Abstract
Multiple tau gene mutations are pathogenic for hereditary frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), with filamentous tau aggregates as the major lesions in the CNS of these patients. Recent studies have shown that bacterially expressed recombinant tau proteins with FTDP-17 missense mutations cause functional impairments, i.e., a reduced ability of mutant tau to bind to or promote the assembly of microtubules. To investigate the biological consequences of FTDP-17 tau mutants and assess their ability to form filamentous aggregates, we engineered Chinese hamster ovary cell lines to stably express tau harboring one or several different FTDP-17 mutations and showed that different tau mutants produced distinct pathological phenotypes. For example, ΔK, but not several other single tau mutants (e.g., V337 M, P301L, R406W), developed insoluble amorphous and fibrillar aggregates, whereas a triple tau mutant (VPR) containing V337M, P301L, and R406W substitutions also formed similar aggregates. Furthermore, the aggregates increased in size over time in culture. Significantly, the formation of aggregated ΔK and VPR tau protein correlated with reduced affinity of these mutants to bind microtubules. Reduced phosphorylation and altered proteolysis was also observed in R406W and ΔK tau mutants. Thus, distinct pathological phenotypes, including the formation of insoluble filamentous tau aggregates, result from the expression of different FTDP-17 tau mutants in transfected Chinese hamster ovary cells and implies that these missense mutations cause diverse neurodegenerative FTDP-17 syndromes by multiple mechanisms.
INTRODUCTION
Tau is an abundant microtubule-associated protein of the CNS that is expressed primarily in neurons and is implicated in the pathogenesis of Alzheimer's disease and related neurodegenerative diseases known as tauopathies (reviewed in Vogelsberg-Ragaglia et al., 1999). The major neuropathological characteristics of tauopathies are numerous neuronal and/or glial cytoplasmic inclusions formed by aggregated paired helical filaments (PHFs) and/or straight filaments composed of aberrantly phosphorylated tau proteins (PHF-tau) in widespread CNS regions (Spillantini and Goedert, 1998; Vogelsberg-Ragaglia et al., 1999). Six alternatively spliced tau isoforms are expressed in the adult human CNS (Goedert et al., 1989; Andreadis et al., 1992), and are localized predominantly in axons (Binder et al., 1985). Tau proteins bind to and stabilize microtubules (MTs) in the polymerized state (Weingarten et al., 1975; Drechsel et al., 1992), but the formation of PHF-tau results in a loss of these important functions (Bramblett et al., 1993; Yoshida and Ihara, 1993). Moreover, unlike normal tau, PHF-tau is insoluble, accumulates in the somatodendritic domain of neurons, and assembles into abnormal filaments that aggregate as neurofibrillary tangles (NFTs; Lee et al., 1991; Goedert et al., 1997). However, the mechanism(s) whereby normal soluble tau assembles into PHFs and aggregates into NFTs remains unknown. This is due, in part, to the inability to develop cell culture and animal models that produce PHFs and NFTs.
Although the massive degeneration of neurons and extensive gliosis associated with progressive accumulations of PHF-tau lesions provided circumstantial evidence implicating filamentous tau pathology in the onset/progression of neurodegenerative disease, the discovery of multiple pathogenic tau gene mutations in many different families with FTDP-17 showed unequivocally that tau abnormalities cause neurodegenerative disease (reviewed in Vogelsberg-Ragaglia et al., 1999). The FTDP-17 tau gene mutations (i.e., missense substitutions, in-frame deletions, intronic substitutions) occur in exons and introns of the tau gene (Clark et al., 1998; Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998; D'Souza et al., 1999; Iijima et al., 1999; Rizzu et al., 1999). They may cause FTDP-17 by altering the functions or levels of specific tau isoforms and/or promoting tau aggregation in the CNS (Hong et al., 1998; Hutton et al., 1998; D'Souza et al., 1999). Indeed, several FTDP-17 missense tau mutations, including Δ280K (ΔK), V337M (VM), P301L (PL), and R406W (RW) have been demonstrated to reduce the ability of bacterially expressed recombinant tau protein to bind to and promote the assembly of MTs (Hong et al., 1998; D'Souza et al., 1999). Therefore, they may cause neurodegenerative disease by inducing a loss of normal tau functions. Other studies showed that recombinant tau with the PL mutation aggregates into filaments more readily than recombinant wild-type (Wt) tau (Goedert et al., 1999; Nacharaju et al., 1999; Gamblin et al., 2000), supporting the idea that some missense mutations may also cause neurodegenerative disease by inducing a gain of toxic function. However, it has not been possible to produce tau aggregates in intact cells even after massively overexpressing Wt tau in cultured neuronal and non-neuronal cells (Kanai et al., 1989; Bramblett et al., 1993; Ebneth et al., 1998).
Thus, to more precisely define how these missense mutations cause tau dysfunction and to assess whether they can cause tau aggregation, we generated stably transfected Chinese hamster ovary (CHO) cell lines that expressed tau harboring one or several topographically distinct FTDP-17 missense mutations. Here, we report that different tau mutants produce distinct pathological phenotypes in transfected CHO cells. More importantly, filamentous tau aggregates were detected in CHO cells expressing the ΔK and VPR mutations.
MATERIALS AND METHODS
Site-directed Mutagenesis of Tau40 to Generate Tau Mutants
Site-directed mutagenesis (Quikchange kit; Stratagene, La Jolla, CA) was used to create a series of single missense mutations (N279K, Δ280K, P301L, S305N, V337M, R406W and a triple mutation, VPR, containing P301L, V337M, and R406W) in the longest human tau isoform (designated Tau40). The sequences of the mutagenized oligonucleotides were as follows: N279K: 5′-GGT GCA GAT AAT TAA GAA GAA GCT GGA TCT TAG C-3′, Δ280K: 5′-GGG AAG GTG CAG ATA ATT AAT AAG CTG GAT CTT AGC AAC GTC C-3′, P301L: 5′-GGA TAA TAT CAA ACA CGT CCT GGG AGG CGG CAG TGT GC-3′, S305N: 5′-CCC GGG AGG CGG CAA TGT GCA AAT AGT CTA C-3′, V337M: 5′-CCA GGA GGT GGC CAG ATG GAA GTA AAA TCT GAG AAG C-3′, and R406W: 5′-GGG GAC ACG TCT CCA TGG CAT CTC AGC AAT GTC TCC-3′. In general, two synthetic oligonucleotide primers containing the desired mutation and complimentary to opposite strands of the vector were incubated with wild-type Tau40 in a pSG5 vector (Stratagene) and Pfu DNA polymerase. After the polymerase chain reaction reaction, the template DNA was digested with DpnI and the remaining mutated cDNA was used to transform Escherichia coli. Each tau construct was subjected to sequence analysis, and the position of each mutation in the largest 441-amino-acid-long tau isoform is shown in Figure 1.
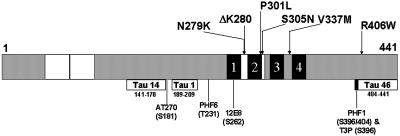
Figure 1.
