Abstract
Coagulopathy and angiogenesis are among the most consistent host responses associated with cancer. These two respective processes, hitherto viewed as distinct, may in fact be functionally inseparable as blood coagulation and fibrinolysis, in their own right, influence tumor angiogenesis and thereby contribute to malignant growth. In addition, tumor angiogenesis appears to be controlled through both standard and non-standard functions of such elements of the hemostatic system as tissue factor, thrombin, fibrin, plasminogen activators, plasminogen, and platelets. “Cryptic” domains can be released from hemostatic proteins through proteolytic cleavage, and act systemically as angiogenesis inhibitors (e.g., angiostatin, antiangiogenic antithrombin III aaATIII). Various components of the hemostatic system either promote or inhibit angiogenesis and likely act by changing the net angiogenic balance. However, their complex influences are far from being fully understood. Targeted pharmacological and/or genetic inhibition of pro-angiogenic activities of the hemostatic system and exploitation of endogenous angiogenesis inhibitors of the angiostatin and aaATIII variety are under study as prospective anti-cancer treatments.
Hemostasis, Angiogenesis, and Tumor Progression
The hemostatic system is anatomically and functionally inseparable from the vasculature. It is therefore somewhat ironic that in the context of cancer, these respective host elements have, until recently, been studied as virtually independent entities. This article is intended to illustrate the ongoing revision of this historical view and to summarize the therapeutic potential of targeting elements of the hemostatic system as a strategy to inhibit tumor angiogenesis.
Close association between malignant disorders and various perturbations in blood coagulation has been recognized for over 130 years [1]. Coagulation dysfunctions of different nature and magnitude, ranging from subtle laboratory abnormalities to overt thromboembolism, thrombophlebitis, and disseminated intravascular coagulation, are routinely found in cancer patients [2–8]. Up to 50% of all patients with malignant disease and up to 90% of those with metastatic lesions demonstrate abnormalities in hemostatic parameters [5]. In this regard, pancreatic cancer, breast cancer, and particularly acute promyelocytic leukemia are the best-described examples [7,9]. However, cancer-related hemostatic complications are usually heterogenous in nature and their pathogenesis is often poorly understood. This is why they are often collectively referred to as “cancer coagulopathy” or “paraneoplastic syndrome” [2], as their manifestations are found at both the systemic level (deregulation of blood coagulation) and locally at the tumor site (crosslinked extravascular fibrin and fibrinogen) [10–13]. There are ample data suggesting that these respective changes are not merely an epiphenomenon of the disease, but rather represent an integral part of the pathobiology of tumor growth and dissemination [2,14–16]. In this regard, the interrelationship between cancer coagulopathy and the onset of tumor angiogenesis is of particular interest.
It is widely accepted that most primary tumors and metastatic lesions cannot grow beyond 2 to 3 mm in size in the absence of vascularization [17]. Regardless of whether the latter is secured by occasional “cooption” of preexisting capillaries [18] or by active recruitment of their new extensions (angiogenesis) [17,19,20], tumor-associated vasculature is essential not only to ensure continued metabolite and oxygen exchange but also as a source of important “paracrine stimulation” [21] through endothelial cell-derived extracellular matrix (ECM) [22], proteases [23] and cytokines, regulating tumor cell growth [24], survival [25], invasion [26], and metastasis [27].
It is now thought that the onset of tumor neovascularization (“angiogenic switch”) results from a shift in balance between angiogenesis stimulators and inhibitors released by both tumor parenchyma and “activated” host stromal cells [28–32]. Among the latter, stromal fibroblasts [33,34], mast cells [35], resident macrophages [36], blood-borne mononuclear leukocytes [15,37,38], and platelets [39,40] are considered the main sources of angiogenesis regulators.
Operationally, pro-angiogenic conditions may be triggered by a gain-of-function and/or a loss-of-function event [28,32,41]. In the former case, angiogenesis stimulators such as vascular endothelial growth factor (VEGF), members of the fibroblast growth factor family (e.g., bFGF, aFGF), hepatocyte growth factor (HGF), or other similarly acting entities are induced or upregulated in the tumor microenvironment, evoking responses of normally quiescent capillary endothelial cells [42,43]. Conversely, such pro-angiogenic state may result from downregulation of constitutively expressed angiogenesis inhibitors acting either locally (e.g., thrombospondin-1 and -2 (TSP-1, -2), maspin, brain-specific angiogenesis inhibitor 1 (BAI1), pigment epithelium-derived factor (PEDF), interferons α, β, and γ, Meth-1, -2) [32,44–47] or systemically (agiostatin, endostatin, vasostatin, aaATIII) [48–51]. The combined, cumulative impact of those various types of influences is believed to be responsible for initiation and maintenance of tumor angiogenesis and resulting escape from a dormant avascular state [28].
In the context of cancer, the imbalance between angiogenesis stimulators and inhibitors can be ultimately traced to direct or indirect influences exerted by genetic events underlying the disease progression [32,52,53]. Thus, loss-of-function mutations in several tumor suppressor genes (e.g., p53) can trigger changes in expression of both angiogenesis inhibitors (e.g., TSP-1, BAI-1) [32] and stimulators (e.g., VEGF) [54–56]. Likewise, gain-of-function genetic events, such as activation of dominant transforming oncogenes in tumor cells (e.g., ras, myc, EGFR, or HER-2), affect expression of multiple molecular effectors of angiogenesis [41]. In both cases, the impact of genetic lesions can be greatly amplified by epigenetic influences such as hypoxia [57–60], cell-cell contact [53,61], paracrine growth factors, and inflammatory cytokines present in the tumor microenvironment [53,62]. Factors produced by transformed cells can also attract, activate, and induce angiogenic phenotype in host stromal cells [35], thereby promoting the process in an “indirect manner.” It is interesting that in a similar fashion, consequences of cellular transformation may also participate in deregulation of the hemostatic system with resulting modulation of vascular responses [63]. In this regard, Rak and Klement [63] have recently proposed that transforming genetic changes can play a causative role in “cancer coagulopathy” by changing the expression of tissue factor (TF), proteases, VEGF, and other mediators. Such genetic influence would likely exert at least an “indirect” effect on tumor angiogenesis. While many scenarios can be envisaged how such genetically driven tumor angiogenesis-hemostasis crosstalk can be realized, it may be helpful to begin by considering some of the relevant consequences of the commonly observed upregulation of VEGF in tumors, an event for which a genetic cause is relatively well defined [41].
In 1979, Dvorak et al. [64] suggested that tumor neovascularization can be explained by apparent capillary hyperpermeability at the tumor site and resulting leakage of fibrinogen into extravascular spaces followed by formation of the pro-angiogenic fibrin matrix. He argued that it is the tumor vascular permeability factor (VPF), rather than then hypothetical tumor angiogenesis factor (TAF) [17,65], that causes formation of the intratumoral capillary network [64]. In 1983, VPF was biochemically identified [66] and in 1989 cloned and found to be identical with the newly discovered endothelial mitogen named VEGF [67,68]. VEGF/VPF (VEGF-A) is now known to be the central endothelial cell-specific growth factor, angiogenesis inducer, survival factor, and permeability regulator, the expression of which is upregulated in the vast majority of solid tumors and leukemias [69]. The corresponding gene (VEGF-A) contains a classical hypoxia-responsive element in its 5′ untranslated region and is normally under physiological control of the hypoxia-inducible factor 1 (HIF-1), a transcription factor regulated by an oxygen-sensing cellular mechanism [70]. However, it is now believed that upregulation of VEGF in many tumor cells is not solely a result of hypoxia, but rather is linked to genetic changes associated with malignant transformation [41]. Some of these changes (expression of activated src oncogene, loss of p53 tumor suppressor gene) act by mimicking the intracellular signals normally induced by hypoxic conditions [70], while others (e.g., mutational activation of ras oncogenes) can amplify the effect of hypoxia [57,62,70] or act in a constitutive manner to stimulate VEGF expression [71]. Once released from tumor cells, VEGF acts specifically on at least five types of endothelial cell receptors, namely, VEGFR-1/flt-1, VEGFR-2/flk-1/KDR, VEGFR-3/flt-4, and non-kinase co-receptors neuropilins-1 and 2 (NP-1, 2) [68,72–74]. The vascular permeability-inducing effects of VEGF/VPF, which are thought to promote fibrinogen extravasation and extravascular clotting, are known to be regulated by a concerted action of VEGFR-2, c-src kinase, focal adhesion kinase, and αvβ5 integrin [75,76]. While the case of VEGF is very instructive, it merely exemplifies the more extensive and complex tripartite interactions that, in different pathological contexts, may occur among tumor parenchyma, microvasculature, and the hemostatic system.
Angiogenic Activities of the Coagulation System
Several constituents of both intrinsic and extrinsic coagulation cascades have been found to possess overt or cryptic angiogenesis-regulating properties [40]. They include, e.g., angiogenesis inhibitory activity expressed by kininostatin, i.e., domain 5 of the high-molecular-weight kininogen (HK) [77], elastase/thrombin-cleaved fragment of antithrombin III [49], and possibly factor XIII [78]. Activities that may stimulate or modulate tumor angiogenesis are linked even more strongly with such key regulators of coagulation as TF, thrombin, and fibrinogen/fibrin, an issue which deserves a more thorough discussion.
Tissue Factor
TF is the principal initiator of the extrinsic pathway of coagulation traditionally recognized to play an important role in “cancer coagulopathy” [79,80]. Increased expression of TF has been observed in many tumor types, including small cell carcinoma, bronchoalveolar carcinoma and large cell carcinoma of the lung, colon adenocarcinoma, head and neck cancer, malignant gliomas, bladder cancer, and prostate cancer [10,81–85]. Tumor cells express TF constitutively [80,81] and possibly trigger production of TF by adjacent host cells, including monocytes and endothelial cells [86].
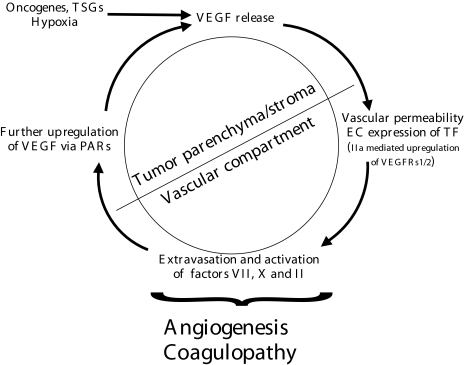
The functional linkage between TF and angiogenesis has been implicated by a number of studies, which invoke existence of several possible complementary mechanisms of action, such as: 1) generation of pericellular thrombin and pro-angiogenic thrombin signaling; 2) extravascular coagulation and formation of pro-angiogenic fibrin matrix; and 3) changes in expression of angiogenic growth factors by autonomous TF-mediated intracellular signals [85,87–93] (Figure 1).
Figure 1.
