Abstract
DC-SIGN and DC-SIGNR are two closely related membrane-associated C-type lectins that bind human immunodeficiency virus (HIV) envelope glycoprotein with high affinity. Binding of HIV to cells expressing DC-SIGN or DC-SIGNR can enhance the efficiency of infection of cells coexpressing the specific HIV receptors. DC-SIGN is expressed on some dendritic cells, while DC-SIGNR is localized to certain endothelial cell populations, including hepatic sinusoidal endothelial cells. We found that soluble versions of the hepatitis C virus (HCV) E2 glycoprotein and retrovirus pseudotypes expressing chimeric forms of both HCV E1 and E2 glycoproteins bound efficiently to DC-SIGN and DC-SIGNR expressed on cell lines and primary human endothelial cells but not to other C-type lectins tested. Soluble E2 bound to immature and mature human monocyte-derived dendritic cells (MDDCs). Binding of E2 to immature MDDCs was dependent on DC-SIGN interactions, while binding to mature MDDCs was partly independent of DC-SIGN, suggesting that other cell surface molecules may mediate HCV glycoprotein interactions. HCV interactions with DC-SIGN and DC-SIGNR may contribute to the establishment or persistence of infection both by the capture and delivery of virus to the liver and by modulating dendritic cell function.
Hepatitis C virus (HCV) is an enveloped, positive-stranded RNA virus classified in the family Flaviviridae. Infection is often associated with chronic disease, sometimes resulting in hepatitis, cirrhosis, and hepatocellular carcinoma. Although chronic infection occurs in up to 70% of individuals, the mechanisms leading to viral persistence have not been defined. The principal site of replication is thought to be the liver, although several laboratories have suggested that HCV may infect a wider range of cell types, including monocytes/macrophages and B cells (28, 33, 44).
HCV encodes two putative envelope glycoproteins, E1 and E2, which are believed to be type I integral transmembrane proteins with C-terminal hydrophobic anchor domains. In vitro expression studies have shown that both glycoproteins associate to form heterodimers, which accumulate in the endoplasmic reticulum, the proposed site for HCV assembly and budding (reviewed in reference 53). Being an enveloped virus, HCV likely interacts with specific cell surface receptors that either induce conformational changes in the E1 and E2 glycoproteins, resulting in fusion between the viral and cellular membranes, or mediate internalization of virus particles to endosomes, where the acidic environment triggers membrane fusion-inducing conformational changes. The E2 glycoprotein is thought to be responsible for initiating virus attachment (29, 54, 67, 71), and we have hypothesized that the E1 glycoprotein contains the fusion peptide responsible for mediating fusion of the virus and cell membranes (31).
The lack of in vitro systems for HCV propagation has hampered biological and physiochemical studies of the virion and its mechanism of cell entry, so that the cellular receptors remain unknown. Difficulties encountered in purifying sufficient quantities of HCV from plasma have limited studies with native virus. In addition, HCV purified from plasma has been reported to exist in association with immune complexes and plasma lipoproteins (2, 6, 57). The association of the virus with lipoproteins has led to the suggestion that HCV may use the low-density lipoprotein receptor to gain entry into cells (3, 71).
In the absence of native HCV particles, virus-like particles expressed in insect cell systems (11, 15, 63, 67) and truncated versions of the E2 glycoprotein have been used as mimics to study virus-cell interactions (29, 54, 58). Truncated E2 binds specifically to human cells and was used to identify CD81 as a putative receptor for some HCV strains (54). Recent reports suggest antigenic differences between the truncated form of E2 and that present on virus-like particles for reactivity with E2-specific monoclonal antibodies and CD81 (15, 63, 67). Since CD81 is expressed on the majority of cell types, it is unlikely to be the sole determinant of viral tropism, and additional cell surface molecules may be required for HCV entry into a target cell (45).
While virus receptors typically play important roles in defining virus tropism, other cell surface molecules can significantly enhance the efficiency of virus infection. For example, the presence of virus attachment factors, while not required for infection, can make infection of receptor-positive cells far more efficient (43). DC-SIGN is one such high-affinity virus attachment factor (32). DC-SIGN is a C-type (calcium-dependent) lectin that is expressed as a homotetrameric type II membrane protein on some dendritic cell subsets and tissue macrophages (32, 60, 61). DC-SIGN binds human immunodeficiency viruses (HIVs) with high affinity, and once bound, the virus can be transferred to adjoining receptor positive cells (20, 32). Alternatively, expression of DC-SIGN on cells that also express the HIV receptors can enhance infection in cis (43). The ability of DC-SIGN to efficiently bind and transmit HIV may help explain how dendritic cells boost infection of T cells in vitro, where dendritic cell-bound virus is efficiently transferred to CD4-positive T cells in the same culture (14).
Binding of DC-SIGN to the HIV envelope protein (Env) is dependent upon the presence of high-mannose N-linked carbohydrate chains (26, 46, 48). The Env protein is heavily glycosylated, as are the other DC-SIGN ligands identified to date, ICAM-2, ICAM-3, and the Ebola virus glycoprotein (5, 32, 59a). The HCV E1 and E2 glycoproteins are heavily glycosylated and predicted to contain high-mannose oligosaccharides, raising the possibility that they may interact with DC-SIGN. In addition, a closely related homologue of DC-SIGN, termed DC-SIGNR or L-SIGN, also functions as an attachment factor for HIV and is expressed on liver sinusoidal endothelial cells (10, 56). Given the localization of DC-SIGNR in the liver and the highly glycosylated nature of the HCV E1 and E2 proteins, we sought to determine if DC-SIGN and DC-SIGNR could bind these viral proteins. We found that soluble E2 glycoprotein bound efficiently to DC-SIGN and DC-SIGNR and that retrovirus pseudotypes bearing chimeric HCV E1 and E2 glycoproteins also bound efficiently to these attachment factors when expressed on cell lines or primary human endothelial cells. Thus, DC-SIGN and DC-SIGNR may serve as HCV attachment proteins. This interaction could play an important role in HCV pathogenesis by influencing infection and modulating dendritic cell function.
MATERIALS AND METHODS
Cell culture.
293-T and QT6 cells were propagated in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, penicillin, and streptomycin. 293 T-REx cells expressing DC-SIGN and DC-SIGNR were described previously and maintained in medium containing DMEM, 10% fetal bovine serum, 100 μg of Zeocin per ml, and 5 μg of blasticidin per ml (55). T-REx cell lines expressing langerin, CD23, CLEC-1, and CLEC-2 were generated as described for DC-SIGN and DC-SIGNR T-REx cells (55) and cultivated in medium containing DMEM, 10% fetal bovine serum, 100 μg of Zeocin per ml, and 5 μg of blasticidin per ml. DC-SIGN and DC-SIGNR expression was induced by culturing the cells in medium containing 0.1 μg of doxycycline per ml or as indicated. 293 T-REx parental cells were maintained in medium containing DMEM, 10% fetal bovine serum, and 5 μg of blasticidin per ml. All cells were grown at 37°C and 5% CO2.
Plasmids.
The plasmid expressing H77 E2661 was described previously (29). The HCV cDNA sequence from strain HC-J4 encoding amino acids 364 to 661 was PCR amplified and cloned into the KpnI and NheI restriction sites of the plasmid vector pCAGGS/MCS (49). The E1/G and E2/G chimeric constructs were generated from the H77 1a consensus sequence with the strategy described by Takikawa and colleagues (40, 62). To generate the Sindbis virus chimeric constructs, amino acids 364 to 711 of E2 and 171 to 340 of E1 from strain HC-J4 were PCR amplified, fused to the transmembrane domains (TMDs) and cytoplasmic tails of Sindbis virus E1 (amino acids 1210 to 1245) and E2 (amino acids 690 to 751), respectively, and inserted into the NheI and XhoI sites of pCAGGS/MCS. The DC-SIGN, DC-SIGNR, and Langerin expression plasmids have been described elsewhere (55, 56, 59a, 65). cDNAs encoding CD23, CLEC-1, and CLEC-2 were obtained by reverse transcription-PCR amplification of total tissue RNA, cloned into pcDNA3, and sequenced to confirm identity.
