Abstract
Chemokines induce the directional migration of targeted populations of leukocytes during periods of inflammation. Moreover, these molecules also regulate T-cell activation and differentiation following antigenic stimulation. In the present study, the contributions of the CC chemokine ligand 3 (CCL3) to the differentiation and migration of effector T cells in response to viral infection of the central nervous system (CNS) were analyzed. CCL3−/− mice infected with mouse hepatitis virus exhibited a significant reduction of virus-specific CD8+ T cells within the CNS, correlating with delayed viral clearance. Decreased infiltration of CD8+ T cells into infected CCL3−/− mice was associated with enhanced accumulation of primed CD8+ T cells in cervical lymph nodes. Although virus-specific CD8+ T cells from CCL3−/− mice were CD44high, they remained CD62Lhigh and CD25low, retained CCR7 expression, and contained limited transcripts of the proinflammatory chemokine receptors CCR5 and CXCR3 compared with virus-specific CD8+ T cells from CCL3+/+ mice. Furthermore, the absence of CCL3 impaired the cytokine production and cytolytic activity of CD8+ T cells. In addition, macrophage accumulation within the CNS was significantly decreased in infected CCL3−/− mice, correlating with reduced demyelination. These results suggest that CCL3 not only mediates macrophage chemotaxis but also significantly enhances differentiation of primed CD8+ T cells into effector cells and their release into circulation, thus potentiating effective migration to the site of infection.
Induction of an effective immune response following viral infection depends on the activation of virus-specific CD8+ T cells that are able to migrate to infected tissues and eliminate virus. Differentiation of naïve T cells into antigen-specific effector T cells is generally accomplished by antigen stimulation and cytokine signaling within the secondary lymphoid tissues. Effector cells exit these tissues and migrate to the site of infection where they are capable of exerting diverse antiviral responses (2, 5, 17, 37).
The distinct migration patterns of naïve and effector T cells depends upon the expression of adhesion molecules called homing receptors on the surface of T cells. Expression of CD62L (l-selectin) and the chemokine receptor CCR7 contribute to the migration of naïve T cells into secondary lymphoid organs (13, 33, 35). Recruitment of effector T cells to sites of infection is accompanied by downregulation of expression of CD62L and CCR7 followed by the upregulation of CD25 (interleukin 2 receptor [IL-2R] α chain) and CD44 as well as increased expression of proinflammatory chemokine receptors such as CXCR3 and CCR5 (27, 34). T-cell differentiation ultimately results in migration to the site of infection and virus elimination via production of cytokines such as gamma interferon (IFN-γ) and/or cell-mediated cytolysis. Although the mechanisms contributing to the generation of effector CD8+ T cells are not completely known, recent evidence indicates that exposure to cytokines, e.g., IL-2 and IL-15, as well as the duration of antigenic stimulation during priming are key factors contributing to effector cell generation (20, 27). However, the mechanisms by which such soluble signals regulate both the generation of effector T cells and their trafficking pattern have not been well characterized. How these factors and their cross-regulation affect T-cell function remains an important question with respect to host defense against viral infection.
We used a model of viral-induced neurologic disease to better understand the relative contributions of chemokines and chemokine receptors in the regulation of immune effector function and leukocyte migration following viral infection of the central nervous system (CNS). Intracranial infection of susceptible mice with mouse hepatitis virus (MHV), a positive-strand RNA virus, results in acute encephalomyelitis with virus replication in both neurons and glia (14). T cells are essential in controlling the acute stage of disease through the release of IFN-γ and perforin, which both aid in virus elimination (21, 32). However, clearance is incomplete and antigen persists in mice that survive the acute disease. The majority of these animals develop a demyelinating disease characterized by mononuclear-cell infiltration and myelin destruction, similar to the pathology of the human demyelinating disease multiple sclerosis (14).
Recent studies from our laboratory have indicated an important role for chemokines and their receptors in the regulation of leukocyte migration and infiltration into the CNS following MHV infection (6, 11, 18, 19, 23, 24). Among the chemokines that are expressed within the CNS during acute disease is the T-cell and macrophage chemoattractant CCL3 (11). To evaluate the functional contributions of CCL3 to host defense and disease, MHV-induced pathogenesis was investigated in mice lacking the ability to express CCL3 (CCL3−/−). The findings indicate that MHV-infected CCL3−/− mice are resistant to virus-induced demyelination, consistent with reduced macrophage trafficking into the CNS. However, infectious virus is not eliminated, coincident with impaired recruitment of CD8+ T cells into the brain. Impaired CD8+-T-cell trafficking is accompanied by the accumulation of virus-specific CD8+ T cells in secondary lymphoid organs, which are characterized by the persistent expression of the lymphoid homing molecules CD62L and CCR7 and the limited appearance of the proinflammatory and activation markers CD25, CXCR3, and CCR5. Virus-specific CCL3−/− CD8+ T cells are also deficient in IFN-γ production and have muted cytolytic activity, further supporting altered activation. These data argue that CCL3 plays an important role in host defense and the pathogenesis of MHV-induced neurologic disease by enhancing CD8+-T-cell differentiation and migration as well as mobilizing macrophage recruitment.
MATERIALS AND METHODS
Animals and virus.
MHV J2.2V-1 was kindly provided by J. Fleming (University of Wisconsin) (14). CCL3−/− mice were generated previously (C57BL/6, H-2b) (7), and control mice (C57BL/6) were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were anesthetized by inhalation of methoxyflurane (Pitman-Moor Inc., Washington Crossing, N.J.) and injected intracranially (i.c.) with 1,000 PFU of MHVJ2.2V-1 suspended in 30 μl of sterile phosphate-buffered saline. Control mice were injected with 30 μl of sterile phosphate-buffered saline alone.
Mononuclear-cell isolation and flow cytometry.
Mononuclear cells were obtained from the brains, cervical lymph nodes (CLN) (two draining CLN per mouse), and spleens of mice, followed by red blood cell lysis as previously described (18). Cell surface expression of phenotypic markers was examined by using the following reagents for four-color flow cytometric analysis: allophycocyanin-conjugated rat anti-mouse CD8; PERCP-conjugated rat anti-mouse CD4; fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD62L, CD44, and CD25 (adhesion molecules associated with T-cell activation) (BD and E Biosciences); phycoerythrin (PE)-conjugated Db/S510-518 major histocompatibility class I tetramer for identification of CD8+ T cells specific for viral spike protein antigen (4). Infiltrating macrophages were determined by using FITC-conjugated rat anti-mouse F4/80 (Serotec, Oxford, England) in combination with PE-conjugated rat anti-mouse CD45 (Pharmingen, San Diego, Calif.). Isotype-matched antibodies were used as controls.
RNase protection assay (RPA).
To detect CCL3 mRNA transcripts within the CNS of MHV-infected mice, total RNA was isolated from brains at days 3, 7, and 12 postinfection (p.i.) by using TRIZOL reagent (Invitrogen, Carlsbad, Calif.) (23). The level of chemokine mRNA transcripts was determined by using the mCK5 probe set (Pharmingen). To detect expression of chemokine receptor mRNA, CD8+ T cells were purified from CLN of CCL3+/+ and CCL3−/− mice at days 7 and 12 p.i. by using MACS microbeads conjugated to anti-CD8 monoclonal antibody (Miltenyi Biotec, Auburn, Calif.) and total RNA was isolated by using TRIZOL reagent. Chemokine receptor mRNA transcripts were analyzed by using the mCK1 probe set (Pharmingen).
