Abstract
Viral gene therapy against malignant tumors holds great promise for tumors that are susceptible to the oncolytic activity of viruses. One advantage of oncolytic viral therapy is that it can potentially be combined with other therapies, such as radiotherapy, to obtain an enhanced tumor response. In the case of prostate cancer, herpes simplex virus-mediated therapies have been shown to be highly effective in animal models; however, studies of the efficacy of combined viral and radiation therapy have not yet been reported. In this study, we have combined G207, a multimutated HSV type 1 vector, with external beam radiation therapy of prostate tumors grown subcutaneously in mice. We examined both the human LNCaP tumor in athymic mice and the mouse transgenic TRAMP tumor in either athymic mice or its syngeneic host, C57BL/6 mice. Virus was delivered either intravenously, in the case of LNCaP, or intratumorally, in the case of TRAMP. We found that individually, either G207 or radiation was effective in delaying tumor growth in these models. However, delivering the treatments simultaneously did not produce an enhanced effect.
Keywords: herpes virus, gene therapy, prostate cancer, radiotherapy, oncolytic virus
Introduction
The use of replication-competent viruses for tumor therapy is a promising strategy that has progressed to early clinical trials [1–5]. Replication-competent or conditionally replicating herpes simplex virus type 1 (HSV-1) vectors have been generated by mutating genes involved in viral DNA synthesis and/or virulence in order to target viral replication and toxicity to tumor cells [6,7]. G207 is a multimutated HSV-1 vector that lacks both copies of the ICP34.5 gene, a gene required for neurovirulence, and contains an insertion of the lacZ gene inactivating the ICP6 gene, encoding the large subunit of ribonucleotide reductase [8]. Studies in both human xenograft and syngeneic mouse tumor models have demonstrated the value of HSV-based therapies in terms of both growth inhibition and cures [9]. These findings await confirmation in human clinical trials.
One advantage of viral gene therapy is that it can potentially be combined with other therapies to obtain an enhanced tumor response. In fact, recent reports about combination viral/radiotherapy and viral/chemotherapy in glioma and head and neck cancer animal models suggest that this may be a viable approach [10–15]. Human prostate tumor cells are particularly sensitive to HSV-1 vectors [16], and this has led to experimental treatment strategies that deliver mutated viruses by either intratumoral or intravenous injection. However, studies on the efficacy of combined viral and radiotherapy have not been reported. In this study, we assess the ability of radiation to affect the activity of G207 against prostate cancer. G207 treatment was combined with external beam radiation therapy of prostate cancer grown subcutaneously in mice. We used both the LNCaP human tumor in athymic mice and the transgenic TRAMP mouse tumor in either athymic mice or its syngeneic host, C57BL/6 mice. Virus was delivered either intravenously, in the case of LNCaP, or intratumorally, in the case of TRAMP. We found that G207 and radiation were each effective in producing growth delay in these models, but simultaneously delivering the treatments did not produce an enhanced effect.
Materials and Methods
Cell Lines and Culture Conditions
The human prostate cancer cell line LNCaP (Georgetown University Medical Center, Lombardi Cancer Center Tissue Culture Shared Resource, Washington, DC) was maintained in RPMI 1640 (Biofluids) containing 10% fetal bovine serum (Life Technologies, Grand Island, NY). The TRAMP-C2 mouse prostate cancer cell line [17] was grown in DMEM high glucose (DMEM-HG; Life Technologies) supplemented with 5% fetal bovine serum (Life Technologies), 5% Nu-serum IV (Collaborative Biomedical Products, Bedford, MA), 5µg/ml bovine insulin (Life Technologies), and 10x8 M dihydrotestosterone (Sigma Chemical, St. Louis, MO). Penicillin-streptomycin (Mediatech, Herndon, VA) and l-glutamine (Mediatech) were added to each culture and the cells were maintained at 37°C with 5% CO2. Both cell lines were confirmed to be free of mycoplasma contamination.
