Abstract
We have identified the Arabidopsis ortholog of barley RAR1 as a component of resistance specified by multiple nucleotide binding/Leu-rich repeat resistance (R) genes recognizing different bacterial and oomycete pathogen isolates. Characterization of partially and fully defective rar1 mutations revealed that wild-type RAR1 acts as a rate-limiting regulator of early R gene–triggered defenses, determining the extent of pathogen containment, hypersensitive plant cell death, and an oxidative burst at primary infection sites. We conclude that RAR1 defense signaling function is conserved between plant species that are separated evolutionarily by 150 million years. RAR1 encodes a protein with two zinc binding (CHORD) domains that are highly conserved across eukaryotic phyla, and the single nematode CHORD-containing homolog, Chp, was found previously to be essential for embryo viability. An absence of obvious developmental defects in null Arabidopsis rar1 mutants favors the notion that, in contrast, RAR1 does not play a fundamental role in plant development.
INTRODUCTION
In countering attack by microbial pathogens or insects, plants have evolved resistance (R) genes that specifically recognize corresponding pathogen avirulence (avr) genes to trigger plant defenses (Dangl and Jones, 2001). Two plant R gene–encoded proteins, tomato Pto and rice Pi-ta, have been shown to interact physically with their pathogen Avr counterparts, AvrPto and Avr-Pita, respectively, in in vitro assays (Scofield et al., 1996; Tang et al., 1996; Jia et al., 2000). Other plant R proteins may associate with pathogen Avr proteins indirectly within a protein complex (Leister and Katagiri, 2000). In the absence of a corresponding R gene, the pathogen is able to colonize its host. Some Avr proteins are virulence factors that facilitate pathogen growth or interfere with basal plant defenses (Nimchuk et al., 2000; Staskawicz et al., 2001). R-Avr protein recognition commonly involves localized programmed plant cell death (the hypersensitive response [HR]), an oxidative burst producing reactive oxygen intermediates (ROI), and the accumulation of salicylic acid (SA), a phenolic molecule necessary for the induction of systemic immunity (systemic acquired resistance) (Feys and Parker, 2000).
Plant R proteins share a limited repertoire of motifs with animal proteins that control innate immunity (Staskawicz et al., 2001). The most prevalent R gene class encodes predicted cytosolic proteins with a central nucleotide binding (NB) domain and C-terminal Leu-rich repeats (LRRs) (Dangl and Jones, 2001). At least one NB-LRR–type protein, Arabidopsis RPM1, is tethered to the plasma membrane, where it may encounter bacterial Avr proteins that are secreted into the plant cell (Boyes et al., 1998; Nimchuk et al., 2000). NB-LRR proteins fall into two subclasses based on their different N-terminal motifs. One group possesses an N-terminal coiled-coil (CC) domain. The second group has N-terminal similarity to the cytoplasmic Toll Interleukin-1 Receptor (TIR) domains of human and Drosophila Toll-like receptors (Dangl and Jones, 2001).
Mutational analyses in plants have led to the identification of genes that are essential for the full expression of R gene–specified resistance, providing an important first step in the elucidation of defense signaling (Feys and Parker, 2000). In Arabidopsis, EDS1 and PAD4, which encode lipase-like proteins (Falk et al., 1999; Jirage et al., 1999), are necessary for resistance conferred by TIR-NB-LRR genes (Aarts et al., 1998; Feys et al., 2001). NDR1, a potentially membrane-associated protein (Century et al., 1997), is dispensable for this resistance but is essential for the function of most, but not all, CC-NB-LRR proteins (Aarts et al., 1998; McDowell et al., 2000). Thus, EDS1/PAD4 and NDR1 appear to specify distinct processes that are required by different R protein structural types.
Another resistance signaling gene, RAR1, was identified in mutational screens for suppressors of Mla12 resistance in barley to the powdery mildew fungus Blumeria (Erysiphe) graminis f.sp. hordei (Freialdenhoven et al., 1994). RAR1 is required by multiple barley Mla genes as well as other unlinked powdery mildew resistance loci (Jørgensen, 1996; Halterman et al., 2001; Zhou et al., 2001). Barley rar1 mutant plants are impaired in whole cell ROI accumulation and in the HR of attacked host epidermal cells in Mla12-specified resistance, suggesting that RAR1 acts early in the plant resistance signaling cascade (Freialdenhoven et al., 1994; Shirasu et al., 1999). RAR1 encodes a 25-kD, putatively cytosolic protein containing two 60–amino acid Cys- and His-rich (CHORD) Zn2+ binding domains that are conserved in sequence and tandem organization in all eukaryotic phyla examined (Shirasu et al., 1999). Plant RAR1 proteins possess an additional 20–amino acid motif with three invariant Cys residues and a His (denoted the CCCH domain) between CHORD domains I and II that is absent from related nonplant sequences. RNA interference of the single CHORD-containing gene in Caenorhabditis elegans, Chp, caused defects in germline development and embryo lethality (Shirasu et al., 1999), suggesting that Chp has a fundamental role in worm development.
It is unresolved whether the molecular functions of plant RAR1 proteins are conserved and whether these are shared with CHORD-containing homologs in metazoans. It was speculated that the lack of copy number expansion of CHORD proteins and the strict tandem organization of CHORD domains I and II across plant and animal phyla may indicate a conserved unit of function (Shirasu et al., 1999). Alternatively, unknown common biochemical function(s) of CHORD units may have been recruited for different biological processes in different species. The lack of definitive null rar1 mutations in barley left open the possibility that a fundamental cellular role of RAR1 may exist in plants that is fulfilled by residual functional protein in the two mutant alleles identified, rar1-1 and rar1-2.
Our mutational screens for genetic suppressors of RPP5 resistance in Arabidopsis accession Landsberg erecta (Ler) to the downy mildew pathogen Peronospora parasitica have identified a series of partially and fully defective mutations within the single RAR1 ortholog. We show that RAR1 is an early component of R gene–triggered resistance against avirulent Peronospora and Pseudomonas syringae pv tomato isolates, exerting rate-limiting control of defense signal fluxes leading to hypersensitive plant cell death. We also find that RAR1 is used by both TIR- and CC-NB-LRR proteins, indicating that its recruitment is not conditioned by a particular R protein structural type. Isolation of definitive null rar1 mutations supports the notion that RAR1 in plants has evolved a signaling capability essential for plant defense against pathogens but dispensable in fundamental processes of plant development.
RESULTS
Isolation of Multiple rar1 Mutants
Expression of the RPP5 resistance gene in accession Ler confers resistance to Peronospora isolate Noco2 (Parker et al., 1997). Infection with Noco2 causes restriction of the pathogen to inoculation sites and an associated HR. Ler rpp5 mutants or the susceptible accession Columbia (Col-0) do not display an HR in response to Noco2. Instead, the pathogen grows beyond inoculation sites to colonize the plant systemically, resulting in asexual sporulation on cotyledons and leaves 5 to 7 days after infection. Mutational screens for suppressors of RPP5 resistance were performed on ∼220,000 M2 seedlings derived from fast neutron (FN)– or ethyl methanesulfonate (EMS)–mutagenized Ler seed. Susceptible mutants, which show substantial levels of pathogen sporulation, and partially susceptible mutants, which permit only low sporulation levels, were isolated.
Crosses between each of the newly identified mutant lines and wild-type Ler produced resistant F1 plants, consistent with all of the mutations being recessive or semidominant. Allelism tests were performed between the mutant lines and with the previously isolated rpp5, eds1, and pad4 mutants (Parker et al., 1997; Falk et al., 1999; Jirage et al., 1999). These analyses revealed two new complementation groups, designated rpr1 and rpr2 (required for RPP5 resistance; Parker et al., 2000). Here, we present our characterization of the rpr2 complementation group, comprising one FN- and five EMS-generated alleles. A comparison of the six isolated rpr2 alleles revealed distinct susceptible infection phenotypes, three showing substantial pathogen sporulation and three (all EMS mutants) showing sparse sporulation (Table 1). F2 seedlings generated from crosses between each of the six independent rpr2 alleles and Ler segregated in a ratio of 3:1 (resistant:susceptible plants; data not shown), indicating that rpr2 is a single recessive mutation.
