Abstract
Phenylbutyrate (PB) is a histone deacetylase inhibitor that has been shown to induce differentiation and apoptosis in various cancer cell lines. Although these effects are most likely due to modulation of gene expression, the specific genes and gene products responsible for the effects of PB are not well characterized. In this study, we used cDNA expression arrays and Western blot to assess the effect that PB has on the expression of various cancer and apoptosis-regulatory gene products. We show that PB attenuates the expression of the apoptosis antagonist Bcl-XL, the double-strand break repair protein DNA-dependent protein kinase, the prostate progression marker caveolin -1, and the pro-angiogenic vascular endothelial growth factor. Furthermore, PB was found to act in synergy with ionizing radiation to induce apoptosis in prostate cancer cells. Taken together, our results point to the possibility that PB may be an effective anti-prostate cancer agent when used in combination with radiation or chemotherapy and for the inhibition of cancer progression.
Keywords: histone deacetylase inhibitor, apoptosis, radiosensitizer, invasion, angiogenesis
Introduction
Phenylbutyrate (PB) is a short-chain fatty acid that induces differentiation and apoptosis in a number of cell lines including prostate cancer cells [1,2]. The mechanism of action of butyrate and butyrate derivatives involves inhibition of the histone deacetylase activity in cells, leading to chromatin modifications and the reprogramming of gene expression [3–7]. Histone deacetylase inhibitors have been shown to upregulate the expression of the cell cycle inhibitor p21WAF1 [8,9], the CD86 receptor involved in tumor immunogeneity [10], and the estrogen [11] and androgen [12] receptors. Butyrate and butyrate derivatives have also been shown to downregulate some genes and gene products such as the apoptotic antagonists Bcl-2 [13–15] and Bcl-XL [16], and urokinase involved in tumor cell invasiveness [17,18].
Due to its low toxicity in vivo and its apoptosis- and differentiation-inducing activity towards cancer cells, PB and other butyrate derivatives have been considered as potential anticancer agents [19–21]. It has been shown that PB reduces the growth of human prostate cancer xenographs in laboratory animals [2]. Moreover, butyrate may act as a chemopreventive agent against colon cancer [22]. As dietary fibers get fermented by bacteria in the bowel, millimolar concentrations of butyrate are liberated, which is thought to lead to the selective elimination of neoplastic colonic cells by apoptosis [22]. Dietary butyrate has also recently been shown to inhibit nitrosomethylurea-induced mammary cancer in the rat [23].
We have previously reported that butyrate attenuates the expression of the apoptosis antagonist BCL-XL in human fibroblasts and acts in synergy with ionizing radiation and cisplatin to induce apoptosis [16]. In this study, we show that at clinically achievable doses, PB attenuates the expression of Bcl-XL, DNA-dependent protein kinase (DNA-PK), caveolin-1, and vascular endothelial growth factor (VEGF). The downregulation of Bcl-XL and DNA-PK by PB correlated to an enhanced sensitivity towards radiation-induced apoptosis in prostate cancer cells. These results suggest the possibility that PB may be a useful anticancer agent when combined with radiation or chemotherapy and against the progression of prostate cancer.
Materials and Methods
Cell Culture
Human PC3, DU-145, and LNCaP cell lines were obtained from American Type Culture Collection (Manassas, VA) and were maintained by in vitro culture at 37°C in a humidified atmosphere containing 5% CO2 in air. Both the PC3 and DU-145 cell lines were grown in MEM (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), whereas the LNCaP cell line was grown in RPMI-1640 (Life Technologies) supplemented with 10% fetal bovine serum. Cells were plated 2 days prior to experiments on 100-mm culture dishes. Cells were about 50% to 70% confluent at the time of the experiments.
RNA Isolation
PC3 cells were treated with 2 mM PB for 18 hours. Following treatment, the dishes were washed with PBS prior to extraction of total RNA from control and treated PC3 cells using TRIZol Reagent according to instructions from the manufacturer (Life Technologies).
cDNA Array Hybridization
Assessment of differentially expressed genes in mock- or PB-treated PC3 cells was performed using the Atlas Human Cancer cDNA Expression Array (Clontech, Palo Alto, CA). Following DNase I digestion of the isolated total RNA, [α-32P]-labeled probe synthesis was undertaken by reverse transcription using the protocol specified in the Clontech manual. The labeled probes were then hybridized to the cDNA filter overnight at 68°C in ExpressHyb buffer (Clontech). After stringent washing at 68°C, filters were analyzed by phosporimaging (Phosphorimager SI; Molecular Dynamics, Sunnyvale, CA). Data analysis was performed using Phosphorimager scans and computational analysis provided by AtlasImage analysis software (Clontech). The data were normalized to the expression of tubulin and ubiquitin. Similar results were obtained when the data were normalized to all the housekeeping genes or total array expression.