Preparation and Injection of Cells into Animals
Four-to 6-week-old C57BL/6 black or NCRNU athymic male mice were obtained from Harlan Laboratories (Indianapolis, IN) or Taconic Laboratories (Germantown, NY), respectively. All animals were quarantined for 1 week before the study and allowed access to food and water ad libitum. The animals were anesthesized by intraperitoneal injection of 0.15 to 0.2 ml of 10% sodium pentobarbital (Abbott Laboratories, North Chicago, IL) in bacteriostatic saline (0.9% sodium chloride; Abbott Laboratories) with 6% ethyl alcohol. C57BL/6 mice were shaved in the rump before injections. Cells, 1x107, in 0.1 ml were injected subcutaneously in the sacral region of each animal to induce tumors in the TRAMP experiments. For the LNCaP experiments, the cells (1x107) were first mixed with an equal volume of Matrigel (Collaborative Biochemical Products) and then injected. Tumors were measured twice weekly by calipers to within 0.5 mm, and volumes were calculated (V=HLW) and recorded. Animals were randomized into treatment groups once their tumor size was in the range of 100 to 320 mm3. After tumor cell injection, LNCaP tumors took 5 to 6 weeks to grow to treatment size, whereas TRAMP tumors grew in 1 to 3 weeks. In the LNCaP experiment, animals were given G207 (2x107 pfu) by tail vein injection on days 0 and 4, and irradiated on days 1 to 5. In both TRAMP experiments, animals were given G207 (2x107 pfu) intratumorally on days 0, 3, and 6, and irradiated daily on days 1 to 5. On days with both virus injection and irradiation, the virus was given to the animals before the irradiation occurred. The animal procedures described here were approved by the Georgetown University Animal Care and Use Committee.
Irradiation of Prostate Tumors LNCaP and TRAMP
A 137Cs Shepherd Mark I irradiator was used to irradiate the tumors in the sacral region of the animal. The mice were restrained in clear plastic holders with a lead cover, which contains a port through which the radiation can enter to irradiate the tumor. The holders were placed behind a lead wall, which shields the mouse's body, exposing the irradiation port of the holder above the edge of the wall. Four animals were irradiated simultaneously. Doses to the tumor under this geometry were confirmed using a phantom mouse and thermoluminescent dosimetry. In the LNCaP experiment, the tumors were given 10 Gy fractionated over days 1 to 5 (i.e., 2 Gy/day). In the TRAMP experiments, the tumors were given a total of 20 Gy over days 1 to 5 (i.e., 4 Gy/day).
Clonogenic Cell Survival Curves
Logarithmically growing cells were harvested and seeded into tissue culture flasks at various cell numbers depending upon the radiation dose which the flask was to receive (i.e., more cells for higher doses), so that the final number of survivors in each flask would be similar. After allowing time for attachment, the flasks were irradiated to various doses and returned to the incubator for 2 weeks. The flasks were stained to reveal colonies produced from the clones of surviving cells and counted. The fraction of survivors relative to the original number of cells seeded was calculated and then normalized to the zero-dose plating efficiency to determine the surviving fraction at each dose. The data were fitted to the single-hit multitarget curve model [18].
Results
Human LNCaP Tumor Treatment
LNCaP is a commonly used hormone-responsive human prostate cell line that grows well, albeit slowly, as subcutaneous tumors in athymic mice [19]. LNCaP tumor cells are particularly sensitive to G207, both in vitro and in vivo [16]. The LNCaP tumor is wild type for p53 and secretes prostatespecific antigen [19]. Its cellular radioresponses have been characterized for both clonogenic survival and apoptosis [20–23]. Because the LNCaP/athymic mouse xenograft model has been prevalent and important in prostate cancer research [24], we chose this as one of our models for studying combined radiation/G207 effects.
We previously found LNCaP tumors to be highly sensitive to intratumorally and intravenously injected G207 [16]. Even a single intratumoral injection of G207 (2x107 pfu) caused a major reduction in tumor volumes with complete eradication of 25% of the tumors. This viral response was too great for combined radiation/viral studies, where partial responses for each agent are needed to assess potential interactions. Even when the viral titers were lowered to 105 pfu, significant cures ensued (data not shown). For this reason, we decided to employ intravenous treatment with G207, which we knew gave a less robust treatment response [16]. We employed two intravenous injections spread over 4 days, and combined it with five daily fractions of radiation, starting on the day after the first viral treatment.
