Abstract
Suc, an end product of photosynthesis, is metabolized by Suc synthase in sink organs as an initial step in the biosynthesis of storage products. Suc synthase activity is known to be regulated by reversible phosphorylation, but the details of this process are unclear at present. Rice SPK, a calcium-dependent protein kinase, is expressed uniquely in the endosperm of immature seed, and its involvement in the biosynthetic pathways of storage products was suggested. Antisense SPK transformants lacked the ability to accumulate storage products such as starch, but produced watery seed with a large amount of Suc instead, as the result of an inhibition of Suc degradation. Analysis of in vitro phosphorylation indicated that SPK phosphorylated specifically a Ser residue in Suc synthase that has been shown to be important for its activity in the degradation of Suc. This finding suggests that SPK is involved in the activation of Suc synthase. It appears that SPK is a Suc synthase kinase that may be important for supplying substrates for the biosynthesis of storage products.
INTRODUCTION
Calcium-dependent protein kinases (CDPKs), which are protein kinases with a calmodulin-like domain, constitute a family of predominantly calcium-dependent Ser/Thr protein kinases and are widespread in the plant kingdom. CDPK is regulated by binding Ca2+ because of the peculiar structure of CDPK; this protein contains a protein kinase domain followed by a unique calmodulin-like domain with four calcium binding EF-hands (Harper et al., 1991; Harmon et al., 2000). There has been no report on CDPKs in yeast, nematode, or animal genomes during the course of the various genome-sequencing projects, suggesting that CDPKs might be absent in animals except for some protozoans (Sanders et al., 1999). Because phylogenetic analysis has shown that there is a tight relationship between CDPKs and the calmodulin-dependent protein kinases (Harmon et al., 2000), it is assumed that CDPKs are analogs of the calmodulin-dependent protein kinases in animals.
Some of the CDPKs have been reported to be regulated by myristoylation, association with lipids, phosphorylation, or connection with 14-3-3 proteins (Camoni et al., 1998; Ellard-Ivey et al., 1999; Farmer and Choi, 1999). The functions of individual CDPKs are proposed to differ depending on their locations and biological properties, and CDPKs have been implicated in biological controls, such as the regulation of water and ion channels (Harmon et al., 2000). However, the physiological roles of CDPKs remain to be elucidated, because little information has been provided by mutagenesis studies and protein–protein interaction analyses (Frattini et al., 1999; Sanders et al., 1999).
In the course of our studies on storage starch biosynthesis in developing rice seed, we found a novel CDPK gene, Spk, the expression of which peaked during the middle stage of seed development (Kawasaki et al., 1993). The pattern of expression of Spk is very similar to that of enzymes involved in storage starch biosynthesis, suggesting that SPK may be involved in the regulation of starch biosynthesis. Starch is the major storage substance in rice seed and is produced from Suc by a series of enzyme activities. On the pathway of starch biosynthesis, some of the enzyme activities have been reported to be regulated by phosphorylation/dephosphorylation (Huber et al., 1996), suggesting that some protein kinases may function as regulatory systems in this pathway. In this article, we report a target protein of SPK and discuss its function.
RESULTS
Spk Is Expressed Specifically in Endosperm of Immature Seed
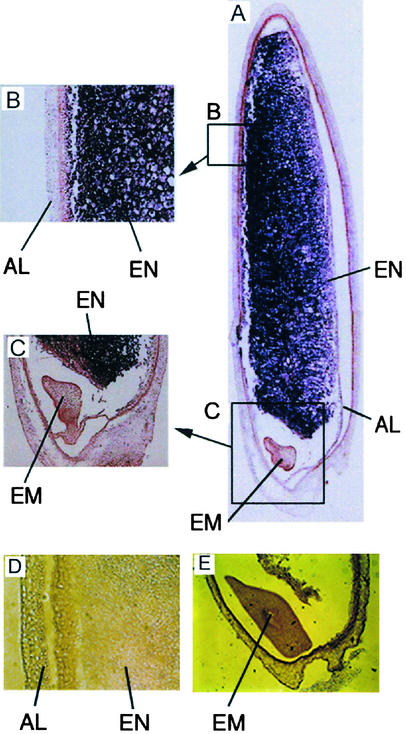
The gene for SPK is expressed exclusively in developing seed 7 to 15 days after flowering, and little of its mRNA is detected in other organs (Kawasaki et al., 1993). To identify the tissue specificity of its expression, we analyzed the localization of the SPK mRNA in immature seed by in situ hybridization. As shown in Figure 1, the expression of Spk occurred exclusively in the endosperm, where a large amount of storage starch is accumulated. Spk expression was not detected in the embryo or the aleurone layer. Thus, it was suggested that the expression patterns of Spk coincided completely with those of enzymes for starch biosynthesis. These results may support the notion that SPK is a unique protein kinase that might be involved in the regulation of storage starch biosynthesis.
Figure 1.
Detection of SPK mRNA in an Immature Rice Seed by in Situ Hybridization.
(A) The region expressing Spk is shown in purple. The specific RNA of the antisense strand was used as a probe.
(B) and (C) Magnified images corresponding to the boxes in (A).
(D) and (E) Results of the control experiment using sense strand RNA as a probe.
AL, aleurone layer; EM, embryo; EN, endosperm.
Defective Biosynthesis of Storage Starch Occurred in Seed of the Antisense SPK Transformants
To determine the function of SPK, we created transgenic rice plants containing the antisense Spk gene. Three and eight transformants of cv Nipponbare and cv Kita-ake, respectively, were isolated independently. DNA gel blot analysis confirmed the presence of the transgene in the transformants, and expression of the gene product was confirmed by RNA gel blot analysis (data not shown). The antisense transformants showed no obvious change in morphology during the vegetative stage and no difference in the timing of heading or flowering compared with the wild type. The spikelets and flowers were normal in shape. Furthermore, there was no difference in the amount of chloroplastic starch in the leaves compared with wild-type plants, as judged by iodine staining (data not shown).