The “vicious circle” of coagulation and angiogenesis in cancer [63]. Tumor cells are thought to acquire angiogenic properties under the influence of transforming changes, i.e., expression of oncogenes and/or tumor suppressor genes (TSG). Resulting onset of VEGF production, along with imbalance in expression of other angiogenesis stimulators and inhibitors, triggers several endothelial cell responses, such as new blood vessel formation, expression of tissue factor (TF), and vascular permeability. This complex reaction allows extensive contact (and activation) of coagulation factors (VII, X, II) in plasma with pro-coagulant extravascular environment (tumor parenchyma, stroma, ECM) and TF-positive tumor endothelium. TF- and PARs-mediated intracellular signals induce further increase in expression of angiogenic factors (e.g., VEGF) by tumor cells. TF signaling also sensitizes endothelial cells to further VEGF stimulation (see text for details).
Studies involving enforced TF downregulation provide compelling evidence for the causative role of this transmembrane receptor protein in developmental and pathological blood vessel formation. Thus, mice harboring the homozygous TF null mutation die in utero with/of apparent abnormalities in their yolk sack vasculature [94,95]. Expression of the antisense TF mRNA in Meth-A sarcoma led to decreased transcription of the VEGF gene, increase in expression of TSP-1, and decline in tumor growth and vascularity [87]. This apparent interrelationship between expression of TF and VEGF is of particular interest because co-localization of these proteins has been documented in several instances, such as human melanoma cells grown as xenografts in immunodeficient mice, human breast cancer, human glioma, and human adenocarcinoma of the lung [83,88,96].
The expression of both TF [97,98] and VEGF [99–101] is controlled by hypoxia. Pentoxifylline treatment downregulates TF expression by monocytes and endothelial cells, and inhibits hypoxia-induced synthesis of both TF and VEGF by three different malignant cell lines [86]. However, while under low oxygen conditions, VEGF promoter responds mainly to HIF-1-induced stimulation [70,102]; TF expression is increased by a mechanism independent of HIF-1 activity, as it readily occurs in the absence of the β-subunit of HIF-1 [103]. Experiments with mice homozygous for a null mutation of the early growth response (Egr-1) gene indicated that this transcription factor is responsible for upregulation of TF in hypoxic lung tissue [97,98]. Interestingly, Egr-1 also appears to be responsible for VEGF-and TNF α-dependent upregulation of TF by endothelial cells [104]. TF is considered to be an early response gene, the expression of which is regulated by serum, growth factors, and cytokines [105].
Structurally, TF gene product is composed of three functionally distinct domains: namely, the extracellular — i.e., the factor VIIa — binding region, the transmembrane domain, and the short cytoplasmatic tail [106]. The relative contribution of these submolecular regions to VEGF upregulation and angiogenesis has been recently a subject of intensive experimental exploration, but also of some debate. Thus, re-expression of the extracellular domain alone was shown to rescue the TF-/- phenotype in mice and prevent both embryonal lethality and vascular defects [107]. This may indirectly imply that, if VEGF is the main mediator of the TF-dependent prenatal angiogenesis, the extracellular domain of TF may be both necessary and sufficient to control VEGF levels. Contrary to this result, Abe et al. [92] demonstrated that transfection of human melanoma cells with the cDNA encoding TF cytoplasmic domain alone resulted in elevated VEGF expression. Furthermore, the cytoplasmatic domain of TF has been recently implicated in triggering protein kinase C-dependent signalling, a mechanism known to mediate VEGF upregulation by a variety of stimuli [108–110]. Although intriguing in itself, this postulated exclusive role of the cytoplasmatic domain in proangiogenic, TF-dependent cellular signalling is probably an exception rather than the rule, if the following observations are taken collectively: 1) aforementioned “rescue” of the TF-/- phenotype in mice by expression of the extracellular TF domain; 2) dependence of TF-induced Ca2+ oscillations and changes in gene expression in several cell types upon binding of fVIIa (to the extracellular domain of TF) [109,111–113]; 3) activation of MAPK pathway, a major inducer of VEGF expression [53,114], by fVIIa/TF interactions [115,116]; 4) fVIIa/TF complex-dependent activation of phosphatidyl inositol 3′-OH kinase (PI3K) and members of src family of kinases, both involved in angiogenesis [117]; and 5) TF-mediated VEGF upregulation in human fibroblasts driven by activation of factor VII-associated procoagulant activity and resulting generation of factor Xa and thrombin [93,118].
The catalytic activity of the TF/VIIa complex triggers activation of factor X and thrombin, both of which can change expression of various genes (likely including angiogenesis regulators), notably through activation of so-called protease-activated receptors (PARs) on the cell surface [107]. This effect can be tamed by the action of the TF pathway inhibitor (TFPI), synthesized by vascular endothelial cells and macrophages [119]. TF can also form a quaternary complex with factor VIIa, factor Xa (fXa), and cell-bound TFPI — an interaction which results in cellular transport of TF to small plasmalemmal vesicles (caveolae), downregulation of its proteolytic activity, and placing it in the proximity of such elements of intracellular signaling as G-protein-coupled receptors, non-receptor tyrosine kinases, and the plasma membrane Ca2+ pumps [8,120–122]. The consequences of these latter events for angiogenesis remain to be elucidated.
Endothelial cells stimulated with VEGF upregulate the expression of TF on their surfaces [104,106,123] — an event likely to induce procoagulant conditions within the tumor microvasculature. This is consistent with the observation that TF is often upregulated on endothelial cells associated with VEGF-positive tumors [88]. It is tempting to speculate that the mutual interrelationship between VEGF and TF expression may constitute a centerpiece of the proangiogenic feedback loop in tumors (compare Figure 1). However, VEGF can also stimulate expression of the elements of the fibrinolytic cascade, including: plasminogen activators (PAs; u-PA and t-PA), urokinase receptor (u-PAR) [124], and plasminogen activator inhibitor 1 (PAI-1) [125]. Collectively, these are not merely uncontrolled changes in proteolytic activities of the host hemostatic and fibrinolytic systems, but rather manifestations of a new equilibrium that favors tumor vascularization invasion and metastasis [20,106,126].
Thrombin
Activation of TF-dependent blood coagulation leads to generation of thrombin, the presence of which has been detected in several tumor types (small cell lung cancer, renal cancer, malignant melanoma, ovarian cancer, laryngeal cancer, and gastric cancer [10–12,127–130]). Thrombin per se is believed to contribute to cancer progression [131,132] through increase in tumor cell adhesiveness, metastatic potential, and tumor cell-induced platelet aggregation [131]. Thrombin increases TF synthesis in different cells and thus facilitates activation of blood coagulation in a self-perpetuating fashion [86]. However, by activating tissue-type plasminogen-activator inhibitor-1 (PAI-1) and thrombin-activatable fibrinolysis inhibitor (TAFI) thrombin also inhibits fibrinolysis [133,134]. Thrombin is pro-migratory [135] and mitogenic for tumor cells, but is also known to amplify the effects of other mitogens [136,137].
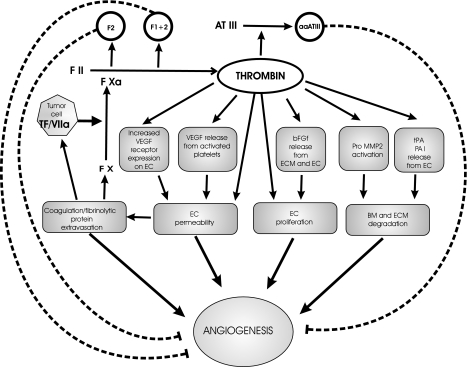
Thrombin is a potent inducer of various processes involved in angiogenesis (Figure 2) [96,138–144]. For example, its action can induce vascular permeability [133] via a VE-cadherin and protein-tyrosine phosphatase SHP2-dependent mechanism [145]. Using the chick chorion allantoic membrane (CAM) assay, Maragoudakis and Tsopanoglou [141] and Tsopanoglou et al. [143] demonstrated that thrombin directly promotes angiogenesis in vivo. This was executed by a proteolytic mechanism independent of fibrin formation because both α-thrombin (containing both the catalytic site and the anionic exosite) and γ-thrombin (catalytically active but lacking the anion-binding exosite required for clotting activity) were active in this model [143]. It is not clear whether the same activity is involved in spontaneous tubule formation by thrombin-treated endothelial cells cultured in Matrigel, an effect dependent on PKC activity [146]. Furthermore, thrombin was shown to be directly mitogenic for endothelial cells [147–149] owing to both proteolytic (i.e., thrombin receptor-dependent) and non-proteolytic (via B loop of the β-thrombin chain) signalling pathways [149].
Figure 2.
Modulation of angiogenesis by thrombin. Several facets of the angiogenic reaction can be influenced by thrombin in a direct or indirect manner, e.g., by formation of provisional fibrin matrix (see text). Proteolysis of prothrombin and antithrombin III gives rise to angiogenesis inhibitors (fragments 1 and 1.2, aaATIII, respectively). Thrombin-activated platelets release a number actual or potential angiogenesis effectors, including VEGF, PF4, TGFα and -β, or FGF. Various direct effects of thrombin on endothelial cells and other cell types that could be involved in the angiogenic process are likely mediated by signals generated from PARs (PAR-1, PAR-3, PAR-4) [247].
Thrombin exerts an indirect pro-angiogenic effect by upregulating VEGF expression in fibroblasts [118], as well as by increasing levels of VEGF receptors on vascular endothelium [142]. Both VEGFR-1/flt-1 and VEGFR-2/KDR/flk-1 were shown to be upregulated in thrombintreated HUVEC cells, through a PKC- and MAPK-dependent mechanism [142]. Thrombin has also been reported to increase the release of VEGF from platelets [150], expression of pro-angiogenic bFGF by endothelial cells [148], and release of bFGF from the ECM stores [151].
In endothelial cell cultures, thrombin causes release of tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1) [152], and activation of progelatinase A (MMP-2) [153]. It has also been proposed that thrombin may affect angiogenesis by promoting degradation of the angiogenesis inhibitor TSP-1 [154].
While active thrombin is, for the most part, believed to be pro-angiogenic, cryptic angiogenesis inhibitory domains do exist within various domains of prothrombin [40]. Thus, prothrombin kringle-2 domain (fragment 2) was shown to inhibit angiogenesis in the CAM assay [155]. Likewise, human prothrombin fragment 1+2 was documented to inhibit bFGF-induced bovine capillary endothelial cell growth and angiogenesis in the CAM [156].
In the presence of heparin, antithrombin III (AT-III) inhibits proteolytic action of 60th, thrombin and activated factor X (fXa) in plasma. Thrombin and neutrophil elastase can cleave the thrombin-binding site of AT-III, thereby generating the anti-angiogenic form of AT-III (aaAT-III), which inhibits bFGF- and VEGF-induced endothelial cell proliferation in vitro and in vivo [49].
Fibrinogen/Fibrin
One of the most important consequences of thrombin activation is the cleavage of plasma fibrinogen to fibrin [7]. Shortened half-life of the plasma fibrinogen and its increased turnover is often observed in cancer patients [7,157]. Tumors are frequently surrounded by fibrin(ogen) matrix also present throughout the tumor stroma (both fibrin I and II) [7,10–14,90,96,127–129,158]. This observation, in conjunction with the extravascular presence of prothrombin fragment 1+2, constitutes the evidence for activation of the extravascular coagulation in the tumor microenvironment [7,13].