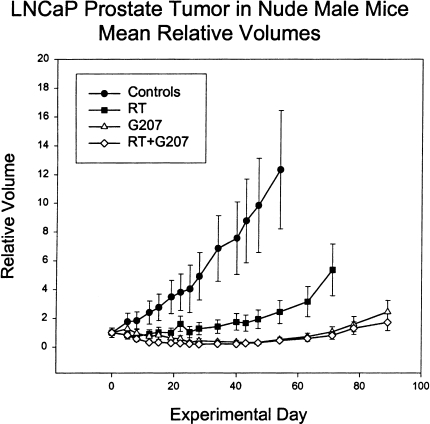
Radiation therapy was effective at inhibiting tumor growth (Figure 1); however, the irradiated tumors grew back relatively quickly. In contrast, volumes of G207-treated tumors were markedly reduced and regrowth occurred much more slowly than for irradiated tumors (Figure 1). Nevertheless, by 40 days, all of the tumors had started to grow back. For the combined radiation and G207 treatment, tumors regressed slightly faster than for G207 alone, but ultimately reached the same minimum volume — again on day 40 — at which point they began to regrow at a rate that was indistinguishable from G207 treatment alone (Figure 1). Nadir tumor volumes were statistically significantly different at P <.05 between all treatment groups, except for radiation plus G207 versus G207 alone, which were not significantly different from each other. There were no cures in any of the groups.
Figure 1.
Mean relative volumes of LNCaP prostate tumors in athymic male mice. The mean relative tumor volumes, compared to the first day of treatment, of tumor-bearing mice treated with virus buffer (Controls), G207, irradiation (RT), or both (RT + G207). Irradiated animals were treated for days 1 to 5 of the experiment with 2-Gy daily fractions for a total dose of 10 Gy. Virus-treated animals were given virus at 2x107 pfu by tail vein injection on days 0 and 4 of the experiment. Each point represents the mean tumor volume ± 1 SEM. In some cases where individual animals were sacrificed due to large tumor burden, the tumor size at the time of sacrifice was used for subsequent calculations of mean tumor volume.
Murine TRAMP Tumor Treatment
TRAMP represents a relatively new animal model for prostate cancer research [17,25]. In this syngeneic mouse model, transgenic mice carry the SV40 large tumor antigen linked to a prostate-specific promoter. Expression of the transgene in prostate tissue caused tumors to arise in situ at about 8 weeks of age, and these tumors resembled naturally arising prostate cancer. Several tumor cell lines have been established in tissue culture from the prostate tumors of TRAMP mice. These tumor lines no longer express large T antigen [17].
TRAMP tumor cell lines grow very well subcutaneously in either athymic mice or in the syngeneic parental mice, C57BL/6, from which the TRAMP transgenic mice were derived. This feature of the model provided major advantages because G207 treatment of murine tumors in syngeneic mice leads to a potent antitumor immune response [26,27]. The TRAMP tumor model allows us to examine the effect of the immune system on both treatment strategies and their combination by comparing efficacy in immunocompromised and immunocompetent mice.
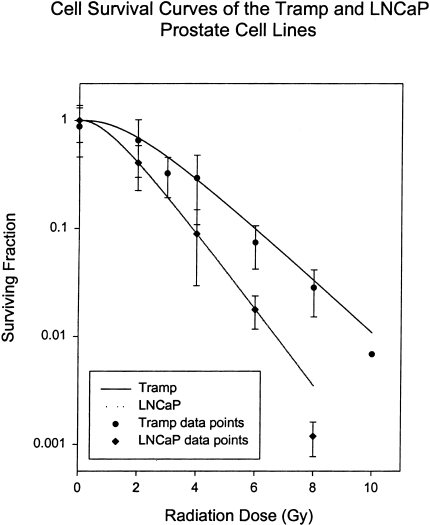
The TRAMP model system is not as well characterized for radiation responses as LNCaP; however, it is known to be wild type for p53, like LNCaP [17]. We were able to confirm the p53 phenotype by showing radiation induction of the p53 transcriptionally activated p21WAF1/CIP1 protein in both LNCaP and TRAMP cells in vitro (data not shown). Preliminary studies with subcutaneous TRAMP tumors suggested that they were twice as resistant to radiation therapy compared to LNCaP. This was consistent with in vitro clonogenic cell survival curve analysis (Figure 2), suggesting that the tumor resistance of TRAMP was an intrinsic property of the cells, and not due to possible tumor physiology differences. Hypoxia, for example, has been reported to affect tumor radioresponses in other prostate cancer animal models [28]. Due to the greater radioresistance of TRAMP tumors, 20 Gy was used for the therapy dose, rather than the 10 Gy that was used for LNCaP.