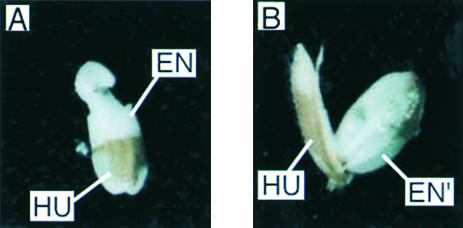
However, the process of seed development in the transformants was quite different from that in the wild-type plants. Together with a delay in seed development, a defect in starch accumulation was evident in most immature seed from the antisense transformants. The seed from antisense transformants were filled with watery sap. This phenotype was observed on all of the antisense transformants from both cultivars. Among them, three Nipponbare transformants, ASPK3, ASPK16, and ASPK19, were analyzed further. The watery seed from these representative transformants contained little starch (1.8 to 2.5 mg/mL), Glc and Glc-6-phosphate (0.3 to 0.9 mg/mL), and Fru and Fru-6-phosphate (1.2 to 2.3 mg/mL) but a large amount of Suc (54.5 to 66.3 mg/mL), whereas the immature seed of the wild-type plants contained an abundance of starch in starch granules (>10 mg/grain) together with 5.7 to 26.7 mg/mL Suc, 0.6 to 5.2 mg/mL Fru (including Fru-6-phosphate), and 18.8 to 23.3 mg/mL Glc (including Glc-6-phosphate) 2 weeks after flowering. This suggested that Suc degradation was reduced in the watery seed of antisense transformants. The outward appearance and sizes of these watery seed were similar to those of wild-type seed, but starch granules were not formed (Figure 2). This observation suggests that SPK is involved in the regulation of storage starch biosynthesis.
Figure 2.
Effect of the Antisense Spk Gene on Its Transformants.
Phenotypic features of immature seed 2 weeks after pollination of the wild type (cv Nipponbare) (A) and of a representative antisense transformant (ASPK19) (B) are shown. HU, hull; EN, endosperm; EN′, watery endosperm.
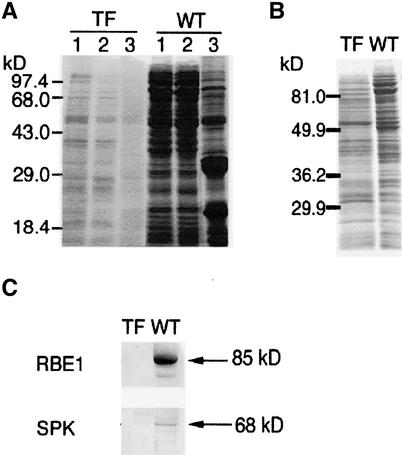
The watery seed of a representative antisense transformant (ASPK19) contained decreased amounts of proteins (Figure 3A) and an altered protein composition (Figure 3B). In particular, reduced accumulation of storage proteins was evident (including the 48-, 32-, and 22-kD proteins in Figure 3A, lane 3). With the absence of SPK, we detected an extreme reduction in several enzymes, such as starch branching enzyme 1 (RBE1), in the immature seed of the transformant (Figure 3C). Similar alterations also were observed in watery seed of the antisense transformant lines ASPK3 and ASPK16 (data not shown). These results suggest that defective SPK also affects the synthesis of several seed proteins.
Figure 3.
Protein Accumulation in Immature Seed of the Transformants and Wild-Type Plants.
(A) Protein accumulated in immature seed of the transformant ASPK19 and the wild type detected by SDS-PAGE. Lane 1, crude extract; lane 2, soluble fraction; lane 3, insoluble fraction. Each lane contained one-fifth of a total crude extract or of each fraction that was prepared independently from a single grain.
(B) Protein composition of immature seed of the transformant ASPK19 or the wild type. Each lane contained 25 μg of total protein from the crude extract.
(C) Detection of RBE1 (85 kD) and SPK (68 kD) in immature seed of the transformant ASPK19 or the wild type by protein gel blot analysis. Each lane contained one-fifth of a total crude extract from a single grain. The proteins corresponding to RBE1 and SPK are indicated by arrows.
TF, transformant ASPK19; WT, wild type.
These seed did not mature, and being sterile, eventually they became shrunken. This phenotype was reproducibly apparent in antisense transformants derived from both cv Nipponbare and cv Kita-ake. The frequency of occurrence of such watery seed was >90% in one of the Nipponbare transformants (ASPK19), although it varied (60 to 90%) depending on the transgenic line. The other seed displayed a phenotype similar to the wild type containing storage starch, in most of which the antisense Spk gene was no longer detected as a result of segregation, resulting in no watery seed being set in subsequent generations. These observations suggest that the phenotype observed is attributable to the antisense Spk gene.
It has been suggested that structural similarity exists among plant CDPK species (Harmon et al., 2000) and that there are several CDPKs in rice (Frattini et al., 1999). Spk is thought to be a single-copy gene, and no fragment was detected that cross-reacted with this gene in DNA gel blot analysis (Kawasaki et al., 1993). Therefore, we infer that the antisense gene specifically inhibited the expression of Spk. When the influence of inhibition on other CDPKs in leaves by the antisense Spk gene was analyzed by protein gel blot analysis using an antibody against AtCDPK1, which can detect several CDPKs because of its low specificity, we observed no difference between the antisense transformants and the wild-type leaves (data not shown).
SPK Is a CDPK
To further elucidate the biochemical properties of SPK, a fusion protein consisting of glutathione-S-transferase (GST) and SPK was prepared. The purified GST–SPK fusion protein showed autophosphorylation activity (Figure 4A). It also phosphorylated histone III-S, but it did not react with casein or myelin basic protein (data not shown). This activity was inhibited strongly in the presence of 1 μM staurosporine, a broad-range protein kinase inhibitor, or 5 μM W-7 [N-(6-aminohexyl)-5-chloro-1-naphthalene sulfonamide], a calmodulin antagonist, but it was not affected by the presence of 10 μM ML-9 (1-[5-chloronaphthalene-1-sulfonyl]-hexahydro-1,4-diazepine), an inhibitor of myosin light chain kinase (Figure 4B). The protein kinase activity was reduced in the presence of EGTA, but it was restored upon the addition of excess Ca2+ (Figure 4A), indicating that its activity is calcium dependent. Because no protein kinase activity was detected in assays examining the GST protein (data not shown), it appears that the SPK portion of the fusion protein has CDPK activity.
Figure 4.