Table 1.
Summary of rar1 Mutations in Ler
| Allele | Mutagenesis | Classa | Nucleotide Changeb | Amino Acid Change | Defect | Predicted Size (Amino Acids) |
|---|---|---|---|---|---|---|
| rar1-10 | FN | S | 5-bp deletion (561 to 565 bp) | Multiple | Frameshift and premature stop | 212 |
| rar1-11 | EMS | S | C to T (154 bp) | Q to STOP | Premature stop | 51 |
| rar1-12 | EMS | PS | G to A (581 bp) | W to STOP | Premature stop | 193 |
| rar1-13 | EMS | S | G to A (3′ splice site in intron 3) | Unknown | Splice site defect | Unknown |
| rar1-14 | EMS | PS | G to A (508 bp) | E to K | Amino acid substitution | 226 |
| rar1-15 | EMS | PS | G to A (590 bp) | C to Y | Amino acid substitution | 226 |
PS, partially susceptible; S, susceptible.
The position of the nucleotide change corresponds to the Rar1 coding sequence (see Figure 1A).
A map-based cloning strategy was used to isolate the RPR2 gene, and its location was narrowed to a 220-kb region on the lower arm of chromosome 5 (see Methods). Within this region, we identified a gene with high sequence similarity to barley RAR1 (Shirasu et al., 1999). Therefore, we determined whether rpr2 contained mutations in Arabidopsis RAR1 by amplification by polymerase chain reaction and sequencing of genomic DNA from the FN-derived rpr2-1 line and wild-type Ler. Alignment of the RAR1 sequences from rpr2-1 and Ler revealed a 5-bp deletion in rpr2-1 (Figure 1A). RAR1 DNA was sequenced in the remaining five rpr2 alleles, and each was found to have a mutation within the RAR1 gene (Figures 1A and 1B). We concluded that RPR2 corresponds to the Arabidopsis ortholog of barley RAR1. Our FN rpr2-1 allele is denoted rar1-10, and the five EMS alleles are denoted, consecutively, rar1-11 to rar1-15, in a convention that accords with that used by J. Dangl, K. Shirasu, and colleagues to distinguish rar1 mutations identified in different Arabidopsis accessions (see Methods).
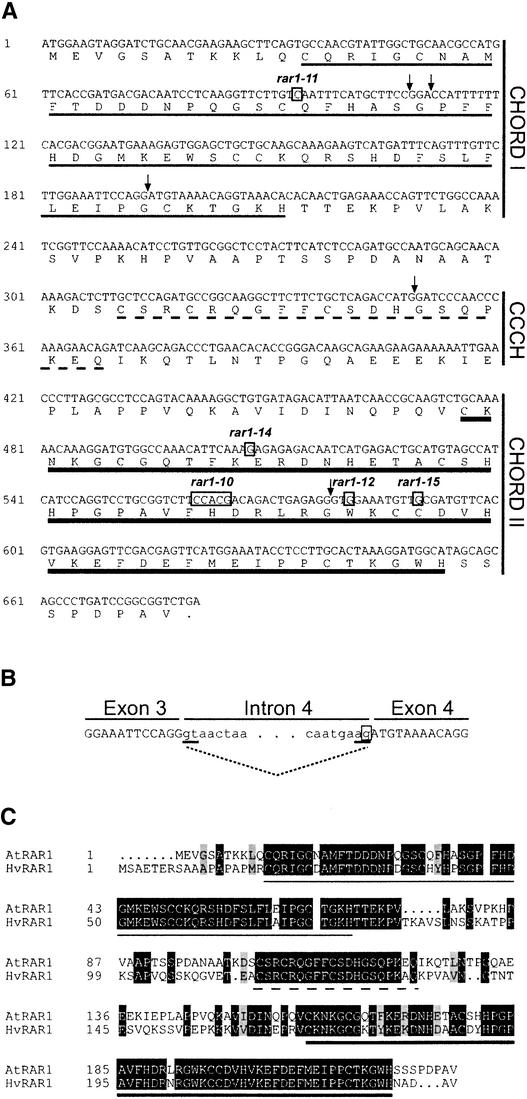
Figure 1.
Sequence Analysis of Wild-Type and Mutant Alleles of Arabidopsis RAR1.
(A) Coding region and deduced amino acid sequence of RAR1 in accession Ler. Arrows mark the positions of introns 1 to 5. The CHORD I, CHORD II, and CCCH domains are indicated by thin, thick, and broken underlines, respectively. Nucleotide changes in rar1-10, rar1-11, rar1-12, rar1-14, and rar1-15 are boxed.
(B) Scheme of the intron 3 splice site defect in rar1-13. The sequence at the exon 3–intron 3–exon 4 boundary is shown. Intron sequences are displayed in lowercase letters, and exon sequences are displayed in uppercase letters. The 5′ and 3′ splice sites of intron 3 are underlined, and splicing of the wild-type RNA is shown with a dotted line. The nucleotide change in rar1-13 is boxed.
(C) Amino acid sequence alignment of Arabidopsis (At) and barley (Hv) RAR1. The proteins have 60% identity. Identical and similar residues are displayed in black and gray boxes, respectively. The CHORD I, CHORD II, and CCCH domains are underlined as in (A).
The mutations found in rar1-10 to rar1-15 are summarized in Table 1. Two of the three mutants showing substantial sporulation contain premature stop codons. rar1-11 has a stop codon at the beginning of the CHORD I domain, whereas rar1-10 (after a frameshift) possesses a stop codon within CHORD II (Figure 1A). In rar1-13, the G nucleotide of the 3′ splice site consensus of intron 3 is mutated (Figure 1B). This consensus sequence is essential for correct processing of primary mRNA transcripts in plants and higher eukaryotes (Goodall and Filipowicz, 1991). Of the mutants classed as partially susceptible, rar1-12 has a stop codon, and rar1-14 and rar1-15 have amino acid substitutions within CHORD II. In rar1-15, a Cys residue (Cys-197) that is invariant in all known plant and animal CHORD domains (Shirasu et al., 1999) is substituted by Tyr. In rar1-14, Glu-170 (acidic) is changed to Lys (basic). Although this residue is not conserved strictly in CHORD I or II, an acidic residue is found invariably at this position in all plant CHORD domains examined.
Expressed sequence tag and genomic DNA database searches show RAR1 to be a probable single-copy gene in all plant species analyzed. We confirmed this in accessions Ler and Col-0 by DNA gel blot analysis (data not shown). The high level of similarity between Arabidopsis and barley RAR1 is shown in Figure 1C. Particularly striking is the almost complete conservation of their CHORD I, CHORD II, and CCCH domains.
Characterization of rar1 Mutant Defects
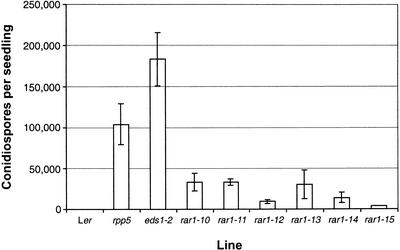
The rar1 mutant lines were backcrossed at least once to parental Ler for phenotypic analysis. We determined the extent of disease susceptibility in the different rar1 mutants by measuring numbers of conidiospores on leaves 7 days after inoculation of plants with Noco2. As shown in Figure 2, sporulation on all rar1 mutant lines was significantly lower than on rpp5 or eds1, which displayed susceptibility and supersusceptibility, respectively (Parker et al., 1997; Feys et al., 2001). Three mutants, rar1-10, rar1-11, and rar1-13, each exhibited ∼50% of the spore levels counted on rpp5 plants. Thus, even the strongest rar1 mutants (see below) do not completely disable RPP5 resistance. Our classification of rar1-12, rar1-14, and rar1-15 as partially susceptible mutants (Table 1) was consistent with lower spore counts on these plants compared with rar1-10, rar1-11, and rar1-13.
Figure 2.
Asexual Sporulation of Peronospora Noco2 on Wild-Type and Mutant Plants.