Adoptive transfer.
Splenocytes from CCL3+/+ or CCL3−/− mice isolated 8 days postimmunization (2 × 105 PFU of MHV-J2.2V-1; intraperitoneal [i.p.] injection) were adoptively transferred (5 × 106 cells suspended in 200 μl of sterile Hanks balanced salt solution) via intravenous (i.v.) injection into the retro-orbital sinus of RAG1−/− or CCL3−/− mice 3 days following i.c. infection with 1,000 PFU of MHV J2.2V-1 (38). Mice were sacrificed 9 days posttransfer, and brains and spinal cords were removed. One-half of each brain was used for flow cytometric analysis, and the remaining half was used to determine viral titers. Spinal cords were stained with Luxol fast blue to assess the severity of demyelination by using the following scoring system: 0, no demyelination: 1, mild inflammation accompanied by loss of myelin integrity; 2, moderate inflammation with increasing myelin damage; 3, numerous inflammatory lesions accompanied by significant increase in myelin stripping; and 4, intense areas of inflammation accompanied by numerous phagocytic cells engulfing myelin debris (18).
Intracellular cytokine staining.
CCL3+/+ and CCL3−/− mice were infected with 1,000 PFU of MHV J2.2V-1, and brains and CLN were removed at 7 days p.i. for analysis of single cell suspensions. A total of 106 cells were stimulated for 6 h in 96-well plates with or without a 5 μM concentration of peptide corresponding to the spike (S) protein-derived S510-518 CD8 epitope, and intracellular staining for IFN-γ was performed by using a previously described procedure (6).
CTL assay.
Cytolytic activity of splenocytes following i.p. immunization with 2.5 × 105 PFU of MHV-J2.2V-1 was analyzed. At 7 days p.i., splenocytes were stimulated in vitro with a 5 μM concentration of S510-518 peptide in RPMI 1640 supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 5 × 10−2 M β-mercaptoethanol, nonessential amino acids, 10% fetal calf serum, and T Stim (Becton Dickinson, Bedford, Mass.) for 6 days. Fluorescence-activated cell sorter analysis of cultured cells was performed to determine the percentage of virus-specific (S510-518) CD8+ T cells present following expansion and was used to normalize the numbers of activated CD8+ T cells. Cytotoxic T-lymphocyte (CTL) assays were performed with Na51CrO4 (New England Nuclear, Boston, Mass.)-labeled RMAS (H-2b) target cells preincubated with a 1 μM concentration of S510-518 peptide. Cr51 release was determined after a 6-h incubation. Specific lysis was defined as follows: 100 × [(experimental release − spontaneous release)/(detergent release − spontaneous release)].
RESULTS
Viral clearance and T-cell recruitment are reduced in MHV-infected CCL3−/− mice.
Following i.c. infection of C57BL/6 mice with MHV, mRNA transcripts for the chemokine CCL3 were present within the brain at days 3, 7, and 12 p.i. (Fig. 1A and B). To determine the contributions of CCL3 to host defense and disease following viral infection of the CNS, inflammation and pathogenesis in CCL3+/+ and CCL3−/− mice infected i.c. with MHV were compared. Infection of CCL3−/− mice did not result in increased mortality compared to what was observed with infected CCL3+/+ mice up to day 12 p.i. Examination of viral titers within the brains of infected mice revealed no differences in viral burden at day 7 p.i. (Table 1, experiment 1). However, by day 12 p.i., virus recovery from the brains of CCL3−/− mice was enhanced (Table 1, experiment 1). Flow cytometric analysis was performed on mononuclear cells isolated from the CNS of infected mice at days 7 and 12 p.i. to determine whether CCL3 was contributing to T-lymphocyte infiltration. The data presented in Table 1 indicate that there is a significant decrease (P ≤ 0.001) in the total numbers of CD8+ T cells infiltrating into the CNS of CCL3−/− mice compared with CCL3+/+ mice at both 7 and 12 days p.i. Moreover, infiltration of CD8+ T cells specific for the immunodominant S510-518 epitope was significantly reduced at both 7 and 12 days p.i. compared to what was seen with CCL3+/+ mice (Table 1). Although the numbers of CD4+ T cells within the brains of CCL3−/− mice were also significantly decreased (P ≤ 0.001) at day 7 p.i., they were significantly higher (P ≤ 0.001) within the brains of CCL3−/− mice compared with CCL3+/+ wild-type mice by day 12 p.i. (Table 1).
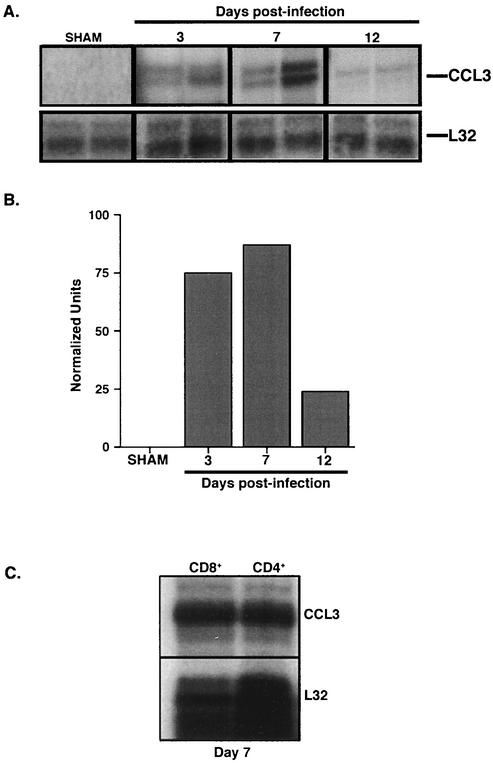
FIG. 1.
CCL3 gene expression within the CNS following MHV infection. (A) CCL3 mRNA transcript levels were determined by RPA analysis of total RNA isolated from the CNS of sham- and MHV-infected CCL3+/+ mice at 3, 7, and 12 days p.i. Each lane represents an individual mouse at the indicated time point. (B) Densitometric analysis of CCL3 mRNA transcripts obtained from the scanned autoradiograph. Data are the average normalized units representing the ratio of band intensity to L32 control. (C) Analysis of CCL3 mRNA production by activated CD4+ and CD8+ T cells. CD4+ and CD8+ T cells were harvested from the spleens of CCL3+/+ mice 7 days after i.p. immunization. Total RNA was isolated from enriched populations of CD4+ and CD8+ T cells, and CCL3 expression was determined by RPA. Both CD4+ and CD8+ T cells expressed CCL3 mRNA. Each lane represents enriched T cells isolated from the spleens of three pooled mice. The total number of CCL3+/+ mice was six.
TABLE 1.
