Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the infectious cause of Kaposi's sarcoma (KS) and certain lymphoproliferations particularly in the context of human immunodeficiency virus (HIV) type 1-induced immunosuppression. The introduction of effective therapies to treat HIV has led to a decline in the incidence of KS, suggesting that immune responses may play a role in controlling KSHV infection and pathogenesis. Cytotoxic-T-lymphocyte (CTL) activity against KSHV proteins has been demonstrated; however, the identification of KSHV CTL epitopes remains elusive and problematic. Although the herpesvirus genomic layout is generally conserved, KSHV encodes a unique hypervariable protein, K1, with intense biological selection pressure at specific amino acid sites. To investigate whether this variability is partly driven by cellular immunity, we designed K1 peptides that match only the unique viral sequence for every individual studied here (autologous peptides). We identified functional CTL epitopes within K1's most variable areas, and we show that a given individual responds only to autologous peptides and not to peptides from other individuals. Furthermore, these epitopes are highly conserved sequences within KSHV isolates from a specific strain but are not conserved between different strains. We conclude that CTL recognition contributes to K1, and therefore to KSHV, evolution.
Cytotoxic-T-lymphocyte (CTL) responses against viral infections are often limited to reactivity against a few immunodominant epitopes, the identity of these being strongly influenced by the major histocompatibility complex (MHC) class I alleles of the host (17, 35). The majority of these epitopes have been identified in conserved sites, and this may be due in part to our inability to detect non-cross-reactive epitopes in variable domains (30). The importance of CTLs in controlling viral replication is supported by models of CD8-depleted animals with AIDS in which decreased numbers of CTLs result in high viral loads (28) and by the frequent selection of human immunodeficiency virus (HIV) or simian immunodeficiency virus mutants in vivo that are no longer recognized by CTLs and therefore escape immune surveillance (5, 24). Kaposi's sarcoma-associated herpesvirus (KSHV) can establish chronic infections, as can other herpesviruses, such as Epstein-Barr virus (EBV), varicella-zoster virus, and cytomegalovirus (26). CTLs against KSHV proteins in HIV-1-positive (22) and HIV-1-negative (32) individuals have been demonstrated; however, there are only four MHC class I-restricted epitopes in KSHV that have been identified thus far (1, 20, 31, 33).
The KSHV K1 open reading frame (ORF-K1) encodes a 46-kDa transmembrane glycoprotein that has been shown to induce foci of transformation in rat fibroblasts (14), enhance KSHV lytic replication (12), cooperate in NF-κB signaling in vitro (27) and in vivo (25), and potentially maintain latent infection by blocking B-cell activation and therefore the induction of lytic replication (13). ORF-K1, located at the extreme left-hand end of the KSHV genome, has positional homology with the gene encoding transforming protein LMP-1 of EBV. K1 is known to contain the two most variable regions (VR1 and VR2) across the entire viral genome, which are used to classify KSHV into four clades (A, B, C, and D) (6, 16, 36). Every viral isolate studied thus far is unique to an infected individual. Unlike the situation in retroviruses, where proteins targeted by the host immune system undergo CTL escape during infection, K1 mutations have not been detected within an infected individual over time (29, 36). There are various hypotheses explaining K1's variability, but evidence for a mechanism that drives its variability has not yet been shown (3, 6, 16, 36). Here, we investigate whether an immunological mechanism is partly responsible for this unique variability.
Initially, we performed computational analyses of K1 variability at the amino acid or codon level using three different measures not previously applied. We investigated the diversity and substitution rates at each amino acid site and then established the ratio of nonsynonymous substitutions (amino acid altering) to synonymous substitutions (silent, noncoding) at each codon position. This analysis allowed us to test whether CTL epitopes are specifically located within positively selected codon sites.
Previously, Osman et al. used a recombinant modified Ankara vaccine expressing K1 (A clade) and identified the presence of CTLs against the whole K1 protein (22). Here, we investigated the regions of K1 that elicit CTL responses, and for this, the patient's own (autologous) K1 sequences were required. Overlapping peptides that corresponded to an individual's exact K1 sequence were synthesized. The immunogenic epitopes of K1 were found to lie only within the highly positive selected region of VR1. We discuss the possibility of diversity in a DNA viral gene being driven by CTL recognition as opposed to CTL escape.
MATERIALS AND METHODS
Subjects.
One hundred twenty HIV-1-positive individuals were identified by positive antibody tests (HIV-1 enzyme-linked immunosorbent assay and HIV-1 Western blotting). All patients were receiving highly active antiretroviral therapy. DNA was extracted from peripheral blood mononuclear cells (PBMCs) for HLA class I typing and nested PCR for ORF-K1. ORK-K1 was amplifiable from 20 patients, and with 10 of these, overlapping peptides were generated for each individual's K1 sequence. The patients were selected on the basis of shared common HLA class I alleles and the availability of PBMCs for multiple immunological assays. All patients signed informed-consent agreements, and the study was approved by the University College and Royal Free Hospital Ethics committees.
