Abstract
The dimer initiation site/dimer linkage sequence (DIS/DLS) region in the human immunodeficiency virus type 1 (HIV-1) RNA genome is suggested to play important roles in various steps of the virus life cycle. However, due to the presence of a putative DIS/DLS region located within the encapsidation signal region (E/psi), it is difficult to perform a mutational analysis of DIS/DLS without affecting the packaging of RNA into virions. Recently, we demonstrated that duplication of the DIS/DLS region in viral RNA caused the production of partially monomeric RNAs in virions, indicating that the region indeed mediated RNA-RNA interaction. We utilized this system to assess the precise location of DIS/DLS in the 5′ region of the HIV-1 genome with minimum effect on RNA packaging. We found that the entire lower stem of the U5/L stem-loop was required for packaging, whereas the region important for dimer formation was only 10 bases long within the lower stem of the U5/L stem-loop. The R/U5 stem-loop was required for RNA packaging but was completely dispensable for dimer formation. The SL1 lower stem was important for both dimerization and packaging, but surprisingly, deletion of the palindromic sequence at the top of the loop only partially affected dimerization. These results clearly indicated that the E/psi of HIV-1 is much larger than the DIS/DLS and that the primary DIS/DLS is completely included in the E/psi. Therefore, it is suggested that RNA dimerization is a part of RNA packaging, which requires multiple steps.
Retrovirus particles contain single-stranded positive-sense RNA as the genome. The genomic RNA always forms dimers in mature virions. These two RNA molecules are noncovalently linked, since incubation at high temperature (∼70°C) or treatment with denaturing agents, such as formamide, easily dissociates them (for a review, see references 10 and 19). Electron microscopic observation revealed that the two RNA molecules are linked symmetrically and the contact point of the dimer, called the dimer linkage sequence (DLS), is located near the 5′ end of each RNA under partially denaturing conditions. It is likely that the presence of two genomes in one virion is advantageous for survival, providing an extra template that can be used when one RNA molecule is damaged and giving genetic variety to the progeny (11, 23). It has been suggested that the DLS in viral genomic RNA usually overlaps with a packaging signal (E/psi) (19). Therefore, it was difficult to assess whether viral RNA dimerization and packaging are independent events.
The partial RNA fragments of the 5′ region of a retrovirus genome transcribed and purified in vitro formed dimer molecules upon incubation in buffer without any proteins or cellular extract (19). Thus, the DLS has been inspected mainly by using in vitro transcription systems. In the case of human immunodeficiency virus type 1 (HIV-1), the 5′ untranslated region just downstream of the splicing signal was first reported to be a DLS, because in in vitro systems, the RNA fragments lacking this region have impaired ability to form a dimer (3, 31, 42). Recently, several groups reported another site within the 5′ untranslated region which was important for RNA dimerization in vitro. This site was located upstream of the 5′ splicing signal and named the dimer initiation site (DIS) (25, 35, 36, 41). The DIS consists of a stem-loop structure with a conserved palindromic sequence at the top of the loop. Two palindromic sites have been suggested to make a contact point, forming a kissing-loop complex to initiate dimer formation (25, 35, 36, 41).
Several studies of RNA dimerization in HIV-1 particles (in vivo) have shown different features of the dimer. The viral nucleocapsid protein (NC) or Gag precursor protein is suggested to alter the secondary structure of the RNA molecule and to stabilize RNA dimers, like molecular chaperons (13, 14, 16, 17). It has been reported that a mutation introduced around the DIS/DLS site did not affect dimer stability in vivo (6, 9, 39) and that a region far from the DIS/DLS affected dimer formation in the retrovirus genome (39, 43). Electron microscopic observation revealed that the HIV-1 RNA genome contains a central DLS and additional loop structures within each monomer subunit (22), suggesting that HIV-1 RNA contains more than one contact point. Thus, there are many discrepancies between the in vitro and in vivo data on HIV-1 RNA dimerization.
Previously, several mutants were generated with a duplicated 5′ region (1,000 bases) of viral genome containing the packaging signal-DLS region (E/DLS) at various ectopic positions of RNA, and their packaging abilities and dimer formation in the particles were examined (38). In the mutant virions, monomeric forms of virion RNA, which were totally absent from the wild-type virions, were observed. This suggested that the 5′ region of the genome indeed plays an important role in RNA-RNA interaction during virion formation. In this study, we utilized this system to assess the exact location of the DIS/DLS of HIV-1. Portions of stem-loop U5/L or stem-loop 1 (SL1) were sequentially deleted, and the effects of these mutations on RNA dimerization and encapsidation efficiency were analyzed.
MATERIALS AND METHODS
Constructs.
The replication-competent HIV-1 proviral clone pNL4-3 (1) and pMSMBA (32), a derivative of pNL4-3, were used as the progenitors for all the mutants described below. pNL4-3 was digested with NheI, treated with T4 DNA polymerase, and self-ligated with T4 DNA ligase to construct pNLNh. Therefore, pNLNh carries a 4-base insertion mutation within the env region, and Env protein expression is abrogated.
To construct pDDNBA, the plasmid pGEM-MM (38) was digested with BamHI and AccI and treated with T4 DNA polymerase, and an ∼0.5-kbp fragment including the 5′ leader region of HIV-1 was isolated. This fragment was ligated into the T4 DNA polymerase-treated NheI site of pNL4-3 to construct pDDNBA. The fragment was in its original orientation.
To construct pdR/U5-BA, pdU5/L-BA, pdPBS-BA, and pdM-BA, the plasmids pdR/U5, pdU5/L, pdPBS, and pdM (34) were digested with NotI, treated with T4 DNA polymerase, and then digested with SpeI. Fragments of ∼1.5 kbp, from the 5′ leader region to the gag region, were isolated from these plasmids and exchanged with the AatII-SphI fragment of pDDNBA to construct pdR/U5-BA, pdU5/L-BA, pdPBS-BA, and pdM-BA, respectively.
To construct pdLA1, a two-step PCR amplification was performed to introduce the mutation. The template for PCR was pNLdSpNc, which was generated by removing the SpeI-NcoI fragment of pNL4-3. The first pair of primers used was a sense primer, 432F (5′-GCAGCTGCTTTTTGCCTGTAC-3′), and an antisense primer, dLA1R (5′-GATTTTCCAACTGATCTGAGGGATCTCTA-3′), and the second pair was a sense primer, dLA1F (5′-TAGAGATCCCTCAGATCAGTTGGAAAATC-3′), and an antisense primer, 783R (5′-CCTTCTAGCCTCCGCTAGTC-3′). Two amplified fragments were isolated, mixed, and used for the second PCR with primers 432F and 783R to generate the mutated fragment. This fragment was digested with SacI and BssHII and inserted into the corresponding position in pNLdSpNc to construct pdLA1sub. The plasmid pdLA1sub was then digested with AatII and SphI, and an ∼3.0-kb fragment was isolated and inserted into the corresponding position in pNLNh and pDDNBA to construct pdLA1 and pdLA1-BA, respectively.