T-lymphocyte and macrophage infiltration into the CNS of MHV-infected CCL3+/+ and CCL3−/− mice
| Experi-ment | Mouse | Day p.i. | Viral titer (log10 PFU/g of tissue) | No. of CD8+ cells | No. of CD8+ (S510-518) tetramer- positive cells | No. of CD4+ cells | No. of F4/80+ CD45high cells | Demyelin- ationb |
|---|---|---|---|---|---|---|---|---|
| 1a | CCL3+/+ | 7 | 3.5 ± 0.2 | (2.9 ± 0.15) × 105 | (9.0 ± 2.0) × 104 | (2.9 ± 0.2) × 105 | (1.0 ± 0.6) × 105 | NDh |
| 12 | <2 | (6.2 ± 0.6) × 104 | (1.0 ± 0.3) × 104 | (8.0 ± 0.2) × 104 | (3.0 ± 2.4) × 105 | 2.9 ± 0.04 | ||
| CCL3−/− | 7 | 3.3 ± 0.7 | (8.0 ± 1.7) × 103e | (2.0 ± 0.5) × 103e | (6.0 ± 0.1) × 104e | (4.0 ± 0.2) × 103e | ND | |
| 12 | 2.9 ± 0.1 | (2.0 ± 0.1) × 103e | (7.0 ± 1.0) × 102e | (3.0 ± 0.4) × 105e | (4.0 ± 0.1) × 103e | 1.6 ± 0.42f | ||
| 2c | RAG1−/− | 12 | 4.5 ± 0.1 | ND | ND | ND | (1.0 ± 0.1) × 104 | 0 |
| CCL3+/+ to RAG1−/− | 12d | 3.0 ± 0.3 | (2.0 ± 0.3) × 105 | (1.6 ± 4.1) × 104 | (1.0 ± 0.02) × 104 | (1.5 ± 0.03) × 105 | 3.1 ± 0.1 | |
| CCL3−/− to RAG1−/− | 12d | 4.8 ± 0.1g | (1.0 ± 0.1) × 104e | (6.0 ± 0.2) × 102e | (2.0 ± 0.1) × 103e | (3.0 ± 0.7) × 104e | 0.8 ± 0.1g | |
| CCL3+/+ to CCL3−/− | 12d | ≤2 | (1.2 ± 0.5) × 105 | (1.2 ± 3.2) × 104 | (1.0 ± 0.3) × 104 | (1.5 ± 0.01) × 105 | 2.9 ± 0.2 |
Characterization of viral clearance and mononuclear-cell infiltration, by flow cytometric analysis, into the CNS following i.c. infection with MHV. The data are means ± standard errors of the means (SEM) and are representative of three separate experiments with a minimum of three mice per group.
Demyelination was determined according to the scoring system described in Materials and Methods.
Characterization of mononuclear-cell infiltration into the CNS following adoptive transfer. The data are means ± SEM and are representative of two separate experiments with a minimum of three mice per group.
Nine days posttransfer of splenocytes.
P ≤ 0.001 compared with results for other mice tested in the same experiment.
P ≤ 0.02 compared with results for CCL3+/+ mice.
P ≤ 0.05 compared with results for other mice tested in the same experiment.
ND, not determined.
MHV-infected CCL3−/− mice exhibit reduced macrophage infiltration and demyelination.
Spinal cords obtained from CCL3+/+ mice at day 12 p.i. exhibited numerous inflammatory foci accompanied by robust demyelination (Fig. 2) (Table 1). Although inflammatory cells (presumably CD4+ T cells, based on flow cytometry results) were present within the spinal cords of CCL3−/− mice, there was a significant reduction (P ≤ 0.02) in myelin destruction (Fig. 2) (Table 1). As macrophage recruitment into the CNS is important in myelin destruction in MHV-infected mice (18, 38), macrophage infiltration into the CNS was investigated in infected CCL3−/− mice. To distinguish between resident microglia and infiltrating monocytes, flow cytometric analysis was performed by using FITC-conjugated anti-F4/80 antibodies and PE-conjugated anti-CD45 antibodies (6). CCL3−/− mice displayed a significant decrease (P ≤ 0.001) in the numbers of infiltrating macrophages at 7 and 12 days p.i., which correlated with reduced demyelination (Table 1).
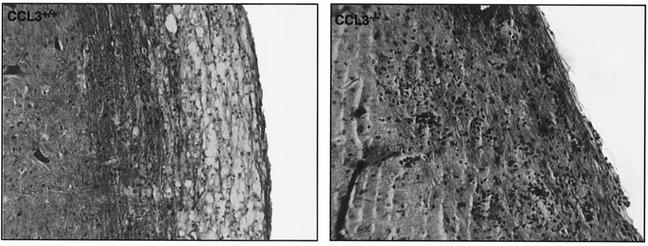
FIG. 2.
Absence of CCL3 results in diminished demyelination. Representative spinal cord sections were isolated from MHV-infected CCL3+/+ and CCL3−/− mice 12 days p.i. and were stained with Luxol fast blue to determine the severity of myelin destruction. CCL3+/+ mice exhibited extensive demyelination accompanied by mononuclear-cell infiltration. Although inflammatory foci are apparent in the white-matter tracts of CCL3−/− mice, myelin destruction was limited. Magnification, ×200.
T cells and macrophages from CCL3−/− mice do not traffic into the CNS following adoptive transfer.
In an attempt to further characterize the contribution of T-cell- versus resident-CNS-cell-derived CCL3 to neuroinflammation following MHV infection of the CNS, a series of adoptive transfer experiments was performed. Immune splenocytes were obtained from CCL3+/+ mice and transferred i.v. to either RAG1−/− or CCL3−/− mice 3 days after i.c. infection (Table 1, experiment 2). Transfer of immune cells from CCL3+/+ mice to MHV-infected RAG1−/− mice resulted in T-cell infiltration into the brain and a reduction in viral titers compared with what was seen with MHV-infected RAG1−/− mice receiving only Hanks balanced salt solution (Table 1). Similarly, transfer of immune CCL3+/+ splenocytes to infected CCL3−/− recipients reduced viral titers, correlating with T-cell infiltration (Table 1). By contrast, RAG1−/− recipients of immune splenocytes obtained from CCL3−/− mice exhibited high viral titers within the brain and limited infiltration of CD4+ and CD8+ T cells (Table 1), although at least twice as many CCL3−/− virus-specific CD8+ and CD4+ T cells were transferred (Table 2). Moreover, the numbers of tetramer-positive CD8+ T cells present within the brains of RAG1−/− recipients of CCL3−/− splenocytes were significantly reduced (P ≤ 0.001) compared with those of recipients of CCL3+/+ donors (Table 1). Both donor CD4+ and CD8+ T cells obtained from MHV-immunized CCL3+/+ mice expressed similar transcript levels for CCL3 at day 7 postimmunization, indicating that differential expression of this chemokine by T-cell subsets does not occur (Fig. 1C). These results suggested that CCL3 produced by T cells may further enhance monocyte recruitment within the CNS. Indeed, adoptive transfer of immune splenocytes from CCL3+/+ mice into either RAG1−/− or CCL3−/− recipients resulted in enhanced F4/80+, CD45high macrophage accumulation within the CNS and myelin destruction (Table 1). These data support the idea that CCL3 production by infiltrating T cells enhances accumulation of F4/80+, CD45high macrophages into the CNS.
