Abstract
Leaf-moving organs, remarkable for the rhythmic volume changes of their motor cells, served as a model system in which to study the regulation of membrane water fluxes. Two plasma membrane intrinsic protein homolog genes, SsAQP1 and SsAQP2, were cloned from these organs and characterized as aquaporins in Xenopus laevis oocytes. Osmotic water permeability (Pf) was 10 times higher in SsAQP2-expressing oocytes than in SsAQP1-expressing oocytes. SsAQP1 was found to be glycerol permeable, and SsAQP2 was inhibited by 0.5 mM HgCl2 and by 1 mM phloretin. The aquaporin mRNA levels differed in their spatial distribution in the leaf and were regulated diurnally in phase with leaflet movements. Additionally, SsAQP2 transcription was under circadian control. The Pf of motor cell protoplasts was regulated diurnally as well: the morning and/or evening Pf increases were inhibited by 50 μM HgCl2, by 2 mM cycloheximide, and by 250 μM phloretin to the noon Pf level. Our results link SsAQP2 to the physiological function of rhythmic cell volume changes.
INTRODUCTION
In view of the function of aquaporins in facilitating water transport through membranes (Maurel, 1997; Agre et al., 1998; Kaldenhoff et al., 1998; Chrispeels et al., 1999; Tyerman et al., 1999; Verkman and Mitra, 2000), it is tempting to assume that these proteins play a significant role in the redistribution of water during the movements of the pulvini of the leguminous Mimosacea tree Samanea saman. These motor organs are responsible for the movement of leaves and leaflets (Figure 1), governed by diurnal and circadian rhythms. The movements result from coordinated and simultaneous volume changes of cortex cells on opposing sides of the pulvinus. Swelling of the extensor cells and shrinking of the flexor cells results in straightening of the pulvinus. During bending, the situation is reversed. According to the current paradigm, the mechanisms of cellular swelling and shrinking are similar in stomatal guard cells and pulvinar motor cells (see reviews by Satter and Moran, 1988; Hedrich and Schroeder, 1989; Schroeder and Hedrich, 1989; Moran et al., 1996). Proton pump–powered fluxes of ions, chiefly KCl, govern the changes in solute content of the motor cells, and these, in turn, drive osmotic water fluxes. The secondary pulvini have become a model for studies of membrane transport regulation because of their massive transcellular ion and water fluxes (Palmer and Asprey, 1958; Satter et al., 1979; Gorton, 1987a, 1987b), their relatively large size, and their convenience of manipulation (reviewed by Satter and Galston, 1981; Moran et al., 1988; Satter et al., 1988; Moran, 1990, 1996).
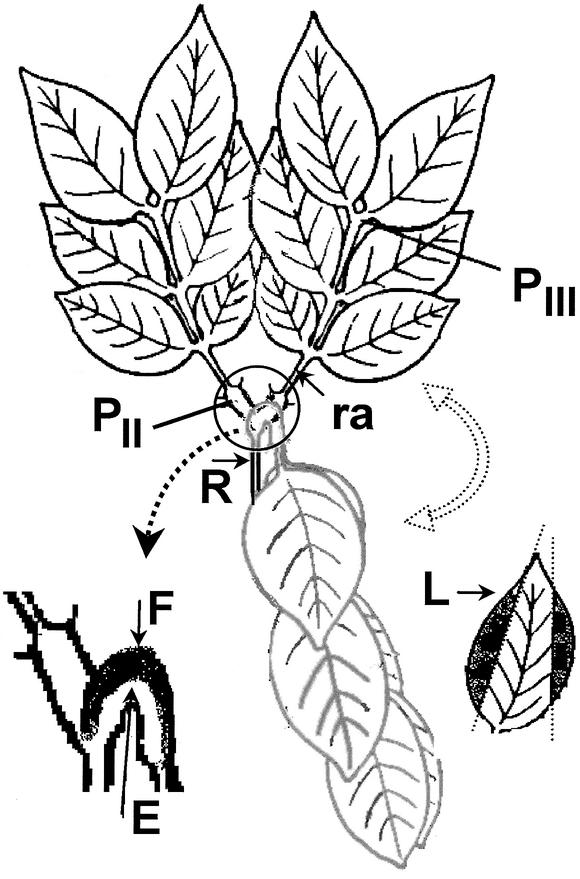
Figure 1.
Scheme of the Top Part of the Leaf of Samanea Displaying Movement between the Open and the Folded Positions (Black and Gray, Respectively).
E and F, extensor and flexor regions, respectively, of the secondary pulvinus (the circled region is enlarged); L (blackened areas), leaf blades (the larger veins are excluded); PII and PIII, secondary and tertiary pulvini, respectively; R, rachis; ra, rachilla. (Modified from Moshelion et al. [2002]).
The notion that aquaporins play a role in pulvinar movements is supported by the correlation between the maturation of the pulvini of Mimosa pudica and the expression of a putative aquaporin related to a tonoplast intrinsic protein in the pulvini (Fleurat-Lessard et al., 1997). However, the plasma membrane aquaporins of the motor organs have not been described, although their function might be even more important for motor cells than that of tonoplast aquaporins (Tyerman et al., 1999).
We addressed the question of the regulation of plasma membrane aquaporins in the context of the rhythmic and reversible cell volume changes in our model system, the pulvini of Samanea. We assumed that the driving force for the transmembrane water movement is the difference in water potential resulting from the fluxes of ions and asked whether the presence and regulation of aquaporins play a role in the rhythmic pulvinar movement.
Our findings appear to support such a notion. Here we present two aquaporins from the pulvinar tissues—new members of the plasma membrane intrinsic protein (PIP) family—representing two classes with strikingly different functional characteristics. Both aquaporins are expressed in a diurnal rhythm. A diurnal rhythm also appears to govern the osmotic water permeability (Pf) of the protoplast membrane, although with a slightly different pattern. In addition, the mRNA of one of these aquaporins, the water-selective aquaporin, is expressed in a circadian rhythm, which could be important for the circadian rhythm of motor cell volume changes.
RESULTS
To study the regulation of water transporters in Samanea motor cells, a pulvinar cDNA library (Moshelion et al., 1998) was screened using a probe derived from Arabidopsis PIP1b (or Pip1;02 according to the recently proposed nomenclature; Johanson et al., 2001). We isolated two new clones of PIP homologs, SsAQP1 (for Samanea saman Aqua-porin 1) and SsAQP2 (for Samanea saman Aquaporin 2).
Sequence Comparison
Sequence comparison of the encoded polypeptides revealed that SsAQP1 belongs to the PIP1 family and SsAQP2 belongs to the PIP2 family of aquaporins (Figure 2). Although SsAQP1 and SsAQP2 are strikingly different from each other at the C terminus, they are ∼62% identical and 73% similar to each other. They are even closer to their respective Arabidopsis homologs, PIP1b (i.e., Pip1;02) and PIP2b (i.e., Pip2;02; Johanson et al., 2001) (83% identical and 90 to 91% similar). The NPA motifs, and the number and positions of cysteine residues, which are possible targets for an interaction with pore-blocking heavy metal ions, are identical to those of PIP1b (Kaldenhoff et al., 1993) and PIP2b (Kammerloher et al., 1994), respectively (Figure 2).
Figure 2.
Alignment of Predicted Amino Acid Sequences of Samanea Aquaporins SsAQP1 and SsAQP2 with Those of PIP1b and PIP2b (i.e., Pip1;02 and Pip2;02) from Arabidopsis.
Identical amino acids are shown in black boxes, and similar amino acids are shown in gray boxes (darker shading indicates a higher level of similarity; for details, see Methods). C, cysteines, the putative sites for inhibition by heavy metals; NPA, conserved pore regions.