The mutant constructs pdLA2 or pdLA2-BA, pdLA3 or pdLA3-BA, and pdLB3 or pdLB3-BA were constructed by the same method as pdLA1 or pdLA1-BA but using different primer pairs. For pdLA2, the first pair was a sense primer, 432F, and an antisense primer, dLA2R (5′-CTGCTAGAGATTTTCGGATCTCTAGTTACC-3′), and the second was a sense primer, dLA2F (5′-GGTAACTAGAGATCCGAAAATCTCTAGCAG-3′), and an antisense primer, 783R. For pdLA3, the first pair was a sense primer, 432F, and an antisense primer, dLA3R (5′-CGAGATCTCCTCTGGCACCAGAGTCACACAAC-3′), and the second was a sense primer, dLA3F (5′-GTTGTGTGACTCTGGTGCCAGAGGAGATCTCG-3′), and an antisense primer, 783R. For pdLB3, the first pair was a sense primer, 432F, and an antisense primer, dLB3R (5′-GGGATCTCTAGTTCGGGCACACAC-3′), and the second was a sense primer, dLB3F (5′-GTGTGTGCCCGAACTAGAGATCCC-3′), and an antisense primer, 783R.
To construct pdLB2 and pdLB2-BA, the plasmid pNLdSpNc was amplified with primers 432F and dLB2R (5′-GTGCGCGCTTCAGCAAGCCGAGTCCTGCGTCGCCAACACAACAGAC-3′), and the amplified fragment was digested with SacI and BssHII and exchanged with the corresponding fragment of pNLdSpNc to construct pdLB2sub. The plasmid pdLB2sub was digested with AatII and SphI, and the resultant 3.1-kb fragment was isolated and inserted into the corresponding position in pNLNh and pDDNBA to construct pdLB2 and pdLB2-BA, respectively. The mutant constructs pdLB4 and pdLB4-BA were constructed by the same method as pdLB2 and pdLB2-BA but using 432F and dLB4R (5′-GTGCGCGCTTCAGCAAGCCGAGTTTACTTTCGCTTTC-3′) as the primers.
The series of mutant constructs pdLC1, pdLC2, and pdLC3 and the series pdLC1-BA, pdLC2-BA, and pdLC3-BA were constructed similarly to pdLA1-BA or pdLA1-BA but with different primer pairs: dLC1F (5′-GGAGATCTCTCTCGGCTTGCTG-3′) and dLC1R (5′-CAGCAAGCCGAGAGAGATCTCC-3′), dLC2F (5′-GGAGATCTCTCAGGACTCGGCTTGCTG-3′) and dLC2R (5′-CAGCAAGCCGAGTCCTGAGAGATCTCC-3′), and dLC3F (5′-GGAGATCTCTCGACGCTCGGCTTGCTG-3′) and dLC3R (5′-CAGCAAGCCGAGCGTCGAGAGATCTCC-3′) were used for pdLC1 or pdLC1-BA, pdLC2 or pdLC2-BA, and pdLC3 or pdLC3-BA, respectively.
The construction of pS1, pBsS1, pBST2, and pBsT6 was described elsewhere (34, 39). These plasmids were digested with NotI, treated with the T4 DNA polymerase, and digested with SpeI. Fragments of ∼1.5 kb, from the 5′ leader region to the gag region, were isolated and ligated with the AatII-SphI fragment of pDDNBA to construct pS1-BA, pBsS1-BA, pBsT2-BA, and pBsT6-BA.
To construct the mutant pdL1, a two-step PCR was performed using two pairs of primers. The first pair was 432F and dL1R (5′-CGCCCCTCGCCTCGCCGAGTCCTGC-3′), and the second pair was dL1F (5′-GCAGGACTCGGCGAGGCGAGGGGCG-3′) and 970R (5′-GTATTTGTCTACAGCCTTCTG-3′). The plasmid pNLdSpNc was used as a template for amplification with these pairs of primers, and two amplified fragments were isolated and mixed. The mixed fragments were then used as templates for the second step of PCR with the primers 432F and 970R, and the amplified fragment was digested with SacI and AccI. The resulting 0.47-kb fragment was isolated and inserted into the corresponding position of pNLdSpNc to construct pdL1sub. The plasmid pdL1sub was digested with AatII and SphI, and the fragment containing the mutation was inserted into the corresponding position in pNLNh and pDDNBA to construct pdL1 and pdL1-BA, respectively.
Three additional plasmids, pGEM634, pGEM634T2, and pGEM634T6, were constructed as templates for in vitro transcription. Each of the plasmids pNLdSpNc, pBsT2, and pBsT6 was used as a template for amplification with the primers PBS-F (5′-AGTGGCGCCCGAACAGGGAC-3′) and 1000R (5′-CTGTCTGAAGGGATGGTTG-3′). The amplified fragments were isolated and ligated with pGEM-Teasy vector (Promega) to construct pGEM634, pGEM634T2, and pGEM634T6, respectively.
Transfection.
293T cells (18) (∼7 × 106) were seeded on dishes (diameter, 150 mm) on the day before transfection. Plasmid DNA (10 μg) was transfected into the cells using the calcium phosphate precipitation method (2). The day after transfection, the medium was replaced with fresh Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum.
Isolation of RNA from cytoplasm and virions.
Forty-eight to 72 h after transfection, the medium and cytoplasmic RNA were collected concurrently as described elsewhere (32). Virions in the medium were collected by centrifugation (100,000 × g; 2 h), and the viral pellet was resuspended in TSE (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, and 1 mM EDTA). The physical virus titer was determined using an enzyme-linked immunosorbent assay kit to quantitate CA-p24 (ZeptoMetrix, Inc.). To isolate RNA from particles, virions were disrupted by the addition of sodium dodecyl sulfate (SDS) to 1% and treated with proteinase K (300 μg/ml) at room temperature for 60 min, followed by Tris-EDTA-saturated phenol-chloroform extraction, chloroform extraction, and ethanol precipitation.
Northern blotting analysis.
Pelleted RNA was resuspended in T buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 1% SDS, 100 mM NaCl, and 10% formamide), and the thermostability of dimeric viral RNA was determined by incubating RNA aliquots for 10 min at the temperatures indicated in Fig. 2 (39). The proportions of the dimer and monomer were measured by electrophoresis at room temperature on a nondenaturing 0.75% native agarose gel in 0.5× Tris-borate-EDTA buffer (29). Field inversion gel electrophoresis was performed for better separation of large RNA molecules. The conditions for field inversion gel electrophoresis were as follows: forward, 5V/cm and 0.6s; reverse, 5V/cm and 0.1s. The agarose gel was then treated with 10% formaldehyde at 65°C before being washed with H2O three times, and the RNA was blotted electrically onto a Hybond-N+ nylon membrane (Amersham Pharmacia Biotech UK, Ltd.).