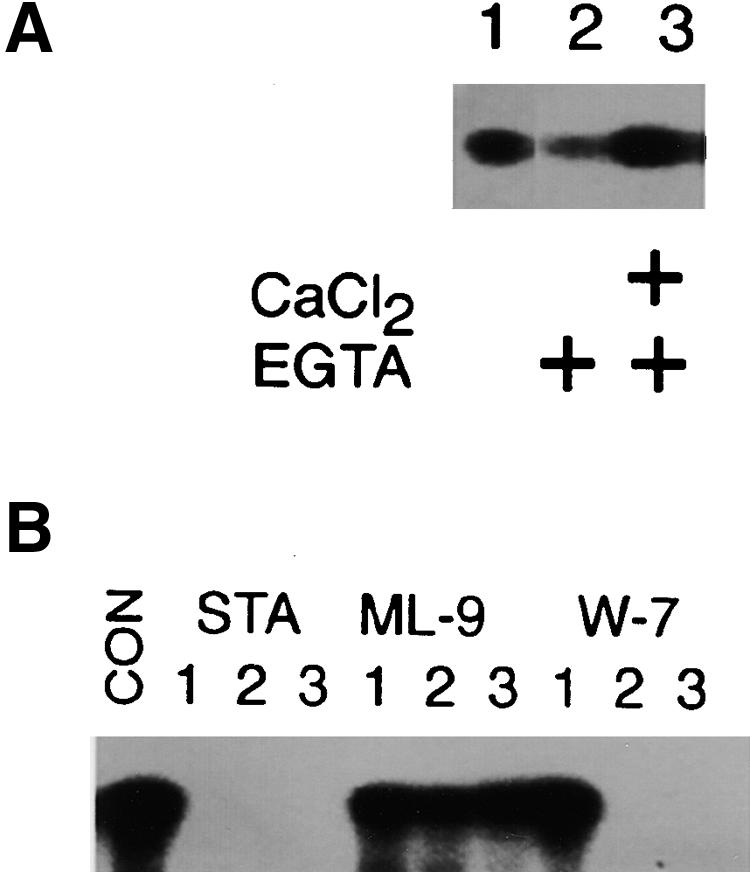
SPK May Have CDPK Activity.
(A) Calcium dependence of SPK autophosphorylation. The effects of the addition of 100 μM Ca2+ (as CaCl2) or 100 μM EGTA to the reaction (+) were examined.
(B) Effects of inhibitors. Results in the presence of 1 μM (lane 1), 5 μM (lane 2), or 10 μM (lane 3) staurosporine (STA), ML-9, W-7, or with no inhibitor (CON) are shown. Each lane contained 3.0 μg of GST–SPK in the reaction mixture.
Suc Synthase Is a Possible Target of SPK
Suc is a major end product of photosynthesis and is transported into sink organs through the phloem. In the sink organs, Suc not only plays a central role in plant growth and development but also is important as a starting material for the production of storage substances (Winter and Huber, 2000). The phenotypic traits displayed by the antisense transformants strongly suggest that SPK is involved in the regulation of the metabolic pathway from Suc to storage starch in immature seed. Suc synthase catalyzes the reversible conversion of Suc, in the presence of UDP, to Fru and UDP-Glc (Weber et al., 1996). This reaction is the initial step in storage starch biosynthesis (Huber et al., 1996; Chourey et al., 1998). It has been reported that Suc synthase in maize leaves is phosphorylated by a CDPK to induce its Suc degradation activity (Huber et al., 1996). Therefore, we examined Suc synthase as a candidate target for SPK using in vitro phosphorylation.
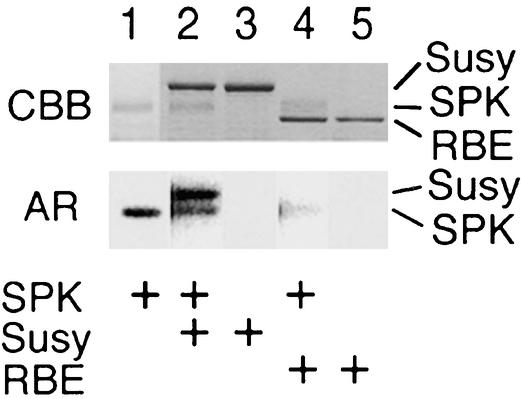
We prepared a GST–Suc synthase fusion protein using a method similar to that used to prepare the GST–SPK fusion protein, that is, by constructing an artificial gene containing a full-length cDNA corresponding to the Suc synthase gene Rsus2 (formerly Rss1) (Su, 1995). As shown in Figure 5, the GST–Suc synthase fusion protein was phosphorylated selectively by GST–SPK in vitro (lane 2), whereas RBE1 was not phosphorylated (lane 4). Autophosphorylation of GST–SPK was detected (lanes 1, 2, and 4), but GST–Suc synthase itself showed no protein kinase activity (lane 3). Because we also confirmed that the GST protein was not phosphorylated by GST–SPK (data not shown), these results imply that Suc synthase is a potential target of SPK.
Figure 5.
In Vitro Phosphorylation of GST–Suc Synthase by SPK.
In vitro phosphorylation of GST–Suc synthase (Susy) and RBE1 (RBE) in the presence or absence of GST–SPK (SPK). The presence of these proteins in the reaction (2.5 μg of GST–SPK, 3.5 μg of GST–Suc synthase, and 3.0 μg of RBE1) is indicated (+). Top, Coomassie blue–stained proteins (CBB); bottom, corresponding autoradiograms (AR).
When in vitro phosphorylation by the GST–SPK fusion protein was performed with a crude extract from an immature rice seed, four proteins of ∼110, 85, 68, and 55 kD were principally phosphorylated (Figure 6). Phosphorylation of these proteins was enhanced (1.2- to 1.5-fold) by the addition of 100 μM Ca2+ to the reaction mixture. These results suggest that these proteins are possible targets of SPK in vivo. Proteins of 85 and 68 kD correspond to those of Suc synthase and SPK, respectively.
Figure 6.
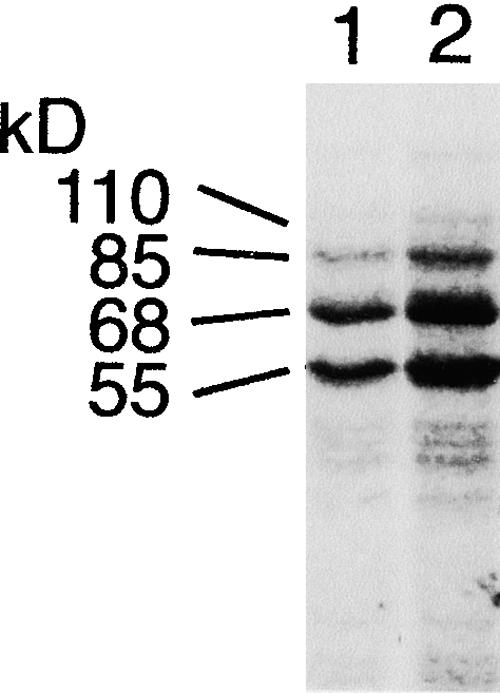
Detection of in Vitro Phosphorylation of Proteins by SPK in Immature Seed.