TABLE 2.
Characterization of T cells used for adoptive transfera
| Mouse | Day p.i. | No. of CD8+ cells | No. of CD8+ (S510-518) tetramer-positive cells | No. of CD4+ cells | No. of CD4+ (M133-147)b cells |
|---|---|---|---|---|---|
| CCL3+/+ | 7 | 7.5 × 105 | 5.3 × 104 | 5.0 × 105 | 4.7 × 104 |
| CCL3−/− | 7 | 7.0 × 105 | 1.1 × 105 | 4.3 × 105 | 8.6 × 104 |
Characterization of mononuclear cells isolated from the spleens of CCL3+/+ or CCL3−/− mice following i.p. immunization with 2 × 105 PFU of MHV. A total of 5 × 106 cells were used for adoptive transfer studies. The data are representative of two separate experiments with a minimum of three mice per group.
The number of virus-specific CD4+ T cells was determined by their ability to produce IFN-γ in response to secondary stimulation with the CD4-immunodominant epitope M133-147. Total numbers were determined by multiplying the percentage of IFN-γ-producing cells by the total number of CD4+ T cells.
Analysis of virus-specific CD8+ T cells by S510-518 tetramer staining.
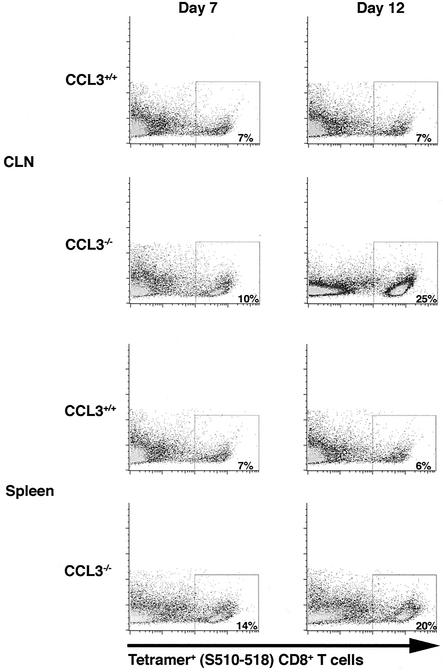
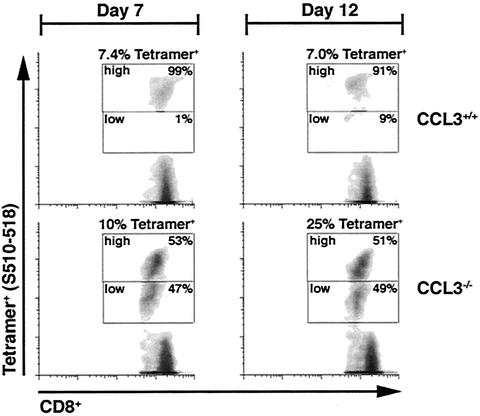
Despite the reduced numbers of CD8+ T cells present within the CNS of CCL3−/− mice, the relative frequencies of S510-518-specific cells within the CD8 population were similar between the groups, indicating impaired overall CD8+-T-cell recruitment rather than reduced expansion in CCL3−/− mice. Therefore, the numbers of S510-518-specific CD8+ T cells present within the CLN or spleens of CCL3+/+ and CCL3−/− mice were determined following either i.c. infection or i.p. immunization, respectively. Similar numbers of CD4+, CD8+, and tetramer-positive CD8+ T cells were present within the CLN of CCL3+/+ and CCL3−/− mice at day 7 following i.c. infection (Fig. 3) (Table 3). However, CCL3−/− mice exhibited increased total numbers of CD4+ and CD8+ T cells and an approximately 25-fold increase in the numbers of tetramer-positive cells within the CLN by 12 days p.i. (Fig. 3) (Table 3). The numbers of tetramer-positive CD8+ T cells were also increased within the spleens of immunized CCL3−/− mice at both 7 and 12 days p.i. compared with those within the spleens of CCL3+/+ mice (Fig. 3) (Table 3). These data clearly indicate that CCL3 is required not for expansion of virus-specific CD8+ T cells but for exit into the circulating pool. Interestingly, although the percentage and overall number of tetramer-positive CD8+ T cells were increased in the secondary lymphoid tissues of CCL3−/− mice, tetramer staining indicated differential fluorescence intensity associated with the tetramer (Fig. 4). In contrast to the high patterns of tetramer staining (tetramerhigh phenotype) observed with CCL3+/+ virus-specific CD8+ T cells within the CLN at 7 (99%) and 12 (91%) days p.i., nearly half of the CCL3−/− virus-specific CD8+ T cells (47 and 49% at 7 and 12 days p.i., respectively) expressed low patterns of tetramer staining (tetramerlow) (Fig. 4). Analysis of CCL3+/+ and CCL3−/− splenocytes following i.p. immunization revealed tetramer populations similar to those in the CLN at both 7 and 12 days p.i. (data not shown).
FIG. 3.
Analysis of S510-518 tetramer-positive CD8+ T cells following infection with MHV. CD8+ T cells were purified from CLN of CCL3+/+ and CCL3−/− mice at days 7 and 12 p.i. by using MACS microbeads conjugated to anti-CD8 monoclonal antibody. To determine the number of CD8+ T cells specific for the immunodominant S510-518 epitope, purified CD8+ T cells were stained with tetramer. Shown are representative dot blots indicating the percentages of S510-518-positive cells compared to side scatter (y axis) present in CLN and spleen of CCL3+/+ and CCL3−/− mice. Data presented are representative of the results obtained from three separate experiments with three mice per experiment.
TABLE 3.
Characterization of infiltrating T cells within secondary lymphoid tissuesa
| Organ | Mouse | Day p.i. | No. of CD4+ cells | No. of CD8+ cells | No. of CD8+ (S510-518) cells |
|---|---|---|---|---|---|
| Lymph nodesb | CCL3+/+ | 7 | (8.4 ± 0.1) × 105 | (5.7 ± 0.4) × 105 | (3.3 ± 0.1) × 104 |
| 12 | (9.6 ± 0.3) × 104 | (7.2 ± 0.3) × 104 | (5.5 ± 0.8) × 103 | ||
| CCL3−/− | 7 | (8.2 ± 0.2) × 105 | (5.0 ± 0.1) × 105 | (4.0 ± 0.1) × 104 | |
| 12 | (9.1 ± 0.2) × 105 | (5.8 ± 0.2) × 105 | (1.3 ± 0.2) × 105d | ||
| Spleenc | CCL3+/+ | 7 | (7.8 ± 0.5) × 106 | (2.3 ± 0.2) × 106 | (1.6 ± 0.3) × 105 |
| 12 | (6.1 ± 0.3) × 105 | (6.6 ± 0.3) × 105 | (2.0 ± 0.4) × 104 | ||
| CCL3−/− | 7 | (5.1 ± 0.1) × 106 | (2.5 ± 0.6) × 106 | (4.2 ± 0.2) × 105d | |
| 12 | (6.3 ± 0.3) × 106 | (2.6 ± 0.3) × 106 | (7.0 ± 0.6) × 105d |
Total cells were determined by multiplying the percentage of doubly positive cells by the number of total lymphocytes isolated.