Topography of FTDP-17 missense mutations in tau- and epitope-specific anti-tau antibodies. Schematic representation of the longest tau isoform, designated Tau40, containing 441 amino acids. The locations of six FTDP-17 missense mutations studied here are identified in the schematic diagram of tau. The two 29-amino-acid-long inserts are defined by white boxes at the amino-terminal region and the MT binding repeats appear as four black blocks near the carboxy terminal of tau. Each MT repeat is numbered 1–4. The defined epitopes recognized by each of the anti-tau antibodies used in this study are shown below the tau schematic with their corresponding amino acid length and position as well as the code name of the specific antibody that recognizes each epitope. The exact site(s) of phosphorylation detected by the phosphorylation-dependent anti-tau MAbs are shown here below the code names.
Stable Expression of Wt and Mutant Tau40 in Transfected CHO Cells
CHO cells were maintained in α-minimum essential medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Rockville, MD) as previously described (Bramblett et al., 1993). CHO Pro5 cells were cotransfected with either the Wt or mutant Tau40 cDNA and pcDNA3 containing the neomycin gene, by using the calcium phosphate precipitation method (Chen and Okayama, 1987) as previously described (Bramblett et al., 1993). After selection in α-minimum essential medium containing 0.8 mg/ml G418, the cells were screened for tau expression by Western blot and indirect immunofluorescence (see below), followed by subcloning. Stably transfected CHO cell lines were established from subclones expressing Wt or mutant tau in >90% of cells at approximately equivalent tau protein levels, and these cell lines were used in the studies reported below unless otherwise stated.
Preparation and Western Blot Analyses of Lysates from Transfected CHO Cells
Transfected CHO cells were washed once with phosphate-buffered saline (PBS) and lysed in ice-cold high salt RAB buffer [0.1 M 2-(N-morpholino)ethanesulfonic acid, 0.5 mM MgSO4, 1 mM EGTA, 2 mM dithiothreitol, and 0.75 M NaCl, pH 6.8] supplemented with 0.1% Triton X-100 and a mixture of protease and phosphatase inhibitors (2 mM phenylmethylsulfonyl fluoride; 20 mM NaF; 0.5 mM sodium orthovanadate; and l-1-tosylamide-2-phenylethylchloromethyl, N-tosyl-l-lysine chloromethyl ketone, leupeptin, pepstatin, and soybean trypsin inhibitor, each at 1 μg/ml). Cell lysates were incubated on ice for 10 min, sonicated, and centrifuged for 20 min at 50,000 × g at 4°C. The supernatants were collected, boiled for 10 min, and then centrifuged for 10 min at 12,000 × g at 4°C. Protein concentration was determined by using the bicinchoninic acid method (Pierce, Rockford, IL). Samples were resolved on 7.5% SDS-PAGE gels and the tau proteins expressed in these cell lines were probed by Western blot analysis by using a variety of epitope-specific anti-tau antibodies (see below). Antibody binding was detected with horseradish peroxidase-conjugated secondary antibody (Jackson Laboratories, West Grove, PA) and the blots were developed either by the enhanced chemiluminescence (Amersham, Piscataway, NJ) or 3,3′-diaminobenzidine method. For quantification, 125I-labeled goat anti-mouse IgG was used as secondary antibody and the blots were exposed to PhosphorImager plates. Quantitative analysis was performed with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Recombinant tau protein corresponding to the largest tau isoform was prepared as described in Hong et al. (1998).
Dephosphorylation and Proteolysis of Tau from Transfected CHO Cells
Heat-stable, high-salt CHO cell lysates were dialyzed overnight in 50 mM Tris, pH 8.0, 0.2 mM EDTA, and protease inhibitors to remove the high salt, inhibit proteolysis, and establish an optimal environment for dephosphorylating tau with alkaline phosphatase (Sigma, St. Louis, MO) as described in Hong et al. (1998). Increased proteolysis was achieved by eliminating protease inhibitors from the high-salt RAB extraction buffer.
Metabolic Labeling and Western Blot Studies of Tau from Transfected CHO Cells
Transfected CHO cells stably expressing Wt or mutant tau proteins were incubated with methionine-free medium for 15 min and pulsed with 100 μCi/ml [35S]methionine (NEN, Boston, MA) for 30 min as described in Merrick et al. (1996). The radiolabeled CHO cells were chased for different lengths of time and harvested in ice-cold RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 5 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS). Radiolabeled tau in cell lysates was immunoprecipitated with a rabbit polyclonal anti-tau antibody (17026), followed by protein A-Sepharose (Pharmacia Biotech, Peapack, NJ) and the antigen–antibody complex was resolved by SDS-PAGE. After the radiolabeled gels were dried, they were exposed to PhosphorImager plates for subsequent analysis.
MT Binding Assay of Tau from Transfected CHO Cells
To determine whether the point mutations alter interactions of tau with tubulin in the MTs of intact cells, an MT binding assay was performed as described in Bramblett et al. (1993) and Merrick et al. (1996). Briefly, CHO cells were harvested in RAB buffer supplemented with 0.1% Triton X-100, 20 μM Taxol, 2 mM GTP, and a mixture of protease inhibitors (as mentioned above) at 37°C. Cell lysates were homogenized with 15 strokes in a warm Dounce homogenizer, and then immediately centrifuged for 20 min at 50,000 × g at 25°C. The supernatant containing unbound tau was removed and the protein concentration determined by the bicinchoninic acid method (Pierce). The remaining pellet was resuspended in a 2× volume of sample buffer corresponding to the total volume of supernatant after normalizing to total protein. The samples were resolved on 7.5% SDS-PAGE gels, transferred onto nitrocellulose replicas, and the amounts of tau and α-tubulin protein were quantified using 125I-labeled secondary antibody. The ratio of tau bound to MTs (pellet) versus soluble or unbound tau (supernatant) was determined by comparing the tau immunoreactivities in these two fractions.
Isolation of Insoluble Tau from Transfected CHO Cells
Low-density CHO cell transfectants were grown to 80% confluency and extracted with high-salt RAB containing 0.1% Triton X-100. The cell lysates were subjected to two or more freeze-thaw cycles to remove tau bound to MTs. The homogenate was centrifuged at 50,000 × g for 20 min to generate a supernatant and a pellet. The supernatant was removed, boiled, and then centrifuged at 50,000 × g for 20 min. The pellet from the original spin was sonicated in 2× sample buffer. Samples containing both the supernatant and the pellet were resolved on 7.5% SDS-PAGE gels and transferred onto nitrocellulose replicas for Western blot analyses.
