Abstract
Kaposi's sarcoma (KS) is the most common tumor affecting AIDS patients with over 20% of these patients afflicted by this disease. Previous studies have demonstrated that KS tumor cells predominantly express the prosurvival protein Bcl-XL compared with Bcl-2. In the current study, we have used an adenoviral vector that expresses Bcl-XS, a functional inhibitor of Bcl-XL, to study the significance of Bcl-XL expression in the KS cell line (SLK) or KS primary cultures. The results demonstrate that 75% to 80% of SLK or KS primary cells were killed by the Bcl-XS containing adenovirus whereas KS cells infected with control adenovirus showed no significant cell death or growth inhibition. Overexpression of Bcl-XL, but not Bcl-2, in SLK cells attenuated apoptosis induced by adenovirus Bcl-XS. Immunoprecipitation experiments revealed that adenoviral Bcl-XS associated with Bcl-XL, but not with Bcl-2. Mutational analysis showed that the α2 helical region of Bcl-XS containing the BH3 motif was critical for killing activity and interaction with Bcl-XL. These results suggest that Bcl-XS is a direct killer and Bcl-XL may act by interacting with and sequestering Bcl-XS. These studies also suggest that targeting Bcl-XL may be of therapeutic benefit for the treatment of tumors that are characterized by inappropriate expression of Bcl-XL.
Keywords: Apoptosis, Bcl-2, Bcl-X, adenovirus, Kaposi's sarcoma
Introduction
Apoptosis, a common form of programmed cell death, is essential during development and tissue homeostasis and plays a role in the pathogenesis of a variety of diseases [1]. Members of the Bcl-2 family that include prosurvival and proapoptotic proteins have been recognized as key regulators of the apoptotic process [2]. Several members of the family including Bcl-2 and Bcl-XL have been shown to protect cells from apoptosis induced by a wide array of stimuli, whereas other members of this family such as Bax, Bak, and Bad have been shown to promote cell death [2]. In addition to prosurvival Bcl-XL, the bcl-x gene can encode several protein products including Bcl-XS as a result of alternative splicing [3–6]. Bcl-XS is a proapoptotic protein that lacks BH1 and BH2 domains [3], which have been shown to be important in mediating the prosurvival function of prosurvival Bcl-2 family members including Bcl-XL [7,8]. bcl-XS mRNA levels have been shown to be upregulated before apoptosis associated with mammary tissue involution in mice and ischemia in rat brain [9,10]. Bcl-XS has been reported to inhibit the protective function of Bcl-2 and Bcl-XL against apoptosis induced by growth factor deprivation and chemotherapeutic drugs [3,11,12]. Furthermore, expression of Bcl-XS has been shown to promote apoptosis or nonapoptotic cell death in several experimental systems [13–16].
It has been postulated that overexpression of cell survival proteins may contribute to neoplasia by blocking apoptosis in premalignant or transformed cells. Many cells overexpress Bcl-2 or Bcl-XL, and this overexpression has been found to correlate with poor prognosis and resistance to treatment in several types of malignancies [17]. Kaposi's sarcoma (KS), a neoplasm with multifocal vascular lesions, is the most common tumor in AIDS patients, afflicting up to 30% of HIV-1-positive patients [18,19]. It has been recently demonstrated that the majority of primary KS tumors overexpress Bcl-XL [20] but also can express Bcl-2 [21]. As these proteins are known to inhibit cell death, expression of these antiapoptosis Bcl-2 family members in KS cells may contribute to the prolonged survival of the tumor cells in vivo.
We previously constructed an adenoviral vector that encodes for the Bcl-XS protein, and showed that primary carcinoma cells as well as cell lines derived from multiple epithelial tumors were killed following infection with this adenovirus in the absence of an exogenous apoptotic signal [14]. However, the effect of Bcl-XS expression in sarcoma cells is unknown. Furthermore, the mechanism by which Bcl-XS promotes cell death remains poorly understood. In this report, we demonstrate that Bcl-XS expression induces apoptosis of KS cells. Significantly, Bcl-XL but not Bcl-2 attenuated the proapoptotic activity of the bcl-XS adenovirus. Consistent with this finding, Bcl-XL but not Bcl-2, associated with Bcl-XS in extracts of KS cells. We also provide evidence that the BH3 motif of Bcl-XS is critical for Bcl-XL binding and killing activity, suggesting that Bcl-XS acts at least in part by antagonizing the prosurvival function of Bcl-XL.