Functional Analysis of Samanea Aquaporins in Oocytes
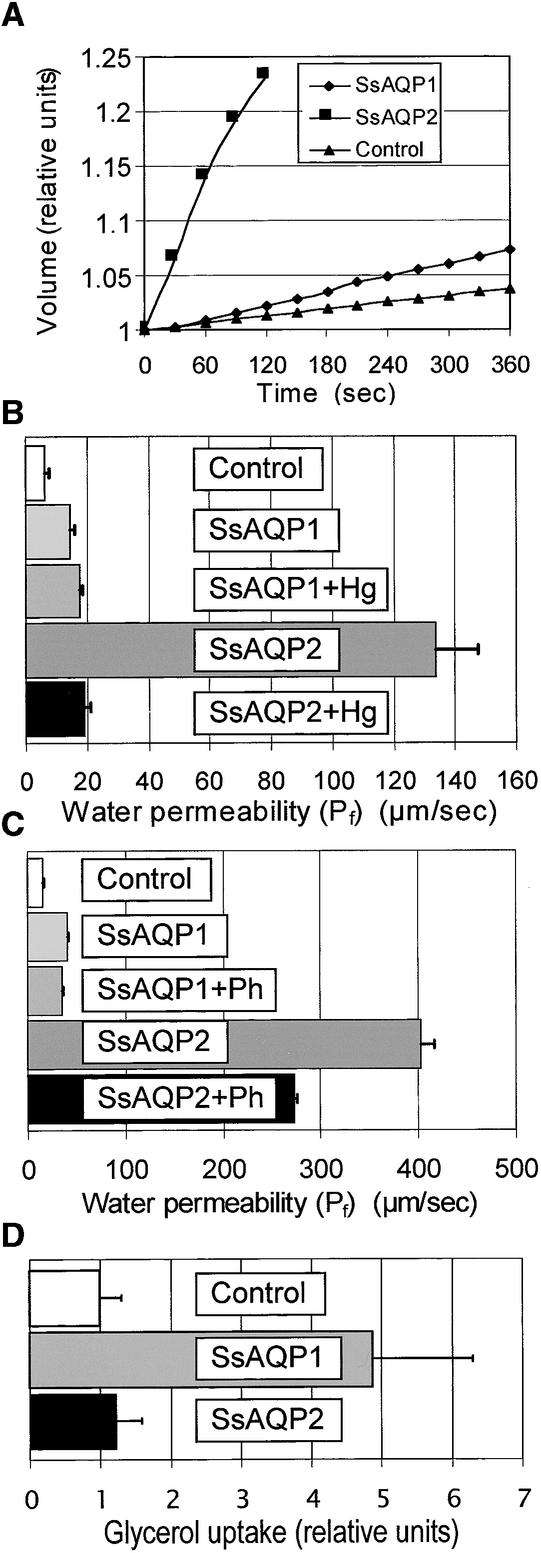
The water transport activities of SsAQP1 and SsAQP2 were assayed in Xenopus laevis oocytes. Three days after complementary RNA (cRNA) injection, we determined the water uptake rates in oocytes exposed to hypoosmotic solutions. From the initial rate of increase in oocyte volume (Figure 3A), we calculated their osmotic Pf values (Figure 3B) and compared them with those of water-injected control oocytes (Table 1). Oocytes expressing SsAQP1 were only approximately twice as permeable to water as water-injected oocytes. Remarkably, oocytes expressing SsAQP2 yielded Pf values approximately 10-fold higher than those expressing SsAQP1. A 30-min preincubation of oocytes injected with SsAQP2 cRNA in a solution containing 0.5 mM HgCl2 decreased the water permeability drastically (P < 0.001). In contrast, oocytes expressing SsAQP1 were unaffected by mercury treatment, indicating that only SsAQP2 is sensitive to mercurials (Table 1).
Figure 3.
Functional Expression in Oocytes.
(A) Swelling kinetics of oocytes in hypoosmotic conditions injected with water (control) or cRNAs of SsAQP1 or SsAQP2, as indicated.
(B) Calculated Pf of oocytes in (A) under hypoosmotic conditions (mean ±sd) with or without 0.5 mM HgCl2.
(C) Calculated Pf of oocytes in (A) under hypoosmotic conditions (mean ±sd) with or without 1 mM phloretin (Ph).
(D) Uptake of 3H-glycerol during the first 10 min of exposure relative to water-injected controls.
Table 1.
Osmotic Water Permeability of Oocytes
| Expression | Nontreated | HgCl2 (0.5 mM) | Phloretin (1 mM) | DMSO (0.4%) |
|---|---|---|---|---|
| Water-injected | ||||
| a | 6.4 ± 0.5 (28) | |||
| b | 15.2 ± 1.6 (11) | 17.0 ± 1.0 (11) | ||
| c | 24.3 ± 2.7 (10) | 28.3 ± 1.7 (10) | ||
| SsAQP1 | ||||
| a | 14.6 ± 0.4 (15) | 17.5 ± 0.4 (12) | ||
| b | 34.5 ± 3.1 (11) | 40.6 ± 5.1 (13) | ||
| SsAQP2 | ||||
| a | 133.6 ± 3.3 (18) | 19.3 ± 0.8 (6) | ||
| b | 402 ± 26 (13) | 273 ± 13.3 (10) | ||
| c | 511.2 ± 31.3 (16) | 649.9 ± 36.1 (11) |
Numbers are mean permeability values (μm/sec) ±se (n) determined in oocytes injected with water or mRNA of the indicated genes under different experimental conditions (columns). a, b, and c denote separate batches of oocytes.
In addition, phloretin, another transport blocker (Dordas et al., 2000), inhibited SsAQP2 selectively (Figure 3C). Although a 30-min preincubation in 1 mM phloretin decreased the Pf of SsAQP2-expressing oocytes by ∼40% (P < 0.001), the Pf of SsAQP1-expressing oocytes did not differ between phloretin-treated and phloretin-untreated cells (Figure 3C, Table 1). Furthermore, phloretin treatment did not affect the water permeability of water-injected oocytes (Table 1). The presence of DMSO in the phloretin solution (see Methods) may have counteracted the phloretin effect; when DMSO was applied alone at the same dilution, it increased the water permeability of SsAP2-expressing oocytes by ∼30% (P < 0.02; Table 1). This may explain why the phloretin block of SsAQP2 was not as effective as the HgCl2 block. The DMSO effect on the basal water permeability of water-injected oocytes was insignificant (Table 1).
To analyze the transport specificity of the Samanea aquaporins, oocytes expressing the cRNA- and water-injected controls were incubated in isotonic medium supplemented with 3H-glycerol. The mean 3H-glycerol uptake of oocytes expressing SsAQP1 or SsAQP2 was compared with the mean 3H-glycerol uptake of controls (Figure 3D). The average accumulation within 10 min was 37 ± 15 (±se) × 10−12 mol/oocyte in controls (n = 11), 181 ± 51 × 10−12 mol/oocyte in oocytes with SsAQP1 (n = 9), and 52 ± 15 × 10−12 mol/oocyte in oocytes with SsAQP2 (n = 8). Thus, the glycerol uptake of SsAQP1-expressing oocytes was fivefold higher than that of the control oocytes (4.9 ± 1.4 in relative units; Figure 3D), demonstrating that SsAQP1 was not selective for water. In contrast, the mean 3H-glycerol uptake of SsAQP2-expressing oocytes did not differ from that of the control oocytes (1.2 ± 0.4 in relative units; Figure 3D), indicating that SsAQP2 was highly specific for water over glycerol.
Diurnal Rhythm in the Pf of Pulvinar Protoplasts
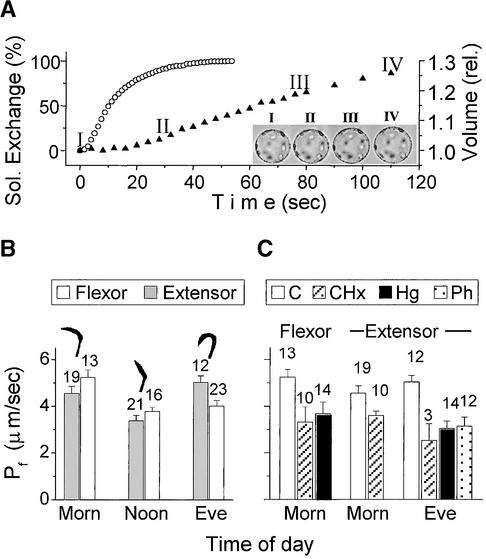
The regulation of the Samanea aquaporins in situ might be revealed through changes of the Pf of the plant cell plasma membrane during the day. To test this hypothesis, we calculated the Pf values of protoplasts challenged by a 30% hypotonic medium (Figure 4). The protoplast swelling response to the hypotonic challenge started 17.6 ± 2 sec (±sd; n = 10) after the beginning of the influx of the hypotonic solution, that is, not until the solution exchange was ∼80 to 90% complete (Figure 4A). Within 110 sec in the hypotonic solution, the protoplast volume increased by 26% ± 3% (mean ±se; n = 10). At this stage, the volume changes were fully reversible, and upon switching back to the isotonic solution, the cells relaxed to 100.9% ± 0.4% (mean ±se; n = 9) of their original size, demonstrating that they were true, nonregulating osmometers. At noon, the mean Pf value of extensor protoplasts was 3.3 ± 0.2 μm/sec and that of flexor protoplasts was 3.7 ± 0.2 μm/sec. These values (corresponding to an open leaf) were significantly smaller than the Pf values of the same-day morning, 4.5 ± 0.3 and 5.2 ± 0.3 μm/sec, respectively (corresponding to an unfolding leaf in motion). In extensors, Pf increased again between noon and evening, to 5.0 ± 0.1 μm/sec (also corresponding to a leaf in motion, in this case, a folding one). In flexors, no such increase was noted. The Pf values did not change further between 9 and 12 pm (4.0 ± 0.3 μm/sec in five flexors and 4.8 ± 0.3 μm/sec in seven extensors).