Indirect Immunofluorescence Studies of Tau and MTs in Transfected CHO Cells
CHO cell transfectants were fixed with 0.3% glutaraldehyde in PEM buffer [80 mM piperazine-N,N′-bis(2-ethanesulfonic acid), pH 6.8, 5 mM EGTA, 1 mM MgCl2] for 10 min, and permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (PBS) for 15 min before quenching the glutaraldehyde with 10 mg/ml sodium borohydride in PBS for 7 min followed by 0.1 M glycine in PBS for 20 min (Black et al., 1996). After a final rinse in PBS, the cells were incubated with 17026, a rabbit polyclonal anti-tau antibody and a monoclonal antibody (MAb) to α-tubulin (Blose et al., 1984) for 2 h at room temperature. Secondary antibodies were fluorescent-labeled donkey anti-rabbit IgG and Texas Red-labeled donkey anti-mouse IgG (Jackson Laboratories).
Transmission and Immuno-electron Microscopy (EM) of Transfected CHO Cells
Transmission and pre-embedding immuno-EM was performed on representative samples of CHO cells expressing Wt or mutant tau (VPR and ΔK; n = 3 samples of each) after fixation with 4% paraformaldehyde and 2% glutaraldehyde or 4% paraformaldehyde and 0.25% glutaraldehyde in PBS buffer, respectively, for 60 min, followed by quenching in 0.1% sodium borohydride in Tris-buffered saline for 10 min and treatment for another 10 min with 20% ethanol. For transmission EM, staining and ultrastructural analysis were performed as described previously (Tu et al., 1995). To facilitate the identification of fibrils in aggregates of mutant tau, grids were also treated with 30% formic acid for 90 s before examination by EM. For immuno-EM, fixed cells were blocked in 5% donor horse serum in PBS with 0.2% cold water fish skin gelatin and 1% ovalbumin for 60 min before incubation with 17026, the anti-tau antiserum (dilution 1:500), in 0.1% BSA and PBS overnight at 4°C. A goat anti-rabbit nanogold-IgG (1:40; Nanoprobes Inc., Yaphank, NY) secondary antibody was applied for 2 h at room temperature. Silver enhancement was performed by incubating cells with silver enhancement reagent (Nanoprobes Inc.) for 8 min in the dark. For the diaminobenzidine (DAB) plus silver-gold-enhancement immuno-EM method (Teclemariam-Mesbah et al., 1997), biotinylated goat anti-rabbit IgG (1:100; Vector, Houston, TX) secondary antibody was applied for 2 h at room temperature for each set of cells. After visualizing the DAB-positive cells labeled by routine immuno-EM methods, silver-gold intensification was performed by incubating the samples in silver methenamine developer (3% methenamine, 5% silver nitrate, and 1% sodium tetraborate) at 60°C for 5 min as described in Teclemariam-Mesbah et al. (1997). The reaction was stopped with 2% sodium acetate and then stabilized in 3% sodium thiosulphate for 5 min. Gold toning was obtained by incubating the cells in 0.1% gold chloride for 5 min, followed by the stabilization step.
CHO cells prepared for immuno-EM by using nanogold and DAB plus silver enhancement were fixed with 2% glutaraldehyde in PBS buffer overnight. Cells were collected and spun down at 1000 × g for 5 min. The pellet from nanogold-labeled cells were postfixed in 0.5% osmium tetroxide for 30 min at 4°C, whereas the pellets of DAB plus silver-gold enhancement-labeled cells were treated in 2% osmium tetroxide for 60 min at 4°C. After dehydration with graded alcohols and propylene oxide, the pellets were embedded in Epon-812 and polymerized at 70°C for 48 h. Sixty-five nanometer thin sections were cut and mounted on 200-mesh copper grids, stained with 1% uranyl acetate in 50% ethanol by bismuth subnitrite and examined with a JEM1010 electron microscope at 80 kV.
Properties of the Epitope Specific Anti-Tau Antibodies Used in These Studies
The following antibodies to tau proteins, including some that recognize specific epitopes in tau, were used in this study: 17026 (a rabbit polyclonal antibody made against the largest human recombinant tau; Ishihara et al., 1999); T3P (a rabbit polyclonal antibody raised to a synthetic peptide containing the phosphorylated Ser396; Lee et al., 1991); MAbs T14 and T46 are phosphorylation-independent anti-tau antibodies (Kosik et al., 1988; Trojanowski et al., 1989); MAb T1 (Binder et al., 1985; Szendrei et al., 1993); MAb PHF1 (specific for phosphorylated serine 396/404; Greenberg and Davies, 1990; Otvos et al., 1994); MAb AT8 (specific for phosphorylated serine 202 and threonine 205; Goedert et al., 1993, 1994); MAb 12E8 (specific for phosphorylated Ser262; Seubert et al., 1995); MAb PHF6 (specific for phosphorylated Thr231; Hoffmann et al., 1997), and MAb AT270 (specific for phosphorylated serine 181; Goedert et al., 1994). The position of the epitope locations for the anti-tau antibodies used in this study are illustrated in Figure 1. T1 was obtained from Dr. L. Binder, PHF1 from Dr. P. Davies, and AT8 and AT270 from Innogenetics (Alharetta, GA). The mouse MAb to α-tubulin was purchased from Amersham (Blose et al., 1984).
RESULTS
Phosphorylated RW and VPR Mutant Tau40 Isoforms Do Not Exhibit Slower SDS-PAGE Mobility
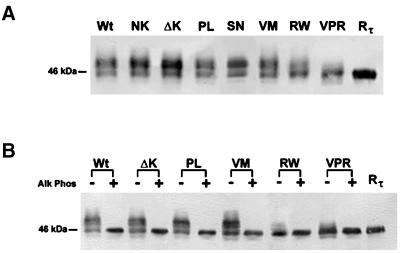
To examine the effects of FTDP-17 mutations on the properties of mutant versus Wt tau, we subjected stably transfected CHO cell lines to SDS-PAGE. Although most of the tau mutants comigrated with Wt tau, the RW and VPR tau mutants predominantly exhibited a faster electrophoretic mobility on SDS-PAGE gel (Figure 2A). To determine whether the faster migrating tau containing the RW mutation corresponded to a reduction in the extent of phosphorylation, high-salt extracts of Wt tau and all of the tau mutants, except the NK and SN, were subjected to enzymatic dephosphorylation with alkaline phosphatase followed by SDS-PAGE (Figure 2B). The NK and SN mutations were not examined in these studies because they alter exon 10 splicing rather than other properties of tau (Hong et al., 1998; D'Souza et al., 1999). Dephosphorylation resulted in the comigration of Wt tau and all the tau mutants examined here, suggesting that CHO cell lines expressing tau with an RW mutation is less phosphorylated than Wt and the other tau transfectants with a single point mutation (Figure 2B).
Figure 2.
SDS-PAGE analysis of the electrophoretic mobility of Wt and mutant tau expressed in CHO cells. Western blot analysis of cell lysates from CHO cells stably expressing either the Wt or mutant tau proteins and probed with a cocktail of MAbs T14 and T46 (T14/46; A). Tau proteins harboring the RW and the triple mutation (VPR) migrate rapidly and nearly in parallel with Wt recombinant tau (Rτ). Extracted Wt and mutant tau proteins were either untreated (−) or treated (+) with alkaline phosphatase (Alk Phos) and probed with T14/46 (B). Note that after treatment with Alk Phos all tau proteins migrate as one strong band with Rτ.
