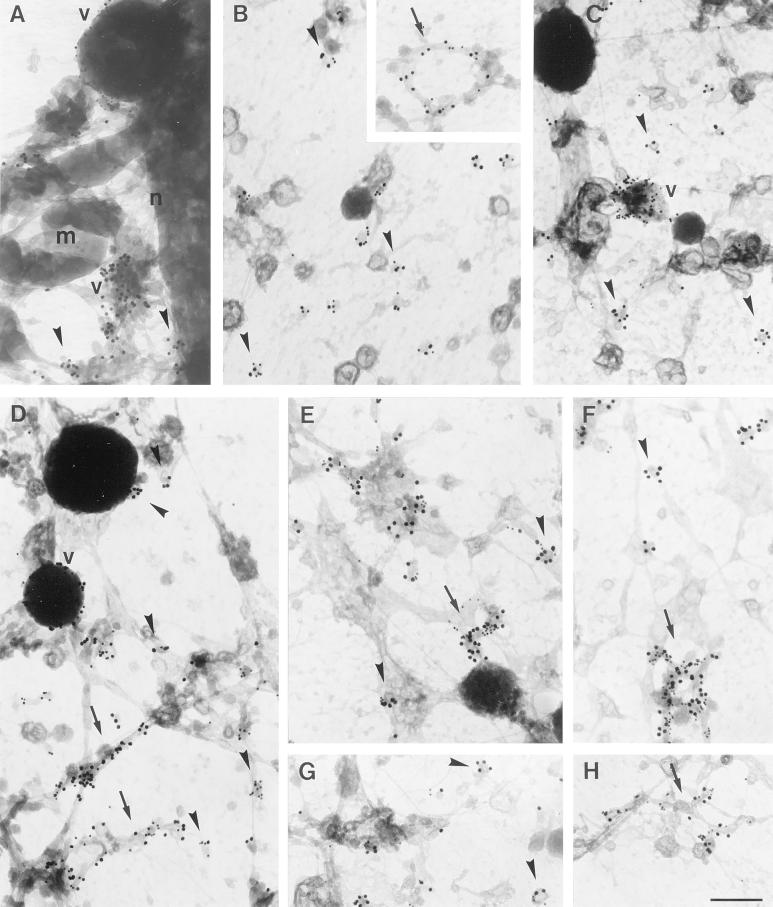
To characterize the morphologically distinct GLUT4-containing compartments further, we double-labeled for GLUT4 and for markers of the endosomal/lysosomal pathway. The high labeling efficiencies allowed us to semiquantitatively assign the distinct compartments. Very distinct labeling of lgp-120, a late endosomal/lysosomal protein, was observed in whole mount preparations. Lgp-120 localized mainly to vacuolar structures that were almost devoid of GLUT4 labeling (Figure 4A). IRAP, a molecule previously demonstrated to localize to GLUT4-carrying membranes (Keller et al., 1995; Malide et al., 1997b; Martin et al., 1997), was found on more than 85% of all three types of GLUT4 compartments (Figure 4B and Table 1). EEA1, a marker of sorting endosomes, was present mainly on distinct vacuoles, as well as on tubular structures with very little labeling of small vesicles (Figure 4C and Table 1), consistent with the localization of EEA1 in other cells (Mu et al., 1995). The majority of GLUT4-containing vacuoles were positive for EEA1, identifying them as early sorting endosomes. However, the majority of GLUT4 was localized to tubules and small vesicles that largely lacked EEA1 (Figure 4C and Table 1). These data indicate that in adipocytes GLUT4 is predominantly targeted to organelles that are distinct from either early sorting endosomes or late endosomes/lysosomes.
Figure 4.
Characterization of intracellular GLUT4 compartments in rat adipocytes. Whole mount preparations of basal (A-F) or insulin stimulated (G, H) adipocytes were obtained as in Figure 2 and double labeled for lgp-120 (A), IRAP (B), EEA1 (C), CD-MPR (D), or VAMP2 (E-H) (10-nm gold particles) and GLUT4 (15-nm gold particles). No significant overlap was observed between lgp-120 and GLUT4 on vacuoles (A). IRAP was highly colocalized with GLUT4 in both vesicles (B) and tubules (B, inset). EEA1 was found on vacuoles, but not on small GLUT4 positive vesicles (C). CD-MPR colocalized with GLUT4 in small vesicles, vacuoles, and tubules, however some vesicles in basal cells did not contain CD-MPR (D). VAMP2 was found on intracellular compartments containing GLUT4, whereas GLUT4 was additionally found in vesicles free of VAMP2 (E, F). After stimulation with insulin both VAMP2-positive and VAMP2-negative GLUT4-vesicles were still observed (G, H). Arrowheads indicate vesicles; v, vacuoles; arrows, tubules; m, mitochondria; n, nucleus. Bar, 200 nm.