Figure 4.
Diurnal Variation in the Pf of Motor Cell Protoplasts.
(A) Comparison between the time courses of bath solution (Sol.) exchange, reported by fluorescence of acridine orange in the incoming solution (open circles), and extensor protoplast volume increase since the start of the hypotonic solution influx (closed triangles). The Pf of this protoplast was 4.7 μm/sec. Note the delay in the start of the volume increase. The inset shows images of the protoplast recorded at the times indicated by the corresponding numerals on the volume-time curve. rel, relative units.
(B) Distribution of Pf values during the day after the day of their isolation. Pulvinus angles between rachis and rachilla (see Figure 1) are shown above the columns.
(C) Treatment with cycloheximide (CHx; 2 mM), HgCl2 (Hg; 50 μM), and phloretin (Ph; 250 μM) compared with the untreated protoplasts (C) from (B).
In (B) and (C), columns denote means ±se, and numerals above columns indicate number of cells. Eve, evening; Morn, morning.
To study the role of protein synthesis in the regulation of water permeability, we assayed the Pf of protoplasts after 7 to 12 hr of preincubation with 2 mM cycloheximide, an inhibitor of protein translation. Cycloheximide decreased the values of Pf in all cases in which water permeability was initially increased, i.e., in the morning and evening in extensors and in the morning in flexors (from 4.6 ± 0.3 to 3.6 ± 0.2 μm/sec, from 5.0 ± 0.3 to 2.5 ± 0.7 μm/sec, and from 5.2 ± 0.3 to 3.3 ± 0.6 μm/sec, respectively; Figure 4C). Cycloheximide did not have any effect at the other times tested (noon in extensors [n = 8] and noon [n = 9] and evening [n = 7] in flexors; data not shown).
In an attempt to determine which type of aquaporin could be involved in the increased protoplast water permeability, we took advantage of the differences in the susceptibility to mercury and phloretin of SsAQP1 and SsAQP2 (Figures 3B and 3C, Table 1). Fourteen flexors were exposed to 50 μM HgCl2 in the morning and 14 extensors were exposed to 50 μM HgCl2 in the evening. HgCl2 slowed the swelling of the protoplasts in the hypotonic medium, decreasing Pf in extensors by ∼40%, to 3.0 ± 0.3 μm/sec (±se), and decreasing Pf in flexors by ∼30%, to 3.7 ± 0.5 μm/sec (Figure 4C). Higher concentrations of HgCl2 (i.e., up to 1 mM) resulted in a pronounced granular appearance and consistently caused severe distortions of cell shape upon exposure to the hypotonic solution. Even in 50 μM HgCl2, cytoplasmic streaming ceased and the protoplasts assumed a somewhat granular appearance with a rather rough circumference.
To address the concern that HgCl2 might disrupt the protoplast membrane and invalidate the Pf measurements, we tested (in the morning) the true, nonregulating osmometer behavior of nine additional flexor protoplasts treated with HgCl2 for 5 to 10 min. They were allowed to swell significantly for ∼1 to 2 min in the hypotonic solution, increasing their volume by an average 9.4% ± 2.1% (P < 0.05), and then to reequilibrate with the isotonic solution, returning (on average) to 97.8% ± 2.2% of their initial volume. This finding indicates that the protoplasts appear to have preserved their true osmometer behavior in 50 μM HgCl2. However, in spite of this result and of earlier observations of the lack of HgCl2 effect on the water permeability of the membrane matrix of liposomes (Zeidel et al., 1992), we cannot exclude other, possibly disruptive HgCl2 effects (Ramahaleo et al., 1999). Like Ramahaleo et al. (1999), we were unable to reverse HgCl2 inhibition by subsequent washing and incubation of the protoplasts in 1 to 2 mM dithiothreitol (DTT) for 3 to 10 min (n = 28; data not shown).
The Pf of extensor protoplasts treated only with DTT (5- to 10-min treatments with 2 mM DTT, in the evening) was 4.9 ± 1.4 (±se; n = 9; data not shown). This treatment introduced almost a four times larger variability into the Pf values, although, on average, DTT per se did not appear to affect the mean Pf (cf. with controls; Figure 4B). Because the inhibitory effect of mercury was only ∼30 to 40% (Figure 4C), such large variability (not overcome even by paired comparisons) was likely to obscure any possible reversibility of HgCl2 inhibition by DTT.
Phloretin, a selective inhibitor of SsAQP2 activity in oocytes (Figure 3C), did not affect the protoplast appearance at all. However, after 10 min of preincubation at 250 μM, it decreased the Pf of extensor protoplasts in the evening by ∼40%, from 5.0 ± 0.3 μm/sec (n = 12) to 3.1 ± 0.4 μm/sec (n = 12; Figure 4C).
Localization of Aquaporin Transcripts in the Leaf
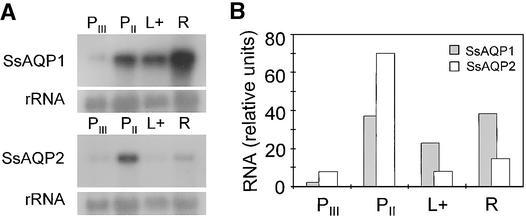
To correlate the regulation of water permeability and the regulation of aquaporin expression, we examined the spatial distribution of aquaporin mRNAs in leaves by RNA gel blot hybridization to mRNA from different leaf parts (Figures 5 to 8). The expression levels of both aquaporins in the secondary pulvini were much higher than in the tertiary pulvini (Figure 5); therefore, we did not continue sampling the tertiary pulvini. The SsAQP1 transcript also was abundant in the leaf blades and the rachis (Figures 5 and 7). In contrast, the SsAQP2 transcript was less abundant in the leaf blades, which do not participate in the mechanism of movement (Figures 6 and 8) (Satter and Galston, 1981). The SsAQP1 and SsAQP2 probes did not yield any signal on mRNA gel blots of Samanea roots (500 ng of poly[A] RNA per lane; data not shown).
Figure 5.
Distribution of Expression of Aquaporins in Morning Samples of the Samanea Leaf.
(A) Autoradiogram of RNA gel blots of total RNA from tertiary pulvini (PIII), secondary pulvini (PII), leaflet blades including midrib veins (L+), and segments of rachis (R) using probes to SsAQP1 or SsAQP2 and 18S rRNA.
(B) Aquaporin signals from (A) quantified by densitometry and normalized to the respective rRNAs.
Figure 8.
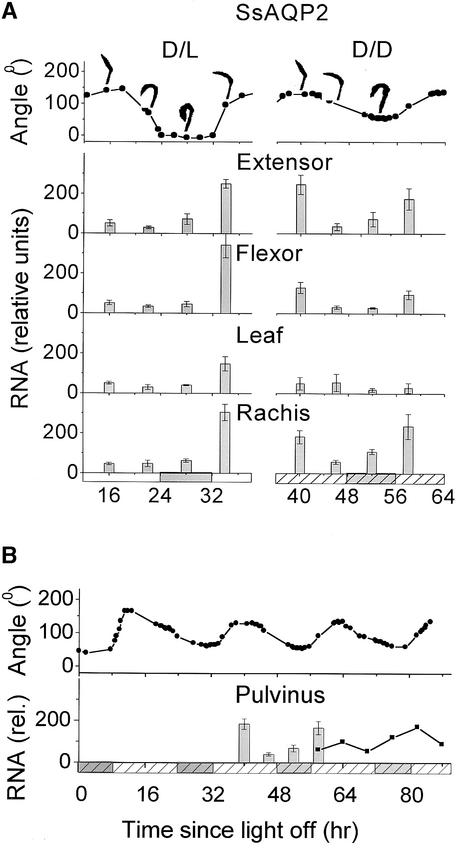
Temporal Pattern of Expression of SsAQP2.