PCR and sequencing.
DNA was extracted from 5 × 106 PBMCs by using a QIAamp blood kit (Qiagen, Hilden, Germany). K1 gene fragments were amplified by nested PCRs with 50-μl mixtures of buffer containing 2 μl of template DNA, 4.0 mM (first round) or 1.5 mM (second round) MgCl2, 1.0 U of Expand polymerase (Roche, Mannheim, Germany), and 10 pmol of the following primers: for the first round with K1 VR1, 5′-GTTCTGCCAGGCATAGTC-3′ and 5′-GTAACATGCTGACCACAAG-3′; for the second round with VR1, 5′-CTGGCGGCCCTTGTGTAAAC-3′ and 5′-GACTGTGTTTGATGGCTGTGC-3′; for the first round with K1 VR2, 5′-CGTCTCGCCTGTCAAATC-3′ and 5′-ACTGGTTGCGTATAGTCTTCC-3′; and for the second round with VR2, 5′-GTATATGTTTTTGGGCGCGTTG-3′ and 5′-CCGTGCACAAATCGTGTAGGG-3′. Alternative primers were also used if VR1 PCR was unsuccessful (in the first round, 5′-GGGTTCTGCCAGGCATAG-3′ and 5′-ACCACAAGTGACTGTGTTTG-3′, and in the second round, 5′-TCAGACCTTGTTGGACATC-3′ and 5′-ACACAAGGTTTGTAAGACAG-3′). The conserved area between VR1 and VR2 from nucleotides 324 to 480 was not amplified; however, overlapping peptides were made for this area. PCR conditions were as follows: 95°C for 2 min, 40 cycles of 94°C for 30 s, 52 to 58°C for 45 s, and 72°C for 1 min; and 72°C for 10 min. PCR products were purified with a QIAgen PCR product purification kit prior to sequencing with a CEQ dye terminator cycle sequencing kit (Beckman Coulter, Fullerton, Calif.).
HLA class I and II typing were performed by the Anthony Nolan Trust (Royal Free Hospital) by using amplification refractory mutation system-PCR with sequence-specific primers (2). Plasma HIV-1 viral loads were determined by the Bayer (Berkshire, United Kingdom) HIV-1 RNA 3.0 (branched DNA) assay or by PCR assay (Cobas Amplicor HIV-1 Monitor test version 1.5; Roche Diagnostics, Sussex, United Kingdom) with a lower level of detection of fewer than 50 HIV-1 RNA copies/ml.
IFN-γ ELIspot assay, CD4 and CD8 depletion, and flow cytometry.
PBMCs were separated from heparinized whole blood by standard density gradient centrifugation on Ficoll-Hypaque (Pharmacia, St. Albans, United Kingdom), and gamma interferon (IFN-γ) release ELIspot assays (MABTECH, Stockholm, Sweden) were carried out as previously described (7). In depletion experiments, assays were carried out with fresh PBMCs after depletion of T cells by using CD4 or CD8 MACS-positive selection beads (Mitenyi Biotec, Auburn, Calif.). Flow cytometry, following labeling with anti-CD4 fluorescein isothiocyanate and anti-CD8 phycoerythrin antibodies (Mitenyi Biotec), was performed on a MoFlow (Fort Collins, Colo.) apparatus, which confirmed that cell subset depletions were >95% in all experiments. Results were considered positive if the number of spot-forming cells per million PBMCs in peptide-stimulated wells was twofold higher than the number of spots per million PBMCs in control wells and if at least 50 spots per million PBMCs were present. The peptide concentration was 5 μM.
Synthetic peptides.
Peptides were synthesized by conventional fluorenylmethoxycarbonyl (f-moc) solid-phase chemistry and analyzed for purity (>95%) by reverse-phase high-pressure liquid chromatography (Shimadzu, Chester, United Kingdom) and mass spectrometry (Thermo Finnigan AQA, San Jose, Calif.). In total, 130 K1 peptides were synthesized, as 50% of these were shared with more than one patient (15-mers overlapping by 5 or 10 nucleotides or 9-mers and 10-mers).
Chromium release assay.
Confirmation of CTL activity was determined by the chromium release assay as previously described (22). Briefly, patients' PBMCs were cultured in 10% AB plasma-RPMI 1640 medium at 2 × 106 cells/ml and stimulated with a 50 μM concentration of the relevant peptide and 50 U of interleukin 2 (Roche Diagnostics Ltd.) per ml, which was added on days 0, 3, and 7 of culture. Effector cells were assessed for specific killing of 51Cr-labeled EBV-transformed autologous B-cell targets (pulsed with each peptide) at effector-to-target ratios of 10:1, 20:1, 40:1, and 80:1 on day 10. Autologous B-cell lines and HLA-matched and -mismatched targets were tested with and without peptide. A spontaneous release of ≤15% of maximum was considered acceptable.
