Abstract
Crd1 (Copper response defect 1), which is required for the maintenance of photosystem I and its associated light-harvesting complexes in copper-deficient (−Cu) and oxygen-deficient (−O2) Chlamydomonas reinhardtii cells, is localized to the thylakoid membrane. A related protein, Cth1 (Copper target homolog 1), is shown to have a similar but not identical function by genetic suppressor analysis of gain-of-function sct1 (suppressor of copper target 1) strains that are transposon-containing alleles at CTH1. The pattern of Crd1 versus Cth1 accumulation is reciprocal; Crd1 abundance is increased in −Cu or −O2 cells, whereas Cth1 accumulates in copper-sufficient (+Cu), oxygenated cells. This expression pattern is determined by a single trans-acting regulatory locus, CRR1 (COPPER RESPONSE REGULATOR 1), which activates transcription in −Cu cells. In +Cu cells, a 2.1-kb Cth1 mRNA is produced and translated, whereas Crd1 is transcribed only at basal levels, leading to Cth1 accumulation in +Cu cells. In −Cu cells, CRR1 function determines the activation of Crd1 expression and the production of an alternative 3.1-kb Cth1 mRNA that is extended at the 5′ end relative to the 2.1-kb mRNA. Synthesis of the 3.1-kb mRNA, which encodes six small upstream open reading frames that possibly result in poor translation, blocks the downstream promoter through transcriptional occlusion. Fluorescence analysis of wild-type, crd1, and sct1 strains indicates that copper-responsive adjustment of the Cth1:Crd1 ratio results in modification of the interactions between photosystem I and associated light-harvesting complexes. The tightly coordinated CRR1-dependent regulation of isoenzymes Cth1 and Crd1 reinforces the notion that copper plays a specific role in the maintenance of chlorophyll proteins.
INTRODUCTION
Copper is a cofactor in a variety of enzymes and electron transfer catalysts and thus is an essential micronutrient for most organisms. Recent molecular genetic analyses have identified components of the pathways for intercellular and intracellular copper distribution in plants. The Arabidopsis COPPER CHAPERONE (CCH) encodes a homolog of the yeast Atx1p and human HAH1 proteins, which have been shown to function in the delivery of copper from the cytoplasm to secretory compartments (reviewed by Askwith and Kaplan, 1998; Himelblau et al., 1998). CCH is expressed ubiquitously in plant organs, but high levels of expression are observed in phloem sap and in senescing leaves, suggesting that the protein is involved in the recovery of copper from senescing organs and its redistribution to other organs via the vascular transport system (Himelblau et al., 1998; Mira et al., 2001). Much less is known about copper chaperones in other cellular compartments, although BLAST searches (June 2001) have revealed that genes encoding homologs of Cox17p, which delivers copper to the mitochondrion, and of yeast Lys7p and human CCS for the insertion of copper into Cu-Zn superoxide dismutase, are present in the Arabidopsis genome (reviewed by La Fontaine et al., 2002).
The Arabidopsis RESPONSE TO ANTAGONIST 1 (RAN1) gene encodes a homolog of yeast Ccc2p and human Menkes and Wilson disease proteins (Hirayama et al., 1999). These proteins are P-type ATPases that interact with Atx1p/HAH and deliver copper to the multicopper oxidases ceruloplasmin, hephaestin, and Fet3p in a post-Golgi compartment (reviewed by Askwith and Kaplan, 1998). The phenotypes of ran1 mutants place the gene product on a pathway for the delivery of copper to ethylene receptor molecules (Hirayama et al., 1999; Woeste and Kieber, 2000). Other candidate transporters are found as homologs of the cyanobacterial P-type ATPase copper transporters CtaA and PacS, which are implicated in the delivery of copper to plastocyanin in the thylakoid lumen (Kanamaru et al., 1994; Phung et al., 1994; Tottey et al., 2001). Kampfenkel et al. (1995) identified an Arabidopsis cDNA, COPT1, that restores high-affinity copper uptake to ctr1 mutants of Saccharomyces cerevisiae, and they proposed that the gene product might be involved in intercellular or intracellular copper transport. Nevertheless, these putative transporters have not yet been submitted to functional analysis in the plant.
Another underinvestigated issue is the question of how plant cells adapt to conditions in which the supply of copper is insufficient to support the biosynthesis of essential copper proteins. A number of studies of plant copper metabolism have focused on the physiological effects of copper deficiency, which deleteriously affects growth, photosynthesis, and respiration. For instance, the leaves of copper-deficient wheat and sugarbeet plants become bleached and the chloroplast ultrastructure becomes disorganized (Henriques, 1989; Casimiro et al., 1990). Decreased photosynthetic electron transport is attributed to reduced plastocyanin accumulation, and Cu-Zn superoxide dismutase activity also is reduced severely in copper-deficient pea plants (Ayala and Sandmann, 1988). In coniferous trees, copper deficiency causes stem and branch deformation attributable to defective lignification of wood (reviewed by Turvey and Grant, 1990). These studies establish the importance of copper as a nutrient but do not describe the molecular events that occur during copper limitation.
We have used the green alga Chlamydomonas reinhardtii as a model for the study of cellular responses to copper deficiency, because this organism is able to thrive under conditions in which copper is limited by substituting a functionally equivalent heme protein, cytochrome c6, for plastocyanin in the photosynthetic electron transfer pathway (reviewed by Merchant, 1998a). Copper-starved Chlamydomonas cells also induce a highly efficient cell surface copper uptake system (Hill et al., 1996) and increase the expression and activity of a tetrapyrrole biosynthetic enzyme, coprogen oxidase, encoded by the Cpx1 gene (Hill and Merchant, 1995). Genetic analysis of the copper deficiency response in Chlamydomonas revealed a new gene, Copper response defect 1 (Crd1), whose expression is required for the maintenance of photosystem I (PSI) and light-harvesting complex proteins (LHCs) in copper-deficient cells (Moseley et al., 2000). The crd1 phenotype is apparent only in copper- or oxygen-deficient growth conditions, because Crd1 is coregulated with the Cpx1 and Cyc6 (encoding cytochrome c6) genes in response to copper or oxygen deficiency (Moseley et al., 2000; Quinn et al., 2000, 2002).
In this work, we undertake functional and expression analysis of Copper target homolog 1 (Cth1), which was identified in the expressed sequence tag (EST) database as a paralog of Crd1 (Moseley et al., 2000). The reciprocal pattern of Cth1 expression relative to Crd1 is established via a novel metalloregulatory mechanism involving transcriptional occlusion to block the production of a translation-competent Cth1 mRNA. We conclude from suppressor analysis that Cth1 function is similar but not identical to that of Crd1 and that the differences relate to PSI function in Cu limitation. Functional analyses of mutants with altered expression of Crd1 and Cth1 reveal that copper deficiency causes dynamic changes in the interactions between PSI and associated LHCs.
RESULTS
Cth1, Encoding a Crd1-Related Sequence, Displays a Distinct Pattern of Expression
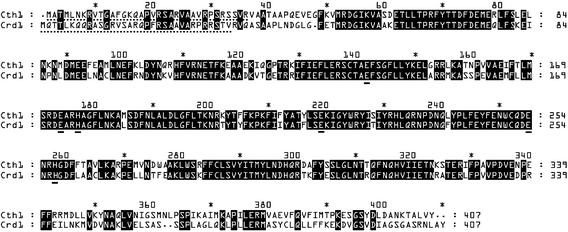
The Chlamydomonas EST database revealed two sets of Crd1-related sequences: one corresponding to the Crd1 gene and a second distinct set corresponding to a paralog, which we named Cth1 (Moseley et al., 2000). The clone from which the longest Cth1 EST was generated was obtained from the Kazusa DNA Research Institute (Kisarazu Chiba, Japan) (Asamizu et al., 1999) and sequenced to reveal a 407–amino acid open reading frame (ORF) that is 66% identical to Crd1, including the putative carboxylate-liganded di-iron binding site and a prospective N-terminal chloroplast-targeting sequence (Figure 1). The degree and extent of sequence relationship suggested that Cth1 might have a function similar to that of Crd1. Crd1 is required for the maintenance of PSI and LHCI in copper-deficient (−Cu) and oxygen-deficient (−O2) Chlamydomonas cultures (Moseley et al., 2000). Like the Cpx1 and Cyc6 genes, the Crd1 gene is activated in −Cu and −O2 cells, which accounts for the conditional phenotype of crd1 mutants (Hill and Merchant, 1995; Moseley et al., 2000; Quinn et al., 2000).
Figure 1.
Cth1 Is Paralogous to Crd1.
ClustalW alignment of Cth1 and Crd1. Identical amino acid residues are shaded in black. Dashed underlines denote the transit sequences predicted by ChloroP 1.1 (Emanuelsson et al., 1999). Putative di-iron center ligands are indicated with solid underlines. The asterisks denote every twentieth position in the alignment. The numbers to the right indicate the position in the alignment at the end of each row.
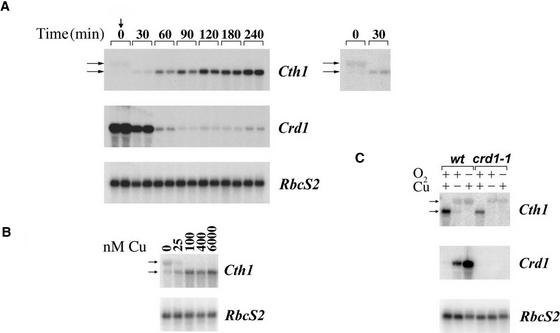
To assess the role of Cth1 in comparison with Crd1, its expression was analyzed with respect to copper nutrition and oxygen supply by RNA gel blot hybridization (Figure 2). Two forms of Cth1 transcripts were observed: a 3.1-kb Cth1 transcript that predominates in RNA from −Cu cells, and a 2.1-kb mRNA, corresponding to the ESTs, that predominates in RNA from copper-sufficient (+Cu) cells (Figure 2A, inset). When copper was added to −Cu cells, the 2.1-kb transcript began to accumulate within 30 min and the 3.1-kb transcript disappeared. The abundance of the 2.1-kb transcript increased up to 10-fold and reached a new steady state representing copper-replete cells after 240 min. During the same period, Crd1 mRNA accumulation was reduced 20-fold, as in the other copper deficiency–induced genes, Cpx1 and Cyc6 (Hill et al., 1991; Hill and Merchant, 1995). Furthermore, expression of the 3.1-kb Cth1 transcript was inhibited fully in cells grown in medium containing 100 μM copper, demonstrating that Cth1 expression is controlled by the same range of copper concentrations as other copper-responsive genes (Figure 2B) (Merchant et al., 1991; Hill and Merchant, 1995; Moseley et al., 2000). The 3.1-kb Cth1 transcript also was induced in copper-replete cells if they were subjected to oxygen deficiency, but in aerated copper-replete cells only the 2.1-kb mRNA was evident (Figure 2C). Cth1, therefore, is another copper-regulated gene of Chlamydomonas. In oxygenated, +Cu cells, a 2.1-kb mRNA accumulated, whereas in −Cu or −O2 conditions, a 3.1-kb mRNA was induced at the expense of the 2.1-kb form.