Table 1.
Characterization of morphologically distinct GLUT4 compartments
| Marker | Value | Compartment
|
||
|---|---|---|---|---|
| Vesicles | Tubules | Vacuoles | ||
| IRAP | % GLUT4 compartments positive for IRAP | 87.5 | 86 | 97.5 |
| Ratio IRAP/GLUT4 | 0.84 | 0.53 | 0.79 | |
| EEA1 | % GLUT4 compartments positive for EEA1 | 9.7 | 28.3 | 75.6 |
| Ratio EEA1/GLUT4 | 0.044 | 0.11 | 1.02 | |
| MPR46 | % GLUT4 compartments positive for MPR46 | 56 | 75.9 | 95 |
| Ratio MPR46/GLUT4 | 0.41 | 0.5 | 0.56 | |
| VAMP-2 | % GLUT4 compartments positive for VAMP-2 | 56.5 | 94.4 | 91.5 |
| Ratio VAMP-2/GLUT4 | 0.38 | 0.83 | 1.33 | |
To analyze colocalization of GLUT4 with the indicated proteins, whole mount adipocyte rims were immuno-double-labeled, as described in Figure 4. Three different intracellular structures were defined by morphology: vesicles (between 60 and 100 nm in diameter), vacuoles (larger spherical structures), and tubules. For all randomly encountered structures gold particles corresponding to GLUT4 labeling and the indicated protein were counted. Only structures labeled for GLUT4 and counting at least two gold particles in total were considered. Compartments containing one or more gold particles for the indicated protein were considered positive for this marker (expressed as percentage of total GLUT4-positive compartments within this category). For the determination of the ratio, the sum of all gold particles counted for the indicated protein was divided by the sum of all gold particles for GLUT4 counted within the same category. For example, 9.7% of all GLUT4 positive vesicles also contained one or more gold particles for EEA1. The ratio for EEA1 to GLUT4 gold particles in these vesicles was as low as 0.044.
CD-MPR shuttles between the TGN and endosomes to transport newly synthesized lysosomal hydrolases from the biosynthetic to the endocytic tract and can thus be considered as a marker of the TGN, early endosomes, and transport vesicles that shuttle between these compartments (Klumperman et al., 1993). We observed significant overlap between GLUT4 and CD-MPR in tubules and vacuoles as well as in small cytoplasmic vesicles. Quantification of this overlap revealed that 56% of the GLUT4-vesicles also contained CD-MPR (Figure 4D and Table 1). Thus, in basal cells, a proportion of GLUT4 seems to traffic together with the CD-MPR from the TGN to endosomes, and/or vice versa, while a considerable amount is segregated from this pathway. VAMP2, a V-SNARE implicated in GLUT4 trafficking also exhibited considerable overlap with GLUT4 in vacuoles, tubules, and vesicles. Of the GLUT4-positive vesicles, 56% were also positive for VAMP2 (Figure 4E and F, and Table 1). Quantitatively, these data suggest there must be some overlap between VAMP2 and CD-MPR. However, in triple-labeling experiments, only 19% of GLUT4-positive vesicles were labeled for both VAMP2 and CD-MPR, suggesting largely distinct origins. Importantly, in these triple-labeling experiments only 21% of the GLUT4 vesicles were not labeled with the VAMP2 or CD-MPR antibodies, indicating that the majority of intracellular GLUT4 vesicles can be accounted for using these markers.