B*27 binding assay.
To confirm that the B*27 peptide identified bound HLA-B*27, we tested binding in an assembly assay with the TAP-defective RMA-S-B*27 line (4, 9, 15). HLA-B*27 molecules stabilized by peptides were monitored by direct staining with fluorescein isothiocyanate-coupled anti-HLA-B*27-specific monoclonal antibodies (HLA-ABCm3; Serotec, Eching, Germany).
Analysis of protein variability at the single-site level.
One hundred forty-five unique nucleotide sequences encoding all of ORF-K1 were collected from the National Center for Biotechnology Information (GenBank) (3, 10, 11, 19). Sequences that contained in-frame insertions (including the putative K1-D strain sequences thus far reported) were excluded from the present analysis, and sequences that clustered weakly within K1-A, -B, and -C strains were also excluded. The amino acid sequences deduced from the nucleotide sequences were aligned with each other by using CLUSTAL X, version 1.6. Alignment was successful for all 289 amino acid sites in 118 different K1 sequences, and no regions were excluded from analysis. Sequences were split into K1-A, -B, or -C strains by computer-based multialign and phylogenetic-tree programs from the National Institutes of Health website (http://bimas.dcrt.nih.gov/molbio /index.html), thus confirming previous reports (6, 16, 36). Programs to analyze changeability and diversity were a kind gift from Yumi Yamaguchi-Kabata (National Institute of Genetics, Mishima, Japan) (34).
In order to analyze the number of amino acid substitutions (changeability), we calculated the number of amino acid changes that have arisen throughout the phylogeny. To further evaluate the level of amino acid variation in this population, we defined the diversity of amino acids at a given position as the proportion of pairs having different amino acids in two sequences randomly chosen from the sample (34). This value was estimated from the frequencies of different amino acids or gaps at each position. This quantity is analogous to the concepts of nucleotide diversity and heterozygosity or gene diversity in population genetics. The amount of diversity estimated by these methods is independent of the number of sequences if the sequences are randomly sampled. Because some sites in K1-C sequences included gaps (within VR2), the diversity of gaps (Dgap) was estimated as
 |
The diversity of amino acids was also estimated as
 |
xi represents the proportion of the ith amino acid (aa) at a given position, and xgap represents the proportion of the gap at a given position.
Elucidation of positively selected individual codon sites in K1.
An excess of nonsynonymous (dn) over synonymous (ds) codon mutations was used to identify sites of positive selection with the CODEML program from Yang's Phylogenetic Analysis Maximum Likelihood (PAML) software (http://abacus.gene.ucl.ac.uk/research.html) (21). K1-A, -B, and -C sequence data sets were analyzed separately to ascertain possible differences. PAML corrects the raw dn and ds values of each site (compared to those of a single consensus sequence) for the likely transition-to-transversion ratio, the empirical base frequency in the sequence groups, and the biased likelihood of each codon to undergo either synonymous or nonsynonymous change. After this correction, the dn-to-ds ratio is termed ω. Previous analyses have observed changes by K1 region but not by site (6, 16). We first tested the null hypothesis by comparing two distinct models of the distribution of ω values between sites. The first was a neutral-selection model (the null hypothesis) that fixes dn/ds at either 0 or 1, and the second was an alternative model with a discrete distribution of ω values (eight classes), including positive selection of values (ω > 1). The likelihood-score ratio of each model was compared to a chi-square distribution with 5 df (as the discrete model has six extra parameters). The alternative model was found to produce a significantly superior fit of the data for the K1-A, -B, and -C data sets. We obtained sequences for the MHC class I B locus (exons 2 and 3 of HLA-B subtypes), KSHV ORF-26 (8), HIV-1 nef (18), and HIV-1 env (subtype B) (34) from GenBank and calculated the dn/ds ratios with the above-described methods (Table 1). The sequences used were obtained by using accession numbers corresponding to different individuals, and all sequences were confirmed to be different.
TABLE 1.
Sequence acquisition numbers for dn/ds ratio analysis
RESULTS
Analysis of K1 variability by substitutions, diversity, and dn-to-ds ratios.