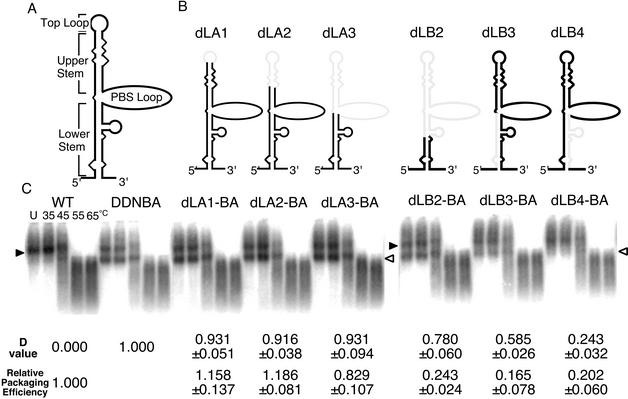
FIG. 2.
Dimerization and packaging efficiencies of various deletion mutants of the U5/L stem-loop. (A) Schematic secondary structure of the HIV-1 U5/L stem-loop. (B) Diagrams of U5/L stem-loop deletion mutants. The lightly shaded lines represent deleted areas of RNA. (C) Representative ImageGauge image of RNA detected by Northern blotting. The positions of dimeric and monomeric viral RNAs are indicated by solid and open arrowheads, respectively. The various temperatures at which aliquots were incubated are indicated for the wild-type (WT) lane. The D value and relative packaging efficiency of each mutant are shown below each blot. The packaging efficiencies were calculated from results for the mutants, which do not contain a second copy of E/DLS. The results represent means ± standard errors from at least three independent experiments. Lane U, unheated sample.
RNA Northern hybridization analysis was performed as described previously (29). The plasmid T7pol (39) and T7 RNA polymerase (New England Biolabs) were used to synthesize the cRNA probe, which detects only unspliced viral RNAs, for Northern hybridization. Approximately 7 × 106 cpm of riboprobe per blot was used in the hybridization reaction. Hybridization was carried out in the presence of Rapid-Hyb buffer (Amersham Pharmacia). Membranes were washed extensively with 0.1× SSC (1× SSC is 150 mM NaCl and 15 mM sodium citrate [pH 7.0])- 0.1% SDS at 70°C. In experiments designed to assess the conversion of dimers to monomers, the relative amounts of both RNA species were quantitated with a BAS1000 imaging plate system and ImageGauge software (Fujifilm Co.).
RNase protection assay.
The antisense probe (∼108 cpm/mg) was synthesized by transcription of pGEM(600-1000) (33), pGEM634, pGEM634T2, and pGEM634T6 with T7 RNA polymerase (New England Biolabs) or by transcription of pT7HIV-1 410-910 (39) with SP6 RNA polymerase (Promega) following linearization with NotI [pGEM(600-1000)], SpeI (pGEM634, pGEM634T2, and pGEM634T6), or SalI (pT7HIV-1 410-910) as described previously (30). To serve as size markers for denaturing polyacrylamide gels, HpaII-digested fragments of pGEM3Zf (+) were 32P end labeled (30). One-fifth of the virion-associated RNA or 10 μg of the cytoplasmic RNA preparation was mixed with 8 × 104 Cerenkov counts of 32P-labeled antisense RNA and precipitated with ethanol. RNase protection assays were performed using an RPA III RNase protection assay kit (Ambion, Inc.). After electrophoresis in 5% polyacrylamide- 8 M urea gels, the quantitation of various protected RNA species was achieved with the BAS1000 imaging plate system and ImageGauge software. The relative packaging efficiency was calculated by the following formula: relative packaging efficiency = (unspliced RNA of the mutant in virion/that in cytoplasm)/(unspliced RNA of the wild type in virion/that in cytoplasm).
The antisense probes from pGEM634, pGEM634T2, and pGEM634T6 were used to distinguish viral RNA of BsS1, BsT2, and BsT6, respectively, from that of the wild type. The probe from pT7HIV-1 410-910 was used to detect viral RNA of dR/U5 and dLB3. Other mutant RNAs were detected by the probe from pGEM(600-1000).
RESULTS
Contribution of 5′ stem-loops to RNA dimerization of HIV-1.
In the 5′ untranslated region of HIV-1 RNA, the RNA is predicted to form seven stem-loop structures (TAR, R/U5, U5/L, SL1, SL2, SL3, and SL4) (7, 34). These stem-loops play important roles in viral genome packaging. On the other hand, it is still unclear whether these stem-loops are required for genome dimerization (6, 9, 39). We recently reported a series of HIV-1 mutant constructs containing an additional E/DLS region at the ectopic position (38). These mutant RNA molecules were packaged into virions as efficiently as the wild-type RNA. Nearly 40% of the mutant RNA molecules in virions, however, appeared to be monomeric, whereas the wild-type RNA molecules in virions were dimeric. We speculated that an additional E/DLS region at the ectopic position binds to the authentic E/DLS region on the same RNA molecules competitively, interfering with intermolecular dimer formation. If a mutation which abolishes RNA-RNA interaction is introduced in one of the E/DLS on this mutant, intramolecular interaction would also be abolished and monomeric RNA would not be observed in the virion. Even if such a mutation negatively affected packaging, the ectopic E/DLS would complement the packaging function. Thus, mutational analysis of this mutant could be used to precisely map the DIS/DLS on the HIV-1 RNA. In this study, we utilized this system to assess the precise location of the DIS/DLS of HIV-1.