Western Blot Analysis
The treated prostate cancer cells were rinsed in PBS, detached by scraping, and collected by centrifugation. The collected cells were lysed by boiling in 100 µL lysis buffer (2% SDS, 10% glycerol, and 62.5 mM Tris, pH 6.8). Samples were subsequently sonicated for 10 seconds using a microtip (Heat Systems-Ultrasonics). Protein concentration was quantified using the Biorad protein assay. To 30 µg protein solution was then added 2-mercaptoethanol and bromophenol blue to 5% and 0.05%, respectively. After a 5-minute boiling, the proteins were separated on 15% SDS-PAGE gels for analysis of Bcl-XL or caveolin-1 or 6% SDS-PAGE gels for analysis of DNA-PK. Protein molecular weight markers were loaded in a separate lane (Biorad, Hercules, CA). After electrophoretic separation, the proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Following blocking of the membranes with 5% dry milk solution (Kroger, Ann Arbor, MI) for 2 hours, they were incubated overnight with anti-caveolin-1, anti-Bcl-XL (Transduction Laboratories, Lexington, KY), or anti-DNA-PK (BD PharMingen, Research Triangle Park, NC) antibodies. Horseradish peroxidase-conjugated anti-mouse secondary antibody (Oncogene Research Products, Boston, MA) and enhanced chemiluminescence (SuperSignal CL; Pierce, Rockford, IL) were used to together with X-ray film to visualize the protein bands. Sample loading was normalized to β-actin expression (anti-β-actin AC-74; Sigma). The quality of total protein transfer was assessed by staining blots with Coomassie brilliant blue following exposure of the membranes to X-ray film. Images were scanned using a flatbed scanner (UMAX) and analyzed using NIH Image software.
Analysis of VEGF Protein Levels in Sample Media
PC-3, DU-145, and LNCaP cells were seeded on 100-mm dishes. When the plates were approximately 60% to 70% confluent, the media was exchanged for fresh media and the cells were either mock-treated or incubated with 100 µM CoCl2 to simulate a hypoxia-like state. To half of the plates, 2 mM PB was added. Following PB treatment for 24 hours, the conditioned media was collected, centrifuged at 6000 rpm for 6 minutes to remove cellular debris, and then stored at -20°C. To calculate the total number of cells per plate, the remaining attached cells were scraped off the plates and an aliquot of the cell suspension was counted using a Coulter counter (Coulter Electronics, Hialeah, FL). Samples stored at -20°C were then thawed and VEGF concentrations were determined using the Quantikine human VEGF Elisa Kit (R&D Systems, Minneapolis, MN). Samples were diluted 1:10 in a dilution solution provided with the ELISA kit. The readings of the VEGF concentration in conditioned media were then expressed as the amount of VEGF in picograms per milliliter per 106 cells. For each set of conditions, the %VEGF was compared to the mock-treated sample, values of which were set to 100%.
Quantification of Apoptosis
Cells were treated with 2 Gy of ionizing radiation and then incubated in the presence or absence of 2 mM PB for 72 hours. Both floating and attached cells (trypsinized) were collected by centrifugation (1000g for 10 minutes), fixed in ethanol, and the cellular DNA was stained with propidium iodide as previously described [16,24]. Cells with sub-G1 DNA content were scored as apoptotic using flow cytometry (Coulter Elite ESP Cell Sorter, Miami, FL) and the Multicycle software package (Phoenix Flow Systems, San Diego, CA).
Results
Butyrate and PB Inhibit the Expression of Bcl-XL in PC3 Cells
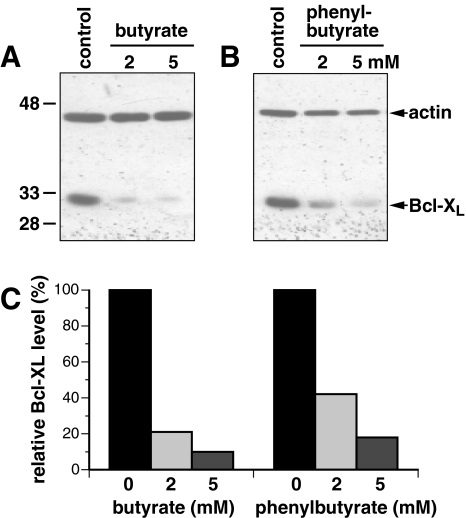
We have previously shown that butyrate reduces the expression of Bcl-XL in human fibroblast cells to about 70% and 40% following a 24-hour incubation with either 5 or 10 mM butyrate, respectively [16]. This attenuated Bcl-XL expression correlated with a sensitization of the cells to radiation-and chemotherapy-induced apoptosis. Here we investigated whether butyrate and its derivative PB could lower the protein levels of Bcl-XL in the prostate cancer cell line PC3. Our results show that the cellular levels of Bcl-XL in PC3 cells were reduced to about 20% and 10% following 24 hours of incubation with 2 or 5 mM butyrate, respectively (Figure 1A and C). Incubation of PC3 cells with 2 or 5 mM PB inhibited the expression of Bcl-XL to about 40% and 15%, respectively (Figure 1B and C). Butyrate and PB similarly attenuated the expression of Bcl-XL in DU-145 and LNCaP cells (data not shown). Thus, our results show that PB can, like butyrate, attenuate the protein levels of Bcl-XL in prostate cancer cells. Furthermore, butyrate appears to reduce the Bcl-XL levels in the prostate cancer cell lines at lower doses than we previously found necessary to reduce Bcl-XL in diploid human fibroblasts [16].
Figure 1.