Proteins on a 10% SDS–polyacrylamide gel are shown as an autoradiogram. Lanes 1 and 2 contained crude extracts from the wild-type plant (cv Nipponbare). Lane 2 shows the results of kinase reactions in the presence of 100 μM Ca2+.
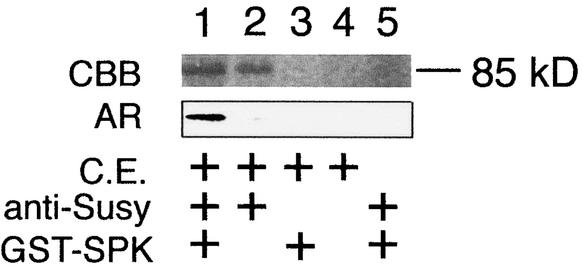
Crude extracts of immature rice seed were immunoprecipitated using anti-GST–Suc synthase antibody. A fraction containing the 85-kD protein that may correspond to Suc synthase was isolated. When in vitro phosphorylation was performed using this fraction as the substrate, the 85-kD protein was phosphorylated specifically by GST–SPK (Figure 7, lane 1). However, no phosphorylated band was detected in other cases (Figure 7, lanes 2 to 5). Therefore, these results indicate that Suc synthase is a potential target of SPK that can be phosphorylated in immature seed. On the other hand, when the fraction from the crude extract isolated by immunoprecipitation with anti-GST–SPK antibody was examined, phosphorylation of the 68-kD protein corresponding to SPK, along with phosphorylation of the added GST–SPK (90 kD), was detected (data not shown). This suggests that in vivo autophosphorylation of SPK also may occur.
Figure 7.
Immunoprecipitation of Suc Synthase in Immature Seed and Phosphorylation by SPK.
Phosphorylation of the constituents of immunoprecipitates obtained with anti-Suc synthase antibody is shown. Lanes show the results of reactions in the presence (+) or absence of a crude extract of immature seed (C.E.), anti-Suc synthase antibody (anti-Susy), and GST–SPK. CBB indicates Coomassie blue–stained proteins, and AR shows the corresponding autoradiograms.
The GST–Suc synthase fusion protein was phosphorylated when a crude extract from immature seed of wild-type plants was used as the enzyme. This activity was reduced when EGTA was added to the reaction mixture (Figure 8, lanes 1 and 2). Therefore, it appears that the activity is caused by some CDPK. When the GST–Suc synthase fusion protein was combined with a crude extract from the watery seed of the antisense transformant (ASPK19), it was phosphorylated as well, but the degree of phosphorylation corresponded approximately to the reduced level seen in wild-type seed reacted in the presence of EGTA (Figure 8, lane 3). These results also were obtained on the other antisense transformants, ASPK3 and ASPK16 (data not shown). Therefore, it is suggested that the watery seed from the antisense transformants may lack the activity of the CDPK.
Figure 8.
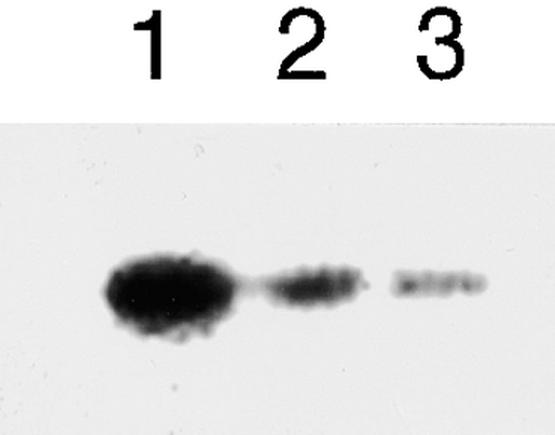
In Vitro Phosphorylation of GST–Suc Synthase by the Crude Extract from the Immature Seed of the Wild-Type Plant and the Watery Seed of the Antisense Transformants.
Each lane contained 3.0 μg of GST–Suc synthase as the substrate. Lanes 1 and 2 contained 8.0 μg of total proteins in the crude extract from the immature seed of cv Nipponbare. Lane 2 also included 1 mM EGTA in the reaction mixture. Lane 3 contained 8.0 μg of total proteins in the crude extract from the watery seed of the antisense transformant ASPK19. Proteins resolved on a 10% SDS–polyacrylamide gel are shown in the autoradiogram.
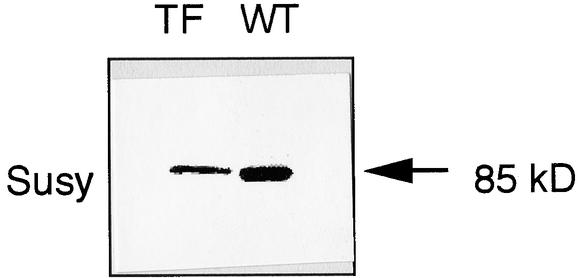
The alteration of Suc synthase was determined in watery seed of the antisense transformants by protein gel blot analysis. As shown in Figure 9, a decreased but sufficient amount of Suc synthase was detected in watery seed of the antisense transformant. This finding suggests that the expression of the Suc synthase gene also occurred in such watery seed.
Figure 9.
Detection of Suc Synthase in the Watery Seed of the Antisense Transformant by Protein Gel Blot Analysis.
Each lane contained one-fifth of a total crude extract from a single grain of the antisense SPK transformant line ASPK19 (TF) and the wild-type plant (WT). The 85-kD protein corresponding to Suc synthase (Susy) is indicated by the arrow.