Production of conidiospores on Ler, rpp5, eds1-2, and six independent rar1 mutants (rar1-11 to rar1-15) was determined 7 days after inoculation. The data and standard error values shown are from three replicate samples per line in a single experiment. Similar results were observed in an independent experiment.
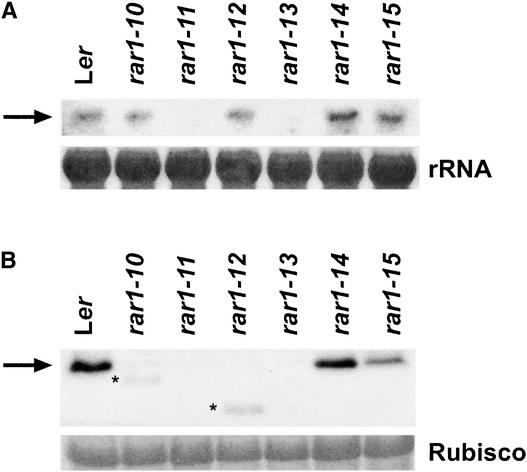
We examined the effects of the different rar1 mutations on RAR1 transcript and RAR1 protein abundance in healthy (pathogen-unchallenged) tissues of mutant and wild-type plants. As shown in Figure 3A, RAR1 mRNA levels in rar1-10, rar1-12, rar1-14, and rar1-15 were similar to those measured in wild-type Ler. In contrast, RAR1 transcripts were undetectable in rar1-11 and were depleted severely in rar1-13. We presume that early protein truncation in rar1-11 and the intron 3 splice defects in rar1-13 (Table 1) led to transcript instability. RAR1 protein amounts in the rar1 mutant lines were determined by probing blots of soluble protein extracts with polyclonal antisera raised against full-length RAR1 protein. Anti-RAR1 antisera detected a single ∼30-kD band in wild-type protein extracts (Figure 3B). RAR1 was not detectable in either rar1-11 (consistent with the transcript analysis) or rar1-13. We deduced from these data that rar1-11 and rar1-13 are complete loss-of-function mutations.
Figure 3.
Molecular Characterization of the rar1 Mutants.
(A) RNA gel blot analysis of RAR1 transcript levels. Total RNA (20 μg) from 4-week-old healthy Ler and rar1 mutant seedlings was probed with a genomic RAR1 DNA fragment. Equal loading was determined by visualization of rRNA on the filter with methylene blue.
(B) Immunoblot analysis of RAR1 protein. Total soluble protein extracts (50 μg) prepared from the same material used for the RNA analysis shown in (A) were probed with polyclonal anti-RAR1 antisera. Asterisks indicate truncated products detected in rar1-10 and rar1-12. Equal loading was determined by Ponceau S staining of the filter. Similar results were obtained in a second independent experiment.
Truncated RAR1 proteins were observed in rar1-10 and rar1-12 at sizes consistent with those predicted by the positions of their premature stop codons (Figure 3B, Table 1). However, their levels were much lower than those in wild-type RAR1, presumably as a result of the reduced stability of the truncated products. Because rar1-10 displayed a level of Noco2 sporulation equivalent to that of the null rar1 alleles, we concluded that rar1-10 is most likely also a null mutant. There was low residual activity associated with the truncated RAR1 protein in rar1-12, reflected by significantly lower Noco2 sporulation levels than on rar1-10, rar1-11, or rar1-13 (Figure 2). Although the E170K CHORD II mutation in rar1-14 did not affect RAR1 protein abundance, the C197Y CHORD II mutation in rar1-15 resulted in a reduction of protein to approximately half the level of the wild type (Figure 3B).
Pathogen-Triggered Cell Death Responses of rar1 Plants
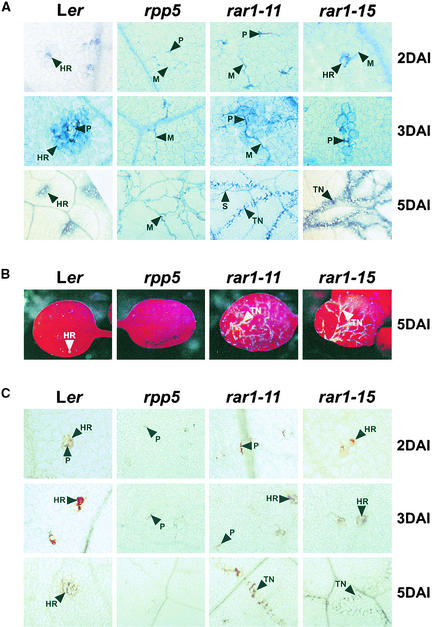
The susceptibility of barley rar1 mutants to powdery mildew was shown to coincide with a failure in epidermal host cell death and ROI accumulation (Freialdenhoven et al., 1994; Shirasu et al., 1999). We investigated whether similar defects were apparent in Arabidopsis rar1 mutant responses to Peronospora. For this analysis, we selected the null mutant rar1-11 and a partially defective mutant, rar1-15, and compared responses with those of Ler (RPP5) and rpp5 mutant plants.
Lactophenol trypan blue staining was used to monitor pathogen growth and plant cell death at various times after inoculation with Noco2 (Rustérucci et al., 2001). As shown in Figure 4A, Ler developed discrete HR lesions on inoculated leaves. Noco2 mycelium did not extend beyond these interaction sites. In contrast, rpp5 mutants permitted Noco2 growth in the absence of plant cell death. At early time points (2 days after inoculation; Figure 4A), the majority (>95%) of interaction sites in null rar1-11 mutant leaves resembled those in rpp5 leaves. A small proportion (<5%) of sites exhibited an attenuated HR, and at later times, trails of collapsed mesophyll cells were found to be associated with the growing mycelium (Figure 4A).
Figure 4.
Host Responses and Peronospora Development in rar1 Mutants.
The images shown are representative of three independent experiments using at least eight leaves per time point for Ler, rpp5, rar1-11, and rar1-15 seedlings after inoculation with Noco2.
(A) Leaves were stained with lactophenol trypan blue at 2, 3, and 5 days after inoculation to reveal necrotic plant cells and pathogen structures.
(B) Cell death–associated autofluorescence viewed under UV light 5 days after Noco2 inoculation.
(C) H2O2 accumulation at plant–pathogen interaction sites monitored by 3,3-diaminobenzidine staining of leaves at the same times as in (A).
DAI, days after inoculation; HR, hypersensitive response; M, mycelium; P, penetration site; S, sporangiophore; TN, trailing necrosis. Magnification ×200 (2 and 3 days after inoculation) and ×100 (5 days after inoculation).
Thus, complete loss of RAR1 function in rar1-11 seemed to cause a strongly delayed HR resulting in trailing plant cell necrosis, which was borne out by the appearance at late time points (5 days after inoculation) of autofluorescing dead cells on leaves of rar1-11 but not rpp5 plants (Figure 4B). In the partially defective rar1-15 plants, an attenuated HR was observed at most plant–pathogen interaction sites, and Noco2 mycelium was seen to emerge from these areas (Figure 4A). At later times, there was less extensive pathogen growth and more extreme trailing plant cell death than was observed in rar1-11 (Figures 4A and 4B).
As a measure of ROI accumulation, we used 3,3-diaminobenzidine polymerization (Thordal-Christensen et al., 1997; Rustérucci et al., 2001) to determine if the rar1 null and partial mutants were compromised in RPP5-triggered H2O2 generation upon Noco2 challenge. In Ler leaves, whole cell H2O2 was detected at the same early time point (2 days after inoculation) as an HR and remained restricted to these discrete areas of dead or dying cells over a 5-day time course (Figure 4C). No whole cell ROI accumulation was observed at interaction sites of rpp5 plants or in the majority of sites in rar1-11 plants (Figure 4C). In contrast, ROI accumulation was readily detectable in most pathogen inoculation sites of rar1-15. At later times, the pattern of whole cell ROI generation followed that of trailing plant cell death in rar1-11 and rar1-15. We concluded from these data that RAR1 acts as a rate-limiting component of early RPP5-conditioned defenses, operating upstream of hypersensitive plant cell death and its accompanying oxidative burst.