Materials and Methods
Cells and Cell Culture
KS cell line (SLK) established from a tumor biopsy from oral mucosa of an iatrogenically immunosuppressed HIV-negative man [22] was obtained from Dr. J. Levy (University of California, San Francisco). The cells were maintained in culture using RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum. For passage, the cells were treated with 0.05% trypsin-0.53 mM EDTA, washed, and replated. Three different human KS cell cultures were isolated from KS tumors of HIV-positive patients and designated as KS21B, KS23B, and KSL100S as described [23,24]. The other primary KS cell line KSPG38 [25] was obtained from Dr. P. Gill (University of Southern California, Los Angeles). Cells were plated on tissue culture dishes (Corning, Corning, NY) coated with attachment factor (Cell Systems, Kirkland, WA) and were maintained in RPMI 1640 and 20% heat-inactivated fetal bovine serum supplemented with 10% nutridoma HU (Boehringer Mannheim Biochemicals, Indianapolis, IN), 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin, 50 µg/ml endothelial cell growth supplement (ICN Biochemicals, Aurora, OH), and 16 U/ml bovine heparin.
Infection of Cells with Recombinant Adenoviruses
The bcl-XS [14] and control E1-deleted adenovirus [26] were prepared as previously described [14] and the number of adenovirus particles in viral stocks was determined by spectrophotometry. SLK cells were infected with a stock of the beta-galactosidase adenovirus [14] of known titer, and then stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside to determine the number of viral particles per cell needed to infect approximately 85% to 95% of the cells. For adenoviral infection, SLK cells or KS primary cultures were exposed to the adenovirus vectors for 4 hours in serum-free medium in a small volume sufficient to cover the monolayer culture. Following incubation, 2% serum containing medium was added and incubated overnight. Next day, the medium was removed and replaced with medium containing 10% serum. Each infection was performed at least in triplicate. At the end of desired length of incubation, cell viability of virally infected cell was determined by trypan blue exclusion and cell growth was assessed by hemocytometry. Statistical significance was calculated by Student's paired t test. P values less than .05 are considered to be statistically significant.
Plasmids
pcDNA3 plasmids containing Flag or HA-tagged bcl-XL or bcl-2 have been described [27]. Plasmids to express wild-type and mutant Bcl-XS were generated by a polymerase chain reaction method using appropriate oligonucleotide primers complementary to the human bcl-XS sequence and ligated to pcDNA-HA [27]. The authenticity of the constructs was confirmed by DNA sequencing.
Transfection, Immunoprecipitation, and Immunoblotting
SLK cells were transfected by the calcium phosphate method with 10 µg of the pcDNA3-neo plasmid containing either Flag-tagged bcl-XL or bcl-2. Individual cell clones were selected for growth in the presence of G418 (1.5 mg/ml). For immunoprecipitation, cells were lysed in Nonidet P-40 isotonic lysis buffer and lysates were incubated with 1 µg/ml anti-Flag antibody or normal rabbit IgG overnight at 4°C with 5% (vol/vol) of protein Asepharose 4B (Zymed Laboratories, South San Francisco, CA). Immune complexes were centrifuged, washed four times with excess cold Nonidet P-40 isotonic lysis buffer, separated on a 12% SDS-polyacrylamide gel and immunoblotted with relevant antibodies as described [27]. Rabbit polyclonal anti-Bcl-X (gift from Dr. C. Thompson, University of Pennsylvania) was used for the detection Bcl-XL and Bcl-XS protein, whereas Bcl-2 protein was detected by anti-human Bcl-2 monoclonal antibody, 6C8 (Pharmingen, San Diego, CA). Immunoprecipitations to assess the binding of Bcl-XS mutants to Bcl-XL were performed in 293 sarcoma cell lysates prepared after transient transfection as described above.
Apoptosis Assay
SLK cells were harvested at day 6 after infection with bcl-XS adenovirus by combining floating cells in the media with adherent cells that were detached with trypsin-EDTA in PBS. Cells were suspended in a hypotonic buffer containing propidium iodide and the percent of cells at sub-Go phase was determined by flow cytometry [28]. For transient assays, wild-type or mutant bcl-XS constructs were cotransfected with a pCMV-µgalactosidase reporter plasmid into 293 kidney sarcoma cells as reported [27]. Apoptosis was determined 18 hours posttransfection in triplicate cultures as reported [27].