We established a phylogenetic analysis and relationship among the three major divergent K1 strains (A, B, and C), as has been previously done with 63 K1 sequences (36); however, in this study we used 118 complete K1 DNA sequences obtained from an updated GenBank database (Fig. 1A). Following translation of the DNA sequences to proteins, the cumulative substitution rates and diversity of the encoded protein at each position in sequence alignments (Fig. 1B) were determined. This analysis showed that all three strains have a high rate of amino acid substitution and diversity across K1 (strain K1-B > K1-A > K1-C for substitutions and diversity). After separating K1 sequences into their respective strains, we created a map of positive selection and calculated the dn/ds ratio (or ω) per codon (21) (Fig. 1C). We observed that the highest probabilities of positive selection in K1 are clustered primarily between codon positions 53 and 94 (VR1) (36), particularly between codons 55 and 71. Figure 1D shows the cumulative dn/ds ratios for all three strains. Overall, there are higher dn/ds ratios for A strains than for B and C strains. The analyses, performed for the first time at the single amino acid level, also show that B strains have more diffuse scattering of positive selection across K1 than A and C strains, where mutations occur specifically in VR1 and VR2 (Fig. 1C). K1-C strains had the highest numbers of substitutions, greatest diversity, and highest probabilities of positive selection but only for the VR2 region (amino acids 191 to 228).
FIG. 1.
(A) Predicted phylogenetic relationships among the 118 complete KSHV ORF-K1 protein sequences (A strain in blue [n = 45]; B strain in red [n = 21], and C strain in green [n = 52]). (B) Cumulative substitution rates and diversity across KSHV ORF-K1 determined with a radial phenogram constructed with alignment (CLUSTAL X, version 1.6) and treeview (Treeview, version 1.5) programs show that A and C strains are the most closely related. For substitutions, the gradient is offset by −1, so a gradient of 0 is equivalent to no substitution. (C) dn-to-ds ratios (ω) across the K1 gene for A, B, and C strains as measured per codon by PAML software. Shown are the results of an analysis of ω values at each position across K1 in all three strains, and specific peaks indicate highly positively selective areas. (D) Cumulative ω values among the three strains showing an overall increase in ω, i.e., the K1 protein is under positive selection throughout the whole sequence (ω > 1). Previously postulated areas of VR1 and VR2 are indicated by light lines; darker dashes (codons 55 to 71) indicate our updated VR1 on the basis of analysis of 118 complete K1 sequences.
Our comparison of the mean dn/ds ratios among ORF-K1, other viral genes, and human MHC class I-B sequences demonstrates that the biological selective pressure observed in K1 is high and comparable to that of the variable areas of other genes studied (Fig. 2). Complete HIV-1 env genes from subtype B strains (and the KSHV ORF-26 gene) were negatively selected. No individual codon sites in the KSHV ORF-26 gene had a dn/ds ratio of >1 (Fig. 2.).
FIG. 2.
Comparison on a logarithmic scale of K1 mean ω values for strains A (45 sequences, 289 codons), B (21 sequences, 289 codons), and C (52 sequences, 284 codons) with the mean ω values of the human MHC class I-B genes (exons 2 and 3; 17 sequences, 262 codons), including the antigen recognition site (17 sequences, 58 codons); HIV-1 env genes (186 sequences, 414 codons), including the V3 loop (34 codons); nef genes (31 sequences, 170 codons); and KSHV ORF-26 genes (9 sequences, 165 codons). The ω value for VR1 is based on codons 55 to 71. Sequence acquisition numbers are provided in Table 1.
Immunogenic epitope analysis by ELIspot.
We synthesized from 10 individuals autologous K1-overlapping peptides covering the whole protein and performed IFN-γ release ELIspot assays (Fig. 3A and B). Two out of 10 did not show any responses (1 of these 2 individuals had received recent systemic cytotoxic treatment for non-Hodgkin's lymphoma). For the eight remaining patients, we observed responses against autologous viral peptide sequences and all responses were in peptides at sites in and around the most variable region of K1 VR1 (Fig. 3A). Furthermore, there were no responses when patients' PBMCs were stimulated with nonautologous viral epitopes and all 10 patients were tested with pooled nonautologous peptides corresponding to the K1 sequence of other patients (Fig. 3B and C).
FIG. 3.