SPK Phosphorylated the Ser Residue at the Activation Site of Suc Synthase
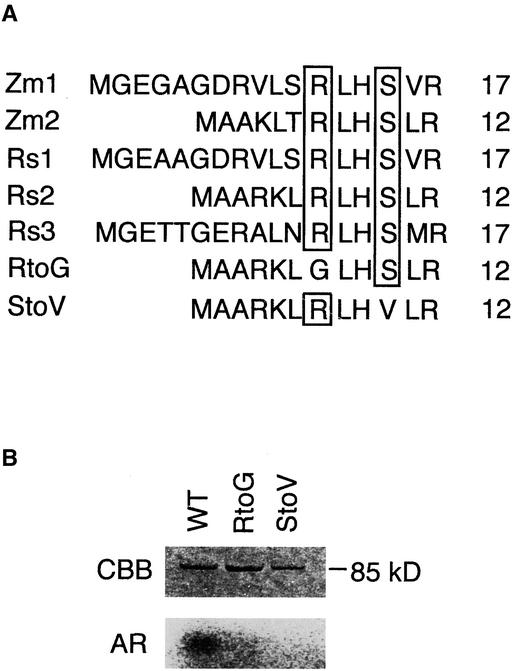
It has been shown that the consensus sequence recognized by Ser/Thr protein kinases, as well as by CDPKs, is RXXS (Figure 10A) (Harper et al., 1994). Huber et al. (1996) reported that phosphorylation of a Ser residue located in the N-terminal region of the maize Suc synthase is required for its activity. The consensus sequence RXXS is present at this site. Because the corresponding amino acid sequence is widely conserved in plant Suc synthases, including those of rice, it seems likely that they are regulated in the same way.
Figure 10.
Determination of the Phosphorylation Site in Suc Synthase.
(A) Alignment of the amino acid sequences of Suc synthases in the region around the potential target site. The consensus amino acid residues, Arg and Ser, are boxed. Zm1, Zm2, Rs1, Rs2, and Rs3 denote Suc synthases encoded by maize Msus1, maize Msus2, rice Rsus1, rice Rsus2, and rice Rsus3, respectively. RtoG, mutant with substitution at the Arg residue; StoV, mutant with substitution at the Ser residue. Numbers indicate the positions of amino acid residues around the potential phosphorylation site. For accession numbers, see Methods.
(B) In vitro phosphorylation of the site-specifically mutated and wild-type Suc synthases. Lanes show the reaction with GST–Suc synthase (wild type [WT]), with GST–RtoG (RtoG), and with GST–StoV (StoV). CBB and AR indicate the Coomassie blue–stained proteins and the corresponding autoradiograms, respectively.
To identify the phosphorylation site in the rice Suc synthase, site-directed mutagenesis was performed. We prepared mutants in which the Ser residue and the Arg residue in the consensus sequence were substituted with a Val residue and a Gly residue, respectively (Figure 10A). In the in vitro phosphorylation reaction, these mutants were not phosphorylated by the GST–SPK fusion protein, whereas the wild-type protein was phosphorylated efficiently (Figure 10B). These results suggested that SPK may recognize the sequence RXXS in Suc synthase and specifically phosphorylate the Ser residue at this site. It also appears that SPK may serve to activate Suc synthase in immature seed.
DISCUSSION
In the present study, we observed that most immature seed from the transformants with defective SPK contained watery sap with a large amount of Suc instead of storage starch. We have obtained preliminary data that, in such transformants, Suc flux in the phloem also was reduced (H. Shimada and H. Hayashi, unpublished results). These observations further support the view that SPK may play a pivotal role in planta in the biosynthesis of storage starch.
It has been shown that ADP-Glc pyrophosphorylase (AGPase) in amyloplasts also is a key enzyme for the biosynthesis of storage starch (Preiss, 1991) and that a decrease in AGPase activity in transgenic potato caused by the antisense gene for a subunit of the AGPase leads to a defect of storage starch in its tubers. In this case, a large quantity of Glc and Suc is accumulated instead (Müller-Röber et al., 1992). This phenotype seems to resemble that of the antisense SPK transformants, but it differs in that only Suc was highly accumulated in immature seed of the antisense SPK transformants. This event may suggest that the function of SPK is tightly involved with the regulation of Suc synthase activity, which catalyzes the initial step of the biosynthesis of storage starch, because Suc transported to the endosperm has been shown to be the starting material for storage starch (Winter and Huber, 2000).
It has been suggested that reversible phosphorylation of Suc synthase occurs after translation, which results in an alteration in its conformation whereby it changes from a soluble protein to a membrane-associated protein (Winter and Huber, 2000). It seems that SPK has no signal sequence for targeting to any organelle or membrane, suggesting that it is localized in the cytosol. SPK might interact with a cytosolic Suc synthase.
The existence of several isoforms of Suc synthase has been reported in many plant species. The isomers have similar molecular masses, ranging from 80 to 90 kD, and usually form a tetramer as the active holoenzyme (Wang et al., 1999). In rice, there are three known isoforms of Suc synthase, whose genes, Rsus1, Rsus2, and Rsus3, are highly homologous (Huang et al., 1996). In immature seed, all three of these genes are expressed and their expression is associated closely with seed development. Among them, Rsus1 and Rsus3 are expressed specifically in the aleurone layer and the endosperm, respectively, whereas Rsus2 is expressed uniformly in immature seed (Wang et al., 1999). In the present study, we used the Rsus2 isoform as a representative isoform of Suc synthase to study the effect of SPK, because the consensus sequence that could be the target sequence recognized by SPK was conserved completely in all three isoforms (Figure 8A). Concerning the results obtained by immunoprecipitation, because we used an antibody to the entire GST–Suc synthase, it seems likely that the fraction obtained by immunoprecipitation contains all isoforms of the Suc synthases expressed in immature seed. Therefore, our findings may reflect the phosphorylation of Suc synthase in immature seed, although it was unclear which isoform(s) is actually phosphorylated in vivo.
In vitro phosphorylation experiments with crude extracts suggest four potential targets of SPK in immature seed (Figure 6). Of these, the 85-kD protein and the 68-kD protein were identified immunologically as Suc synthase and SPK itself, respectively, although the identities of the 110- and 55-kD proteins are unclear. They also could be involved in Suc metabolism in sink organs.
The accumulation of both storage proteins and enzymes involved in storage starch synthesis was reduced in immature seed of the antisense transformants. Suc synthase activity supplies hexoses to the sink organ, and these are not only the starting materials for the synthesis of storage starch but also are important for cell metabolism, including the provision of carbon skeletons for amino acid synthesis. Low activity of Suc synthase also may influence protein biosynthesis. The phosphorylation of other proteins also was implicated, and the combined loss of phosphorylation of these proteins could contribute to the transformant phenotype. It has been suggested that Suc synthase expression in potato tuber requires SNF1-related protein kinase activity (Halford and Hardie, 1998). Similarly, Suc synthase expression might require SPK in immature rice seed. In this study, we found that the Suc synthase gene was expressed in the watery seed of the antisense transformants (Figure 9). This also suggests that the activation of Suc synthase by its phosphorylation is important for Suc degradation in immature seed.