Crosslinked fibrin is believed to provide a provisional matrix that promotes angiogenesis [7,96,158,159] by supporting endothelial cell adhesion, migration, and survival [158]. Fibrinogen interacts directly with integrin αvβ3 [160], an effect which likely provides endothelial cells with survival signals [161] and is mediated through β572–574 region of the human fibrinogen chain [162]. Also, factor XIII, which participates in fibrin crosslinking, can serve as a ligand for this anti-apoptotic integrin [78]. Stabilization and pro-angiogenic action of bFGF, and possibly other soluble growth factors, may be promoted by binding to fibrinogen and fibrin [163–166]. In vitro, fibrin induces expression of TF by HUVECs [167], expression of pro-angiogenic chemokine IL-8 in calf pulmonary artery endothelial cells [159], and expression of PAs and TSP-1 in corneal endothelial cells [167]. Likewise, fibrin degradation products — mainly fragment E — may promote angiogenesis [168]. It has been shown that fibrinopeptide B cleavage and exposure of the β15–42 region of the fibrin molecule by thrombin are necessary for various biological effects of fibrin such as stimulation of cellular mitogenesis (in both HUVECs and human skin fibroblasts) [169] and endothelial cell spreading [170]. However, it is noteworthy that in mice deficient for fibrinogen (Fib-/-), growth of B16 and LLC tumors after subcutaneous injection was found to be uninhibited, which may suggest that all the various functions ascribed to fibrin during tumor angiogenesis could be redundant [16].
Components of the Fibrinolysis System as Regulators of Tumor Angiogenesis
Numerous studies have demonstrated enhanced activity of u-PA in breast, gastric, colon, lung, prostate, and ovarian cancer, as well as in malignant melanoma and brain tumors, mostly in association with high grade, clinical malignancy, and poor prognosis [171–176]. High expression of t-PA in highly malignant gliomas and breast cancers often correlates with unfavorable prognosis as well [177,178]. Paradoxically, elevated PAI-1 also indicates poor prognosis in several types of cancers [171,176,177], a notion suggesting that change in balance, rather than utter deregulation and exuberance of the pericellular proteolysis, is compatible with aggressive tumor growth and angiogenesis [126].
Proangiogenic growth factors, such as VEGF and bFGF, are known to increase the expression of PAs, their receptors (e.g., u-PAR), and PAI-1 by endothelial cells [124,125, 179–181]. VEGF and bFGF synergize in stimulating both angiogenesis [182,183] and expression of u-PA and its receptor, u-PAR, in bovine adrenal cortex-derived microvascular endothelial cells [184]. These results are consistent with co-expression of VEGF and tPA transcripts [185] and co-localization of t-PA, u-PA, PAI-1, and VEGF in rat gliomas [186]. Moreover, co-expression of VEGF and u-PA was found in human colorectal cancer [187].
The ability of endothelial cells to express fibrinolytic activity is largely attributed to plasminogen and PAs: t-PA and u-PA. PAs convert a zymogen-plasminogen to plasmin, an enzyme capable of degrading ECM both directly and indirectly (e.g., through the activation of latent matrix metalloproteinases) [152,188]. Regulation of u-PA activity involves binding to the cell surface receptor (u-PAR) [124]. The single-chain proenzyme u-PA (sc-u-PA) can be activated more efficiently in the context of the u-PAR. Active two-chain u-PA is quickly inhibited by PAI-1. The u-PA/PAI-1 complex is internalized together with u-PAR and degraded in lysosomes, after which u-PAR is recycled to the cell surface (see Refs. [189,190] for review). The role of u-PA/u-PAR system in tumor progression is supported by the observation that expression of u-PAR is localized to the leading front of migrating monocytes and invading tumor cells [124] and that tumor growth and metastasis can be blocked by anti-u-PA antibodies [191].
Plasmin and PAs have been implicated in angiogenesis-promoting phenomenon of “pericellular fibrinolysis” [192]. This process, which facilitates endothelial cells invasion into the fibrin matrix, can be antagonized by plasmin inhibitors, α2-antiplasmin and α2-macroglobulin [192]. Recent study suggests, however, that PA/plasminogen system is not essential for endothelial cell penetration into fibrin gels because such function could be performed by the membrane type-1 matrix metalloproteinase [193]. Again, it should be born in mind that both inhibition of the PA/Plg/MMP-dependent proteolysis and its supraoptimal stimulation (in PAI-1 deficiency) are incompatible with effective angiogenesis [106,126,194].
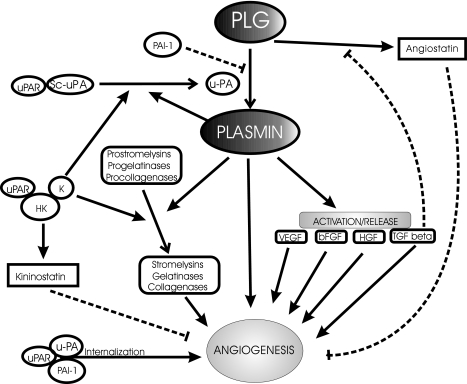
PA system acts in a complex and pleiotropic fashion so that it can simultaneously influence, in many ways, endothelial cell adhesion, proteolysis, and signaling (Figure 3). Cell surface u-PAR binds to vitronectin in a PAI-1 inhibitable manner — an interaction which could clearly promote cell adhesion and angiogenesis [195]. Moreover, domain 2/3 of u-PAR expressed on endothelial cells serves as a receptor for high-molecular-weight kininogen (HK) and this binding is, in turn, inhibited by interaction with vitronectin [196]. Formation of the HK/kallikrein/u-PAR complex can further promote fibrinolytic activity and possibly angiogenic competence of endothelial cells [196]. This action can be antagonized by domain 5 of HK (kininostatin), which is known to downregulate endothelial cell proliferation and migration and thereby inhibit angiogenesis [77]. In a recent study, two-chain form of HK (HKa), HK domain 6, or corresponding peptides, but not the intact HK, were shown to directly bind to and inhibit endothelial cell proliferation, survival, and angiogenesis in a manner independent of u-PAR or vitronectin binding [197]. The latter effects should not be viewed in isolation from the proangiogenic effects exerted by the entire kinin system. Thus, release of bradykinin from HK and enforced overexpression of kallikrein were both found to be pro-angiogenic under certain conditions [198–200]. Moreover, interaction between HK and sc-u-PA with or without factor XII contribution may lead to plasmin generation on endothelial cell surfaces [201] and further barrage of complex proteolytic influences on the progress of angiogenesis. Finally, binding of u-PA to its receptor triggers intracellular signals [202], which could modulate angiogenesis in a proteolysis-dependent or -independent manner [189].
Figure 3.
Pro-and anti-angiogenic activities encoded within the fibrinolytic system. A multitude of effects on angiogenesis has been attributed to various effectors of fibrinolysis. Additional detailed studies are warranted on some of the more recently uncovered modulators of this system, e.g., TAFI [248] or endostatin [221]. The view emerging from “gene knockout” studies is that both excessive activation (PAI-1 deficiency) as well as excessive inhibition of fibrinolysis (PA deficiency) are incompatible with effective angiogenic response [20].
Another activity associated with the PA system, which is relevant to angiogenesis regulation, is related to the proteolytic processing of soluble growth factors and cytokines. For example, u-PA can activate pro-HGF, while plasmin cleaves bFGF, activates latent TGFβ (transforming growth factor β) [203,204], and can liberate the membrane-bound isoforms of VEGF (VEGF189) [68]. HGF is known to possess intrinsic pro-angiogenic properties, but also the ability to stimulate VEGF production [205,206], and to promote motility and cellular invasiveness. TGFβ, while growth-inhibitory for endothelial cells in vitro, is thought to promote angiogenesis in vivo by a variety of mechanisms, such as regulation of ECM formation and proteolysis [171], participation in blood vessel maturation [20,207,208], stimulation of VEGF release [209], and inhibition of angiostatin generation [210].
Discovery of angiostatin is one of the most spectacular events that illustrate the link between the PA system and regulation of angiogenesis [211]. Angiostatin, a 38-kDa cleavage product of mature plasminogen, made up of the first four of the five highly homologous kringle domains, has been detected in tumor-bearing mice as a systemically acting, circulating angiogenesis inhibitor [211]. At least two sources of proteases, i.e., matrix metalloelastases (MME) released from tumor-infiltrating macrophages and serine proteases produced directly by tumor cells, catalyze plasminogen conversion to angiostatin or its isoforms [212]. Several such isoforms have been identified. For example, the 52-kDa isoform of angiostatin containing kringles 1 through 4 and a portion of kringle 5 — so-called “angiostatin 4.5” (apparently generated by plasmin) — was detected in brain tumors and in malignant ovarian ascites [213]. Another 55-kDa angiogenesis inhibitor was obtained by digestion of plasminogen with urokinase-activated plasmin, and contains intact kringles 1–4 and most of the kringle 5 (denoted as K1–5) [214]. In this case, the endothelial cell-specific inhibitory effect appears to be 50-fold more potent than that of angiostatin [214]. Also kringle 5 of plasminogen, in itself, exerts a negative effect on angiogenesis [212]. Recently, a recombinant protein composed of kringles 1–3 (rPK1–3) has been reported to inhibit growth of human glioma xenografts in nude mice [215]. Several studies confirmed the anti-angiogenic effect of angiostatin or its derivatives in various systems [216], albeit to a different degree and with various modifications of the drug delivery. Also, gene therapy approaches utilizing liposomes complexed to plasmids encoding angiostatin were found to be effective against breast cancer in nude mice [217]. Interestingly, radiation therapy combined with angiostatin treatment can, in some cases, act synergistically to inhibit expansion of the tumor vasculature [218].
The molecular mechanism, by which angiostatin inhibits angiogenesis, remains unclear. In this regard, it is known that angiostatin binds to the α/β-subunits of the ATP synthase on the surface of endothelial cells, potentially inducing H+ cytoplasmic influx and cytolysis [219]. Furthermore, angiostatin may inhibit endothelial cell invasion via complex formation with t-PA and resulting blockade of the PA/plasminogen system [220]. In this context, it is thought-provoking that another inhibitor of angiogenesis, endostatin, apparently acts through an opposite mechanism, i.e., by stimulation of plasmin generation and removal of proangiogenic fibrin matrix [221]. Clearly, further studies are warranted to elucidate and predict the net effect of the various fibrinolytic and procoagulant proteases implicated in angiogenesis.
The Role of Platelets in Tumor Angiogenesis
Analysis of platelets in cancer patients often reveals quantitative and qualitative abnormalities [39,222,223]. Among those, thrombocytosis; increased, reduced, or spontaneous aggregation; impaired adhesion; and hypersensitivity to different agonists are the most frequently cited examples [39,223]. Various types of tumor cells can activate platelets in vitro by virtue of direct contact, release of ADP, production of thromboxane A2 or cancer procoagulant, generation of thrombin, or activation of the tumor-associated proteinases [223]. In the presence of VEGF, endothelial cells promote platelet activation [224]. Adhesion and aggregation of activated platelets are accompanied by the release (mainly from α-granules) of many potential angiogenesis regulators, such as: VEGF-A [145], VEGF-C [225], bFGF [226], HGF [227], insulin-like growth factor-1 and -2 [228,229], epidermal growth factor [230], and platelet-derived endothelial cell growth factor [231]. These sorts of observations led Pinedo et al. [39] to hypothesize that platelets may play an active and causative role in tumor angiogenesis.