Figure 2.
Radiation survival curves for TRAMP and LNCaP cells. Points represent the mean of four independent experiments, each performed on different days; bars represent the standard deviation of the means. The data were fitted to the single-hit multitarget survival model and D0 and Dq parameters, respectively, measured in Gy, for each cell line, which were as follows: Tramp, 1.763 and 1.219; LNCaP, 1.015 and 1.631.
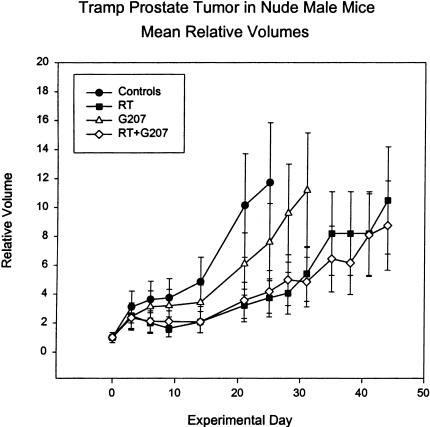
Mouse tumor cells, in general, are less susceptible to G207 replication and cytotoxicity than human tumor cells. Growth curves for untreated TRAMP tumors in athymic mice showed that they grow twice as fast as LNCaP tumors, and the delay produced by G207 was much less than that for LNCaP (Figure 3). TRAMP tumors treated with radiation therapy alone produced about twice the growth delay of G207 alone. Combined radiation and G207 produced no greater delay than radiation therapy alone.
Figure 3.
Mean relative volumes of TRAMP prostate tumors in athymic male mice. The mean relative tumor volumes, compared to the first day of treatment, of tumor-bearing mice treated with virus buffer (Controls), G207, irradiation (RT), or both (RT + G207). Irradiated animals were treated for days 1 to 5 of the experiment with 4-Gy daily fractions for a total dose of 20 Gy. Virus-treated animals were given virus at 2x107 pfu by intratumoral injections on days 0, 3, and 6 of the experiment. Each point represents the mean tumor volume ± 1 SEM. In some cases where individual animals were sacrificed due to large tumor burden, the tumor size at the time of sacrifice was used for subsequent calculations of mean tumor volume.
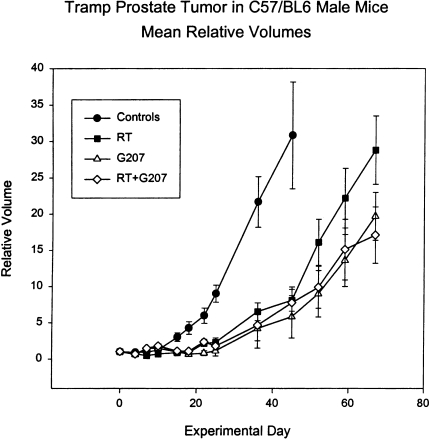
In order to assess the possible influence of an intact immune system in the TRAMP response to G207 and radiation, the TRAMP tumor experiment was also conducted in C57BL/6 mice (Figure 4). Compared to the athymic mouse host, G207 was much more effective in inhibiting TRAMP tumor growth in C57BL/6 mice (Figure 3 versus Figure 4). In fact, the G207 delay was now greater than the delay for radiation alone. This increased efficacy of G207 in syngeneic mouse models is consistent with results obtained with CT26 colon carcinoma in BALB/c mice, N18 neuroblastoma in A/J mice, and M3 melanoma in DBA/2 mice [26,27], suggesting that immune responses contribute to the antitumor activity of G207.