Insulin Recruits GLUT4 Mainly from Small Vesicles
To localize the insulin-responsive GLUT4 pool, we next compared the subcellular distribution of GLUT4 in whole mount rims from basal and insulin treated cells. Using the same antibody to label cryo-sections of isolated rat adipocytes, we have previously observed a redistribution of GLUT4 from intracellular membranes to the plasma membrane without loss of total label, indicating that the results were not affected by epitope masking (Malide et al., 2000). After insulin treatment of the cells GLUT4 associated with small vesicles, membrane tubules, vacuoles, plasma membrane, and a nondefined pool (Figure 5). Plasma membrane-associated GLUT4 was defined as label on the membrane sheet that was not associated with any obvious structure. Stereo images confirmed that this label localized just above the formvar film (not shown). We also observed significant labeling of this structure using antibodies specific for syntaxin 4 and caveolin1, two plasma membrane proteins (our unpublished results). The nondefined pool included distinct structures, which could not be clearly assigned to one of the other pools described above. Under basal conditions, 61% of the total GLUT4 labeling was associated with small vesicles, 25% with tubules, 6% with vacuoles, and 4% with the plasma membrane (Figure 5). Upon insulin treatment, GLUT4 label at the plasma membrane increased to 18%. This 4.4-fold increase of GLUT4 at the plasma membrane is probably an underestimation in view of the likelihood of background labeling of this structure in basal cells. Unfortunately, it is not possible to accurately measure background labeling of the plasma membrane using this whole mount technique.
Upon insulin-treatment we observed a slight, but significant increase of GLUT4 on early endosomal vacuoles, consistent with previous studies in brown adipose tissue (Slot et al., 1991). The total amount of GLUT4 associated with tubules and the nondefined pool was not significantly different between basal and insulin treated cells. In contrast, GLUT4 label on small vesicles decreased by 37% in response to insulin. These data pinpoint the small vesicle pool as the insulin-responsive compartment in rat adipocytes.
Insulin-triggered Release of a Subpopulation of Small GLUT4-Carrying Vesicles
Although the small vesicles were identified as the major intracellular insulin-responsive GLUT4 pool, they were heterogeneous with respect to VAMP2 and CD-MPR content (see above). To investigate whether only a specific subpopulation of these vesicles was recruited after 20 min stimulation with insulin, we double-labeled whole mount rims using antibodies specific for VAMP2 and GLUT4 (see for example, Figure 4, E-H) or for CD-MPR and GLUT4 (see for example Figure 4D). The gold particles representing these markers were counted for individual vesicles, and only vesicles that contained ≥ 3 gold particles for the combined markers were considered in a quantitative analysis.
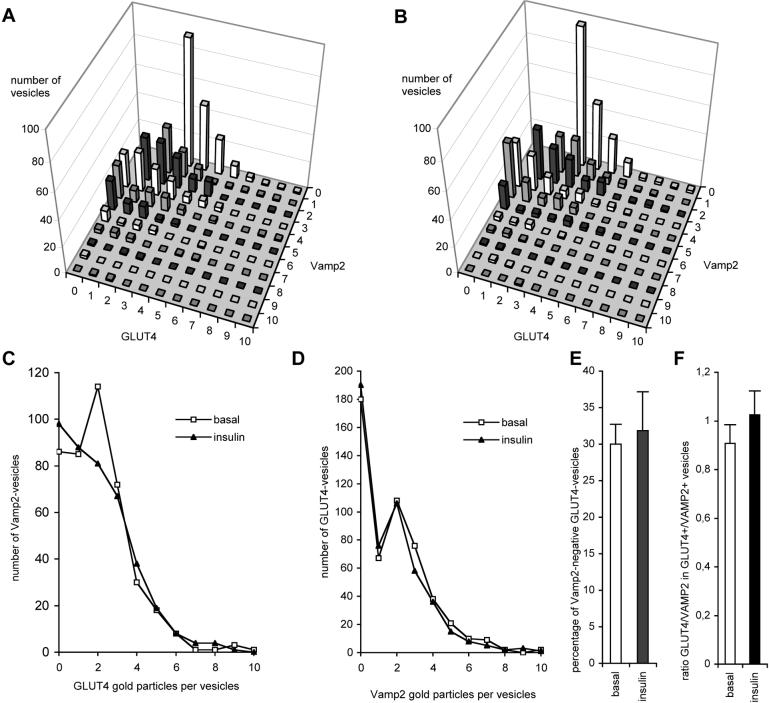