Results
Bcl-XS is Expressed After Infection of SLK Tumor Cells with bcl-XS Adenovirus
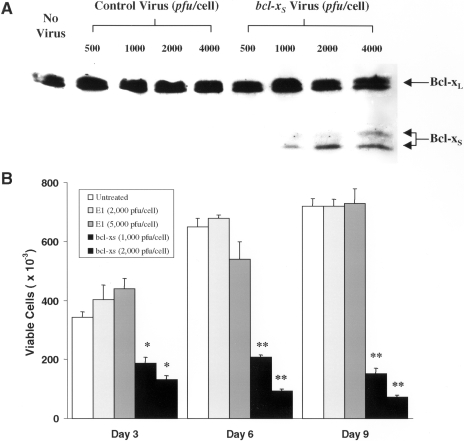
To verify that Bcl-XS protein is expressed after treatment of the SLK cell line with the bcl-XS adenovirus, extracts from cells infected with various concentrations of control and bcl-XS adenovirus were immunoblotted with an antibody that recognizes both Bcl-XS and Bcl-XL. Forty-eight hours after infection, a dose-dependent increase in Bcl-XS expression was observed (Figure 1A). The Bcl-XS protein was detected as a doublet, which is consistent with a previous report [12]. Bcl-XS was undetectable in extracts from uninfected cells or cells infected with equivalent titers of control adenovirus (Figure 1A). After treatment of SLK cells with control or bcl-XS adenovirus, the levels of endogenous Bcl-XL were unaffected when compared with those of uninfected SLK cells (Figure 1A).
Figure 1.
Adenoviral Bcl-XS kills SLK cells. (A) Cells were infected either with control or bcl-XS adenovirus for 72 hours and the expression of Bcl-XS protein was analyzed by immunoblotting. Note that the cells infected with the virus expressed Bcl-XS protein whereas the level of endogenous Bcl-XL protein remained unaltered. (B) SLK cells were infected with adenovirus and at day 3, 6, or 9, the number of viable cells (adhering and nonadhering cells) were determined by trypan blue exclusion. Results are shown as the mean±SEM of triplicate cultures. */**Indicates P values less than .05/.01, respectively compared with the results of control virus infected cells.
The bcl-XS Adenovirus Inhibits Cell Growth and Induces Cell Death in SLK Tumor Cells
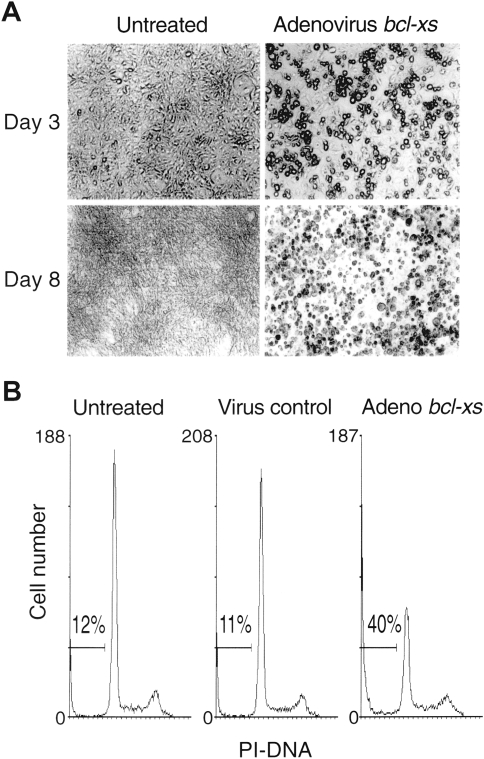
SLK cells infected with control adenovirus at 2000 or 5000 pfu/cell showed no cytostatic effects on growth as compared with untreated cells when assessed at days 3, 6, and 9 after infection (Figure 1B). In contrast, exposure of SLK cells to the bcl-XS adenovirus at 1000 or 2000 pfu/cell resulted in progressive loss of cell growth and reduced cell viability (Figure 1B). By day 3 after infection, a significant percentage of the SLK tumor cells infected with the bcl-XS adenovirus displayed rounded-up morphology; by day 8, the great majority of the cells become fragmented and floated in the dish, whereas cells treated with control adenovirus (data not shown) or cells left untreated remained attached to the dish and were viable (Figure 2A). Apoptotic cells undergo DNA fragmentation, an event that can be assessed by staining of nuclear DNA [31]. To determine if induction of cell death by the bcl-XS adenovirus was associated with loss of DNA integrity, we performed flow cytometric analysis of nuclei stained with propidium iodide. In a representative experiment, we found that about 40% of the cells infected with the Bcl-XS adenovirus for 72 hours exhibited a sub-Go DNA profile, which is characteristic of apoptotic cells, as opposed to about 10% apoptotic cells in untreated cultures or SLK cells infected with control virus (Figure 2B). These results suggest that the bcl-XS adenovirus induces apoptosis in KS cells.
Figure 2.
Viability and morphologic changes of SLK cells following treatment with control adenovirus or bcl-XS adenovirus (A). Photomicrographs (400x) of a representative field of SLK cells were taken on days 3 and 8 after virus infection. Morphology of cells infected with control adenovirus (not shown) were not different from untreated control. (B) Cell cycle profile was determined in untreated cells, cell infected with control adenovirus, or with bcl-XS adenovirus using 2000 pfu/cell at 72 hours post infection.