Platelets are the source of angiogenesis stimulators, but also of angiogenesis inhibitors (Table 1) [39,40,232]. With regard to the latter, platelet factor-4 (PF4) was the first hemostatic protein demonstrated to be an inhibitor of angiogenesis in vivo [40]. PF4 interacts with surface heparin-like glycosaminoglycans on endothelial cells, thereby blocking the binding sites for heparin-binding endothelial growth factors [233–235]. PF4 also directly inhibits bFGF dimerization and activity [236]. In addition, PF4 inhibits the endothelial stimulatory activity of bFGF, EGF, and VEGF121 by a mechanism independent of their interactions with heparin sulfate proteoglycans [235]. This is consistent with the observation that the analogue of PF4, which lacks the heparin-binding capacity (rPF4-241), is also able to inhibit tumor angiogenesis [237]. This notion was explored further by using various peptides derived from PF4 [238]. Thus, the peptide derived from the C-terminus of PF4 (residues 47–70) interferes with biological functions of both bFGF and VEGF. It is puzzling that a peptide derived from the central PF4 region (17–58), containing a potential heparin-binding domain, apparently does not interfere with endothelial cell responses to these respective angiogenic ligands (i.e., bFGF or VEGF). However, this peptide inhibits heparin-dependent interactions of these growth factors with their high-affinity receptors, when these receptors are expressed “ectopically”, i.e., in non-endothelial cells (e.g., CHO cells) [239]. More recently, gene therapy approaches have been developed to use modified PF-4 as an anti-tumor agent [240].
Table 1.
Examples of Platelet-Derived Angiogenesis Stimulators and Inhibitors.
| Platelet-Derived Angiogenesis | Stimulators Platelet-Derived Angiogenesis Inhibitors |
| VEGF-A [150,225], VEGF-C [225], bFGF [226], HGF/SF [227], IGF-1 [228], IGF-2 [229], EGF [230], PD-ECGF [249], Ang-1 [250] | PF-4 and PF-4 fragments [235,236,238], HGF domains NK1 and NK2 [245,251–253], TSP-1 [241,254,255] |
TSP-1, a potent angiogenesis inhibitor, is also a constituent of platelet α granules [241]. The central stalk of TSP-1 interacts with CD36 receptor on the surface of endothelial cells, resulting in angiogenesis inhibition [241,242]. However, very high concentrations of TSP-1 can also stimulate angiogenesis via interaction with the integrin-activating protein IAP/CD37 [241]. TSP-1 is also involved in activation of the latent TGF-β, thereby triggering angiogenesis-regulating effects of this cytokine [243]. Platelet-derived HGF can serve as another example of this “one-mediator-two-functions” paradigm. While essentially pro-angiogenic, HGF contains cryptic anti-angiogenic subdomains in its α-chain [40,244]. Thus, alternative splicing of the HGF mRNA can result in expression of the first kringle domain inhibitor (NK1) or first two kringle domain (NK2) inhibitor, both of which suppress HGF-induced angiogenesis [233], as does the recombinant HGF/NK4 variant [245].
Activated platelets stimulate endothelial cells to express TF [246] and to form tubular networks in Matrigel, an effect apparently independent of platelet aggregation or release processes [232]. It was argued that adhesion of platelets to endothelium via surface glycoproteins may be responsible for this morphogenetic response [232]. Collectively, as with other components of the hemostatic system, the role of platelets in various aspects, stages, and forms of tumor angiogenesis is complex and not fully amenable to simple generalizations and predictions.
Summary
Processes regulating vascular expansion, homeostasis and blood clotting intersect at many critical points. In the context of cancer, this anatomical and functional mutual interdependence is manifested by consistent co-incidence of “cancer coagulopathy” and activation of tumor angiogenesis. It is an open question to what extent one is the cause or the consequence of the other. Nevertheless, the explosion of findings implicating various hemostatic mechanisms in tumor growth and neovascularization is a foundation of at least two novel therapeutic approaches to treat cancer, namely: 1) derivation of hemostatic proteins as angiogenesis inhibitors, and 2) using antithrombotic pharmacotherapy (e.g., heparin or low-molecular-weight heparin) to control blood vessel formation. However, the great complexity of the hemostatic system and the multitude of pro- and anti-angiogenic activities it encodes (even within individual constituent proteins) suggest that for each therapeutic action, a proper clinical context should be precisely defined and validated. In this regard, we have previously proposed that both the molecular nature of “cancer coagulopathy” [63] and the operating angiogenic mechanism [21] likely vary as a function of tumor progression (angiogenesis progression) as well as genetic and epigenetic variables characteristic of each tumor type. We believe that it is important to consider such tumor specific, heterogenous and evolving nature of these respective host responses (i.e., coagulopathy and angiogenesis), while designing anti-angiogenic cancer therapies based on interference with the hemostatic system.
Acknowledgements
We thank our families for their support and many of our colleagues for helpful discussions.
Footnotes
This work was supported by the Terry Fox grant from the National Cancer Institute of Canada and the HCHRC grant to J.R., and by the grant 6 P05A 096 21 from the Polish Committee of Scientific Research (KBN) to M.W.
References
- 1.Trousseau A. Phlegmasia alba dolens. Clinique Medicale de l′Hotel-Dieu de Paris: The Sydenham Society; 1865. pp. 94–96. [Google Scholar]
- 2.Rickles FR, Levine M, Edwards RL. Hemostatic alterations in cancer patients. Cancer Metastasis Rev. 1992;11:237–248. doi: 10.1007/BF01307180. [DOI] [PubMed] [Google Scholar]
- 3.Bick RL. Coagulation abnormalities in malignancy. Semin Thromb Hemostasis. 1992;18:353–372. doi: 10.1055/s-2007-1002575. [DOI] [PubMed] [Google Scholar]
- 4.Luzzato G, Schafer AI. The prethrombotic state in cancer. Semin Oncol. 1990;17:147–159. [PubMed] [Google Scholar]
- 5.Nand S. Hemostasis and cancer. Cancer J. 1993;6:54–58. [Google Scholar]
- 6.Donati MB. Cancer and thrombosis. Haemostasis. 1994;24:54–58. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak FH. Abnormalities of hemostasis in malignant disease. In: Coleman RB, Hirsh J, Marder VJ, Salzman JB, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia: Lippincott; 1994. pp. 1238–1254. [Google Scholar]
- 8.Ruf W, Mueller BM. Tissue factor in cancer angiogenesis and metastasis. Curr Opin Hematol. 1996;3:379–384. doi: 10.1097/00062752-199603050-00008. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan R, DeLa Cadena RA. Mechanism of the coagulopathy associated with acute promyelocytic leukemia — clinical conference. Am J Hematol. 1998;59:234–237. doi: 10.1002/(sici)1096-8652(199811)59:3<234::aid-ajh9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Wojtukiewicz MZ, Zacharski LR, Memoli VA, Kisiel W, Kudryk BJ, Rousseau SM, Stump DC. Abnormal regulation of coagulation/fibrinolysis in small cell carcinoma of the lung. Cancer. 1990;65:481–485. doi: 10.1002/1097-0142(19900201)65:3<481::aid-cncr2820650318>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Wojtukiewicz MZ, Zacharski LR, Memoli VA, Kisiel W, Kudryk BJ, Rousseau SM, Stump DC. Malignant melanoma. Interaction with coagulation and fibrinolysis pathways in situ. Am J Clin Pathol. 1990;93:516–521. doi: 10.1093/ajcp/93.4.516. [DOI] [PubMed] [Google Scholar]
- 12.Zacharski LR, Memoli VA, Ornstein DL, Rousseau SM, Kisiel W, Kudryk BJ. Tumor cell procoagulant and urokinase expression in carcinoma of the ovary. J Natl Cancer Inst. 1993;85:1225–1230. doi: 10.1093/jnci/85.15.1225. [DOI] [PubMed] [Google Scholar]
- 13.Wojtukiewicz MZ, Rucinska M, Zimnoch L, Jaromin J, Piotrowski Z, Rozanska-Kudelska M, Kisiel W, Kudryk BJ. Expression of prothrombin fragment 1+2 in cancer tissue as an indicator of local activation of blood coagulation. Thromb Res. 2000;97:335–342. doi: 10.1016/s0049-3848(99)00169-3. [DOI] [PubMed] [Google Scholar]
- 14.Dvorak HF. Tumors: wounds that do not heal. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 15.Senger DR. Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines [comment] Am J Pathol. 1996;149:1–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 17.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 18.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 21.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumor cells and endothelial cells. The “angiogenesis progression” hypothesis. Eur J Cancer. 1996;32A:2438–2450. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- 22.Nicosia RF, Tchao R, Leighton J. Angiogenesis-dependent tumor spread in reinforced fibrin clot culture. Cancer Res. 1983;43:2159–2166. [PubMed] [Google Scholar]
- 23.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Anti-integrin αbβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rak JW, Hegmann EJ, Lu C, Kerbel RS. Progressive loss of sensitivity to endothelium-derived growth inhibitors expressed by human melanoma cells during disease progression. J Cell Physiol. 1994;159:245–255. doi: 10.1002/jcp.1041590208. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Nicolson GL, Fidler IJ. Direct in vitro lysis of metastatic tumor cells by cytoline-activated murine vascular endothelial cells. Cancer Res. 1991;51:245–254. [PubMed] [Google Scholar]
- 26.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 27.Hamada J, Cavanaugh PG, Miki K, Nicolson GL. A paracrine migration-stimulating factor for metastatic tumor cells secreted by mouse hepatic sinusoidal endothelial cells: identification as complement component C3b. Cancer Res. 1993;53:4418–4423. [PubMed] [Google Scholar]
- 28.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 29.Folkman J. What is the evidence that tumors are angiogenesis-dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 30.Plate KH, Breier G, Risau W. Molecular mechanism of developmental and tumor angiogenesis. Brain Pathol. 1994;4:207–221. doi: 10.1111/j.1750-3639.1994.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 32.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 33.Hlatky L, Tsionou C, Hahnfeldt P, Coleman CN. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994;54:6083–6086. [PubMed] [Google Scholar]