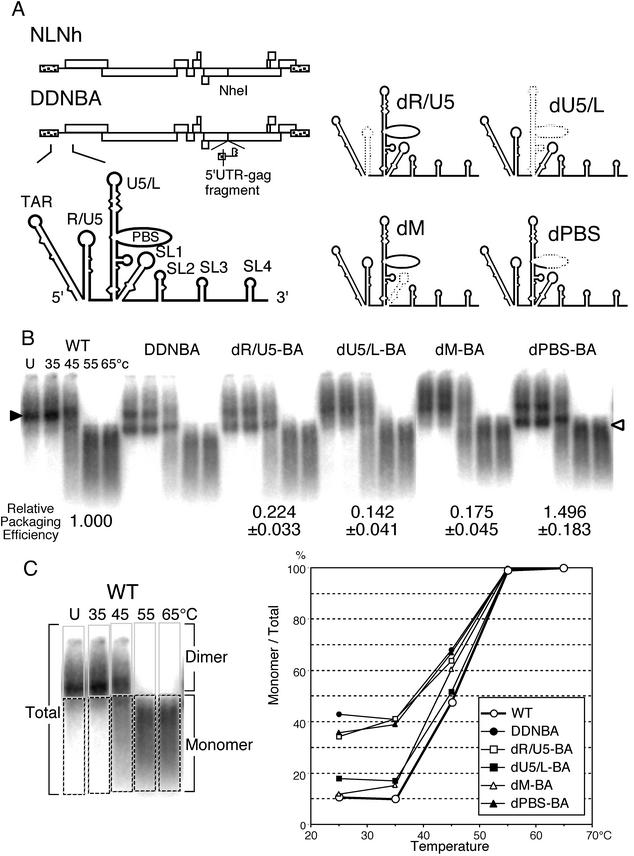
We first constructed a plasmid called pDDNBA, a derivative of pDDN, for clearer identification of the DIS/DLS and easier manipulation of the mutant construction. The duplicated region of pDDNBA was 405 bases long, including all seven stem-loops at the 5′ end of the HIV-1 genome, whereas that of pDDN was >1,000 bases long, from the 5′ end to the middle of the gag gene. Polyadenylation signals and primer binding sites (PBS) in the ectopic fragment on pDDNBA were deleted to abolish undesired polyadenylation and ectopic initiation of reverse transcription. 293T cells were transfected with pDDNBA, and progeny virions were collected from the supernatant. The RNA encapsidation and dimerization abilities of the virions were compared with those of pDDN and the wild-type, pNLNh. The RNA-packaging efficiency of DDNBA was comparable to those of DDN and NLNh, and its RNA dimerization profile was indistinguishable from that of DDN. Therefore, the first 405 bases from the 5′ end of the HIV-1 RNA were sufficient to promote RNA-RNA interaction in virions (data not shown). Thus, we decided to use pDDNBA instead of pDDN as a progenitor of the mutants in the RNA dimerization assay. There were two E/DLS sites on the genome RNA of DDNBA, and we needed to decide which one would be mutated in this study. In a previous paper, it was speculated that the original E/DLS site on the mutant molecules worked more efficiently than the ectopic sites for the RNA-RNA interaction in the virion (37). In addition, we intended to observe the effect of the mutations on both dimerization and packaging efficiency. For packaging analysis, we have to use the mutants, which have only one E/DLS at the original position. Therefore, we decided to introduce mutations in the original E/DLS site of pDDNBA. For this purpose, we constructed the pDDNBA derivative plasmids pdR/U5-BA, pdU5/L-BA, pdM-BA, and pdPBS-BA, in which stem-loops R/U5, U5/L, and SL1 and the PBS, respectively, were deleted (Fig. 1A). Each mutant contained the deletion in the 5′ authentic E/DLS, whereas an additional E/DLS at the ectopic position was intact except for polyadenylation signals and the PBS, like pDDNBA. We compared the RNA dimerization profiles of these mutant virions to those of DDNBA and the wild type (Fig. 1B and C). The virion RNAs from dPBS-BA and dR/U5-BA formed monomeric RNA at low temperature, similar to that of DDNBA. This result indicated that the PBS and stem-loop R/U5 do not help to promote RNA-RNA interaction in virions, and we concluded that these regions were not included in the DIS/DLS at all. On the other hand, the RNAs produced by dU5/L-BA and dM-BA contained very little monomer, similar to that of the wild type. This result indicated that the U5/L and SL1 stem-loop regions are important for the formation of dimeric RNA in virions.
FIG. 1.
Impact of a stem-loop deletion of the 5′ region of HIV-1 RNA on genome dimerization. (A) Diagram of the prototype plasmids NLNh and DDNBA and the 5′ regions of the stem-loop deletion mutants. The dotted lines represent deleted areas of RNA. (B) Representative ImageGauge image of RNA detected by Northern blotting. Aliquots of RNA extracted from virions were resuspended in T buffer, incubated for 10 min in parallel reactions at various temperatures, and then analyzed on a native agarose gel. The positions of dimeric and monomeric viral RNAs are indicated by a solid and open arrowhead, respectively. The various temperatures at which aliquots were incubated are indicated for the wild-type (WT) lane. Three or more independent experiments gave similar results. The relative packaging efficiency of each mutant is shown below its blot. The packaging efficiencies were calculated from the results for the mutants, which do not contain a second copy of E/DLS. The relative encapsidation efficiencies were calculated as the ratio of the amount of mutant genomic RNA to that of the wild type (pNLNh) in the virions, with normalization to the cytoplasmic levels of the two RNAs. The results represent means ± standard errors from at least three independent experiments. (C) Thermal-dissociation kinetics of dimeric viral RNAs shown in panel B. A schematic figure for dimer/monomer calculation is shown on the left. Each lane of the blot was separated into two parts, dimer or larger and monomer or smaller molecules. The relative amounts of monomeric and total RNAs in each lane were quantitated with ImageGauge, and the percentage of the total represented by monomeric RNA was calculated for each RNA sample. Lanes U, unheated samples.
Precise location of DIS/DLS within the U5/L stem-loop.
To identify the exact region responsible for RNA-RNA interaction within the U5/L stem-loop region, we constructed a series of U5/L stem-loop deletion mutants, dLA1-BA, dLA2-BA, and dLA3-BA. The U5/L stem-loop could be divided into four parts, the top loop, the upper stem, the PBS loop, and the lower stem (Fig. 2A). The top loop (9 bases; nucleotides [nt] 604 to 612 [the numbering starts at position +1 of the 5′ end of the U3 region]) of the U5/L stem-loop was deleted in dLA1-BA, the top loop and half of the upper stem (23 bases; nt 598 to 620) were deleted in dLA2-BA, and the top loop, the entire upper stem, and the PBS loop (85 bases; nt 586 to 670) were deleted in dLA3-BA (Fig. 2B). The virion RNAs from all dLA mutants formed monomeric RNA at room temperature, quite similar to that of DDNBA (Fig. 2C). This indicated that the top loop, the upper stem, and the PBS loop of the U5/L stem-loop do not contribute to RNA-RNA interaction in virions. We next introduced a deletion in the lower stem of the U5/L stem-loop (Fig. 2B). The mutant dLB2-BA lacks 109 bases (nt 578 to 686), corresponding to the top loop, the entire upper stem, the PBS loop, and half of the lower stem. The 19 bases (nt 577 to 585) of the 5′ side of the lower stem were deleted in dLB3-BA, and the 26 bases (nt 671 to 696) of the 3′ side of the lower stem were deleted in dLB4-BA. The RNA isolated from dLB2-BA particles showed a profile similar to that of DDNBA (Fig. 2C). The dLB3-BA RNA also formed monomeric RNA, but the amount of monomer tended to be reduced compared to that of DDNBA (Fig. 2C). On the other hand, dLB4-BA RNA showed that the mutations caused severe impairment in monomer formation. These results suggested that the region affecting RNA dimer formation was located in the deleted part of dLB4-BA and that the area absent in dLB3-BA might partially contribute to dimer formation. Therefore, the 11 bases (nt 577 to 587) of the 5′ side and the 10 bases (nt 687 to 696) of the 3′ side of the lower stem appeared to affect RNA-RNA interaction in the HIV-1 genome.