Antibodies.
The mouse monoclonal antibodies (MAbs) m507 (immunoglobulin G2b [IgG2b], anti-DC-SIGN), m604 (IgG2b, anti-DC-SIGNR), and m526 and m612 (IgG2a, anti-DC-SIGN and DC-SIGNR) were purchased from R&D Systems, Inc., Minneapolis, Minn., and have been characterized previously (9, 35). Rat MAbs specific for HCV E2 (6/1a, 9/75, and 3/11) and E1 (3/8ow) were described previously (29). The anti-CD81 MAb 5A6 was kindly provided by S. Levy (Stanford University).
Purification of dendritic cells.
Human peripheral blood mononuclear cells were isolated from leukocyte concentrates by density gradient centrifugation on Ficoll-Paque (Amersham Pharmacia Biotech). CD14+ monocytes were positively selected with CD14-MicroBeads (Miltenyi Biotec), and the cells were cultured in RPMI 1640 with 1% single-donor plasma, 500 U of recombinant human interleukin-4 (IL-4; R&D Systems) per ml, and 1,000 U of recombinant human granulocyte-macrophage colony-stimulating factor (Immunex) per ml for 5 days. Cells were matured by additional culture for 2 to 3 days in the presence of IL-1β, IL-6, tumor necrosis factor, and prostaglandin E2. Maturation cytokines were added at a final concentration of IL-1β of 10 ng/ml; IL-6 of 1,000 U/ml; tumor necrosis factor of 10 ng/ml, and prostaglandin E2 of 1 μg/ml (all from R&D Systems except prostaglandin E2, which was from Sigma-Aldrich). Maturation of dendritic cells was verified by CD83, CD25, and major histocompatibility complex (MHC) types I and II marker staining.
Soluble E2 binding assay.
293-T cells were transiently transfected with plasmids expressing H77, HC-J4 E2661, or vector alone with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Tissue culture supernatants containing E2661 were harvested 48 h posttransfection, and the amount of E2 antigen was quantified by enzyme immunoassay, as described previously (29). Cells expressing DC-SIGN and DC-SIGNR were harvested, washed with phosphate-buffered saline (PBS), and blocked with PBS-1% fetal bovine serum-0.05% sodium azide (fluorescence-activated cell sorting [FACS] buffer). Approximately 2 × 105 cells were incubated with mock antigen or E2661 at a saturating concentration in PBS-1% fetal bovine serum-0.05% sodium azide-1 mM CaCl2 for 1 h at room temperature, washed, and subjected to flow cytometry analysis for staining of cell-associated E2 with the indicated MAbs.
Flow cytometric analysis.
To assess cell surface expression of DC-SIGN and other cell surface-expressed molecules, flow cytometric analyses were performed. Transiently transfected BHK or 293-T cells or stable T-REx cell lines were harvested, washed with PBS, and resuspended in ice-cold FACS buffer. Approximately 2 × 105 cells were incubated with the various MAbs at 5 μg/ml in a total volume of 100 μl for 30 min at room temperature. Cells were washed and incubated in 100 μl of phycoerythrin-conjugated anti-species IgG (Jackson Laboratories) diluted 1:1,000 in FACS buffer for 30 min at room temperature, washed, and reconstituted in FACS buffer. Flow cytometric analyses were performed with a FACSCalibur flow cytometer (Becton Dickinson).
Pseudotype production and virus binding assay.
HIV particles harboring the HCV glycoproteins were generated by cotransfection of 293-T cells with equal amounts of the HCV E1 and/or E2 expression plasmids and the env-defective pNL4-3-Luc-R−E− proviral genome (18). The supernatant was harvested 48 h posttransfection, aliquoted, and stored at −80°C. The p24 antigen content of the supernatants was assessed with a commercially available enzyme immunoassay (Coulter Beckman). To investigate virus binding to DC-SIGN and the various other lectins, 293-T cells were transfected with plasmids encoding the various lectins and pcDNA3 control vector, and the cells were seeded into 96-well plates 24 h after transfection. Alternatively, 293 T-REx cells were induced to express DC-SIGN and DC-SIGNR by doxycycline treatment. Equal volumes of p24 normalized viral supernatants were incubated with target cells for 3 h, and the cells were washed three times with DMEM-10% fetal bovine serum and lysed in 1% Triton X-100. The p24 content of the lysates was assessed by antigen capture enzyme-linked immunosorbent assay.
Western blot analysis of HCV E2 binding to various lectins and E2 incorporation into HIV particles.
QT6 quail cells were infected with vaccinia virus encoding T7 polymerase, vTF1.1, at a multiplicity of infection of 10 for 1 h. Cells were subsequently transfected with expression plasmids encoding the different lectins and a pcDNA3 control vector, incubated overnight in DMEM with 10% fetal bovine serum and rifampin (100 μg/ml), detached, and incubated with H77 E2661 antigen for 1 h at 37°C. Cells were washed three times with cold PBS containing Ca2+ and Mg2+, lysed in 1% NP-40-150 mM NaCl-50 mM Tris (pH 8.0)-protease inhibitor cocktail (Roche Molecular Biochemicals). Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and samples were analyzed for HCV E2 (with a 1:100 dilution of rat anti-E2 MAbs 6/1a and 9/75) and lectin expression (with a 1:1,000 dilution of mouse anti-DC-SIGN m28 or anti-AU1 antibody) by Western blot.
To investigate the incorporation of E2 glycoproteins into HIV particles, equal volumes of viral supernatants containing 20 ng of p24 antigen were separated by SDS-PAGE, and HCV glycoproteins as well as p24 antigen were detected by Western blot as described above. Alternatively, viral supernatants were concentrated through a 30% sucrose cushion and analyzed by gradient centrifugation. Then 250 μl of the concentrated sample was layered onto the top of a 7 to 41% continuous sucrose gradient and separated by centrifugation at 35,000 rpm for 3 h at 4°C. After centrifugation, the gradient was fractionated from the bottom into 1-ml aliquots, and the protein was precipitated by the addition of 250 μl of cold 50% trichloroacetic acid (TCA). The pellets were washed in 2% TCA, resuspended in sample buffer, and separated on a Novex NuPAGE 4% to 12% Bis-Tis gel. Protein was detected with anti-HCV E2 MAb 3/11 at a dilution of 1:40 and anti-p24 MAb at a dilution of 1:500.
RESULTS
Soluble HCV E2 binds DC-SIGN and DC-SIGNR.
Since the HCV E1 and E2 glycoproteins are retained in the endoplasmic reticulum via motifs in their C-terminal TMDs (16, 24, 30), truncated forms of the E2 glycoprotein lacking the TMD have been used as mimics to study HCV-cell interactions (Fig. 1). We have previously characterized secreted forms of the H77 genotype 1a E2 glycoprotein and found that a protein terminating at amino acid 661 (E2661) is efficiently secreted, binds CD81 with high affinity, and is recognized by a series of conformation-dependent MAbs (29). To investigate whether E2 can bind DC-SIGN and DC-SIGNR, we utilized T-REx cells, which express high levels of DC-SIGN and DC-SIGNR upon induction with doxycycline (Fig. 2A) (55, 56). However, T-REx cells constitutively express CD81 (Fig. 2A), which could complicate interpretation of the E2 cell binding assays. Therefore, to investigate specific interactions of E2 with DC-SIGN and DC-SIGNR, we selected E2 glycoproteins with high (H77) and low (HC-J4) binding affinities for a recombinant form of CD81 (57a).