The cellular level of Bcl-XL is reduced in PC3 cells by treatment with butyrate or PB. (A) PC3 cells were mock-treated (control) or exposed to 2 or 5 mM butyrate for 24 hours before being collected and the level of Bcl-XL protein determined by Western blotting. (B) Same as (A) but cells were treated with PB. (C) The bands in (A) and (B) were scanned and quantified using NIH Image 1.62. Following normalization to the actin levels, the values were expressed relative to the untreated control cells. These blots are representative of at least four similar experiments.
The mechanism of inhibition of Bcl-XL expression by PB observed in this study may be transcriptional [5] or due to caspase-mediated cleavage of the Bcl-XL protein [25]. However, reverse transcription polymerase chain reaction experiments did not detect a transcriptional affect of PB on the Bcl-XL mRNA levels, nor did we observe cleavage products of the Bcl-XL protein on Western blots (data not shown). Thus, the mechanism by which PB reduces the Bcl-XL levels in prostate cancer cells needs to be elucidated.
PB Affects the Gene Expression Profile in PC3 Cells
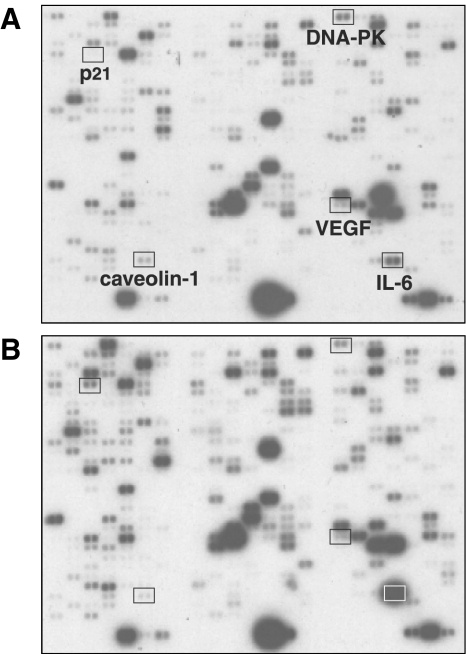
To explore the effect of PB on the gene expression profile in PC3 cells, we used the Atlas human cancer cDNA expression array from Clontech to analyze the abundance of specific mRNAs in mock-or PB-treated cells. As can be seen in Figure 2, treatment with 2 mM PB resulted in the increased expression of numerous genes. This is to be expected because PB is a histone deacetylase inhibitor that would reverse the histone deacetylase-mediated suppression of transcription of certain genes [26,27]. We note that the p21WAF1 and interleukin-6 (IL-6) genes are greatly upregulated by PB (Figure 2), as previously reported for other cells incubated with histone deacetylation inhibitors [8,28]. Taking the average of two independent array experiments using RNA isolated from two independent experiments, the signal for the p21WAF1 and IL-6 genes increased following PB exposure to 521% (S.E.M.±4) and 603% (S.E.M.±103), respectively. A few genes were found to be downregulated by PB. One of these genes was DNA-PK, the gene product of which is an important component of the DNA double-strand break repair machinery [29,30]. Another gene was caveolin-1, which in some cancers is considered a tumor-suppressor gene [31] but appears to be involved in the progression of the metastatic development in prostate cancer [32]. Finally, the VEGF gene was downregulated in PC3 cells by PB. VEGF is an important factor involved in tumor angiogenesis [33]. The expression of DNA-PK, caveolin-1, and VEGF decreased to 37% (S.E.M.±8), 18% (interpretable data from only one experiment was obtained), and 59% (S.E.M.±3), respectively.
Figure 2.
Effect of PB on the gene expression profile in PC3 cells. (A) Mock-treated PC3 cells. (B) PC3 cells treated with 2 mM PB for 18 hours. Whereas the expression of the IL-6 and p21WAF1 genes was upregulated by PB treatment, DNA-PK, caveolin-1, and VEGF gene expression decreased.
PB Inhibits the Protein Expression of DNA-PK and Caveolin-1 in Prostate Cancer Cells
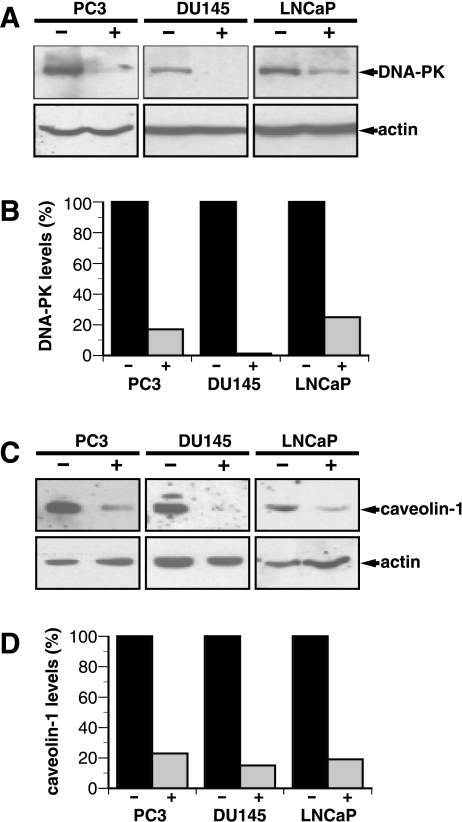
Because DNA-PK and caveolin-1 could potentially act as useful therapeutic targets for prostate cancer treatment, we wanted to further explore whether the reduced gene expression following treatment with PB translated into a lower protein expression. Using Western blot technique combined with specific antibodies and chemiluminescence detection, we show that PB greatly reduced both DNA-PK and caveolin-1 protein levels in PC3, DU-145, and LNCaP prostate cancer cells (Figure 3). Following a 48-hour incubation with 2 mM PB, the protein levels of DNA-PK were reduced to 17%, 1%, and 25% in PC3, DU-145, and LNCaP cells, respectively. The corresponding numbers for caveolin-1 were 23%, 15%, and 19%, respectively. In contrast, the protein levels of actin were not affected by PB and we found that p21WAF1 protein levels increased by PB as previously described using other cancer cells [34–36].