Analysis of Different R Gene Requirements for RAR1
We examined the requirements of different Arabidopsis R genes for RAR1 by challenging wild-type and mutant plants with avirulent Peronospora or Pseudomonas isolates that are recognized by particular R genes (Table 2). Besides testing the responses of R genes present in Ler, several R genes not found in Ler but expressed in accessions Col-0 and Wassileskija were included. For the latter tests, the wild-type accessions were crossed with either rar1-10 or rar1-11, and F2 generation plants were selected that were homozygous for wild-type or mutant rar1 and that segregated for the respective R gene (Table 2; see Methods).
Table 2.
R Gene Requirements for RAR1
| Pathogen | Isolate/ Strain |
R gene | NB-LRR Class |
rar1 Phenotype |
|---|---|---|---|---|
| P. parasitica | Noco2 | RPP5 | TIR | S |
| Hiks1 | RPP7a | ? | R | |
| Emco5 | RPP8 | CC | R | |
| Maks9 | RPP21 | ? | (S) | |
| Cala2 | RPP2b | TIR | R | |
| Emwa1 | RPP4b | TIR | S | |
| Cala2 | RPP1Ac | TIR | R | |
| P. syringae pv | avrRpm1 | RPM1 | CC | S |
| tomato (DC3000) | avrRpt2 | RPS2 | CC | S |
| avrRps4 | RPS4 | TIR | S | |
| avrPphB | RPS5b | CC | S |
All pathogen tests were carried out using rar1-10. Plant lines were spray inoculated with P. parasitica isolates and resistance or susceptibility scored seven days after inoculation by measuring the extent of host cell death and pathogen sporulation on inoculated leaves. Suspensions of the different P. syringae strains were infiltrated into leaves and pathogen growth and disease symptom development recorded over five days. The structures of RPP8 (McDowell et al., 1998), RPP2 (Holub, 2001); RPP1A (Botella et al., 1998), RPM1 (Grant et al., 1995), RPS2 (Bent et al., 1994), RPS4 (Gassmann et al., 1999) and RPS5 (Warren et al., 1998) have been described.
RPP7 in Ler is defined as a locus cosegregating with Col-0 RPP7 in >4000 Col-0 × Ler F2 seedlings but may differ from Col-0 RRP7 (E. Holub, personal communication).
R gene introduced from Col-0 by crossing with Ler rar1-10.
R gene introduced from Ws-0 by crossing. CC, coiled coil; TIR, Toll-Interleukin-1 Receptor; ?, unknown; R, resistant; S, susceptible; (S), partially susceptible.
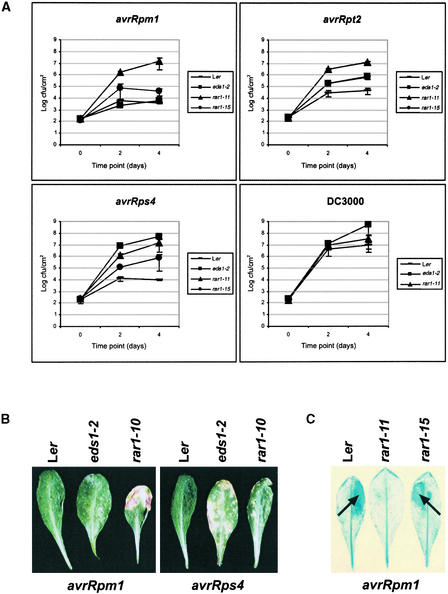
We found that RAR1 was essential for resistance conferred by RPP4, a recognitionally distinct Col-0 ortholog of RPP5 (van der Biezen et al., 2002). RAR1 also was required for full expression of resistance mediated by RPP21 (a locus not yet defined) but was dispensable for the function of other RPP genes examined (Table 2). All four R genes (RPM1, RPS2, RPS4, and RPS5) tested against corresponding avirulent Pseudomonas isolates (expressing avrRpm1, avrRpt2, avrRps4, and avrPphB, respectively) required RAR1 (Table 2). Figure 5A shows that the growth of Pseudomonas DC3000 harboring avrRpm1, avrRpt2, or avrRps4 was similar in rar1-11 leaves to that of virulent DC3000 (containing an empty vector) in either rar1-11 or Ler. Although rar1-11 exhibited full susceptibility to these avirulent bacteria, it did not display a supersusceptibility phenotype that is characteristic of eds1-2 mutants to either DC3000 expressing avrRps4 or DC3000 alone (Figure 5A).
Figure 5.
Bacterial Growth and Disease Symptom Formation on rar1 Leaves.
(A) Growth of Pseudomonas strain DC3000 expressing avrRpm1, avrRps2, or avrRps4 or containing an empty vector (DC3000) was measured over 4 days in leaves of Ler, eds1-2, rar1-11, and rar1-15. Data shown are averages of two independent experiments ±se. cfu, colony-forming units.
(B) Disease symptoms in Ler, eds1-2, and rar1-10 caused by Pseudomonas expressing either avrRpm1 or avrRps4 at 5 days after leaves were dipped in bacterial suspensions (5 × 107 colony-forming units/mL).
(C) HR development (arrows) in leaves of Ler, rar1-11, and rar1-15 after hand infiltration of 5 × 106 colony-forming units/mL DC3000/avrRpm1 and staining at 16 hr with lactophenol trypan blue.
We found in all cases that disease symptom development correlated with bacterial growth in the different plant lines, as shown for plants inoculated with DC3000 expressing either avrRpm1 or avrRps4 (Figure 5B). In these assays, a distinction could be made between the susceptibility of rar1-11 and the enhanced susceptibility of eds1-2 to DC3000/avrRps4. In an accompanying article, Tornero et al. (2002) report a much lower dependence of RPS4 on RAR1 in accession Col-0 than we found for Ler RPS4. We assessed whether rar1-15, which was partially defective in RPP5 resistance (Figure 2), also suppressed bacterial resistance more weakly than the null rar1-11 allele. The growth of all of the avirulent Pseudomonas strains was intermediate between that observed in rar1-11 and resistant Ler (Figure 5A).
These results demonstrate that RAR1, as in RPP5 resistance, exerts a rate-limiting control of plant defenses to avirulent bacteria. In rar1-15, the timing and intensity of an RPM1-induced HR (Figure 5C) was not obviously different from that in wild-type plants after infiltration of concentrated suspensions of DC3000/avrRpm1, whereas HR (Figure 5C) were strongly attenuated in rar1-11. Expression of an RPS2-conditioned HR was not observed in leaves of either rar1-11 or rar1-15 (data not shown). We were unable to observe consistent HR development in wild-type or mutant plants after challenge with DC3000/avrRPS4.
Defense-Related Gene Expression in rar1
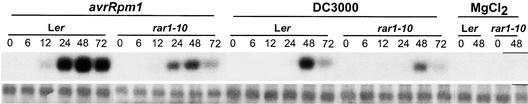
Our analyses of rar1 responses to Peronospora or Pseudomonas revealed deficiencies in defenses conditioned by certain R genes but no relaxation of resistance to virulent pathogens (Figures 2 and 5). We wanted to explore whether RAR1 function is restricted to plants undergoing an R gene–triggered HR. SA accumulation is required for the expression of basal resistance and systemic immune responses to virulent pathogens in the absence of an HR (Gaffney et al., 1993; Cao et al., 1994; Reuber et al., 1998; Feys et al., 2001). Therefore, we compared induction profiles of the SA-responsive gene PR1 in wild-type (Ler) and rar1-10 null mutant leaves after inoculation with avirulent (DC3000/avrRpm1) or virulent (DC3000) bacteria. We found that PR1 expression was induced more rapidly in wild-type plants responding to DC3000/avrRpm1 than to DC3000 alone over a 72-hr period (Figure 6). In rar1-10 leaves, PR1 induction was reduced strongly compared with that in wild-type leaves after inoculation with avirulent DC3000/avrRpm1, but it was similar to the wild-type response after inoculation with virulent DC3000 (Figure 6). Therefore, defects in RAR1 appear not to be registered by plants in basal resistance.