(A) Top, angle between the rachis and the terminal rachilla in an intact, tree-attached leaf (same as in Figure 7). Bottom, SsAQP2 transcript levels, with four repeats in dark/light alternations. Other details as in Figure 7.
(B) Top, the angle record of (A), top, D/D, prolonged further in continuous darkness. Bottom, SsAQP2 transcript fluctuations in a quasi-whole pulvinus (averaged extensor, flexor, and rachis data of [A], D/D, columns) and in a true whole pulvinus (squares). Other details as in Figure 7. rel., relative units.
Figure 7.
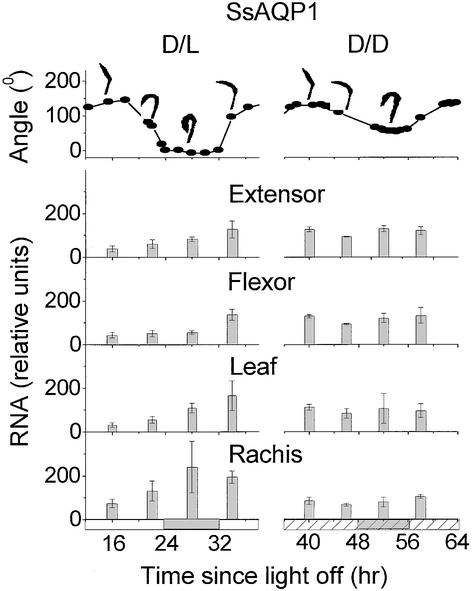
Temporal Pattern of Expression of SsAQP1.
Top, angle between the rachis and the terminal rachilla in an intact, tree-attached leaf (see Figure 1) in continuous darkness (D/D) and dark/light alternations (D/L). Pulvinus angles are illustrated above the traces. Bottom, SsAQP1 transcript levels in various leaf parts (leaf = leaflet blades without the major veins) detected by a probe and sampled during dark/light alternations or continuous darkness. The time count is in hours starting from when lights went off for the last time at the end of a normal day. Open horizontal bars, day illumination; closed horizontal bars, night; hatched horizontal bars, subjective day; closed hatched horizontal bars, subjective night. Columns (means ±se of three repeats) are clustered around the time of sampling (see Methods for details).
Figure 6.
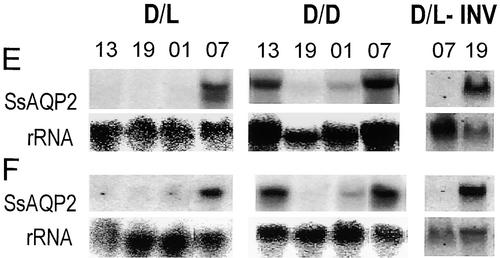
Rhythmic Variation of SsAQP2 Expression in the Motor Tissues.
Phosphorimager scans of RNA gel blots of total RNA from extensor or flexor parts of secondary pulvini with probes to SsAQP2 and 18S rRNA. Numbers are abbreviations of the time of sampling: day (13:00), evening (19:00), night (01:00), and morning (07:00). D/D, continuous darkness, measurements between 39 and 64 hr after lights went off at the end of a normal day; D/L, diurnal alternations of dark and light; D/L-INV, dark/light illumination inverted (leaves were harvested 7 days after the inversion); E, extensor; F, flexor.
Temporal Regulation of Aquaporin Expression
In our experimental conditions, leaf angle changed rhythmically, as described in previous reports (Palmer and Asprey, 1958; Satter and Morse, 1990), both during diurnal alterations of light and dark (Figures 7 and 8A, D/L, top) and during constant darkness (Figures 7 and 8A, D/D, top). In continuous darkness, the leaf angle continued to fluctuate rhythmically with a diminished, but constant, amplitude for at least 85 hr. The mean duration of the last two periods in this record was 23.3 hr (Figure 8B, top).
To detect a possible rhythm in aquaporin mRNA regulation in the leaf by diurnal dark/light alternations, we analyzed RNA gel blots of material sampled during three to four repeats of 24-hr series (16 samples each). These samples were from extensors and flexors of the secondary pulvinus, leaf blades, and parts of the rachis below the pulvinus, including the vascular bundle from within the pulvinus (illustrated in Figures 1 and 6 and summarized in Figures 7 and 8A). To test the circadian rhythms, we analyzed similarly the RNA of pulvinar tissues starting at 39 hr after the onset of darkness (i.e., 31 hr after the regular signal of “lights on” was omitted). At this time, the acute effects of the missing lights-on signal appear to have largely subsided and the rhythm of the leaf movement became free running (Figure 8B, top).
SsAQP1
Two-way analysis of variance of the transcript levels of SsAQP1 confirmed the observation that in dark/light conditions the morning levels of SsAQP1 mRNA were significantly higher than those in the preceding noon (P < 0.01) and that the levels were intermediate in other leaf parts (Figure 7, D/L; n = 3). In dark/light conditions, the rachis yielded more specific mRNA than the other leaf parts, whereas in continuous darkness, more SsAQP1 transcript was detected in the extensor and flexor than in the rachis and leaf. However, we did not detect significant fluctuations in the transcript level of SsAQP1 during continuous darkness (Figure 7, D/D; n = 3).
SsAQP2
The morning signal intensity of SsAQP2 transcript was lowest in the leaf and highest in flexor and extensor (two-way analysis of variance; P < 10−4) (Figure 8B, D/L; n = 4). In each of the four leaf parts, the mRNA level in the morning sample in dark/light conditions was by far the highest of all four time points (P < 10−4), and the preceding noon, evening, and midnight levels were similar to one another. Notably, when the dark/light cycle was inverted, both the leaf angle and the mRNA abundance of both aquaporins, assayed 7 days later, were inverted in adjustment to the new illumination pattern (data for SsAQP2 in extensors are shown in Figure 6, D/L-INV; n = 1). In continuous darkness, the subjective morning transcript level in flexor and rachis decreased relative to their respective levels in dark/light conditions, and in the leaf the transcript level became nondifferent from zero. The time of the highest transcript level in continuous darkness (in all but the leaf) was subjective noon and subjective morning, whereas the subjective evening and midnight levels remained considerably lower (P < 0.05; Figure 8A, D/D).
To examine the correlation between the leaf movement and the transcript level of SsAQP2 in continuous darkness, we extended our measurements by sampling whole pulvini at 6-hr intervals starting at 58 hr of continuous darkness (a subjective morning) until 88 hr (a subjective noon; Figure 8B, bottom, squares; n = 1). This 58- to 88-hr series was concatenated with a “quasi-whole pulvinus” series, reconstructed from the 39- to 58-hr series of extensor, flexor, and rachis data of Figure 8A (Figure 8B, bottom, columns; n = 3). Thus, in continuous darkness, the fluctuations of the SsAQP2 mRNA levels in the whole pulvinus remained related to the fluctuations of the leaflet angle (the exceptionally high first noon transcript level may be attributable to the residual acute effects of a missed lights-on signal at 31 hr).
DISCUSSION
Functional Characteristics of Heterologously Expressed SsAQP1 and SsAQP2
The two new aquaporins found in Samanea represent two subgroups of plant aquaporins with strikingly different functional characteristics, as determined by heterologous expression in X. laevis oocytes. SsAQP1, which belongs to the PIP1 aquaporin subfamily, is not specific for water but is permeable to glycerol. In contrast, SsAQP2, which is similar to PIP2 aquaporins, is highly specific for water (i.e., not permeable to glycerol). In addition, SsAQP1 is resistant to HgCl2 and phloretin, whereas SsAQP2 is inhibited by these compounds (Figure 3). Thus, SsAQP1 is another member of the PIP1 aquaporin subfamily, like NtAQP1 from tobacco, which is permeable to small solutes and is insensitive to mercurials (Biela et al., 1999).
Like its closest homolog, Arabidopsis PIP2b (Kammerloher et al., 1994), SsAQP2 also induced much higher water permeability in oocytes than did members of the PIP1 subfamily (Figures 3B and 3C) (Biela et al., 1999; Chaumont et al., 2000). Because SsAQP1, unlike many of the other tested PIP1 subfamily members, is a functional aquaporin, together with SsAQP2 it offers a suitable model for the study of the structure–function relationship of aquaporins and of their post-translational regulation.