First, we investigated if the insulin-responsive GLUT4 vesicles were enriched in VAMP2 (Figure 6). Based on the distribution of these two markers we were able to dissect two separate populations of GLUT4 vesicles (Figure 6D). In basal cells, 70% of vesicle-linked GLUT4 associated with VAMP2, while the remaining GLUT4 was found in VAMP2-negative vesicles. In response to insulin, the relative amount of GLUT4 associated with small vesicles was decreased by 37% (Figure 5). If GLUT4 were selectively recruited from either VAMP2-positive or VAMP2-negative vesicles, one would expect to observe an increase in the relative amount of GLUT4 on VAMP2-negative or VAMP2-positive vesicles respectively. However, we did not observe a significant change in the amount of GLUT4 associated with either vesicle population. Because there is a net decrease in total GLUT4 labeling of small vesicles following insulin treatment (Figure 5), these data suggest that both the VAMP2-positive and VAMP2-negative GLUT4 vesicle populations contribute equally to the insulin effect (Figure 6, A-D). However, quantitatively the vast majority of GLUT4 (70%) was derived from the VAMP2-positive pool (Figure 6E). We observed significant heterogeneity in the number of gold particles per vesicle for both VAMP2 and GLUT4 (Figure 6, A, C and D). However, as indicated in Figure 6C, we did not observe a selective effect of insulin on any particular subpopulation of VAMP2-carrying GLUT4, indicating that all of the vesicles within this population are equally insulin responsive. Also, the ratio of total GLUT4 to total VAMP2 within this pool remained constant in response to insulin (Figure 6F). These data argue against sorting of GLUT4 from VAMP2 en route to the cell surface, and instead are consistent with a model in which these vesicles fuse directly with the plasma membrane in response to insulin.
Figure 6.
Role of VAMP2 in GLUT4 translocation. Vesicles from adipocyte rims double labeled for VAMP2 and GLUT4 as shown in Figure 4, E-H were used for quantitative analysis. Counting was done in a random manner, similar to Figure 5, but now only vesicles containing ≥ 3 gold particles in total were considered. For 100 individual vesicles the number of gold particles representing VAMP2 and GLUT4 were counted (For each condition 6 × 100 vesicles were counted from two independent experiments). The distributions of all 600 counted vesicles in basal (A) or insulin treated (B) rat adipocytes are shown. Each bar represents the number of vesicles having a certain labeling characteristic. For example, the highest bar in (A) stands for 91 vesicles, each labeled with three gold particles for GLUT4 and no gold particles for VAMP2. No differences in the overall distribution of vesicles in basal (A) and insulin-stimulated (B) adipocytes were observed. Upon insulin stimulation, neither the average number of GLUT4 gold particles in VAMP2-positive vesicles (C) nor the average number of VAMP2 in GLUT4 vesicles (D) was changed. Clearly two distinct peaks representing VAMP2-negative and VAMP2-positive GLUT4-vesicles can be discerned (D). Insulin did not significantly change the percentage of VAMP2-negative GLUT4-vesicles (E). In contrast to Table 1, these quantifications include GLUT4-negative vesicles, explaining the relative lower percentage of VAMP2-negative vesicles in basal cells. The ratio of GLUT4-gold particles over VAMP2-gold particles in GLUT4-positive/VAMP2-positive vesicles remained unchanged after insulin treatment (F).