Figure 6.
Effect of rar1 on Pathogen-Induced PR1 Transcript Accumulation.
Leaves of 5-week-old Ler, eds1-2, and rar1-10 plants were hand infiltrated with Pseudomonas DC3000 expressing avrRpm1 or DC3000 containing an empty vector in 10 mM MgCl2 or with 10 mM MgCl2 alone. Total RNA was extracted at 0, 6, 12, 24, 48, and 72 hr after infection, and 20-μg samples were probed on gel blots with a genomic PR1 DNA fragment. Equal loading was determined by visualization of rRNA on the filter with methylene blue (bottom gel). Three independent experiments gave similar results.
DISCUSSION
RAR1 Function Is Conserved between Barley and Arabidopsis
We have identified the single Arabidopsis RAR1 ortholog as an essential component of resistance conferred by NB-LRR–type R genes recognizing avirulent bacterial and oomycete pathogens. In RPP5 resistance against Peronospora (Figure 4) and in RPM1 resistance against Pseudomonas (Figure 5), RAR1 functions at an early stage of plant defense, controlling expression of the HR and whole cell ROI generation at plant–pathogen interaction sites. In barley, RAR1 operates upstream of epidermal cell ROI accumulation and the HR in Mla12-conditioned resistance to an ascomycete fungus (Shirasu et al., 1999). We conclude that the function of RAR1 in disease resistance is conserved between monocotyledonous and dicotyledonous plant species that are separated evolutionarily by ∼150 million years.
RAR1 Is Required by TIR- and CC-NB-LRR Resistance Genes
An important finding from this study is the identification of RAR1-dependent and RAR1-independent R genes in both the TIR- and CC-NB-LRR classes (Table 2). Thus, genetic recruitment of RAR1 is not determined by a particular NB-LRR structural type, in contrast with the observed signaling preferences of TIR-NB-LRR–type genes for EDS1 and PAD4 and the majority of CC-NB-LRR genes for NDR1 (Aarts et al., 1998; McDowell et al., 2000; Peart et al., 2002). This distinction raises questions about the mechanism(s) that control RAR1 participation in the resistance pathway. We think it unlikely that the speed or intensity with which certain R genes induce an HR influence their RAR1 signaling mode, because RPM1, which conditions a rapid and strong HR and efficient pathogen containment, had a RAR1 requirement equivalent to that of RPS4, which triggers a weaker HR and restricts pathogen growth less effectively (Figure 5) (Rustérucci et al., 2001). Moreover, barley Mla1 and Mla6 specify temporally and spatially similar rapid epidermal cellular defenses against powdery mildew infection yet differ strikingly in their RAR1 dependence (Zhou et al., 2001; Halterman et al., 2001).
It is significant that the Mla1- and Mla6-encoded CC-NB-LRR proteins are 92% identical, implying that other discriminatory mechanisms regulate the engagement of RAR1 (see below). Our results suggest that RAR1 activity is restricted to R-avr gene–triggered responses and do not extend to its involvement in basal resistance against virulent pathogens. Thus, the susceptibility of rar1 to DC3000 was equivalent to that of wild-type plants and was not as extreme as in eds1 plants exhibiting hypersusceptibility to this bacterial strain (Figure 5). Consistent with the bacterial growth data, the induced expression of PR1, a marker of SA-dependent defenses in plant immunity (Gaffney et al., 1993; Cao et al., 1994; Reuber et al., 1998; Feys et al., 2001), was similar in rar1 and wild-type responses to virulent DC3000 (Figure 6).
RAR1 Exerts Rate-Limiting Control of R Gene–Triggered Defenses
The characterization of null and partially defective Arabidopsis rar1 mutants revealed a quantitative function of RAR1 in R gene–mediated defenses. Particularly instructive were analyses of RPP5 resistance in the various rar1 mutant backgrounds. Here, the extent of plant cell necrosis and pathogen colonization could be quantified readily using a combination of trypan blue staining and visualization of autofluorescence under UV light (Figure 4). We noted that complete loss of RAR1 function in rar1-10, rar1-11, and rar1-13 strongly delayed but did not completely abolish the HR or its accompanying oxidative burst. Similarly, it restricted but failed to halt Peronospora growth. The partial loss of RAR1 function in rar1-14 and rar1-15 resulted in intermediate effects on all of these parameters. The tight correlation between HR intensity, ROI accumulation, and the extent of pathogen containment in RPP5 resistance suggests that these processes are tightly linked in the defense cascade.
Although we cannot assume that the trailing plant cell necrosis seen at later stages of infection in rar1 plants (Figure 4) is qualitatively the same as the hypersensitive cell death of wild-type plants, we interpret it as a manifestation of a delayed HR. Consistent with this idea, necrosis occurred only in cells in contact with the invading pathogen. The RPP5 gene was shown previously to act in a semidominant manner in wild-type plants (Parker et al., 1993). Therefore, both RPP5 and RAR1 are capable of limiting defense signal flux. This raises the question of whether the dependence on RAR1 could vary with different RPP5 gene dosages. If RAR1 is rate limiting in homozygous RPP5 plants, could it be dispensed with if RPP5 expression were upregulated? In this regard, it will be useful to test the effect of rar1 on RPP5 heterozygotes and on plants overexpressing RPP5. We found that the resistance conferred by different R genes (RPM1, RPS2, and RPS4) to Pseudomonas also was controlled quantitatively by RAR1 (Figure 5), although the extent of hypersensitive plant cell death could not be quantified as clearly as in the RPP5 response.
We conclude that RAR1 exerts strict control of early signal fluxes leading to the HR triggered by different R genes. Whereas the loss of RAR1 partially suppressed RPP5 resistance (50% of the loss seen in rpp5; Figure 2), it resulted in the complete suppression of RPM1, RPS2, and RPS4 resistance to avirulent Pseudomonas. This finding may reflect the different modes of infection by the two pathogens. Alternatively, it might indicate different degrees of R gene reliance on RAR1, perhaps related to different amounts of RAR1 protein being engaged at a particular regulatory step. Tornero et al. (2002) show that the stability of RPM1 is reduced in a Col-0 null rar1 mutant. Their finding implies that RAR1 acts at the level of R protein stability before pathogen challenge. This model is not inconsistent with the early, rate-limiting function of RAR1 demonstrated by ourselves and Tornero et al. (2002). Therefore, different degrees of genetic dependence on RAR1 by R genes may reflect the intrinsic efficiency in triggering resistance by a particular R protein. Efficiency could be dictated by R protein abundance in the cell, as implied by the semidominance of RPP5 (see above), and other factors such as R protein affinity for pathogen avirulence and/or additional signaling components. Resistance mediated by the RAR1-independent genes RPP8 and RPP1A resembled that of the wild type in null rar1 plants in both the timing and extent of the HR and ROI accumulation (data not shown). These R proteins may use different cell death and oxidative burst mechanisms, or they may be sufficiently abundant in the cell to trigger defenses without the involvement of RAR1.
RAR1 Interactions with Other Defense Components
The isolation of definitive null rar1 mutants (rar1-11 and rar1-13) has helped clarify the issue of whether RAR1 in plants has functions conserved with animal CHORD proteins (Shirasu et al., 1999). The low frequency with which rar1 mutations were isolated relative to R gene mutations in barley (Jørgensen, 1996), coupled with uncertainties about residual barley rar1-2 activity (Shirasu et al., 1999), left open the possibility that plant RAR1 shares with animal CHORD proteins a vital developmental role, as demonstrated for the C. elegans CHORD protein CHP (Shirasu et al., 1999). Because our homozygous null rar1 mutant lines did not display obvious developmental defects, we favor the idea that plant RAR1 has evolved a distinct capability in plant defense.