The RNA profile from dLB3-BA showed that the introduced deletion had an intermediate effect on dimer formation (Fig. 2C). Since the ratio of monomer content to the total RNA of the virion produced from DDNBA varied slightly from experiment to experiment, we developed the following formula to give an index value of the dimer formation ability (D) of each mutant, to compare the effects of the mutation of the DLS area more quantitatively: D = (Mu − W)/(BA − W), where W, BA, and Mu represent the ratios of the monomer content to total the RNA of virions produced from the wild type, DDNBA, and each mutant based on the DDNBA construct at room temperature. The D value of the wild type is 0, and that of DDNBA is 1. When the mutation severely affects dimer formation, the monomeric genome content of the virion is reduced, and D becomes close to zero. Thus, the D value was expected to represent the magnitude of the effect of the mutation on RNA-RNA interaction in virions. We applied this formula to the results of the dimerization assay of the mutants dLA1-BA, dLA-2BA, dLA3-BA, dLB1-BA, dLB2-BA, and dLB3-BA. As shown in Fig. 2C, the values for the dLA1-BA, dLA2-BA, and dLA3-BA mutants were close to 1, while that for dLB2-BA was ∼0.8 and that for dLB4-BA was ∼0.25. The D value for dLB3-BA was ∼0.6. These results indicate that the formula is useful to show the relative efficiency of the RNA dimerization of each mutant quantitatively.
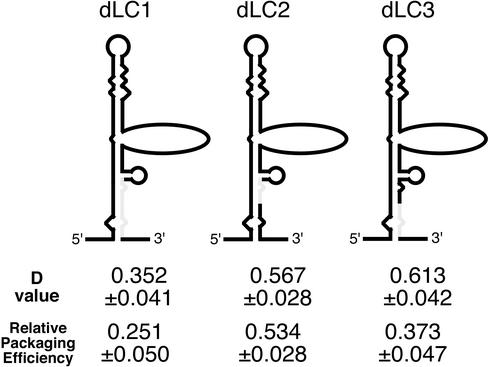
We also constructed three mutants which contained deletions within the 10 bases of the 5′ side of the lower stem to assess the precise location of the DLS/DIS (Fig. 3). The deleted area of dLC1-BA is 10 bases of the 3′ side of the lower stem (nt 687 to 696), which is the region deleted from dLB4-BA but still present in dLB2-BA. The upper and lower 5 bases of this area were deleted in dLC2-BA (nt 687 to 691) and dLC3-BA (nt 692 to 696). The RNA profiles of these mutant viruses were compared to those of DDNBA and the wild type, and D values were calculated (Fig. 3). The D value of the dLC1-BA mutant was ∼0.35. This value was slightly higher than that of dLB4-BA but still much lower than that of DDNBA. The D values of both the dLC2-BA and dLC3-BA mutants were ∼0.6, showing partial inhibition of dimerization. Thus, 10 bases just upstream of the SL1 stem-loop play an important role in mediating RNA-RNA interaction and/or in forming overall structure necessary for dimerization in virions.
FIG. 3.
Dimerization and packaging efficiencies of lower-stem deletion mutants of the U5/L region. Diagrams of deletion mutants are shown. The lightly shaded lines represent deleted areas of RNA. The D value and relative packaging efficiency of each mutant is shown below its diagram. The packaging efficiencies were calculated from results for the mutants, which do not contain a second copy of E/DLS. The results represent means ± standard errors from at least three independent experiments.
Functional areas for RNA dimerization within SL1.
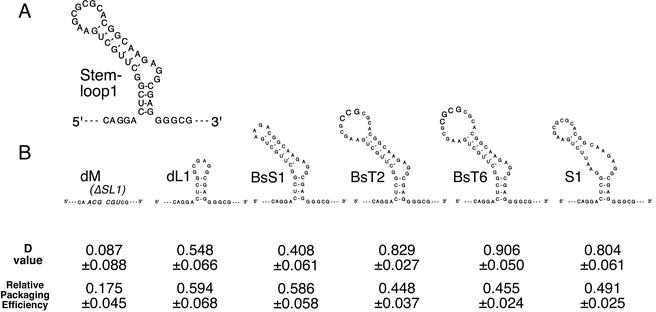
The palindromic sequence on the loop of SL1 is believed to be the dimer initiation sequence in vitro. We tried to identify the region within the SL1 stem-loop which is responsible for RNA-RNA interaction in vivo. We constructed five SL1 mutants as derivatives of the pDDNBA construct. The mutants BsS1-BA, BsT2-BA, and BsT6-BA contain a 5-base deletion, a 3-base insertion, and a 4-base insertion, respectively, at the BssHII recognition site in the hairpin loop of SL1 (Fig. 4B). The loop and top stem of the authentic SL1 is deleted in the mutant dL1-BA (21 bases; nt 703 to 723), and the base pairing of the top stem is partially abolished in S1-BA (Fig. 4B). We examined the dimerization efficiencies of the genomic RNAs of these mutants. As shown in Fig. 4B, the D value of dM-BA, in which the entire SL1 was deleted, was almost 0, whereas those of dL1-BA and BsS1-BA were ∼0.5. These results indicate that the hairpin loop and upper-stem region only partially contribute to dimer formation and that the entire stem-loop formation of the SL1 region is required for mediating RNA-RNA interaction in vivo. On the other hand, the ability to form a dimer was not altered by the hairpin loop mutations introduced into BsT2-BA or BsT6-BA (Fig. 4B). This means that enlargement of the hairpin loop does not affect dimerization. The ability to form dimers was also maintained after the upper-stem disruption introduced into the mutant S1-BA. This suggests that the upper-stem formation of SL1 is not required for RNA-RNA interaction.
FIG. 4.
Dimerization and packaging efficiencies of various SL1 mutants. (A) Schematic secondary structure of HIV-1 SL1 stem-loop. (B) Diagrams of SL1 stem-loop mutants. The inserted bases in BsT2 and BsT6 are indicated in large letters. The MluI recognition site introduced at the position where SL1 is deleted in the mutant dM is indicated in large italic letters. The D value and relative packaging efficiency of each mutant are shown below its diagram. The packaging efficiencies were calculated from results for the mutants, which do not contain a second copy of E/DLS. The results represent means ± standard errors from at least three independent experiments.
The encapsidation signal expands to a much larger area than the DIS/DLS.