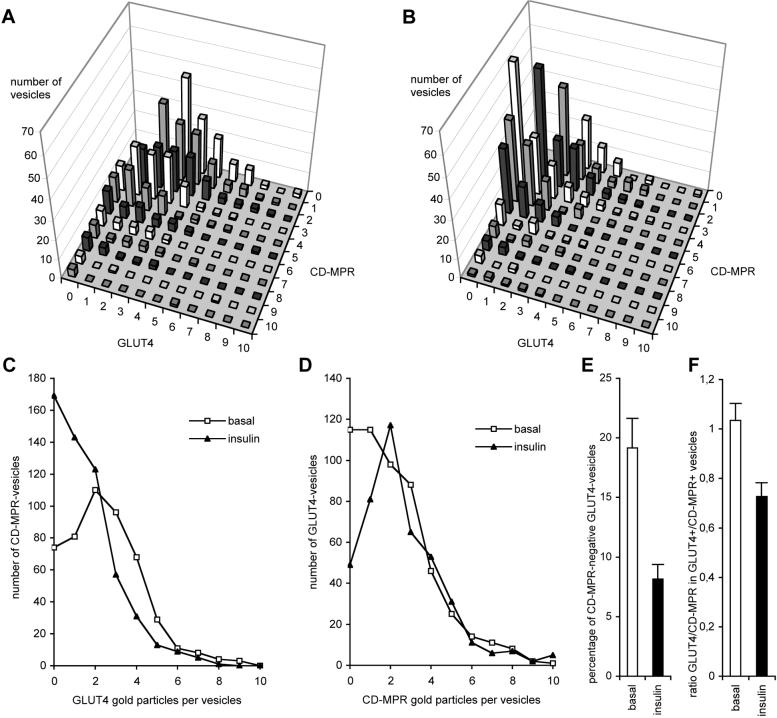
To determine the relationship of the insulin-responsive vesicles to the endosomal/TGN system, we performed double-labeling experiments with antibodies specific for GLUT4 and the CD-MPR. In basal cells, GLUT4 was present in CD-MPR-negative and CD-MPR-positive vesicles (Figure 7,A and D). In contrast to our observations for VAMP2 (Figure 6), we observed a relative decrease of GLUT4-positive/CD-MPR-negative vesicles in response to insulin (Figure 7E). Thus, consistent with a selective recruitment of GLUT4 in response to insulin, we observed an increase in the total number of GLUT4-negative/CD-MPR-positive vesicles. Despite this selective effect, and again in contrast to that observed for VAMP2, in response to insulin we observed a 30% decrease in the GLUT4 concentration within in the CD-MPR-positive population (Figure 7F). A decrease of GLUT4 in CD-MPR-positive vesicles is also evident in Figure 7E and is indicative for active sorting of GLUT4 from the dynamic TGN/endosome system. Collectively, these data show that insulin primarily recruits GLUT4 from a population of GLUT4-vesicles that contain VAMP2, but that some GLUT4 is also recruited from the CD-MPR pathway.
Figure 7.
Insulin recruits GLUT4 mainly from a CD-MPR-negative GLUT4-vesicle pool, but also from CD-MPR-positive vesicles. Preparation, as shown in Figure 4D, was quantified similar to that shown in Figure 6. Compared with basal adipocytes (A) and after insulin stimulation (B), a clear change in the distribution of vesicles is visible. The average number of GLUT4 gold particles in CD-MPR-positive vesicles decreased upon insulin stimulation (C), and GLUT4 disappeared from the CD-MPR-negative vesicle pool (D). The number of vesicles containing only GLUT4 and no CD-MPR decreased upon insulin treatment (E, p < 0.005). Insulin caused a decrease of the ratio of GLUT4-gold particles over CD-MPR-gold particles in GLUT4-positive/CD-MPR-positive vesicles (F, p < 0.01).
DISCUSSION
Earlier studies, in which immuno-electron microscopy was performed on cryosections, demonstrated that in basal muscle or fat cells the majority of GLUT4 is localized to intracellular vesicular-tubular membranes (Slot et al., 1991a). However, due to the lack of 3-d information, it was not possible to quantitatively distinguish vesicles from tubules. In addition, the relatively low labeling efficiency, which is characteristic of this technique, has limited a precise characterization of the intracellular GLUT4 compartments. In the present study, we have developed a novel morphological technique to overcome these problems. This technique takes advantage of the unusual architecture of the adipocytes. These cells are comprised of a thin cytoplasmic rim surrounding a large lipid droplet. By sandwiching the cells between an EM grid and a nitrocellulose membrane we were able to segregate the cytoplasmic rims from the central lipid droplets. Because the rims are only ∼500 nm thick, it has been possible to obtain a 3-d image of all the organelles found in the cytoplasm without the need for making sections. Experiments in which such preparations were judged by morphological criteria and organelles identified by immuno-labeling of specific markers revealed that all major organelles were present in these preparations including nuclei, ER, Golgi, intermediate compartment, TGN, early and late endosomes, plasma membrane, and mitochondria (our unpublished results). In addition, all major cytoskeletal elements remained intact. This procedure has been optimized by morphological criteria for retention of organelles, including small vesicles. With the current whole mount procedure we find 86% of all GLUT4 in basal cells associated with small vesicles (61%) or tubules (25%), consistent with another study in which we determined the distribution of GLUT4 by immunocytochemistry on cryosections (Malide et al., 2000). On cryosections, 85% of GLUT4 associated with either small vesicles or tubules. This indicates that it is unlikely that we selectively lost GLUT4-carrying membranes with our current new procedure. A significant advance achieved with this technique is the improvement in labeling efficiency. We routinely observe 5–10 gold particles per vesicle using our GLUT4 antibody. A disadvantage of this technique is the difficulty in correcting for background labeling associated with the PM. Despite this, we did observe GLUT4 movement to the membrane in response to insulin. However, numerous groups have quantified the effect of insulin on cell surface levels of GLUT4, and so this was not the objective of this endeavor.