Figure 2.
Cth1 and Crd1 Are Expressed Reciprocally in Response to Copper and Oxygen Deficiency.
(A) RNA gel blot analysis of a time course of Cth1 and Crd1 expression during the transition from copper deficiency to copper sufficiency. The vertical arrow indicates the addition of 6 μM CuSO4 to –Cu cultures. Duplicate RNA samples were prepared at each time point. The 0-min time point was sampled immediately after the addition of copper to the culture. Total RNA (8.1 μg) was loaded in each lane. RbcS2 accumulation is shown as a loading control. Exposure times were 39.5 hr for Cth1 and Crd1 and 1 hr for RbcS2. The 3.1- and 2.1-kb Cth1 transcripts are indicated with horizontal arrows. The inset shows Cth1 expression at the 0- and 30-min time points at higher contrast to reveal the less abundant transcripts.
(B) RNA gel blot analysis of Cth1 mRNA accumulation in crd1-1 cultures grown with 0, 25, 100, 400, and 6000 nM CuSO4 supplemented in the medium.
(C) Cth1 versus Crd1 expression as a function of copper and oxygen availability in wild-type (wt) and crd1-1 strains. +Cu, 6 μM supplemented CuSO4; –Cu, no supplemented CuSO4; +O2, aerated culture (100% air); –O2, unaerated culture (0% air).
The conditional chlorotic phenotype of crd1 mutants in either −Cu or −O2 growth conditions was attributed to the absence of the Crd1 gene product under these conditions (Figure 2C). Because the 3.1-kb Cth1 mRNA was present under these conditions, we conclude that this form of the RNA does not encode a protein that can substitute functionally for Crd1. On the other hand, because mutations at the CRD1 locus had no apparent effect in +Cu, oxygenated cells, we conclude that the 2.1-kb Cth1 transcript encodes a polypeptide that can functionally replace Crd1.
The 3.1-kb mRNA Is Extended at the 5′ End Relative to the 2.1-kb mRNA
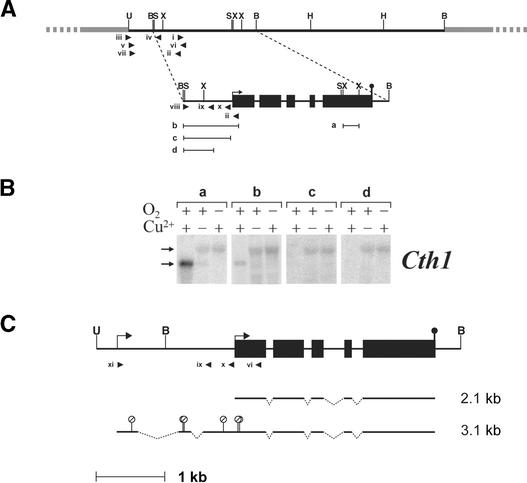
Because the two forms of mRNA appeared to be functionally different, we sought to understand the physical difference between them and the mechanism that gives rise to these species. A genomic DNA fragment encoding Cth1 was isolated from a λEMBL3 library (Goldschmidt-Clermont and Rahire, 1986) and a region of 5249 bp including the Cth1 coding region, 2 kb upstream of the initiator Met and 375 bp downstream of the polyadenylation site, was sequenced (scheme in Figure 3A). Probes corresponding to regions upstream and downstream of the Cth1 ORF were used in RNA gel blot analysis to determine whether the 3.1-kb mRNA was extended at the 5′ versus the 3′ end relative to the 2.1-kb form (Figure 3B). Probes a and b hybridized to both the 2.1- and 3.1-kb Cth1 transcripts, whereas probes c and d hybridized solely to the 3.1-kb Cth1 transcript, leading to the conclusion that the 3.1-kb mRNA is extended at the 5′ end relative to the 2.1-kb species.
Figure 3.
Cth1 Structural Gene and Transcripts.
(A) Cth1 λEMBL3 (λ30-1) and plasmid (pCth1-2) clones. The λEMBL3 arms are represented by gray lines (not to scale), whereas solid black lines denote Chlamydomonas genomic DNA. The 5′ end of the longest cDNA representing the 2.1-kb +Cu Cth1 mRNA is illustrated with an arrow. Black rectangles represent exons encoding the Cth1 protein. The mRNA polyadenylation site is represented by a closed circle. Roman numerals indicate primers (see Table 1). Restriction sites that were verified by digestion are shown as vertical lines: B, BamHI; H, HindIII; S, SalI; U, Sau3AI; X, XhoI. a, b, c, and d represent DNA fragments used as probes in (B). Arrowheads illustrate the positions of oligonucleotide primers used to amplify regions of the 5′ end of Cth1.
(B) RNA gel blot analysis of the Cth1 transcripts expressed in the indicated growth conditions. +Cu, 6 μM supplemented CuSO4; –Cu, no supplemented CuSO4; +O2, aerated culture (100% air); –O2, unaerated culture (0% air). Probes a, b, c, and d are illustrated in (A). Horizontal arrows indicate the positions of migration of the 3.1- and 2.1-kb Cth1 transcripts.
(C) Transcript models of the 2.1- and 3.1-kb Cth1 mRNAs deduced from the sequences of cDNA clone CM079h12 (Asamizu et al., 1999) and from Cth1-specific amplification products from a –Cu cDNA library (see Methods). Open circles with diagonal bars denote the positions of stop codons within the first three exons of the 3.1-kb Cth1 transcript. Arrow, black rectangles, closed circle, roman numerals, B, and U are the same as (A). The broken line indentations indicate positions of sequences that are removed by splicing in the corresponding mRNA.
The sequence corresponding to the extension was amplified from a library of cDNAs prepared from −Cu cells (Merchant and Bogorad, 1987a) using Cth1-specific primers (see Methods). The longest product, ∼9 × 102 bp, accounts almost completely for the size difference between the two Cth1 mRNAs, suggesting that the amplification product is close to representing a full-length cDNA. Sequence analysis of the amplification products suggests a transcript model in which a 3.1-kb mRNA is generated in −Cu cells by the use of a different promoter, followed by splicing of seven exons (Figure 3C; see also Figure 9). When the rest of the transcript (the downstream 3′ portion corresponding to the 2.1-kb mRNA) was analyzed by reverse transcriptase–mediated polymerase chain reaction (PCR) of RNA from −Cu or −O2 cells, the products were exactly the same size as for the +Cu 2.1-kb mRNA from +Cu, aerated cells. Therefore, we conclude that the 3.1-kb RNA differs from the 2.1-kb mRNA by virtue of a 962-bp extension at the 5′ end. Conceptual translation of the 3.1-kb mRNA revealed that the Cth1 ORF is preceded by a 1059-nucleotide 5′ untranslated region (UTR). This 1059-nucleotide sequence contains multiple short ORFs (Met followed by a termination codon), which could prevent the translation of the Cth1 polypeptide (Figure 3C). We hypothesized that the 3.1-kb mRNA would not produce the Cth1 polypeptide. In this case, Crd1 would be induced in −Cu cells by the increased abundance of Crd1 mRNAs, whereas Cth1 would be more highly expressed in +Cu cells, which produce the translatable 2.1-kb Cth1 mRNA.
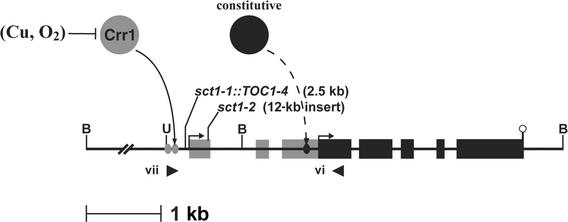
Figure 9.
Inserted Elements in CTH1 Prevent Normal Regulation of the Gene.
Putative copper-responsive promoter elements with the core sequence GTAC (gray ovals) in the 5′ flanking region of CTH1 stimulate initiation of the 3.1-kb transcript in –Cu or –O2 cells. Insertion of the 2.5-kb TOC1-4 element in sct1-1 strains between the transcription start site and the copper-responsive promoter elements disrupts transcriptional activation at the upstream initiation site, allowing constitutive expression of the 2.1-kb transcript from the downstream initiation site. sct1-2 strains contain a 12-kb insert in the first exon, preventing normal splicing of the 3.1-kb Cth1 mRNA and also blocking transcriptional interference between the –Cu/–O2 and +Cu transcription start sites. Candidate transcription initiation sites are indicated by arrows. Transcribed sequences exclusive to the 3.1-kb Cth1 mRNA are represented by gray rectangles, and black rectangles illustrate exons present in the 2.1-kb Cth1 transcript. An open circle designates the polyadenylation site. A Crr1-dependent activator is represented by a gray circle, whereas the black circle and the black oval denote a constitutive activator of transcription and a constitutive promoter, respectively. Arrowheads illustrate the primers used to amplify a 2.4-kb Cth1 PCR product, which provided the template for the probe used for DNA gel blot analysis (see Figure 7). B, BamHI; U, Sau3AI. Roman numerals indicate primers (see Table 1).