Phosphorylation of Tau with the RW Mutation Is Reduced at Ser396 and Ser404
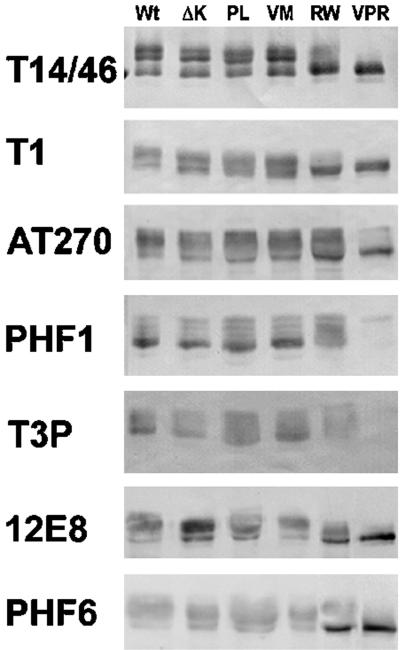
To identify the exact phosphorylation site(s) affected by the RW mutation, we performed immunoblot analysis with a panel of phosphorylation site- or epitope-specific anti-tau antibodies on lysates of CHO cells expressing Wt tau and several different tau mutants (Figure 3). The phosphorylation-independent anti-tau MAbs T14 and T46 were used in combination (T14/46) and detected at least three distinct phosphoisoforms in cells expressing Wt, ΔK, PL, and the VM mutation, but not in cells expressing the RW and the triple VPR mutations. The pattern of tau immunobands detected by the phosphorylation-dependent MAbs T1, AT270, PHF1, T3P, 12E8, and PHF6 also do not differ significantly in cells expressing Wt versus ΔK, PL, and VM mutations. However, CHO cells expressing the RW and the triple VPR mutations showed a significant reduction in the extent of phosphorylation at Ser396/404 (as detected by T3P and the PHF1 MAb) without affecting phosphorylation at the Thr181 (as detected by AT270), Ser262 (as detected by 12E8), and Thr231 (as detected by PHF6) sites, suggesting that the RW mutation selectively reduces tau phosphorylation at Ser396 and Ser404 (Figure 3). The greater reduction in PHF1 and T3P immunoreactivities of the VPR tau mutant relative to the RW tau mutant suggests that the V337 M and P301L mutations act synergistically with the RW mutation to reduce tau phosphorylation at Ser396 and Ser404.
Figure 3.
RW and VPR tau mutants exhibit reduced phosphorylation at Ser396 and Ser404. The phosphorylation of Wt and mutant tau at multiple sites was determined by immunoblot analysis with a panel of anti-tau antibodies. Note that the phosphorylation of the RW and VPR tau mutants expressed in CHO cells is reduced at the Ser 396/404 site (as detected by PHF1 and T3P antibodies), but not at Thr181 (AT270), Ser262 (12E8), or Thr231 (PHF6).
Tau Mutations Do Not Alter the Turnover of Tau Phosphoisoforms in Transfected CHO Cells
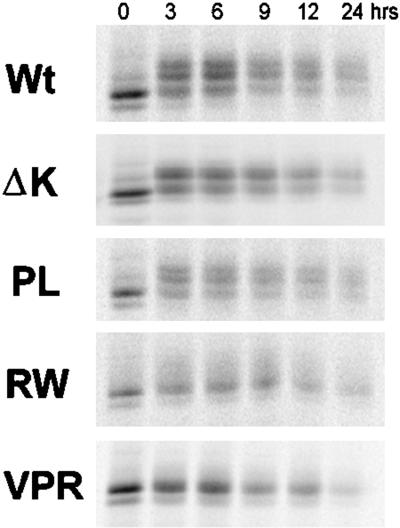
We also assessed whether the lack of the slower migrating and more phosphorylated tau isoforms in the RW and VPR mutants might be due to the inability of CHO cells to phosphorylate tau at sites that lead to the mobility shift or a faster turnover of phosphate groups present in the slower migrating tau isoforms. To do this, we examined the turnover of the phosphoisoforms in Wt and mutant tau transfectants by using a pulse-chase paradigm. After the cells were pulsed with [35S]methionine for 30 min, they were chased for different lengths of time (Figure 4). At time zero, the majority of the radiolabeled tau proteins migrated as a single poorly phosphorylated band. However, a chase of 3 h generated slower migrating tau isoforms that we have shown are more phosphorylated (Merrick et al., 1996). Significantly, these slower migrating tau isoforms were detected at all chase time points in CHO cells expressing Wt tau, PL, and ΔK, but not RW and VPR tau mutants, suggesting that the latter tau mutants are not phosphorylated by CHO cells to generate these phosphoisoforms (Figure 4). As with PL and Wt tau, ΔK mutants were also modified by phosphorylation within 3 h after labeling. However, the slowest species of the ΔK tau mutants were not as prominent, suggesting that these mutant forms of tau are not phosphorylated to the same extent as Wt and PL tau. Thus, our pulse chase studies support the idea that the RW and VPR, as well as the ΔK, tau mutants are not phosphorylated to the same extent as Wt and PL in CHO cells. Finally, these pulse-chase studies also did not reveal any significant differences between the turnover rates of the Wt versus the mutant tau isoforms.
Figure 4.
Turnover of Wt and mutant tau in CHO cells is similar. Wt and mutant tau proteins were immunoprecipitated at various times after pulse labeling with [35S]methionine. No significant difference in the turnover rate of mutant versus Wt tau was apparent in these cells. However, although Wt tau and PL tau mutants migrated more slowly over the chase period, an indication of increase phosphorylation, the slower migrating tau isoforms are not detected in cells harboring the RW and VPR mutations.
Proteolytic Processing of Tau Is Altered by the ΔK, RW, and VPR Mutations
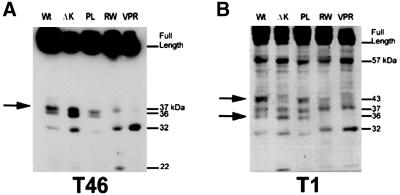
The reduction in tau phosphorylation at Ser396 and Ser404 in the RW mutants suggests the possibility that a conformational change, due to the substitution of arginine with tryptophan at residue 406 of the 441-amino-acid-long tau protein, could result in a differential accessibility of kinases and/or endogenous protease(s) to this mutant form of tau. To test this possibility, we prepared cell lysates from Wt and mutant tau CHO cell transfectants in the absence of protease inhibitors and analyzed the tau fragments by Western blotting with multiple anti-tau antibodies (Figure 5, A and B). Overall, the pattern of proteolytic tau fragments generated were similar among Wt tau and PL mutant-expressing cells, but differed from those detected in cells expressing ΔK, RW, and VPR tau mutants. Specifically, a fragment of ∼36 kDa appeared to be absent from the RW and VPR tau mutants, and this 36-kDa fragment was most likely derived from the carboxy half of tau because it was detected by both the T46 and T1 Mabs, which are specific for epitopes, including residues 404–441 and 189–209, respectively (Figure 5, A and B). Another ∼43-kDa tau fragment cleaved at the carboxy terminus was also not detected in the RW, VPR, and ΔK tau mutants. And a 57-kDa fragment cleaved at the carboxy terminus (because it was detected by T1, but not T46) was not affected by any of the tau mutations examined here. The differences in the pattern of proteolytic fragments are not due to the extent of tau phosphorylation because dephosphorylation did not have any effect on the presence or absence of these fragments (our unpublished results). Taken together, these data support the notion that a conformational change induced by the ΔK and RW mutations could account for the changes in phosphorylation and proteolysis of these tau mutants.