Bcl-XS Containing Adenovirus Kills Primary KS Cells
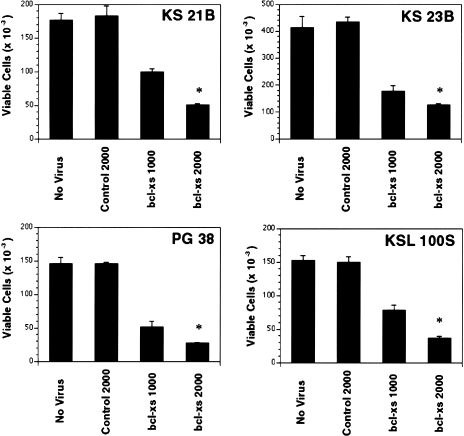
As SLK cells could be killed efficiently by adenovirus bcl-Xs, we extended our studies to primary cultures of KS tumor cells to validate our observations made with the KS cell line. Infection of four independent primary cultures of KS tumor cells with the bcl-XS adenovirus resulted in marked cytotoxicity of tumor cells when compared to uninfected cells or cells treated with control adenovirus (Figure 3). To determine the expression levels of Bcl-2 and Bcl-XL in these KS tumor cells, extracts were prepared and subjected to immunoblotting with anti-Bcl-2 and anti-Bcl-X antibodies. The analysis showed that all the KS tumor cell cultures tested (KS21B, KS23B, KS PG38, and KSL 100S) expressed Bcl-XL at varying levels, whereas Bcl-2 was detectable only in KS 23B cells (data not shown).
Figure 3.
Cell viability of primary cultures of KS tumor cells infected with bcl-XS adenovirus and expression of Bcl-XL and Bcl-2 proteins in primary cultures of KS tumors. KS cells (KS21B, KS23B, PG38, and KSL100S) were infected with either control adenovirus (2000 pfu/cell) or bcl-XS (pfu/cell indicated in the figure) as described in the Materials and Methods section. At day 8, cell viability of untreated or virally infected cells were determined by trypan blue exclusion and cell numbers were assessed by hemocytometry. *Indicates P values less than .01 compared with cells infected with control virus.
Bcl-XL but Not Bcl-2 Protects SLK Tumor Cells from Cell Death Mediated by the bcl-XS Adenovirus
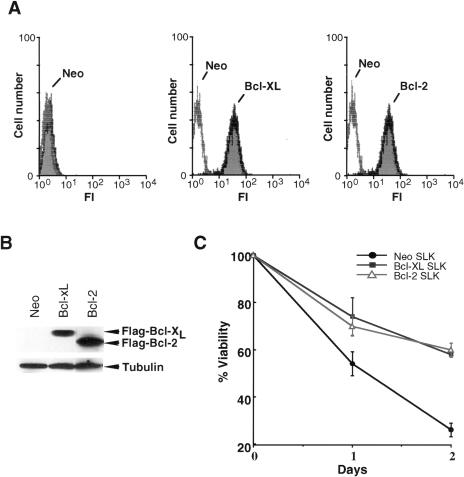
Primary cultures of KS cells can express Bcl-XL (Ref. [20] and in this study) and Bcl-2 [21]. To determine if Bcl-XL or Bcl-2 can regulate killing mediated by the bcl-XS adenovirus, we transfected the SLK cell line with expression plasmids producing Flag-tagged Bcl-XL, Flag-tagged Bcl-2 or control plasmid and identified two stable clones that overexpress Bcl-XL or Bcl-2. Flow cytometric analysis using anti-Flag antibody showed that the clones expressed Flag-tagged Bcl-XL or Bcl-2 in virtually all cells (Figure 4A). Western blot analysis confirmed that the clones expressed Flag-Bcl-XL or Flag-Bcl-2, although the levels of Bcl-2 were higher than those of Bcl-XL (Figure 4B). To determine if exogenous Bcl-XL and Bcl-2 provide increased survival, SLK cells transfected with plasmids producing these prosurvival proteins or plasmid control were exposed to the chemotherapeutic drug etoposide and cell viability was assessed after drug exposure. Approximately 70% to 75% of the SLK cells lost their viability after incubation with etoposide for 2 days (Figure 4C). By contrast, SLK cells overexpressing Bcl-XL or Bcl-2 showed increased survival after treatment with the drug (Figure 4C). To determine whether Bcl-XL or Bcl-2 could modulate cell killing induced by the bcl-XS adenovirus, SLK tumor cells stably transfected with these prosurvival genes or control plasmid were infected with the bcl-XS adenovirus and cell viability was assessed at different times after adenovirus infection. As seen in Figure 5, expression of Bcl-XL promoted the survival of SLK cells after infection with the bcl-XS adenovirus whereas Bcl-2 did not.
Figure 4.