Figure 3.
Expressions of DNA-PK and caveolin-1 proteins are down-regulated in prostate cancer cells. (A) PC3, DU-145, and LNCaP cells were mock-treated or incubated with 2 mM PB for 48 hours. Western blots show that DNA-PK protein levels are reduced by PB. The blots in (A) are representative of blots from three independent experiments. (B) The bands in (A) were scanned and quantified using NIH Image 1.62 and, following normalization to the actin levels, expressed relative to the untreated control. (C) Cells were treated as in (A). Western blots show that caveolin-1 protein levels are reduced by PB. (D) Quantification of the bands in (C) was performed as described in (B). The blots in (C) are representative of three similar experiments.
It has been shown that DNA-PK is a substrate for caspase-3 during apoptosis [37]. However, we do not believe that this mechanism was responsible for the loss of DNA-PK following PB treatment because 1) we only used attached cells that did not yet display any apoptotic characteristic and 2) we did not observe the cleavage products expected to be induced by caspase-3 [37]. Because the reductions observed in the protein levels of DNA-PK and caveolin-1 are similar to the reductions found in mRNA expression (Figure 2), the mechanism by which PB attenuates the expression of these proteins is most likely regulated at the level of transcription.
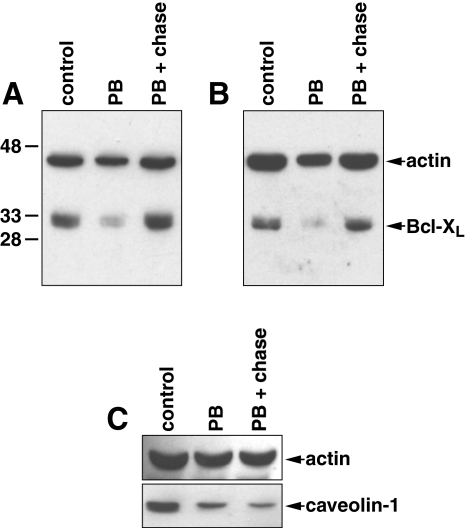
We next investigated whether the suppressing effect that PB had on the protein expression of Bcl-XL and caveolin-1 was reversible; we allowed cells treated with PB to recover in drug-free media for 24 hours prior to the analysis of protein content. It was found that PB-treated PC3 or DU-145 cells fully recovered the protein expression of Bcl-XL following a 24-hour chase in drug-free media (Figure 4A and B). However, we did not observe a similar recovery of the expression of caveolin-1 in DU-145 cells (Figure 4C). Thus, the reversibility of the suppressive effect that PB has on protein expression may be gene- or gene product-specific.
Figure 4.
Reversible suppression of Bcl-XL but not caveolin-1 by PB. Western blots of Bcl-XL from (A) PC3 cells or (B) DU-145 cells that were ether mock-treated (control), treated with 2 mM PB for 24 hours (PB), or treated with 2 mM PB for 24 hours followed by a 24-hour chase in the absence of PB (PB+chase). (C) Same treatment as in (A) and (B) but with Western blot analysis of caveolin-1 using DU-145 cells.
Secretion of VEGF from Prostate Cancer Cells is Attenuated by PB
The VEGF is critical in tumor angiogenesis [33]. As cells become hypoxic in a growing tumor, secretion of VEGF stimulates vascularization. Prostate cancer cell lines have been shown to express high levels of VEGF [38] and its expression positively correlates to the stage, grade, and clinical outcome of prostate cancer [39]. The PC3 cells have been shown to express more cytosolic VEGF than DU-145 cells, which in turn express more VEGF than LNCaP cells [38]. In this study, we used an ELISA technique to measure the amount of VEGF secreted by these cells in culture. We found that compared to PC3 cells, the DU-145 cells secreted 64% and the LNCaP cells secreted about 20% of VEGF into the culture media when normalized to the same number of cells (data not shown). Thus, the ranking order of VEGF secretion observed in this study matched the ranking order of VEGF cell staining of these cells as shown previously [38]. Because the LNCaP cells secreted low levels of VEGF and were less reliably measured, we decided not to include them in the PB experiments described below.