This notion is supported by the recent characterization of barley rar1-2 as a probable null mutant (Azevedo et al., 2002). Analysis of the partially defective, but full-length, rar1-14 and rar1-15 alleles identified in this study permitted further molecular dissection of RAR1 function. The amino acid exchanges in these mutants were both within the CHORD II domain (Figure 1A, Table 1). In rar1-15, the exchange of an invariant Cys for a Tyr in the zinc binding motif led to reduced protein stability (Figure 3), which may contribute substantially to its partial loss of function. In contrast, the rar1-14 protein was as abundant as wild-type RAR1 (Figure 3), indicating that the Glu-to-Lys exchange may perturb a structural or functional role without destabilizing the protein. Regardless of the precise molecular defects of rar1-14, its retention of some activity (Figure 2) suggests that the activities of CHORD domains I and II may be at least partially separable. Consistent with this idea, a higher level of sequence conservation between individual CHORD I or II domains of different species than between CHORDs I and II of the same organism suggests a divergence in function (Shirasu et al., 1999). The rar1-14 mutant line will be a useful tool to test whether CHORD I and CHORD II participate in distinct molecular associations.
Several recent pieces of data provide a link between RAR1 and a second gene, SGT1, in plant resistance. Metazoan CHORD proteins contain a C-terminal extension not found in plant RAR1 sequences that has homology (the CS domain) with a portion of animal SGT1 (Shirasu et al., 1999). In yeast, SGT1 is required for assembly of the kinetochore (CBF3) complex and for SCF (Skp1-Cullin/cdc53-F box) E3 ligase activation through interaction with Skp1 (Kitagawa et al., 1999). In animals cells, SCF E3 ligases recognize specific phosphorylated substrates through their particular F box proteins and target them for ubiquitilation (Deshaies, 1999).
Ubiquitilation of proteins involved in transcription, cell cycle regulation, and other vital cellular functions normally targets them for degradation by the 26S proteasome (Deshaies, 1999; Callis and Vierstra, 2000). In plants, the ubiquitilation of proteins as a means of cellular regulation is poorly understood. However, a molecular connection was established recently between an Arabidopsis SCF complex containing the F box component TIR1 and regulated protein degradation in response to the phytohormone auxin (Gray et al., 2001).
We found that the rpr1 mutations identified in our RPP5 suppressor screens (Parker et al., 2000) are defective alleles of SGT1b, one of two highly homologous SGT1 genes in Arabidopsis, suggesting another link between resistance signaling and ubiquitilation of proteins (Austin et al., 2002). SGT1b also is required for RPP7 resistance in Col-0 to Peronospora isolate Hiks1 (Tör et al., 2002). Moreover, transient silencing of SGT1 in barley single-cell bombardment assays reveals a requirement for SGT1 in Mla-specified powdery mildew resistance (Azevedo et al., 2002).
Although the Arabidopsis rar1 mutations described here were isolated in the same screens as sgt1b, we found only a partial overlap in the spectrum of R genes requiring RAR1 and SGT1b, implying that they have both combined and distinct roles in defense (Austin et al., 2002). It is notable that Tornero et al. (2002) present genetic evidence for both cooperative and separate functions of RAR1 and NDR1 in different R gene–mediated responses. Together, these data support the existence of a complex matrix of signals that possibly are influenced by the extent of molecular association between various components.
METHODS
Plant Material, Pathogen Strains, and Pathology Tests
The null rpp5 (Parker et al., 1997) and eds1-2 (Falk et al., 1999) mutants of Arabidopsis thaliana have been described previously. Six rpr2 (rar1) mutants were isolated from fast neutron– or ethyl methanesulfonate–mutagenized Landsberg erecta (Ler) M2 seed obtained from Lehle Seeds (Round Rock, TX). In agreement with the groups of J. Dangl (University of North Carolina, Chapel Hill) and K. Shirasu (Sainsbury Laboratory, Norwich, UK), we have designated Ler rar2-1 to rpr2-6 as rar1-10 to rar1-15. Wassileskija (Ws-0) rar1 alleles are designated rar1-1 to rar1-9, and Columbia (Col-0) rar1 alleles are designated rar1-20 onward. For pathogenicity tests, plants were grown under a 10-hr photoperiod at 22°C ± 1°C with light intensity of 180 to 250 μE·m−2·sec−1 and ∼65% RH.
Peronospora parasitica and Pseudomonas syringae pv tomato DC3000 isolates were cultured and prepared for inoculations as described previously (Dangl et al., 1992; Innes et al., 1993). Three-week-old seedlings were sprayed to imminent runoff with 4 × 104 Peronospora conidiospores/mL in sterile distilled water. Conidiospores on leaves of infected plants were quantified 7 days after inoculation by vortexing 20 to 30 seedlings in 2 mL of distilled water. Conidiospores were counted with a hemocytometer using a light microscope at ×200 magnification. Bacterial growth tests were performed by vacuum infiltration of a bacterial suspension (1 × 105 colony-forming units/mL) in 10 mM MgCl2 and 0.01% Silwet L77 (Union Carbide Chemicals and Plastics, Versoix, Switzerland) into leaves of 4- to 5-week-old plants. Disease symptom development was monitored after dipping leaves in a bacterial suspension (5 × 107 colony-forming units/mL) in 10 mM MgCl2 and 0.01% Silwet L77.
Genomic DNA and RNA Preparations
Genomic DNA was prepared from fresh leaf material using the DNeasy 96 Plant Kit (Qiagen, Valencia, CA) in combination with the Retsch MM300 mixer mill (Retsch, Haan, Germany), according to the manufacturer's instructions. Total RNA was extracted from 4-week-old plants using Tri-Reagent (Sigma). Total RNA (20 μg) was loaded per lane on denaturing RNA gels and transferred to nylon membranes. Membranes were stained with 0.03% methylene blue in 0.5 M sodium acetate, pH 5.0, to ensure equal loading. A 1.3-kb RAR1 genomic DNA fragment (from exons 2 to 6; derived from the cloned RAR1 gene digested with SspI) was used as a hybridization probe.
RPR2 Mapping Analysis
A backcross mapping population was made by crossing Ler rpr2-1 (rar1-10) with Col-0 glabrous. Resulting F1 progeny then were crossed to rpr2-1. Progeny segregated in a ratio of 1:1 (Noco2-resistant:Noco2-susceptible plants). Susceptible individuals were rescued (sprayed with 0.2 mg/mL Ridomil [Norartis, Greensboro, NC]), and DNA preparations were made of resistant and susceptible plants. Additional populations were generated from resistant individuals that were homozygous for RPP5 (determined using codominant amplified polymorphic DNA [CAPS] marker g4539 [Parker et al., 1997]) and segregating at rpr2. CAPS and simple sequence length polymorphism (SSLP) markers identified in The Arabidopsis Information Resource (TAIR; http://www.aribidopsis.org) were used to position RPR2 onto the lower arm of chromosome 5. Additional CAPS and SSLP markers were generated from single nucleotide polymorphisms (SNPs) and simple dinucleotide repeats listed on the TAIR World Wide Web site.
In the final stages of mapping, markers were generated from the newly available Cereon Arabidopsis Polymorphism Collection (available through TAIR). RPR2 was narrowed down to a region of ∼220 kb (comprising overlapping transformation-competent artificial chromosome and P1 clones K17N15, K10D11, MIO24, MJM18, and MSG15), based on three remaining recombinant lines. Markers defining this region were as follows: proximal SSLP on K17N15 (41,219 bp), PM57 (5′-TAGGGGAAAATGTAGGATCA-3′) and PM58 (5′-AGCACCTCGTATACACCATC-3′), with polymerase chain reaction (PCR) product sizes of 194 bp (Col-0 glabrous) and 181 bp (Ler); distal SSLP on MSG15 (66,387 bp), PM65 (5′-TCCCTTACTGTCTTGTGGTT-3′) and PM66 (5′-AAAACATGTCATTCGTTTCC-3′), with PCR product sizes of 266 bp (Col-0 glabrous) and 243 bp (Ler). Other markers are available on request.