Characterization, by flow cytometric analysis, of mononuclear cells within the CLN following i.c. infection with MHV. The data are representative of three separate experiments with a minimum of three mice per group and are means ± SEM.
Characterization, by flow cytometric analysis, of mononuclear cells within the spleen following i.p. immunization with MHV. The data are representative of three separate experiments with a minimum of three mice per group and are means ± SEM.
P ≤ 0.01 compared with results for CCL3+/+ mice.
FIG. 4.
Differential tetramer staining on CCL3−/− virus-specific CD8+ T cells. Analysis of tetramer (S510-518) fluorescence intensity on virus-specific CD8+ T cells. Total lymphocytes were harvested from the CLN of i.c. infected mice at 7 and 12 days p.i. and stained directly with CD8 and tetramer (S510-518) ex vivo. Dot blots represent tetramer staining on gated CD8+ T cells. The intensity of tetramer staining separated CD8+ T cells into three populations: tetramer positive (either tetramerhigh or tetramerlow) and tetramer negative. Density blots show the results with cells isolated from one representative mouse from each group at the indicated time points. The experiment was performed in triplicate with three mice per group.
Phenotypic characterization of S510-518 virus-specific CD8+ T cells.
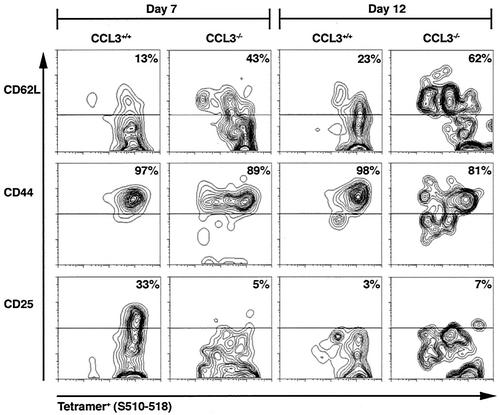
The barely detectable levels of tetramer-positive CD8+ T cells within the brains of infected CCL3−/− mice suggested possible alterations in the homing receptor and/or activation phenotype. To address this possibility, the receptor profile of CD8+ T cells isolated from the CLN and spleen following i.c. infection and i.p. immunization, respectively, were analyzed. T cells obtained from the spleens and lymph nodes of uninfected mice expressed high levels of CD62L and low levels of CD25 and CD44, characteristic of naïve T cells (data not shown). Following i.c. infection with MHV, tetramer-positive cells isolated from the CLN of CCL3+/+ mice at day 7 p.i. acquired an expression pattern typically associated with activation: CD62Llow (13%), CD44high (97%), and CD25high (33%) (Fig. 5). In contrast, although tetramer-positive CD8+ T cells isolated from CCL3−/− mice expressed high levels of CD44 (89%), they failed to upregulate CD25 (5%) and retained expression of CD62L (43%) (Fig. 5). At day 12 p.i., CCL3+/+ tetramer-positive cells remained CD44high (98%) and expression of CD62L was slightly increased, indicating an overall reduction in the activation state of these cells compared to day 7 p.i. This was supported by dramatically reduced CD25 expression (3%). Similarly, tetramer-positive cells isolated from the CLN of CCL3−/− mice at day 12 p.i. expressed high levels of CD44 (81%), increased levels of CD62L (62%), and very little CD25 (7%) (Fig. 5).
FIG. 5.
Altered receptor phenotype on CCL3−/− virus-specific CD8+ T cells. CCL3+/+ and CCL3−/− mice were infected i.c. with MHV, and the activation state of virus-specific CD8+ T cells was analyzed at 7 and 12 days p.i. Cells isolated from the CLN were stained for CD8, tetramer (S510-518), and either CD25, CD62L, or CD44. Contour blots represent CD62L, CD44, and CD25 expression on gated tetramer (S510-518)-positive, CD8+ T cells. The percentage of tetramer-positive cells expressing each receptor is shown in the upper right quadrant for each blot. The contour blots shown represent gated populations of tetramer-positive CD8+ T cells isolated from one representative mouse from each group. The experiment was performed in duplicate with three mice per group.
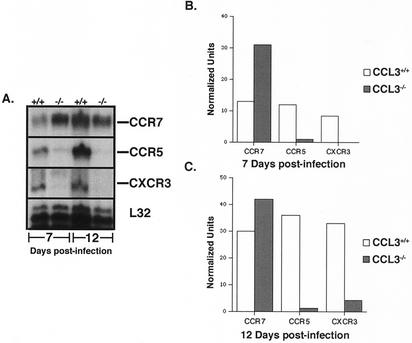
Analysis of chemokine receptor mRNA expression revealed the appearance of transcripts for the proinflammatory chemokine receptors CCR5 and CXCR3 as well as reduced transcripts for the lymphoid homing receptor CCR7 in CD8+ T cells from CCL3+/+ mice (Fig. 6). This correlated with their activated receptor expression profile. In contrast, neither CCR5 nor CXCR3 mRNA transcripts increased significantly in CD8+ T cells from CCL3−/− mice at either day 7 or 12 p.i. Furthermore, CCR7 mRNA expression remained elevated compared with what was seen with CCL3+/+ mice. Although previous reports have shown that cytokines, such as IL-2 and IL-15, play a role in the development of effector T cells (27), no significant difference in mRNA levels for these cytokines was detected within the CLN of MHV-infected CCL3−/− mice compared with CCL3+/+ mice (data not shown). These results indicated a defect in CCL3−/− mice that restricted the migration potential of CD8+ T cells. Furthermore, the absence of CD8+ T cells within the CNS of CCL3−/− mice may be due, at least in part, to an altered receptor phenotype that reduces trafficking out of secondary lymphoid organs and into the periphery.
FIG. 6.
Chemokine receptor gene expression in CCL3+/+ and CCL3−/− mice. (A) CCR7, CXCR3, and CCR5 mRNA transcript levels were determined by RPA analysis of total RNA obtained from CD8+ T cells isolated from CLN of MHV-infected CCL3+/+ and CCL3−/− mice at 7 and 12 days p.i. (B and C) Densitometric analysis of chemokine receptor mRNA transcripts obtained from the scanned autoradiograph. CCL3−/− CD8+ T cells exhibited a reduction in transcript levels for CXCR3 and CCR5 and an increase in CCR7 expression at both time points. Data are the average normalized units representing the ratio of band intensity to L32 control. Nine CCL3+/+ mice and nine CCL3−/− mice were used. A similar receptor profile was observed in spleens of CCL3+/+ and CCL3−/− mice (data not shown). Sham-infected CCL3+/+ and CCL3−/− mice displayed receptor profiles similar to those of MHV-infected CCL3−/− mice at day 7 p.i. (data not shown).
Decreased CD8+-T-cell effector function in CCL3−/− mice.