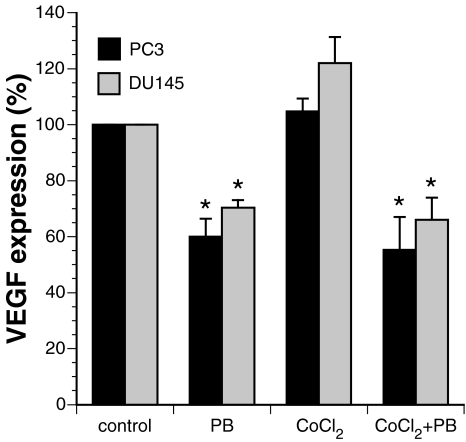
We next investigated whether PB affects the expression of secreted VEGF in the media of cultured PC3 and DU-145 cells. Cells were mock-treated or incubated in the presence of 2 mM PB for 24 hours. The conditioned media was then collected and the abundance of VEGF was determined using an ELISA kit (R&D Systems). It was found that PB reduced the amount of VEGF to 60% and 70% compared to the mock-treated samples for the PC3 and DU-145 cells, respectively (Figure 5). We also simulated hypoxia by treating the cells for 24 hours with cobalt chloride [40]. This marginally increased the amount of secreted VEGF to 104% in the PC3 cells and to 122% in DU-145 cells. When cells were incubated simultaneously with both cobalt chloride and PB, the VEGF levels dropped to 55% and 66% for PC3 and DU-145 cells, respectively (Figure 5). Thus, PB significantly reduced the amount of VEGF secreted from these prostate cancer cells both under normal and hypoxia-simulated conditions.
Figure 5.
PB reduces the secretion of VEGF from cells grown under normal or hypoxic-simulated conditions. Cells were mock-treated, treated with 2 mM PB, treated with 100 µM CoCl2 or with the combination of 100 µM CoCl2 and 2 mM PB. The levels of VEGF secreted into the growth media were determined using ELISA. The values are expressed as relative values of the mock-treated cells and they represent the average of three independent experiments with error bars showing the standard error of the mean (S.E.M.). Statistical analysis showed that the PB-treated cells secreted significantly less amounts of VEGF (stars) than controls (P<.05).
Interaction between Ionizing Radiation and PB in the Induction of Apoptosis
We have previously shown that butyrate and ionizing radiation can act in synergy to induce apoptosis in human fibroblasts [16]. This radiosensitizing effect by butyrate correlated with a reduction in the protein levels of Bcl-XL. In this study, we show that in addition to an attenuation of the expression of Bcl-XL in prostate cancer cells, the doublestrand break repair protein DNA-PK is greatly reduced by PB. The reduced protein levels of Bcl-XL and DNA-PK would be expected to sensitize these cells further to the cytotoxic affects of ionizing radiation because cells that lack DNA-PK activity are extremely sensitive to ionizing radiation [41]. However, PB induces cell cycle arrest by increasing the expression of p21WAF1 [42] and this may partially protect cells from subsequent exposure to ionizing radiation. In fact, we have observed that more apoptosis is induced if the butyrate exposure is scheduled to occur after, rather then prior to, irradiation [16]. Furthermore, it has been shown that the duration of butyrate treatment prior to radiation is important for whether butyrate acts as a radiosensitizer (72-hour pretreatment) or radioprotector (24-hour pretreatment) [43].
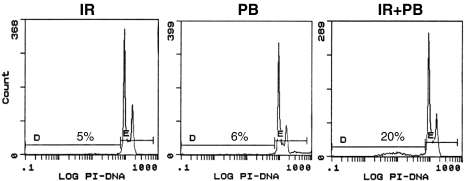
To test whether the apoptosis-inducing ability of ionizing radiation could be potentiated in prostate cancer cells by PB, we irradiated DU-145 cells with 2 Gy and then incubated the cells for 72 hours in the presence or absence of 2 mM PB. Flow cytometric analysis of DNA content revealed that neither PB nor irradiation alone induced significant amounts of apoptosis during this time period (Figure 6). However, when the irradiated cells were subsequently incubated in with PB for 72 hours, an increased amount of apoptosis was observed. Thus, ionizing radiation and PB appear to act in synergy to induce apoptosis in prostate cancer cells.
Figure 6.
PB acts in synergy with ionizing radiation to induce apoptosis in DU-145 prostate cancer cells. DU-145 cells were treated with 2 mM PB or irradiated with 2 Gy of ionizing radiation and incubated for 72 hours in the absence or presence of 2 mM PB. Cells were then collected and fixed, stained with propidium iodide, and analyzed for the percentage of cells with a sub-G1 DNA content using flow cytometry.
Discussion
In this study, we show that the histone deacetylase inhibitor PB attenuated the protein expression of Bcl-XL, DNA-PK, caveolin-1, and VEGF in prostate cancer cell lines. Incubation of the PC3, DU-145, and LNCaP cell lines with the clinically achievable dose of 2 mM PB [21,44] reduced the protein levels of Bcl-XL to about 40% within 24 hours (Figure 1 and data not shown). Furthermore, the protein expression of DNA-PK and caveolin-1 was reduced to below 20% within 48 hours (Figure 3) and the secretion of VEGF was reduced by about 40% within 24 hours in PC3 and DU-145 cells.