Figure 5.
Alteration in proteolytic processing by FTDP-17 tau mutations. Immunoblot analysis of tau extracted from CHO cells expressing Wt and FTDP-17 mutant tau proteins showing the intact, full-length species of each tau isoform and the profile of breakdown products generated, as visualized by the T46 MAb (A) and the T1 MAb (B). The banding pattern is altered for the ΔK, RW, and VPR tau mutants relative to Wt tau, suggesting that these mutations induce a conformational change that modifies the proteolysis of these proteins.
ΔK and VPR Tau Mutants Develop Tau Aggregates as Detected by Indirect Immunofluorescence
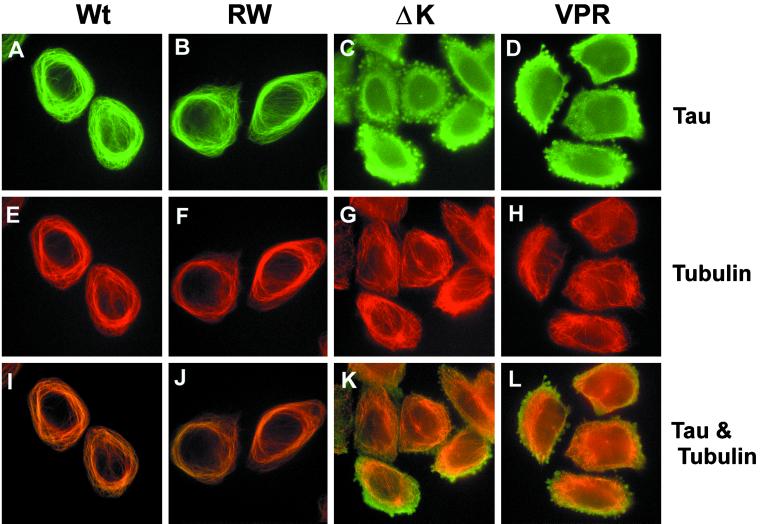
To assess whether any of the overexpressed tau mutants in CHO cells develop tau aggregates, indirect immunofluorescence studies were conducted. As observed previously for Wt tau (Kanai et al., 1989; Bramblett et al., 1993), the expression of RW mutant tau in CHO cells resulted in the bundling of MTs and the formation of MT cables around the nucleus, as detected by anti-α-tubulin and anti-tau antibodies (compare Figure 6A with B, and E with F). In fact, the staining of Wt tau and RW tau mutants colocalized almost exactly with that of α-tubulin (Figure 6, A and B). Moreover, the staining pattern of the PL and VM tau mutant expressing CHO cell clones looked comparable to the CHO cells expressing Wt tau (our unpublished results). However, the tau and MT staining pattern was dramatically different in cells expressing either the ΔK or VPR tau mutants. Specifically, focal tau immunoreactivities were detected in ∼70–80% of the CHO cell transfectants with the ΔK and the VPR tau mutations, and these tau immunoreactive aggregates were present throughout the perinuclear cytoplasm, although they varied in size and shape (Figure 6, C and D). In addition to these tau aggregates, tau staining in these cells was mostly diffuse and did not colocalize with the MT network (Figure 6, C and D). Furthermore, the aggregates seen in CHO cells expressing the ΔK and VPR mutant tau were also immunostained by the MAb Alz50 and other phosphorylation-dependent and -independent anti-tau antibodies (our unpublished results). The inclusions did not contain f-actin (our unpublished results) or β-tubulin (Figure 8, C and D). The lack of MT bundling in these CHO cells suggests that the ΔK and VPR tau mutants do not bind very well to MTs (Figure 6, G and H).
Figure 6.
FTDP-17 missense mutations induce tau aggregation and disrupt MT bundling. CHO cells expressing Wt and mutant tau were plated onto glass coverslips and allowed to settle overnight. After fixation in 0.3% glutaraldehyde, cells were permeabilized with Triton X-100 and immunostained with recombinant tau (17026) (A–D and I–L) and α-tubulin (E–H and I–L). Colocalization and bundling of tau and tubulin are readily apparent in cells with Wt (A, E, and I) and RW (B, F, and J) tau. This pattern is completely disrupted with the expression of the ΔK and VPR tau mutants. Note that in CHO cells expressing these tau mutants, tau and tubulin no longer colocalize (K and L), the tubulin network is not bundled (G and H), and aggregates of tau are apparent (C and D). Bar, 30 μm at 20×.
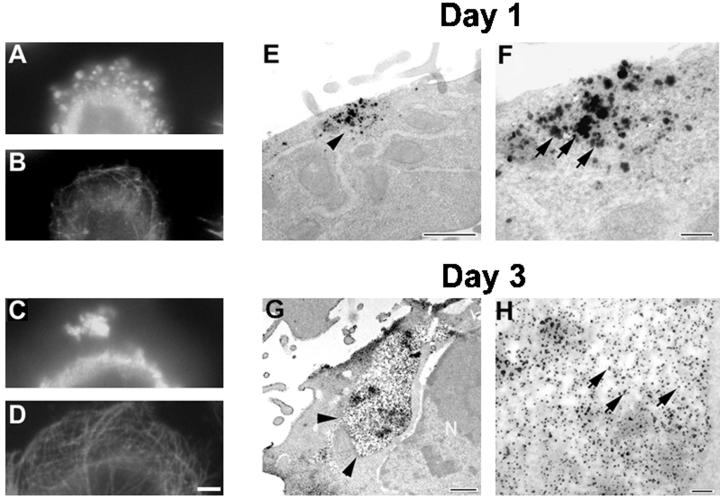
Figure 8.
Aggregates of FTDP-17 tau mutants grow larger with time. CHO cells stably expressing Wt tau and mutant tau protein were plated at low density on coverslips and grown for either 1 or 3 d. (A–D) Double-labeled immunofluorescence images of CHO cells expressing the VPR tau mutants immunostained with rabbit antirecombinant tau (17026; A and C) and a MAb to α-tubulin (B and D) in cells grown for 1 d (A and B) or 3 d (C and D). (E–H) Immuno-EM detection of tau by using 17026 as the primary antibody visualized either with secondary antibody and silver-enhanced DAB (E and F) or nanogold conjugated secondary antibody (G and H). Bar, 10 μm at 60× for immunofluorescence photomicrographs; bar, 100 nm for immuno-EM.