Expression of functional Bcl-XL and Bcl-2 in SLK cell clones. (A) Immunoblotting analysis for Flag-Bcl-2 and Flag-Bcl-XL using anti-Flag monoclonal antibody. Immunoblotting for beta-tubulin was used as a loading control. Note that the SLK-Neo control does not express either Flag-Bcl-XL or Flag-Bcl-2. (B) Fluorescence histograms of SLK clones. Clones expressing Flag-Bcl-XL or Flag-Bcl-2 were analyzed for Flag-epitope expression by flow cytometry with anti-Flag monoclonal antibody. Controls are SLK clones expressing Flag-Bcl-XL or Flag-Bcl-2 stained with isotype-matched control IgG. (C) Cell survival of SLK clones after treatment with etoposide. SLK clones were treated with etoposide (2 µg/ml) and cell viability was determined on days 1 and 2 after continuous exposure to the drug. Results shown are the mean±SD of triplicate cultures and are representative of at least two separate experiments.
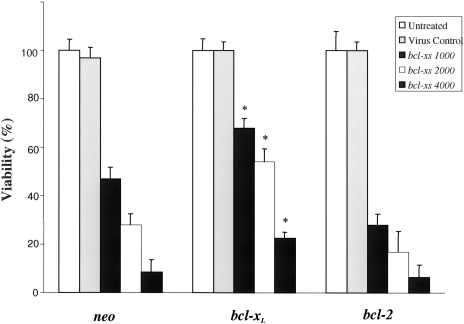
Figure 5.
Viability of SLK cells overexpressing Bcl-XL or Bcl-2. Cells were infected with control adenovirus or bcl-XS adenovirus at different pfu/cell (indicated in the figure) and cell viability was determined at day 8. The results are presented as percent of viable cells relative to that of untreated cells that was considered as being 100%. *Indicates P values less than .01 compared with neo-transfected SLK cells.
Bcl-XL but Not Bcl-2 Interact with Bcl-XS After Infection of SLK Cells with Adenovirus bcl-XS
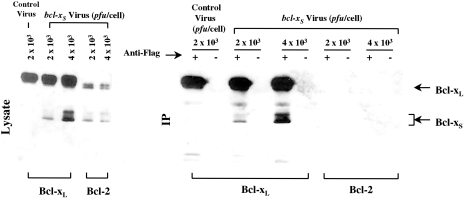
To gain insight into the mechanism by which Bcl-Xs mediates killing of KS cells, we prepared protein extracts from control and adenovirus bcl-XS-infected SLK cells and immunoprecipitated Bcl-XL and Bcl-2 complexes with anti-Flag antibody. Immunoblotting analysis of control, Bcl-XL, and Bcl-2 immunoprecipitates with anti-Bcl-X antibody revealed that Bcl-XS associated with Bcl-XL but not with Bcl-2 (Figure 6). Immunoblotting of total lysates with anti-Bcl-X antibody showed that adenoviral Bcl-XS was expressed in both Bcl-XL and Bcl-2 transfectants indicating that the lack of association of Bcl-XS with Bcl-2 cannot be explained by differential expression of Bcl-XS in SLK cells (Figure 6).
Figure 6.
Differential interaction of Bcl-XL and Bcl-2 with Bcl-XS. SLK cells expressing Flag-tagged Bcl-XL or Bcl-2 were exposed to bcl-XS adenovirus and for control, SLK cells expressing Flag-tagged Bcl-XL were exposed to E1-deleted adenovirus (pfu/cell indicated in the figure) for 72 hours. Extracts from infected cells were immunoprecipitated with anti-Flag or control antibody as described in methods and immunoprecipitates were immunoblotted with rabbit polyclonal against Bcl-X.
The BH3 Motif and the C-Terminal Transmembrane Domain of Bcl-XS are Critical for Killing Activity
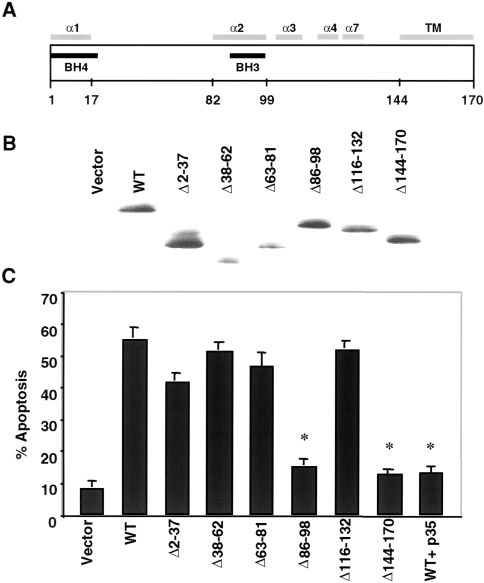
We generated a panel of Bcl-XS deletion mutants based on the known crystal structure of Bcl-XL [29] to determine the regions that are important for killing activity. These included Bcl-XS mutants with internal deletion of the BH4 domain (residues 2 to 37), flexible loop region (residues 38 to 62), BH3 domain (residue 86 to 98), most of the α2, α4, and α7 helical region (residues 116 to 132), and the C-terminal transmembrane domain (residues 144 to 170) (Figure 7A). Immunoblotting analysis of cell extracts from human 293 kidney sarcoma cells transiently transfected with Bcl-XS expression constructs revealed that the mutants were expressed in transfected cells (Figure 7B). Functional analysis showed that residues 86 to 98 (BH3 domain) and the membrane anchoring domain of Bcl-XS were required for killing activity (Figure 7C). Expression of baculovirus p35, a caspase inhibitor, suppressed apoptosis induced by wild-type Bcl-XS (Figure. 7C), suggesting that the proapoptotic activity of Bcl-XS is mediated through caspases.