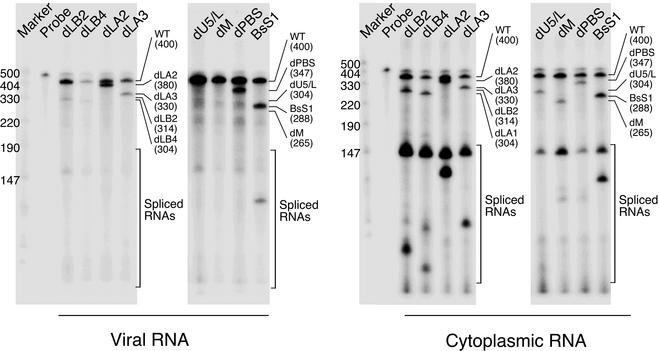
The E/psi site and the DIS/DLS site have been believed to overlap, and they may cooperate during the particle formation process. We tried to find the location of the E/psi site by RNase protection assay using 5′-region deletion mutants that do not contain a second copy of the E/DLS region within their genomes. First, 293T cells were cotransfected with equal amounts of pNLNh (the wild type) and a plasmid containing various mutations examined above. The cytoplasmic RNA was isolated from these cells, and the virion RNA was isolated from the medium as previously described (32); both were subjected to RNase protection analysis with a riboprobe capable of detecting both wild-type and mutant RNAs (Fig. 5). The relative encapsidation efficiencies of the mutants were determined by calculating the ratio of the mutant RNA to the wild-type RNA in the virion relative to the ratio of the two RNAs in the cytoplasm. Quantitation of the unspliced viral RNA in the cytoplasm by RNase protection assay indicated that all mutants expressed similar levels of steady-state cytoplasmic RNA relative to the wild type (Fig. 5 and data not shown). As shown in Fig. 1B and previous reports (33, 34), complete deletion of the R/U5, U5/L, and SL1 stem-loop regions resulted in harsh reduction of packaging efficiency. On the other hand, deletion of the PBS and the upper stem-loop region of U5/L (dLA1, dLA2, and dLA3) did not affect packaging (Fig. 2B). The packaging efficiencies of the mutants dLB2, dLB3, and dLB4 were seriously impaired, showing that the entire lower stem of the U5/L region plays an important role in RNA encapsidation (Fig. 2B). These results indicated that the packaging signal does not completely coincide with the DIS/DLS, since the R/U5 stem-loop and the areas deleted from the mutant dLB2 contributed little to the dimer formation function. The mutant dLC1 showed a significant impairment in packaging, whereas the mutants dLC2 and dLC3 did not (Fig. 3). Top-stem truncation, top-stem corruption, or hairpin loop deletion of the SL1 stem-loop region only partially reduced packaging ability (Fig. 4B). Enlargement of the hairpin loop of SL1 also only partially affected encapsidation (Fig. 4B, BsT2 and BsT6).
FIG. 5.
RNase protection assay measuring packaging efficiencies of pNLNh derivatives. The mutants were cotransfected into 293T cells along with the wild-type (WT), pNLNh. The cytoplasmic RNA from the transfected cells and the virion RNA were subjected to RNase protection analysis. The probe used was generated from pGEM(600-1000). This probe is complementary to a portion of the 5′ untranslated region and the gag gene from pNLNh and is capable of distinguishing the wild-type and each mutant RNA. The amount of probe loaded on the gel was 1/500 of the amount of probe used in the experiment. The position of each diagnostic band for the viral RNA is indicated on the right of each gel. The numbers in parentheses represent the sizes (in nucleotides) of the protected bands of the mutants.
DISCUSSION
In this work, we used a previously reported system (38) to study the RNA-RNA interaction that occurs in mature HIV-1 virions. This system has several advantages for analyzing the RNA dimerization of HIV in vivo. First, we can see the in vivo dimerization of the mutant of the packaging signal region without affecting the encapsidation ability of RNA. Second, we can limit the area for analyzing dimerization to the 5′ leader region. There are several reports suggesting that the area other than the primary 5′ DIS/DLS affects retroviral genome dimerization in vivo (39, 43). Even in the reports describing the importance of the primary DIS/DLS site of HIV-1, a significant amount of dimerized genome from the DIS/DLS mutants is usually observed (9, 26, 40). These facts obviously suggest the presence of the secondary or tertiary DLS within viral RNA. Our system facilitates the analysis of the 5′ DIS/DLS directly and independently of these secondary or tertiary DLSs.
The first experiment gave clear-cut results (Fig. 1). The R/U5 loop and the PBS were not included in the DIS/DLS, whereas the SL1 and U5/L stem-loop regions played critical roles in genome dimerization. In subsequent experiments, we anatomized the U5/L stem-loop to identify the region responsible for dimer formation. Most of the U5/L stem-loop is not required, and only 10 bases of lower stem play an important role in inducing RNA dimerization. These results are consistent with recent reports suggesting the participation of the U5/L region in RNA dimerization in vivo (27, 40). We also found that the 5′ side of the lower stem contributes partially to dimer formation (Fig. 2).
Unexpected results were obtained from the experiments with the SL1 stem-loop. Surprisingly, all SL1 mutants except dM-BA partially or fully retained dimerization activity. The mutant BsS1-BA lacks a palindromic sequence in the hairpin loop, so the initial interaction between the authentic and ectopic SL1 sequences on the same RNA would be ablated. BsT6-BA has a longer palindromic sequence (10 bases), which could potentially lead to more stable interaction between the two SL1 hairpins intermolecularly while intramolecule loop interaction would still be mediated by 6-base palindromic sequences. BsT2-BA contains an interrupted palindrome that might interfere with stable association between the two hairpins. Based on a previous analysis of mutants containing lesions in the hairpin loop (15, 28), the mutations in BsS1-BA and BsT2-BA would abrogate the homodimer formation of SL1 in vitro. Since it is believed that the palindromic sequence on the top of SL1 initiates dimer formation, BsS1-BA and BsT2-BA were expected to have a lethal defect in dimer formation. Nevertheless, BsT2-BA completely retained the ability to form dimers, and BsS1-BA partially retained the function. BsT6-BA and S1-BA were also unchanged in terms of dimerization. These results suggested that the contributions of the top hairpin loop and upper stem of SL1 to dimer formation are limited. Instead, the entire SL1 stem-loop, or possibly the lower stem of SL1, is required for complete RNA dimerization. In addition, the result for BsT2-BA clearly showed that the palindromic sequence on the hairpin loop of SL1 is not required for RNA-RNA interaction in the virion. In contrast, all SL1 mutations resulted in a reduction of packaging efficiency (Fig. 4B). Even the mutations that did not affect dimerization (BsT2, BsT6, and S1) caused some defect of encapsidation. These results indicate that the hairpin loop and top stem of SL1 are much more important in the generation of a packaging signal than in RNA dimerization. Consistent with this finding, Harrison et al. reported that SL1 stem corruption or loop deletion caused partial packaging and replication defects (20).