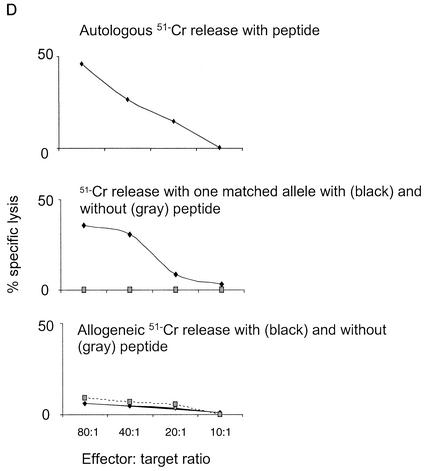
Epitopes that elicit CTL responses as demonstrated by IFN-γ release ELIspot assays, with HLA class I restriction, were identified in eight HIV-1-infected patients on highly active antiretroviral therapy. (A) Patients' K1 amino acid sequences from codons 50 to 101. Epitope sequences that elicit responses and HLA class I-restricted alleles (Cw3, B*27, B*51, and B*55) are shaded. The viral strain of each patient is also indicated. The same class II HLA types were not shared between individuals that responded to the same epitope, and CD8+ depletion eliminated responses. (B) ELIspot data for 10 patients in whom autologous peptides were made. (C) Summary of ELIspot and chromium release assays. The number of responding patients tested is shown in brackets with the number of spot-forming cells per 106 PBMCs (ELIspot) and the highest mean percentage of specific lysis (51Cr release) for each patient. E:T, effector-to-target ratio. (D) Representative Cr release assay demonstrating percentage of specific lysis with the B*27-restricted peptide HRQSIWITW (data for patient 4 are shown). Results of experiments with an autologous effector and target (top), an effector and target matched at B*27 (middle), and an unmatched effector and target (with and without the peptide) are shown.
Chromium release assay.
Confirmation of cell killing of targets presenting all four peptides was determined by using a standard chromium release assay (Fig. 3C). K1-specific CTL responses were greatest to the B*27-restricted nonamer HRQSIWITW, with a mean specific cytotoxicity of 43% (±5%) at an effector/target ratio of 80:1 (Fig. 3D). The mean specific cytotoxicities for the other three peptides at this effector/target ratio ranged from 17 to 34% (Fig. 3C). The correlation between ELIspot results and KSHV-specific cytotoxicity in the chromium release assay was 0.373 (r value). Responses were greatest for effector-mediated killing of autologous B-cell targets followed by HLA-matched targets. Background counts were observed by using effectors against HLA-unmatched targets, with and without peptides (Fig. 3D).
Epitope frequency among strains.
To investigate whether the four epitopes identified here were shared among the three strains, we aligned 118 K1 protein sequences from the GenBank database. It appears that all the epitopes are relatively conserved within each strain and are not shared with the other strains; e.g., the B*27-restricted epitope exists in over 90% of the sequences from the A strain but is not present in either B or C strains (Table 2). However, two amino acid changes within this epitope resulted in a 9-mer, highly conserved in clade C strain viruses (90.4%), which did not induce any CTL responses in two tested patients carrying the A strain.
TABLE 2.
Intrastrain conservation of identified epitopesa
Epitope occurrence within the three viral strains as deduced from the analysis of the 118 complete K1 protein sequences. 9-mer peptides correspond to the epitopes identified in this study, and the HLA class I restriction is indicated. Amino acids in bold represent changes in alternative peptide sequences in the patients indicated as determined by ELIspot assay. Peptides corresponding to KSHV strain A are in blue; alternative peptides corresponding to strain C are in green.
B*27 binding assay.
Transfected RMA-S-B*27 cells incubated at 37°C without peptide showed no HLA-B*27 expression but did so at 27°C. However, when the temperature was raised from 27 to 37°C, we found that after 8 h, the cell line no longer showed empty heavy-chain expression (i.e., it was thermolabile). In contrast, when the RMA-S-B*27 line was incubated with exogenous peptide at 27°C, stable expression of heavy chains was maintained at 37°C (i.e., it was thermostable). Incubation with an unrelated control peptide with HLA-B*27 revealed no stabilization of HLA-B*27 on the surface, whereas the B*27 nonamer (sequence HRQSIWITW) and decamer (sequence HRQSIWITWH), both of which induce IFN-γ release in ELIspot assays with samples from B*27-positive patients infected with KSHV strains that contain this epitope, showed stabilization of the molecule (Fig. 4).
FIG. 4.
B*2702 binding assay on RMS-S-B*27-expressing cells. The left-hand panels show RMA-S-B*27 (expressing B*27) cells without peptide or with negative control peptide at 27°C (thermostable) to 37°C (thermolabile). The right panels show results for positive controls and binding to the B*27-restricted epitope (nonamer or decamer).
We identified HLA class I restriction within B*27, B*51, B*55, and Cw*3 alleles (Fig. 3), as responses to these epitopes were observed in more than one patient possessing that allele and were not observed in mismatched cases (Fig. 3A). ELIspot, chromium release, and B*27 binding assays confirmed that these epitopes are immunogenic. We also used an HLA-binding, motif-based epitope prediction algorithm which indicated high scoring of these epitopes (23). In CD8 depletion experiments, all positive responses were eliminated (data not shown). CD8 depletions were confirmed by fluorescence-activated cell sorter analysis (data not shown).