Reciprocal Copper-Responsive Accumulation of Crd1 Versus Cth1
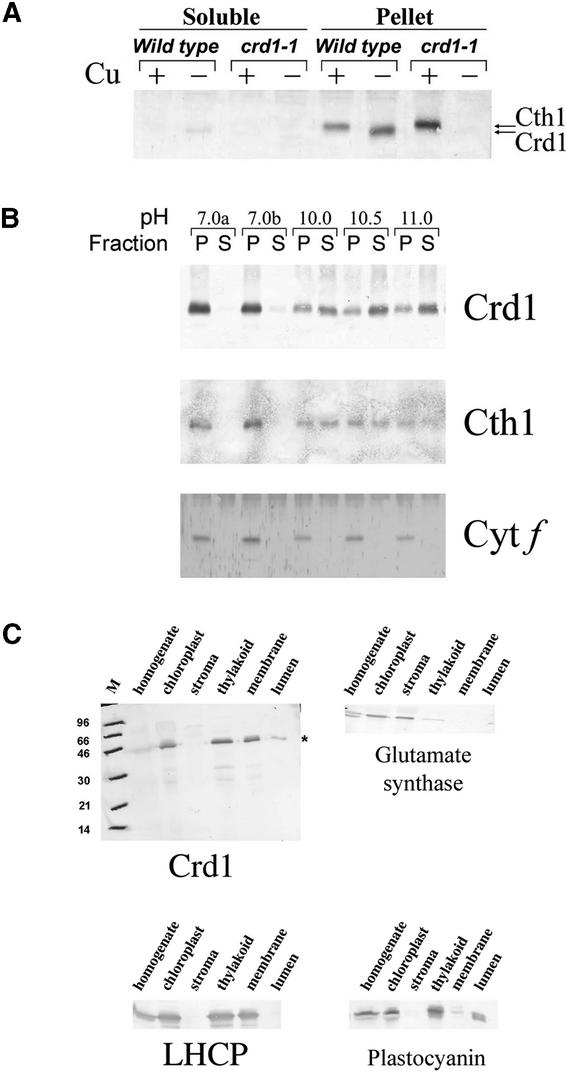
The abundance of Crd1 and Cth1 was monitored by immunoblot analysis. The antiserum (see Methods) recognizes both polypeptides, but they can be distinguished by a slight mobility difference (apparent mass of 40.7 versus 39.8 kD) and Crd1 can be defined unambiguously as the faster migrating species (corresponding to 39.8 kD) because it is absent in the crd1 strains. Cth1 and Crd1 are found in the pellet fraction and are concluded to be membrane associated (Figure 4A). Comparison of extracts from +Cu versus −Cu cells showed that, as hypothesized, Crd1 accumulated primarily in −Cu cells, whereas Cth1 accumulated primarily in +Cu cells. Because sequence analysis suggested that the proteins were highly hydrophilic, the prevalence of the proteins in the pellet fractions was unexpected. Therefore, we determined whether they might be associated peripherally with the membrane by carbonate extraction (Figure 4B). Both proteins showed parallel release as the pH of the wash was increased. In some experiments, the proteins were released almost quantitatively from the membrane at pH ∼10.5. In the same situation, a hydrophilic protein that is anchored to the membrane, cytochrome f, remained completely pellet associated. Given that crd1 mutations affect PSI and LHCI accumulation in −Cu cells, we also determined whether the abundance of Crd1 or Cth1 was altered in PSI-deficient mutants (data not shown). The regulation of Cth1 versus Crd1 remains copper responsive, and both proteins accumulate in several different PSI-deficient mutants, such as psaBΔ (Fischer et al., 1996), ycf4::aadA (Boudreau et al., 1997), and F15 (Stampacchia et al., 1997). In fact, Crd1 function is required even in a PSI-deficient strain because copper deficiency–induced chlorosis was observed in a crd1 ycf4::aadA double mutant (data not shown).
Figure 4.
Localization and Expression of Cth1 and Crd1.
(A) Immunodetection of Cth1 and Crd1 in soluble and pellet fractions from wild-type and crd1-1 cells grown with (+; 6 μM CuSO4) or without (−; 0 μM CuSO4) supplemental copper.
(B) Comparable alkaline release of Cth1 and Crd1 from pellet fractions of wild-type cells. Cytochrome (Cyt) f is used as a marker for the behavior of integral membrane proteins under these conditions. P, pellet; S, soluble.
(C) Immunolocalization of Crd1 in pea chloroplast fractions. Glutamate synthase, LHCP, and plastocyanin also were immunolocalized as markers for stroma, thylakoid membrane, and thylakoid lumen, respectively. The lanes marked “thylakoid” represent thylakoid membranes with intact lumen. These were fractionated to produce “membrane” versus “lumen” components, as described in Methods. M, molecular mass (in kD).
We had proposed previously that Crd1 should be localized to the plastid on the basis that (1) the crd1 phenotype affects the post-translational accumulation of chloroplast-localized complexes (PSI and LHCs), (2) Crd1 homologs are encoded in the plastid genomes of chromophytic algae, and (3) the algal and vascular plant Crd1s have N-terminal transit sequence–like extensions relative to bacterial homologs (Moseley et al., 2000). The antibodies were used to confirm the localization of Crd1 to the plastid. Pea chloroplasts were isolated and fractionated, and fractions were analyzed for the presence of marker proteins (glutamate synthase for stroma, LHC for thylakoid membrane, and plastocyanin for lumen) or Crd1. Crd1 cofractionated with markers for the thylakoid membrane (Figure 4C). This finding is consistent with its function in the maintenance of PSI and LHCs.
CRR1-Dependent Expression of Crd1 and Cth1
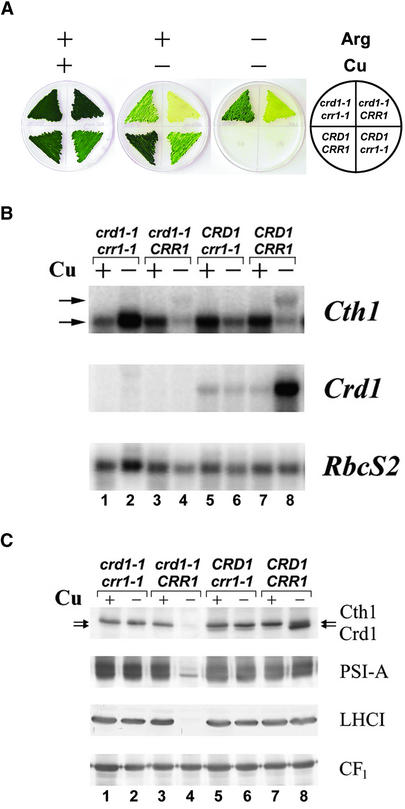
Previously, we had characterized the expression of genes that are induced in copper deficiency and, based on a number of criteria, proposed a model in which the expression of Crd1, Cpx1, and Cyc6 requires copper-responsive elements and the product of the COPPER RESPONSE REGULATOR 1 (CRR1) locus (Quinn and Merchant, 1995; Quinn et al., 1999, 2000, 2002; Moseley et al., 2000; M. Eriksson, unpublished data). The CRR1 locus is defined by strains crr1-1 and crr1-2, in which all known responses to copper deficiency are blocked, including the transcriptional activation of Cpx1 and Cyc6 (M. Eriksson, unpublished data). Because the 3.1-kb form of Cth1 mRNA accumulates in copper and oxygen deficiency (Figure 2), like Cyc6 and Cpx1 mRNAs, it is reasonable to hypothesize that production of the 3.1-kb form of Cth1 mRNA might be CRR1 dependent. To test this possibility, six tetratype tetrads generated from a cross between crr1-1 and crd1-1::ARG7 were analyzed for the crd1 phenotype. Representative analyses are shown in Figure 5. The 3.1-kb Cth1 mRNA was not produced in any strain carrying the crr1-1 allele (Figure 5B, lanes 2 and 6 compared with lane 8); instead, only the 2.1-kb form accumulated. The corresponding immunoblots show high accumulation of Cth1 in both +Cu and −Cu crr1 strains compared with wild-type (CRR1) strains, whereas Cth1 abundance was higher in +Cu relative to −Cu cells (Figure 5C). We conclude that Cth1 is another target of the CRR1 pathway. The resulting constitutive (i.e., copper-independent) accumulation of the polypeptide also is consistent with the regulatory model for Cth1 translation solely from the 2.1-kb mRNA (Figure 3C).
Figure 5.
The CRR1 Locus Controls Both Crd1 and Cth1 Expression.
(A) Representative tetratype tetrad from the cross of an ARG7-tagged crd1 mutant (arg7crd1-1::ARG7CRR1) with an arg7crr1-1 strain (arg7CRD1crr1-1). The genotype of the strain in each sector is indicated at right. The strains were grown for 2 weeks at 18°C on TAP medium with (+) or without (–) 6 μM supplemented CuSO4 and with (+) or without (–) 500 μg/mL Arg.
(B) RNA gel blot analysis of Cth1 and Crd1 expression in –Cu and +Cu cells of the tetrad described in (A). The horizontal arrows indicate the positions of the 3.1- and 2.1-kb Cth1 transcripts. RbcS2 expression was monitored to control for equal loading.
(C) Immunoblot analysis of Cth1, Crd1, PSI-A, and LHCI accumulation in –Cu or +Cu cells. Horizontal arrows indicate the migration of Cth1 and Crd1. Equal numbers of cells were loaded in each lane. CF1 (coupling factor) abundance was used as a control for equal loading.
When the spores were analyzed for the copper-conditional chlorotic phenotype of crd1-1::ARG7 (Moseley et al., 2000), we noted that the crd1 phenotype did not penetrate in the crd1-1::ARG7 crr1-1 double mutant (Figures 5A and 5C, compare lanes 2 and 4). The double mutant displayed only the characteristic crr1 slow growth phenotype in −Cu medium (Figure 5A). Specifically, the double mutant was green in −Cu medium relative to the crd1 single mutant, which is consistent with normal accumulation of PSI and LHCI in the double mutant relative to the crd1 single mutant. We attribute the crr1-dependent “rescue” of the crd1 phenotype to the misexpression of Cth1 in −Cu cells of crr1 mutants. This result indicates that Cth1 and Crd1 have similar or overlapping functions.
Cth1 Misexpression Can Suppress the crd1 Phenotype
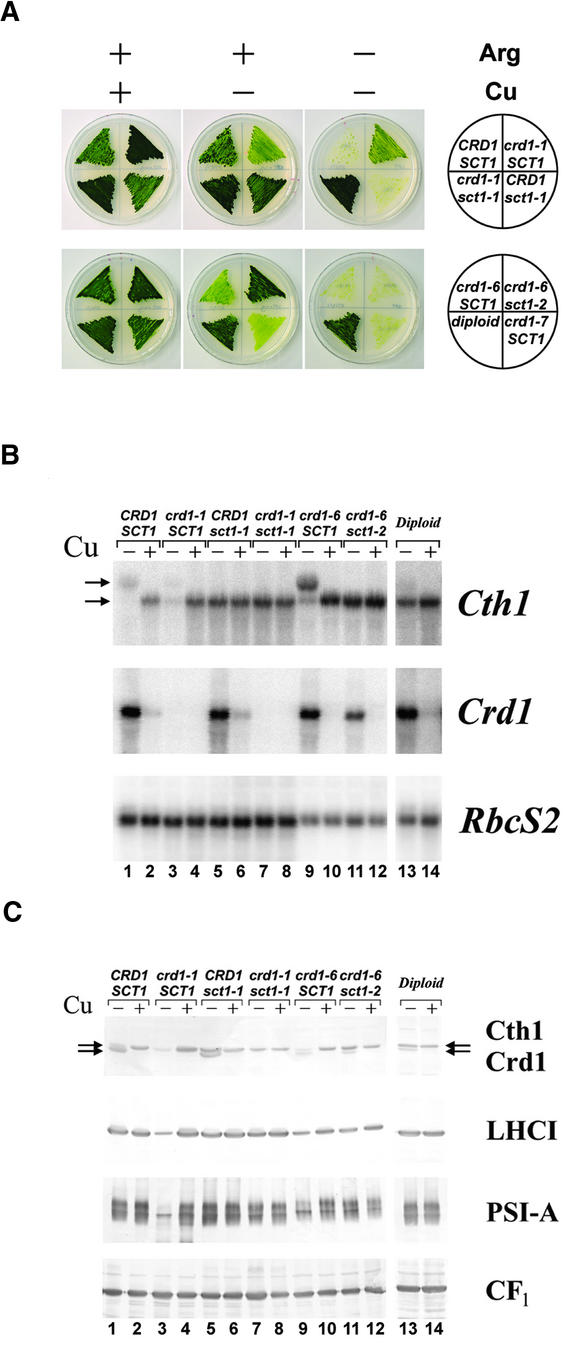
When crd1 mutant strains are maintained in −Cu medium, the cultures frequently accumulate spontaneous suppressors that no longer display the crd1 phenotype. Two independent suppressor of copper target 1 (sct1) strains, sct1-1 and sct1-2, were isolated from cultures of crd1-1 and crd1-6 (derived originally from different genetic backgrounds), respectively. Genetic analysis showed that sct1-1 and sct1-2 segregate independently of the CRD1 locus (Figure 6A and data not shown), indicating that they do not represent true revertants. No progeny showing the crd1 phenotype resulted from a cross of crd1-1 sct1-1 to crd1-6 sct1-2, demonstrating that these second-site suppressor mutations are not crd1 allele specific and revealing that sct1-1 and sct1-2 are either allelic or tightly linked (data not shown). To understand the mechanism of suppression, we analyzed vegetative diploids homozygous for crd1 but heterozygous for sct1-2 for the accumulation of chlorophyll and chlorophyll proteins (Figures 6A and 6C) and concluded that a single copy of sct1-2 in a homozygous crd1 diploid is sufficient to suppress the crd1 phenotype. This result is consistent with a model in which sct1 mutations confer a gain of function that enables cells to bypass the requirement for Crd1 in −Cu conditions.