- 34.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 35.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polverini PJ, Cotran RS, Gimbrone MA, Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- 37.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58(4):313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 39.Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 40.Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chem. 2000;275:1521–1524. doi: 10.1074/jbc.275.3.1521. [DOI] [PubMed] [Google Scholar]
- 41.Rak J, Yu JL, Klement G, Kerbel RS. Oncogenes and angiogenesis: signaling three-dimensional tumor growth [In Process Citation] J Invest Dermatol Symp Proc. 2000;5:24–33. doi: 10.1046/j.1087-0024.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 42.Denekamp J. Endothelial cell proliferation as a novel approach to targeting tumor therapy. Br J Cancer. 1982;45:136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999;59:4770–4775. [PubMed] [Google Scholar]
- 44.Iruela-Arispe ML, Vazquez F, Ortega MA. Antiangiogenic domains shared by thrombospondins and metallospondins, a new family of angiogenic inhibitors. Ann NY Acad Sci. 1999;886:58–66. doi: 10.1111/j.1749-6632.1999.tb09400.x. [DOI] [PubMed] [Google Scholar]
- 45.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 46.Schmid EF, Binder K, Grell M, Scheurich P, Pfizenmaier K. Both tumor necrosis factor receptors, TNFR60 and TNFR80, are involved in signaling endothelial tissue factor expression by juxtacrine tumor necrosis factor alpha. Blood. 1995;86:1836–1841. [PubMed] [Google Scholar]
- 47.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 48.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 49.O'Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin [see comments] Science. 1999;285:1926–1928. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 50.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 51.Pike SE, Yao L, Jones KD, Cherney B, Appella E, Sakaguchi K, Nakhasi H, Teruya-Feldstein J, Wirth P, Gupta G, Tosato G. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J Exp Med. 1998;188:2349–2356. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel RS. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- 53.Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, Mansour SJ, Ahn NG, Kerbel RS. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490–498. [PubMed] [Google Scholar]
- 54.Kieser A, Weich HA, Brandner G, Marme D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene. 1994;9:963–969. [PubMed] [Google Scholar]
- 55.Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996;56:2299–2301. [PubMed] [Google Scholar]
- 56.Harada H, Nakagawa K, Iwata S, Saito M, Kumon Y, Sakaki S, Sato K, Hamada K. Restoration of wild-type p16 down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human gliomas. Cancer Res. 1999;59:3783–3789. [PubMed] [Google Scholar]
- 57.Mazure NM, Chen EY, Yeh P, Laderoute KR, Giaccia AJ. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996;56:3436–3440. [PubMed] [Google Scholar]
- 58.Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- 59.Laderoute KR, Alarcon RM, Brody MD, Calaoagan JM, Chen EY, Knapp M, Yun Z, Denko NC, Giaccia AJ. Opposing effects of hypoxia on expression of the angiogenic inhibitor thrombospondin 1 and the angiogenic inducer vascular endothelial growth factor. Clin Cancer Res. 2000;6:2941–2950. [PubMed] [Google Scholar]
- 60.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers HR, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koura AN, Liu W, Kitadai Y, Singh RK, Radinsky R, Ellis LM. Regulation of vascular endothelial growth factor expression in human colon carcinoma cells by cell density. Cancer Res. 1996;56:3891–3894. [PubMed] [Google Scholar]
- 62.Viloria-Petit AM, Rak J, Hung M-C, Rockwell P, Goldstein N, Kerbel RS. Neutralizing antibodies against EGF and ErbB-2/neu receptor tyrosine kinases down-regulate VEGF production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 63.Rak J, Klement G. Impact of oncogenes and tumor suppressor genes on deregulation of hemostasis and angiogenesis in cancer. Cancer Metastasis Rev. 2000;19:93–96. doi: 10.1023/a:1026516920119. [DOI] [PubMed] [Google Scholar]
- 64.Dvorak HF, Dvorak AM, Manseau EJ, Wiberg L, Churchill WH. Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J Natl Cancer Inst. 1979;62:1459–1472. [PubMed] [Google Scholar]
- 65.Folkman J. Tumor angiogenesis factor. Cancer Res. 1974;34:2109–2113. [PubMed] [Google Scholar]
- 66.Senger DR, Galli S, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 67.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 69.Brown LF, Detmar M, Claffey KP, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. In: Goldberg ID&REM, editor. Regulation of Angiogenesis. Basel, Switzerland: Birkhauser Verlag; 1997. pp. 233–269. [Google Scholar]
- 70.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 71.Okada F, Rak J, St. Croix B, Lieubeau B, Kaya M, Roncari L, Sasazuki S, Kerbel RS. Impact of oncogenes on tumor angiogenesis: mutant K-ras upregulation of VEGF/VPF is necessary but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 73.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 74.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 75.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RI, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7(2):222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 76.Cheresh DA. Src kinase regulation of VEGF signaling and vascular permeability following cerebral ischemia. Keystone Symposia Abstract Book Keystone Symposium X1, 4/24–29/2001. 2001:28. [Angiogenesis and Chronic Disease] [Google Scholar]
- 77.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) downregulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–550. [PubMed] [Google Scholar]
- 78.Dallabrida SM, Falls LA, Farrell DH. Factor XIIIa supports microvascular endothelial cell adhesion and inhibits capillary tube formation in fibrin. Blood. 2000;95:2586–2592. [PubMed] [Google Scholar]
- 79.Gordon SG. Cancer cell procoagulants and their role in malignant disease. Semin Thromb Hemostasis. 1992;18:424–433. doi: 10.1055/s-2007-1002580. [DOI] [PubMed] [Google Scholar]
- 80.Rao LV. Tissue factor as a tumor procoagulant. Cancer Metastasis Rev. 1992;11:249–266. doi: 10.1007/BF01307181. [DOI] [PubMed] [Google Scholar]
- 81.Callander NS, Varki N, Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70:1194–1201. doi: 10.1002/1097-0142(19920901)70:5<1194::aid-cncr2820700528>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 82.Wojtukiewicz MZ, Zimnoch L, Kloczko J, Bielawiec M, Jaromin J, Dib AH, Lewko J, Mariak Z. Heterogenous expression of endothelial cell associated proteins in gliomas of different malignancy. In: Messmer K, Kubler WM, editors. Proceedings of the 6th World Congress for Microcirculation; Monduzzi Editore; Bologna, Italy. 1996. pp. 1007–1010. [Google Scholar]
- 83.Takano S, Tsuboi K, Tomono Y, Mitsui Y, Nose T. Tissue factor, osteopontin, alphavbeta3 integrin expression in microvasculature of gliomas associated with vascular endothelial growth factor expression. Br J Cancer. 2000;82:1967–1973. doi: 10.1054/bjoc.2000.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wojtukiewicz MZ, Zacharski LR, Rucinska M, Zimnoch L, Jaromin J, Rozanska-Kudelska M, Kisiel W, Kudryk BJ. Expression of tissue factor and tissue factor pathway inhibitor in situ in laryngeal carcinoma. Thromb Haemostasis. 1999;82:1659–1662. [PubMed] [Google Scholar]
- 85.Abdulkadir SA, Carvalhal GF, Kaleem Z, Kisiel W, Humphrey PA, Catalona WJ, Milbrandt J. Tissue factor expression and angiogenesis in human prostate carcinoma [see comments] Hum Pathol. 2000;31:443–447. doi: 10.1053/hp.2000.6547. [DOI] [PubMed] [Google Scholar]
- 86.Zacharski LR, Wojtukiewicz MZ, Costantini V, Ornstein DL, Memoli VA. Pathways of coagulation/fibrinolysis activation in malignancy. Semin Thromb Hemostasis. 1992;18:104–116. doi: 10.1055/s-2007-1002415. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, Stern DM, Nawroth PP. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease [see comments] Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 89.Folkman J. Tumor angiogenesis and tissue factor. Nat Med. 1996;2:167–168. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 90.Shoji M, Abe K, Nawroth PP, Rickles FR. Molecular mechanisms linking thrombosis and angiogenesis in cancer. Trends Cardiovasc Med. 1997;7:52–59. doi: 10.1016/S1050-1738(96)00142-9. [DOI] [PubMed] [Google Scholar]
- 91.Amirkhosravi A, Meyer T, Warnes G, Amaya M, Malik Z, Biggerstaff JP, Siddiqui FA, Sherman P, Francis JL. Pentoxifylline inhibits hypoxia-induced upregulation of tumor cell tissue factor and vascular endothelial growth factor. Thromb Haemostasis. 1998;80:598–602. [PubMed] [Google Scholar]
- 92.Abe K, Shoji M, Chen J, Bierhaus A, Danave I, Micko C, Casper K, Dillehay DL, Nawroth PP, Rickles FR. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc Natl Acad Sci USA. 1999;96:8663–8668. doi: 10.1073/pnas.96.15.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ollivier V, Bentolila S, Chabbat J, Hakim J, de Prost D. Tissue factor-dependent vascular endothelial growth factor production by human fibroblasts in response to activated factor VII. Blood. 1998;91:2698–2703. [PubMed] [Google Scholar]
- 94.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van V, I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 95.Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MJ, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shoji M, Hancock WW, Abe K, Micko C, Casper KA, Baine RM, Wilcox JN, Danave I, Dillehay DL, Matthews E, Contrino J, Morrissey JH, Gordon S, Edgington TS, Kudryk B, Kreutzer DL, Rickles FR. Activation of coagulation and angiogenesis in cancer: immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152:399–411. [PMC free article] [PubMed] [Google Scholar]
- 97.Yan SF, Zou YS, Gao Y, Zhai C, Mackman N, Lee SL, Milbrandt J, Pinsky D, Kisiel W, Stern D. Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA. 1998;95:8298–8303. doi: 10.1073/pnas.95.14.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan SF, Lu J, Zou YS, Kisiel W, Mackman N, Leitges M, Steinberg S, Pinsky D, Stern D. Protein kinase C-beta and oxygen deprivation. A novel Egr-1-dependent pathway for fibrin deposition in hypoxemic vasculature. J Biol Chem. 2000;275:11921–11928. doi: 10.1074/jbc.275.16.11921. [DOI] [PubMed] [Google Scholar]