In sink tissues, Suc is hydrolyzed by Suc synthase or invertase (Chourey et al., 1998; Winter and Huber, 2000). The invertases include three isozymes that differ in their pH optima for activity (acidic, neutral, and alkaline) (Sampietro, 1995). Of these, the neutral and alkaline invertases are cytosolic (Lee and Sturm, 1996; Winter and Huber, 2000). It is assumed that these invertases are maintenance enzymes that function mainly when the activities of cell wall acid invertase and Suc synthase are low (Winter and Huber, 2000). In sugarcane, the alkaline invertase and the neutral invertase have been shown to have subunits of ∼125 and 57 kD, respectively (Lee and Sturm, 1996). These may contribute the other targets of SPK.
Suc synthase also occurs in other sink organs, such as stems and roots (Winter and Huber, 2000). Studies examining the effects of the overexpression and antisense inhibition of Suc synthase in potato plants have confirmed its crucial role in carbon partitioning and sink strength (Zrenner et al., 1995). It has been suggested that Suc synthase is phosphorylated by an SNF1-related protein kinase in Arabidopsis seedlings (Chikano et al., 2001). There may be different systems for the regulation of Suc synthase activity. Suc synthase is involved critically in cell development and Suc metabolism. It also is a key enzyme for cellulose biosynthesis (Amor et al., 1995; Chourey et al., 1998). A defect in Suc synthase activity could affect cell metabolism, protein synthesis, and the development of the endosperm.
In conclusion, SPK functions as a Suc synthase kinase and is required to induce Suc synthase activity in the degradation of Suc in immature seed, although it may be regulated by several protein kinases. Hexoses are supplied by Suc synthase and are used as the starting materials in the biosynthetic pathways of storage products. It is assumed that the loss of Suc synthase activity could result in a lack of accumulated storage products in immature seed. It is suggested as well that SPK activity regulates gene expression in general in the developing endosperm, and its loss results in low enzyme activity in starch biosynthetic pathways as well as little storage protein synthesis. This model is supported by the results of studies using antisense SPK plants.
METHODS
In Situ Hybridization
Sections of immature seed were prepared from japonica rice (Oryza sativa cv Nipponbare), which was grown in the open field of the National Institute of Agrobiological Sciences in Tsukaba, Japan. A digoxigenin-labeled antisense RNA probe was prepared from a 1.0-kb EcoRI fragment that corresponded to the coding region. Hybridization assays were performed as described by Kouchi and Hata (1993).
Construction of the Antisense Spk Gene, and Transformation of Rice Plants
The full-length SPK cDNA (Kawasaki et al., 1993) was inserted downstream of the cauliflower mosaic virus 35S promoter of pBI221 (a derivative of pUC19 with insertion of the sequence for the 35S promoter of cauliflower mosaic virus; purchased by Toyobo, Osaka, Japan) in the antisense direction. The transformants containing the antisense gene were prepared by electroporation using rice protoplast cells (cv Nipponbare or cv Kita-ake) as described by Shimada et al. (1993), and regenerated plants were grown in a greenhouse. DNA gel blot analysis and RNA gel blot analysis for determination of the transgene were performed as described by Ausubel et al. (1987).
Determination of Starch, Glc, Fru, and Suc Contents
Watery sap was collected from immature seed (2 weeks after flowering) of the antisense transformants. Milky endosperm solution was prepared from immature seed of the wild-type plants (cv Nipponbare). They were used for the determination of sugar and starch contents. Starch, Glc, Fru, and Suc concentrations were measured by enzymatic assay methods using an F-kit (Boehringer Mannheim). In this procedure, Glc was converted initially to Glc-6-phosphate by hexokinase, and the resultant Glc-6-phosphatase was converted with NADP to gluconate-6-phosphate and with NADPH to Glc-6-phosphate dehydrogenase. The amount of Glc, including Glc-6-phosphate, was determined by measurement of the generated NADPH. Fru was converted initially to Fru-6-phophate by hexokinase. Generated Fru-6-phosphate was converted to Glc-6-phosphate by phosphoglucose isomerase. Then, using the generated Glc-6-phosphate, Fru (with Fru-6-phosphate) content was measured as for the determination of Glc content. This value was compensated by the amount of Glc in the solution. Suc was hydrolyzed initially into Glc and Fru by invertase, and then they were measured as described. The value of the Suc content was compensated by the values of the Glc and Fru contents. Soluble starch was converted initially to Glc by amyloglucosidase, and generated Glc was measured as described. The value of the soluble starch content was compensated by the value of the content of free Glc in the solution.
Analysis of Proteins in Immature Seed
A crude extract was prepared from an immature rice seed (2 weeks after flowering) of each plant (cv Nipponbare) by grinding it in 30 μL of 10% glycerol and 100 mM Mes-NaOH, pH. 7.0, after removal of its lemma and palea. It was fractionated by centrifugation (15,000g) into soluble and insoluble fractions. Proteins were separated by 10% SDS-PAGE (Laemmli, 1970) and transferred onto a polyvinylidene difluoride membrane (Immobilon; Amersham Pharmacia Biotech). Protein gel blots were blocked with 5% fat-free powdered milk solution in 20 mM Tris-HCl, pH. 7.5, and 500 mM NaCl. Blots were incubated with rabbit antiserum raised against RBE1, glutathione-S-transferase (GST)–SPK fusion protein, GST–Suc synthase fusion protein, or calcium-dependent protein kinase from Arabidopsis (Urao et al., 1994) for the detection of each protein. Immunodecorated proteins were visualized with horseradish peroxidase–conjugated anti-goat secondary antibody using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and DAB substrates (Funakoshi, Tokyo, Japan). The amount of total protein was estimated using a protein assay kit (Bio-Rad).
Preparation of a GST–SPK Fusion Protein
SPK cDNA (Kawasaki et al., 1993) was digested partially with EcoRI to generate a 1.9-kb fragment that included the protein-coding region. Through this operation, the initial amino acid residue (Met) of SPK was deleted. The resultant fragment was ligated to the EcoRI site of the pGX3X vector (Amersham Pharmacia Biotech) to generate a GST–SPK fusion gene. The construct was sequenced to confirm that the respective coding regions had been joined in frame, with 5′-GGG/AATTCTTGCCAA-3′ encoding the amino acid sequence (GST)-GIP/NSCQ-(SPK) in the junction region. The GST–SPK fusion protein was prepared using an Escherichia coli expression system as described by Seo et al. (1995). During purification of the fusion protein, 1 mM phenylmethylsulfonyl fluoride was added to the buffer solution.
