Abstract
It is believed that flavivirus assembly occurs by intracellular budding of the nucleocapsid into the lumen of the endoplasmic reticulum (ER). Recombinant expression of tick-borne encephalitis (TBE) virus envelope proteins prM and E in mammalian cells leads to their incorporation into enveloped recombinant subviral particles (RSPs), which have been used as a model system for studying assembly and entry processes and are also promising vaccine candidates. In this study, we analyzed the formation and secretion of TBE virus RSPs and of a membrane anchor-free E homodimer in mammalian cells. Immunofluorescence microscopy showed that E was accumulated in the lumen of the ER. RSPs were observed by electron microscopy in the rough and smooth ER and in downstream compartments of the secretory pathway. About 75% of the particles appeared to be of the size expected for RSPs (about 30 nm in diameter), but a number of larger particles and tubular structures were also observed in these compartments. Secretion of membrane anchor-free E dimers was detected 30 min after synthesis of prM and E, and secretion of RSPs was detected 1 h after synthesis of prM and E. We also found that the presence of the single N-linked oligosaccharide side chain on the E protein and its trimming by glucosidases was necessary for secretion of RSPs and truncated E dimers. Our results suggest that incorporation of prM and E into RSPs occurs at the ER membrane without other viral elements being required, followed by rapid transport along the compartments of the secretory pathway and secretion. Moreover, the carbohydrate side chain of E is involved in at least one assembly or transport step.
Before enveloped viruses leave the host cell after replication, they acquire a lipid bilayer by budding at the plasma membrane or at the membrane of an intracellular organelle such as the endoplasmic reticulum (ER), the ER-to-Golgi intermediate compartment (ERGIC), or the Golgi complex. This implies that the viral envelope proteins as well as the viral genome have to be transported to the site of virion formation. Moreover, molecular signals or determinants are usually required for coordinated virus assembly.
Flaviviruses, a genus of small enveloped RNA viruses within the family Flaviviridae, are assembled intracellularly, probably by budding into the ER of the infected cell (reviewed in reference 36). Virus particles were detected by electron microscopy in the lumen of the rough ER and in either the lumen of the smooth ER or the ERGIC (32, 56). However, budding intermediates at the ER membrane have not been observed, and the molecular mechanisms of flavivirus assembly are largely unknown.
During flavivirus infections, noninfectious subviral particles that contain the viral envelope proteins but lack the nucleocapsid are released in addition to infectious virions (45). Similar particles are also produced when the flavivirus envelope proteins are expressed without any other viral proteins (5, 41). They were shown to be excellent immunogens and thus promising vaccine candidates (see reference 27 and references therein). Other studies have demonstrated the utility of flavivirus subviral particles as a model system for virus assembly and entry (3, 12, 19, 48).
The envelope of tick-borne encephalitis (TBE) virus, the member of the Flavivirus genus used in this study, consists of a regular lattice formed by two viral membrane proteins, namely, the major envelope glycoprotein E (molecular mass, 52 kDa) and the small membrane protein M (molecular mass, 7 to 8 kDa). They are synthesized as part of a polyprotein precursor that is co- and posttranslationally cleaved into the individual chains (reviewed in reference 36).
E mediates virus entry into the cell via receptor-mediated endocytosis, and it carries the major antigenic epitopes leading to a protective immune response (reviewed in reference 28). It is a type I membrane glycoprotein (carrying one N-linked oligosaccharide) with two transmembrane segments at its carboxy terminus linked by a short cytoplasmic loop. The solution of the X-ray structure of the ectodomain of E showed that the protein forms head-to-tail homodimers on the viral surface (43). When exposed to low pH, the E proteins undergo an irreversible rearrangement leading to dissociation of the dimers followed by formation of trimers, and these transitions are apparently required for fusion (4, 52, 53). Recent experimental data provided evidence that the disulfide-linked loop at the tip of the E protein functions as an internal fusion peptide (3).
The N-linked glycosylation consensus sequence of E at position 154 is present in other flaviviruses as well (10). It has been suggested that the carbohydrate side chain may stabilize the dimer contacts between two E molecules (43). Another study has revealed that the N-linked glycan does not play a major role in the antigenic structure of the TBE virus E protein (57). In general, it is not clear whether the carbohydrate side chain of E is involved in flavivirus maturation or not, since the protein is not glycosylated in other flaviviruses, such as some strains of Kunjin virus and West Nile virus (1, 11).
M is synthesized as a precursor protein, prM (molecular mass, 25 kDa) containing one carbohydrate side chain. Members of our group and others have shown that prM has a chaperone-like role in the folding and maturation of E (34, 38). Heterodimer formation between prM and E starts soon after synthesis, a process that seems to be essential for E to reach its final native conformation. The interaction between prM and E is also important for later processing steps. It has been suggested that prM holds E in an inactive conformation to prevent low-pH rearrangements during transport through the acidic compartments of the trans-Golgi network (reviewed in reference 27). Shortly before the virus is released from the cell, the pr portion is cleaved from prM by the cellular protease furin, leading to mature virions consisting of E and M molecules (51).
A few years ago, a plasmid vector system for the expression of prM and E of TBE virus was developed that leads to the formation and secretion of recombinant subviral particles (RSPs) in mammalian cells (5). Studies using purified RSPs demonstrated that they were smaller in size than virions (30 nm in diameter instead of 50 nm) (48), and they had similar surface properties and fusion activity as infectious viruses (12, 48). Recently, the structure and protein arrangement of TBE virus RSPs were resolved by cryoelectron microscopy (19). This study revealed that 60 molecules each of M and E were arranged in a T=1 symmetry on the surface of the particle.
Data from experiments using prM and C-terminally truncated variants of E revealed that the first of the two transmembrane segments of E was sufficient for incorporation of E into RSPs and that deletion of the entire transmembrane region led to the secretion of soluble homodimeric E proteins (5, 6).
In this study, we have investigated the assembly and secretion of RSPs as well as a membrane anchor-free variant of E by recombinant expression of the TBE virus envelope proteins. We found that the majority of E was localized to the lumen of the ER. RSPs were detected in the rough and smooth ER, the ERGIC, and the Golgi complex. Secreted RSPs were detected in the cell culture medium 1 h after synthesis, whereas membrane anchor-free E was present in the medium already 30 min after synthesis. Inhibition of N-linked glycosylation of E, or glucose trimming of the carbohydrate side chain of E, resulted in a significant decrease in the secretion of RSPs and membrane anchor-free E, suggesting a critical role for this glycan in one or more assembly and/or secretion steps.
MATERIALS AND METHODS
Plasmids and virus.