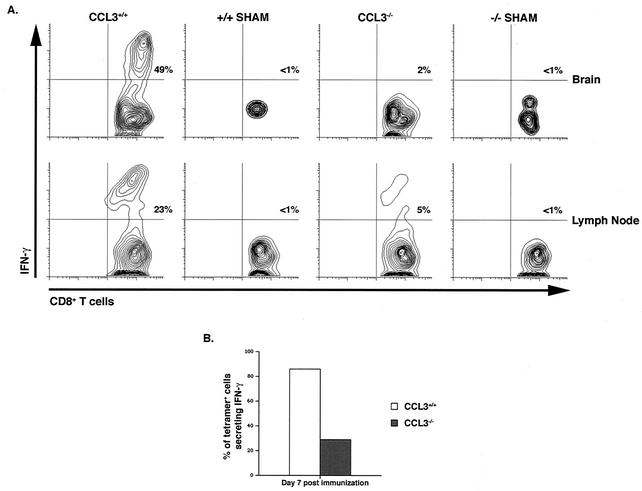
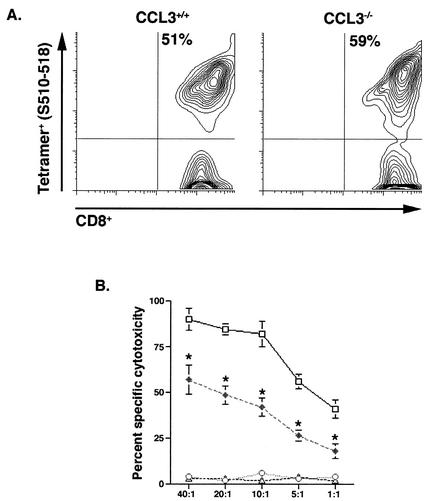
To further characterize the contributions of CCL3 to the activation state of CD8+ T cells, IFN-γ production was measured by intracellular cytokine staining. Upon peptide restimulation, 23% of CD8+ T cells within the CLN of i.c. infected CCL3+/+ mice produced IFN-γ (Fig. 7A). These activated, IFN-γ-producing CD8+ T cells were preferentially recruited to the CNS, where nearly half (49%) of all infiltrating CD8+ T cells were capable of secreting IFN-γ in response to peptide stimulation (Fig. 7A). In contrast, only 5% of the CD8+ T cells isolated from the CLN of i.c. infected CCL3−/− mice were capable of producing IFN-γ. The altered activation state of CCL3−/− CD8+ T cells correlated with sequestered migration of CD8+ T cells to the inflamed CNS, as only 2% of T cells within the CNS produced IFN-γ (Fig. 7A). Furthermore, the ratio of tetramer-positive CD8+ T cells producing IFN-γ within the spleen was dramatically decreased in CCL3−/− mice (∼30%) compared with wild-type mice (∼85%) (Fig. 7B). To identify additional functional differences between the two populations of CD8+ T cells, CTL activity was assessed. CCL3+/+ and CCL3−/− mice were immunized with MHV, and splenocytes were collected at 7 days p.i. Virus-specific CD8+ T cells were expanded for 6 days in the presence of the S510-518 peptide and used for CTL analysis. Although the frequencies of virus-specific CD8+ T cells were similar in both CCL3+/+ and CCL3−/− mice following expansion (Fig. 8A), there was a pronounced impairment in the cytolytic activity of CCL3−/− compared with CCL3+/+ CD8+ T cells (Fig. 8B).
FIG. 7.
IFN-γ production is diminished in CCL3−/− mice. CCL3+/+ and CCL3−/− mice were infected i.c. with MHV, and the CD8+-T-cell response to virus was analyzed at 7 days p.i. (A) Cells isolated from either the CLN or CNS were stained for CD8 and intracellular IFN-γ following stimulation with S510-518. Representative contour blots from flow cytometric analysis are shown. The overall percentages of doubly positive cells are indicated in the upper right quadrants. (B) The percentages of tetramer-positive CD8+ T cells capable of IFN-γ production within the spleen of CCL3+/+ and CCL3−/− mice at day 7 postimmunization are compared. Data represent three experiments with four mice in each group. Twelve CCL3+/+ mice and 12 CCL3−/− mice were used (P ≤ 0.05).
FIG. 8.
Reduced cytolytic activity in CCL3−/− CD8+ T cells. (A) To determine the cytolytic activity of virus-specific CD8+ T cells, splenocytes were isolated from i.p. immunized mice at 7 days p.i. Based on tetramer analysis, the frequencies of virus-specific CD8+ T cells were comparable in both groups of mice following expansion in the presence of S510-518 peptide for 6 days. (B) The cytolytic activities of CCL3+/+ and CCL3−/− cells were tested on RMAS target cells at the indicated effector-to-target cell ratios (x axis). Open squares and open circles represent CTL activity of CCL3+/+ CD8+ T cells following stimulation with S510-518 and irrelevant peptide, respectively. Results for CCL3−/− mice following stimulation with S510-518 and irrelevant peptide are represented by closed diamonds and open triangles, respectively. Cytolysis is shown as the percentage of specific cell lysis of peptide-treated target cells minus that of untreated target cells (n = 6; P ≤ 0.01).
DISCUSSION
Previous studies have demonstrated an important role for CCL3 in host defense as well as in contributing to inflammatory pathology following microbial infection. Mice deficient in CCL3 production exhibit increased susceptibility to disease following infection with paramyxovirus (9), Aspergillus spp. (28), Klebsiella pneumoniae (22), influenza virus (7), and coxsackievirus (7). In all cases, alterations in an effective host response correlated with a paucity in leukocyte accumulation at sites of infection. In addition, impaired CCL3 production also has been shown to result in reduced lung pathology following respiratory syncytial virus infection of mice (12). Therefore, the collective evidence clearly indicates an important role for CCL3 in both host defense and disease by virtue of its potent chemotactic properties.
Recent reports have also suggested a role for CCL3 in the development of a proinflammatory Th1 response. Following infection with Cryptococcus neoformans, mice lacking CCL3 are unable to generate a protective Th1 response, and these mice developed a skewed polarization toward a Th2 response, as indicated by increased numbers of IL-4- and IL-10-producing T cells (30). Other studies have suggested that CCL3 can directly inhibit IL-4 production (25), indicating that CCL3 may be able to enhance Th1 responses while inhibiting Th2 polarization. However, following MHV infection, there was no increase in IL-4 or IL-10 transcript levels within the CNS, spleen, or lymph nodes, indicating that a compensatory Th2 response does not occur in the absence of a protective Th1 response (data not shown). One possible reason for the discrepancy is that following C. neoformans infection, both Th1 and Th2 responses can be generated, and this is dictated by the dose and strain used for infection. However, following MHV infection, Th1 cytokines are prominently induced, with little to no production of Th2 cytokines (31). Therefore, the absence of CCL3 may lead to the generation of virus-specific T cells that are nonpolarized rather than skewed toward a Th2 phenotype.