Role of Membrane Water Permeability in the Function of the Pulvinus
The flexor volume changes in a moving pulvinus are almost opposite in phase to those of extensors (reviewed by Satter and Galston, 1981; Gorton, 1987a, 1987b; Satter et al., 1988; Moran et al., 1996). Irrespective of this phase difference, the largest transcellular water fluxes between flexors and extensors occur twice daily, during the movement of the pulvinus (i.e., in the morning and toward the evening). Therefore, if the facilitated movement of water into and out of the cells is important for this phenomenon, this is when increased cellular water permeability would be expected. Our findings support this hypothesis. In both types of cells, Pf was smaller at noon, when pulvini are stationary, than in the preceding morning, when pulvini unbend. Curiously, only in extensors did the evening Pf values increase relative to the preceding noon values (Figure 4B). Thus (with similar driving forces), during a folding motion, water efflux from the shrinking extensors could exceed water influx into the swelling flexors. This is in accord with the more pronounced volume changes of extensors, relative to flexors, in the moving intact pulvini reported for Samanea (Satter et al., 1979), for Robinia pseudoaccacia, a related Mimosacea tree (Moysset et al., 1991), or for another legume, Phaseolus vulgaris (Irving et al., 1997).
Do the Low Pf Values Found in Our Experiments Represent Those of the Intact Motor Cells in Situ in Physiological Conditions?
The low values of the Pf in the pulvinar protoplasts are surprising. We were unable to find a bias in our methodological approaches that would lead to a severe underestimation of Pf. In particular, in view of the low Pf values obtained in our experiments, an unstirred layer effect is unlikely, as discussed by Ramahaleo et al. (1999).
Direct Pf determinations in the pulvinus are lacking because of the complications caused by the extensive plasmodesmatal connections between the cells and the nondetermined compound elasticity of the cell wall in the different regions of the pulvinus. The transmembrane differences in water potential in the pulvinus also are not known with any degree of confidence (reviewed by Fleurat-Lessard, 1990; Gorton, 1990; see also Irving et al., 1997). Therefore, at present, extrapolating quantitatively from the protoplast Pf to the actual water permeability of cells in moving pulvini would be highly inaccurate. Thus, whether or not the Pf in intact cells is as low as in isolated protoplasts or considerably higher, and whether it is rate limiting for the volume changes, remain to be resolved. Nevertheless, the similarity between the pharmacological properties of the protoplasts and the SsAQP2-expressing oocytes suggests a qualitative extrapolation: that the water permeability of intact motor cells could be partially regulated via their SsAQP2 aquaporins.
Role of SsAQP1 and SsAQP2 in Protoplast Swelling
What is the predominant water pathway across the protoplast plasma membrane, the lipid matrix or the aquaporins? Do the diurnal changes in the osmotic water membrane permeability of Samanea protoplasts reflect changes in the activity of plasma membrane aquaporins or in the properties of lipid matrix alone? The inhibitory effect of cycloheximide does not exclude the possibility that the diurnal changes in water permeability reflect the diurnal regulation of the translation of enzymes that determine the lipid content or other properties of the membrane matrix.
The notion that, in our experiments, aquaporins, and not only the lipid matrix, were involved in the water permeability changes is supported primarily by the significant decrease of extensor and flexor Pf by exposure to mercury ions and the decrease of extensor Pf by exposure to phloretin (Figure 4C). We are aware that, as cautioned by others (Tyerman et al., 1999; Eckert and Kaldenhoff, 2000), HgCl2 could have other effects, preventing the reversal by DTT of the aquaporin inhibition, because the protoplasts appeared somewhat granular even at the lowest concentration of HgCl2 (10 μM). In fact, treatment with DTT does not always restore aquaporin activity in protoplasts preexposed to HgCl2 (Ramahaleo et al., 1999). Phloretin, already shown to block aquaporins in animals (Abrami et al., 1996; Echevarria et al., 1996; Nagelhus et al., 1998; Tsukaguchi et al., 1999; Ford et al., 2000; Saliba and Kirk, 2001) and even in plants (Dordas et al., 2000), inhibited selectively the Samanea SsAQP2 expressed in oocytes (Figure 3C) and decreased the osmotic permeability of extensors (Figure 4C). The fact that phloretin treatment did not alter the Pf values of water-injected oocytes but significantly decreased the Pf of SsAQP2-expressing oocytes (Table 1) emphasizes the selectivity of its aquaporin inhibition. No cell-disruptive properties have been attributed to phloretin, and eventually it may replace HgCl2 as a tool of choice.
Together, these results suggest that the morning and evening increases in protoplast Pf over its noon values are the result of an increase in the activity of aquaporins. It is possible that, although the stress of protoplast isolation could have caused a partial loss of aquaporin function, the fluctuations of the residual Pf values in protoplasts at the different times may reflect, to some extent, the fluctuations of the original aquaporin activity. The confirmation of this notion awaits direct measurements of water channel activity in the intact tissues.
Role of SsAQP1 and SsAQP2 in Pulvinar Movement
In view of the striking abundance of aquaporin genes predicted in Arabidopsis (Arabidopsis Genome Initiative, 2000) and those already cloned from various plants (recently reviewed by Chaumont et al., 2000, 2001; Santoni et al., 2000), it is remarkable that the use of nonstringent conditions resulted in the cloning of only two aquaporin genes from the Samanea pulvini (of 12 positively identified plaques). This in itself suggests that both, or at least one of them, play(s) a specific role in pulvinar function. Moreover, if the localization of aquaporin expression is taken as a guide to their role in plant–water relations (Schaffner, 1998; Eckert and Kaldenhoff, 2000), detection of their mRNA in the pulvini suggests that they are important for the large water fluxes responsible for the motor cell volume changes. Even more suggestive of this role is the correlation between the rhythms in the aquaporin transcript levels and the rhythm of the bending of the pulvinus (Figures 6 to 8). Thus, under constant temperature conditions, SsAQP1 and SsAQP2 were both regulated by light, and the correlation of their transcript levels with the pulvinus angle was maintained even when the light/dark regimen was inverted (Figure 6, D/L-INV). Diurnal regulation of aquaporin expression along with diurnal variability in root hydraulic conductance have been observed in Lotus japonicus (Henzler et al., 1999). Thus, it is likely that both cases represent a general phenomenon.
The transcript level of the flexor and extensor aquaporins peaked once during the day, occurring in both cell types in a similar phase relative to the leaf angle (Figures 7 and 8) and coinciding approximately with the morning peak of osmotic permeability in flexors and extensors (Figure 4). This, and the fact that cycloheximide abolished this permeability increase in both flexors and extensors, suggests that protein synthesis is a necessary step between the increased transcript level and the increased permeability in the morning. The evening increase of permeability in extensors (unmatched by that in flexors) occurred in the absence of an evening transcript increase. However, the Pf-decreasing effect of cycloheximide on extensors in the evening suggests that protein translation is a prerequisite for Pf increase even then. Further work will clarify whether the translated protein is an aquaporin itself or a modulator of an aquaporin.
Which Aquaporin Is More Likely to Be Involved in Pulvinar Movement?
Judging by the transcript level (Figures 5 to 8), SsAQP2 seemed more specific than SsAQP1 to those parts of the leaf that are involved in the hydraulic mechanism of movement, the secondary pulvini and the vascular bundle. In addition, the fluctuations of the SsAQP2 transcript were much more dramatic than those of SsAQP1 (Figures 7 and 8). Therefore, it seems likely that SsAQP2, rather than SsAQP1, is involved in the rhythmic movement of the pulvini. Notably, SsAQP2 also was expressed rhythmically in continuous darkness (Figures 8A, D/D, and 8B, bottom). This was in stark contrast to SsAQP1, the transcript level of which (in the same motor tissues) remained invariably high in the absence of light (Figure 7, D/D). The tight correlation between the fluctuations of SsAQP2 transcript level and the leaf angle (Figures 8A, D/D, and 8B) indicates that, like the entire pulvinus, the SsAQP2 transcript is a target for both diurnal and circadian regulation. It suggests further that this aquaporin is part of the hydraulic mechanism of the pulvinus movement. This notion is supported by the HgCl2 and phloretin inhibition of the Pf of extensors, paralleling HgCl2 and phloretin effects on SsAQP2 in oocytes (Figures 3B, 3C, and 4C).