The major observations made in this study are as follows. First, we present morphological evidence that the majority of GLUT4 in rat adipocytes is targeted to a small vesicular compartment distinct from early and late endosomes and TGN. This observation is consistent with several previous studies using both biochemical and morphological techniques (Livingstone et al., 1996; Malide et al., 1997a; Martin et al. 1996; Hashiramoto and James, 2000). Second, in response to insulin we have observed a decrease in the number of GLUT4 vesicles, suggesting that insulin stimulates exocytosis of at least a subpopulation of these vesicles. Third, small GLUT4 vesicles also contain IRAP and VAMP2, consistent with these molecules being closely associated with GLUT4 either as cargo or in regulating its trafficking. The concentration of GLUT4 in VAMP2-positive vesicles did not change upon insulin treatment, indicating that GLUT4 was not recruited from VAMP2-positive compartments by a sorting process but rather by fusion of preformed vesicles, possibly directly with the plasma membrane. Fourth, by comparing the distribution of GLUT4 with CD-MPR, we observed a population of vesicles that contained both proteins and a separate population that contained GLUT4 only. That these vesicles represent distinct functional pools is supported by the observation that insulin had a much more potent effect on the GLUT4-positive/CD-MPR-negative population. Finally, we show that insulin also decreased the GLUT4 concentration in the CD-MPR-positive vesicle population, indicating insulin-induced sorting of GLUT4 from the dynamic CD-MPR pathway either to the plasma membrane or by generating insulin responsive VAMP2-positive vesicles.
Observations similar to those described here have been made using a completely different approach in 3T3-L1 adipocytes (Martin et al., 2000). In this study vesicles were isolated from adipocytes and labeled on an EM grid using a whole mount approach. Here it was reported that insulin caused a decrease in the total number of GLUT4 positive vesicles, rather than a decrease in GLUT4 per vesicle, and that this population of insulin- responsive vesicles did not contain CD-MPR. Collectively, these studies suggest a model in which GLUT4 is targeted from either endosomes or the TGN into a population of small cytoplasmic vesicles that are also enriched in VAMP2 and IRAP. Insulin may stimulate direct fusion of these vesicles with the plasma membrane. It should be noted, however, that we cannot exclude the possibility that these vesicles fuse with another organelle, such as endosomes or the TGN, as an intermediate step for GLUT4 en route to the cell surface. Our morphological study is consistent with recent biochemical studies that also suggest two distinct intracellular sources of insulin responsive GLUT4 (Foran et al., 1999; Lee et al., 1999; Millar et al., 1999; Hashiramoto and James, 2000). First, it has been demonstrated that protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving VAMP2 (Foran et al., 1999). Second, using iodixanol density gradient sedimentation, it has been possible to isolate a GLUT4 compartment that is distinct from both endosomes and TGN and that is highly insulin responsive (Hashiramoto and James, 2000). Third, a peptide encompassing the cytosolic tail of the v-SNARE cellubrevin inhibited GTPγS-stimulated GLUT4 translocation by ∼ 40% but had no effect on the insulin response. Conversely, a fusion protein encompassing the cytosolic tail of VAMP2 had no significant effect on GTPγS-stimulated GLUT4 translocation but inhibited the insulin response by ∼ 40% (Millar et al., 1999). Possibly, recruitment of GLUT4 from the CD-MPR pathway relies on cellubrevin.
A major focus should be to establish the molecular features of GLUT4 that allow it to be incorporated selectively into the insulin-responsive vesicles, compared with proteins that exclusively traffic the constitutive recycling pathways such as CD-MPR and the transferrin receptor.