We used a simian virus 40 (SV40)-based recombinant plasmid vector system derived from TBE virus strain Neudoerfl (GenBank accession no. U27495) expressing either prM and full-length E (SV-PEwt) (2), prM and a membrane anchor-free form of E (SV-PE400) (6), or prM and full-length E containing a single amino acid mutation within its glycosylation consensus sequence (Ser156Ala) (SV-PE156A). The vector included the SV40 early promoter and an SV40 origin of replication for amplification in COS cells. TBE virus strain Neudoerfl (40) grown in primary chicken embryo cells was purified in two cycles by sucrose gradient centrifugation as described previously (29).
Infection of mammalian cells with TBE virus.
COS-1 cells (ATCC CRL 1650) were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 4 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml at 37°C in 5% CO2. At 60 to 70% confluence, the cells were infected with TBE virus at a multiplicity of infection of ca. 1 in DMEM containing 2% FCS, glutamine, and antibiotics. The virus-containing medium was replaced by fresh medium 1 h after infection. Twenty-four hours postinfection, the cells were used for further experiments.
Transfection of mammalian cells with recombinant plasmids.
COS-1 cells grown to 50 to 60% confluence were transiently transfected with SV-PEwt, SV-PE400, or SV-PE156A plasmid DNA complexed to Lipofectamine (Life Technologies) in DMEM without serum or antibiotics. Five hours after transfection, an equal amount of DMEM containing 20% FCS, 200 U of penicillin/ml, and 200 μg of streptomycin/ml was added to the cells without removing the transfection mixture. Twenty-four hours posttransfection, the cells expressing recombinant TBE virus envelope proteins were used for further experiments.
Immunofluorescence.
COS-1 cells grown on 15-mm coverslips were transfected with SV-PEwt or SV-PE400 plasmid DNA as described above. Twenty-four hours posttransfection, the cells were fixed with 2.5% formaldehyde in serum-free medium supplemented with 20 mM HEPES (pH 7.4) for 20 min at room temperature. The cells were washed twice with serum-free medium, followed by incubation with phosphate-buffered saline containing 1 mM Mg2+ and 0.5 mM Ca2+ (PBS++) at 4°C for 10 min. Subsequently, the coverslips carrying the cells were transferred to a humid chamber and permeabilized in PBS++ containing 20% goat serum, 15 mM glycine, and 0.05% saponin for 15 min at room temperature. All subsequent incubation and washing steps were carried out with this buffer. The cells were incubated at room temperature for 45 min with an antibody directed against TBE virus envelope proteins and an antibody that recognizes marker proteins of various cellular organelles at dilutions between 1:100 and 1:500. After extensive washing, the cells were incubated at room temperature in the dark for 30 min with fluorescence-label conjugated secondary antibodies diluted 1:200. The cells were washed five times with permeabilization buffer and twice with water, followed by mounting of the coverslips on glass slides with Mowiol. Fluorescence microscopy was done with a Zeiss Axiovert microscope, and image processing was done with a Hamamatsu charge-coupled-device camera and the OpenLab software (InVision).
Electron microscopy.
COS-1 cells were transfected with SV-PEwt plasmid DNA as described above. Twenty-four hours posttransfection, the cells were fixed with 2.5% glutaraldehyde in 50 mM cacodylate buffer (50 mM KCl and 2.5 mM MgCl2) (pH 7.2) for 30 min at room temperature. After the cells were washed five times with cacodylate buffer, they were incubated in 2% OsO4 in cacodylate buffer for 2 h on ice, followed by washing with water. The cells were incubated overnight in a 0.5% aqueous solution of uranyl acetate. Subsequently, the cells were dehydrated with increasing concentrations of ethanol, treated twice with propylene oxide for 5 min, and incubated in a 1:1 propylene oxide-Epon mixture overnight. The cells were transferred into pure Epon and incubated for 6 h at room temperature followed by embedding in fresh Epon at 63°C for 36 h. The Epon blocks were cut with a Reichert-Jung microtome (Ultracut). Electron microscopy was carried out by using a Zeiss EM 910 electron microscope (Zeiss, Oberkochen, Germany).
Pulse-chase analysis of TBE virus envelope protein transport and secretion.
Subconfluent 60-mm-diameter dishes of virus-infected or plasmid-transfected COS-1 cells were washed twice with PBS++. After starvation in cysteine/methionine (Cys/Met)-free medium at 37°C for 30 min, the cells were pulse-labeled with 0.2-mCi35S-labeled methionine-cysteine (ProMix, Amersham Pharmacia) in Cys/Met-free medium containing 20 mM HEPES (pH 7.4) at 37°C for 2 or 5 min. After removal of the pulse medium, the cells were washed twice with 5 mM unlabeled Cys/Met in DMEM supplemented with 10% FCS, glutamine, antibiotics, and 20 mM HEPES (pH 7.4) followed by incubation with this medium at 37°C for chase times between 30 min and 4 h.
The reaction was stopped by flooding the cells with ice-cold PBS++ containing 20 mM N-ethylmaleimide (NEM). After incubation at 4°C for 5 min, the cells were lysed with 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) in HEPES-buffered saline (HBS) (pH 7.4) containing 20 mM NEM, 1 mM phenylmethylsulfonyl fluoride, and 10 μg (per milliliter) each of chymostatin, leupeptin, antipain, and pepstatin to inhibit proteolysis. The nuclei and the cellular debris were pelleted by centrifugation, and the postnuclear supernatants were used for further experiments.
Chase media were collected and supplemented with NEM to a final concentration of 20 mM. After detached cells and debris were pelleted, the chase media were subjected to immunoprecipitation to detect prM/M and E.
Temperature shift pulse-chase analysis.
COS-1 cells expressing recombinant prM and E were starved and pulse-labeled as described above. After incubation in chase medium at 37°C for 45 min to allow the proteins to fold completely, the dishes were transferred to a 15 or 20°C water bath, followed by incubation at this temperature for 75 min. Alkylation, cell lysis, and collection of the chase media were done as described above.
Pulse-chase experiments with inhibitory drugs.
To block protein transport across the organelles of the secretory pathway, brefeldin A was added to starvation, pulse, and chase media to a final concentration of 5 μg/ml. Glycosylation of newly synthesized proteins was prevented by supplementing the starvation, pulse, and chase media with 5 μg of tunicamycin/ml. Glucose trimming of carbohydrate side chains was inhibited by the addition of 50 mM N-butyl-deoxynojirimycin to the starvation, pulse, and chase media.
Immunoprecipitation and SDS-PAGE.