Figure 7.
The BH3 and membrane-anchoring domains of Bcl-XS are required for proapoptotic activity in 293 kidney sarcoma cells. (A) Schematic diagram of Bcl-XS structure. Predicted alpha helices and transmembrane region (TM) are shown. Numbers indicate position of amino acids. (B) Immunobloting analysis using extracts of 293 cells transiently transfected with Bcl-XS plasmids. Expression was detected with anti-HA antibody. (C) Apoptosis in 293 cells transiently transfected with Bcl-XS plasmids and control plasmid. Assay was performed 28 hours posttransfection. p35 denotes expression of baculovirus p35. Values are means±SD of triplicate cultures. *Indicates P value less than .01 compared with cells transfected with wild-type plasmid.
The BH3 Domain of Bcl-XS is Required for Interaction with Bcl-XL
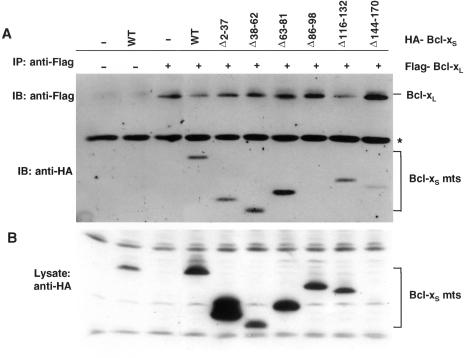
We next determined the region of Bcl-XS that is required for Bcl-XL binding. In these experiments, we coexpressed Bcl-XS and Bcl-XL by transient transfection in 293 cells and immunoprecipitated Bcl-XL with anti-Flag antibody. The analysis in Figure 8 revealed that all mutants except Bcl-XS (Δ86–98) coimmunoprecipitated with Bcl-XL, indicating that the BH3 domain of Bcl-Xs is required for the interaction with Bcl-XL.
Figure 8.
The BH3 domain is required for binding to Bcl-Xl. (A) Immunoprecipitation of Bcl-XL with wild-type and mutant Bcl-XS. 293 cells were cotransfected with plasmids encoding Bcl-XL and Bcl-XS as indicated. Bcl-XL/Bcl-XS complexes were immunoprecipitated with anti-Flag and immunoblotted with anti-HA and anti-Flag. (C) Immunoblotting of total lysates used for above immunoprecipitations. Note that although deletion mutant Δ144–170 is expressed (see panel A, last lane), it is not visible on this exposure.
Discussion
We report here that KS tumor cells undergo cell death after infection with a replication-deficient adenoviral vector that expresses Bcl-XS. Adenoviral vector expressing Bcl-XS caused cell death as early as 3 days after infection of the SLK cell line and several primary cultures of KS cells. Bcl-XS lacks BH1 and BH2 but contains BH3 and BH4 domains. As it has been shown for BH3-only Bcl-2 family members, such as Bad and Hrk [27,30], the BH3 domain of Bcl-XS may mediate apoptosis by its ability to heterodimerize, with and inactivate prosurvival Bcl-2 family proteins. Consistent with this hypothesis, our mutational analyses revealed a correlation between Bcl-XS killing activity and the ability of Bcl-Xs to bind Bcl-XL. In these experiments, the BH3 domain of Bcl-XS was required for both Bcl-XL binding and killing activity. In agreement with our results, it has been shown that only mutant forms of Bcl-XS that contain the BH3 domain can antagonize the prosurvival function of Bcl-XL against Bax-mediated apoptosis [12]. The ability of Bcl-XS to heterodimerize with Bcl-XL has been controversial [11], although recent experiments support our observation that Bcl-XS interacts with Bcl-XL [12]. In contrast, Bcl-XS did not associate with Bcl-2 in SLK cells infected with the Bcl-XS adenovirus. Consistent with these findings is our observation that cell death induced by the bcl-XS adenovirus was partially inhibited by Bcl-XL but not by Bcl-2.