Conclusion
Our results suggest that membrane water permeability in extensors and flexors is under pronounced temporal regulation, which differs between these cell types, although in both cell types the transcript level of SsAQP1 and SsAQP2 aquaporins is regulated strongly and similarly in a diurnal manner. Of the two aquaporins, SsAQP2 appears to be the most important one for pulvinar movement, based on four lines of evidence: (1) SsAQP2 expression imparted to oocytes a 10-fold higher permeability to water than that of SsAQP1; (2) SsAQP2 is the HgCl2- and phloretin-sensitive aquaporin, and HgCl2 and phloretin inhibited protoplast swelling; (3) its transcript distribution is limited to the movement-associated parts of the leaf; and (4) its encoding gene is the only one regulated in a circadian manner.
While this article was at its final revision stages, the mRNA level of an Arabidopsis tonoplast aquaporin, δ-TIP, was shown to cycle in a circadian rhythm (Harmer et al., 2000), becoming the first aquaporin gene in any organism shown to be regulated in this manner. SsAQP2 is the first plasma membrane aquaporin shown to be regulated both diurnally and by the circadian clock. Although the ultimate resolution of its indispensability (van Os et al., 2000) awaits its controlled in situ on and off switching, our results suggest a physiological function for SsAQP2 in rhythmic cell volume changes.
Our findings regarding the rhythmic regulation of aquaporins may have implications beyond our model system. For example, knowing that water permeability is regulated by the biological clock invites, on the one hand, adjustment of irrigation regimens to reduce water costs, and, on the other hand, attempts to entrain a plant to optimize its water use efficiency.
METHODS
Plant Material and Leaf Movement
Samanea saman trees (also known as Pithecellobium saman) were grown in a greenhouse under an 8-hr-dark/16-hr-light (D/L) regimen at temperatures of 26°C ± 3°C/35°C ± 7°C (±sd), respectively, with light intensity of 300 to 700 μmol m−2 sec−1 and humidity of 75% ± 7%. For the determination of transcript level rhythm, trees were transferred to a growth chamber with humidity of 77% ± 3% (±sd) during D/L alternations and 80% ± 3% (±sd) during constant darkness (D/D) at a constant temperature of 28°C ± 1°C. Either the same D/L regimen was continued for 3 to 5 days until leaf harvest (light intensity was 50 to 100 μmol m−2 sec−1, depending on the exact location in the chamber), or after 3 days of accommodation in the chamber, the lights were turned off and leaves were sampled between 39 and 56 hr (and once between 56 and 86 hr) of constant darkness using a green safelight (Suh et al., 2000). Leaf parts were harvested directly into liquid nitrogen. Leaf angles were measured in sequential frames of a digital videotape of an undetached, intact moving leaf. During the video recording, flashes of the same green safelight were used for exposures in the dark (Figures 4, 7A, and 7D).
cDNA Library Construction and Screening
Secondary pulvini of Samanea, harvested at 7 am, 1 pm, and 7 pm from greenhouse-kept plants, were pooled before isolation of total RNA as described by Logemann et al. (1987). Poly(A)+ RNA was purified using oligo(dT)-coated particles (Dyna beads; Dynal Biotech ASA, Oslo, Norway). cDNA was synthesized with the λZapII system (Stratagene) and cloned into λ phages. All steps were performed according to the manufacturers' protocols. The amplified cDNA library was screened by plaque hybridization using a radioactively labeled probe, which was derived from a 1064-bp PIP1b (Pip1;02) restriction fragment, containing conserved regions. Twelve positively identified plaques were excised from agar plates, and cDNA-containing plasmids were isolated by in vivo excision. Subsequent sequence analysis revealed two different cDNA clones with complete open reading frames and high homologies to known aquaporins.
DNA and protein sequence data were analyzed using Mac DNASIS (Hitachi, Tokyo, Japan). Multiple sequence alignments were performed using ClustalW 1.6 and edited with Genedoc (http://www.cris.com/~ketchup/genedoc.shtml).
RNA Gel Blot Analysis and Quantification of mRNA Levels
For the study of the in planta expression rhythm, series of four consecutive samples were collected at 6-hr intervals from four different leaf parts: the two motor tissues, extensor and flexor, from the secondary pulvini; leaflet blades without the midrib/middle veins; and parts of rachis below the terminal secondary pulvini, including the central portion of the pulvinus remaining after excision of the extensor and flexor (i.e., mainly the vascular bundle). Each 16-sample series is considered a repeat. One additional D/D series consisted of RNA extracted from a whole, undivided pulvinus between 56 and 86 hr of D/D. The D/L samples of both aquaporins consisted of one repeat of mRNA gel blots and two (SsAQP1) or three (SsAQP2) repeats of total RNA gel blots. The D/D series all consisted of total RNA gel blots. RNA gel blot experiments were performed according to standard protocols (Sambrook et al., 1989) with cDNA probes specific for SsAQP1 and SsAQP2 labeled using the Ready to Go kit (Amersham Pharmacia Biotech) using either total RNA of each leaf part or mRNA isolated from the total RNA.
The signals from the mRNA series were quantified by densitometry of autoradiograms and normalized to dot blot analysis of the mRNA samples, and signals from the total RNA series were digitized directly with a phosphorimager, corrected for background, and normalized to 18S rRNA from the same samples. The 16 samples from each series (and the five samples from the whole pulvinus series) then were expressed as percentages of the mean level of aquaporin mRNA in their own series. The corresponding percentage values of all of the repeated series were averaged subsequently over each separate time point. A “quasi-whole pulvinus” series was reconstructed from pooled individual percentage values of mRNA of extensor, flexor, and rachis during hours 39 to 56 of D/D, averaged over each separate time point.
Oocyte Preparation, in Vitro Complementary RNA Synthesis, and Injection
Oocytes (stages V and VI) were isolated from Xenopus laevis and incubated in ND 96 solution (96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes-NaOH, pH 7.4) supplemented with penicillin and streptomycin (200 units/mL each; Gibco BRL) at 16°C. For oocyte expression, the two cDNAs were cloned into vector pGemHe (Liman et al., 1992) using the flanking restriction sites BamHI and HindIII of the multicloning site of pBluescript SK+. Complementary RNA was synthesized in vitro by T7 RNA polymerase (MegaScript; Ambion, Austin, TX) using the T7 promoter of pGemHe, which provides X. laevis translation signal sequences adjacent to the multicloning site to promote translation of the plant complementary RNA (cRNA) (Hoth et al., 1997). cRNA (10 to 50 ng) or an equivalent volume of water was injected into each oocyte.
Radioactive Glycerol Uptake Assay
Tritiated glycerol transport was assayed at room temperature in ND 96 solution containing 0.5 mM 3H-glycerol (final activity, 3.7 MBq/mL) adjusted to 220 mosmol/L. Individual oocytes were bathed for 10 min in a 1.5-mL reaction tube containing 50 μL of incubation medium underlaid with silicon oil. Incubation was stopped by short centrifugation. The supernatant was removed, and oocytes were lysed overnight in 10% SDS at room temperature. Radioactivity was measured by liquid scintillation.
Oocyte Osmotic Water Permeability Assay
Single oocytes were transferred 3 days after cRNA injection from ND 96 solution (220 mosmol) to a ND 96 solution diluted to 70 mosmol with distilled water. Changes in oocyte volume were monitored at room temperature with a microscope video system by taking digital images at 30-sec intervals. Oocyte volumes (V) were calculated from the measured area of each oocyte (NIH Image 1.61; http://rsb.info.nih.gov/nih-image). The osmotic permeability coefficient (Pf) was calculated for the first 6 or 2 min (SsAQP2) according to Yang and Verkman (1997) using the formula Pf = V0[d(V/V0)/dt]/[S0 × Vw(Osmin − Osmout)], where the initial oocyte volume (V0) and the initial oocyte surface area (S0) were calculated from every single oocyte 5 sec after transferring into half-strength ND 96. The molar volume of water (Vw) is given as 18 cm3/mol. For determination of sensitivity to mercurials, the oocytes were incubated in ND 96 solution containing 0.5 mM HgCl2 for 10 min before and during the uptake measurements. Phloretin was solubilized by sonication for more than 1 hr from a stock solution of 250 mM in DMSO to a final concentration of 1 mM. Oocytes were preincubated in the phloretin-containing ND 96 solution for 30 min. The hypotonic solution did not include phloretin.