Postnuclear supernatants and chase media from pulse-chase experiments were precleared on protein A coupled to Sepharose CL-4B beads (Sigma) for 1 to 2 h at 4°C. Precleared lysate (80 to 200 μl) or 1 ml of precleared chase medium was loaded on protein A-Sepharose CL-4B beads (10-μl bead volume), and 1 μl of antibody was added. The proteins were precipitated by incubation with end-over rotation at 4°C overnight. Immune complexes were pelleted at 8,500 × g for 4 min, followed by two washes with 0.5% CHAPS in HBS and one wash with HBS alone with intermittent shaking at 4°C for 10 min. Subsequently, the precipitated material was solubilized by adding 40 μl of sample buffer and heated to 95°C for 5 min. After the protein A-Sepharose beads were pelleted, the supernatant was divided in two equal aliquots, and the reducing agent dithiothreitol was added to one of them to give a final concentration of 100 mM. The proteins were analyzed either by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or by both nonreducing and reducing SDS-PAGE on minigels followed by autoradiography.
Antibodies.
To detect TBE virus envelope proteins in immunoprecipitation and immunofluorescence experiments, a rabbit polyclonal antiserum [k-PM(2)] recognizing both prM and E as well as the nonstructural protein NS1 (5), a mouse monoclonal anti-E antibody (B1) (23), and a mouse monoclonal anti-prM antibody (8H1) (31) were used. For the immunofluorescence colocalization studies, anti-calnexin polyclonal antiserum (26), anti-protein disulfide isomerase mouse monoclonal antibody (StressGen), anti-ERGIC53 (49), and anti-GM130 (37) mouse monoclonal antibodies were applied. Alexa dyes (Molecular Probes) were used as fluorescence label-conjugated secondary antibodies in immunofluorescence experiments.
RESULTS
Intracellular localization of TBE virus envelope proteins expressed recombinantly.
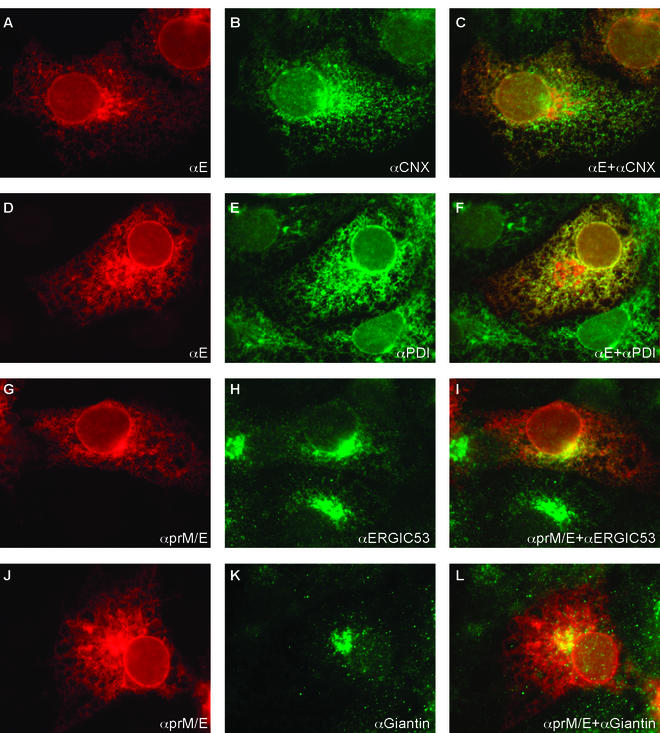
To determine the intracellular distribution of prM and E, COS-1 cells were transfected with a plasmid that codes for both of the glycoproteins (SV-PEwt) (2). The cells were fixed, permeabilized, and double stained for one or both of the envelope proteins and for cellular marker antigens. Anti-calnexin (55) and anti-protein disulfide isomerase (PDI) (20) were used as markers for the ER, anti-ERGIC-53 was used as a marker for the intermediate compartment (49), and anti-giantin was used as a marker for the Golgi complex (37). A mouse monoclonal anti-E (Fig. 1A and D), a mouse monoclonal anti-prM (not shown), and a rabbit polyclonal anti-prM/E antiserum (Fig. 1G and J) were all found to stain the nuclear envelope and a reticular network extending throughout the cytoplasm. The finding that prM and E had the same distribution was consistent with the previous observation that they occur as a tight heterodimeric complex (5, 38). The distribution overlapped almost completely with the two ER markers (Fig. 1C and F). In addition, prM and E showed some overlap with ERGIC-53 (Fig. 1I) and, to a minor extent, with giantin (Fig. 1L). No staining was observed at the plasma membrane.
FIG. 1.
Intracellular localization of TBE virus envelope proteins. COS-1 cells transfected with SV-PEwt plasmid DNA were fixed and subjected to indirect immunofluorescent costaining with primary antibodies that recognize TBE virus envelope proteins and antibodies against a marker protein of a cellular organelle. The proteins were then labeled with secondary antibodies conjugated to red or green light-emitting fluorophores. Shown are cells stained with a monoclonal anti-E antibody (A and D), cells labeled with a polyclonal antiserum recognizing both prM and E (G and J), immunofluorescent staining of the ER with polyclonal anti-calnexin (CNX) (B) and anti-protein disulfide isomerase (PDI) antisera (E), cells stained with a monoclonal antibody against a protein in the ERGIC (H), and immunostaining of the Golgi with a monoclonal anti-giantin antibody (K). Also shown is colocalization of viral envelope proteins with the organelle markers, which is represented by the yellow regions within each cell in the merged images (C, F, I, and L).
Intracellular localization of prM and transmembrane anchor-free E.
To investigate whether the localization of prM and E depended on the transmembrane domain of E, we analyzed the intracellular distribution of SV-PE400. This construct encodes prM and a truncated, soluble E protein that contains the ectodomain but lacks the C-terminal membrane anchor and leads to the secretion of soluble E dimers (5, 6). Figure 2 shows an immunofluorescence experiment carried out in analogy to the experiment shown in Fig. 1 for SV-PEwt. The anti-E mouse monoclonal antibody (Fig. 2A and D) as well as the anti-prM/E polyclonal antiserum (Fig. 2G and J) stained the nuclear envelope and the ER, as seen for SV-PEwt in Fig. 1. The distribution of prM and E400 overlapped mostly with the two ER markers calnexin and PDI (Fig. 2C and F, respectively) but not to the same extent as observed for SV-PEwt. A portion of prM and E400 clearly colocalized with ERGIC-53 (Fig. 2I) and with giantin (Fig. 2L).
FIG. 2.