Figure 6.
Second-Site Suppressors Rescue the crd1 Phenotype.
(A) The top row shows the segregation of Arg auxotrophy, the crd1 phenotype, and an sct1 allele in a representative tetratype tetrad from the cross of a suppressed crd1-1 strain (arg7crd1-1::ARG7sct1-1) to an Arg auxotrophic strain (arg7CRD1SCT1). The bottom row compares the phenotypes of a strain harboring an independently generated sct1 allele (arg2crd1-6sct1-2), the parental strain (arg2crd1-6SCT1), another crd1 strain (arg7crd1-7SCT1), and a vegetative diploid that is homozygous for crd1 and heterozygous for the sct1-2 allele (arg2crd1-6sct1-2/arg7crd1-7SCT1). The illustration at right indicates the genotype of the strain in each sector. Strains were grown at 25°C on solid TAP medium with 6 μM CuSO4 (+) and transferred once (top) or twice (bottom) to medium without added copper (–) and with (+) or without (–) 500 μg/mL Arg, as indicated.
(B) RNA gel blot analysis of Cth1 and Crd1 expression in the strains shown in (A) with (+) or without (–) copper supplementation. The horizontal arrows indicate the position of the 3.1- and 2.1-kb Cth1 transcripts.
(C) Immunoblot analysis of Cth1, Crd1, LHCI, PSI-A, and CF1 accumulation in the same strains. Horizontal arrows indicate the migration of Cth1 and Crd1.
Because misexpression of Cth1 in crr1 mutants can suppress the crd1 phenotype (Figure 5), we determined whether sct1 mutations function through altered expression of Cth1. RNA gel blot analysis showed that strains harboring the sct1-1 or sct1-2 alleles do not accumulate the 3.1-kb Cth1 mRNA; rather, the 2.1-kb Cth1 message is produced in both +Cu and −Cu cells (Figure 6B, compare lanes 5, 7, 11, and 13, corresponding to sct1 strains, with lanes 1 and 9, corresponding to SCT1 strains), a situation similar to that observed for crr1 strains. The sct1-1 strain also does not induce the 3.1-kb transcript in response to hypoxia (data not shown), indicating that production of this transcript by any mechanism is blocked. Immunoblot analysis indicates that the Cth1 protein is overexpressed in −Cu cells of sct1 strains relative to SCT1 strains (Figure 6C), suggesting that the spontaneous suppressors, which were chosen phenotypically for chlorophyll accumulation, result from Cth1 function in −Cu conditions. It is not likely that sct1 represents a crr1 allele or a regulatory component of the copper-responsive signal transduction pathway because sct1 strains adapt normally to copper and oxygen deficiency in all other respects. For instance, Crd1 expression is not affected in sct1 mutants (Figure 6B, lanes 5 and 6).
Constitutive Expression of the 2.1-kb Cth1 mRNA in sct1 Strains Results from Insertions of Transposable Elements
The altered pattern of Cth1 expression in sct1 strains could result either from a mutation in Cth1 affecting the use of the putative upstream promoter responsible for the production of the 3.1-kb RNA or from a mutation in a trans-acting factor affecting the pattern of transcription of the Cth1 gene. DNA gel blot analysis revealed restriction fragment length polymorphisms in the 5′ region of Cth1 in sct1-1 and sct1-2 strains compared with wild type (Figure 7, lanes 3, 4, 6, and 8 compared with lanes 1, 2, and 5), which is consistent with the former hypothesis. We deduced that the sct1-1 and sct1-2 strains contain ∼2.5- and ∼12-kb inserts, respectively, at the CTH1 locus. When sct1-1 was backcrossed to the wild-type strain, the presence of the insert (tested by amplification of the 5′ region of Cth1) and the absence of a fragment corresponding to the wild-type sequence cosegregated with the sct1-1 phenotype (six complete tetrads analyzed; probability of independent assortment < 5%). A revertant of sct1-2, called rsc1 (revertant of suppressor of copper target 1), was isolated by screening mutagenized cells for restoration of the crd1 phenotype. Molecular characterization of rsc1 indicated that normal copper-responsive expression of Cth1 was restored in the rsc1 strain (data not shown) and that this was correlated with the loss of the ∼12-kb insert at the 5′ end of Cth1 (Figure 7, lane 7 compared with lane 6). Together, these results confirm that sct1-1 and sct1-2 are alleles of Cth1 and suggest that large insertions in the 5′ end of Cth1 allow copper-independent, constitutive production of the 2.1-kb mRNA.
Figure 7.
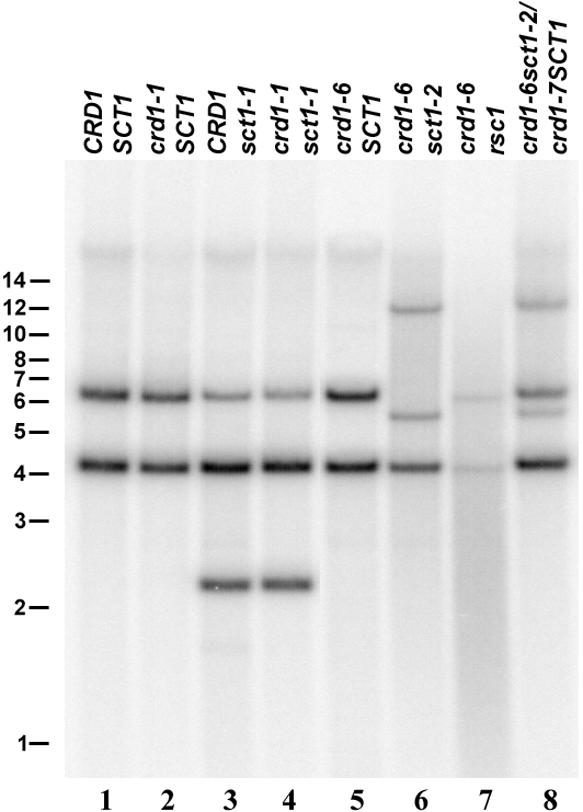
Restriction Fragment Length Polymorphisms at the CTH1 Locus in sct1 Strains.
DNA gel blot analysis of the CTH1 locus in wild-type (CRD1SCT1) and mutant strains containing the crd1-1, sct1-1, crd1-6, and sct1-2 alleles. Genomic DNA (3 μg) was digested with BamHI and probed with a 2.4-kb PCR product hybridizing to the 5′ end of Cth1 (see Methods and Figure 9). The ∼6.5-kb hybridizing bands in lanes 3 and 4 represent fusions between the inserted element and the wild-type sequence that are similar in size to the wild-type fragment. The positions of molecular mass markers (in kb) are shown at left.
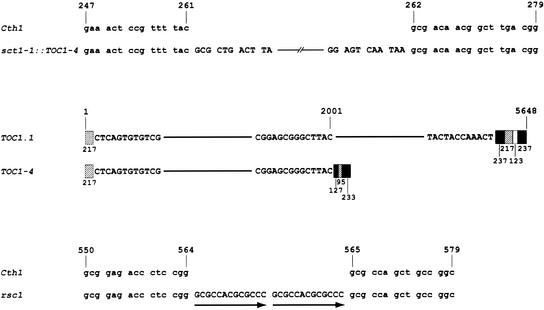
The inserted sequence in sct1-1 was identified by partial sequence analysis of an ∼3.5-kb PCR product covering the insert plus flanking DNA (see Methods). The sequence of the junction region between Cth1 and the inserted DNA revealed that the inserted sequence is very similar to an element called TOC1.1 (Figure 8) (Day et al., 1988). Nevertheless, the 2.5-kb TOC1.1-like element in sct1-1, now called TOC1-4, is smaller than TOC1.1, which is 5.7 kb. A characteristic direct repeat region at the 3′ end of TOC1.1, consisting of several blocks of repeated sequences, is truncated in TOC1-4. Each block is truncated at its 5′ boundary relative to the corresponding block in TOC1.1 (with +1 defining the 5′ border of the element). For sct1-2, we sequenced the relevant region of the Cth1 gene in strain rsc1 to test the notion that rsc1 resulted from deletion of the 12-kb insert in sct1-2. Indeed, we found two 13-bp direct repeats in the Cth1 gene at a position corresponding to the first exon of the 3.1-kb transcript. This 26-bp “footprint” is highly suggestive of excision of a transposon from this position (Figure 8). The positions of the elements in sct1 strains support the model that downregulation of the 2.1-kb mRNA in −Cu cells results from transcriptional occlusion (Figure 9; see Discussion).
Figure 8.
sct1 Alleles Contain Transposon Insertions at the CTH1 Locus.