- 99.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 100.Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- 101.Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269:4355–4359. [PubMed] [Google Scholar]
- 102.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 103.O'Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ. Identification of hypoxically inducible mRNAs in HeLa cells using differential display PCR. Role of hypoxia-inducible factor-1. Eur J Biochem. 1996;241:403–410. doi: 10.1111/j.1432-1033.1996.00403.x. [DOI] [PubMed] [Google Scholar]
- 104.Mechtcheriakova D, Wlachos A, Holzmuller H, Binder BR, Hofer E. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood. 1999;93:3811–3823. [PubMed] [Google Scholar]
- 105.Mackman N. Regulation of tissue factor gene expression in human monocytic and endothelial cells. Haemostasis. 1996;26(Suppl 1):17–9. doi: 10.1159/000217234. [DOI] [PubMed] [Google Scholar]
- 106.Carmeliet P, Moons L, Dewerchin M, Mackman N, Luther T, Breier G, Ploplis V, Muller M, Nagy A, Plow E, Gerard R, Edgington T, Risau W, Collen D. Insights in vessel development and vascular disorders using targeted inactivation and transfer of vascular endothelial growth factor, the tissue factor receptor, and the plasminogen system. Ann NY Acad Sci. 1997;811:191–206. doi: 10.1111/j.1749-6632.1997.tb52002.x. [DOI] [PubMed] [Google Scholar]
- 107.Parry GC, Mackman N. Mouse embryogenesis requires the tissue factor extracellular domain but not the cytoplasmic domain. J Clin Invest. 2000;105:1547–1554. doi: 10.1172/JCI9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zioncheck TF, Roy S, Vehar GA. The cytoplasmic domain of tissue factor is phosphorylated by a protein kinase C-dependent mechanism. J Biol Chem. 1992;267:3561–3564. [PubMed] [Google Scholar]
- 109.Rottingen JA, Enden T, Camerer E, Iversen JG, Prydz H. Binding of human factor VIIa to tissue factor induces cytosolic Ca2+ signals in J82 cells, transfected COS-1 cells, Madin-Darby canine kidney cells and in human endothelial cells induced to synthesize tissue factor. J Biol Chem. 1995;270:4650–4660. doi: 10.1074/jbc.270.9.4650. [DOI] [PubMed] [Google Scholar]
- 110.Camerer E, Rottingen JA, Iversen JG, Prydz H. Coagulation factors VII and X induce Ca2+ oscillations in Madin-Darby canine kidney cells only when proteolytically active. J Biol Chem. 1996;271:29034–29042. doi: 10.1074/jbc.271.46.29034. [DOI] [PubMed] [Google Scholar]
- 111.Camerer E, Gjernes E, Wiiger M, Pringle S, Prydz H. Binding of factor VIIa to tissue factor on keratinocytes induces gene expression. J Biol Chem. 2000;275:6580–6585. doi: 10.1074/jbc.275.9.6580. [DOI] [PubMed] [Google Scholar]
- 112.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prydz H, Camerer E, Rottingen JA, Wiiger MT, Gjernes E. Cellular consequences of the initiation of blood coagulation. Thromb Haemostasis. 1999;82:183–192. [PubMed] [Google Scholar]
- 114.Milanini J, Vinals F, Pouyssegur J, Pages G. p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J Biol Chem 273, 1998:18165–18172. doi: 10.1074/jbc.273.29.18165. [DOI] [PubMed] [Google Scholar]
- 115.Poulsen LK, Jacobsen N, Sorensen BB, Bergenhem NC, Kelly JD, Foster DC, Thastrup O, Ezban M, Petersen LC. Signal transduction via the mitogen-activated protein kinase pathway induced by binding of coagulation factor VIIa to tissue factor. J Biol Chem. 1998;273:6228–6232. doi: 10.1074/jbc.273.11.6228. [DOI] [PubMed] [Google Scholar]
- 116.Sorensen BB, Freskgard PO, Nielsen LS, Rao LV, Ezban M, Petersen LC. Factor VIIa-induced p44/42 mitogen-activated protein kinase activation requires the proteolytic activity of factor VIIa and is independent of the tissue factor cytoplasmic domain. J Biol Chem. 1999;274:21349–21354. doi: 10.1074/jbc.274.30.21349. [DOI] [PubMed] [Google Scholar]
- 117.Versteeg HH, Hoedemaeker I, Diks SH, Stam JC, Spaargaren M, van Bergen En Henegouwen PM, van Deventer SJ, Peppelenbosch MP. Factor VIIa/tissue factor-induced signaling via activation of Src-like kinases, phosphatidylinositol 3-kinase, and Rac. J Biol Chem. 2000;275:28750–28756. doi: 10.1074/jbc.M907635199. [DOI] [PubMed] [Google Scholar]
- 118.Ollivier V, Chabbat J, Herbert JM, Hakim J, de Prost D. Vascular endothelial growth factor production by fibroblasts in response to factor VIIa binding to tissue factor involves thrombin and factor Xa. Arterioscler Thromb Vasc Biol. 2000;20:1374–1381. doi: 10.1161/01.atv.20.5.1374. [DOI] [PubMed] [Google Scholar]
- 119.Novotny WF. Tissue factor pathway inhibitor. Semin Thromb Hemostasis. 1994;20:101–108. doi: 10.1055/s-2007-1001894. [DOI] [PubMed] [Google Scholar]
- 120.Sevinsky JR, Rao LV, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruf W, Fischer EG, Huang HY, Miyagi Y, Ott I, Riewald M, Mueller BM. Diverse functions of protease receptor tissue factor in inflammation and metastasis. Immunol Res. 2000;21:289–292. doi: 10.1385/IR:36:1:289. [DOI] [PubMed] [Google Scholar]
- 122.Ruf W, Mueller BM. Tissue factor signaling. Thromb Haemostasis. 1999;82:175–182. [PubMed] [Google Scholar]
- 123.Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, de Martin R, Binder BR, Hofer E. Specificity, diversity, and convergence in VEGF and TNF-{alpha} signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J. 2001;15:230–242. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- 124.Mandriota SJ, Seghezzi G, Vassalli JD, Ferrara N, Wasi S, Mazzieri R, Mignatti P, Pepper MS. Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J Biol Chem. 1995;270:9709–9716. doi: 10.1074/jbc.270.17.9709. [DOI] [PubMed] [Google Scholar]
- 125.Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181:902–906. doi: 10.1016/0006-291x(91)91276-i. [DOI] [PubMed] [Google Scholar]
- 126.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 127.Wojtukiewicz MZ, Zacharski LR, Memoli VA, Kisiel W, Kudryk BJ, Rousseau SM, Stump DC. Fibrinogen-fibrin transformation in situ in renal cell carcinoma. Anticancer Res. 1990;10:579–582. [PubMed] [Google Scholar]
- 128.Wojtukiewicz MZ, Zimnoch L, Kloczko J, Bielawiec M, Jaromin J, Rozanska-Kudelska M, Matuszewska E. Fibrinogen-fibrin conversion in laryngeal cancer in situ: a tissue factor dependent process. Thromb Haemostasis. 1995;73:128. [Google Scholar]
- 129.Wojtukiewicz MZ, Zimnoch L, Jaromin J, Kloczko J, Bielawiec M, Matuszewska EA. Immunohistochemical demonstration of fibrin II in gastric cancer tissue. Pol J Pharmacol. 1996;48:229–232. [PubMed] [Google Scholar]
- 130.Zacharski LR, Memoli VA, Morain WD, Schlaeppi JM, Rousseau SM. Cellular localization of enzymatically active thrombin in intact human tissues by hirudin binding. Thromb Haemostasis. 1995;73:793–797. [PubMed] [Google Scholar]
- 131.Wojtukiewicz MZ, Tang DG, Nelson KK, Walz DA, Diglio CA, Honn KV. Thrombin enhances tumor cell adhesive and metastatic properties via increased alpha IIb beta 3 expression on the cell surface. Thromb Res. 1992;68:233–245. doi: 10.1016/0049-3848(92)90081-k. [DOI] [PubMed] [Google Scholar]
- 132.Wojtukiewicz MZ, Tang DG, Ben Josef E, Renaud C, Walz DA, Honn KV. Solid tumor cells express functional ”tethered ligand‘ thrombin receptor. Cancer Res. 1995;55:698–704. [PubMed] [Google Scholar]
- 133.Zacharski LR, Costantini V, Wojtukiewicz MZ, Memoli VA, Kudryk BJ. Anticoagulants as cancer therapy. Semin Oncol. 1990;17:217–227. [PubMed] [Google Scholar]
- 134.Gaffney PJ, Edgell TA, Whitton CM. The haemostatic balance — Astrup revisited. Haemostasis. 1999;29:58–71. doi: 10.1159/000022461. [DOI] [PubMed] [Google Scholar]
- 135.Zhou H, Gabazza EC, Takeya H, Deguchi H, Urano H, Adachi Y, Suzuki K. Prothrombin and its derivatives stimulate motility of melanoma cells. Thromb Haemostasis. 1998;80:407–412. [PubMed] [Google Scholar]
- 136.Wojtukiewicz MZ, Tang DG, Ciarelli JJ, Nelson KK, Walz DA, Diglio CA, Mammen EF, Honn KV. Thrombin increases the metastatic potential of tumor cells. Int J Cancer. 1993;54:793–806. doi: 10.1002/ijc.2910540514. [DOI] [PubMed] [Google Scholar]
- 137.Fischer EG, Ruf W, Mueller BM. Tissue factor-initiated thrombin generation activates the signaling thrombin receptor on malignant melanoma cells. Cancer Res. 1995;55:1629–1632. [PubMed] [Google Scholar]
- 138.Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol. 1996;16:488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- 139.Ellis CA, Tiruppathi C, Sandoval R, Niles WD, Malik AB. Time course of recovery of endothelial cell surface thrombin receptor (PAR-1) expression. Am J Physiol. 1999;276:C38–C45. doi: 10.1152/ajpcell.1999.276.1.C38. [DOI] [PubMed] [Google Scholar]
- 140.Maragoudakis ME, Tsopanoglou NE, Andriopoulou P, Maragoudakis MM. Effects of thrombin/thrombosis in angiogenesis and tumour progression. Matrix Biol. 2000;19:345–351. doi: 10.1016/s0945-053x(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 141.Maragoudakis ME, Tsopanoglou NE. On the mechanism(s) of thrombin-induced angiogenesis. Adv Exp Med Biol. 2000;476:47–55. doi: 10.1007/978-1-4615-4221-6_4. [DOI] [PubMed] [Google Scholar]
- 142.Tsopanoglou NE, Maragoudakis ME. On the mechanism of thrombin-induced angiogenesis. Potentiation of vascular endothelial growth factor activity on endothelial cells by up-regulation of its receptors. J Biol Chem. 1999;274:23969–23976. doi: 10.1074/jbc.274.34.23969. [DOI] [PubMed] [Google Scholar]
- 143.Tsopanoglou NE, Pipili-Synetos E, Maragoudakis ME. Thrombin promotes angiogenesis by a mechanism independent of fibrin formation. Am J Physiol. 1993;264:C1302–C1307. doi: 10.1152/ajpcell.1993.264.5.C1302. [DOI] [PubMed] [Google Scholar]
- 144.Walz DA, Fenton JW. The role of thrombin in tumor cell metastasis. Invasion Metastasis. 1994;14:303–308. [PubMed] [Google Scholar]
- 145.Ukropec JA, Hollinger MK, Salva SM, Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem. 2000;275:5983–5986. doi: 10.1074/jbc.275.8.5983. [DOI] [PubMed] [Google Scholar]
- 146.Haralabopoulos GC, Grant DS, Kleinman HK, Maragoudakis ME. Thrombin promotes endothelial cell alignment in Matrigel in vitro and angiogenesis in vivo. Am J Physiol. 1997;273:C239–C245. doi: 10.1152/ajpcell.1997.273.1.C239. [DOI] [PubMed] [Google Scholar]
- 147.Belloni PN, Carney DH, Nicolson GL. Organ-derived microvessel endothelial cells exhibit differential responsiveness to thrombin and other growth factors. Microvas Res. 1992;43:20–45. doi: 10.1016/0026-2862(92)90004-9. [DOI] [PubMed] [Google Scholar]