We have summarized the results in schematic functional maps (Fig. 6). It should be emphasized that there are parallel interrelations between DIS/DLS and E/psi in some regions presented. For example, complete deletion of SL1 resulted in a marked reduction of both dimerization and encapsidation abilities, whereas a top-stem truncation or top-loop deletion produced only a partial reduction of these two functions (Fig. 4B). Similarly, the mutations introduced in dLB4, dLC1, dLC2, and dLC3 affected, almost in parallel, the magnitudes of the packaging and dimerization functions (Fig. 2 and 3). Moreover, we could not find a region that is functional only for dimerization and not for packaging, whereas we observed regions functional for packaging but not dimerization (e.g., the entire R/U5 stem-loop or the upper region of the U5/L lower stem). In other words, the regions important for dimerization were always important for packaging. Previously, we reported that two E/DLS molecules are required but that dimerized RNA strands do not need to be packaged into HIV-1 virions (37). Taken together, our results strongly suggest that the RNA linkage structures and/or their interaction is required for packaging. We hypothesize that the RNA-packaging event consists of multiple steps and that RNA dimerization is one of the steps. We also assume that E/DLS RNA must be recognized as an intact packaging signal by viral protein only after a secondary or higher structure is formed by two E/DLS molecules. It should be noted, however, that the assumption that in our experimental system the intermolecular and intramolecular dimers are selected equally well for packaging is untested.
FIG. 6.
Functional mapping of the 5′ region of HIV-1. The functional maps of RNA dimerization (top) and genome packaging (bottom) are shown separately. The shadowed open lines represent the areas dispensable for each function. The solid bold lines represent the areas essential for each function. The shaded lines represent the areas important for efficient functions. The dashed lines represent the areas not yet analyzed.
It has been suggested that the upper-stem-loop parts of the U5/L region form a relatively complicated structure for the uptake of tRNA primer into the PBS (5, 24). Our results showed that this region is not necessary for dimer formation or the encapsidation of RNA (Fig. 2) and might suggest that the region exists primarily for primer uptake, even though it is located at the center of the E/DLS. We also found that the PBS site itself is required for neither dimerization nor packaging (Fig. 1), and these facts might imply that the primer binding event occurs independently of the packaging-dimerization event.
Our result with the mutants dLB3 and dLB4 (Fig. 2) showed that the contributions to dimerization of each side of the U5/L lower stem were not identical. This suggested that the primary structure rather than the stem structure in this region is important for mediating RNA dimer formation and/or that the lower stem region of the U5/L stem-loop might form differently. In addition, we could not deny the possibility that the large-deletion mutations might change the overall structure of the E/DLS region. The RNA structure model we used in this study was based on the Mfold program and is a relatively simple one (34). There is accumulating evidence that the HIV-1 leader RNA folds into a more complicated secondary, and probably tertiary, structure (4, 12, 21). However, the models currently suggested are based on in vitro experiments and/or computational analysis, and the HIV-1 RNA structure in vivo is still unclear. As our experiments were performed in an in vivo system, our data would thus be one of the clues to unveil the in vivo structures of E/DLS. The results of our DIS/DLS analysis contain some discrepancies from other reports using different systems (40). On the other hand, in a recent paper studying packaging signals in the U5/L stem-loop region (8), the regions shown to be necessary for encapsidation were quite similar to those in our results. These facts might also imply the complexity of E/DLS formation and structure in vivo.
HIV dimeric RNA is apparently stabilized by the action of NC protein in mature virions (13, 14, 16, 17). While we measured the extent of dimerization of the RNA in mature particles, the RNA is undoubtedly packaged without the stabilizing effect of free NC protein. It is at least a theoretical possibility that some RNAs are packaged without RNA-RNA interactions but are then converted to dimers by the action of NC when the virus matures. According to this strict approach, our data would suggest that the ability of the E/DLS region to form RNA dimers in mature particles is necessary but not sufficient for packaging.
In conclusion, we have demonstrated that the HIV-1 U5/L and SL1 regions could be a multifunctional domain and suggested that E/DLS dimerization might be an essential step for the genome packaging of HIV-1. As the region we have discussed was necessary but not sufficient for dimerization and/or packaging, more studies are under way to elucidate the minimum region required and sufficient for RNA dimerization of HIV-1 in vivo.
Acknowledgments
We thank Antonito T. Panganiban for helpful discussion and advice and Sayuri Sakuragi for encouragement.
J.-I.S. was supported by The Organization for Pharmaceutical Safety and Research. This work was supported by grants from the Organization for Pharmaceutical Safety and Research; the Human Health Foundation; the Ministry of Education, Culture, Sports, Science and Technology; and the Ministry of Health, Labour, and Welfare, Japan.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldovini, A., and B. D. Walker. 1990. Techniques in HIV research. Stockton Press, New York, N.Y.
- 3.Awang, G., and D. Sen. 1993. Mode of dimerization of HIV-1 genomic RNA. Biochemistry 32:11453-11457. [DOI] [PubMed] [Google Scholar]
- 4.Beerens, N., F. Groot, and B. Berkhout. 2001. Initiation of HIV-1 reverse transcription is regulated by a primer activation signal. J. Biol. Chem. 276:31247-31256. [DOI] [PubMed] [Google Scholar]
- 5.Beerens, N., B. Klaver, and B. Berkhout. 2000. A structured RNA motif is involved in correct placement of the tRNA(3)(Lys) primer onto the human immunodeficiency virus genome. J. Virol. 74:2227-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkhout, B., and J. L. van Wamel. 1996. Role of the DIS hairpin in replication of human immunodeficiency virus type 1. J. Virol. 70:6723-6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clever, J., C. Sassetti, and T. G. Parslow. 1995. RNA secondary structure and binding sites for gag gene products in the 5′ packaging signal of human immunodeficiency virus type 1. J. Virol. 69:2101-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clever, J. L., D. Miranda, Jr., and T. G. Parslow. 2002. RNA structure and packaging signals in the 5′ leader region of the human immunodeficiency virus type 1 genome. J. Virol. 76:12381-12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clever, J. L., and T. G. Parslow. 1997. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J. Virol. 71:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffin, J. 1984. Genome structure, p. 261-368. In R. Weiss, N. Teich, H. Vermus, and J. Coffin (ed.), RNA tumor viruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Coffin, J. M. 1979. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J. Gen. Virol. 42:1-26. [DOI] [PubMed] [Google Scholar]