Intracellular localization of prM and membrane anchor-free E. COS-1 cells transfected with SV-PE400 plasmid DNA were fixed and subjected to indirect immunofluorescent costaining with primary antibodies that recognize TBE virus envelope proteins and antibodies against a marker protein of a cellular organelle, as shown in Fig. 1 for SV-PEwt. The legend for the panels, indicating the antibodies used, is analogous to that for Fig. 1.
From the data of our immunofluorescence experiments, we conclude that the majority of prM and Ewt was located in the ER. Only a small fraction of the membrane-bound Ewt protein colocalized with the intermediate compartment and possibly with the Golgi complex. In contrast, the membrane anchor-free E400 was more dispersed along the secretory pathway, with the major portion of the protein still being detected in the ER but with significant amounts localizing to the intermediate compartment and the Golgi complex.
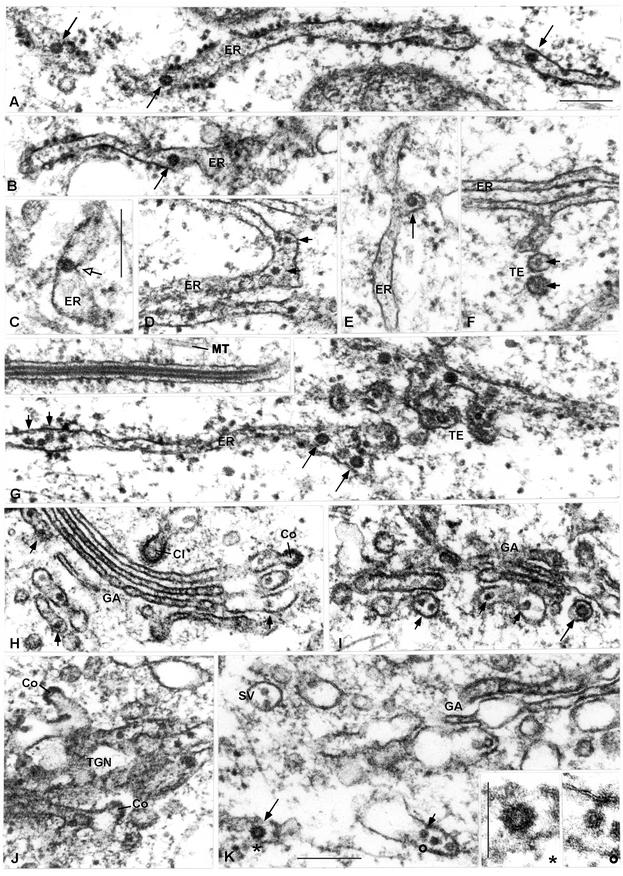
Assembly and secretion of recombinant subviral particles visualized by electron microscopy.
The transfected cells were also analyzed by thin-section electron microscopy as shown in Fig. 3. In contrast to nontransfected cells (not shown), the transfected COS-1 cells contained virus-like particles in the lumen of various compartments of the secretory pathway. They were most frequently observed within the lumen of the rough ER (Fig. 3A and B). A possible late-budding intermediate at the ER membrane is shown in Fig. 3C. Although less frequently, the particles were also present in the smooth ER (Fig. 3D and E), in transitional elements or ERGIC (Fig. 3F and G), in the rims of the Golgi cisternae (Fig. 3H and I), in the trans-Golgi network (TGN), Fig. 3J), and in secretory vesicles (Fig. 3K).
FIG. 3.
Electron micrographs of COS-1 cells transfected with SV-PEwt. RSPs of two different sizes are seen in the lumen of the rough and smooth ER (A to G), in transitional elements (TE) (F and G), the Golgi complex (H and I), the trans-Golgi network (TGN) (J), and in a large vesicle, probably representing a secretory vesicle (SV) (K). Short solid and long solid arrows point to small and large particles, respectively. The open arrow in panel C points to a possible late-budding intermediate at the ER membrane. The inset in panel G shows a long tubular structure (diameter, 50 nm) found inside a cisterna of the rough ER. The insets in panel K show magnifications of RSPs of the large (*) and small (○) size. Cl, clathrin-coated vesicle; Co, COP-coated vesicle; MT, microtubule. Magnification is identical (see the bar in panel A) for all panels (including the inset in panel G) except panels C and K and the inset in panel K. All bars are 0.2 μm except in the inset to panel K (0.1 μm).
About 75% of the particles were calculated to have had a diameter of 25 to 31 nm. This is consistent with the size observed earlier for TBE virus RSPs (19, 48), taking into account that some shrinking occurred during the chemical fixation. In their secreted form, these have been shown to have T=1 icosahedral symmetry and to contain a lipid envelope and 60 copies each of E and M (19). The remaining 25% of the particles had a diameter of 45 to 51 nm, corresponding to the size of intact TBE virus. These also displayed low electron density at the center, indicating that there was an empty space where normally the nucleocapsid would be located. Particles of both sizes were found within the same cisterna (Fig. 3G).
In addition, the electron microscopy analysis revealed the occasional presence of long tubular structures in the ER (see the inset in Fig. 3G). Their diameter (approximately 50 nm) and overall appearance suggested that they were most likely related to the larger of the RSPs except that they had grown into extended tubes instead of spherical particles. The longest tubular particle observed was 3.5 μm in length. Since such particles have not been seen in cells infected with TBE virus, they most likely represented products of abnormal budding. They were not observed in the Golgi complex, suggesting that they may not undergo secretion.
Taken together, the morphological studies indicated that prM and E were located mainly in the ER but were also present in other exocytic compartments, consistent with the idea that RSPs are synthesized in the ER and then transported through the secretory pathway.
Secretion block of TBE virus envelope proteins expressed recombinantly.
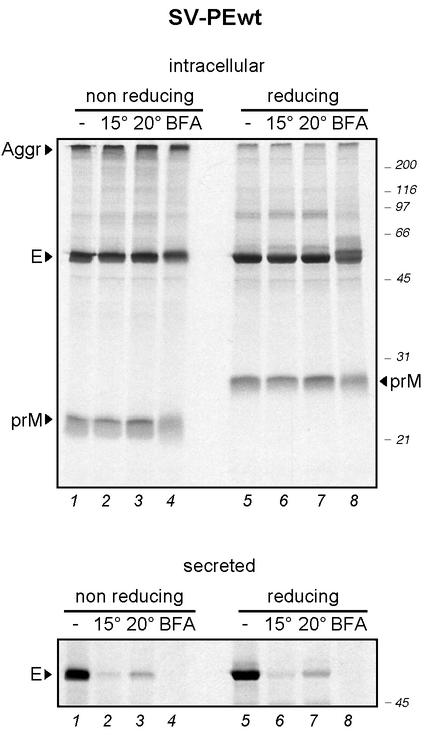
Passage through the secretory pathway can be inhibited at different levels by temperature blocks and by drug treatments. To determine their effect on the secretion of RSPs, COS-1 cells transfected with SV-PEwt were pulse-labeled with [35S]Cys/Met for 5 min and chased for 45 min at 37°C to allow folding and dimerization of prM and E (38). To induce a block between the intermediate compartment and the cis-Golgi, the cells were cooled to 15°C for 75 min, and to block exit from the TGN, 20°C incubation was similarly used (47). Moreover, to block exit from the ER, brefeldin A was added to one sample at 37°C during starvation, pulse, and chase (16). The postnuclear supernatants of the cells and the chase media were subjected to immunoprecipitation with a polyclonal anti-prM/E antiserum, followed by nonreducing and reducing SDS-PAGE and autoradiography.