- 148.Herbert JM, Dupuy E, Laplace MC, Zini JM, Bar SR, Tobelem G. Thrombin induces endothelial cell growth via both a proteolytic and a non-proteolytic pathway. Biochem J. 1994;303:227–231. doi: 10.1042/bj3030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schaeffer P, Riera E, Dupuy E, Herbert JM. Nonproteolytic activation of the thrombin receptor promotes human umbilical vein endothelial cell growth but not intracellular Ca2+, prostacyclin, or permeability. Biochem Pharmacol. 1997;53:487–491. doi: 10.1016/s0006-2952(96)00735-6. [DOI] [PubMed] [Google Scholar]
- 150.Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Benezra M, Vlodavsky I, Ishai-Michaeli R, Neufeld G, Bar-Shavit R. Thrombin-induced release of active basic fibroblast growth factor-heparan sulfate complexes from subendothelial extracellular matrix. Blood. 1993;81:3324–3331. [PubMed] [Google Scholar]
- 152.Levin EG, Stern DM, Nawroth PP, Marlar RA, Fair DS, Fenton JW, Harker LA. Specificity of the thrombin-induced release of tissue plasminogen activator from cultured human endothelial cells. Thromb Haemostasis. 1986;56:115–119. [PubMed] [Google Scholar]
- 153.Zucker S, Conner C, DiMassmo BI, Ende H, Drews M, Seiki M, Bahou WF. Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. J Biol Chem. 1995;270:23730–23738. doi: 10.1074/jbc.270.40.23730. [DOI] [PubMed] [Google Scholar]
- 154.Bouck N, Polverini PJ, Tolsma SS, Frazier WA, Good D. Tumor suppressor gene control of angiogenesis. J Cell Biochem Suppl. 1991;15F:216. [Google Scholar]
- 155.Lee TH, Rhim T, Kim SS. Prothrombin kringle-2 domain has a growth inhibitory activity against basic fibroblast growth factor-stimulated capillary endothelial cells. J Biol Chem. 1998;273:28805–28812. doi: 10.1074/jbc.273.44.28805. [DOI] [PubMed] [Google Scholar]
- 156.Rhim TY, Park CS, Kim E, Kim SS. Human prothrombin fragment 1 and 2 inhibit bFGF-induced BCE cell growth. Biochem Biophys Res Commun. 1998;252:513–516. doi: 10.1006/bbrc.1998.9682. [DOI] [PubMed] [Google Scholar]
- 157.Yoda Y, Abe T. Fibrinopeptide A (FPA) level and fibrinogen kinetics in patients with malignant disease. Thromb Haemostasis. 1981;46:706–709. [PubMed] [Google Scholar]
- 158.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Review: vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 159.Qi J, Goralnick S, Kreutzer DL. Fibrin regulation of interleukin-8 gene expression in human vascular endothelial cells. Blood. 1997;90:3595–3602. [PubMed] [Google Scholar]
- 160.Cheresh DA, Berliner SA, Vicente V, Ruggeri ZM. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell. 1989;58:945–953. doi: 10.1016/0092-8674(89)90946-x. [DOI] [PubMed] [Google Scholar]
- 161.Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 162.Thiagarajan P, Rippon AJ, Farrell DH. Alternative adhesion sites in human fibrinogen for vascular endothelial cells. Biochemistry. 1996;35:4169–4175. doi: 10.1021/bi952532b. [DOI] [PubMed] [Google Scholar]
- 163.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- 164.Sahni A, Baker CA, Sporn LA, Francis CW. Fibrinogen and fibrin protect fibroblast growth factor-2 from proteolytic degradation. Thromb Haemostasis. 2000;83:736–741. [PubMed] [Google Scholar]
- 165.Sahni A, Sporn LA, Francis CW. Potentiation of endothelial cell proliferation by fibrin(ogen)-bound fibroblast growth factor-2. J Biol Chem. 1999;274:14936–14941. doi: 10.1074/jbc.274.21.14936. [DOI] [PubMed] [Google Scholar]
- 166.Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem. 1998;273:7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- 167.Contrino J, Goralnick S, Qi J, Hair G, Rickles FR, Kreutzer DL. Fibrin induction of tissue factor expression in human vascular endothelial cells. Circulation. 1997;96:605–613. [PubMed] [Google Scholar]
- 168.Thompson WD, Smith EB, Stirk CM, Marshall FI, Stout AJ, Kocchar A. Angiogenic activity of fibrin degradation products is located in fibrin fragment E. J Pathol. 1992;168:47–53. doi: 10.1002/path.1711680109. [DOI] [PubMed] [Google Scholar]
- 169.Sporn LA, Bunce LA, Francis CW. Cell proliferation on fibrin: modulation by fibrinopeptide cleavage. Blood. 1995;86:1802–1810. [PubMed] [Google Scholar]
- 170.Bunce LA, Sporn LA, Francis CW. Endothelial cell spreading on fibrin requires fibrinopeptide B cleavage and amino acid residues 15–42 of the beta chain. J Clin Invest. 1992;89:842–850. doi: 10.1172/JCI115663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Foekens JA, Peters HA, Look MP, Portengen H, Schmitt M, Kramer MD, Brunner N, Janicke F, Meijer-van Gelder ME, Henzen-Logmans SC, van Putten WL, Klijn JG. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res. 2000;60:636–643. [PubMed] [Google Scholar]
- 172.Okusa Y, Ichikura T, Mochizuki H. Prognostic impact of stromal cell-derived urokinase-type plasminogen activator in gastric carcinoma. Cancer. 1999;85:1033–1038. [PubMed] [Google Scholar]
- 173.Harvey SR, Sait SN, Xu Y, Bailey JL, Penetrante RM, Markus G. Demonstration of urokinase expression in cancer cells of colon adenocarcinomas by immunohistochemistry and in situ hybridization. Am J Pathol. 1999;155:1115–1120. doi: 10.1016/S0002-9440(10)65214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oka T, Ishida T, Nishino T, Sugimachi K. Immunohistochemical evidence of urokinase-type plasminogen activator in primary and metastatic tumors of pulmonary adenocarcinoma. Cancer Res. 1991;51:3522–3525. [PubMed] [Google Scholar]
- 175.Evans CP, Elfman F, Parangi S, Conn M, Cunha G, Shuman MA. Inhibition of prostate cancer neovascularization and growth by urokinase-plasminogen activator receptor blockade. Cancer Res. 1997;57:3594–3599. [PubMed] [Google Scholar]
- 176.Kuhn W, Schmalfeldt B, Reuning U, Pache L, Berger U, Ulm K, Harbeck N, Spathe K, Dettmar P, Hofler H, Janicke F, Schmitt M, Graeff H. Prognostic significance of urokinase (uPA) and its inhibitor PAI-1 for survival in advanced ovarian carcinoma stage FIGO IIIc. Br J Cancer. 1999;79:1746–1751. doi: 10.1038/sj.bjc.6690278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Witte JH, Sweep CG, Klijn JG, Grebenschikov N, Peters HA, Look MP, van Tienoven TH, Heuvel JJ, Bolt-De Vries J, Benraad TJ, Foekens JA. Prognostic value of tissue-type plasminogen activator (tPA) and its complex with the type-1 inhibitor (PAI-1) in breast cancer. Br J Cancer. 1999;80:286–294. doi: 10.1038/sj.bjc.6690353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Franks AJ, Ellis E. Immunohistochemical localisation of tissue plasminogen activator in human brain tumours. Br J Cancer. 1989;59:462–466. doi: 10.1038/bjc.1989.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gualandris A, Presta M. Transcriptional and posttranscriptional regulation of urokinase-type plasminogen activator expression in endothelial cells by basic fibroblast growth factor. J Cell Physiol. 1995;162:400–409. doi: 10.1002/jcp.1041620312. [DOI] [PubMed] [Google Scholar]
- 180.Mignatti P, Mazzieri R, Rifkin DB. Expression of the urokinase receptor in vascular endothelial cells is stimulated by basic fibroblast growth factor. J Cell Biol. 1991;113:1193–1201. doi: 10.1083/jcb.113.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 182.Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 183.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:365–371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 184.Pepper MS, Mandriota SJ. Regulation of vascular endothelial growth factor receptor-2 (Flk-1) expression in vascular endothelial cells. Exp Cell Res. 1998;241:414–425. doi: 10.1006/excr.1998.4072. [DOI] [PubMed] [Google Scholar]
- 185.Lindgren M, Johansson M, Sandstrom J, Jonsson Y, Bergenheim AT, Henriksson R. VEGF and tPA co-expressed in malignant glioma. Acta Oncol. 1997;36:615–618. doi: 10.3109/02841869709001324. [DOI] [PubMed] [Google Scholar]
- 186.Sandstrom M, Johansson M, Sandstrom J, Bergenheim AT, Henriksson R. Expression of the proteolytic factors, tPA and uPA, PAI-1 and VEGF during malignant glioma progression. Int J Dev Neurosci. 1999;17:473–481. doi: 10.1016/s0736-5748(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 187.Nakata S, Ito K, Fujimori M, Shingu K, Kajikawa S, Adachi W, Matsuyama I, Tsuchiya S, Kuwano M, Amano J. Involvement of vascular endothelial growth factor and urokinase-type plasminogen activator receptor in microvessel invasion in human colorectal cancers. Int J Cancer. 1998;79:179–186. doi: 10.1002/(sici)1097-0215(19980417)79:2<179::aid-ijc14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 188.Schleef RR, Loskutoff DJ. Fibrinolytic system of vascular endothelial cells. Role of plasminogen activator inhibitors. Haemostasis. 1988;18:328–341. doi: 10.1159/000215815. [DOI] [PubMed] [Google Scholar]
- 189.Kroon ME, Koolwijk P, van Goor H, Weidle UH, Collen A, van der PG, van Hinsbergh VW. Role and localization of urokinase receptor in the formation of new microvascular structures in fibrin matrices. Am J Pathol. 1999;154:1731–1742. doi: 10.1016/S0002-9440(10)65429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Blasi F. Proteolysis, cell adhesion, chemotaxis, and invasiveness are regulated by the u-PA-u-PAR-PAI-1 system. Thromb Haemostasis. 1999;82:298–304. [PubMed] [Google Scholar]
- 191.Ossowski L, Clunie G, Masucci MT, Blasi F. In vivo paracrine interaction between urokinase and its receptor: effect on tumor cell invasion. J Cell Biol. 1991;115:1107–1112. doi: 10.1083/jcb.115.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fukao H, Ueshima S, Okada K, Matsuo O. The role of the pericellular fibrinolytic system in angiogenesis. Jpn J Physiol. 1997;47:161–171. doi: 10.2170/jjphysiol.47.161. [DOI] [PubMed] [Google Scholar]
- 193.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 194.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nubetae O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 195.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest. 1997;100:1481–1487. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang JC, Claffey K, Sakthivel R, Darzynkiewicz Z, Shaw DE, Leal J, Wang YC, Lu FM, McCrae KR. Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: partial activity within domain 5. FASEB J. 2000;14:2589–2600. doi: 10.1096/fj.99-1025com. [DOI] [PubMed] [Google Scholar]
- 198.Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, Straino S, Tozzi MG, Smith R, Gaspa L, Bianchini G, Stillo F, Capogrossi MC, Madeddu P. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- 199.Emanueli C, Zacheo A, Minasi A, Chao J, Chao L, Salis MB, Stacca T, Straino S, Capogrossi MC, Madeddu P. Adenovirus-mediated human tissue kallikrein gene delivery induces angiogenesis in normoperfused skeletal muscle. Arterioscler Thromb Vasc Biol. 2000;20:2379–2385. doi: 10.1161/01.atv.20.11.2379. [DOI] [PubMed] [Google Scholar]