Figure 4.
Mean relative volumes of TRAMP prostate tumors in male C57BL/6 mice. The mean relative tumor volumes, compared to the first day of treatment, of tumor-bearing mice treated with virus buffer (Controls), G207, irradiation (RT), or both (RT + G207). Irradiated animals were treated for days 1 to 5 of the experiment with 4-Gy daily fractions for a total dose of 20 Gy. Virus-treated animals were given virus at 2x107 pfu by intratumoral injections on days 0, 3, and 6 of the experiment. Each point represents the mean tumor volume ± 1 SEM. In some cases where individual animals were sacrificed due to large tumor burden, the tumor size at the time of sacrifice was used for subsequent calculations of mean tumor volume.
In contrast to the G207 response, tumor growth with radiation alone was similar in athymic and C57BL/6 mice, suggesting that the immune system was not affecting TRAMP tumor regression caused by radiotherapy. Although tumor radioresponses are largely thought to be driven by intrinsic properties of tumors [29], recently it has been reported that T cells and natural killer cells may promote radiotherapy tumor regression in some tumors [30]. Nevertheless, in our model, the presence or absence of a thymus did not affect tumor radioresponse, and the combined radiation and G207 treatment produced no greater tumor delay than the best single therapy alone in either host. Also, an interaction between G207 and radiation treatment was not seen for the TRAMP tumor regardless of the host strain.
Discussion
It has been reported that the efficacy of therapeutic HSV-1 R3616 against subcutaneous and intracranial human U87 malignant glioma xenografts in athymic mice is significantly enhanced by radiation [10,13]. Similar findings were reported for HSV-1 R7020 against subcutaneous human head and neck cancer cell line, SQ20b [11]. In the current study, G207, another therapeutic herpes vector, is evaluated for combination therapy against prostate cancer. When the combination of G207 and radiation did not enhance efficacy in the TRAMP syngeneic tumor model, we hypothesized that this might be due to species differences or the effect of cellular immunity. Therefore, we examined a human xenograft in athymic mice, as was the case in the studies of Advani et al. [10,11] and Bradley et al. [13]. In neither a human nor a mouse tumor model system was there a benefit from combining radiation with G207.
In our experiments, radiation treatments were fractionated over five consecutive days (i.e., experimental days 1 to 5).We find that this fractionation regimen is amenable to a wide variety of subcutaneous tumor models, regardless of differences in tumor growth rates or intrinsic cellular radiosensitivities, and it allows easier cross comparisons between the radioresponses of tumors because the number and timing of fractions are always constant. For intratumoral injections, we used three injections, timed to be before (day 0), during (day 3), and after (day 6) radiation therapy, in order to help ensure that we would cover all possible temporal sensitivity windows for radiation/virus interactions. For intravenous injections, we injected on days 0 and 4 because we had previously shown that this injection regimen worked well with LNCaP tumors [16]. It was not our intention to directly duplicate the protocols of Advani et al. [10,11] and Bradley et al. [13] because different experimental regimens were used in each of their three publications, and no particular protocol appeared to be critical. Also, because radiation treatments with different doses and fractionation schemes were seen to enhance the antitumor activity of both R7020 and R3616 on glioma and carcinoma, we did not expect that it would be necessary to exactly imitate a particular treatment regimen in order to see an effect with G207. Furthermore, Advani et al. and Bradley et al. did not directly compare the effect of different time delays of irradiation postinfection; however, in the one study where different delays were used, there were no indications that a shorter time (4 hours) was better than a longer delay (24 hours). Therefore, we do not believe that the failure of radiation and G207 to act synergistically or additively in our prostate tumor models can be attributed merely to minor temporal differences between virus and irradiation treatments. Rather, it is more likely that differences in tumor type and biology play a role in the phenomenon reported by Advani et al. and Bradley et al.