PrM and E were present in cell lysates under all conditions, with no significant differences in the expression level (Fig. 4, upper panel). Some aggregates detected at the top of the nonreducing lanes could be dissolved by reduction, suggesting that they were caused by intermolecular disulfide cross-linking (38). prM was less efficiently detected in the autoradiograms because the protein contains fewer Cys and Met residues than the E protein and thus incorporates lower amounts of [35S]Cys/Met (7 Cys residues and 6 Met residues in prM as opposed to 12 Cys residues and 13 Met residues in E). For prM, a clear difference in the electrophoretic mobility between nonreducing and reducing conditions was visible. This shift was identical for all samples, indicating that disulfide bond formation occurred normally even when the temperature was lowered or when the samples were treated with brefeldin A. As previously reported (38), the corresponding shift in the E protein band was much smaller but was consistently observed in all samples, as further determined by 7.5% SDS-PAGE to enhance the resolution of the bands around 50 kDa (data not shown).
FIG. 4.
Blockage of the secretory pathway by temperature shifts and brefeldin A. COS-1 cells expressing recombinant E and prM were pulse-labeled for 5 min and chased for 2 h. For temperature shift experiments, the cells were chased at 37°C for 45 min to ensure proper protein folding, followed by a 75-min chase at 15 or 20°C to block transport at the ERGIC or Golgi level, respectively. Brefeldin A (BFA) was added to another sample during starvation, pulse, and chase, and the cells were kept at 37°C during the entire pulse and chase period. TBE virus envelope proteins from total cellular extracts (upper panel) and chase media (lower panel) were immunoprecipitated with a polyclonal antiserum recognizing prM and E, analyzed by SDS-PAGE, and detected by autoradiography. Positions of the individual proteins are indicated; molecular size standards are on the right. Aggr, aggregates.
In the sample treated with brefeldin A (lanes 4 and 8), a slight shift in the mobility of E was observed. It may be caused by the brefeldin A-induced fusion of the ER with the Golgi, which exposes ER proteins to the ER-to-Golgi mannosidases and other modifying enzymes (30). Moreover, the E protein migrated as a double band under reducing conditions (lane 8). Since the SV-PEwt construct contains the first 30 amino acid residues of NS1 (NS1*) at its C terminus, the upper band might correspond to the noncleaved E-NS1* form (38). Signal peptidase cleavage of this short amino acid stretch may be delayed or less efficient in cells treated with brefeldin A.
In each case, the amount of extracellular E decreased dramatically when the secretory pathway was blocked (Fig. 4, lower panel). Thus, the secretion of the RSPs was efficiently blocked when transport through the organelles of the secretory pathway was inhibited.
Kinetics of RSP and membrane anchor-free E dimer secretion.
To compare the secretion kinetics of infectious virions, RSPs, and membrane anchor-free E dimers, we pulse-labeled cells infected with TBE virus or transfected either with SV-PEwt or SV-PE400 plasmid DNA for 2 min. After chase times between 30 min and 4 h, cell-associated and secreted TBE virus envelope proteins were immunoprecipitated and analyzed as described above. In addition to prM and E, the polyclonal antiserum used recognized the nonstructural protein NS1, which is expressed in infected cells and secreted as multimers independently of the virion (14).
As shown in Fig. 5A, postnuclear supernatants from virus-infected cells showed three bands corresponding to prM, E, and NS1 (upper panel). The amount of prM and E in the cells decreased between 2 and 4 h (lanes 3 and 4). E was detected in the cell culture medium after 2 h, with a dramatic increase 4 h after synthesis (lower panel, lanes 3 and 4, respectively). The intracellular turnover of NS1 was faster, and the protein was clearly detectable in the medium 2 h after synthesis. A nonspecific band at 45 kDa was visible in the cell-associated samples throughout, probably corresponding to labeled actin (upper panel, lanes 1 to 4).
FIG. 5.
Kinetics of secretion of TBE virus, RSPs, and membrane anchor-free E dimers. COS-1 cells infected with TBE virus (A) or transfected with plasmids expressing recombinant prM and full-length E (B) or prM and E lacking the two transmembrane segments (C) were pulse-labeled with [35S]Cys/Met for 2 min and chased between 30 min and 4 h, as indicated above the lanes. Postnuclear supernatants (upper panels) and chase media (lower panels) were subjected to immunoprecipitation with a polyclonal anti-prM/E antiserum recognizing both TBE virus envelope proteins, followed by reducing SDS-PAGE and autoradiography. Positions of the individual proteins are marked on the side. The nonspecific band at 45 kDa seen in all lanes in virus-infected cells (A, upper panel) probably corresponds to labeled actin. Molecular weight standards are indicated on the right.
When expressed from a recombinant construct (SV-PEwt), prM and E were still visible in the cell lysate after 4 h of chase (Fig. 5B, upper panel), the amount decreasing slightly after 2 to 4 h (lanes 3 and 4). A faint band of secreted E was detected after 1-h chase (lower panel, lane 2), with a gradual increase after 2 and 4 h (lanes 3 and 4, respectively). Small amounts of prM appeared in the chase medium after 2 to 4 h, probably due to incomplete cleavage by furin (data not shown).
When prM and membrane anchor-free E were pulse-labeled (SV-PE400), the labeled prM and E were detected intracellularly in decreasing amounts from one-half to 4 h of chase (Fig. 5C, upper panel). Secretion of soluble E400 dimers was detected after a 30-min chase, increasing gradually until 2 h of chase (lower panel).
Taken together, the pulse-chase analysis indicated that the viruses, the RSPs, and E lacking the transmembrane segments differed in their kinetics of secretion, with the membrane anchor-free E variant being the fastest and the intact virus being the slowest. Our results also suggested that it took the labeled viral envelope proteins at least 1 h to fold, assemble into virus particles in the ER, and traverse the secretory pathway.
Role of the N-linked glycan in E.