The first pair of sequences show the junctions between Cth1 and TOC1-4 in sct1-1. Cth1 sequences are shown in lowercase, and the 11 bases at each terminus of TOC1-4 are shown in uppercase. The corresponding wild-type Cth1 sequence is shown on the first line. The second alignment compares the arrangement of long terminal repeat sequences in TOC1.1 (Day et al., 1988) and TOC1-4. Repeated sequences are represented by shaded boxes, with the length of each repeat indicated below. The repeats at the 3′ terminus of TOC1-4 are all truncated at their 5′ ends relative to the repeats in TOC1.1. The third pair of sequences aligns the Cth1 sequence from the rsc1 strain and the wild-type sequence. The rsc1 allele contains a 26-bp insertion composed of two 13-bp direct repeats at position 564 of the Cth1 gene. The direct repeats are shown in uppercase and are illustrated with arrows.
Cth1 Cannot Substitute Fully for All Functions of Crd1
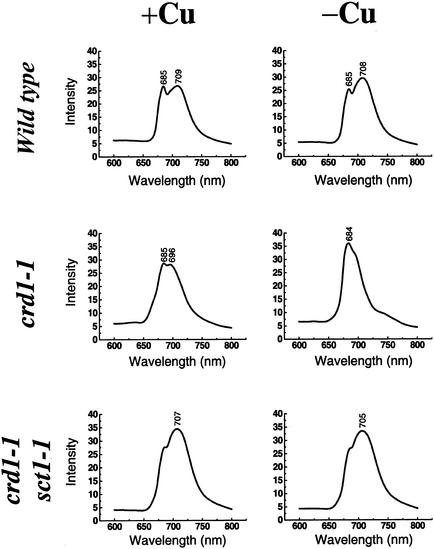
The conditional phenotype of crd1 strains (i.e., chlorosis only in copper deficiency) is consistent with the replacement of Crd1 by Cth1 in +Cu conditions (Figure 2). The suppression of the chlorotic phenotype of crd1 strains through the misexpression of Cth1 also indicates that Cth1 can functionally replace Crd1 (Figures 5 and 6). However, the fact that the two paralogous genes are regulated reciprocally by the CRR1 pathway suggested that there might be subtle functional differences. We also wondered whether the basal expression of Crd1 (Figure 2A) might indicate a function for Crd1 in +Cu cells as well. Because immunoblot analysis of chlorophyll proteins did not reveal a phenotype for crd1 mutants in +Cu conditions, we probed for a photosynthetic function of chlorophyll proteins (i.e., interactions between reaction centers and their antenna) by collecting low-temperature (77K) fluorescence emission spectra of thylakoid membranes (Figure 10).
Figure 10.
Low-Temperature (77K) Fluorescence of Strains Expressing Cth1 versus Crd1.
77K fluorescence emission spectra of thylakoid membranes prepared from wild-type, crd1-1, and crd1-1sct1-1 cells grown in the presence (+Cu) or absence (–Cu) of 6 μM copper. The excitation wavelength was 435 nm. Fluorescence emission is shown in arbitrary units. The peaks are labeled with the wavelength of the maximum.
The fluorescence emission spectrum from copper-replete wild-type cells shows characteristic peaks at 685 and 709 nm, resulting from fluorescence from LHCII attached to PSII and from PSI and LHCI, respectively (Wollman and Bennoun, 1982; Wollman and Delepelaire, 1984). Surprisingly, the spectrum was changed in −Cu cells: the intensity of the peak at 708 nm was increased relative to the 685-nm peak in −Cu wild-type cells. One interpretation of the spectrum is that this alteration reflects a state transition in response to excess excitation (Wollman and Delepelaire, 1984), but room temperature fluorescence measurements indicate that −Cu wild-type cells are in state I (J.-D. Rochaix, personal communication). An alternative interpretation is that the increase in fluorescence intensity of the 708-nm peak reflects partial disconnection of the LHCI antennae from the PSI reaction center or a modification in its interaction, suggesting a physical difference in photosynthetic complexes of +Cu versus −Cu cells. For the crd1 mutant, the PSI/LHCI peak was absent (or greatly reduced) in membranes isolated from −Cu cells. This was as expected, but surprisingly, the spectrum of membranes from +Cu crd1 cells was consistently different from that of +Cu wild-type cells. The PSI/LHCI peak was blue-shifted by 13 nm, suggesting that the connection between the reaction center and the antennae is abnormal, even though wild-type amounts of the complex-specific polypeptides accumulated. This indicates a function for Crd1 in +Cu cells.
The definition of a phenotype of the crd1 mutant in +Cu cells presented an opportunity to determine whether Cth1 can fully replace Crd1. Thylakoid membranes were prepared from +Cu or −Cu cultures of a suppressed crd1-1 strain (crd1-1 sct1-1) that expressed Cth1 in place of Crd1, and fluorescence spectra were collected. Comparison of the spectra from the +Cu crd1-1 sct1-1 versus wild-type or a crd1 mutant showed that Crd1 function cannot be bypassed fully. The PSI/LHCI fluorescence peak (maximum at 707 nm) from crd1-1 sct1-1 cells had a much higher intensity relative to wild type (Figure 10). On this basis, we propose that Crd1 and Cth1 have similar but not identical roles in the maintenance and function of PSI and antenna proteins.
DISCUSSION
Cth1 Function Overlaps with That of Crd1
Cth1 was identified in the EST database as a paralog of Crd1 (Figure 1). Here we show that Cth1 and Crd1 show complementary expression patterns with respect to copper and oxygen availability so that one protein, Crd1, is induced in −Cu cells and the other protein, Cth1, is upregulated in +Cu cells (Figures 2 and 3). A role for Crd1 in the adaptation of the photosynthetic apparatus to copper deficiency had been suggested in previous work based on the conditional PSI- and LHCI-deficient phenotype of crd1 mutants (Moseley et al., 2000). Although misexpression of Cth1 can suppress this phenotype (Figures 5 and 6), suggesting that Cth1 has a function similar to Crd1, suppressed strains show subtle differences in the function of the peripheral antenna-PSI reaction center complexes (Figure 10). Differences also are evident in crd1 mutants versus wild-type strains maintained in copper-replete conditions when Cth1 function is normal (Figure 10). On this basis, we propose that Cth1 and Crd1 have nonidentical but overlapping functions.
Based on the pleiotropic post-translational effect on the maintenance of PSI and LHCs, the presence of a putative transit sequence, and the occurrence of Crd1 homologs in some plastid genomes, we had suggested that Crd1 was localized to the plastid (Moseley et al., 2000). The restricted pattern of expression of the Crd1 homolog in plants (Zheng et al., 1998) suggested further that its gene product functioned in the chloroplast, and this was verified experimentally (Figure 4C). Although its association with the membrane was unexpected, the results of the alkaline release experiments and the fractionation studies are not inconsistent with the model proposed recently for another di-iron protein, Coq7p, which is modeled as an interfacial integral membrane protein (Stenmark et al., 2001). A recent study of a Crd1/Cth1 homolog in Rubrivivax gelatinosus implicates it in the cyclase reaction in which Mg-protoporphyrin IX monomethyl ester is converted to divinyl protochlorophyllide a (Pinta et al., 2002). Biochemical studies of the eukaryotic cyclase reaction suggest a multicomponent thylakoid enzyme that is fully membrane associated in Chlamydomonas preparations but composed of membrane and soluble components in preparations from cucumber cotyledons (reviewed by Beale, 1999). The fractionation studies in this work suggest that Crd1 may correspond to the soluble component in cucumber cotyledons that remains membrane associated in Chlamydomonas.
Although the replacement of plastocyanin in −Cu cells by cytochrome c6 is well established (reviewed by Merchant, 1998b), other adaptations of the photosynthetic apparatus are not well described. Even in wild-type cells, copper deficiency alters the interaction between PSI and its peripheral antenna (Figure 10). The increase in fluorescence from PSI/LHCI in −Cu relative to copper-replete cells is suggestive of a reduction in energy transfer from the peripheral antennae to the reaction center. Perhaps structural alterations are necessary in the PSI/LHCI complex to reduce the rate of oxidation of the reaction center to compensate for a reduced rate of electron transfer by cytochrome c6 relative to plastocyanin, which is the preferred electron transfer catalyst in vivo. The finding that Crd1 isoforms are required under different copper nutritional states is suggestive of a role for copper in pathways leading to and determining PSI function. These results reinforce the notion of a dynamic photosynthetic apparatus responding to environmental and nutritional cues.
A survey of the presence of Crd1 homologs in the genome databases reveals single-copy sequences in some genomes, such as Arabidopsis, and multiple copies in others, such as Anabaena sp 7120 (three copies; http://www.kazusa.or.jp/cyano/anabaena/),), Synechocystis sp 6803 (two copies; for accession numbers, see Methods), Nostoc punctiforme (two copies; http://www.jgi.doe.gov/JGI_microbial/html/index.html), and Synechococcus sp 7002 (two copies; D. Bryant, personal communication). Although the pattern of expression of the Crd1 homologs has not been addressed for these cyanobacterial species, it is interesting that many cyanobacteria, like some of the green algae, have a well-defined system for the adaptation of the photosynthetic apparatus to copper deficiency. The presence of multiple Crd1-like genes may reflect a specialization of function for the adaptation of the photosynthetic apparatus.
CRR1 Determines Copper-Responsive Cth1 Expression through Transcriptional Occlusion, Preventing Production of the 2.1-kb mRNA
In previous work, we identified several processes that are turned on during acclimation to copper deficiency; specifically, expression of Cyc6, Cpx1, and Crd1, degradation of plastocyanin, and copper assimilation (reviewed by Merchant, 1998b; Moseley et al., 2000). None of these processes can be activated in crr1 mutants (M. Eriksson, unpublished data). In this work, we identify another copper-regulated gene, Cth1 (Figure 2). Its gene product, Cth1, accumulated in +Cu cells, in which Crd1 abundance was low. In −Cu cells, Cth1 abundance was low but Crd1 accumulated (Figures 4 to 6). This pattern of expression is the opposite of the pattern for the other CRR1 targets, whose gene products increase substantially in abundance in copper deficiency. How can one rationalize this observation at the molecular level?
The Cth1 gene produces two transcripts: a 2.1-kb mRNA that is responsible for production of the Cth1 polypeptide in +Cu cells, and a longer 3.1-kb mRNA whose accumulation in −Cu cells is dependent on a functional CRR1 locus (Figures 2 and 5), presumably through the action of copper response elements (Figure 9). Sequence analysis of amplified cDNAs corresponding to the ∼1-kb 5′ extension on the 3.1-kb mRNA revealed multiple short ORFs upstream of the pre-Cth1 ORF (Figure 3C) that may prevent the ribosomes from reaching the downstream pre-Cth1 ORF. A similar mechanism has been reported to mediate the translational control of yeast GCN4 (reviewed by Hinnebusch, 1997). Therefore, the 3.1-kb mRNA may not be functional as a template for pre-Cth1 synthesis. An alternative model, in which the 3.1-kb mRNA is less available for translation because of reduced abundance (resulting from decreased half-life relative to the 2.1-kb form), cannot be excluded completely. Nevertheless, we do not favor this suggestion, because in experiments in which we analyzed RNA and protein from the same sample, we noted that Cth1 protein abundance correlated with the abundance of the 2.1-kb mRNA rather than with the sum of the 2.1- and 3.1-kb mRNAs, implying that the 3.1-kb form is not used effectively as a translation template. Transcriptional activity in −Cu cells at the CRR1-dependent promoter for this 3.1-kb mRNA is expected to prevent the use of the downstream promoter responsible for the 2.1-kb RNA as a result of transcriptional occlusion (Cullen et al., 1984). Hence, Cth1 is not produced in −Cu cells but is produced solely in +Cu cells from the 2.1-kb mRNA. By controlling the use of the upstream promoter, CRR1 regulates the reciprocal production of Cth1 in +Cu cells versus Crd1 in −Cu cells.