DISCUSSION
In this study, we explored the relationship between positive selection in KSHV K1 and sites of CTL recognition. It was previously shown that K1 has two hypervariable regions (3, 6, 16, 36). Here, we mapped the positive selection and variability of K1 at the codon rather than averaging within a window (16) (Fig. 1B and C). This enabled us to compare precisely sites of positive selection by exhaustive CTL epitope mapping. Given this level of analysis, we suggest a redefinition of VR1 as extending from codons 55 to 71 rather than the previously described extension from codons 53 to 94 (Fig. 1D) (6). The level of positive selection within VR1 is comparable to, or possibly higher than, a number of well-known positively selected immunogenic regions in other genes studied (Fig. 2).
We next tested whether this variability is partly driven by cellular immunity. To identify class I-restricted epitopes within K1, we required autologous peptides corresponding exactly to the patient's own viral sequence and individuals of an appropriate HLA class I type that were infected with a specific viral strain. We tested the entire K1 proteins of 10 individuals by using autologous overlapping peptides, yet we could find no CTL responses outside the VR1 region (Fig. 3A). We show that CTL responses occurred in 8 out of 10 patients tested and that the responses were specifically against positively selected sites within KSHV as identified by ELIspot, chromium release, and binding assays (Fig. 1C, 3, and 4). Epitopes located at equivalent K1 regions in other KSHV strains did not show binding (or antagonism in ELIspot assays) when the peptides were tested in patients with a different K1 strain and HLA class I allele (Fig. 3B). This result provides evidence to suggest that interstrain antigenic diversity between K1 subtypes exists and may promote viral-host “coevolution.”
We also aligned 118 K1 protein sequences from the GenBank database to investigate whether the four epitopes identified here are conserved among the three strains. We observed that all the epitopes were relatively conserved within each strain and were not shared with the other strains (Table 2). This finding suggests intrastrain conservation of epitope antigenicity and might partly explain why each strain predominates in a specific population. KSHV A strain is found in the United States and in northwestern Europe, C is found mainly in Mediterranean and Middle Eastern populations, and B dominates in sub-Saharan Africa (6, 16, 36).
We and others have thus far been able to identify only four other epitopes that are all HLA-class I A*02 restricted in the complete KSHV genome. This was done by using peptides that overlap a number of conserved proteins or by using computer programs to predict HLA-binding epitopes (1, 20, 31, 33). We also evaluated K1 using a binding prediction algorithm program to identify peptides that could bind HLA-A*02 alleles, but the scores were low (23). Previous attempts to identify HLA-binding motifs by computational analysis with consensus K1 sequences also failed to predict CTL responses (1). Further studies will show why uncommon alleles are preferentially selected for CTL responses against K1 and whether this is a result of a viral immune evasion mechanism towards common HLA class I molecules.
To confirm that K1 does not change over time in an individual, we sequenced K1 in DNA samples from various time points (up to 5 years apart) in 16 patients, and no change in their specific K1 nucleotide sequences was observed (data not shown). Furthermore, K1 does not appear to change in different tumor sites within an individual. For example, in one patient with KSHV-infected primary effusion lymphoma, Castleman's disease, and KS, identical K1 sequences were reported in all three tumors (29).
The genetic changes occurring in K1 are probably a result of mutations that accumulated over epochs of viral evolution. CTL recognition is clearly associated with K1 variability, but further studies will show whether other immunological factors like humoral responses also contribute to this. Herpesviruses have evolved through cospeciation with their hosts. Evasion of all host immune control mechanisms will lead to overwhelming viral infection with subsequent death of the host and, therefore, of the virus. For viruses to persist as latent infections without causing harm, a pathogen-host equilibrium must be established (35). K1 is an early-lytic-phase antigen; given the cell-mediated responses shown here, it is possible that continual immune surveillance, even in the context of HIV-1 infection, serves to recognize cells reactivating from latency, which limits viral dissemination, thus preserving the host and therefore the virus. Our data show a clustering of functional CTL epitopes in the most positively selected region of the most variable gene of KSHV. We consider it possible that the pressure causing this selection could be a mechanism associated with virus-host natural adaptation and, therefore, survival.
Acknowledgments
We thank Tony Drinkwater, Zoe Cuthbertson, Pat Byrne, and Diana Aldam for recruiting study subjects and Mary Collins, Robin Weiss, and Paul Klenerman for discussion. We thank Richard Campbell for help with peptide synthesis. We also thank Anne-Margaret Little and Tawna Pitts for HLA typing and fluorescence-activated cell sorter analysis. We are grateful to Yumi Yamaguchi-Kabata and Ziheng Yang for computational programs and to the patients who participated in this study.
J.S. and N.W. are Medical Research Council Ph.D. students, and the work was also supported by Cancer Research U.K. and The Leukemia Research Fund.