It is expected that both the cellular levels of Bcl-XL and DNA-PK would influence the response of cancer cells to radiation or chemotherapy. Bcl-XL, a member of the Bcl-2 family of apoptosis regulators, antagonizes apoptosis by promoting mitochondrial membrane permeability, thus preventing the release of cytochrome c [45,46]. Bcl-XL is frequently overexpressed in tumors, making these cells more resistant to chemotherapy [47–49]. The DNA-PK is a critical component of both the DNA double-strand breakrepair machinery and in V(D)J recombination [41]. Cells lacking DNA-PK catalytic activity are defective in DNA double-strand break-repair and extremely sensitive to the effects of ionizing radiation. Increased cellular levels of DNA-PK have been shown to protect cells against the toxic effects of ionizing radiation, adriamycin, bleomycin, and cisplatin [50]. Interestingly, it was recently reported that sodium butyrate severely inhibits DNA double-strand breakrepair in human lymphocytes [51]. Furthermore, in this study, we show that PB sensitizes prostate cancer cells to ionizing radiation-induced apoptosis (Figure 6). Thus, it is possible that the attenuation of the expression of DNA-PK in prostate cancer cells by PB contributes to a reduced repair of DNA double-strand breaks induced by ionizing radiation and that the reduced expression of Bcl-XL contributes to the lowering of the apoptotic threshold. Taken together, these findings should stimulate efforts to investigate the efficacy of combining PB with radiation or chemotherapy for the treatment of prostate cancer.
Caveolin-1 is highly expressed in prostate cancer cells and has been shown to positively correlate to stage, grade, and clinical outcome of prostate cancer [52]. Caveolin-1 is thought to regulate integrin signaling pathways [53], and the reduction of its expression by antisense cDNA has been shown to convert androgen-insensitive metastatic prostate cancer cells into androgen-sensitive cells [54]. In preliminary studies using the sea urchin embryo invasion assay [55,56], we have found that PB efficiently blocks the invasive properties of both DU-145 and PC3 cells (E. Dyer et al., unpublished observation). Whether this is due to the downregulation of caveolin-1 or the inhibition of some other gene product involved in invasiveness, such as urokinase [18], has to be elucidated.
VEGF is critical for tumor angiogenesis [33]. Similar to caveolin-1, VEGF is highly expressed in prostate cancer cells and has been shown to positively correlate to stage, grade, and clinical outcome of prostate cancer [39]. The downregulation of VEGF by PB found in this study, together with the findings that butyrate can directly inhibit the proliferation of endothelial cells without affecting viability [57], suggests that butyrate and PB may have anti-angiogenic properties. Furthermore, it has been shown that anti-VEGF treatments potentiate the sensitivity of tumor cells to ionizing radiation both in vitro and in vivo [58–60]. Thus, PB may sensitize tumor cells to ionizing radiation by its effects on both angiogenesis by downregulation of VEGF and DNA damage processing and apoptosis by down-regulation of DNA-PK and Bcl-XL.
PB and other butyrate derivatives have been recognized as potential anticancer agents [19–21]. Our results that PB suppresses the expression of Bcl-XL, DNA-PK, caveolin-1, and VEGF should stimulate further studies investigating the potential usefulness of PB as an anticancer agent especially when combined with radiation or other cancer therapeutics.
Acknowledgements
We thank the members of the Ljungman laboratory for valuable contributions to this work.
Abbreviations
- DNA-PK
NA-dependent protein kinase
- VEGF
vascular endothelial growth factor
- PB
phenylbutyrate
Footnotes
This work was supported by a Career Development grant and a Student Development award from the NIH/NCI prostate SPORE grant, University of Michigan.
References
- 1.Carducci MA, Nelson JB, Chan-Tack KM, Ayyagari SR, Sweatt WS, Campbell PA, Nelson WG, Simons JW. Phenylbutyrate induces apoptosis in human prostate cancer and is more potent than phenylacetate. Clin Cancer Res. 1996;2:379–387. [PubMed] [Google Scholar]
- 2.Melchior SW, Brown LG, Figg WD, Quinn JE, Santucci RA, Brunner J, Thuroff JW, Lange PH, Vessella RL. Effects of phenylbutyrate on proliferation and apoptosis in human prostate cancer cells in vitro and in vivo. Int J Oncol. 1999;14:501–508. doi: 10.3892/ijo.14.3.501. [DOI] [PubMed] [Google Scholar]
- 3.Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in cultures. Mol Cell Biochem. 1982;42:65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- 4.Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Exp. 1996;5:245–253. [PMC free article] [PubMed] [Google Scholar]
- 5.Eickhoff B, Ruller S, Laue T, Kohler G, Stahl C, Schlaak M, van der Bosch J. Trichostatin A modulates expression of p21 (waf1/cip1), Bcl-xL, ID1, ID2, ID3, CRABP, GATA-2, hsp86 and TFIID/TAFII31 mRNA in human lung adenocarcinoma cells. Biol Chem. 2000;381:107–112. doi: 10.1515/BC.2000.015. [DOI] [PubMed] [Google Scholar]