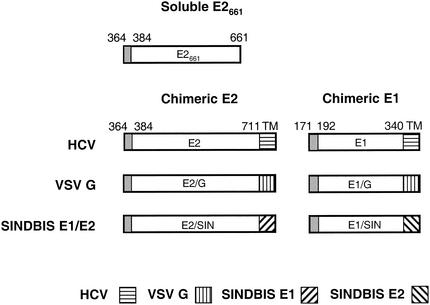
FIG. 1.
Schematic diagram of HCV E1 and E2 constructs. The soluble E2661 and chimeric E2 and E1 constructs containing HCV cDNA sequences encoding the indicated regions were cloned into expression vectors as described in Materials and Methods. The shaded areas represent the signal peptide, and the hatched areas represent transmembrane (TM) domain swaps.
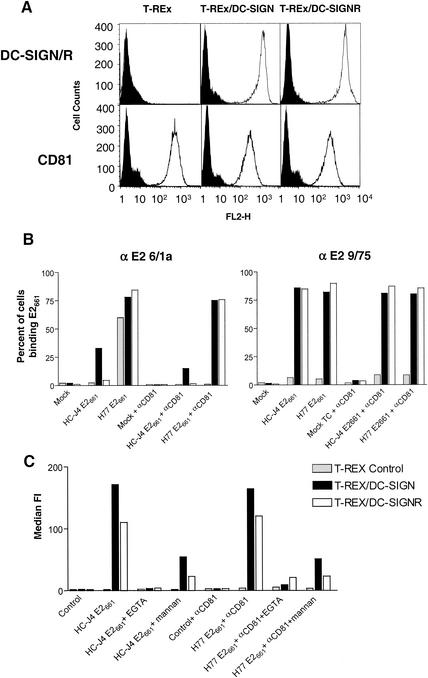
FIG. 2.
Binding of soluble E2 to cells expressing DC-SIGN and DC-SIGNR. (A) Human 293-T and T-REx cell lines expressing DC-SIGN or DC-SIGNR (DC-SIGN/R) under an inducible promoter were incubated with 1 μg of doxycycline per ml to induce receptor expression. Parental T-REx cells, which do not express DC-SIGN and DC-SIGNR, were also used. Expression of DC-SIGN, DC-SIGNR, and CD81 was monitored by FACS analysis with MAbs specific for DC-SIGN and DC-SIGNR (m612) or anti-CD81 MAb 5A6 (open histograms). An isotype-matched mouse IgG was used as a negative control (solid shaded histograms). (B) Parental T-REx cells or cells expressing DC-SIGN or DC-SIGNR were incubated with the indicated E2 glycoprotein in the presence and absence of the anti-CD81 MAb 5A6. After washing, cell surface-bound E2 was detected with anti-E2 MAbs 6/1a and 9/75. MAb 6/1a can detect E2 when complexed with CD81, whereas the epitope recognized by MAb 9/75 is occluded when E2 binds CD81. The results are expressed as the percentage of cells binding E2 and are representative of two independent experiments. (C) Parental T-REx cells or cells expressing DC-SIGN or DC-SIGNR were incubated with the indicated E2 glycoprotein (H77 E661 binding was evaluated in the presence of anti-CD81 MAb 5A6) in the presence and absence of EGTA and mannan, and cell-bound E2 was detected with MAb 9/75. The results are expressed as the median fluorescence intensity (FI).
Soluble H77 and HC-J4 E2 glycoproteins were incubated with cells, unbound protein was removed by washing, and cell surface-bound E2 was detected with anti-E2 MAbs 6/1a and 9/75 (Fig. 2B). These MAbs were chosen because 6/1a can detect CD81-complexed and noncomplexed E2, whereas MAb 9/75 recognizes an epitope that is masked when E2 binds CD81 and can therefore distinguish between CD81-dependent and -independent forms of cell-bound E2 (29). With MAb 6/1a, we found that H77 E2 bound well to the parental T-REx cells and to those expressing DC-SIGN and DC-SIGNR. In contrast, HC-J4 E2 only bound to T-REx cells expressing DC-SIGN (Fig. 2B). However, preincubation of cells with the anti-CD81 MAb 5A6 inhibited H77 E2 binding to parental T-REx cells but not to those expressing DC-SIGN and DC-SIGNR (Fig. 2B). These data are consistent with our previous observations that H77 E2661 binds CD81 with high affinity (29) but also show that H77 E2 can bind DC-SIGN and DC-SIGNR.
This interpretation was confirmed by the use of MAb 9/75, which does not recognize CD81-complexed E2. This MAb only detected H77 and HC-J4 E2 bound to cells expressing DC-SIGN and DC-SIGNR and not to the parental T-REx cells, confirming that both E2 proteins bind DC-SIGN and DC-SIGNR independently of CD81. In addition, preincubation of cells with the anti-CD81 MAb had no effect on E2 binding to cells expressing DC-SIGN and DC-SIGNR (Fig. 2C). In contrast, E2 binding to DC-SIGN and DC-SIGNR was inhibited by mannan and EGTA, agents that are known to block ligand interactions with C-type lectins (32) (Fig. 2C). Finally, it is interesting that MAb 6/1a detected HC-J4 E2 bound to DC-SIGN but not to DC-SIGNR, suggesting subtle differences in how these lectins interact with HC-J4 E2.
HIV pseudotypes bearing HCV E1 and E2 chimeras bind DC-SIGN and DC-SIGNR.
To evaluate the binding of HCV glycoproteins to DC-SIGN in the context of a virus particle, we used an HIV packaging system in which chimeric HCV E1 and E2 glycoproteins were pseudotyped into retroviral particles. Since HIV assembles at the plasma membrane, it was necessary to express the HCV glycoproteins at this location for efficient pseudotype formation. We (31) and others (13, 42, 47, 62) have reported that truncated forms of HCV E1 and E2 fused to the TMD and cytoplasmic tail of influenza virus hemagglutinin or vesicular stomatitis virus (VSV) G glycoprotein are efficiently expressed at the cell surface.
The determinants of HCV glycoprotein heterodimer formation are found in their TMDs (21). In contrast, VSV-G and influenza virus hemagglutinin form trimers via ectodomain interactions (22, 68, 69). We therefore generated chimeric proteins containing the Sindbis virus E1 and E2 TMDs, which are known to form heterodimers stabilized via motifs in their TMDs (Fig. 1) (72). Chimeric H77 E1 and E2 glycoproteins fused with the TMDs of VSV-G (E1/G and E2/G) or Sindbis virus glycoproteins E1 and E2 (E1/SIN and E2/SIN) were tested for cell surface expression. All chimeric HCV glycoproteins were expressed at the cell surface and able to bind a panel of conformation-dependent MAbs specific for HCV E1 and E2 (Fig. 3A and data not shown).
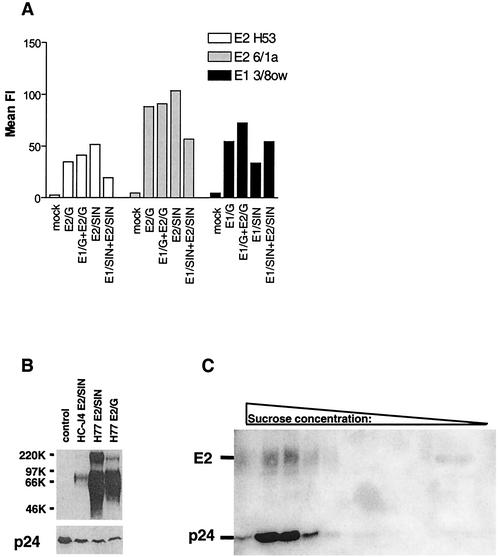
FIG. 3.