Protein Kinase Assay
Protein kinase activity was assayed according to Harper et al. (1993). The kinase reaction was performed using γ-32P-ATP (111 TBq/mmol; DuPont–New England Nuclear Life Science Products) in 20 mM Tris-HCl, pH 8.0, 2 mM MgCl2, and 1 mM DTT. Calcium (as CaCl2) and inhibitors for the protein kinases, staurosporine (Kyowa Medix, Tokyo, Japan), W-7 [N-(6-aminohexyl)-5-chloro-1-naphthalene sulfonamide; Sigma], ML-9 [1-(5-chloronaphthalene-1-sulfonyl)-hexahydro-1,4-diazepine; Sigma], and EGTA (Sigma), were added to the corresponding reaction cocktails in appropriate concentrations. When a crude extract of an immature rice seed from cv Nipponbare was used as a substrate, GST–SPK was preincubated in unlabeled 25 μM ATP for 10 min to reduce the extent of the autocatalytic 32P-labeling reaction. When a crude extract of immature rice seed from cv Nipponbare or an antisense transformant was used as an enzyme, it was preincubated with unlabeled 25 μM ATP for 10 min before the addition of the GST–Suc synthase fusion protein as a substrate. After 20 min of incubation at 30°C, the ATP remaining in the reaction mixture was removed by means of a Sephadex G-50 spin column (Pharmacia Amersham Biotech). Phosphorylated proteins were separated on a 10% SDS–polyacrylamide gel and stained with Coomassie Brilliant Blue R250. Their radioactivity was detected by autoradiography using a BAS2000 Bio-image analyzer (Fuji Film Co., Tokyo, Japan).
Immunoprecipitation
Immunoprecipitation and the subsequent protein kinase reactions were performed according to Seo et al. (1999). Fifteen micrograms of total protein in the crude extract of immature Nipponbare seed was used for the immunoprecipitation with 5 μg of the anti-GST–Suc synthase antibody (for the detection of Suc synthase) or the anti-GST–SPK antibody (for the detection of SPK). Rabbit polyclonal antibody raised against the GST–Suc synthase fusion protein or the GST–SPK fusion protein was purified using a protein G–Sepharose column (Amersham Pharmacia Biotech) before use in the immunoprecipitation experiment. The immune complexes from the crude extract were used for the protein kinase reactions.
Preparation of a GST–Suc Synthase Fusion Protein
A GST–Suc synthase fusion gene was constructed as follows. By using a polymerase chain reaction–amplified fragment corresponding to Rsus2 (Yu et al., 1992) as the probe, a cDNA encoding a Suc synthase was isolated from a cDNA library constructed from immature rice seed. From the cDNA, a 2.5-kb fragment was prepared by treatment with NheI, XbaI, and the Klenow enzyme, ligated to the pGX3X vector, which had been digested with EcoRI, and then blunt-ended by the Klenow enzyme. This 2.5-kb fragment contained the region encoding most of the Suc synthase, although the initial four amino acid residues had been lost. When the GST–Suc synthase fusion gene was constructed, an unexpected deletion occurred at the EcoRI site; fortunately, however, this event generated a functional fusion gene because both parts were connected in frame. The fusion gene was sequenced and found to have the following structure: 5′-GGGAAT/CTAGCTCGCCTCCACAGTCTCC-3′, encoding the amino acid sequence (GST)-GN/LARLHSLR-(Suc synthase) in the junction region. The GST–Suc synthase fusion protein was prepared using the E. coli expression system, as in the preparation of the GST–SPK fusion protein.
Site-Directed Mutagenesis of the Suc Synthase Gene
Site-directed mutagenesis of the Suc synthase gene was performed on the GST–Suc synthase fusion gene by the oligonucleotide-directed dual amber method (Hashimoto-Gotoh et al., 1995) using the Mutan-Super Express Km kit (Takara Shuzo Co., Kyoto, Japan). To achieve substitution of Gly with Arg and Val with Ser, synthetic DNAs (5′-GAATCTAGCCGGCCTCCACAG-3′ and 5′-GCCTCCACGTGC-TCCGCGAAC-3′) were used, and mutants were screened on the basis of the generation of NaeI and BbrPI sites, respectively. After confirmation of their nucleotide sequences, the corresponding mutant proteins were prepared using the E. coli expression system.
Accession Numbers
The accession number for the SPK cDNA is E07613. The accession numbers for the sequences shown in Figure 10A are L22296 (maize Msus1), X02400 (maize Msus2), X59046 (rice Rsus1), Z15028 (rice Rsus2), and L03366 (rice Rsus3).
Acknowledgments
We thank Kouichi Mizuno, Kazuo Shinozaki, and Shigemi Seo for providing RBE1, Arabidopsis CDPK antibody, and protocols on immunodetection, respectively. This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan (Grant Nos. 9274224, 10170226, 11151227, and 12556003), a grant from the Green Frontier Program from the Ministry of Agriculture, Forestry, and Fisheries, and grants from the Mishima-Kaiun Memorial Foundation, the Kurata Foundation, and the TORAY Science Foundation to H.S.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010454.
References
- Amor, Y., Haigler, C.H., Johnson, S., Wainscott, M., and Delmer, D.P. (1995). A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc. Natl. Acad. Sci. USA 92, 9353–9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, E.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K., eds (1987). Current Protocols in Molecular Biology. (New York: John Wiley & Sons).