- 6.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 7.Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60:4561–4572. [PubMed] [Google Scholar]
- 8.Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, Fujita T, OhtaniFujita N, Matsukawa Y, Tokino T, Yamagishi H, Oka T, Nomura H, Sakai T. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J Biol Chem. 1997;272:22199–22206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- 9.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation [In Process Citation] Proc Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda T, Towatari M, Kosugi H, Saito H. Upregulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96:3847–3856. [PubMed] [Google Scholar]
- 11.Yang X, Ferguson AT, Nass SJ, Phillips DL, Butash KA, Wang SM, Herman JG, Davidson NE. Transcriptional activation of estrogen receptor alpha in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000;60:6890–6894. [PubMed] [Google Scholar]
- 12.Sadar MD, Gleave ME. Ligand-independent activation of the androgen receptor by the differentiation agent butyrate in human prostate cancer cells. Cancer Res. 2000;60:5825–5831. [PubMed] [Google Scholar]
- 13.Mandal M, Kumar R. Bcl-2 expression regulates sodium butyrate-induced apoptosis in human MCF-7 breast cancer cells. Cell Growth Differ. 1996;7:311–318. [PubMed] [Google Scholar]
- 14.Hague A, Diaz GD, Hicks DJ, Krajewski S, Reed JC, Paraskeva C. bcl-2 and bak may play a pivotal role in sodium butyrate-induced apoptosis in colonic epithelial cells; however, overexpression of bcl-2 does not protect against bak-mediated apoptosis. Int J Cancer. 1997;72:898–905. doi: 10.1002/(sici)1097-0215(19970904)72:5<898::aid-ijc30>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Ng AY, Bales W, Veltri RW. Phenylbutyrate-induced apoptosis and differential expression of Bcl-2, Bax, p53 and Fas in human prostate cancer cell lines. Anal Quant Cytol Histol. 2000;22:45–54. [PubMed] [Google Scholar]
- 16.Chung DH, Zhang FF, Chen F, McLaughlin WP, Ljungman M. Butyrate attenuates Bcl-XL expression in human fibroblasts and acts in synergy with ionizing radiation to induce apoptosis. Radiat Res. 1998;149:187–194. [PubMed] [Google Scholar]
- 17.Gibson PR, Rosella O, Rosella G, Young GP. Butyrate is a potent inhibitor of urokinase secretion by normal colonic epithelium in vitro. Gastroenterology. 1994;107:410–419. doi: 10.1016/0016-5085(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 18.Engelhard HH, Homer RJ, Duncan HA, Rozental J. Inhibitory effects of phenylbutyrate on the proliferation, morphology, migration and invasiveness of malignant glioma cells. J Neuro-Oncol. 1998;37:97–108. doi: 10.1023/a:1005865125588. [DOI] [PubMed] [Google Scholar]
- 19.Samid D, Hudgins WR, Shack S, Liu L, Prasanna P, Myers CE. Phenylacetate and phenylbutyrate as novel, nontoxic differentiation inducers. Adv Exp Med Biol. 1997;400A:501–505. doi: 10.1007/978-1-4615-5325-0_67. [DOI] [PubMed] [Google Scholar]
- 20.Thibault A, Figg W, Samid D. Phase I study of the differentiation agent phenylbutyrate in patients with cancer. Proc Am Soc Clin Oncol. 1996;15:484–491. [Google Scholar]
- 21.Gore SD, Carducci MA. Modifying histones to tame cancer: clinical development of sodium phenylbutyrate and other histone deacetylase inhibitors. Exp Opin Invest Drugs. 2000;9:2923–2934. doi: 10.1517/13543784.9.12.2923. [DOI] [PubMed] [Google Scholar]
- 22.Scheppach W, Bartram HP, Richter F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur J Cancer. 1995;31A:1077–1080. doi: 10.1016/0959-8049(95)00165-f. [DOI] [PubMed] [Google Scholar]
- 23.Belobrajdic DP, McIntosh GH. Dietary butyrate inhibits NMU-induced mammary cancer in rats. Nutr Cancer. 2000;36:217–223. doi: 10.1207/S15327914NC3602_11. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman M, Zhang F. Blockage of RNA polymerase as a possible trigger for UV light-induced apoptosis. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- 25.Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pazin MJ, Kadonaga JT. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 27.Weidle UH, Grossmann A. Inhibition of histone deacetylases: a new strategy to target epigenetic modifications for anticancer treatment. Anticancer Res. 2000;20:1471–1485. [PubMed] [Google Scholar]
- 28.Wang LS, Chow KC, Wu CW. Expression and upregulation of interleukin-6 in oesophageal carcinoma cells by n-sodium butyrate. Br J Cancer. 1999;80:1617–1622. doi: 10.1038/sj.bjc.6690571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirchgessner C, Patil C, Evans J, Cuomo C, Fried L, Carter T, Oettinger M, Brown J. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 30.Lees-Miller SP, Godbout R, Chan DW, Weinfeld M, Day RS, III, Barron GM, Allalunis-Turner J. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995;267:1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- 31.Razani B, Schlegel A, Lisanti MP. Caveolin proteins in signaling, oncogenic transformation and muscular dystrophy. J Cell Sci. 2000;113:2103–2109. doi: 10.1242/jcs.113.12.2103. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–1880. [PubMed] [Google Scholar]