Amplification and Sequencing of RAR1
A 2.2-kb genomic fragment spanning the RAR1 coding region was amplified from DNA using primers ARAR1 (5′-CCTACCTTCTCAATTCGTCCGATTTCTTC-3′; MIO24, 7095 bp) and ARAR2 (5′-AGAGAGATTCGAGCCGTTCGTTGAGAGTA-3′; MIO24, 4934 bp) prepared from Ler and the rpr2 (rar1) mutant alleles. PCR errors were minimized by using the Expand High Fidelity PCR System (Roche, Basel, Switzerland). PCR products were sequenced using DNA primer sets spanning the RAR1 gene. Arabidopsis RAR1 and barley RAR1 protein sequences were aligned with Clustal W (http://www2.ebi.ac.uk/clustalw) and shaded using BoxShade (http://www.ch.embnet.org/software/BOX_form.html).
Selection of rar1 in Combination with R Genes from Other Accessions
Individual lines segregating for Ws-0 RPP1A and homozygous for rar1 were selected from F2 plants derived from a Ws-0 × rar1-10 cross. A PCR primer pair that detects an SSLP between Ws-0 and Ler within the Ws-0 RPP1 locus (5′-GGAATGATGATGTACTGTCCCAACCTCAC-3′ and 5′-ATTCTTGGATCCGCCATATTC-3′) was used in conjunction with rar1-10 mutant-specific primers. Primers PM81 (5′-CCAGTACAAAAGGCTGTGAT-3′) and PM82 (5′-ACAGTGAAAGAAAAGGGTCA-3′) gave a 195-bp product in the wild type and a 190-bp product in rar1-10 attributable to a 5-bp deletion (Table 1). Independent lines segregating for RPP2, RPS5, or RPP4 in Col-0 and homozygous for rar1 were selected from Col-0 × rar1-11 F2 plants using SSLP and CAPS makers within or closely flanking the desired genes. The RAR1 CAPS marker was derived from a SNP between the Ler and Col-0 RAR1 sequence that creates an additional MaeIII site in Col-0 (intron 5). Primers used were PM67 (5′-AAGAACAGATCAAGCAGACC-3′) and PM68 (5′-TCCTTTAGTGCAAGGAGGTA-3′).
Digestion of PCR products with MaeIII gave the following fragment sizes (in base pairs): Col-0, 187, 27, and 138; Ler, 187 and 165. The RPP2 locus was selected using PCR primer sets AG and g8300 (http://www.aribidopsis.org). PCR primer sets flanking the RPS5 locus were SSLP marker nga63 and CAPS marker SNP190 (polymorphism detected with RsaI; M.J. Austin, unpublished data). PCR primer sets flanking RPP4 were AG (http://www.aribidopsis.org) and set 2 (5′-GGGAGATTAAAGAAGCCTTTGC-3′ and 5′-GTGCGG–TTAACTGTTCGGTTACC-3′) detecting an SSLP (Col-0, 1.5 kb; Ler, 0.9 kb). To test the Col-0 RPP4 requirement for RAR1, it was necessary to select lines that were homozygous Col-0 rpp8, because Ler RPP8 also mediates resistance to Peronospora isolate Emwa1 (McDowell et al., 1998). Homozygous Col-0 rpp8 lines were selected using a RPP8-specific CAPS marker (McDowell et al., 1998). Multiple independent lines segregating for the respective R genes and homozygous for rar1 were inoculated with diagnostic Peronospora and Pseudomonas isolates (Table 2).
Protein Immunoblot Analysis
Total protein was extracted from 4-week-old seedlings by grinding with buffer (10 mM Na2HPO4, 1.8 mM KH2PO4, 2.5 mM KCl, 0.14 M NaCl, 5% [v/v] glycerol, 0.2% [v/v] Nonidet P-40 [IGEPAL CA-630, Sigma, Dorset, UK], and protease inhibitor cocktail [Roche]) and centrifugation at 16,000g at 4°C. Supernatants were collected, and 30 μg of each sample was resolved on 12% SDS–polyacrylamide gels and blotted onto polyvinylidene difluoride membranes (Amersham). RAR1-specific antibodies raised in rabbits using the full-length recombinant RAR1 protein as antigen have been described elsewhere (Azevedo et al., 2002). Membranes were probed with anti-RAR1 at a dilution of 1:5000, as described by Feys et al. (2001).
Histochemical Analysis of Host–Pathogen Interaction Sites
Plant cell necrosis and Peronospora development were monitored by staining with lactophenol trypan blue as described by Koch and Slusarenko (1990). Staining was performed on leaves of intact plants that had been spray inoculated with Peronospora or on excised leaves on which a 10-μL droplet of conidiospores had been placed. Examination of trypan blue–stained material and detection of H2O2 by staining with 3,3-diaminobenzidine was performed as described by Rustérucci et al. (2001).
Accession Numbers
The GenBank accession numbers for the sequences described in this article are AF192262 (Arabidopsis RAR1) and AF192261 (barley RAR1).
Acknowledgments
We thank Cereon Genomics for access to its Arabidopsis Polymorphism Collection. We are grateful to Jennifer Tedman for sequencing rar1-15 and to Paul Schulze-Lefert for helpful comments on the manuscript. This work was funded by The Gatsby Charitable Foundation and by a Biological and Biotechnology Research Council grant to J.E.P.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.001040.
References
- Aarts, N., Metz, M., Holub, E., Staskawicz, B.J., Daniels, M.J., and Parker, J.E. (1998). Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 10306–10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, M., Muskett, P.R., Kahn, K., Feys, B.J., Jones, J.D.G., and Parker, J.E. (2002). Regulatory role of SGT1 in early R gene-mediated plant defences. Science 295, 2077–2080. [DOI] [PubMed] [Google Scholar]
- Azevedo, C., Sadanandom, A., Kitagawa, K., Freialdenhoven, A., Shirasu, K., and Schulze-Lefert, P. (2002). The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295, 2073–2076. [DOI] [PubMed] [Google Scholar]
- Bent, A.F., Kunkel, B.N., Dahlbeck, D., Brown, K.L., Schmidt, R., Giraudat, J., Leung, J., and Staskawicz, B.J. (1994). RPS2 of Arabidopsis thaliana: A leucine-rich repeat class of plant disease resistance protein. Science 265, 1856–1860. [DOI] [PubMed] [Google Scholar]
- Botella, M.A., Parker, J.E., Frost, L.N., Bittner-Eddy, P.D., Beynon, J.L., Daniels, M.J., Holub, E.B., and Jones, J.D.G. (1998). Three genes of the Arabidopsis RPP1 complex resistance locus recognize distinct Peronospora parasitica avirulence determinants. Plant Cell 10, 1847–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes, D.C., Nam, J., and Dangl, J.L. (1998). The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proc. Natl. Acad. Sci. USA 95, 15849–15854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis, J., and Vierstra, R.D. (2000). Protein degradation in signaling. Curr. Opin. Plant Biol. 3, 381–386. [DOI] [PubMed] [Google Scholar]
- Cao, H., Bowling, S.A., Gordon, S., and Dong, X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century, K.S., Shapiro, A.D., Repetti, P.P., Dahlbeck, D., Holub, E., and Staskawicz, B.J. (1997). NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278, 1963–1965. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L., and Jones, J.D.G. (2001). Plant pathogens and integrated defence responses to infection. Nature 411, 826–833. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L., Holub, E.B., Debener, T., Lehnackers, H., Ritter, C., and Crute, I.R. (1992). Genetic definition of loci involved in Arabidopsis-pathogen interactions. In Methods in Arabidopsis Research, C. Koncz, N.H. Chua, and J. Schell, eds (Singapore: World Scientific Publishers), pp. 393–418.