To generate an effector CD8+-T-cell response, a complex combination of signals is required. It has become increasingly clear that although signaling through the T-cell receptor (TCR) can induce proliferation through both IL-2-dependent and -independent pathways, it is not sufficient to instruct dividing T cells to differentiate. A second signal, either cell bound or soluble, provided during TCR stimulation is required to instruct virus-specific T cells to differentiate and become activated effector cells (1). Importantly, our results indicate a novel mechanism whereby CCL3 signaling contributes to the activation and differentiation of effector CD8+ T cells following viral infection. CD8+ T cells appeared to be selectively affected, as CD4+ T cells were capable of infiltrating into the CNS of CCL3−/− mice by day 12 p.i. Whether this is the result of differential regulation of CCL3 receptor expression and/or differences in chemokine signaling requirements of this T-cell subset is unknown. Recent studies by Karpus and colleagues demonstrating that CCL3 can induce both nonpolarized and naïve T cells to produce IFN-γ in an IL-12- and IFN-γ-independent pathway further support a role for CCL3 in T-cell activation (15, 16). Indeed, our study indicates that in the absence of CCL3 signaling, tetramer-positive CD8+ T cells exhibit considerably muted IFN-γ production and limited CTL activity.
One possible explanation for the reduced effector activity observed in virus-specific CD8+ T cells from MHV-infected CCL3−/− mice is the overall reduction in CD25 (IL-2Rα) expression at all time points analyzed. Studies utilizing IL-2R-deficient mice revealed that IL-2 signaling is required for the generation of effector CD8+ T cells (26). Interestingly, although IL-2 signaling has a prominent role in enhancing CD4+- and CD8+-T-cell proliferation (3, 10, 29), the lack of IL-2R did not completely inhibit anti-CD3- and anti-CD28-induced proliferation, revealing an IL-2-independent, TCR-mediated pathway of CD8+-T-cell proliferation. However, perforin and granzyme levels were considerably reduced and these cells lacked the ability to produce IFN-γ, suggesting that although IL-2 can contribute to T-cell proliferation, it is also critical in the development of effector CD8+ T cells. Studies utilizing lymphocytic choriomeningitis virus infection in vivo further support a role for IL-2 and IL-2R in the development and maintenance of IFN-γ-producing CD8+ T cells (8, 36). Although IL-2 production was not compromised in the absence of CCL3 (data not shown), expression of IL-2R was severely impaired (Fig. 6), suggesting that CCL3-mediated upregulation of IL-2R may be the critical step in the development of effector CD8+ T cells during MHV infection.
One interesting finding was the presence of numerous tetramerlow cells within the secondary lymphoid organs of CCL3−/− mice following MHV infection. Analysis of TCR-β chain expression indicated comparable expression of TCR on the surfaces of both tetramerhigh and tetramerlow cells, suggesting that the two populations of tetramer cells could be separated by affinity to the S510-518 antigen (M. J. Trifilo and T. E. Lane, unpublished observations). Alternatively, the inability to clear virus from the CNS in CCL3−/− mice may also lead to persistent antigen presentation within the CLN that could participate in the downregulation of tetramer on the surface of CD8+ T cells. The absence of tetramerlow cells in lymphoid tissues of CCL3+/+ mice at day 7 p.i. and the preferential expansion of these cells in the absence of CCL3 suggest that in addition to the ability to influence the development of high-affinity (tetramerhigh) CD8+ T cells, CCL3 can also negatively select against low-affinity virus-specific CD8+ T cells.
Regardless of effector T-cell function, tetramer-positive CD8+ T cells were not able to migrate to the CNS in the absence of CCL3. Although expression of CD62L is critical for the recruitment of T cells into and within the secondary lymphoid organs, its downregulation is not required for migration into peripheral tissue. Furthermore, CCL3 expression within the CNS was not essential for T-cell recruitment, as determined by the ability of adoptively transferred CCL3+/+ T cells to migrate to the CNS within CCL3−/− mice. Therefore, additional modifications in the receptor profile of CCL3−/− CD8+ T cells must exist. Analysis of chemokine receptor transcripts on CD8+ T cells within the secondary lymphoid organs of CCL3−/− mice revealed increased expression of the lymphoid homing receptor CCR7 and the absence of the proinflammatory chemokine receptors CCR5 and CXCR3. Like that of CD62L, downregulation of CCR7 is not necessary for migration of T cells out of secondary lymphoid organs and into the periphery. However, both CXCR3 and CCR5 and their respective ligands (CXCL10 and CCL5, respectively) have been previously shown to be critical in the recruitment of activated CD4+ and CD8+ T cells into the CNS following MHV infection (11, 18, 23). The results of these previous studies, in addition to the observation that CD8+ T cells lacking CXCR3 and CCR5 do not infiltrate the CNS, indicate that upregulation of proinflammatory chemokine receptors is a critical step required for the migration of activated T cells into the CNS in response to MHV infection. Although it is possible that chemokine receptor expression is also linked to IL-2-mediated differentiation, the time course of chemokine receptor expression is not known at this time. Further analysis of chemokine receptor expression within the secondary lymphoid organs should delineate the precise sequence of events and demonstrate whether CCL3 is able to directly or indirectly control their transcription.
Our study also clearly indicates that CCL3 expression contributes to macrophage accumulation within the CNS and demyelination following MHV infection of the CNS. This is consistent with earlier findings showing that macrophages amplify the severity of MHV-induced demyelination (6, 11, 18, 23, 24, 38). A recent study by Glass et al. (11) demonstrated that macrophage migration and demyelination is limited in mice lacking the CCL3 receptor CCR5. Therefore, the results presented in this study indicate that one mechanism by which CCL3 attracts macrophages into the CNS may be through signaling of CCR5 expressed on the surface of these cells. Furthermore, the adoptive transfer studies support the idea that CCL3 expression by activated T cells present within the CNS is important in signaling macrophage recruitment to the brain.
Collectively, these findings indicate that in the absence of CCL3 signaling, CD8+ T cells are capable of generating an antigen-specific response but are unable to generate T cells that are effective in host defense following viral infection of the CNS by virtue of their (i) altered migration potential, (ii) inability to produce IFN-γ, and (iii) deficient cytolytic activity. The data presented are the first to our knowledge that have demonstrated an important role for CCL3 with regard to T-cell differentiation and emigration in the context of viral infection. In conclusion, the results presented support earlier studies indicating that chemokine expression is crucial not only in leukocyte recruitment to the site of infection but also in CD8+-T-cell activation (6, 11, 18, 23, 24). The observation that CCL3 expression is necessary to amplify CD8+-T-cell effector function as well as specifically affect CD8+-T-cell and macrophage trafficking following viral infection points to a nonredundant role for CCL3 in host defense and disease.
Acknowledgments
This work was supported by National Institutes of Health grants NS37336 and NS41429 and National Multiple Sclerosis Society grant R63278-A-3 (T.E.L.). W.A.K. is supported by a grant from the American Heart Association Texas Affiliate. C.C.B. is supported by NIH grants NS18146 and AI47249.