Primary cultures of KS tumor cells or SLK cells, although expressing constitutive levels of Bcl-XL were susceptible to apoptosis induced by adenovirus Bcl-XS. Similarly, the bcl-XS adenovirus induced apoptosis in neuroblastoma cells that overexpress Bcl-XL [31]. It is possible that the constitutive levels of Bcl-XL may not be sufficiently high to bind all the exogenous Bcl-XS. However, even after transfection of Bcl-XL into SLK cells, the bcl-XS adenovirus induced cell death although at a reduced rate compared to controls. Another possibility is that Bcl-XS is a direct killer that mediates cell death that is in part independent of Bcl-XL. Although this latter mechanism needs to be further investigated, it may involve binding to mitochondria and disruption of mitochondrial integrity [16], as postulated for other BH3-containing proapoptotic molecules [32,33]. In this model, Bcl-XL inhibits Bcl-XS activity by interacting with and sequestering Bcl-XS. It is most likely that Bcl-XS acts directly to kill cells, because if it acted through other proapoptotic molecules, such as Bax or Bak, Bcl-2 might be expected to interfere with Bcl-XS. Our studies have demonstrated that all the primary cultures tested thus far have been effectively killed by the adenovirus bcl-XS. Considering the aggressive nature of AIDS-KS and the lack of currently available effective treatment, inhibition of Bcl-XL activity may be of benefit for the treatment of this potentially fatal disease. Although the bcl-XS adenovirus may be effective in the treatment of superficial tumors, effective inactivation of Bcl-XL will require different approaches with improved delivery of therapeutic molecules to tumor cells. Recent studies have identified small molecules that induce apoptosis by binding to the BH3-binding pocket of Bcl-XL and blocking BH3 domain-mediated heterodimerization between Bcl-2 family members [34,35]. These studies suggest that it may be possible to effectively target the Bcl-XL survival pathway in diseases such as KS that are characterized by aberrant expression of prosurvival Bcl-2 family members.
Acknowledgements
The authors thank Dr. P. Gill for KSPG38 cells and Dr. Leventon-Kriss for SLK cell line. The authors also thank Dr. J. E. Nor, Dr. Y. Hu and Felicia Chen for their help and a reviewer for insightful comments.
Abbreviations
- BH3
Bcl-2 homology domain 3
- KS
Kaposi's sarcoma
Footnotes
This work was supported by grants R01 CA70057 from the National Institutes of Health (NIH) and DAMD17-96-6019 from the Department of the U. S. Army to GN.
References
- 1.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Adams J, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 3.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindstern T, Turka LA, Mao X, Nuñez G, Thompson CB. Bcl-X, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 4.Fang W, Rivard JJ, Mueller DL, Behrens TW. Cloning and molecular characterization of mouse bcl-x in B and T lymphocytes. J Immunol. 1994;153:4388–4398. [PubMed] [Google Scholar]
- 5.Grillot DA, Gonzalez-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin MF, Nuñez G. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol. 1997;158:4750–4757. [PubMed] [Google Scholar]
- 6.Yang XF, Weber GF, Cantor H. A novel Bcl-x isoform connected to the T cell receptor regulates apoptosis in T cells. Immunity. 1997;7:629–639. doi: 10.1016/s1074-7613(00)80384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter JJ, Bond BL, Parslow TG. Functional dissection of the human Bcl-2 protein: sequence requirements for inhibition of apoptosis. Mol Cell Biol. 1996;16:877–883. doi: 10.1128/mcb.16.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin XM, Oltval ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 9.Dixon EP, Stephenson DT, Clemens JA, Little SP. Bcl-xshort is elevated following severe global ischemia in rat brains. Brain Res. 1997;776:222–229. doi: 10.1016/s0006-8993(97)01040-8. [DOI] [PubMed] [Google Scholar]
- 10.Heermeier KM, Benedict M, Li M, Furth P, Nuñez G, Hennighausen L. Bax and Bcl-XS are induced at the onset of apoptosis in involuting mammary epithelial cells. Methods Dev. 1996;56:197–207. doi: 10.1016/0925-4773(96)88032-4. [DOI] [PubMed] [Google Scholar]
- 11.Minn AJ, Boise LH, Thompson CB. Bcl-Xs antagonizes the protective effects of Bcl-XL. J Biol Chem. 1996;271:6306–6312. doi: 10.1074/jbc.271.11.6306. [DOI] [PubMed] [Google Scholar]