- 200.Parenti A, Morbidelli L, Ledda F, Granger HJ, Ziche M. The bradykinin/B1 receptor promotes angiogenesis by up-regulation of endogenous FGF-2 in endothelium via the nitric oxide synthase pathway. FASEB J. 2001;15:1487–1489. [PubMed] [Google Scholar]
- 201.Madeddu P, Emanueli C. Can knockout mice help dissect relevant genes in hypertension? Evidence and confounding factors. Hypertension. 1999;34:e14–e15. doi: 10.1161/01.hyp.34.6.e14. [DOI] [PubMed] [Google Scholar]
- 202.Koshelnick Y, Ehart M, Stockinger H, Binder BR. Mechanisms of signaling through urokinase receptor and the cellular response. Thromb Haemostasis. 1999;82:305–311. [PubMed] [Google Scholar]
- 203.Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, Tsubouchi H, Blasi F, Comoglio PM. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gille J, Khalik M, Konig V, Kaufmann R. Hepatocyte growth factor/scatter factor (HGF/SF) induces vascular permeability factor expression by cultured keratinocytes. J Invest Dermatol. 1998;111:1160–1165. doi: 10.1046/j.1523-1747.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 206.Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1) Oncogene. 1994;9:2683–2690. [PubMed] [Google Scholar]
- 207.Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 208.Antonelli-Orlidge A, Saunders KB, Smith SR, D'Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci USA. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Petrovaara L, Kaipanen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- 210.O'Mahony CA, Albo D, Tuszynski GP, Berger DH. Transforming growth factor-beta 1 inhibits generation of angiostatin by human pancreatic cancer cells. Surgery. 1998;124:388–393. [PubMed] [Google Scholar]
- 211.O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces canal sustains dormancy of human tumors in mice. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 212.Cao Y. Therapeutic potentials of angiostatin in the treatment of cancer. Haematologica. 1999;84:643–650. [PubMed] [Google Scholar]
- 213.Soff GA, Hong J, Fishman D, Kleinman M, Kaplan E, Zagzag D, Schultz D, Cundiff D, Park S, Enghild J, Stack MS, Gately S. Angiostatin 4,5: a naturally occurring human angiogenesis inhibitor. Blood. 1998;92(Suppl):174a. [Google Scholar]
- 214.Cao R, Wu HL, Veitonmaki N, Linden P, Farnebo J, Shi GY, Cao Y. Suppression of angiogenesis and tumor growth by the inhibitor K1–5 generated by plasmin-mediated proteolysis. Proc Natl Acad Sci USA. 1999;96:5728–5733. doi: 10.1073/pnas.96.10.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Joe YA, Hong YK, Chung DS, Yang YJ, Kang JK, Lee YS, Chang SI, You WK, Lee H, Chung SI. Inhibition of human malignant glioma growth in vivo by human recombinant plasminogen kringles 1–3. Int J Cancer. 1999;82:694–699. doi: 10.1002/(sici)1097-0215(19990827)82:5<694::aid-ijc12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 216.Sim BK, MacDonald NJ, Gubish ER. Angiostatin and endostatin: endogenous inhibitors of tumor growth. Cancer Metastasis Rev. 2000;19:181–190. doi: 10.1023/a:1026551202548. [DOI] [PubMed] [Google Scholar]
- 217.Chen QR, Kumar D, Stass SA, Mixson AJ. Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res. 1999;59:3308–3312. [PubMed] [Google Scholar]
- 218.Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M, Soff GA, Sukhatme VP, Kufe DW, Weichselbaum RR. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 219.Moser TL, Stack MS, Asplin I, Enghild JJ, Jrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stack MS, Gately S, Bafetti LM, Enghild JJ, Soff GA. Angiostatin inhibits endothelial and melanoma cellular invasion by blocking matrix-enhanced plasminogen activation. Biochem J. 1999;340:77–84. [PMC free article] [PubMed] [Google Scholar]
- 221.Reijerkerk A, Mosnier LO, Bouma BN, Meijers JCM, Voest EE, Gebbink MFBG. Endostatin stimulates tPA mediated plasmin formation. Meeting Proceedings 2000, Keystone Symposium. Exp Clin Regul Angiog. 2000;87:341. [Google Scholar]
- 222.Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Olas B, Mielicki WP, Wachowicz B, Krajewski T. Cancer procoagulant stimulates platelet adhesion. Thromb Res. 1999;94:199–203. doi: 10.1016/s0049-3848(98)00214-x. [DOI] [PubMed] [Google Scholar]
- 224.Verheul HM, Jorna AS, Hoekman K, Broxterman HJ, Gebbink MF, Pinedo HM. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood. 2000;96:4216–4221. [PubMed] [Google Scholar]
- 225.Wartiovaara U, Salven P, Mikkola H, Lassila R, Kaukonen J, Joukov V, Orpana A, Ristimaki A, Heikinheimo M, Joensuu H, Alitalo K, Palotie A. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemostasis. 1998;80:171–175. [PubMed] [Google Scholar]
- 226.Brunner G, Nguyen H, Gabrilove J, Rifkin DB, Wilson EL. Basic fibroblast growth factor expression in human bone marrow and peripheral blood cells. Blood. 1993;81:631–638. [PubMed] [Google Scholar]
- 227.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Karey KP, Sirbasku DA. Human platelet-derived mitogens: II. Subcellular localization of insulin-like growth factor I to the alpha-granule and release in response to thrombin. Blood. 1989;74:1093–1100. [PubMed] [Google Scholar]
- 229.Karey KP, Marquardt H, Sirbasku DA. Human platelet-derived mitogens: I. Identification of insulin-like growth factors I and II by purification and N alpha amino acid sequence analysis. Blood. 1989;74:1084–1092. [PubMed] [Google Scholar]
- 230.Ben Ezra J, Sheibani K, Hwang DL, Lev-Ran A. Megakaryocyte synthesis is the source of epidermal growth factor in human platelets. Am J Pathol. 1990;137:755–759. [PMC free article] [PubMed] [Google Scholar]
- 231.Griffiths L, Stratford IJ. Platelet-derived endothelial cell growth factor thymidine phosphorylase in tumour growth and response to therapy. Br J Cancer. 1997;76:689–693. doi: 10.1038/bjc.1997.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pipili-Synetos E, Papadimitriou E, Maragoudakis ME. Evidence that platelets promote tube formation by endothelial cells on matrigel. Br J Pharmacol. 1998;125:1252–1257. doi: 10.1038/sj.bjp.0702191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sato Y, Shimada H, Takaki R. Autocrinological role of basic fibroblast growth factor on tube formation of vascular endothelial cells in vitro. Biochem Biophys Res Commun. 1991;180:1098–1102. doi: 10.1016/s0006-291x(05)81179-9. [DOI] [PubMed] [Google Scholar]
- 234.Pluda JM, Parkinson DR. Clinical implications of tumor-associated neovascularization and current antiangiogenic strategies for the treatment of malignancies of pancreas. Cancer. 1996;78:680–687. doi: 10.1002/(SICI)1097-0142(19960801)78:3<680::AID-CNCR49>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 235.Gengrinovitch S, Greenberg SM, Cohen T, Gitay-Goren H, Rockwell P, Maione TE, Levi B-Z, Neufeld G. Platelet factor-r inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. J Biol Chem. 1995;270:15059–15065. doi: 10.1074/jbc.270.25.15059. [DOI] [PubMed] [Google Scholar]
- 236.Perollet C, Han ZC, Savona C, Caen JP, ikfalvi A. Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood. 1998;91:3289–3299. [PubMed] [Google Scholar]
- 237.Maione TE, Gray GS, Hunt AJ, Sharpe RJ. Inhibition of tumor growth in mice by an analogue of platelet factor 4 that lacks affinity for heparin and retains potent angiostatic activity. Cancer Res. 1991;51:2077–2083. [PubMed] [Google Scholar]
- 238.Gupta SK, Hassel T, Singh JP. A potent inhibitor of endothelial cell proliferation is generated by proteolytic cleavage of the chemokine platelet factor 4. Proc Natl Acad Sci USA. 1995;92:7799–7803. doi: 10.1073/pnas.92.17.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jouan V, Canron X, Alemany M, Caen JP, Quentin G, Plouet J, Bikfalvi A. Inhibition of In vitro angiogenesis by platelet factor-4-derived peptides and mechanism of action. Blood. 1999;94:984–993. [PubMed] [Google Scholar]
- 240.Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 241.Dawson DW, Bouck NP. Thrombospondin as an inhibitor of angiogenesis. In: Teicher BA, editor. Antiangiogenic Agents in Cancer Therapy. Totowa, NJ: Humana Press; 1999. pp. 185–203. [Google Scholar]
- 242.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 243.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 244.Rosen EM, Lamszus K, Laterra J, Polverini P, Rubin JS, Goldberg ID. HGF/SF in angiogenesis. Ciba Found Symp. 1997;212:215–226. doi: 10.1002/9780470515457.ch14. [DOI] [PubMed] [Google Scholar]
- 245.Date K, Matsumoto K, Kuba K, Shimura H, Tanaka M, Nakamura T. Inhibition of tumor growth and invasion by a four-kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene. 1998;17:3045–3054. doi: 10.1038/sj.onc.1202231. [DOI] [PubMed] [Google Scholar]
- 246.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemostasis. 1998;80:1008–1014. [PubMed] [Google Scholar]
- 247.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 248.An SS, Greenfield R. Activation of thrombin activatable fibrinolysis inhibitor (TAFI) by uPAR may cause thrombotic complications in cancer. Meet Proc, 2001:8. [Google Scholar]
- 249.Moghaddam A, Zhang HT, Fan TP, Hu DE, Lees VC, Turley H, Fox SB, Gatter KC, Harris AL, Bicknell R. Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc Natl Acad Sci USA. 1995;92:998–1002. doi: 10.1073/pnas.92.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li JJ, Huang YQ, Basch R, Karpatkin S. Thrombin induces the release of angiopoietin-1 from platelets. Thromb Haemostasis. 2001;85:204–206. [PubMed] [Google Scholar]
- 251.Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997;420:1–6. doi: 10.1016/s0014-5793(97)01475-0. [DOI] [PubMed] [Google Scholar]
- 252.Cioce V, Csaky KG, Chan AM, Bottaro DP, Taylor WG, Jensen R, Aaronson SA, Rubin JS. Hepatocyte growth factor (HGF)/NK1 is a naturally occurring HGF/scatter factor variant with partial agonist/antagonist activity. J Biol Chem. 1996;271:13110–13115. doi: 10.1074/jbc.271.22.13110. [DOI] [PubMed] [Google Scholar]
- 253.Chan AM, Rubin JS, Bottaro DP, Hirschfeld DW, Chedid M, Aaronson SA. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991;254:1382–1385. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- 254.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441–452. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]