Cell surface expression of chimeric HCV glycoproteins and incorporation into virus pseudotypes. (A) BHK cells transfected with plasmids expressing the H77 chimeric glycoproteins depicted in Fig. 1 were stained for glycoprotein expression with anti-E2 MAbs H53 and 6/1a and anti-E1 MAb 3/8ow and analyzed by FACS. The mean fluorescence intensity (FI) is shown and is relative to an isotype-matched irrelevant MAb. (B) 293-T cells were cotransfected with plasmid DNA encoding the indicated E2 chimeric glycoproteins and NL4-3-Luc-R−E−, the supernatant was harvested 48 h after transfection and clarified by passage through a 0.45-μm filter, and incorporation of HCV glycoproteins and HIV core p24 antigen was analyzed by Western blot with anti-E2 MAbs 6/1a and 9/75 (upper panel) and anti-p24 MAb (lower panel). Similar results were obtained when virus particles were pelleted through a 20% sucrose cushion by ultracentrifugation (data not shown). (C) HC-J4 E2/SIN and NL4-3-Luc-R−E− were cotransfected into 293-T cells, and the supernatant was processed as described above and concentrated through a 30% sucrose cushion. The concentrated supernatant was centrifuged into a continuous sucrose gradient, and fractions were analyzed by Western blotting as described in Materials and Methods.
To produce virus pseudotypes, 293-T cells were cotransfected with plasmids encoding an envelope-defective HIV-1 proviral genome expressing a luciferase reporter gene (NL4-3-Luc-R−E−) and the chimeric HCV glycoproteins. Extracellular supernatants were collected 48 h after transfection and separated by SDS-PAGE, and HIV core antigen (p24) and HCV glycoprotein incorporation were analyzed directly by Western blot (Fig. 3B) or the supernatants were first subjected to gradient centrifugation followed by Western blot analysis (Fig. 3 C). The HC-J4 E2/SIN, H77 E2/SIN, and H77 E2/G chimeric glycoproteins as well as p24 antigen were readily detectable, suggesting the formation of pseudotyped particles. However, the E2-p24 ratio varied between virus preparations, possibly due to differences in transfection efficiency during the preparation of different pseudotype stocks (Fig. 3B). Upon gradient analysis of supernatants obtained by cotransfection of HC-J4 E2/SIN and NL4-3-Luc-R−E− a predominant signal for E2 and p24 was detected at sucrose densities of 34.5 to 39%, confirming the incorporation of the chimeric glycoprotein into HIV particles (Fig. 3C). A similar migration pattern was observed upon analysis of supernatants containing wild-type HIV-1 NL4-3 virions (data not shown). We were unable to monitor incorporation of HCV E1 into virus particles due to the low affinity of the E1-specific MAbs available.
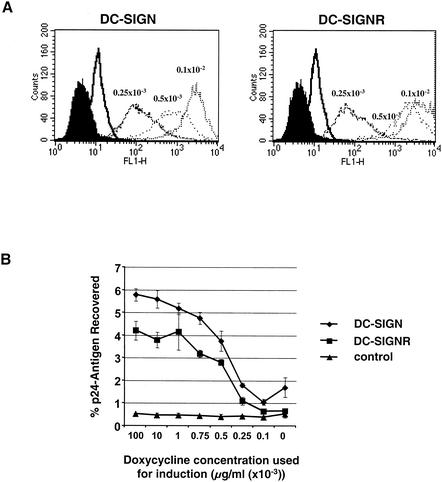
Cell-binding assays were performed with virus pseudotypes expressing the H77 E2/SIN and E2/G glycoproteins or HC-J4 E1/SIN and E2/SIN glycoproteins, either singly or in combination (Fig. 4A and B). Parental T-REx cells and those induced to express DC-SIGN or DC-SIGNR were incubated with equal amounts of each virus pseudotype, unbound virus was removed by washing, the cells were lysed, and p24 antigen was quantified by enzyme-linked immunosorbent assay. For a positive control, virus pseudotypes expressing the HIV-1 NL4-3 Env protein were used and shown to bind cells expressing DC-SIGN and DC-SIGNR, as previously reported (55, 56). Virus pseudotypes harboring chimeric HCV glycoproteins bound cells expressing DC-SIGN or DC-SIGNR at levels comparable to those of virus bearing the NL4-3 Env protein and approximately 10-fold more efficiently than parental T-REx cells not expressing DC-SIGN and DC-SIGNR (Fig. 4A).
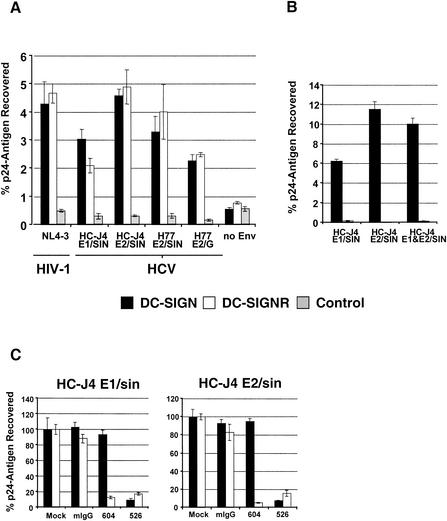
FIG. 4.
Binding of HIV-HCV pseudotype particles to T-REx cells expressing DC-SIGN and DC-SIGNR. (A and B) DC-SIGN, DC-SIGNR, and parental T-REx cells were induced with 0.1 μg of doxycycline per ml overnight and incubated with equal volumes of medium containing 1 ng of p24 antigen-normalized virus pseudotypes. Cells were washed and lysed, and p24 antigen content was determined by enzyme-linked immunosorbent assay. The percentage of recovered antigen is indicated. Representative experiments carried out in quadruplicate are shown, with standard deviations indicated. Similar results were obtained in three independent experiments. (C) The binding experiment was carried out as described for A, but prior to addition of virus, T-REx cells were incubated with 20 μg of the indicated MAbs per ml. The results are presented as a percentage of pseudotype binding to control untreated cells. Comparable results were obtained in an independent experiment.
The ability of virus particles produced in cells expressing HC-J4 E1/SIN to bind DC-SIGN- and DC-SIGNR-positive cells suggests that this chimeric glycoprotein was incorporated into virus. None of the viruses bound to the parental T-REx cells in the absence of DC-SIGN and DC-SIGNR (Fig. 4A). It was of interest that viruses expressing H77 E2/SIN or E2/G failed to bind the parental cells, suggesting minimal interaction with CD81 in the context of these virus particles. HIV expressing both HC-J4 E1/SIN and E2/SIN glycoproteins failed to show any difference in cell binding compared to viruses expressing either chimeric glycoprotein alone (Fig. 4B). Furthermore, viruses produced in the absence of viral glycoproteins failed to interact with DC-SIGN- and DC-SIGNR-positive cells, implying that the virus-cell interaction was not mediated via cell membrane proteins incorporated into the particles (Fig. 4A).
The DC-SIGN and DC-SIGNR dependency of cell binding was further assessed with MAbs specific for DC-SIGNR (MAb 604) or DC-SIGN and DC-SIGNR (MAb 526) (9, 35). MAb 604 blocked virus binding to DC-SIGNR-positive cells, while MAb 526 prevented binding to both DC-SIGN- and DC-SIGNR-positive cells (Fig. 4C). Similar results were obtained with viral supernatants that were concentrated through a 30% sucrose cushion (data not shown). Thus, both HCV E1 and E2 bind to DC-SIGN and DC-SIGNR when expressed in the context of virus particles. HIV-HCV pseudotype preparations expressing the various chimeric glycoproteins were tested for their ability to infect T-REx cells expressing DC-SIGN and DC-SIGNR and to transfer infectivity to Huh-7 and HepG2 hepatoma cells. Although luciferase was routinely detected in cell types incubated with HIV NL4-3 reporter viruses, no such activity was detected in any cell type exposed to HIV-HCV pseudotypic particles.