Despite the fact that the combination of G207 and radiation did not enhance the tumor response over the most effective individual therapy, there are several findings that are important for designing tumor therapy. The TRAMP tumor maintained the same radiosensitivity in either the athymic or immunocompetent host. This was expected because immune responses are not thought to be a major factor in tumor radioresponses [30]. G207, however, worked worse than radiation in athymic mice, but better than radiation in C57BL/6 mice. This illustrates the dual factors contributing to the viral antitumor response — direct oncolytic activity and induction of tumor-specific immune responses.
Although radiation did not sensitize the tumors to virus, it also did not decrease the efficacy of the virus either. These results, combined with our earlier published results [16] indicating that tumors that recurred following radiation therapy remained sensitive to virus, suggest that G207 therapy might be a useful therapy for tumors that recur in the radiation field. Also, because localized radiotherapy would not result in systemic immunosuppression, it is less likely than chemotherapy to interfere with subsequent immune-mediated tumor cell killing by G207. Along these lines, intravenous administration of herpes virus following irradiation might have the added benefit of targeting metastases that local radiation therapy alone cannot reach. Conversely, these results suggest that little benefit is derived from delivering both treatments simultaneously. Because the radiation adds little to the cytotoxicity of prostate tumors produced by the virus alone, delivering radiation at the time of viral treatment might even preclude later use of radiotherapy to treat recurring local disease. These results, taken together with our earlier findings that recurring irradiated tumors remained fully sensitive to G207 [16], suggest that sequential, rather than simultaneous, treatment with radiation followed by G207 may produce the best results in terms of delaying tumor regrowth and prolonging survival.
The discrepancy between these findings in prostate and those previously reported with glioma and head and neck cancer may reflect more than simple tumor type differences. R3613 and R7020 are more virulent viruses that replicate better than G207. If, as the previous investigators suggest, the enhanced effect is due to increased viral replication in irradiated cells [10] — possibly as the result of host cell DNA damage [31] — then viruses with a high replication capacity might be better able to capitalize on this. Alternatively, the relatively high sensitivity of prostate tumors to G207 herpes virus [16] may mean that they are already fully sensitized to the cytotoxic effects of the virus, and irradiation cannot further enhance killing by virus. Regardless, our findings suggest that tumor sensitization to herpes viral therapy by radiation is not a universal phenomenon, and that both viral- and tissue-specific factors may be strong determinants of the effect, and warrant further study.
Footnotes
This study was supported by grants from the Association for the Cure of Cancer of the Prostate (CaP Cure) and the United States Department of Defense DAMD17-98-1-8490 (Army Prostate Cancer Research Program). S. D. R. and R. L. M. are consultants to MediGene, which has a license from Georgetown University for G207.
References
- 1.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 2.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, MacLean A, Harland J, McKie E, Mabbs R, Brown M. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma [see comments] Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 3.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. A controlled trial of intratumoral ONYX-015, a selectively replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 4.Ganly I, Eckhardt SG, Rodriguez GI, Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C, Von Hoff DD, Kaye SB. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer [published erratum appears in Clin Cancer Res 2000;6(5):2120] Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 5.Lamont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) [In Process Citation] Ann Surg Oncol. 2000;7:588–592. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs A, Breakefield XO, Fraefel C. HSV-1-based vectors for gene therapy of neurological diseases and brain tumors: Part II. Vector systems and applications. Neoplasia. 1999;1:402–416. doi: 10.1038/sj.neo.7900056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabkin SD, Mineta T, Miyatake S, Yazaki T. Gene therapy: Targeting tumor cells for destruction. Hum Cell. 1996;9:265–276. [PubMed] [Google Scholar]