- 12.Damgaard, C. K., H. Dyhr-Mikkelsen, and J. Kjems. 1998. Mapping the RNA binding sites for human immunodeficiency virus type-1 gag and NC proteins within the complete HIV-1 and -2 untranslated leader regions. Nucleic Acids Res. 26:3667-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darlix, J. L., C. Gabus, M. T. Nugeyre, F. Clavel, and F. Barre-Sinoussi. 1990. cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J. Mol. Biol. 216:689-699. [DOI] [PubMed] [Google Scholar]
- 14.De Rocquigny, H., C. Gabus, A. Vincent, M.-C. Fournie-Zaluski, B. Roques, and J.-L. Darlix. 1992. Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc. Natl. Acad. Sci. USA 89:9373-9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirac, A. M., H. Huthoff, J. Kjems, and B. Berkhout. 2001. The dimer initiation site hairpin mediates dimerization of the human immunodeficiency virus type 2 RNA genome. J. Biol. Chem. 276:32345-32352. [DOI] [PubMed] [Google Scholar]
- 16.Feng, Y. X., S. Campbell, D. Harvin, B. Ehresmann, C. Ehresmann, and A. Rein. 1999. The human immunodeficiency virus type 1 Gag polyprotein has nucleic acid chaperone activity: possible role in dimerization of genomic RNA and placement of tRNA on the primer binding site. J. Virol. 73:4251-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, Y. X., T. D. Copeland, L. E. Henderson, R. J. Gorelick, W. J. Bosche, J. G. Levin, and A. Rein. 1996. HIV-1 nucleocapsid protein induces “maturation” of dimeric retroviral RNA in vitro. Proc. Natl. Acad. Sci. USA 93:7577-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 19.Greatorex, J., and A. Lever. 1998. Retroviral RNA dimer linkage. J. Gen. Virol. 79:2877-2882. [DOI] [PubMed] [Google Scholar]
- 20.Harrison, G. P., G. Miele, E. Hunter, and A. M. L. Lever. 1998. Functional analysis of the core human immunodeficiency virus type 1 packaging signal in a permissive cell line. J. Virol. 72:5886-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huthoff, H., and B. Berkhout. 2001. Two alternating structures of the HIV-1 leader RNA. RNA 7:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höglund, S., Å. Öhagen, J. Goncalves, A. T. Panganiban, and D. Gabuzda. 1997. Ultrastructure of HIV-1 genomic RNA. Virology 233:271-279. [DOI] [PubMed] [Google Scholar]
- 23.Jones, J. S., R. W. Allan, and H. M. Temin. 1994. One retroviral RNA is sufficient for synthesis of viral DNA. J. Virol. 68:207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang, S. M., and C. D. Morrow. 1999. Genetic analysis of a unique human immunodeficiency virus type 1 (HIV-1) with a primer binding site complementary to tRNAMet supports a role for U5-PBS stem-loop RNA structures in initiation of HIV-1 reverse transcription. J. Virol. 73:1818-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laughrea, M., and L. Jette. 1994. A 19-nucleotide sequence upstream of the 5′ major splice donor is part of the dimerization domain of human immunodeficiency virus 1 genomic RNA. Biochemistry 33:13464-13474. [DOI] [PubMed] [Google Scholar]
- 26.Laughrea, M., L. Jette, J. Mak, L. Kleiman, C. Liang, and M. A. Wainberg. 1997. Mutations in the kissing-loop hairpin of human immunodeficiency virus type 1 reduce viral infectivity as well as genomic RNA packaging and dimerization. J. Virol. 71:3397-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laughrea, M., N. Shen, L. Jette, J. L. Darlix, L. Kleiman, and M. A. Wainberg. 2001. Role of distal zinc finger of nucleocapsid protein in genomic RNA dimerization of human immunodeficiency virus type 1: no role for the palindrome crowning the R-U5 hairpin. Virology 281:109-116. [DOI] [PubMed] [Google Scholar]
- 28.Laughrea, M., N. Shen, L. Jette, and M. A. Wainberg. 1999. Variant effects of non-native kissing-loop hairpin palindromes on HIV replication and HIV RNA dimerization: role of stem-loop B in HIV replication and HIV RNA dimerization. Biochemistry 38:226-234. [DOI] [PubMed] [Google Scholar]
- 29.Lear, A. L., M. Haddrick, and S. Heaphy. 1995. A study of the dimerization of Rous sarcoma virus RNA in vitro and in vivo. Virology 212:47-57. [DOI] [PubMed] [Google Scholar]
- 30.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Marquet, R., F. Baudin, C. Gabus, J. L. Darlix, M. Mougel, C. Ehresmann, and B. Ehresmann. 1991. Dimerization of human immunodeficiency virus (type 1) RNA: stimulation by cations and possible mechanism. Nucleic Acids Res. 19:2349-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride, M. S., and A. T. Panganiban. 1996. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J. Virol. 70:2963-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride, M. S., and A. T. Panganiban. 1997. Position dependence of functional hairpins important for human immunodeficiency virus type 1 RNA encapsidation in vivo. J. Virol. 71:2050-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride, M. S., M. D. Schwartz, and A. T. Panganiban. 1997. Efficient encapsidation of human immunodeficiency virus type 1 vectors and further characterization of cis elements required for encapsidation. J. Virol. 71:4544-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muriaux, D., P. M. Girard, B. Bonnet-Mathoniere, and J. Paoletti. 1995. Dimerization of HIV-1Lai RNA at low ionic strength. An autocomplementary sequence in the 5′ leader region is evidenced by an antisense oligonucleotide. J. Biol. Chem. 270:8209-8216. [DOI] [PubMed] [Google Scholar]
- 36.Paillart, J. C., R. Marquet, E. Skripkin, B. Ehresmann, and C. Ehresmann. 1994. Mutational analysis of the bipartite dimer linkage structure of human immunodeficiency virus type 1 genomic RNA. J. Biol. Chem. 269:27486-27493. [PubMed] [Google Scholar]
- 37.Sakuragi, J.-I., A. Iwamoto, and T. Shioda. 2002. Dissociation of genome dimerization from packaging functions and virion maturation of human immunodeficiency virus type 1. J. Virol. 76:959-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakuragi, J.-I., T. Shioda, and A. T. Panganiban. 2001. Duplication of the primary encapsidation and dimer linkage region of HIV-1 RNA results in the appearance of monomeric RNA in virions. J. Virol. 75:2557-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakuragi, J.-I., and A. T. Panganiban. 1997. Human immunodeficiency virus type 1 RNA outside the primary encapsidation and dimer linkage region affects RNA dimer stability in vivo. J. Virol. 71:3250-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen, N., L. Jette, M. A. Wainberg, and M. Laughrea. 2001. Role of stem B, loop B, and nucleotides next to the primer binding site and the kissing-loop domain in human immunodeficiency virus type 1 replication and genomic-RNA dimerization. J. Virol. 75:10543-10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skripkin, E., J. C. Paillart, R. Marquet, B. Ehresmann, and C. Ehresmann. 1994. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc. Natl. Acad. Sci. USA 91:4945-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundquist, W. I., and S. Heaphy. 1993. Evidence for interstrand quadruplex formation in the dimerization of human immunodeficiency virus 1 genomic RNA. Proc. Natl. Acad. Sci. USA 90:3393-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tchénio, T., and T. Heidmann. 1995. The dimerization/packaging sequence is dispensable for both the formation of high-molecular-weight RNA complexes within retroviral particles and the synthesis of proviruses of normal structure. J. Virol. 69:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]