ACKNOWLEDGMENTS
We are grateful to Dr.G. Posthuma for his help with the isolation of epididymal fat pads. G.R. especially thanks Dr.D. Malide and Dr.S.W. Cushman for teaching isolation of rat adipocytes and [U-14C]-glucose uptake experiments. R. Scriwanek is thanked for excellent photographic assistance. We thank Dr.Lienhard, Dr.Hopkins, Dr.Hille-Rehfeld, Dr.Shermin, Dr.Toh, and Dr.Chaponnier for the provided antibodies. G.R. was supported by a fellowship (no. ERBCHBGCT940692) from the Commission of the European Communities (Human Capital and Mobility), and D.E.J. is a research fellow of the National Health and Medical Research Council of Australia.
REFERENCES
- Appell KC, Simpson IA, Cushman SW. Characterization of the stimulatory action of insulin on insulin-like growth factor II binding to rat adipose cells. Differences in the mechanism of insulin action on insulin-like growth factor II receptors and glucose transporters. J Biol Chem. 1988;263:10824–10829. [PubMed] [Google Scholar]
- Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Bogan JS, Lodish HL. Two compartments for insulin-stimulated exocytosis in 3T3–L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderhead DM, Kitagawa K, Tanner LI, Holman GD, Lienhard GE. Insulin regulation of the two glucose transporters in 3T3–L1 adipocytes. J Biol Chem. 1990;265:13801–13808. [PubMed] [Google Scholar]
- Charron MJ, Brosius III FC, Alper SL, Lodish HF. A glucose transporter protein expressed predominantly in insulin-responsive tissues. Proc Natl Acad Sci USA. 1989;86:2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell: apparent translocation of intracellular transport system to the cell surface. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- Edelmann L, Hanson PJ, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf JS, Boeglin DJ, Pessin JE. Temporal separation of insulin-stimulated GLUT4/IRAP vesicle plasma membrane docking and fusion in 3T3L1 adipocytes. J Biol Chem. 1999;274:37357–37361. doi: 10.1074/jbc.274.52.37357. [DOI] [PubMed] [Google Scholar]
- Foley JE, Kashiwagi A, Verso ME, Reaven G, Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. J Clin Invest. 1983;72:1901–1909. doi: 10.1172/JCI111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran PGP, Fletcher LM, Oatey PB, Mohammed N, Dolly JO, Tavare JM. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3–L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J Biol Chem. 1999;274:28087–28095. doi: 10.1074/jbc.274.40.28087. [DOI] [PubMed] [Google Scholar]
- Garza LA, Birnbaum MJ. Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J Biol Chem. 2000;275:2560–2567. doi: 10.1074/jbc.275.4.2560. [DOI] [PubMed] [Google Scholar]
- Hashiramoto M, James DE. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3–L1 adipocytes. Mol Cell Biol. 2000;20:416–427. doi: 10.1128/mcb.20.1.416-427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman GD, Lo Leggio L, Cushman SW. Insulin-stimulated GLUT4 glucose transporter recycling. J Biol Chem. 1994;269:17516–17524. [PubMed] [Google Scholar]
- James DE, Strube M, Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Hille A, Veenendaal T, Oorschot V, Stoorvogel W, von Figura K, Geuze HJ. Differences in the endosomal distribution of the two mannose 6-phosphate receptors. J Cell Biol. 1993;121:997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard G. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J Biol Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- Lee W, Ryu J, Souto RP, Pilch PF, Jung CY. Separation and partial characterization of three distinct intracellular GLUT4 compartments in rat adipocytes. Subcellular fractionation without homogenization. J Biol Chem. 1999;274:37755–37762. doi: 10.1074/jbc.274.53.37755. [DOI] [PubMed] [Google Scholar]
- Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3–L1 adipocytes. Biochem J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malide D, Dwyer NK, Blanchette-Mackie EJ, Cushman SW. Immunocytochemical evidence that GLUT4 resides in a specialized translocation post-endosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin. J Histochem Cytochem. 1997a;45:1083–1096. doi: 10.1177/002215549704500806. [DOI] [PubMed] [Google Scholar]
- Malide D, St-Denis JF, Keller SR, Cushman SW. Vp165 and GLUT4 share similar vesicle pools along their trafficking pathway in rat adipose cells. FEBS Lett. 1997b;409:461–468. doi: 10.1016/s0014-5793(97)00563-2. [DOI] [PubMed] [Google Scholar]