This model is substantiated by the analysis of two independent sct1 strains in which 2.1-kb Cth1 mRNA accumulation and Cth1 production were restored as a consequence of transposon insertion downstream of the CRR1-dependent promoter (Figures 8 to 10). The introduction of transcriptional terminators between two promoters can inhibit transcriptional interference (Proudfoot, 1986), and in this case it enabled the synthesis of the 2.1-kb Cth1 mRNA. Loss of the transposon in strain rsc1-1 reestablished the copper-responsive accumulation of Cth1 (Figures 8 and 9).
The proposed mechanism of regulation is an elegant device to effect both upregulation and downregulation in a pathway through a single transcriptional element, and it has the decided benefit of obligatory coordination. Copper-responsive accumulation of plastocyanin and cytochrome c6 in Pediastrum boryanum uses a similar principle (Nakamura et al., 2000). An alternative mRNA is produced from the plastocyanin-encoding gene in −Cu cells. In this case, the mRNA cannot be translated because it is truncated at the 5′ end and lacks the initiator Met. The similarity lies in the fact that translational regulation is effected through transcriptional control.
Transposable Elements in Cth1
Two independently generated suppressors of crd1 alleles contained DNA insertions at the 5′ end of CTH1. A 2.5-kb insert in the sct1-1 strain was confirmed by sequence analysis to be a member of the family of TOC1 transposable elements (Day et al., 1988). These are unusual elements with features of both retrotransposons and nonviral retroposons (Day et al., 1988; Day and Rochaix, 1991). A different sequence was inserted in Cth1 in sct1-2. This insert is possibly the Gulliver transposon, because it is similar in size (∼12 kb) and it leaves a 26-bp footprint composed of two 13-bp direct repeats upon excision, which is a common feature of class II transposable elements (Ferris, 1989; Schnell and Lefebvre, 1993).
A number of Chlamydomonas transposons have been identified because of the phenotype resulting from their insertion into a gene of interest. For instance, the TOC1.1 element was found in an intron of the OEE1 gene (Day et al., 1988), and Tcr3 was isolated by virtue of its disruption of the NIT8 locus (Wang et al., 1998). These elements can be useful markers for cloning genes as well. Integration of Gulliver into the NIT2 locus enabled the cloning and characterization of that gene (Schnell and Lefebvre, 1993); similarly, the Fus1 gene was isolated using a Tcr1-tagged mutant allele (Ferris et al., 1996). In each of these cases, expression of the gene at the locus of insertion was disrupted; however, we found that transposon insertions at the CTH1 locus resulted in gain-of-function phenotypes. It is possible that suppressors of pcy1-ac208 also arise from transposon insertions in other components of the copper-responsive signal transduction pathway (Merchant and Bogorad, 1987b). It has been suggested that Chlamydomonas transposons mobilize too infrequently to be useful for insertional mutagenesis (Graham et al., 1995). Nevertheless, this study suggests that they may be useful in directed suppressor screens if a strong selection is available.
METHODS
Growth Conditions
Copper-deficient (−Cu) Tris-acetate-phosphate (TAP) medium was prepared as described by Quinn and Merchant (1998). Copper-supplemented medium was prepared by the addition of an aqueous solution of CuSO4 to −Cu medium. Cells were grown in liquid cultures and on plates as described by Moseley et al. (2000). Anoxic cultures were grown by bubbling TAP medium for 25 hr with a mixture of 98% nitrogen and 2% CO2.
Strains and Genetic Analysis
The crd1 alleles have been described by Moseley et al. (2000). The crr1-1 strain was generated in a CC425 background (M. Eriksson, unpublished data). For genetic crosses, strains of opposite mating types were mated and zygotes were dissected according to the methods described by Harris (1989). Six tetratype tetrads were obtained from a cross between crd1-1 (arg7crd1-1::ARG7CRR1mt−) and crr1-1 (arg2CRD1crr1-1mt+) strains, demonstrating that the CRD1 and CRR1 loci are not linked. An sct1-1 strain (arg7crd1-1::ARG7sct1-1mt−) was crossed to an arg7 strain (arg7CRD1SCT1mt+) to investigate the segregation of CRD1 and SCT1. Seven complete tetrads from the cross were scored for Arg auxotrophy and the crd1 phenotype. Primers vi and vii were used for polymerase chain reaction (PCR) amplification of the proximal 2.5 kb of Cth1 from the spores of six tetrads. Arg auxotrophic spores that are mutant at the CTH1 locus (see Results) were backcrossed to crd1-1 strains to verify the presence of the sct1-1 mutation in a wild-type (CRD1) background. Because the crd1-1 alleles were tagged with the Arg7 gene, another suppressor (sct1-2) was isolated in an arg2crd1-6::ble background to enable the selection of vegetative diploids according to the method of Harris (1989). The crd1-1::ARG7sct1-1 and crd1-6::blesct1-2 strains were crossed to determine whether the suppressor mutations were at the same locus. No crd1 progeny were observed among 52 spores from 13 zygotes, indicating that sct1-1 and sct1-2 are tightly linked.
Analysis of Cth1 cDNA and Genomic Sequences in Wild-Type, sct1, and rsc1 Strains
Cth1 cDNA clone CM079h12 (Asamizu et al., 1999) was provided by S. Tabata (Kazusa DNA Research Institute, Kisarazu Chiba, Japan). A 114-bp PCR amplification product containing the Cth1 5′ untranslated region (UTR) was amplified from genomic DNA using primers i and ii (Table 1, Figure 3). This DNA fragment was cloned into pGEM-T Easy (Promega, Madison, WI) to make plasmid pCth1-1. The 5′ UTR fragment and a 345-bp XhoI fragment from the 3′ UTR of Cth1 were used to probe a Chlamydomonas reinhardtii wild-type genomic library in λEMBL3 (Goldschmidt-Clermont and Rahire, 1986) by plaque hybridization. Clone λ30-1 containing an ∼13-kb insert that hybridized to both Cth1 probes was isolated, and a 4.2-kb BamHI fragment containing 1 kb of upstream DNA, the entire Cth1 coding region, and the 3′ UTR was cloned into pBluescript KS+ (Stratagene, La Jolla, CA) to produce plasmid pCth1-2. The plasmid insert was sequenced completely on both strands. An additional 1 kb of upstream DNA was amplified from λ30-1 using primers iii and iv, and the sequence of this PCR product was combined with that of the pCth1-2 insert to produce the 5.3-kb Cth1 genomic sequence.
Table 1.
Oligonucleotide Primers Used for Amplification of Cth1 cDNA and Genomic Sequences
| Primer Number |
Sequence | Position Relative to Cth1 Genomic Sequence | Position Relative to λEMBL3 Sequence |
|---|---|---|---|
| i | CTTAATAGGCTAGCTCGCTAC | 2039 to 2059 | |
| ii | CTTGTTCAGCATGGTGGCCAT | 2153 to 2133 | |
| iii | CCCGAGAAGATGTTGAGCAAACTT | 19951 to 19974 | |
| iv | GAGAGGATATCGTGGGCT | 1364 to 1347 | |
| v | GATCTTGCAACAATGTATGCTGTG | 1 to 21 | |
| vi | TTCAGCTCCAGGCTGAAC | 2386 to 2369 | |
| vii | GTGTACAGGCTCACAAGCGAAGACA | 22 to 46 | |
| viii | CAAGGATCCGCGGTTGGCGTC | 1017 to 1034 | |
| ix | GAGCTGTGCTGCTGCGTA | 1652 to 1635 | |
| x | CAATGCAAGGATCTGGAG | 1985 to 1968 | |
| xi | GCTCAGCCGGCAATTCACTG | 298 to 317 |
The 5′ end of a cDNA corresponding to the Cth1 transcript from −Cu cells was amplified from DNA isolated from a λgt11 cDNA library (Merchant and Bogorad, 1987a). Approximately 5 × 105 plaques were grown for 8 hr, and then phage were eluted overnight into phage diluent and storage (SM) buffer (Sambrook et al., 1989). Phage DNA was prepared according to the method of Sambrook et al. (1989). Cth1 sequences were amplified from 0.5 μg of this DNA preparation using primer x (Table 1) and either the λgt11-F (5′-TTG-ACACCAGACCAACTGGTA-3′) or the λgt11-R (5′-CGTCAGTAT-CGGCGGAATT-3′) primer, which hybridize to the right and left arms of λgt11, respectively. PCR products from the primer x/λgt11-R reaction were purified on a QIAQUICK column (Qiagen, Valencia, CA), and 1 μL of these products, diluted 100-fold, was used as a template for amplification using λgt11-R and a nested primer, ix (Table 1). The longest PCR product (575 bp) was cloned into the pGEM-T Easy vector to produce plasmid pCth1-3, and the insert was sequenced using M13 forward and reverse sequencing primers (Invitrogen, Carlsbad, CA). A 1.3-kb fragment from the 5′ end of the −Cu Cth1 cDNA was amplified from the cDNA pool with primers xi and vi and sequenced to verify the transcript model (Table 1, Figure 2C). Reverse transcriptase–mediated PCR amplification of Cth1 cDNA sequences from copper-supplemented and oxygen-deficient mRNA was performed according to the method of Xie and Merchant (1996).
To determine the position of the TOC1-4 transposable element in the 5′ region of CTH1 in sct1-1, primers v and iv were used to amplify a 3.8-kb DNA fragment from the genomic DNA of a crd1-1 sct1-1 strain. Three independent plasmids, pSct1-1A, pSct1-1B, and pSct1-1C, were generated by cloning the PCR product into pGEM-T Easy, and the insert in pSct1-1A was partially sequenced. No discrepancies were found between the Cth1 sequences from wild type and sct1-1 on either side of the junction with TOC1-4. Primers v and iv also were used to amplify a 1.3-kb DNA fragment from the genomic DNA of rsc1, which subsequently was cloned in a similar manner and sequenced.