The TBE virus E protein contains a single N-linked glycan located close to the dimerization interface (43). We tested whether this carbohydrate side chain was critical for assembly and secretion of RSPs and membrane anchor-free E dimers by interfering with oligosaccharide addition and processing. To inhibit glycosylation, we made use of tunicamycin, which is an inhibitor of core glycan synthesis (17), and we generated an SV-PEwt construct (called SV-PE156A) in which the glycosylation consensus sequence in E was mutated (Ser156Ala) so that it would not be recognized by the oligosaccharyltransferase. In the latter case, glycosylation of prM, which also contains a single glycan, was expected to remain unaltered.
Cells transfected with SV-PEwt, SV-PE400, or SV-PE156A were pulse-labeled for 5 min and chased for 4 h. The viral glycoproteins were immunoprecipitated with a polyclonal anti-prM/E antiserum and analyzed by reducing SDS PAGE. As shown in Fig. 6A, the expression was normal for each construct in the presence and absence of tunicamycin (upper panel). Ewt was somewhat less efficiently detected when glycosylation was prevented (lane 2) or in the glycosylation mutant (lanes 5 and 6). The epitopes on Ewt recognized by these antibodies may be altered upon inhibition of glycosylation or deglucosylation, or some degradation may have occurred after failure to pass the ER quality control machinery. This effect was not observed for SV-PE400 (compare lanes 3 and 4).
FIG. 6.
Effect of glycosylation inhibition on secretion of TBE virus envelope proteins. COS-1 cells transfected with SV-PEwt, SV-PE400, or SV-PE156A (Ser156Ala; i.e., prM and E carrying an amino acid point mutation which abolishes glycosylation of E) were pulse-labeled for 5 min and chased for 2 h in the presence (+) or absence (−) of tunicamycin (Tun). The intracellular and secreted fractions of E (upper and lower panels, respectively) were immunoprecipitated with a polyclonal anti-prM/E antiserum (A) or with a mouse monoclonal anti-E antibody (B) and analyzed by reducing SDS-PAGE as described above, followed by autoradiography. Molecular weight standards are indicated on the right.
Relative to secretion in the control samples (Fig. 6A, lower panel, lanes 1 and 3), secretion of the nonglycosylated E was dramatically reduced (lanes 2, 4, 5, and 6). Quantitation by phosphorimager analysis (not shown) indicated that secretion was reduced by about 90% relative to that of the control sample in lane 1 and normalized to the corresponding intracellular amounts. The membrane anchor-free variant of E was less affected (lanes 3 and 4), with secretion reduced to 30% of that of the control.
To analyze whether the secretion effect was caused by inefficient folding of E, we precipitated the lysates with a conformation-specific anti-E monoclonal antibody previously used to assess the antigenic structure of E (23). E was detected in all samples after precipitation with the B1 monoclonal antibody (Fig. 6B), and prM was coprecipitated with E. Intrachain disulfide bond formation in prM and E was normal, as determined by SDS-PAGE under nonreducing and reducing conditions (data not shown). However, some slight quantitative differences were observed compared to the precipitation of E with the polyclonal anti-prM/E antiserum shown in Fig. 6A. These results suggested that prevention of glycosylation had only a minor effect on the folding of E, and heterodimerization between prM and E occurred normally.
N-linked glycans undergo glucose trimming in the ER as a prerequisite for interaction with the calnexin-calreticulin chaperone cycle (18). To test whether inhibition of the glucosidases involved in this process had an effect on secretion, cells transfected with SV-PEwt or SV-PE400 were pulse-labeled for 5 min and chased for 4 h in the presence of N-butyl-deoxynojirimycin (bDNJ), an alpha-glucosidase inhibitor (17), which was added during starvation, pulse, and chase, or after 15 min of chase, or after 30 min of chase. Cell-associated and secreted viral envelope proteins were immunoprecipitated and analyzed on reducing SDS-PAGE.
As shown in Fig. 7, a dramatic decrease in E protein secretion was again observed when glucose trimming was blocked. Quantitation by phosphorimager analysis (not shown) indicated that when bDNJ was present throughout starvation, pulse, and chase, secretion of the E protein was reduced by about 80% relative to that of the control sample and normalized to the corresponding intracellular amounts (Fig. 7A, lanes 1 and 2). In the case of membrane anchor-free E dimers, the effect was again somewhat less (reduction to 30% of the level of the control) (Fig. 7B, lanes 1 and 2). When bDNJ was added after 15 min of chase, secretion of Ewt and E400 was reduced to 30 and 50%, respectively (Fig. 7A and B, lanes 3), relative to the level of the control after normalization. After 30 min of chase, secretion was still reduced to 45 and 60%, respectively (Fig. 7A and B, lanes 4). This suggested that the effect of bDNJ was likely to involve late folding or assembly steps, because according to previous studies, prM and E fold and associate within 20 min (38). Consistent with this interpretation, we found that bDNJ, like tunicamycin, did not inhibit either oxidative folding of prM and E or the formation of prM-E heterodimers (data not shown).
FIG. 7.
Secretion of TBE virus envelope proteins after inhibition of glucose trimming with bDNJ. COS-1 cells transfected with SV-PEwt (A) or SV-PE400 (B) were pulse-labeled for 5 min and chased for 2 h. To prevent glucose trimming on the carbohydrate side chains, the glucosidase inhibitor N-butyl-deoxynojirimycin (bDNJ) was added to starvation, pulse, and chase media (+) or to the chase medium after 15 or 30 min as indicated. −, no bDNJ added. Immunoprecipitates of postnuclear supernatants and chase media (upper and lower panels, respectively) were analyzed by reducing SDS-PAGE, followed by autoradiography. Molecular weight standards are indicated on the right.
However, the Ewt (but not E400) forms for which glucose trimming was prevented were less efficiently detected by the polyclonal anti-prM/E serum (Fig. 7A, lanes 2 and 3), similar to our observation in the tunicamycin experiment shown in Fig. 6.
Our results thus indicated that the single N-linked glycan in the TBE virus E glycoprotein, and its trimming by ER glucosidases, is essential for efficient secretion of RSPs and membrane anchor-free E dimers. The glycan is not required for oxidative folding and heterooligomeric assembly of prM/E dimers.
DISCUSSION
In this study, we have analyzed the assembly and secretion of TBE virus envelope proteins prM and E in the form of recombinant subviral particles (RSPs) and as membrane anchor-free E dimers. We found that prM and full-length E were localized to the ER, where assembly of virus-like particles probably occurred. The small amount of viral proteins seen in the intermediate compartment and Golgi complex was probably due to RSPs in transit to the extracellular space.