- 8.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 9.Martuza RL. Conditionally replicating herpes vectors for cancer therapy. J Clin Invest. 2000;105:841–846. doi: 10.1172/JCI9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, Weichselbaum RR. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: A new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- 11.Advani SJ, Chung SM, Yan SY, Gillespie GY, Markert JM, Whitley RJ, Roizman B, Weichselbaum RR. Replication-competent, non-neuroinvasive genetically engineered herpes virus is highly effective in the treatment of therapy-resistant experimental human tumors. Cancer Res. 1999;59:2055–2058. [PubMed] [Google Scholar]
- 12.Chahlavi A, Todo T, Martuza RL, Rabkin SD. Replication-competent herpes simplex virus vector G207 and cisplatin combination therapy for head and neck squamous cell carcinoma. Neoplasia. 1999;1:162–169. doi: 10.1038/sj.neo.7900016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, Whitley RJ, Markert JM, Roizman B, Weichselbaum RR. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. 1999;5:1517–1522. [PubMed] [Google Scholar]
- 14.Markert JM, Gillespie GY, Weichselbaum RR, Roizman B, Whitley RJ. Genetically engineered HSV in the treatment of glioma: A review. Rev Med Virol. 2000;10:17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Chmura SJ, Advani SJ, Kufe DW, Weichselbaum RR. Strategies for enhancing viral-based gene therapy using ionizing radiation. Radiat Oncol Invest. 1999;7:261–269. doi: 10.1002/(SICI)1520-6823(1999)7:5<261::AID-ROI1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Walker JR, McGeagh KG, Sundaresan P, Jorgensen TJ, Rabkin SD, Martuza RL. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum Gene Ther. 1999;10:2237–2243. doi: 10.1089/10430349950017211. [DOI] [PubMed] [Google Scholar]
- 17.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 18.Albright N. Computer programs for the analysis of cellular survival data. Radiat Res. 1987;112:331–340. [PubMed] [Google Scholar]
- 19.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 20.Kimura K, Bowen C, Spiegel S, Gelmann EP. Tumor necrosis factor-alpha sensitizes prostate cancer cells to gamma-irradiation-induced apoptosis. Cancer Res. 1999;59:1606–1614. [PubMed] [Google Scholar]
- 21.Garzotto M, Haimovitz-Friedman A, Liao WC, White-Jones M, Huryk R, Heston WD, Cardon-Cardo C, Kolesnick R, Fuks Z. Reversal of radiation resistance in LNCaP cells by targeting apoptosis through ceramide synthase. Cancer Res. 1999;59:5194–5201. [PubMed] [Google Scholar]
- 22.Nava VE, Cuvillier O, Edsall LC, Kimura K, Milstien S, Gelmann EP, Spiegel S. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 2000;60:4468–4474. [PubMed] [Google Scholar]
- 23.Colletier PJ, Ashoori F, Cowen D, Meyn RE, Tofilon P, Meistrich ME, Pollack A. Adenoviral-mediated p53 transgene expression sensitizes both wild-type and null p53 prostate cancer cells in vitro to radiation. Int J Radiat Oncol Biol Phys. 2000;48:1507–1512. doi: 10.1016/s0360-3016(00)01409-7. [DOI] [PubMed] [Google Scholar]
- 24.Navone NM, Logotheis CJ, Von Eschenbach AC, Troncoso P. Model systems of prostate cancer: Uses and limitations. Cancer Metastasis Rev. 1999;17:361–371. doi: 10.1023/a:1006165017279. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todo T, Rabkin SD, Sundaresan P, Wu A, Meehan KR, Herscowitz HB, Martuza RL. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 27.Todo M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 28.Leith JT, Quaranto L, Padfield G, Michelson S, Hercbergs A. Radiobiological studies of PC-3 and DU-145 human prostate cancer cells: X-ray sensitivity in vitro and hypoxic fractions of xenografted tumors in vivo. Int J Radiat Oncol Biol Phys. 1993;25:283–287. doi: 10.1016/0360-3016(93)90350-5. [DOI] [PubMed] [Google Scholar]
- 29.Steel GG, Adams GE, Horwich A, editors. The Biological Basis of Radiotherapy. Amsterdam: Elsevier; [Google Scholar]
- 30.Mason K, Staab A, Hunter N, McBride W, Petersen S, Terry N, Milas L. Enhancement of tumor radioresponse by docetaxel: Involvement of immune system. Int J Oncol. 2001;18:599–606. doi: 10.3892/ijo.18.3.599. [DOI] [PubMed] [Google Scholar]
- 31.Miller CS, Smith KO. Enhanced replication of herpes simplex virus type 1 in human cells. J Dent Res. 1991;70:111–117. doi: 10.1177/00220345910700020301. [DOI] [PubMed] [Google Scholar]