The ability of DC-SIGN to support HIV binding is dependent upon the levels at which it is expressed (55). In T-REx cells, approximately 100,000 copies of DC-SIGN are needed to support maximal HIV transmission. This level of expression is well below what is seen on the surface of immature monocyte-derived dendritic cells, which typically have in excess of 150,000 copies of DC-SIGN (9). To study the relationship between HCV binding and DC-SIGN and DC-SIGNR expression, cells expressing either DC-SIGN or DC-SIGNR were induced with different concentrations of doxycycline. FACS analysis confirmed variable levels of DC-SIGN and DC-SIGNR expression (Fig. 5A). Binding of HC-J4 E2/SIN-containing virus particles was dependent upon DC-SIGN and DC-SIGNR expression and occurred efficiently at DC-SIGN and DC-SIGNR expression levels comparable to those found on immature human monocyte-derived dendritic cells (MDDCs) (Fig. 5B).
FIG. 5.
Effect of cell surface DC-SIGN and DC-SIGNR expression levels on HIV-HCV pseudotype binding. DC-SIGN, DC-SIGNR, and control T-REx cells were induced overnight with the indicated concentrations of doxycycline, and receptor expression and binding of HC-J4 E2/SIN viruses were assessed in parallel. (A) Surface expression of DC-SIGN and DC-SIGNR. DC-SIGN- and DC-SIGNR-positive cells were incubated in medium containing the indicated concentrations of doxycycline, stained with anti-AU-1 antibody, and analyzed by FACS. (B) HC-J4 E2/SIN pseudotype binding to DC-SIGN, DC-SIGNR, and control cells. The indicated cell lines were incubated with equal volumes of medium containing 1.5 μg of p24 antigen-normalized virus, and binding was quantified as described in Materials and Methods. The percentage of bound p24 antigen is indicated. A representative experiment carried out in triplicate is shown, and the standard deviation is indicated. Similar results were obtained in an independent experiment.
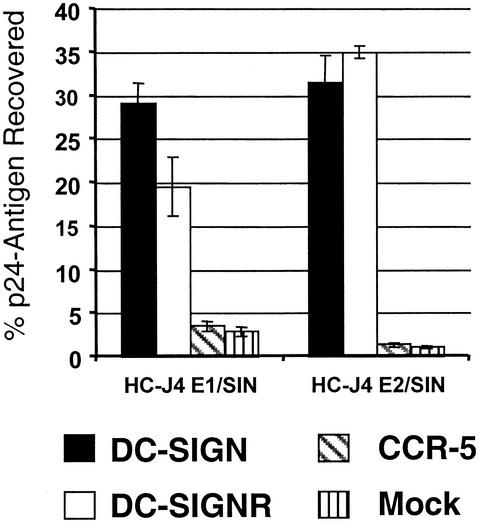
HIV/HCV pseudotypes bind DC-SIGN and DC-SIGNR expressed on human umbilical vein endothelial cells.
DC-SIGNR is expressed on human liver sinusoidal endothelial cells, where it may interact with HCV, enhancing infection of endothelial cells or trapping virus and presenting it to adjacent hepatocytes (10, 56). To determine if DC-SIGN and DC-SIGNR expressed on endothelial cells can bind HCV glycoproteins, primary human umbilical vein endothelial cells (HUVECs) were transduced with lentivirus vectors expressing DC-SIGN or DC-SIGNR. The expression level of DC-SIGN and DC-SIGNR on the HUVECs was comparable to that seen upon induction of T-REx cells (data not shown). A lentivirus encoding the chemokine receptor CCR-5 was used as a negative control. HIV pseudotypes expressing HC-J4 E1/SIN or HC-J4 E2/SIN glycoprotein bound specifically to HUVECS expressing DC-SIGN or DC-SIGNR (Fig. 6). The relatively high level of virus recovery in these experiments (approximately 25% of input virus) was also observed in some experiments with T-REx cells and is probably due to high levels of HCV glycoprotein incorporation in some virus preparations resulting from efficient transfection. However, all pseudotype virus stocks showed at least a 10-fold increase in binding to cells expressing DC-SIGN and DC-SIGNR compared to parental cells.
FIG. 6.
Binding of HIV-HCV pseudotype particles to HUVECs transduced to express DC-SIGN, DC-SIGNR, and CCR-5. HUVECs were infected overnight with lentiviruses encoding DC-SIGN, DC-SIGN and DC-SIGNR, and CCR-5 and maintained for 3 days as previously described (59a). The level of DC-SIGN and DC-SIGNR expression was similar to that obtained with T-REx cells (data not shown). Thereafter, binding of 2 ng of p24 antigen-normalized HC-J4 E1/SIN and E2/SIN pseudotype viruses was assessed as described in Materials and Methods. The percentage of recovered viral p24 antigen is indicated. A representative experiment carried out in quadruplicate is shown, and the standard deviation is indicated. Similar results were obtained in an independent experiment.
HCV E2 binds DC-SIGN on dendritic cells.
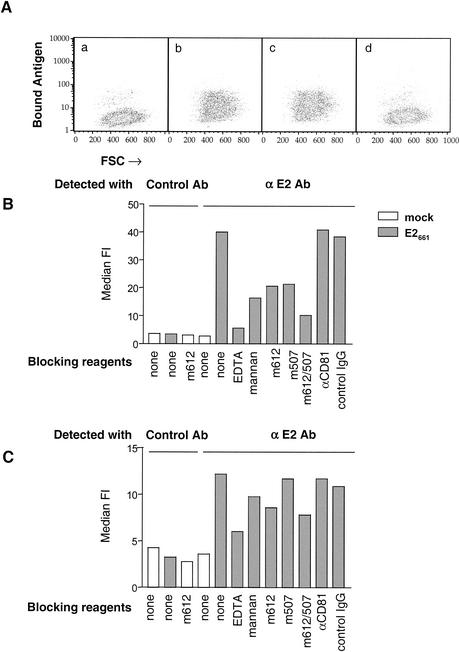
To determine if HCV E2 can interact with DC-SIGN on dendritic cells, immature MDDCs were generated by IL-4 and granulocyte-macrophage colony-stimulating factor treatment, and their phenotype was confirmed by FACS analysis as described in Materials and Methods (data not shown). Immature MDDCs were incubated with HC-J4 E2661, and bound antigen was detected with MAb 9/75 by FACS analysis. E2 bound to immature MDDCs, and MAbs specific for DC-SIGN (m612 and m507) inhibited the binding by >75%, whereas the anti-CD81 MAb had no detectable effect (Fig. 7A and B). This binding could also be inhibited by incubation with EDTA or mannan (Fig. 7B). These data suggest that E2 interaction with immature MDDCs is largely dependent upon DC-SIGN (Fig. 7B). However, maturation of MDDCs with IL-1β, IL-6, tumor necrosis factor, and prostaglandin E2 for 2 to 3 days resulted in reduced binding of E2, concomitant with reduced expression of DC-SIGN (Fig. 7C and data not shown). Interaction of E2 with mature dendritic cells was only partially inhibited by MAbs to DC-SIGN, suggesting that other cell surface molecules may contribute to E2 binding under these conditions (Fig. 7C).
FIG. 7.
Binding of E2 to immature and mature MDDCs. (A) Binding of mock (a) and HC-J4 E2661 (b) antigens to immature MDDCs in the presence of anti-CD81 (c) and anti-DC-SIGN MAbs m612 and m507 (d). Bound E2 antigen was visualized with anti-E2 MAb 9/75 and analyzed by FACS. Immature (A and B) and mature (C) MDDCs were incubated with mock (open bars) and HC-J4 E2661 (shaded bars) antigens in the presence and absence of the indicated blocking reagents, and the bound antigen was detected with the E2-specific MAb 9/75 or an isotype-matched irrelevant control antibody; results are shown as the median fluorescence intensity (FI). Data from a representative experiment are shown, and similar results were obtained in two independent experiments.