PrM and E are the only viral proteins needed for production of RSPs (5). Within a few minutes after synthesis, translocation, and cleavage from the polyprotein, they reach their fully oxidized state, heterodimerize, and complete their folding program (38). Particle formation requires further interactions between these complexes, which most likely arise before or during particle budding in the ER. However, they were unfortunately difficult to demonstrate experimentally in cell lysates.
We found that 75% of the particles formed by recombinant expression of prM and E had the expected diameter of approximately 30 nm, corresponding to the already well-characterized RSPs with T=1 symmetry (19), while others had the dimensions of native virus particles (50 nm in diameter). The larger particles were detected not only in the lumen of the ER, but also in other secretory compartments, suggesting that they, like the smaller RSPs, are transported normally. Although secreted RSPs of this size class have not yet been reported in the literature, a recent study has revealed that large and small RSPs are secreted from cells in approximately the same ratios observed here when prM cleavage is prevented by mutagenesis (Allison et al., manuscript in preparation).
In addition, we observed some rod-like particles in the ER of transfected cells. They most likely represented products of abnormal budding in which the vesicle failed to pinch off, allowing the bud to grow into an empty, inflexible, tubular structure with a diameter similar to that of the virus. However, we cannot exclude that they represent a host cell-derived structure induced by the recombinant expression of prM and E in these cells.
Soluble, anchor-free E dimers were secreted quite rapidly. Pulse-chase experiments indicated that they started to appear in the medium already 30 min after synthesis. This rate was similar to that previously reported for anchor-free forms of influenza hemagglutinin (50), vesicular stomatitis virus G protein (22), and human immunodeficiency virus gp160 (24). In all of these cases, the rate-limiting step is probably folding (15). In comparison, secreted RSPs were detected 1 to 2 h after glycoprotein synthesis, suggesting that budding and particle assembly were rate limiting. Appearing in the medium only 2 to 4 h after a radioactive pulse, intact virus particles released from infected cells were even more slowly secreted, suggesting that the formation of the larger capsid-containing particles needed more time than formation of the smaller T=1 RSPs. Since budding intermediates in the ER could rarely be observed by electron microscopy, it seems likely that budding itself is a rapid process. Thus, the time-consuming part of the process was probably the assembly of a prebudding lattice.
Ultrastructural studies, temperature shift experiments, and the effects of brefeldin A treatment showed that the RSPs are transported through the normal secretory pathway. This is consistent with recent findings on the assembly and secretion of Kunjin virus (39). Immunofluorescence and electron microscopy studies showed that assembly of this virus occurs in the rough ER, followed by transport and maturation along the secretory pathway. A recent report showed, moreover, that hepatitis C virus-like particles form at the ER membrane when only the structural proteins are recombinantly expressed (7), strongly implicating a similar budding site for this virus.
The ER membrane is not normally known to form vesicles that bud into the lumen. The formation of RSPs and native viruses is therefore an unusual, nonphysiological process. It is possible that once folded, the prM-E heterodimers may alone possess the intrinsic capacity to assemble laterally into an isometric lattice in the ER membrane and its lumenal surface, as previously suggested (48). Like elements of the clathrin coat, they may be able to introduce the membrane curvature needed to form a bud and to trigger the fusion reaction needed to pinch off the membrane vesicle (21). Alternatively, they may rely on additional host cell-derived factors, such as lumenal chaperones and folding enzymes. Since host cell proteins have not been identified as part of mature viruses or RSPs (48), cellular proteins, if they are involved, might play a role as chaperones during virion assembly. The mechanism by which they would be excluded from the mature virus or RSP, however, remains unclear.
Previous immunofluorescence studies, together with earlier biochemical studies (38), showed that the majority of intracellular prM and E were localized to the ER as stable heterodimers. Although membrane anchor-free E400 appears to be secreted more efficiently than Ewt, and E400 is distributed more evenly along the secretory pathway compared to Ewt, it is evident that the TBE virus envelope proteins have the properties of resident ER membrane proteins. It remains to be elucidated whether ER localization is mediated by prM or E, since neither of the two have typical dibasic signals in their cytoplasmic domains for ER retrieval (reviewed in reference 54). In fact, they have cytoplasmic loops consisting of only a few amino acid residues between their two transmembrane segments. It is possible that signals are located (i) in the ectodomain, (ii) in the short spacer on the lumenal side that connects the ectodomain to the transmembrane domain, or (iii) in the transmembrane segments. That the glycoproteins can leave the ER when assembled into virions or RSPs could mean that such ER localization signals are either masked, modified, or no longer interact with retention factors. Another possibility is that formation of aggregates prevents the proteins from being transported.
The data presented in this paper as well as previous studies have shown that prM forms a tight complex with E that is required both for acquisition of a native structure of E and for transport of the immature virions across the secretory pathway (34, 38). However, E400 is released from the cell as a soluble homodimer (6). Thus, it remains unclear at which stage prM dissociates from the membrane anchor-free E400, what triggers the homodimerization of E, and whether oligomerization also occurs for E400. While a tight association between prM and E is required for folding and transport, cleavage of prM by furin in the trans-Golgi network may cause a destabilization of the prM-E400 interaction where the stem-anchor region of E is lacking, thereby leading to the dissociation of prM and E400 and to the homodimerization of two E400 molecules.
Although E contains only a single N-linked glycan, glycosylation and carbohydrate trimming were found to play an important role in the maturation and secretion of RSPs and membrane anchor-free E dimers. Prevention of glycosylation by tunicamycin, or by mutating the glycosylation consensus sequence in E, drastically reduced the amount of secreted RSPs and membrane anchor-free E dimers. A similar drop in secretion was seen when glucose trimming of N-linked glycans was inhibited. Our analysis suggested that unlike the effects of such treatments on most other viral glycoproteins within the flaviviruses (13) or other virus genera (25, 42), the reason for lack of secretion in the case of TBE virus products was not defective folding. Oxidative folding and generation of conformational epitopes on prM and E were in fact normal by all available criteria, and the prM/E heterodimers were formed normally. The step at which the oligosaccharide was needed was therefore downstream from initial folding. Our guess is that it involves formation of E-E dimers, because membrane anchor-free E, which is normally secreted as dimers, was poorly secreted unless it was properly glycosylated and glucose trimmed.
The Asn154 residue that carries the oligosaccharide moiety is located in the central domain of E. The glycan itself could not be visualized in the crystal structure. However, it was suggested that it may interact with the dimerization domain of the other E subunit and thus contribute to the stability of the dimeric structure (43). However, the function of the sugar moiety on E in the ER may be linked to prM, because a recent image reconstruction study of dengue virus places M in a pocket between the two E subunits exactly where the glycan in TBE virus is located (35). Thus, it could be that the glycan is essential in the formation of the proper interface between the two E subunits and prM or between two E subunits.