REFERENCES
- 1.Brander, C., P. O'Connor, T. Suscovich, N. G. Jones, Y. Lee, D. Kedes, D. Ganem, J. Martin, D. Osmond, S. Southwood, A. Sette, B. D. Walker, and D. T. Scadden. 2001. Definition of an optimal cytotoxic T lymphocyte epitope in the latently expressed Kaposi's sarcoma-associated herpesvirus kaposin protein. J. Infect. Dis. 184:119-126. [DOI] [PubMed] [Google Scholar]
- 2.Bunce, M., C. M. O'Neill, M. C. Barnardo, P. Krausa, M. J. Browning, P. J. Morris, and K. I. Welsh. 1995. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens 46:355-367. [DOI] [PubMed] [Google Scholar]
- 3.Cook, P. M., D. Whitby, M. L. Calabro, M. Luppi, D. N. Kakoola, H. Hjalgrim, K. Ariyoshi, B. Ensoli, A. J. Davison, T. F. Schulz, et al. 1999. Variability and evolution of Kaposi's sarcoma-associated herpesvirus in Europe and Africa. AIDS 13:1165-1176. [DOI] [PubMed] [Google Scholar]
- 4.De Silva, A. D., A. Boesteanu, R. Song, N. Nagy, E. Harhaj, C. V. Harding, and S. Joyce. 1999. Thermolabile H-2Kb molecules expressed by transporter associated with antigen processing-deficient RMA-S cells are occupied by low-affinity peptides. J. Immunol. 163:4413-4420. [PubMed] [Google Scholar]
- 5.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 6.Hayward, G. S. 1999. KSHV strains: the origins and global spread of the virus. Semin. Cancer Biol. 9:187-199. [DOI] [PubMed] [Google Scholar]
- 7.Herr, W., E. Ranieri, A. Gambotto, L. S. Kierstead, A. A. Amoscato, L. Gesualdo, and W. J. Storkus. 1999. Identification of naturally processed and HLA-presented Epstein-Barr virus peptides recognized by CD4(+) or CD8(+) T lymphocytes from human blood. Proc. Natl. Acad. Sci. USA 96:12033-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakoola, D. N., J. Sheldon, N. Byabazaire, R. J. Bowden, E. Katongole-Mbidde, T. F. Schulz, and A. J. Davison. 2001. Recombination in human herpesvirus-8 strains from Uganda and evolution of the K15 gene. J. Gen. Virol. 82:2393-2404. [DOI] [PubMed] [Google Scholar]
- 9.Kuon, W., H. G. Holzhutter, H. Appel, M. Grolms, S. Kollnberger, A. Traeder, P. Henklein, E. Weiss, A. Thiel, R. Lauster, P. Bowness, A. Radbruch, P. M. Kloetzel, and J. Sieper. 2001. Identification of HLA-B27-restricted peptides from the Chlamydia trachomatis proteome with possible relevance to HLA-B27-associated diseases. J. Immunol. 167:4738-4746. [DOI] [PubMed] [Google Scholar]
- 10.Lacoste, V., J. G. Judde, J. Briere, M. Tulliez, B. Garin, E. Kassa-Kelembho, J. Morvan, P. Couppie, E. Clyti, J. Forteza Vila, B. Rio, A. Delmer, P. Mauclere, and A. Gessain. 2000. Molecular epidemiology of human herpesvirus 8 in Africa: both B and A5 K1 genotypes, as well as the M and P genotypes of K14.1/K15 loci, are frequent and widespread. Virology 278:60-74. [DOI] [PubMed] [Google Scholar]
- 11.Lacoste, V., E. Kadyrova, I. Chistiakova, V. Gurtsevitch, J. G. Judde, and A. Gessain. 2000. Molecular characterization of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 strains from Russia. J. Gen. Virol. 81:1217-1222. [DOI] [PubMed] [Google Scholar]
- 12.Lagunoff, M., D. M. Lukac, and D. Ganem. 2001. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 75:5891-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, B. S., X. Alvarez, S. Ishido, A. A. Lackner, and J. U. Jung. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 192:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, H., R. Veazey, K. Williams, M. Li, J. Guo, F. Neipel, B. Fleckenstein, A. Lackner, R. C. Desrosiers, and J. U. Jung. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat. Med. 4:435-440. [DOI] [PubMed] [Google Scholar]
- 15.Ljunggren, H. G., and K. Karre. 1985. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J. Exp. Med. 162:1745-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeoch, D. J. 2001. Molecular evolution of the gamma-herpesvirinae. Philos. Trans. R. Soc. Lond. B 356:421-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 18.McNearney, T., Z. Hornickova, A. Templeton, A. Birdwell, M. Arens, R. Markham, A. Saah, and L. Ratner. 1995. Nef and LTR sequence variation from sequentially derived human immunodeficiency virus type 1 isolates. Virology 208:388-398. [DOI] [PubMed] [Google Scholar]