Protoplast Osmotic Water Permeability Assay
Protoplasts were isolated during the early hours of the day, as described by Moshelion and Moran (2000), in sterile conditions and kept at ∼23 ± 2°C under a regular D/L regimen with light intensity of 2 to 5 μmol cm−2 sec−1 in maintenance buffer (half-strength B5–Gamborg's medium supplemented with 335 mM sorbitol, 230 mM sucrose, 12 mM Mes, 2.5 mM KOH, 3 mM N-methyl-d-glucamine, and 1 mM CaCl2, pH 6.1) until use. Before the assay, the protoplasts were equilibrated for 10 to 20 min in isotonic experimental solution (5 mM KOH and 1 mM CaCl2, pH 6.0, adjusted to 650 mosmol with sorbitol). The hypotonic solution (450 mosmol) differed from the isotonic solution only in the concentration of sorbitol. A protoplast-containing drop was added to the ∼250-μL elongated experimental chamber and allowed to settle on the glass bottom (pretreated with protamine sulfate [1% in H2O, w/v]). Healthy-looking protoplasts (round, with randomly distributed chloroplasts and visible cytoplasmic strands) with diameters in the range of 27 to 42 μm were selected for the experiments.
The protoplasts were videotaped during a constant flow of solution (∼4 mL/min, or, given the bath dimensions, ∼4 mm/sec) before and after a change from isotonic to hypotonic solution (and, in some experiments, also during a return to the isotonic solution). The image focus was adjusted manually a few times during the recording. The assays were conducted at room temperature. The two-dimensional images of the protoplasts, separated by 4 sec, were converted to volumes, assuming that they were perfectly spherical. We verified this using the micrometer-calibrated focusing knob of the microscope (Diaphot; Nikon, Tokyo, Japan) and, additionally, the z-scanning capability of the confocal laser scanning microscope (Axiovert 135M, LSM510; Zeiss, Jena, Germany) combined with the image-reconstruction algorithms of the system (data not shown). We also verified (using calcofluor white staining [0.1%] for ∼20 min) that, within the permeability assay period (until the end of the second day after protoplast isolation), the protoplasts did not regenerate cell walls. Fragments of similarly stained pulvinar cell walls, fluorescing brightly under the same illumination (excitation, 350 nm; emission, 450 to 510 nm) (Galbraith, 1981), served as positive controls (data not shown).
The initial cross-sectional area of each protoplast was measured before the hypotonic challenge (using ImageJ, version 1.18, a public domain program by W. Rasband, National Institutes of Health, Bethesda, MD) and used for the calculation of the surface area and volume of the protoplast. Osmotic water permeability was calculated from the rate of the protoplast volume change during the first ∼20 sec after the delay using the same equation used above. Each repeat consisted of protoplasts isolated at the same session, assayed during the second day after isolation, at four periods during the day: morning, between 6:00 am and 9:00 am; noon, from 10:00 am to 2:00 pm; evening, from 3:00 pm to 9:00 pm; and night, from 9:00 pm to 12:00 pm.
To test the sensitivity to HgCl2, a 5- to 10-min preincubation in the isotonic medium with 50 μM HgCl2, dissolved in the isotonic experimental solution, preceded the exposure to the hypotonic solution with the same additive. To test the reversibility of HgCl2 inhibition, the protoplasts were washed off, incubated for 3 to 10 min in an isotonic solution containing 1 to 2 mM DTT, and exposed to a DTT-containing hypotonic solution.
Phloretin was dissolved initially as a stock of 125 mM in DMSO, diluted directly to the final concentration of 250 μM in the isotonic incubation solution, and sonicated for more than 1 hr. Protoplasts were preincubated in phloretin for 10 min before the hypoosmotic challenge with a phloretin-free solution.
To test the effect of the inhibition of translation, protoplasts were incubated in 2 mM cycloheximide in maintenance solution for ∼7 to 12 hr before the assays. Cycloheximide was not included in the actual assays.
Statistics
To analyze the rhythm of mRNA levels simultaneously for all individual (already normalized) samples, we used two-way analysis of variance as implemented in the program JMP (SAS Institute, Cary, NC). Data are presented as means ±se unless indicated otherwise. The criterion for rejecting the null hypothesis (that the compared values do not differ) was P < 0.05 unless specified otherwise.
Accession Numbers
The accession numbers for SsAQP1 and SsAQP2 are AF067184 and AF067185, respectively.
Acknowledgments
We are grateful to Prof. Elisha Tel-Or, Dr. Fania Ingel, and the Otto Warburg Center for Agricultural Biotechnology (Faculty of Agricultural, Food, and Environmental Quality Sciences) for the use of their facilities, to Dr. Hillary Foot and Vivian Zuaretz for help with the analyses of variance, to Dr. Hillel Fromm for help with the initial RNA preparation, and to Prof. Arie Moran for suggesting the use of phloretin. We thank Deutsche Forschungsgemeinschaft (SFB 251) and Fonds der Chemischen Industrie for providing support to R.K. and the Germany-Israel Foundation (Grant No. G-384-193.13/94) for support to N.M. and R.H.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010351.
References
- Abrami, L., Berthonaud, V., Deen, P.M.T., Rousselet, G., Tacnet, F., and Ripoche, P. (1996). Glycerol permeability of mutant aquaporin 1 and other AQP-MIP proteins: Inhibition studies. Pflugers Arch. 431, 408–414. [DOI] [PubMed] [Google Scholar]
- Agre, P., Bonhivers, M., and Borgnia, M.J. (1998). The aquaporins, blueprints for cellular plumbing systems. J. Biol. Chem. 273, 14659–14662. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Biela, A., Grote, K., Otto, B., Hoth, S., Hedrich, R., and Kaldenhoff, R. (1999). The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J. 18, 565–570. [DOI] [PubMed] [Google Scholar]
- Chaumont, F., Barrieu, F., Jung, R., and Chrispeels, M.J. (2000). Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 122, 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont, F., Barrieu, F., Wojcik, E., Chrispeels, M.J., and Jung, R. (2001). Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 125, 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels, M.J., Crawford, N.M., and Schroeder, J.I. (1999). Proteins for transport of water and mineral nutrients across the membranes of plant cells. Plant Cell 11, 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordas, C., Chrispeels, M.J., and Brown, P.H. (2000). Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 124, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria, M., Windhager, E.E., and Frindt, G. (1996). Selectivity of the renal collecting duct water channel aquaporin-3. J. Biol. Chem. 271, 25079–25082. [DOI] [PubMed] [Google Scholar]
- Eckert, M., and Kaldenhoff, R. (2000). Light-induced stomatal movement of selected Arabidopsis thaliana mutants. J. Exp. Bot. 51, 1435–1442. [PubMed] [Google Scholar]
- Fleurat-Lessard, P. (1990). Structure and ultrastructure of the pulvinus in nyctinastic legumes. In The Pulvinus: Motor Organ for Leaf Movement. Current Concepts in Plant Physiology, Vol. 3, R.L. Satter, H.L. Gorton, and T.C. Vogelmann, eds (Rockville, MD: American Society of Plant Physiologists), pp. 101–129.
- Fleurat-Lessard, P., Frangne, N., Maeshima, M., Ratajczak, R., Bonnemain, J., and Martinoia, E. (1997). Increased expression of vacuolar aquaporin and H+-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol. 114, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, P., Merot, J., Jawerbaum, A., Gimeno, M.A.F., Capurro, C., and Parisi, M. (2000). Water permeability in rat oocytes at different maturity stages: Aquaporin-9 expression. J. Membr. Biol. 176, 151–158. [DOI] [PubMed] [Google Scholar]
- Galbraith, D.W. (1981). Microfluorimetric quantitation of cellulose biosynthesis by plant protoplasts using calcofluor white. Physiol. Plant. 53, 111–116. [Google Scholar]
- Gorton, H.L. (1987. a). Water relations in pulvini from Samanea saman. I. Intact pulvini. Plant Physiol. 83, 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton, H.L. (1987. b). Water relations in pulvini from Samanea saman. II. Effects of excision of motor tissues. Plant Physiol. 83, 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton, H.L. (1990). Pulvinar water relations in nyctinastic plants. In The Pulvinus: Motor Organ for Leaf Movement. Current Concepts in Plant Physiology, Vol. 3, R.L. Satter, H.L. Gorton, and T.C. Vogelmann, eds (Rockville, MD: American Society of Plant Physiologists), pp. 214–222.