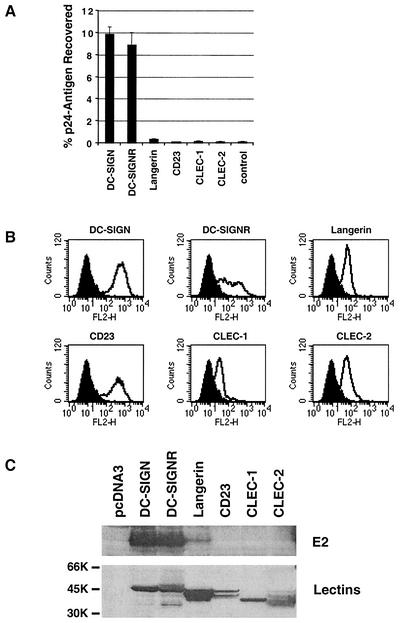
Interactions of HIV-HCV pseudotypes and truncated E2 with additional C-type lectins.
While DC-SIGN can efficiently bind the HIV-1 Env protein, other molecules on the surface of dendritic cells can also support this interaction (9, 65, 70). Since binding of E2 to immature MDDCs was only partially inhibited by DC-SIGN-specific MAbs and binding to mature MDDCs was not inhibited, we examined the ability of E2 to interact with other C-type lectins known to be expressed on dendritic cells. T-REx cells expressing langerin, CD23, CLEC-1, or CLEC-2 were tested for their ability to bind HC-J4 E2/SIN-bearing HIV particles. All of the lectins were engineered to contain the AU1 antigenic tag so that expression could be confirmed by FACS analysis (Fig. 8B) or Western blot (Fig. 8C, lower panel). CLEC-1 was expressed at relatively low levels, confirming that this protein is mainly localized in the cytoplasm (17). Only cells expressing DC-SIGN and DC-SIGNR bound detectable levels of virus (Fig. 8A), and binding to langerin was slightly above background. Similar results were obtained when binding of soluble H77 E2661 to transiently transfected quail cells was examined. Thus, E2 protein bound well to cells expressing DC-SIGN and DC-SIGNR and weakly to cells expressing langerin (Fig. 8C, top panel).
FIG. 8.
Interaction of HCV E2 with cells expressing various C-type lectins. (A) Binding of HC-J4 E2/SIN pseudotype virus to T-REx cells expressing different lectins. The T-REx cells were induced to express the indicated lectins, and binding was assessed as described in Materials and Methods. The percentage of recovered antigen is shown, and similar results were obtained in an independent experiment. (B) Surface expression of the lectin proteins. The T-REx cell lines were induced overnight to express the indicated lectins, and surface expression was assessed by FACS analysis with a monoclonal antibody against the AU-1 antigen tag. Results are shown relative to staining of control T-REx cells. Comparable results were obtained in an independent experiment. (C) Binding of H77 E2661 to quail cells expressing different lectins. Quail cells were infected with vaccinia virus encoding T7 polymerase and transfected with plasmid DNA encoding the indicated lectins. Cells were incubated with soluble H77 E2661, washed extensively, and lysed, and bound protein was detected by anti-E2 MAbs 6/1a and 9/75 by Western blot (upper panel). In parallel, lectin expression was confirmed by Western blot with a combination of anti-DC-SIGN/DC-SIGNR and anti-AU-1 antibody (lower panel).
DISCUSSION
The lack of an in vitro system for the propagation of HCV makes the identification of cell surface proteins that may serve as virus receptors or attachment factors difficult. Putative receptors have been identified (54), although it is not apparent at present how their authenticity can be confirmed. However, identification of cellular ligands to which HCV glycoproteins bind can help define the mechanisms that contribute to viral pathogenesis and may suggest new therapeutic approaches. The chemokine receptors that are utilized by HIV to infect cells, for example, are targets of small-molecule inhibitors that are now in clinical development (23). It is therefore important to identify potential virus receptors (proteins required for virus entry) and attachment factors (proteins to which virus binds but which are not required for infection) even in the absence of replication-competent HCV systems.
To overcome the problems associated with the production of native HCV particles, we used soluble versions of the E2 glycoprotein and HIV pseudotypes expressing membrane-associated chimeric E1 and E2 glycoproteins, which contain the VSV-G or Sindbis virus E1 or E2 transmembrane domains. While our systems are unlikely to completely mimic the native structures of E1 and E2 on HCV particles, the observations that E2661 is efficiently secreted from cells, binds a number of conformation-dependent MAbs, and interacts with a number of human cell types suggest that it folds in a manner comparable to the full-length protein (29). Furthermore, Takikawa and colleagues (62) reported that chimeric HCV E1/G and E2/G glycoproteins were able to induce cell-cell fusion, although a recent report was unable to confirm this observation in the context of VSV pseudotypes (13).
We noted differences in the ability of H77 E2661 and pseudotyped viruses expressing H77 E2/SIN or E2/G to bind CD81 (Fig. 2 and 4) in that viruses failed to show detectable binding to parental T-REx cells shown to express CD81. This may simply reflect different sensitivities of the two assay formats. However, these data are consistent with recent reports of similar differences in the ability of truncated E2, virus-like particles, and plasma-purified HCV to bind CD81 (67, 71). The observation that E2 glycoproteins from different HCV strains vary in their ability to bind CD81 (57a) coupled with its almost universal expression argue that CD81 is unlikely to be the primary receptor for HCV infection. More recently, the E2-CD81 interaction has been reported to provide a costimulatory signal for the activation of T cells (66) and to modulate NK cell function (19, 64) suggesting that the HCV-CD81 interaction could have an immunomodulatory role.
We found that both truncated soluble E2 and virus-associated E1 and E2 glycoproteins bound to cells expressing DC-SIGN and DC-SIGNR. These interactions were observed in cells of different lineages and were shown to be dependent upon DC-SIGN and DC-SIGNR expression levels. The ability of both mannan and EGTA to inhibit E2 binding to DC-SIGN and DC-SIGNR is consistent with a carbohydrate recognition event. The C-type lectin langerin has also been shown to bind HIV Env in a mannan-inhibitable manner (65); however, langerin did not efficiently bind E2, suggesting that although the presence of high-mannose carbohydrates determines to some degree the specificity of binding for these C-type lectins, other determinants may exist between different lectins.
Although DC-SIGN binds high-mannose N-linked carbohydrate structures, modulation of the content of immature carbohydrate chains on a glycoprotein can affect DC-SIGN binding (26, 46, 48). Conditions in which all carbohydrate chains of HIV Env remain in the high-mannose form resulted in a more efficient interaction with DC-SIGN, suggesting that multiple N-linked high-mannose chains need to be present on a protein surface to allow optimal engagement of a DC-SIGN tetramer. Langerin may have an additional and/or slightly different requirements than DC-SIGN for efficient glycoprotein binding.
Since DC-SIGN and DC-SIGNR are not expressed on hepatocytes, they are unlikely to function as true HCV receptors; however, they may serve as HCV attachment factors. Recruitment of virus to the cell surface, either by a viral receptor or via an attachment factor, can be rate limiting for infection (50). Therefore, factors such as DC-SIGN and DC-SIGNR that promote virus binding may enhance the rate and efficiency of virus infection (10, 43, 55). An additional property that distinguishes DC-SIGN from other attachment factors is its ability to efficiently transmit captured virus to an adjoining cell expressing the principal receptor (32). The mechanisms that account for DC-SIGN-mediated transfer of virus are incompletely understood but may involve virus internalization and recycling to the cell surface (25, 41).