RNA Gel Blot Analysis
RNA was isolated and analyzed by hybridization as described previously (Quinn et al., 1999; Moseley et al., 2000). Eight micrograms of total RNA was loaded in each lane. Gene-specific Cth1 probes were made from (1) a 345-bp XhoI fragment corresponding to bp 4305 to 4650 of the Cth1 genomic sequence, and (2) PCR products from amplification of pCth1-2 using the 5′ primer viii and the 3′ primers ii, x, and ix (Table 1, Figure 2A). Hybridization was detected by exposure of blots to Biomax MS film (Eastman Kodak Co., Rochester, NY) at −80°C with two intensifying screens or to PhosphorImager screens (Molecular Dynamics, Sunnyvale, CA).
Antiserum Production
An ∼1.2-kb fragment was amplified from an Arabidopsis Crd1 cDNA clone corresponding to amino acid residues 1 to 409 (the precursor protein sequence). The PCR product was gel purified, digested with BamHI, cloned in frame to the C terminus of the thioredoxin-encoding sequence of the expression vector pTrxFus (Invitrogen), and introduced into Escherichia coli host strain GI724 for Trp-inducible expression. The fusion protein localized to inclusion bodies; therefore, a preparation of enriched inclusion bodies was solubilized (Marston, 1987) and used directly for antiserum production. Polyclonal antibodies were raised in rabbits at Covance Research Products (Denver, PA) by two subcutaneous nodal injections of the purified antigen (0.25 and 0.15 mg) followed by three boosts (0.15 mg). The resulting antiserum was designated α-AtCrd1.
Analysis of Chlamydomonas Proteins
Immunoblot analysis of Chlamydomonas whole cell extracts was as described by Moseley et al. (2000) except that samples were heated at 60°C for 10 min before discontinuous SDS-PAGE (10% acrylamide on separating gels). Proteins were transferred to nitrocellulose for detection of Cth1, Crd1, and cytochrome f (Figures 4A and 4B) or to polyvinylidene difluoride membranes for detection of Cth1, Crd1, PSI-A, LHCI, and CF1 (Figures 5C and 6C). For all strains, the amount of sample loaded was normalized on the basis of cell counts. For Crd1 or LHCI detection, samples equivalent to 2 × 106 cells were loaded; for PSI-A,105 cells were loaded; and for CF1, equivalent to 8 × 105 cells were loaded. Antibody dilutions were 1:300 or 1:1000 for α-AtCrd1, 1:1000 for α-LHCI (p14.1; Hippler et al., 2001), and 1:5000 for α-CF1. Alkaline extraction of peripheral membrane proteins was performed as follows. Harvested cells, equivalent to 1 mg/mL chlorophyll, were washed once with 0.01 M sodium phosphate, pH 7.0, and divided into aliquots. These were then placed in Eppendorf microfuge tubes such that after centrifugation the pellets had a volume of 50 μL. These pellets were resuspended in 0.5 mL of extraction buffer (0.1 M sodium hydrogen carbonate, pH 10.0, 10.5, 11.0, or 11.5) or control buffer (0.01 or 0.1 M sodium phosphate, pH 7.0) and subjected to three cycles of freezing and thawing. Samples were then centrifuged (5 min at 20,800g, 4°C). The supernatant, containing released proteins, was aspirated, and the extracted pellets were resuspended in 0.5 mL of 0.01 M sodium phosphate, pH 7.0.
Fractionation and Analysis of Pea Chloroplasts
Chloroplasts and thylakoids were isolated from pea (Pisum sativum cv Progress #9; Harris Seed, Rochester, NY) as described by Musser and Theg (2000). Crude homogenate was collected after blended pea leaves were filtered through Miracloth (Calbiochem, San Diego, CA). Stromal extract was prepared as described by Abad et al. (1989). Thylakoid membranes were separated from thylakoid lumen by treatment of intact thylakoids with 0.1% Triton X-100 (Sigma/Aldrich, St. Louis, MO) followed by centrifugation for 10 min at 100,000g. Crude homogenate, stromal extract, and thylakoid lumen proteins were concentrated by trichloroacetic acid precipitation, and lanes were loaded with fractions derived from chloroplasts containing 1 μg of chlorophyll, with the exception of stromal and thylakoid lumen fractions, which contained twice that amount. Samples were analyzed by SDS-PAGE followed by colorimetric immunoblotting using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Sigma/Aldrich) as described by Harlow and Lane (1988).
DNA Preparation and Hybridization
To prepare Chlamydomonas genomic DNA minipreparations for PCR amplification, individual colonies were scraped up from TAP plates with sterile pipette tips and resuspended in 20 μL of water. The cell suspensions were mixed with 50 μL of SDS-EB (100 μM Tris-HCl, pH 8, 40 μM EDTA, pH 8, 40 μM NaCl, 2% SDS, and 100 μg/mL RNase A) and heated at 50°C for 15 min. Four hundred eighty microliters of 6 M sodium iodide (Fisher Scientific, Tustin, CA) and 10 μL of silica beads (Sigma/Aldrich) were then added, and the cell lysates were incubated for 5 min at room temperature. The silica beads were pelleted by centrifugation at 20,800g for 10 sec in a tabletop centrifuge and then resuspended and washed three times with 120 μL of 80% ethanol and 20% Tris-EDTA (10:1), pH 8, pelleting the beads by centrifugation between each wash. Bound DNA was eluted into 25 μL of water by heating for 5 min at 55°C.
Genomic DNA midipreparations were prepared using a modified protocol based on the method of Rochaix (1980). Cells from 50-mL cultures were pelleted by centrifugation (3,696g, 5 min) and lysed for 2 hr at 50°C in proteinase K buffer (10 mM Tris-HCl, pH 8, 10 mM EDTA, 10 mM NaCl, 0.5% SDS, and 200 μg/mL proteinase K). Lysates then were extracted once with phenol:chloroform. RNase A was added to the aqueous phases to a concentration of 62.5 μg/mL and incubated at 37°C for 1 hr. After phenol:chloroform extraction was repeated once, polysaccharides were precipitated selectively from the aqueous phases by slow addition of 0.35 volume of 100% ethanol and incubation for 15 min on ice. Precipitated polysaccharides were removed by centrifugation at 12,096g for 10 min. Nucleic acids were precipitated from the supernatants by the addition of equal volumes of isopropanol, collected by centrifugation (7,840g, 10 min), dissolved in water, and reprecipitated with 20% polyethylene glycol 8000 (Fisher Scientific) to remove contaminating RNA. For DNA gel blot analysis, 2 to 3 μg of genomic DNA was digested for 4 hr at 37°C with 100 units of BamHI (New England Biolabs, Beverly, MA) and separated by electrophoresis on gels with 0.7% agarose and 1 × Tris-acetate-EDTA buffer. A probe corresponding to the 5′ 2.4 kb of Cth1 was made from a PCR amplification product using primers vii and vi (Figures 3A and 10). Transfer to nylon membranes and hybridization with radiolabeled probes were as described previously (Merchant and Bogorad, 1987a), and the blot was exposed to a PhosphorImager screen (Molecular Dynamics) for 25 hr.
Measurements of Low-Temperature Fluorescence Emission Spectra
Measurements were performed with thylakoid membranes isolated according to the method of Chua and Bennoun (1975) suspended in 20 mM Hepes, pH 7.5, and 60% glycerol to a density equivalent to 5 μg/mL chlorophyll. Low-temperature (77K) fluorescence emission spectra were recorded using a Fluoromax-2 spectrofluorometer (Instruments S.A. Inc., Munich, Germany).
Accession Numbers
The accession numbers for the genes/clones described in this article are AV391947 (Cth1 EST), AF337037 (Cth1 +Cu cDNA clone), AF426027 (Cth1 −Cu cDNA clone), AF337038 (Cth1 nuclear gene), AF237671 (Crd1 cDNA clone), D90899 and D90912 (Crd1 homologs in Synechocystis sp. 6803), AF236101 (Arabidopsis Crd1 cDNA clone), and U02425 (λEMBL3).
Acknowledgments
We thank Sebastian Herzog, Rebecca Nelson, Elke Wehinger, Luisita Dolfini, Margarita Gordus, and John Perea for their excellent technical assistance. This research was supported by Grant GM42143 from the National Institutes of Health. J.L.M. was supported, in part, by the molecular biology Ph.D. program and a Dissertation Year Fellowship from the Graduate Division of the University of California Los Angeles, and M.E. was supported, in part, by a European Molecular Biology Organization Long-Term Fellowship. S.M.T. and N.P.A. were supported, in part, by U.S. Department of Agriculture and Department of Energy grants to S.M.T. M.H. was supported, in part, by Grant Hi739/1-1 from the Deutsche Forschungsgemeinschaft.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010420.