- Harmer, S.L., Hogenesch, L.B., Straume, M., Chang, H.S., Han, B., Zhu, T., Wang, X., Kreps, J.A., and Kay, S.A. (2000). Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113. [DOI] [PubMed] [Google Scholar]
- Hedrich, R.J., and Schroeder, J.I. (1989). The physiology of ion channels and electrogenic pumps in higher plant cells. Annu. Rev. Plant Physiol. 40, 539–569. [Google Scholar]
- Henzler, T., Waterhouse, R.N., Smyth, A.J., Carvajal, M., Cooke, D.T., Schaffner, A.R., Steudle, E., and Clarkson, D.T. (1999). Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 210, 50–60. [DOI] [PubMed] [Google Scholar]
- Hoth, S., Dreyer, I., Dietrich, P., Becker, D., Mueller-Roeber, B., and Hedrich, R. (1997). Molecular basis of plant-specific acid activation of K+ uptake channels. Proc. Natl. Acad. Sci. USA 94, 4806–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving, M.S., Ritter, S., Tomos, A.D., and Koller, D. (1997). Phototropic response of the bean pulvinus: Movement of water and ions. Bot. Acta 110, 118–126. [Google Scholar]
- Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjövall, S., Fraysse, F., Weig, A.R., and Kjellbom, P. (2001). The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 126, 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff, R., Kolling, A., and Richter, G. (1993). A novel blue light-inducible and abscisic acid-inducible gene of Arabidopsis thaliana encoding an intrinsic membrane protein. Plant Mol. Biol. 23, 1187–1198. [DOI] [PubMed] [Google Scholar]
- Kaldenhoff, R., Grote, K., Zhu, J.J., and Zimmermann, U. (1998). Significance of plasmalemma aquaporins for water transport in Arabidopsis thaliana. Plant J. 14, 121–128. [DOI] [PubMed] [Google Scholar]
- Kammerloher, W., Fischer, U., Piechottka, G.P., and Schaffner, A.R. (1994). Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 6, 187–199. [DOI] [PubMed] [Google Scholar]
- Liman, E.R., Tytgat, J., and Hess, P. (1992). Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871. [DOI] [PubMed] [Google Scholar]
- Logemann, J., Schell, J., and Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163, 16–20. [DOI] [PubMed] [Google Scholar]
- Maurel, C. (1997). Aquaporins and water permeability of plant membranes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 399–429. [DOI] [PubMed] [Google Scholar]
- Moran, N. (1990). The role of ion channels in osmotic volume changes in Samanea motor cells analysed by patch-clamp methods. In The Pulvinus: Motor Organ for Leaf Movement. Current Concepts in Plant Physiology, Vol. 3, R.L. Satter, H.L. Gorton, and T.C. Vogelmann, eds (Rockville, MD: American Society of Plant Physiologists), pp. 142–158.
- Moran, N. (1996). Membrane-delimited phosphorylation enables the activation of the outward-rectifying K channels in a plant cell. Plant Physiol. 111, 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, N., Ehrenstein, G., Iwasa, K., Mischke, C., Bare, C., and Satter, R.L. (1988). Potassium channels in motor cells of Samanea saman: A patch-clamp study. Plant Physiol. 88, 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, N., Yueh, Y.G., and Crain, R.C. (1996). Signal transduction and cell volume regulation in plant leaflet movements. News Physiol. Sci. 11, 108–114. [Google Scholar]
- Moshelion, M., and Moran, N. (2000). K+-efflux channels in extensor and flexor cells of Samanea saman are not identical: Effects of cytosolic Ca2+. Plant Physiol. 124, 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion, M., Becker, D., Hedrich, R., Mueller-Roeber, B., and Moran, N. (1998). In search of the plant outward-rectifying K channel molecule: the “Ca+2 test.” In 11th International Workshop on Plant Membrane Biology, Cambridge, UK, M. Tester, C. Morris, and J. Davies, eds (Berlin: Springer Verlag/Experimental Biology Online; http://link.springer.de/link/service/journals/00898/meeting/ cmbrdg98/sess05.htm#05sess49), p. 160.
- Moshelion, M., Becker, D., Czempinski, K., Mueller-Roeber, B., Hedrich, R., Attali, B., and Moran, N. (2002). Diurnal and circadian regulation of putative potassium channels in a leaf–moving organ. Plant Physiol. 128, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moysset, L., Sugranes, S.L., and Simon, E. (1991). Changes in morphometry and elemental composition of Robinia pseudoaccacia pulvinar motor cells during leaflet movements. J. Exp. Bot. 42, 1315–1324. [Google Scholar]
- Nagelhus, E.A., Veruki, M.L., Torp, R., Haug, F.M., Laake, J.H., Nielsen, S., Agre, P., and Ottersen, O.P. (1998). Aquaporin-4 water channel protein in the rat retina and optic nerve: Polarized expression in Muller cells and fibrous astrocytes. J. Neurosci. 18, 2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, J.H., and Asprey, G.F. (1958). Studies in the nyctinastic movement of the leaf pinnae of Samanea saman (Jacq.) Merrill. II. The behaviour of upper and lower half-pulvini. Planta 51, 770–785. [Google Scholar]
- Ramahaleo, T., Morillon, R., Alexandre, J., and Lassalles, J.P. (1999). Osmotic water permeability of isolated protoplasts: Modifications during development. Plant Physiol. 119, 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba, K.J., and Kirk, K. (2001). H+-coupled pantothenate transport in the intracellular malaria parasite. J. Biol. Chem. 276, 18115–18121. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Santoni, V., Gerbeau, P., Javot, H., and Maurel, C. (2000). The high diversity of aquaporins reveals novel facets of plant membrane functions. Curr. Opin. Plant Biol. 3, 476–481. [DOI] [PubMed] [Google Scholar]
- Satter, R.L., and Galston, A.W. (1981). Mechanisms of control of leaf movements. Annu. Rev. Plant Physiol. 32, 83–110. [Google Scholar]
- Satter, R.L., and Moran, N. (1988). Ionic channels in plant cell membranes. Physiol. Plant. 72, 816–820. [Google Scholar]
- Satter, R.L., and Morse, M.J. (1990). Light-modulated, circadian rhythmic leaf movements in nyctinastic legumes. In The Pulvinus: Motor Organ for Leaf Movement. Current Topics in Plant Physiology, Vol. 3, R.L. Satter, H.L. Gorton, and T.C. Vogelmann, eds (Rockville, MD: American Society of Plant Physiologists), pp. 10–24.
- Satter, R.L., Geballe, G.T., Applewhite, P.B., and Galston, A.W. (1979). Relationship between motor cell ultrastructure and leaf movements in Samanea saman. Physiol. Plant. 46, 338–346. [Google Scholar]
- Satter, R.L., Morse, M.J., Lee, Y., Crain, R.C., Cote, G., and Moran, N. (1988). Light- and clock-controlled leaflet movements in Samanea saman: A physiological, biophysical and biochemical analysis. Bot. Acta 101, 205–213. [Google Scholar]
- Schaffner, A.R. (1998). Aquaporin function, structure, and expression: Are there more surprises to surface in water relations? Planta 204, 131–139. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., and Hedrich, R. (1989). Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem. Sci. 14, 187–192. [DOI] [PubMed] [Google Scholar]
- Suh, S., Moran, N., and Lee, Y. (2000). Blue light activates depolarization-dependent K+ channels in flexor cells from Samanea saman motor organs via two mechanisms. Plant Physiol. 123, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaguchi, H., Weremowicz, S., Morton, C.C., and Hediger, M.A. (1999). Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am. J. Physiol. Renal Physiol. 277, F685–F696. [DOI] [PubMed] [Google Scholar]
- Tyerman, S.D., Bohnert, H.J., Maurel, C., Steudle, E., and Smith, J.A.C. (1999). Plant aquaporins: Their molecular biology, biophysics and significance for plant–water relations. J. Exp. Bot. 50, 1055–1071. [Google Scholar]
- van Os, C.H., Kamsteeg, E.J., Marr, N., and Deen, P.M. (2000). Physiological relevance of aquaporins: Luxury or necessity? Pflugers Arch. 440, 513–520. [DOI] [PubMed] [Google Scholar]
- Verkman, A.S., and Mitra, A.K. (2000). Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 278, F13–F28. [DOI] [PubMed] [Google Scholar]
- Yang, B.X., and Verkman, A.S. (1997). Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 272, 16140–16146. [DOI] [PubMed] [Google Scholar]
- Zeidel, M.L., Ambudkar, S.V., Smith, B.L., and Agre, P. (1992). Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry 31, 7436–7440. [DOI] [PubMed] [Google Scholar]