- 19.Meng, Y. X., T. J. Spira, G. J. Bhat, C. J. Birch, J. D. Druce, B. R. Edlin, R. Edwards, C. Gunthel, R. Newton, F. R. Stamey, C. Wood, and P. E. Pellett. 1999. Individuals from North America, Australasia, and Africa are infected with four different genotypes of human herpesvirus 8. Virology 261:106-119. [DOI] [PubMed] [Google Scholar]
- 20.Micheletti, F., P. Monini, C. Fortini, P. Rimessi, M. Bazzaro, M. Andreoni, M. Giuliani, S. Traniello, B. Ensoli, and R. Gavioli. 2002. Identification of cytotoxic T lymphocyte epitopes of human herpesvirus 8. Immunology 106:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen, R., and Z. Yang. 1998. Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics 148:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osman, M., T. Kubo, J. Gill, F. Neipel, M. Becker, G. Smith, R. Weiss, B. Gazzard, C. Boshoff, and F. Gotch. 1999. Identification of human herpesvirus 8-specific cytotoxic T-cell responses. J. Virol. 73:6136-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker, K. C., M. A. Bednarek, and J. E. Coligan. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163-175. [PubMed] [Google Scholar]
- 24.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. Bangham, C. R. Rizza, et al. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354:453-459. [DOI] [PubMed] [Google Scholar]
- 25.Prakash, O., Z. Y. Tang, X. Peng, R. Coleman, J. Gill, G. Farr, and F. Samaniego. 2002. Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J. Natl. Cancer Inst. 94:926-935. [DOI] [PubMed] [Google Scholar]
- 26.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 27.Samaniego, F., S. Pati, J. Karp, O. Prakash, and D. Bose. 2000. Human herpesvirus 8 k1-associated nuclear factor-kappa b-dependent promoter activity: role in Kaposi's sarcoma inflammation? J. Natl. Cancer Inst. Monogr. 28:15-23. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 29.Stebbing, J., N. Wilder, S. Ariad, and M. Abu-Shakra. 2001. Lack of intra-patient strain variability during infection with Kaposi's sarcoma-associated herpesvirus. Am. J. Hematol. 68:133-134. [DOI] [PubMed] [Google Scholar]
- 30.Walker, B. D., and B. T. Korber. 2001. Immune control of HIV: the obstacles of HLA and viral diversity. Nat. Immunol. 2:473-475. [DOI] [PubMed] [Google Scholar]
- 31.Wang, Q. J., X.-L. Huang, G. Rappocciolo, F. J. Jenkins, W. H. Hildebrand, Z. Fan, E. K. Thomas, and C. R. Rinaldo. 2002. Identification of an HLA A*0201-restricted CD8+ T-cell epitope for the glycoprotein B homolog of human herpesvirus 8. Blood 99:3360-3366. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Q. J., F. J. Jenkins, L. P. Jacobson, L. A. Kingsley, R. D. Day, Z. W. Zhang, Y. X. Meng, P. E. Pellet, K. G. Kousoulas, A. Baghian, and C. R. Rinaldo, Jr. 2001. Primary human herpesvirus 8 infection generates a broadly specific CD8(+) T-cell response to viral lytic cycle proteins. Blood 97:2366-2373. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson, J., A. Cope, J. Gill, D. Bourboulia, P. Hayes, N. Imami, T. Kubo, A. Marcelin, V. Calvez, R. Weiss, B. Gazzard, C. Boshoff, and F. Gotch. 2002. Identification of Kaposi's sarcoma-associated herpesvirus (KSHV)-specific cytotoxic T lymphocyte epitopes and evaluation of reconstitution of KSHV-specific responses in human immunodeficiency virus type 1-infected patients receiving highly active antiretroviral therapy. J. Virol. 76:2634-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi-Kabata, Y., and T. Gojobori. 2000. Reevaluation of amino acid variability of the human immunodeficiency virus type 1. gp120 envelope glycoprotein and prediction of new discontinuous epitopes. J. Virol. 74:4335-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zinkernagel, R. M., and H. Hengartner. 2001. Regulation of the immune response by antigen. Science 293:251-253. [DOI] [PubMed] [Google Scholar]
- 36.Zong, J.-C., D. M. Ciufo, D. J. Alcendor, X. Wan, J. Nicholas, P. J. Browning, P. L. Rady, S. K. Tyring, J. M. Orenstein, C. S. Rabkin, I.-J. Su, K. F. Powell, M. Croxson, K. E. Foreman, B. J. Nickoloff, S. Alkan, and G. S. Hayward. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73:4156-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]