References
- Abad, M.S., Clark, S.E., and Lamppa, G.K. (1989). Properties of a chloroplast enzyme that cleaves the chlorophyll a/b binding protein precursor: Optimization of an organelle-free reaction. Plant Physiol. 90, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamizu, E., Nakamura, Y., Sato, S., Fukuzawa, H., and Tabata, S. (1999). A large scale structural analysis of cDNAs in a unicellular green alga, Chlamydomonas reinhardtii. I. Generation of 3433 non-redundant expressed sequence tags. DNA Res. 6, 369–373. [DOI] [PubMed] [Google Scholar]
- Askwith, C., and Kaplan, J. (1998). Iron and copper transport in yeast and its relevance to human disease. Trends Biochem. Sci. 23, 135–138. [DOI] [PubMed] [Google Scholar]
- Ayala, M.B., and Sandmann, G. (1988). Activities of Cu-containing proteins in Cu-depleted pea leaves. Physiol. Plant. 72, 801–806. [Google Scholar]
- Beale, S.I. (1999). Enzymes of chlorophyll biosynthesis. Photosynth. Res. 60, 43–73. [Google Scholar]
- Boudreau, E., Takahashi, Y., Lemieum, C., Turmel, M., and Rochaix, J.-D. (1997). The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J. 16, 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro, A., Barroso, J., and Pais, M.S. (1990). Effect of copper deficiency on photosynthetic electron transport in wheat plants. Physiol. Plant. 79, 459–464. [Google Scholar]
- Chua, N.H., and Bennoun, P. (1975). Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: Wild-type and mutant strains deficient in photosystem II reaction center. Proc. Natl. Acad. Sci. USA 72, 2175–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, B.R., Lomedico, P.T., and Ju, G. (1984). Transcriptional interference in avian retroviruses: Implications for the promoter insertion model of leukaemogenesis. Nature 307, 241–245. [DOI] [PubMed] [Google Scholar]
- Day, A., and Rochaix, J.-D. (1991). A transposon with an unusual LTR arrangement from Chlamydomonas reinhardtii contains an internal tandem array of 76 bp repeats. Nucleic Acids Res. 19, 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, A., Schirmer-Rahire, M., Kuchka, M.R., Mayfield, S.P., and Rochaix, J.D. (1988). A transposon with an unusual arrangement of long terminal repeats in the green alga Chlamydomonas reinhardtii. EMBO J. 7, 1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson, O., Nielsen, H., and von Heijne, G. (1999). ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 8, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, P.J. (1989). Characterization of a Chlamydomonas transposon, Gulliver, resembling those in higher plants. Genetics 122, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, P.J., Woessner, J.P., and Goodenough, U.W. (1996). A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol. Biol. Cell 7, 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, N., Stampacchia, O., Redding, K., and Rochaix, J.-D. (1996). Selectable marker recycling in the chloroplast. Mol. Gen. Genet. 251, 373–380. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont, M., and Rahire, M. (1986). Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J. Mol. Biol. 191, 421–432. [DOI] [PubMed] [Google Scholar]
- Graham, J.E., Spanier, J.G., and Jarvik, J.W. (1995). Isolation and characterization of Pioneer1, a novel Chlamydomonas transposable element. Curr. Genet. 28, 429–436. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Harris, E.H. (1989). The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. (San Diego, CA: Academic Press). [DOI] [PubMed]
- Henriques, F.S. (1989). Effects of copper deficiency on the photosynthetic apparatus of sugar beet (Beta vulgaris L.). J. Plant Physiol. 135, 453–458. [Google Scholar]
- Hill, K.L., and Merchant, S. (1995). Coordinate expression of coproporphyrinogen oxidase and cytochrome c6 in the green alga Chlamydomonas reinhardtii in response to changes in copper availability. EMBO J. 14, 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, K.L., Li, H.H., Singer, J., and Merchant, S. (1991). Isolation and structural characterization of the Chlamydomonas reinhardtii gene for cytochrome c6: Analysis of the kinetics and metal specificity of its copper-responsive expression. J. Biol. Chem. 266, 15060–15067. [PubMed] [Google Scholar]
- Hill, K.L., Hassett, R., Kosman, D., and Merchant, S. (1996). Regulated copper uptake in Chlamydomonas reinhardtii in response to copper availability. Plant Physiol. 112, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau, E., Mira, H., Lin, S.-J., Culotta, V.C., Penarrubia, L., and Amasino, R.M. (1998). Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol. 117, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch, A.G. (1997). Translational regulation of yeast GCN4. J. Biol. Chem. 272, 21661–21664. [DOI] [PubMed] [Google Scholar]
- Hippler, M., Klein, J., Fink, T., Allinger, T., and Hoerth, P. (2001). Towards functional proteomics of membrane protein complexes: Analysis of thylakoid membranes from Chlamydomonas reinhardtii. Plant J. 28, 595–607. [DOI] [PubMed] [Google Scholar]
- Hirayama, T., Kieber, J.J., Hirayama, N., Kogan, M., Guzman, P., Nourizadeh, S., Alonso, J.M., Dailey, W.P., Dancis, A., and Ecker, J.R. (1999). RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 97, 383–393. [DOI] [PubMed] [Google Scholar]
- Kampfenkel, K., Kushnir, S., Babiychuk, E., Inzé, D., and Van Montagu, M. (1995). Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J. Biol. Chem. 270, 28479–28486. [DOI] [PubMed] [Google Scholar]
- Kanamaru, K., Kashiwagi, S., and Mizuno, T. (1994). A copper-transporting P-type ATPase found in the thylakoid membrane of the cyanobacterium Synechococcus species PCC7942. Mol. Microbiol. 13, 369–377. [DOI] [PubMed] [Google Scholar]
- La Fontaine, S., Quinn, J., and Merchant, S. (2002). Comparative analysis of copper and iron metabolism in photosynthetic eukaryotes versus yeast and mammals. In Handbook of Copper Pharmacology and Toxicology, E.J. Massaro, ed (Totowa, NJ: Humana Press), in press.
- Marston, F.A.O. (1987). The purification of eukaryotic polypeptides expressed in Escherichia coli. In DNA Cloning, Vol. III: A Practical Approach, D.M. Glover, ed (Oxford, UK: IRL Press), pp. 59–88.
- Merchant, S. (1998a). Reciprocal, copper-responsive accumulation of plastocyanin and cytochrome c6 in algae and cyanobacteria: A model for metalloregulation of metalloprotein synthesis. In Metal Ions in Gene Regulation, S. Silver and W. Walden, eds (New York: Chapman & Hall), pp. 450–467.
- Merchant, S. (1998b). Synthesis of metalloproteins involved in photosynthesis: Plastocyanin and cytochromes. In The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas, J.-D. Rochaix, M. Goldschmidt-Clermont, and S. Merchant, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 597–611.
- Merchant, S., and Bogorad, L. (1987. a). The Cu(II)-repressible plastidic cytochrome c: Cloning and sequence of a complementary DNA for the pre-apoprotein. J. Biol. Chem. 262, 9062–9067. [PubMed] [Google Scholar]
- Merchant, S., and Bogorad, L. (1987. b). Metal ion regulated gene expression: Use of a plastocyanin-less mutant of Chlamydomonas reinhardtii to study the Cu(II)-dependent expression of cytochrome c-552. EMBO J. 6, 2531–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant, S., Hill, K., and Howe, G. (1991). Dynamic interplay between two copper-titrating components in the transcriptional regulation of cyt c6. EMBO J. 10, 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira, H., Martínez-García, F., and Peñarrubia, L. (2001). Evidence for the plant-specific intercellular transport of the Arabidopsis copper chaperone CCH. Plant J. 25, 521–528. [DOI] [PubMed] [Google Scholar]
- Moseley, J., Quinn, J., Eriksson, M., and Merchant, S. (2000). The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper-deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J. 19, 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser, S.M., and Theg, S.M. (2000). Characterization of the early steps of OE17 precursor transport by the thylakoid ΔpH/Tat machinery. Eur. J. Biochem. 267, 2588–2598. [DOI] [PubMed] [Google Scholar]
- Nakamura, M., Yoshizaki, F., and Sugimura, Y. (2000). Accumulation of plastocyanin mRNA lacking 5′ region in the green alga Pediastrum boryanum grown under copper-deficient conditions. Plant Cell Physiol. 41, 33–41. [DOI] [PubMed] [Google Scholar]
- Phung, L.T., Ajlani, G., and Haselkorn, R. (1994). P-type ATPase from the cyanobacterium Synechococcus 7942 related to the human Menkes and Wilson disease gene products. Proc. Natl. Acad. Sci. USA 91, 9651–9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinta, V., Picaud, M., Reiss-Husson, F., and Astier, C. (2002). Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear-iron-cluster-containing protein involved in aerobic oxidative cyclization of Mg protoporphyrin IX monomethylester. J. Bacteriol. 184, 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot, N.J. (1986). Transcriptional interference and termination between duplicated α-globin gene constructs suggests a novel mechanism for gene regulation. Nature 322, 562–565. [DOI] [PubMed] [Google Scholar]
- Quinn, J., Eriksson, M., Moseley, J., and Merchant, S. (2002). Oxygen deficiency-responsive gene expression in Chlamydomonas through a copper-sensing signal transduction pathway. Plant Physiol. 128, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, J.M., and Merchant, S. (1995). Two copper-responsive elements associated with the Chlamydomonas Cyc6 gene function as targets for transcriptional activators. Plant Cell 7, 623–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, J.M., and Merchant, S. (1998). Copper-responsive gene expression during adaptation to copper deficiency. Methods Enzymol. 297, 263–279. [DOI] [PubMed] [Google Scholar]
- Quinn, J.M., Nakamoto, S.S., and Merchant, S. (1999). Induction of coproporphyrinogen oxidase in Chlamydomonas chloroplasts occurs via transcriptional regulation of Cpx1 mediated by copper response elements and increased translation from a copper deficiency-specific form of the transcript. J. Biol. Chem. 274, 14444–14454. [DOI] [PubMed] [Google Scholar]
- Quinn, J.M., Barraco, P., Eriksson, M., and Merchant, S. (2000). Coordinate copper- and oxygen-responsive Cyc6 and Cpx1 expression in Chlamydomonas is mediated by the same element. J. Biol. Chem. 275, 6080–6089. [DOI] [PubMed] [Google Scholar]
- Rochaix, J.-D. (1980). Restriction fragments from Chlamydomonas chloroplast DNA. Methods Enzymol. 65, 785–795. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schnell, R.A., and Lefebvre, P.A. (1993). Isolation of the Chlamydomonas regulatory gene NIT2 by transposon tagging. Genetics 134, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampacchia, O., Girard-Bascou, J., Zanasco, J.-L., Zerges, W., Bennoun, P., and Rochaix, J.-D. (1997). A nuclear-encoded function essential for translation of the chloroplast psaB mRNA in Chlamydomonas. Plant Cell 9, 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, P., Grunler, J., Mattsson, J., Sindelar, P.J., Nordlund, P., and Berthold, D.A. (2001). A new member of the family of di-iron carboxylate proteins, Coq7 (clk-1), a membrane-bound hydroxylase involved in ubiquinone biosynthesis. J. Biol. Chem. 276, 33297–33300. [DOI] [PubMed] [Google Scholar]
- Tottey, S., Rich, P.R., Rondet, S.A.M., and Robinson, N.J. (2001). Two Menkes-type ATPases supply copper for photosynthesis in Synechocystis sp. 6803. J. Biol. Chem. 276, 19999–20004. [DOI] [PubMed] [Google Scholar]
- Turvey, N.D., and Grant, B.R. (1990). Copper deficiency in coniferous trees. For. Ecol. Manage. 37, 95–122. [Google Scholar]
- Wang, S.-C., Schnell, R.A., and Lefebvre, P.A. (1998). Isolation and characterization of a new transposable element in Chlamydomonas reinhardtii. Plant Mol. Biol. 38, 681–687. [DOI] [PubMed] [Google Scholar]
- Woeste, K.E., and Kieber, J.J. (2000). A strong loss-of-function mutation in RAN1 results in constitutive activation of the ethylene response pathway as well as a rosette-lethal phenotype. Plant Cell 12, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman, F.-A., and Bennoun, P. (1982). A new chlorophyll-protein complex related to photosystem I in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 680, 352–360. [Google Scholar]
- Wollman, F.-A., and Delepelaire, P. (1984). Correlation between changes in light energy distribution and changes in the thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J. Cell Biol. 98, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z., and Merchant, S. (1996). The plastid-encoded ccsA gene is required for heme attachment to chloroplast c-type cytochromes. J. Biol. Chem. 271, 4632–4639. [DOI] [PubMed] [Google Scholar]
- Zheng, C.C., Porat, R., Lu, P., and O'Neill, S.D. (1998). PNZIP is a novel mesophyll-specific cDNA that is regulated by phytochrome and the circadian rhythm and encodes a protein with a leucine zipper motif. Plant Physiol. 116, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]