- 12.Chang BS, Kelekar A, Harris MH, Harlan JE, Fesik SW, Thompson CB. The BH3 domain of Bcl-XS is required for inhibition of the antiapoptotic function of Bcl-XL. Mol Cell Biol. 1999;10:6673–6681. doi: 10.1128/mcb.19.10.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumantran VN, Ealovega MW, Nuñez G, Clarke MF, Wicha MS. Overexpression of Bcl-XS sensitizes MCF-7 cells to chemotherapy-induced apoptosis. Cancer Res. 1995;55:2507–2510. [PubMed] [Google Scholar]
- 14.Clarke MF, Apel IJ, Benedict MA, Eipers PG, Sumantran V, Gonzalez-Garcia M, Doedens M, Fukunaga N, Davidson B, Dick JE, Minn AJ, Boise LH, Thompson CB, Wicha M, Nuñez G. A recombinant bcl-Xs adenovirus selectively induces apoptosis in cancer cells but not normal bone marrow cells. Proc Natl Acad Sci USA. 1995;92:11024–11028. doi: 10.1073/pnas.92.24.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pena JC, Fuchs E, Thompson CB. Bcl-x expression influences keratinocyte cell survival but not terminal differentiation. Cell Growth Differ. 1997;6:619–629. [PubMed] [Google Scholar]
- 16.Fridman JS, Benedict MA, Maybaum J. Bcl-X(S)-induced cell death in 3T3 cells does not require or induce caspase activation. Cancer Res. 1999;59:5999–6004. [PubMed] [Google Scholar]
- 17.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 18.Haverkos HW, Dortman DP, Morgan M. Prevalence of Kaposi's sarcoma among patients with AIDS. N Engl J Med. 1985;321:1518–1518. [PubMed] [Google Scholar]
- 19.Gill PS, Hamilton A, Naidu Y. Epidemic (AIDS-related) Kaposi's sarcoma: epidemiology, pathogenesis and treatment. In: Devita VT, Hellman S, Rosenberg SA, editors. AIDS. Vol. 7. Philadelphia, PA: Lippincott; 1994. pp. 1–11. [Google Scholar]
- 20.Foreman KE, Wrone-Smith T, Boise LH, Thompson CB, Polverini PJ, Simonian PJ, Nuñez G, Nickoloff BJ. Kaposi's sarcoma tumor cells preferentially express Bcl-XL. Am J Pathol. 1996;149:795–803. [Google Scholar]
- 21.Morris CB, Gendelman R, Marrogi AJ, Lu M, Lockyer JM, Alperin-Lea W, Ensoli B. Immunochemical detection of Bcl-2 in AIDS-associated and classical Kaposi's sarcoma. Am J Pathol. 1996;148:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 22.Herndier BG, Werner A, Arntein P, Abbey NW, Dermatis F, Cohen RL, Shuman MA, Levy JA. Characterization of a human Kaposi's sarcoma cell line that induces angiogenic tumors in animals. AIDS. 1994;8:575–581. doi: 10.1097/00002030-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Huang YQ, Friedman-Kien AE, Li JJ, Nickoloff BJ. Cultured Kaposi's sarcoma cell line express factor XIIIa, CD14 and VCAM-1, but not factor VIII or ELAM-1. Arch Dermatol. 1993;129:1291–1296. [PubMed] [Google Scholar]
- 24.Foreman KE, Trinh D, Nestle FO, Mitra RS, Nickoloff BJ. Cultured Kaposi's sarcoma tumor cells fail to stimulate T cell proliferation. Clin Immunol Immunopathol. 1996;78:172–179. doi: 10.1006/clin.1996.0026. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZY, Ganju RK, Wang JF, Ona MA, Hatch WC, Zheng T, Avraham S, Gill P, Groopman JE. Cytokine signaling through the novel tyrosine kinase RAFTK in Kaposi's sarcoma cells. J Clin Invest. 1997;99:1798–1804. doi: 10.1172/JCI119344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inohara N, Ding L, Chen S, Nuñez G. Harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-XL. EMBO J. 1997;16:1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonian PL, Grillot DAM, Nuñez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208–1216. [PubMed] [Google Scholar]
- 29.Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 30.Kelekar A, Chang BS, Harlan JE, Fesik SW, Thompson CB. Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL. Mol Cell Biol. 1997;17:7040–7046. doi: 10.1128/mcb.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dole MG, Clarke MF, Holman P, Benedict M, Lu J, Jasty R, Eipers P, Thompson CB, Rode C, Bloch C, Nuñez G, Castle VP. Bcl-XS enhances adenoviral vector-induced apoptosis in neuroblastoma cells. Cancer Res. 1996;56:5734–5740. [PubMed] [Google Scholar]
- 32.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzung S-P, Kim KM, Basañez G, Giedt CD, Simon J, Zimmerberg J, Zhang KYJ, Hockenbery DM. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 35.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-XL. Nat Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]