DC-SIGNR expression in vivo is restricted to specific types of endothelial cells, including liver sinusoidal endothelial cells, lymph node sinuses, and some capillaries in the human placenta and gastrointestinal tract (10, 35, 56, 60). Recently, Breiner and colleagues hypothesized that hepatocytes are not directly exposed to blood-borne viruses because sinusoidal endothelial cells, which line the hepatic sinusoids, although fenestrated, physically separate the sinusoidal blood from the hepatocytes (12). A number of reports support this model, in that a variety of molecules appear to be selectively transported across the sinusoidal endothelia (38, 39, 51, 52, 59). The expression of DC-SIGNR on liver sinusoidal endothelial cells suggests that this cell type may capture and concentrate circulating HCV in the liver and present virus to the adjoining hepatocytes, in a manner analogous to DC-SIGN presentation of HIV on dendritic cells to adjoining T lymphocytes.
HCV infection persists in the majority of individuals and, in addition to causing liver disease, may also affect B-cell proliferation. More recently, HCV has been implicated as the causative agent of mixed cryoglobulinemia and in the pathogenesis of B-cell non-Hodgkin's lymphoma (1, 27, 28, 34). The high frequency of chronic infection suggests that an effective antiviral immune response is not initiated or maintained and that virus-mediated immune evasion strategies may be operating. Several studies have reported impaired maturation, reduced allostimulatory ability, and decreased gamma interferon production of blood-derived dendritic cells in HCV-infected individuals, which is restored during successful interferon therapy and subsequent viral clearance (4, 7, 8, 36, 37). Since the major site of HCV replication is thought to be hepatocytes within the liver and association of HCV RNA with dendritic cells appears to occur at low frequency, their reported dysfunction may not be attributable to direct infection (44). The data presented here support a model in which HCV interaction with DC-SIGN on dendritic cells may affect their ability to signal and stimulate T cells.
In conclusion, we report that HCV E2, in the form of a truncated soluble protein and HIV pseudotypes expressing chimeric VSV and Sindbis virus fusion glycoproteins, interacts specifically with primary cell types and cell lines expressing DC-SIGN and DC-SIGNR but not with the other C-type lectins tested. It will be important to confirm that these glycoprotein-cell interactions occur in the context of authentic HCV particles and primary cell types expressing DC-SIGN and DC-SIGNR. Such interactions may contribute to the establishment or persistence of HCV infection both by the capture and delivery of virus to the liver and by exerting immunomodulatory effects.
Acknowledgments
The first two authors contributed equally to this work.
We are grateful to Peter Balfe and Ralph Steinman for reading the manuscript and for helpful comments. We also thank Christian Munz for helpful comments and insight. We thank Karin Strecker for expert technical assistance.
J.Z., C.M.R., and J.A.M. were supported by the Greenberg Medical Research Foundation and by PHS grants CA57973 and AI40034. R.W.D. was supported by NIH R01 AI50469, Burroughs Wellcome Fund Translational Research award, and an Elizabeth Glaser Scientist award from the Pediatric AIDS Foundation. S.P. was supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG). G.J.L. was supported by a grant from the NIH Medical Scientist Training Program. Research at the University of Pennsylvania was also supported by the Penn Center for AIDS Research and NIH grant P30 AI45008.
REFERENCES
- 1.Abel, G., Q. X. Zhang, and V. Agnello. 1993. Hepatitis C virus infection in type II mixed cryoglobulinemia. Arthritis Rheum. 36:1341-1349. [DOI] [PubMed] [Google Scholar]
- 2.Agnello, V. 1997. Immune complexes in hepatitis C. Hepatology 26:1687-1688. [DOI] [PubMed] [Google Scholar]
- 3.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q. X. Zhang. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbar, S. M., N. Horiike, M. Onji, and O. Hino. 2001. Dendritic cells and chronic hepatitis virus carriers. Intervirology 44:199-208. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muñiz, A. L. Corbí, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andre, P., F. Komurian-Pradel, S. Deforges, M. Perret, J. L. Berland, M. Sodoyer, S. Pol, C. Brechot, G. Paranhos-Baccala, and V. Lotteau. 2002. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J. Virol. 76:6919-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. [DOI] [PubMed] [Google Scholar]
- 8.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. [DOI] [PubMed] [Google Scholar]
- 9.Baribaud, F., S. Pöhlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashirova, A. A., T. B. Geijtenbeek, G. C. v. Duijnhoven, S. J. v. Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. v. Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumert, T. F., S. Ito, D. T. Wong, and T. J. Liang. 1998. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J. Virol. 72:3827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breiner, K. M., H. Schaller, and P. A. Knolle. 2001. Endothelial cell-mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms. Hepatology 34:803-808. [DOI] [PubMed] [Google Scholar]
- 13.Buonocore, L., K. J. Blight, C. M. Rice, and J. K. Rose. 2002. Characterization of vesicular stomatitis virus recombinants that express and incorporate high levels of hepatitis C virus glycoproteins. J. Virol. 76:6865-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 15.Clayton, R. F., A. Owsianka, J. Aitken, S. Graham, D. Bhella, and A. H. Patel. 2002. Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J. Virol. 76:7672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocquerel, L., J.-C. Meunier, A. Pillez, C. Wychowski, and J. Dubuisson. 1998. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J. Virol. 72:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colonna, M., J. Samaridis, and L. Angman. 2000. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur. J. Immunol. 30:697-704. [DOI] [PubMed] [Google Scholar]
- 18.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 19.Crotta, S., A. Stilla, A. Wack, A. D'Andrea, S. Nuti, U. D'Oro, M. Mosca, F. Filliponi, R. M. Brunetto, F. Bonino, S. Abrignani, and N. M. Valiante. 2002. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deleersnyder, V., A. Pillez, C. Wychowski, K. Blight, J. Xu, Y. S. Hahn, C. M. Rice, and J. Dubuisson. 1997. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 71:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doms, R. W., A. Helenius, and W. Balch. 1987. Role for ATP in the assembly and transport of VSV G protein trimers. J. Cell Biol. 105:1957-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duvet, S., L. Cocquerel, A. Pillez, R. Cacan, A. Verbert, D. Moradpour, C. Wychowski, and J. Dubuisson. 1998. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 273:32088-32095. [DOI] [PubMed] [Google Scholar]
- 25.Engering, A., T. B. Geijtenbeek, S. J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118-2126. [DOI] [PubMed] [Google Scholar]
- 26.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 27.Ferri, C., L. La Civita, G. Longombardo, F. Greco, and S. Bombardieri. 1993. Hepatitis C virus and mixed cryoglobulinaemia. Eur. J. Clin. Investig. 23:399-405. [DOI] [PubMed] [Google Scholar]
- 28.Ferri, C., L. La Civita, and A. L. Zignego. 1996. Extrahepatic manifestations of hepatitis C virus infection. Ann. Intern. Med. 125:344. [DOI] [PubMed] [Google Scholar]
- 29.Flint, M., C. Maidens, L. D. Loomis-Price, C. Shotton, J. Dubuisson, P. Monk, A. Higginbottom, S. Levy, and J. A. McKeating. 1999. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 73:6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flint, M., and J. A. McKeating. 1999. The C-terminal region of the hepatitis C virus E1 glycoprotein confers localization within the endoplasmic reticulum. J. Gen. Virol. 80:1943-1947. [DOI] [PubMed] [Google Scholar]