- Deshaies, R.J. (1999). SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467. [DOI] [PubMed] [Google Scholar]
- Falk, A., Feys, B.J., Frost, L.N., Jones, J.D.G., Daniels, M.J., and Parker, J.E. (1999). EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA 96, 3292–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, B.J., and Parker, J.E. (2000). Interplay of signaling pathways in plant disease resistance. Trends Genet. 16, 449–455. [DOI] [PubMed] [Google Scholar]
- Feys, B.J., Moisan, L.J., Newman, M.A., and Parker, J.E. (2001). Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J. 20, 5400–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freialdenhoven, A., Scherag, B., Hollricher, K., Collinge, D.B., Thordal-Christensen, H., and Schulze-Lefert, P. (1994). Nar-1 and Nar-2, two loci required for Mla12-specified race-specific resistance to powdery mildew in barley. Plant Cell 6, 983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., Ward, S., Kessmann, H., and Ryals, J. (1993). Requirement of salicylic acid in the induction of systemic acquired resistance. Science 261, 754–756. [DOI] [PubMed] [Google Scholar]
- Gassmann, W., Hinsch, M.E., and Staskawicz, B.J. (1999). The Arabidopsis RPS4 bacterial-resistance gene is a member of the TIR-NBS-LRR family of disease-resistance genes. Plant J. 20, 265–277. [DOI] [PubMed] [Google Scholar]
- Goodall, G.J., and Filipowicz, W. (1991). Different effects of intron nucleotide composition and secondary structure on pre-mRNA splicing in monocot and dicot plants. EMBO J. 10, 2635–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, M.R., Godiard, L., Straube, E., Ashfield, T., Lewald, J., Sattler, A., Innes, R.W., and Dangl, J.L. (1995). Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269, 843–846. [DOI] [PubMed] [Google Scholar]
- Gray, W.M., Kepinski, S., Rouse, D., Leyser, O., and Estelle, M. (2001). Auxin regulates SCFTIR-dependent degradation of AUX/IAA proteins. Nature 414, 271–276. [DOI] [PubMed] [Google Scholar]
- Halterman, D., Zhou, F., Wei, F., Wise, R.P., and Schulze-Lefert, P. (2001). The MLA6 coiled-coil, NBS-LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f.sp. hordei in barley and wheat. Plant J. 25, 335–348. [DOI] [PubMed] [Google Scholar]
- Holub, E. (2001). The arms race is ancient history in Arabidopsis, the wildflower. Nat. Genet. Rev. 2, 516–527. [DOI] [PubMed] [Google Scholar]
- Innes, R.W., Bisgrove, S.R., Smith, N.M., Bent, A.F., Staskawicz, B.J., and Liu, Y.-C. (1993). Identification of a disease resistance locus in Arabidopsis that is functionally homologous to the RPG1 locus of soybean. Plant J. 4, 813–820. [DOI] [PubMed] [Google Scholar]
- Jia, Y., McAdams, S.A., Bryan, G.T., Hershey, H.P., and Valent, B. (2000). Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19, 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage, D., Tootle, T.L., Reuber, T.L., Frost, L.N., Feys, B.J., Parker, J.E., Ausubel, F.M., and Glazebrook, J. (1999). Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc. Natl. Acad. Sci. USA 96, 13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, J.H. (1996). Effect of three suppressors on the expression of powdery mildew resistance genes in barley. Genome 39, 492–498. [DOI] [PubMed] [Google Scholar]
- Kitagawa, K., Skowyra, D., Elledge, S.J., Harper, J.W., and Heiter, P. (1999). SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a subunit of the SCF ubiquitin ligase complex. Mol. Cell 4, 21–33. [DOI] [PubMed] [Google Scholar]
- Koch, E., and Slusarenko, A. (1990). Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister, R.T., and Katagiri, F. (2000). A resistance gene product of the nucleotide binding site:leucine rich repeats class can form a complex with bacterial avirulence proteins in vivo. Plant J. 22, 345–354. [DOI] [PubMed] [Google Scholar]
- McDowell, J.M., Dhandaydham, M., Long, T.A., Aarts, M.G.M., Goff, S., Holub, E.B., and Dangl, J.L. (1998). Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell 10, 1861–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, J.M., Cuzick, A., Can, C., Beynon, J., Dangl, J.L., and Holub, E.B. (2000). Downy mildew (Peronospora parasitica) resistance genes in Arabidopsis vary in functional requirements for NDR1, EDS1, NPR1 and salicylic acid accumulation. Plant J. 22, 523–529. [DOI] [PubMed] [Google Scholar]
- Nimchuk, Z., Marois, E., Kjemtrup, S., Leister, R.T., Katagiri, F., and Dangl, J.L. (2000). Eukaryotic fatty acylation drives plasma membrane targeting and enhances function of several type III effector proteins from Pseudomonas syringae. Cell 101, 353–363. [DOI] [PubMed] [Google Scholar]
- Parker, J.E., Szabò, V., Staskawicz, B.J., Lister, C., Dean, C., Daniels, M.J., and Jones, J.D.G. (1993). Phenotypic characterization and molecular mapping of the Arabidopsis thaliana locus RPP5, determining disease resistance to Peronospora parasitica. Plant J. 4, 821–831. [Google Scholar]
- Parker, J.E., Coleman, M.J., Szabò, V., Frost, L.N., Schmidt, R., van der Biezen, E.A., Moores, T., Dean, C., Daniels, M.J., and Jones, J.D.G. (1997). The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the Toll and Interleukin-1 receptors with N and L6. Plant Cell 9, 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J.E., Feys, B.J., van der Biezen, E.A., Noël, N., Aarts, N., Austin, M.J., Botella, M.A., Frost, L.N., Daniels, M.J., and Jones, J.D.G. (2000). Unravelling R gene-mediated disease resistance pathways in Arabidopsis. Mol. Plant Pathol. 1, 17–24. [DOI] [PubMed] [Google Scholar]
- Peart, J.R., Cook, G., Feys, B.J., Parker, J.E., and Baulcombe, D.C. (2002). An EDS1 orthologue is required for N-mediated resistance against tobacco mosaic virus. Plant J. 29, 569–579. [DOI] [PubMed] [Google Scholar]
- Reuber, T., Plotnikova, J.M., Dewdney, J., Rogers, E.E., Wood, W., and Ausubel, F.M. (1998). Correlation of defence gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J. 16, 473–485. [DOI] [PubMed] [Google Scholar]
- Rustérucci, C., Aviv, D.H., Holt, B.F., Dangl, J.L., and Parker, J.E. (2001). The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13, 2211–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield, S.R., Tobias, C.M., Rathjen, J.P., Chang, J.F., Lavelle, D.T., Michelmore, R.W., and Staskawicz, B.J. (1996). Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274, 2063–2065. [DOI] [PubMed] [Google Scholar]
- Shirasu, K., Lahaye, T., Tan, M.W., Zhou, F.S., Azevedo, C., and Schulze-Lefert, P. (1999). A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans. Cell 99, 355–366. [DOI] [PubMed] [Google Scholar]
- Staskawicz, B.J., Mudgett, M.B., Dangl, J.L., and Galan, J.E. (2001). Common and contrasting themes of plant and animal diseases. Science 292, 2285–2289. [DOI] [PubMed] [Google Scholar]
- Tang, X., Frederick, R.D., Zhou, J., Halterman, D.A., Jia, Y., and Martin, G.B. (1996). Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274, 2060–2063. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen, H., Zhang, Z.G., Wei, Y.D., and Collinge, D.B. (1997). Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Tör, M., Gordon, P., Cuzick, A., Eulgem, T., Sinapidou, E., Mert, F., Can, C., Dangl, J.L., and Holub, E.B. (2002). Arabidopsis SGT1b is required for defence signaling conferred by several downy mildew resistance genes. Plant Cell 14, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero, P., Merritt, P., Sadanandom, A., Shirasu, K., Innes, R.W., and Dangl, J.L. (2002). RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Biezen, E.A., Freddie, C.T., Kahn, K., Parker, J.E., and Jones, J.D.G. (2002). Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB-LRR genes and confers downy mildew resistance through multiple signaling components. Plant J. 29, 439–451. [DOI] [PubMed] [Google Scholar]
- Warren, R.F., Henk, A., Mowery, P., Holub, E., and Innes, R.W. (1998). A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 10, 1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F., Kurth, J., Wei, F., Elliott, C., Valè, G., Yahiaoui, N., Keller, B., Somerville, S., Wise, R., and Schulze-Lefert, P. (2001). Cell-autonomous expression of barley Mla1 confers race-specific resistance to the powdery mildew fungus via a Rar1-independent signaling pathway. Plant Cell 13, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]