ΔK and VPR Mutant Tau Show Significantly Reduced MT Binding
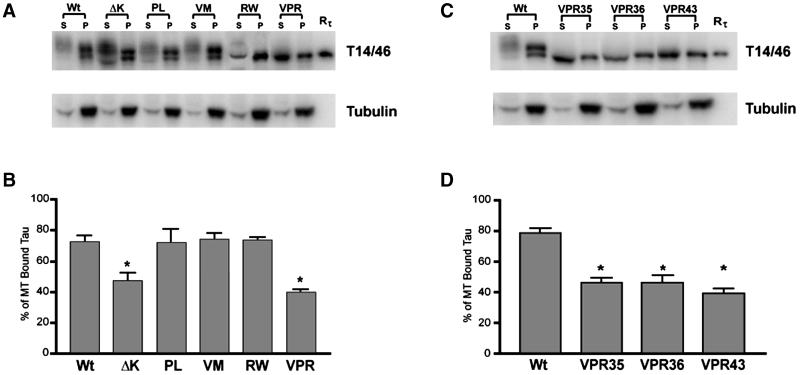
To further confirm that ΔK and VPR tau mutants expressed in CHO cells lead to reduced binding to MTs, we compared the ability of Wt and mutant tau proteins extracted from CHO cells to bind to endogenous MTs. As shown in Figure 7, A and B, the amount of tau bound to MTs and recovered from the pellets comprised ∼75% of the total tau in CHO cell transfectants expressing the Wt tau, and PL, VM, and RW tau mutants, whereas only 48 and 40% of bound tau proteins were recovered from cells expressing the ΔK and VPR tau mutants, respectively. The specific reduction of the VPR tau mutants to MTs was further substantiated by similar data from three different subclones of VPR transfectants (Figure 7, C and D). Finally, we showed that ∼80% of the tubulin was recovered in the pellet in all tau transfectants (Figure 7, A and C).
Figure 7.
FTDP-17 missense mutations decrease the binding of tau to MT. Cell lysates from CHO cells stably expressing Wt or mutant tau were separated into cytoskeletal (P) and soluble fractions (S) after MT assembly. The amount of tau and α-tubulin present in each fraction was determine by immunoblot analysis with I125-conjugated secondary antibody for quantification (A and C). (B and D) Quantitation of the percentage of tau in the soluble fraction relative to the total amount of tau present. Note that the ΔK and VPR mutations significantly reduce the binding of tau to MT, leaving more of these mutant proteins free in the soluble fraction. (n = 4, *p < 0.01), and that the dramatic loss of MT binding was evident in three different CHO cell clones that expressed the VPR tau mutants (C and D).
Abundant Tau Aggregates Are Present in ΔK- and VPR-Expressing CHO Cells
To further investigate the inclusions formed by the ΔK and VPR tau mutants, transfected CHO cells were maintained on coverslips for either 1 or 3 d. After 1 d in culture, numerous small tau-positive aggregates were detected (Figure 8A), which did not disrupt the MT network (Figure 8B). However, after 3 d in culture, much larger, variably shaped tau-positive aggregates (∼1–3 per cell) appeared in >80% of the CHO cells expressing the ΔK and VPR tau mutations (Figure 8C). These tau aggregates also did not perturb the MT network (Figure 8D). To determine whether tau filaments were found in the small and large tau inclusions, transmission EM and immuno-EM studies were conducted. Strong tau-positive staining was localized to both the small and large tau aggregates (Figure 8, E–H). Occasional filaments were also detected in the aggregates by transmission EM (Figure 9, A and B), but they were better visualized after formic acid extraction (Figure 9, C and D). The tau-positive aggregates were not detected by histochemical dye such as Thioflavin S, indicating that there is probably not sufficient numbers of filaments formed and/or that there is insufficient cross β-pleated sheet structures in these inclusions.
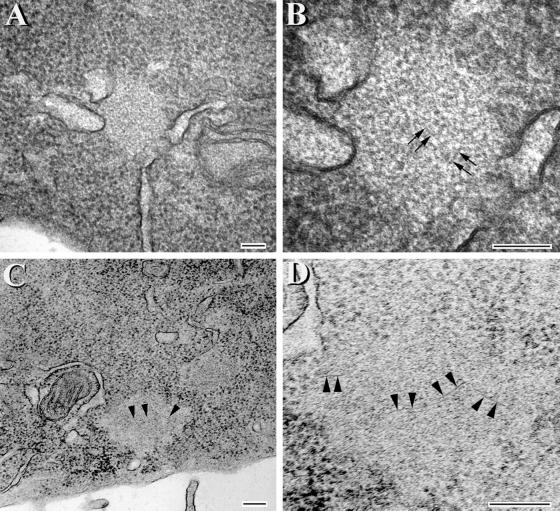
Figure 9.
Aggregates with fibrillar structures are detected in CHO cells expressing the VPR tau mutants. Transmission EM of tau aggregates at low (A and C) and corresponding high (B and D) magnifications. (C and D) Images of aggregates that were treated with formic acid. The image in D is a higher magnification of the lower left portion of the inclusion seen in C. Arrowheads in C correspond to the same region that is marked by arrowheads at higher magnification in D. Arrows in B and D highlight fibril-like structures within the aggregates. Bar, 100 nm.
Tau with ΔK and VPR Mutations Becomes More Insoluble than Other Tau Isoforms Expressed in Transfected CHO Cells
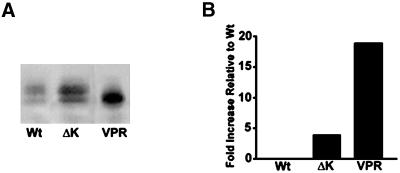
To assess whether the formation of tau aggregates correlated with the accumulation of insoluble mutant tau proteins, we compared the amount of insoluble tau recovered from tau transfectants expressing either Wt tau or the ΔK or VPR tau mutants after extraction with high-salt RAB buffer containing 0.1% Triton X-100. We found a significant increase in the amount of insoluble tau recovered from CHO cells expressing the ΔK (∼5 fold) and the VPR tau mutants (∼17 fold) compared with the amount detected in Wt tau-expressing CHO cells (Figure 10, A and B).
Figure 10.
FTDP-17 tau mutant become increasingly insoluble in CHO cells. Soluble and insoluble fractions of tau from CHO cells expressing Wt and FTDP-17 mutant tau protein were isolated and evaluated by immunoblot analysis with Tau14/46 (A). Quantitative analysis (n = 3) of the percentage of insoluble tau relative to the soluble fraction in Wt-, ΔK-, and VPR-expressing CHO cells (B). There is an increase in the amount of insoluble tau present in cells expressing the ΔK and VPR tau mutants relative to Wt tau.