- 31.Flint, M., J. M. Thomas, C. M. Maidens, C. Shotton, S. Levy, W. S. Barclay, and J. A. McKeating. 1999. Functional analysis of cell surface-expressed hepatitis C virus E2 glycoprotein. J. Virol. 73:6782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geijtenbeek, T. B. H., D. S. Kwon, R. Torensma, S. J. v. Vliet, G. C. F. v. Duijnhoven, J. Middel, I. L. M. H. A. Cornelissen, H. S. L. M. Nottet, V. N. Kewalramani, D. R. Littman, C. G. Figdor, and Y. v. Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 33.Hamaia, S., C. Li, and J. P. Allain. 2001. The dynamics of hepatitis C virus binding to platelets and 2 mononuclear cell lines. Blood 98:2293-2300. [DOI] [PubMed] [Google Scholar]
- 34.Hausfater, P., E. Rosenthal, and P. Cacoub. 2000. Lymphoproliferative diseases and hepatitis C virus infection. Ann. Med. Interne (Paris) 151:53-57. [PubMed] [Google Scholar]
- 35.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakumu, S., S. Ito, T. Ishikawa, Y. Mita, T. Tagaya, Y. Fukuzawa, and K. Yoshioka. 2000. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J. Gastroenterol. Hepatol. 15:431-436. [DOI] [PubMed] [Google Scholar]
- 37.Kanto, T., N. Hayashi, T. Takehara, T. Tatsumi, N. Kuzushita, A. Ito, Y. Sasaki, A. Kasahara, and M. Hori. 1999. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 162:5584-5591. [PubMed] [Google Scholar]
- 38.Kataoka, M., and M. Tavassoli. 1985. The role of liver endothelium in the binding and uptake of ceruloplasmin: studies with colloidal gold probe. J. Ultrastruct. Res. 90:194-202. [DOI] [PubMed] [Google Scholar]
- 39.Kempka, G., and V. Kolb-Bachofen. 1988. Binding, uptake, and transcytosis of ligands for mannose-specific receptors in rat liver: an electron microscopic study. Exp. Cell Res. 176:38-48. [DOI] [PubMed] [Google Scholar]
- 40.Kolykhalov, A. A., E. V. Agapov, K. J. Blight, K. Mihalik, S. M. Feinstone, and C. M. Rice. 1997. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277:570-574. [DOI] [PubMed] [Google Scholar]
- 41.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 42.Lagging, L. M., K. Meyer, R. J. Owens, and R. Ray. 1998. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J. Virol. 72:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerat, H., S. Rumin, F. Habersetzer, F. Berby, M. A. Trabaud, C. Trepo, and G. Inchauspe. 1998. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood 91:3841-3849. [PubMed] [Google Scholar]
- 45.Levy, S., S. C. Todd, and H. T. Maecker. 1998. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu. Rev. Immunol. 16:89-109. [DOI] [PubMed] [Google Scholar]
- 46.Lin, G., G. Simmons, S. Pöhlmann, F. Baribaud, H. Ni, G. J. Leslie, B. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuura, Y., H. Tani, K. Suzuki, T. Kimura-Someya, R. Suzuki, H. Aizaki, K. Ishii, K. Moriishi, C. S. Robison, M. A. Whitt, and T. Miyamura. 2001. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology 286:263-275. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell, D. A., A. J. Fadden, and K. Drickamer. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939-28945. [DOI] [PubMed] [Google Scholar]
- 49.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 50.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Omoto, E., J. J. Minguell, and M. Tavassoli. 1992. Endothelial transcytosis of iron-transferrin in the liver does not involve endosomal traffic. Pathobiology 60:284-288. [DOI] [PubMed] [Google Scholar]
- 52.Omoto, E., and M. Tavassoli. 1989. The role of endosomal traffic in the transendothelial transport of ceruloplasmin in the liver. Biochem. Biophys. Res. Commun. 162:1346-1350. [DOI] [PubMed] [Google Scholar]
- 53.Op De Beeck, A., L. Cocquerel, and J. Dubuisson. 2001. Biogenesis of hepatitis C virus envelope glycoproteins. J. Gen. Virol. 82:2589-2595. [DOI] [PubMed] [Google Scholar]
- 54.Pileri, P., Y. Uematsu, S. Compagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 55.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Münch, F. Kirchoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pöhlmann, S., E. J. Soilleux, F. Baribaud, G. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prince, A. M., T. Huima-Byron, T. S. Parker, and D. M. Levine. 1996. Visualization of hepatitis C virions and putative defective interfering particles isolated from low-density lipoproteins. J. Viral Hepatol. 3:11-17. [DOI] [PubMed] [Google Scholar]
- 57a.Roccasecca, R., H. Ansuini, A. Vitelli, A. Meola, E. Scarselli, S. Acali, M. Pezzanera, B. B. Ercole, J. McKeating, A. Yagnik, A. Lahm, A. Tramontano, R. Cortese, and A. Nicosia. 2003. Binding of the hepatitis C virus E2 glycoprotein to CD81 is strain specific and is modulated by a complex interplay between hypervariable regions 1 and 2. J. Virol. 77:1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosa, D., S. Campagnoli, C. Moretto, E. Guenzi, L. Cousens, M. Chin, C. Dong, A. Weiner, J. Y. N. Lau, Q.-L. Choo, D. Chien, P. Pileri, M. Houghton, and S. Abrignani. 1996. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc. Natl. Acad. Sci. USA 93:1759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlepper-Schafer, J., D. Hulsmann, A. Djovkar, H. E. Meyer, L. Herbertz, H. Kolb, and V. Kolb-Bachofen. 1986. Endocytosis via galactose receptors in vivo. Ligand size directs uptake by hepatocytes and/or liver macrophages. Exp. Cell Res. 165:494-506. [DOI] [PubMed] [Google Scholar]
- 59a.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleaux, J. L. Riley, R. W. Doms, P. Bates, and S. Pohlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 60.Soilleux, E. J., L. S. Morris, B. Lee, S. Pöhlmann, J. Trowsdale, R. W. Doms, and N. Coleman. 2001. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 195:586-592. [DOI] [PubMed] [Google Scholar]
- 61.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. 2002. DC-SIGN is expressed on subsets of dendritic cells and specialized macrophages in tissue, and on a sub-population of plasmacytoid blood dendritic cells. J. Leukoc. Biol. 71:445-457. [PubMed] [Google Scholar]
- 62.Takikawa, S., K. Ishii, H. Aizaki, T. Suzuki, H. Asakura, Y. Matsuura, and T. Miyamura. 2000. Cell fusion activity of hepatitis C virus envelope proteins. J. Virol. 74:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Triyatni, M., J. Vergalla, A. R. Davis, K. G. Hadlock, S. K. Foung, and T. J. Liang. 2002. Structural features of envelope proteins on hepatitis C virus-like particles as determined by anti-envelope monoclonal antibodies and CD81 binding. Virology 298:124-132. [DOI] [PubMed] [Google Scholar]
- 64.Tseng, C. T., and G. R. Klimpel. 2002. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 195:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pöhlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 66.Wack, A., E. ldaini, C. Tseng, S. Nuti, G. Klimpel, and S. Abrignani. 2001. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur. J. Immunol. 31:166-175. [DOI] [PubMed] [Google Scholar]
- 67.Wellnitz, S., B. Klumpp, H. Barth, S. Ito, E. Depla, J. Dubuisson, H. E. Blum, and T. F. Baumert. 2002. Binding of hepatitis C virus-like particles derived from infectious clone H77C to defined human cell lines. J. Virol. 76:1181-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiley, D. C., J. J. Skehel, and M. Waterfield. 1977. Evidence from studies with a cross-linking reagent that the haemagglutinin of influenza virus is a trimer. Virology 79:446-448. [DOI] [PubMed] [Google Scholar]
- 69.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 289:366-373. [DOI] [PubMed] [Google Scholar]
- 70.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wunschmann, S., J. D. Medh, D. Klinzmann, W. N. Schmidt, and J. T. Stapleton. 2000. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J. Virol. 74:10055-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao, J. S., E. G. Strauss, and J. H. Strauss. 1996. Interactions between PE2, E1, and 6K required for assembly of alphaviruses studied with chimeric viruses. J. Virol. 70:7910-7920. [DOI] [PMC free article] [PubMed] [Google Scholar]