- Malide, D., Ramm, G., Cushman, S.W., and Slot, J.W. (2000). Immuno-EM evidence that GLUT4 translocation explains the stimulation of glucose transport in isolated rat white adipose cells. J. Cell Sci. (in press). [DOI] [PubMed]
- Martin L, Shewan A, Millar CA, Gould GW, James DE. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3–L1 adipocytes. J Biol Chem. 1998;273:1444–1452. doi: 10.1074/jbc.273.3.1444. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Rice JE, Gould GW, Keller SR, Slot JW, James DE. The glucose transporter GLUT4 and the aminopeptidase vp165 colocalize in tubulo-vesicular elements in adipocytes and cardiomyocytes. J Cell Sci. 1997;110:2281–2291. doi: 10.1242/jcs.110.18.2281. [DOI] [PubMed] [Google Scholar]
- Martin, S., Meerloo, T., Millar, C.A., Lyttle, C.T., Marsh, B.J., Gould, G.W., and James, D.E. (2000). Effects of insulin on intracellular GLUT4 vesicles in adipocytes: Evidence for a secretory mode of regulation. J. Cell Sci., in press. [DOI] [PubMed]
- Millar CA, Shewan A, Hickson GR, James DE, Gould GW. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar CA, Meerloo T, Martin S, Hickson GRX, Shimwell NJ, Wakelam MJO, James DE, Gould GW. Adipsin and the glucose transporter GLUT4 traffic to the cell surface via independent pathways in adipocytes. Traffic. 2000;1:141–151. doi: 10.1034/j.1600-0854.2000.010206.x. [DOI] [PubMed] [Google Scholar]
- Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EPC, Toh BH. EEA1, an early endosome-associated protein. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Hess LJ, James DE. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991;260:C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston E, Ploug T. GLUT4 in cultured skeletal myocytes is segregated from the transferrin receptor and stored in vesicles associated with the TGN. J Cell Sci. 1996;109:2967–2978. doi: 10.1242/jcs.109.13.2967. [DOI] [PubMed] [Google Scholar]
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Slot JW, Studelska DR, Hanpeter DE, Robinson LJ, Geuze HJ, James DE. Immunocytochemical and biochemical studies of GLUT4 in rat skeletal muscle. J Biol Chem. 1992;267:6278–6285. [PubMed] [Google Scholar]
- Ross SA, Scott HM, Morris NJ, Leung WY, Mao F, Lienhard GE, Keller SR. Characterization of the insulin-regulated membrane aminopeptidase in 3T3–L1 adipocytes. J Biol Chem. 1996;271:3328–3332. doi: 10.1074/jbc.271.6.3328. [DOI] [PubMed] [Google Scholar]
- Ross SA, Herbst JJ, Keller SR, Lienhard GE. Trafficking kinetics of the insulin-regulated membrane aminopeptidase in 3T3–L1 adipocytes. Biochem Biophys Res Commun. 1997;239:247–251. doi: 10.1006/bbrc.1997.7459. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991a;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A. 1991b;88:7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Garruti G, Martin S, Oorschot V, Posthuma G, Kraegen EW, Laybutt R, Thibault G, James DE. Glucose transporter (GLUT-4) is targeted to secretory granules in rat atrial cardiomyocytes. J Cell Biol. 1997;137:1243–1254. doi: 10.1083/jcb.137.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Charron MJ, Shah N, Lodish HF, Jarett L. IEM demonstration of insulin-stimulated translocation of glucose transporters to the plasma membrane of isolated rat adipocytes and masking of the carboxyl-terminal epitope of intracellular GLUT4. Proc Natl Acad Sci USA. 1991;88:6893–6897. doi: 10.1073/pnas.88.15.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner LI, Lienhard GE. Insulin elicits a redistribution of transferrin receptor in 3T3–L1 adipocytes through an increase in the rate constant for receptor externalization. J Biol Chem. 1987;262:8975–8980. [PubMed] [Google Scholar]
- Weber, T.M., Joost, H.G., Simpson, I.A., and Cushman, S.W. (1988). Methods for assessment of glucose transport activity and the number of glucose transporters in isolated rat adipose cells and membrane fractions. In: The insulin receptor (ed. C.R. Kahn and L.C. Harrison) part B, pp. 171–187. Alan R Liss Inc, New York.
- Wei ML, Bonzelius F, Scully RM, Kelly RB, Herman GA. GLUT4 and transferrin receptor are differentially sorted along the endocytic pathway in CHO cells. J Cell Biol. 1998;140:565–575. doi: 10.1083/jcb.140.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]