DISCUSSION
Our study demonstrates for the first time that the overexpression of specific FTDP-17 tau mutants in CHO cells leads to the formation of intracytoplasmic tau aggregates that can be detected by indirect immunofluorescence, transmission EM, and immuno-EM. Furthermore, the presence of tau aggregates correlates with mutant tau proteins that remain insoluble in nonionic detergents such as Triton X-100. Other evidence also supports the view that topographically separate missense mutations in the tau gene pathogenic for FTDP-17 differentially alter the biochemical properties and/or functions of the corresponding tau mutants in transfected non-neuronal cells. Specifically, our data show that some missense mutations alter the phosphorylation of tau at specific sites and other mutations reduce the MT binding ability of tau. Although evidence is emerging to suggest that topographically distinct intronic and exonic FTDP-17 tau gene mutations result in losses of different tau functions and/or gains of toxic properties by tau isoforms (Clark et al., 1998; Hasegawa et al., 1998; Hong et al., 1998; Hutton et al., 1998; D'Souza et al., 1999; Dayanandan et al., 1999; Matsumura et al., 1999), the study reported here comprehensively analyzed and compared the consequences of diverse FTDP-17 missense substitutions on the biochemical and functional properties of tau mutants expressed in stably transfected cells. Significantly, we demonstrated a direct correlation between missense substitutions that reduce the ability of the corresponding tau mutants to bind MTs and the formation of intracytoplasmic accumulations of insoluble tau aggregates. Thus, our findings support the hypothesis that a reduction in the binding of tau to MTs, concomitant with increased levels of unbound tau proteins, could initiate a pathological cascade leading to the aggregation and assembly of tau into abnormal filaments.
It is well known that phosphorylation regulates the binding of tau to MTs, that increased phosphorylation at specific sites in tau reduces MT binding, and that hyperphosphorylated PHF-tau is completely unable to bind MTs, but that this function can be restored by enzymatic dephosphorylation of PHF-tau (Bramblett et al., 1993; Yoshida and Ihara, 1993). However, it is unclear whether phosphorylation plays a role in the pathogenesis of the topographically separate FTDP-17 missense tau mutations. Indirect evidence from our studies showed that a change in the secondary structure of tau rather than phosphorylation mediates the pathogenicity of some of the missense mutations. For example, we demonstrated that the RW mutation causes a selective reduction in tau phosphorylation at Ser396 and Ser404 and that this reduction is most likely a consequence of altered secondary structure around the site of R406W mutation. The data to support this idea are as follows. First, the Ser396 and Ser404 residues are the only phosphorylation sites that are affected and they are located close to the R406W residue. This implies that a change in local secondary structure could impede phosphorylation by specific kinases. Second, differences in the pattern of proteolytic fragments generated from the RW mutant compared with Wt tau suggest differential accessibility of Wt and RW mutant tau to endogenous proteases. Third, the observation that nonphosphorylated, bacterially expressed recombinant RW mutant tau bind less well to MTs compared with Wt tau suggests that phosphorylation is not responsible for this reduction (Hasegawa et al., 1998; Hong et al., 1998). Fourth, because the R406W mutation is not located on a MT binding repeat or an inter-repeat region, it should not alter MT binding directly. Finally, the reduction in phosphorylation at Ser396, which we observed in tau proteins extracted from the brains of affected members of a kindred with a RW tau gene mutation, lend further support to our experimental results (Reed et al., 1997; our unpublished observation). Thus, our data are consistent with a change in the secondary structure induced by the RW mutation.
Previous in vitro studies have shown that recombinant PL, VM, RW, and ΔK tau mutants isolated from genetically engineered E. coli have a reduced binding affinity for MTs (Hasegawa et al., 1998; Hong et al., 1998; D'Souza et al., 1999). However, when expressed in transfected CHO cells, none of these tau mutants, except for the ΔK tau mutant, exhibited reduced MT binding. The reason for this discrepancy is most likely due to technical limitations in the ability to control precisely the expression of tau protein in transfected cells such that a small reduction in the affinity of tau for MT cannot be detected. This hypothesis is supported by the observation that introduction of three mutations (VPR) in a single tau isoform amplifies this reduction to levels that can be readily observed. In contrast, the single ΔK mutation caused a significant reduction in the MT binding ability of the corresponding tau mutant. Indeed, the 280K residue, which is located in the inter-repeat region of tau between MT binding repeat 1 and 2, was identified previously as one of three lysine residues that is most critical in modulating the binding of tau to MTs (Goode and Feinstein, 1994). Furthermore, our previous in vitro data on MT binding showed that the ΔK mutation caused the most dramatic reduction in the MT binding affinity of tau compared with other tau mutants harboring one of several different missense substitutions (Hong et al., 1998; D'Souza et al., 1999). Additionally, the ΔK mutation perturbs the alternative splicing of tau resulting in the diminished inclusion of exon 10 (D'Souza et al., 1999). Thus, the ΔK missense mutation may be pathogenic for FTDP-17 by disrupting mechanisms that regulate the expression of the tau gene and/or by altering biochemical properties of tau isoforms that are critical for the function and viability of CNS neurons and glia.
Indeed, multiple pathogenic mechanisms have been proposed for the diverse FTDP-17 mutations and several FTDP-17 mutations appear to cause tau dysfunction by reduced MT binding and/or promoting filament formation. We speculate that the reduced binding of tau to MTs is an initiating event that leads to the formation of abnormal tau filaments and/or the aggregation of tau. This hypothesis is supported by several lines of evidence from our studies of the ΔK and VPR tau mutants. First, the ΔK and VPR tau mutants were the only two mutants that evidenced a reduction in the ability to bind MTs, and they also were the only tau mutants that aggregated into tau-rich inclusions in the cytoplasm of CHO cells. Second, although other FTDP-17 missense mutations (e.g., PL) were shown to facilitate the aggregation of bacterially expressed recombinant tau in the presence of heparin (Goedert et al., 1999; Nacharaju et al., 1999; Gamblin et al., 2000), none of the other tau mutants (including PL) we studied in CHO cells developed detectable intracytoplasmic tau aggregates by using the criteria established here. Finally, because the turnover rate of Wt tau and all the tau mutants in CHO transfectants was similar, it seems unlikely that the tau pathologies caused by the ΔK and VPR mutations are the result of decreased turnover of the mutant tau proteins. Instead, our data are consistent with the interpretation that these mutations may be pathogenic because they cause a reduction in the binding of tau to MTs, resulting in an increase in the cytosolic concentration of tau that culminates in aggregation of tau in the cytoplasm. However, we cannot rule out the possibility that these specific mutations also could promote tau aggregation and filament formation by other mechanisms.