- 33.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 34.McGrath-Morrow SA, Stahl JL. G(1) Phase growth arrest and induction of p21 (Waf1/Cip1/Sdi1) in IB3-1 cells treated with 4-sodium phenylbutyrate. J Pharmacol Exp Ther. 2000;294:941–947. [PubMed] [Google Scholar]
- 35.DiGiuseppe JA, Weng LJ, Yu KH, Fu S, Kastan MB, Samid D, Gore SD. Phenylbutyrate-induced G1 arrest and apoptosis in myeloid leukemia cells: structure-function analysis. Leukemia. 1999;13:1243–1253. doi: 10.1038/sj.leu.2401471. [DOI] [PubMed] [Google Scholar]
- 36.Davis T, Kennedy C, Chiew YE, Clarke CL, deFazio A. Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clin Cancer Res. 2000;6:4334–4342. [PubMed] [Google Scholar]
- 37.Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper ME, Glynne-Jones E, Goddard L, Thurston VJ, Griffiths K. Vascular endothelial growth factor (VEGF) expression in prostatic tumours and its relationship to neuroendocrine cells. Br J Cancer. 1996;74:910–916. doi: 10.1038/bjc.1996.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strohmeyer D, Rossing C, Bauerfeind A, Kaufmann O, Schlechte H, Bartsch G, Loening S. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45:216–224. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 40.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1 alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 41.Muller C, Calsou P, Frit P, Salles B. Regulation of the DNA-dependent protein kinase (DNA-PK) activity in eukaryotic cells. Biochimie. 1999;81:117–125. doi: 10.1016/s0300-9084(99)80044-3. [DOI] [PubMed] [Google Scholar]
- 42.Archer SY, Meng SF, Shei A, Hodin RA. p21 (Waf1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AC, Whittaker T, Thibault A, Samid D. Modulation of radiation response of human tumour cells by the differentiation inducers, phenylacetate and phenylbutyrate. Int J Radiat Biol. 1997;72:211–218. doi: 10.1080/095530097143437. [DOI] [PubMed] [Google Scholar]
- 44.Piscitelli SC, Thibault A, Figg WD, Tompkins A, Headlee D, Lieberman R, Samid D, Myers CE. Disposition of phenylbutyrate and its metabolites, phenylacetate and phenylacetylglutamine. J Clin Pharm. 1995;35:368–373. doi: 10.1002/j.1552-4604.1995.tb04075.x. [DOI] [PubMed] [Google Scholar]
- 45.Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 46.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 47.Dole MG, Jasty R, Cooper MJ, Thompson CB, Nunez G, Castle VP. Bcl-x (L) is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res. 1995;55:2576–2582. [PubMed] [Google Scholar]
- 48.Lebedeva I, Rando R, Ojwang J, Cossum P, Stein CA. Bcl-XL in prostate cancer cells: effects of overexpression and downregulation on chemosensitivity. Cancer Res. 2000;60:6052–6060. [PubMed] [Google Scholar]
- 49.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 50.Xu WM, Liu LZ, Smith GCM, Charles IG. Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. Nat Cell Biol. 2000;2:339–345. doi: 10.1038/35014028. [DOI] [PubMed] [Google Scholar]
- 51.Stoilov L, Darroudi F, Meschini R, van der Schans G, Mullenders LHF, Natarajan AT. Inhibition of repair of X-ray-induced DNA double-strand breaks in human lymphocytes exposed to sodium butyrate. Int J Radiat Biol. 2000;76:1485–1491. doi: 10.1080/09553000050176243. [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59:5719–5723. [PubMed] [Google Scholar]
- 53.Chapman HA, Wei Y, Simon DI, Waltz DA. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb Haemostasis. 1999;82:291–297. [PubMed] [Google Scholar]
- 54.Nasu Y, Timme TL, Yang G, Bangma CH, Li L, Ren C, Park SH, DeLeon M, Wang J, Thompson TC. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nat Med. 1998;4:1062–1064. doi: 10.1038/2048. [DOI] [PubMed] [Google Scholar]
- 55.Livant DL, Linn S, Markwart S, Shuster J. Invasion of selectively permeable sea urchin embryo basement membranes by metastatic tumor cells, but not by their normal counterparts. Cancer Res. 1995;55:5085–5093. [PubMed] [Google Scholar]
- 56.Livant DL, Brabec RK, Pienta KJ, Allen DL, Kurachi K, Markwart S, Upadhyaya A. Anti-invasive, antitumorigenic, and antimetastatic activities of the PHSCN sequence in prostate carcinoma. Cancer Res. 2000;60:309–320. [PubMed] [Google Scholar]
- 57.Tse CS, Williams DM. Inhibition of human endothelial cell proliferation in vitro in response to n-butyrate and propionate. J Periodontal Res. 1992;27:506–510. doi: 10.1111/j.1600-0765.1992.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 58.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 59.Gorski DH, Mauceri HJ, Salloum RM, Gately S, Hellman S, Beckett MA, Sukhatme VP, Soff GA, Kufe DW, Weichselbaum RR. Potentiation of the antitumor effect of ionizing radiation by brief concomitant exposures to angiostatin. Cancer Res. 1998;58:5686–5689. [PubMed] [Google Scholar]
- 60.Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M, Soff GA, Sukhatme VP, Kufe DW, Weichselbaum RR. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:91. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]