REFERENCES
- 1.Altman, A., K. M. Coggeschall, and T. Mustelin. 1990. Molecular events mediating T cell activation. Adv. Immunol. 48:227-360. [DOI] [PubMed] [Google Scholar]
- 2.Ando, K., L. G. Guidotti, S. Wirth, T. Ishikawa, G. Missale, T. Moriyama, R. D. Schreiber, H. J. Schlicht, S. Huang, and F. V. Chisari. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152:3245-3253. [PubMed] [Google Scholar]
- 3.Appleman, L. J., A. Berezovskaya, I. Grass, and V. A. Boussiotis. 2000. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J. Immunol. 164:144-151. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. [PubMed] [Google Scholar]
- 5.Biron, C. A. 1994. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr. Opin. Immunol. 6:530-538. [DOI] [PubMed] [Google Scholar]
- 6.Chen, B. P., W. A. Kuziel, and T. E. Lane. 2001. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J. Immunol. 167:4585-4592. [DOI] [PubMed] [Google Scholar]
- 7.Cook, D. N., M. A. Beck, T. M. Coffman, S. L. Kirby, J. F. Sheridan, I. B. Pragnell, and O. Smithies. 1995. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 269:1583-1585. [DOI] [PubMed] [Google Scholar]
- 8.Cousens, L. P., J. S. Orange, and C. A. Biron. 1995. Endogenous IL-2 contributes to T cell expansion and IFN-γ production during lymphocytic choriomeningitis virus infection. J. Immunol. 155:5690-5699. [PubMed] [Google Scholar]
- 9.Domachowske, J. B., C. A. Bonville, J. L. Gao, P. M. Murphy, A. J. Easton, and H. F. Rosenberg. 2000. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J. Immunol. 165:2677-2682. [DOI] [PubMed] [Google Scholar]
- 10.Gillis, S., and K. A. Smith. 1977. In vitro generation of tumor-specific cytotoxic lymphocytes. Secondary allogeneic mixed tumor lymphocyte culture of normal murine spleen cells. J. Exp. Med. 146:468-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass, W. G., M. T. Liu, W. A. Kuziel, and T. E. Lane. 2001. Reduced macrophage and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology 288:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeberle, H. A., W. A. Kuziel, H. J. Dieterich, A. Casola, Z. Gatalica, and R. P. Garofalo. 2001. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J. Virol. 75:878-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamann, A., K. Klugewitz, F. Austrup, and D. Jablonski-Westrich. 2000. Activation induces rapid and profound alterations in the trafficking of T cells. Eur. J. Immunol. 30:3207-3218. [DOI] [PubMed] [Google Scholar]
- 14.Houtman, J. J., and J. O. Fleming. 1996. Pathogenesis of mouse hepatitis virus induced demyelination. J. Neurovirol. 2:361-376. [DOI] [PubMed] [Google Scholar]
- 15.Karpus, W. J., and K. J. Kennedy. 1997. MIP-1α and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J. Leukoc. Biol. 62:681-687. [PubMed] [Google Scholar]
- 16.Karpus, W. J., N. W. Lukacs, K. J. Kennedy, W. S. Smith, S. D. Hurst, and T. A. Barrett. 1997. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 158:4129-4136. [PubMed] [Google Scholar]
- 17.Kundig, T. M., H. Hengartner, and R. M. Zinkernagel. 1993. T cell-dependent IFN-γ exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J. Immunol. 150:2316-2321. [PubMed] [Google Scholar]
- 18.Lane, T. E., M. T. Liu, B. P. Chen, V. C. Asensio, R. M. Samawi, A. D. Paoletti, I. L. Campbell, S. L. Kunkel, H. S. Fox, and M. J. Buchmeier. 2000. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 74:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane, T. E., V. C. Asensio, N. Yu, A. D. Paoletti, I. L. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of α- and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160:970-978. [PubMed] [Google Scholar]
- 20.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T lymphocyte responses: intermediates, effectors and memory cells. Science 290:92-97. [DOI] [PubMed] [Google Scholar]
- 21.Lin, M. T., S. A. Stohlman, and D. R. Hinton. 1997. Mouse hepatitis virus is cleared from the central nervous system of mice lacking perforin-mediated cytolysis. J. Virol. 71:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindell, D. M., T. J. Standiford, P. Mancuso, Z. J. Leshen, and G. B. Huffnagle. 2001. Macrophage inflammatory protein 1α/CCL3 is required for clearance of an acute Klebsiella pneumoniae pulmonary infection. Infect. Immun. 69:6364-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2001. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurologic function in a viral model of multiple sclerosis. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 24.Liu, M. T., D. Armstrong, T. A. Hamilton, and T. E. Lane. 2001. Expression of Mig (monokine induced by interferon-γ) is important in T lymphocyte recruitment and host defense following viral infection of the central nervous system. J. Immunol. 166:1790-1795. [DOI] [PubMed] [Google Scholar]
- 25.Lukacs, N. W., S. W. Chensue, W. J. Karpus, P. Lincoln, C. Keefer, R. M. Strieter, and S. L. Kunkel. 1997. C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am. J. Pathol. 150:1861-1868. [PMC free article] [PubMed] [Google Scholar]
- 26.Malek, T. R., A. Yu, P. Scibelli, M. G. Lichtenheld, and E. K. Codias. 2001. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J. Immunol. 166:1675-1683. [DOI] [PubMed] [Google Scholar]
- 27.Manjunath, N., P. Shankar, J. Wan, W. Weninger, M. A. Crowley, K. Hieshima, T. A. Springer, X. Fan, H. Shen, J. Lieberman, and U. H. von Andrian. 2001. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Investig. 108:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehrad, B., T. A. Moore, and T. J. Standiford. 2000. Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary Aspergillosis in neutropenic hosts. J. Immunol. 165:962-968. [DOI] [PubMed] [Google Scholar]
- 29.Morgan, D. A., F. W. Ruscetti, and P. Gallo. 1976. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193:1007-1008. [DOI] [PubMed] [Google Scholar]
- 30.Olszewski, M. A., G. B. Huffinagle, R. A. McDonald, D. M. Lindell, B. B. Moore, D. N. Cook, and G. B. Toews. 2000. The role of macrophage inflammatory protein-1α/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J. Immunol. 165:6429-6436. [DOI] [PubMed] [Google Scholar]
- 31.Parra, B., D. R. Hinton, M. T. Lin, D. J. Cua, and S. A. Stohlman. 1997. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology 233:260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-γ is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 33.Potsch, C., D. Vohringer, and H. Pircher. 1999. Distinct migration patterns of naïve and effector CD8 T cells in the spleen: correlation with CCR7 receptor expression and chemokine reactivity. Eur. J. Immunol. 29:3562-3570. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto, F., E. Kremmer, B. Palermo, A. Hoy, P. Ponath, S. Qin, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur. J. Immunol. 29:2037-2045. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 36.Su, H. C., L. P. Cousens, L. D. Fast, M. K. Slifka, R. D. Bungiro, R. Ahmed, and C. A. Biron. 1998. CD4+ and CD8+ T cell interactions in IFN-γ and IL-4 responses to viral infections: requirements for IL-2. J. Immunol. 160:5007-5017. [PubMed] [Google Scholar]
- 37.Walsh, C. M., M. Matloubain, C. C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. F. Huang, J. D. E. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, G. F., A. A. Dandekar, L. Powe, and S. Perlman. 2000. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 165:2278-2286. [DOI] [PubMed] [Google Scholar]