- Camoni, L., Harper, J.F., and Palmgren, M.G. (1998). 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett. 430, 381–384. [DOI] [PubMed] [Google Scholar]
- Chikano, H., Ogawa, M., Ikeda, Y., Koizumi, N., Kusano, T., and Sano, H. (2001). Two novel genes encoding SNF1-related protein kinases from Arabidopsis thaliana: Differential accumulation of AtSR1 and AtSR2 transcripts in response to cytokinins and sugars, and phosphorylation of sucrose synthase by AtSR2. Mol. Gen. Genet. 264, 674–681. [DOI] [PubMed] [Google Scholar]
- Chourey, R.S., Talircio, W.W., Carlson, S.J., and Ruan, Y.-L. (1998). Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol. Gen. Genet. 259, 88–96. [DOI] [PubMed] [Google Scholar]
- Ellard-Ivey, M., Hopkins, R.B., White, T.J., and Lomax, T.L. (1999). Cloning, expression and N-terminal myristoylation of CpCPK1, a calcium-dependent protein kinase from zucchini (Cucurbita pepo L.). Plant Mol. Biol. 39, 199–208. [DOI] [PubMed] [Google Scholar]
- Farmer, P.K., and Choi, J.H. (1999). Calcium and phospholipid activation of a recombinant calcium-dependent protein kinase (DcCPK1) from carrot (Daucus carota L.). Biochim. Biophys. Acta 1434, 6–17. [DOI] [PubMed] [Google Scholar]
- Frattini, M., Morello, L., and Breviario, D. (1999). Rice calcium-dependent protein kinase isoforms OsCDPK2 and OsCDPK11 show different responses to light and different expression patterns during seed development. Plant Mol. Biol. 41, 753–764. [DOI] [PubMed] [Google Scholar]
- Halford, N.G., and Hardie, D.G. (1998). SNF1-related protein kinases: Global regulators of carbon metabolism in plants? Plant Mol. Biol. 37, 735–748. [DOI] [PubMed] [Google Scholar]
- Harmon, A.C., Gribskov, M., and Harper, J.F. (2000). CDPKs, a kinase for every Ca2+ signal? Trends Plant Sci. 5, 154–159. [DOI] [PubMed] [Google Scholar]
- Harper, J.F., Sussman, M.R., Schaller, G.E., Putnam-Evans, C., Charbonneau, H., and Harmon, A.C. (1991). A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science 252, 951–954. [DOI] [PubMed] [Google Scholar]
- Harper, J.F., Binder, B.M., and Sussman, M.R. (1993). Calcium and lipid regulation of an Arabidopsis protein kinase expressed in Escherichia coli. Biochemistry 32, 3282–3290. [DOI] [PubMed] [Google Scholar]
- Harper, J.F., Fuang, J.-F., and Lloyd, S.J. (1994). Genetic identification of an autoinhibitor in CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 33, 7267–7277. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh, T., Mizuno, T., Ogasahara, Y., and Nakagawa, M. (1995). An oligodeoxyribonucleotide-directed dual amber method for site-directed mutagenesis. Gene 152, 271–275. [DOI] [PubMed] [Google Scholar]
- Huang, J.W., Chen, J.T., Yu, W.P., Shyur, L.F., Wang, A.Y., Sung, H.Y., Lee, P.D., and Su, J.C. (1996). Complete structures of three rice sucrose synthase isogenes and differential regulation of their expressions. Biosci. Biotechnol. Biochem. 60, 233–239. [DOI] [PubMed] [Google Scholar]
- Huber, S.C., Huber, J.L., Liao, P.-C., Gage, D.A., McMichael, R.W., Jr., Chourey, P.S., Hannah, L.C., and Koch, K. (1996). Phosphorylation of serine-15 of maize leaf sucrose synthase: Occurrence in vivo and possible regulatory significance. Plant Physiol. 112, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, T., Hayashida, N., Baba, T., Shinozaki, K., and Shimada, H. (1993). The gene encoding a calcium-dependent protein kinase located near the sbe1 gene encoding starch branching enzyme I is specifically expressed in developing rice seeds. Gene 129, 183–189. [DOI] [PubMed] [Google Scholar]
- Kouchi, H., and Hata, S. (1993). Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol. Gen. Genet. 238, 106–119. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lee, H.-S., and Sturm, A. (1996). Purification and characterization of neutral and alkaline invertase from carrot. Plant Physiol. 112, 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röber, B., Sonnewald, U., and Willmitzer, L. (1992). Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 11, 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss, J. (1991). Biology and molecular biology of starch synthesis and its regulation. In Surveys of Plant Molecular and Cell Biology, Vol. 7, B.J. Miflin, ed (Oxford, UK: Oxford University Press), pp. 59–114.
- Sampietro, A.R. (1995). The plant invertases. In Sucrose Metabolism: Biochemistry, Physiology and Molecular Biology, H.G. Pontis, G.L. Salerno, and E.J. Echeverria, eds (Rockville, MD: American Society of Plant Physiologists), pp. 65–71.
- Sanders, D., Brownlee, C., and Harper, J.F. (1999). Communicating with calcium. Plant Cell 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H., and Ohashi, Y. (1995). Tobacco MAP kinase: A possible mediator in wound signal transduction pathways. Science 270, 1988–1992. [DOI] [PubMed] [Google Scholar]
- Seo, S., Sano, H., and Ohashi, Y. (1999). Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, H., Tada, Y., Kawasaki, T., and Fujimura, T. (1993). Antisense regulation of the rice waxy gene expression using a PCR-amplified fragment of the rice genome reduces the amylose content in grain starch. Theor. Appl. Genet. 86, 665–672. [DOI] [PubMed] [Google Scholar]
- Su, J.-C. (1995). Metabolic roles of sucrose synthase: Example of rice isozymes encoded by three isogenes. In Sucrose Metabolism: Biochemistry, Physiology and Molecular Biology, H.G. Pontis, G.L. Salerno, and E.J. Echeverria, eds (Rockville, MD: American Society of Plant Physiologists), pp. 40–48.
- Urao, T., Katagiri, T., Mizoguchi, T., Yamaguchi-Shinozaki, K., Hayashida, N., and Shinozaki, K. (1994). An Arabidopsis thaliana cDNA encoding Ca2+-dependent protein kinase. Plant Physiol. 105, 1461–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A.-Y., Kao, M.-H., Yang, W.-H., Sayion, Y., Liu, L.-F., Lee, P.-D., and Su, J.-C. (1999). Differentially and developmentally regulated expression of three rice sucrose synthase genes. Plant Cell Physiol. 40, 800–807. [DOI] [PubMed] [Google Scholar]
- Weber, H., Buchner, P., Borisjuk, L., and Wobus, U. (1996). Sucrose metabolism during cotyledon development of Vicia faba L. is controlled by the concerted action of both sucrose-phosphate synthase and sucrose synthase: Expression patterns, metabolic regulation and implications for seed development. Plant J. 9, 841–850. [DOI] [PubMed] [Google Scholar]
- Winter, H., and Huber, S.C. (2000). Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit. Rev. Plant Sci. 19, 41–67. [DOI] [PubMed] [Google Scholar]
- Yu, W.-P., Wang, A.-Y., Juang, R.-H., Sung, H.-Y., and Su, J.-C. (1992). Isolation and sequences of rice sucrose synthase cDNA and genomic DNA. Plant Mol. Biol. 18, 139–142. [DOI] [PubMed] [Google Scholar]
- Zrenner, R., Salanoubat, M., Willmitzer, L., and Sonnewald, U. (1995). Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 7, 97–107. [DOI] [PubMed] [Google Scholar]