Although filaments were detected within aggregates that also were decorated by anti-tau antibodies, these filaments are not identical to authentic PHFs in Alzheimer's disease or tau filaments in tangles of FTDP-17 patients. This is not surprising because tau tangles undoubtedly develop over a long period of time and they are found in postmitotic neurons and nondividing glial cells. Indeed, our observations that the aggregates become larger in mutant CHO transfectants cultured for 3 versus 1 d support the idea that the formation of tau tangles may be a slow process. Nevertheless, our ability to demonstrate the formation of some filaments within the tau aggregates in dividing non-neuronal CHO cells provides proof of the concept that increasing levels of cytosolic mutant tau proteins can lead to the assembly of tau that aggregate into inclusions, and the identification of specific tau mutants that form insoluble fibrillar aggregates will facilitate future efforts to develop in vitro models of PHFs in postmitotic neurons and glial cells. Finally, because we link the formation of tau aggregates to reduced MT binding caused by specific mutations, these data support the hypothesis that both gains of toxic properties and losses of normal tau functions are involved in the onset/progression of neurodegenerative tauopathies. Thus, the CHO cell mutant tau model system described here will be useful in studies designed to further elucidate mechanisms leading to the formation of tau pathology in these diseases.
ACKNOWLEDGMENTS
We thank the Biomedical Imaging Core Facility of the University of Pennsylvania for their assistance in the EM studies. Supported in part by grants from the National Institute on Aging and the John H. Ware III Endowed Chair for Alzheimer's Disease Research.
REFERENCES
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–10633. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Slaughter T, Moshiach S, Obrocka M, Fischer I. Tau is enriched on dynamic microtubules in the distal region of growing axons. J Neurosci. 1996;16:3601–3619. doi: 10.1523/JNEUROSCI.16-11-03601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose SH, Meltzer DI, Feramisco JR. 10 nm Filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984;98:847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency. transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Poorkaj P, Wszolek ZK, Geschwind DH, Nasreddine ZS, Miller B, Payami H, Awert F, Markopoulou K, D'Souza I, Lee VM-Y, Reed LA, Trojanowski JQ, Zhukareva V, Bird TD, Schellenberg GD, Wilhelmsen K. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related chromosome 17-linked neurodegenerative disorders. Proc Natl Acad Sci USA. 1998;95:13103–13107. doi: 10.1073/pnas.95.22.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza I, Poorkaj P, Hong M, Nochlin D, Lee VM-Y, Bird TD, Schellenberg GD. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci USA. 1999;96:5598–5603. doi: 10.1073/pnas.96.10.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanandan R, Van SM, Mack TG, Ko L, Yen SH, Leroy K, Brion JP, Anderton BH, Hutton M, Lovestone S. Mutations in tau reduce its microtubule binding properties in intact cells and affect its phosphorylation. FEBS Lett. 1999;446:228–232. doi: 10.1016/s0014-5793(99)00222-7. [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobbs MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinsesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implication for Alzheimer's disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, King ME, Dawson H, Vitek MP, Kuret J, Berry RW, Binder LI. In vitro polymerization of tau proteins monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry. 2000;39:6136–6144. doi: 10.1021/bi000201f. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes RA, Crowther RA. Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett. 1999;450:306–311. doi: 10.1016/s0014-5793(99)00508-6. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Cohen P, Vanmechelen E, Vandermeeren M, Cras P. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer's disease: identification of phosphorylation sites in tau protein. Biochem J. 1994;301:871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Six J, Lubke U, Vandermeeren M, Cras P, Trojanowski JQ, Lee VM-Y. The abnormal phosphorylation of tau protein at Ser202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci USA. 1993;90:5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M, Trojanowski JQ, Lee VM-Y. The neurofibrillary pathology of Alzheimer's disease. In: Prusiner SB, Rosenberg RN, Di Mauro S, Barchi RL, editors. The Molecular and Genetic Basis of Neurological Diseases. Boston: Butterworth Heineman Press; 1997. pp. 613–627. [Google Scholar]
- Goode BL, Feinstein SC. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J Cell Biol. 1994;124:769–782. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 1998;437:207–210. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Lee VM-Y, Leight S, Varga I, Otvos LJ. Unique Alzheimer's. disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry. 1997;36:8114–8124. doi: 10.1021/bi970380+. [DOI] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM-Y. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett A, Adamson J, Lincoln S, Dickson D, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Iijima M, Tabira T, Poorkaj P, Schellenberg GD, Trojanowski JQ, Lee VM-Y, Schmidt ML, Takahashi K, Nabika T, Matsumoto T, Yamashita Y, Yoshioka S, Ishino H. A. distinct familial presenile dementia with a novel missense mutation in the tau gene. NeuroReport. 1999;10:497–501. doi: 10.1097/00001756-199902250-00010. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM-Y. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Takemura R, Oshima T, Mori H, Ihara Y, Yanagisawa M, Masaki T, Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989;109:1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM-Y, Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68. A major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Matsumura N, Yamazaki T, Ihara Y. Stable expression in Chinese hamster ovary cells of mutated tau genes causing frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) Am J Pathol. 1999;154:1649–1656. doi: 10.1016/S0002-9440(10)65420-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick SE, Demoise DC, Lee VM-Y. Site-specific dephosphorylation of tau protein at Ser202/Thr205 in response to microtubule depolymerization in cultured human neurons involves protein phosphatase 2A. J Biol Chem. 1996;271:5589–5594. doi: 10.1074/jbc.271.10.5589. [DOI] [PubMed] [Google Scholar]
- Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, Yen SH. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett. 1999;447:195–199. doi: 10.1016/s0014-5793(99)00294-x. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM-Y. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind R, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van HG, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ. Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol. 1997;42:564–572. doi: 10.1002/ana.410420406. [DOI] [PubMed] [Google Scholar]
- Rizzu P, Van Swieten JC, Joosse M, Hasegawa M, Stevens M, Tibben A, Niermeijer MF, Hillebrand M, Ravid R, Oostra BA, Goedert M, Van DC, Heutink P. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet. 1999;64:414–421. doi: 10.1086/302256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P, Mawal-Dewan M, Barbour B, Jakes R, Goedert M, Johnson GVW, Litersky JM, Schenk D, Lieberburg I, Trojanowski JQ, Lee VM-Y. Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem. 1995;270:18917–18922. doi: 10.1074/jbc.270.32.18917. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- Szendrei GI, Lee VM-Y, Otvos LJ. Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J Neurosci Res. 1993;34:243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R, Wortel J, Romijn HJ, Buijs RM. A simple silver-gold intensification procedure for double DAB labeling studies in electron microscopy. J Histochem Cytochem. 1997;45:619–621. doi: 10.1177/002215549704500414. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Schuck T, Schmidt ML, Lee VM-Y. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989;37:209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Tu P-H, Elder G, Lazzarini RA, Nelson D, Trojanowski JQ, Lee VM-Y. Overexpression of the human NFM subunit in transgenic mice modifies the level of endogenous NFL and the phosphorylation state of NFH subunits. J Cell Biol. 1995;129:1629–1640. doi: 10.1083/jcb.129.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Trojanowski JQ, Lee VM-Y. Cell biology of tau and cytoskeletal pathology in Alzheimer's disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer's Disease. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 359–371. [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Ihara Y. Tau in paired helical filaments is functionally distinct from fetal tau: assembly incompetence of paired helical filament-tau. J Neurochem. 1993;61:1183–1186. doi: 10.1111/j.1471-4159.1993.tb03642.x. [DOI] [PubMed] [Google Scholar]