However, in either case, it is difficult to see why not only the presence of the oligosaccharide but also its glucose-trimming status is essential. The inhibition by bDNJ suggests that the calnexin-calreticulin cycle may be involved in the maturation of prM and E. Calnexin and calreticulin are ER lectins that interact with partially glucose-trimmed glycoproteins in the ER and serve as important molecular chaperones (18). They promote correct folding of their substrate proteins, and they are involved in quality control and ER retention of incompletely folded and assembled proteins.
Inhibition of ER glucosidases has been shown to inhibit the production of several different viruses of various families (8, 33, 46, 59). In the case of flaviviruses, iminosugar derivatives were shown to have an antiviral effect on dengue and Japanese encephalitis virus infection (13, 58). In many of these cases, the reason for inhibition has been shown to involve incomplete maturation of viral glycoproteins. For dengue virus, Courageot et al. reported that prM-E heterodimers were less stable (13), which may lead to defects in virus assembly. The inhibition of TBE virus RSP secretion shown here is most likely caused by similar mechanisms, although in our hands, initial folding of both prM and E seemed normal, and prM and E associated stably with each other.
Although calnexin colocalized with E in the ER of cells, we were not able to detect any interaction between the TBE virus prM and E and calnexin or calreticulin by immunoprecipitation. This does not necessarily mean that such interactions do not occur in situ, because the complexes with glycoproteins that contain only a single glycan are often weak and difficult to demonstrate (9, 44). However, in a recent report, the E protein from Japanese encephalitis virus, which also contains a single N-linked glycan in the same position, was shown to interact with calnexin (58). In contrast, E from dengue virus serotypes 1 and 2, which carries two carbohydrate side chains, did not (13, 58). These observations indicate that association of flavivirus envelope protein E with calnexin can occur but varies between members of the genus. If calnexin and/or calreticulin do bind to the TBE virus E protein in the ER, they may participate as cellular cofactors during RSP assembly and/or homodimerization of E. Without them, one might speculate that E dimers do not form properly and that the envelope proteins fail to form the lattice interactions needed for particle budding.
In summary, we have provided evidence that TBE virus envelope proteins are incorporated into RSPs at the ER membrane, followed by transport along the secretory pathway. Assembly of prM-E heterodimers into larger complexes seems to be rate limiting for secretion of RSPs and the native virus. The presence of the N-linked oligosaccharide and the proper glucose trimming of the carbohydrate side chain of E are required for secretion. It remains to be elucidated whether any cellular factors participate in flavivirus assembly.
Acknowledgments
The authors thank Connie Schmaljohn (USAMRIID, Fort Detrick, Md.) for kindly providing anti-prM antibody; Hans-Peter Hauri for kindly providing anti-ERGIC53 and anti-giantin antibodies; and Karin Mench, Silvia Röhnke, and Walter Holzer for excellent technical assistance.
This work was supported by a grant from the Swiss Federal Institute of Technology to A.H. I.C.L. was a recipient of a Short-Term Fellowship from the European Molecular Biology Organization (EMBO).
REFERENCES
- 1.Adams, S. C., A. K. Broom, L. M. Sammels, A. C. Hartnett, M. J. Howard, R. J. Coelen, J. S. Mackenzie, and R. A. Hall. 1995. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 206:49-56. [DOI] [PubMed] [Google Scholar]
- 2.Allison, S. L., C. W. Mandl, C. Kunz, and F. X. Heinz. 1994. Expression of cloned envelope protein genes from the flavivirus tick-borne encephalitis virus in mammalian cells and random mutagenesis by PCR. Virus Genes 8:187-198. [DOI] [PubMed] [Google Scholar]
- 3.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, and F. X. Heinz. 2001. Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. J. Virol. 75:4268-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J. Virol. 69:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allison, S. L., K. Stadler, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J. Virol. 69:5816-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison, S. L., K. Stiasny, K. Stadler, C. W. Mandl, and F. X. Heinz. 1999. Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E. J. Virol. 73:5605-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard, E., D. Brand, S. Trassard, A. Goudeau, and P. Roingeard. 2002. Hepatitis C virus-like particle morphogenesis. J. Virol. 76:4073-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolt, G., I. R. Pedersen, and M. Blixenkrone-Moller. 1999. Processing of N-linked oligosaccharides on the measles virus glycoproteins: importance for antigenicity and for production of infectious virus particles. Virus Res. 61:43-51. [DOI] [PubMed] [Google Scholar]
- 9.Cannon, K. S., D. N. Hebert, and A. Helenius. 1996. Glycan-dependent and -independent association of vesicular stomatitis virus G protein with calnexin. J. Biol. Chem. 271:14280-14284. [DOI] [PubMed] [Google Scholar]
- 10.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649-688. [DOI] [PubMed] [Google Scholar]
- 11.Chambers, T. J., M. Halevy, A. Nestorowicz, C. M. Rice, and S. Lustig. 1998. West Nile virus envelope proteins: nucleotide sequence analysis of strains differing in mouse neuroinvasiveness. J. Gen. Virol. 79:2375-2380. [DOI] [PubMed] [Google Scholar]
- 12.Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37-46. [DOI] [PubMed] [Google Scholar]
- 13.Courageot, M. P., M. P. Frenkiel, C. D. Dos Santos, V. Deubel, and P. Despres. 2000. Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J. Virol. 74:564-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crooks, A. J., J. M. Lee, L. M. Easterbrook, A. V. Timofeev, and J. R. Stephenson. 1994. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J. Gen. Virol. 75:3453-3460. [DOI] [PubMed] [Google Scholar]
- 15.Doms, R. W., R. A. Lamb, J. K. Rose, and A. Helenius. 1993. Folding and assembly of viral membrane proteins. Virology 193:545-562. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson, J. G., D. Finazzi, and R. D. Klausner. 1992. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360:350-352. [DOI] [PubMed] [Google Scholar]
- 17.Elbein, A. D. 1987. Glycosylation inhibitors for N-linked glycoproteins. Methods Enzymol. 138:661-709. [DOI] [PubMed] [Google Scholar]
- 18.Ellgaard, L., M. Molinari, and A. Helenius. 1999. Setting the standards: quality control in the secretory pathway. Science 286:1882-1888. [DOI] [PubMed] [Google Scholar]
- 19.Ferlenghi, I., M. Clarke, T. Ruttan, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 20.Freedman, R. B. 1989. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell 57:1069-1072. [DOI] [PubMed] [Google Scholar]
- 21.Garoff, H., R. Hewson, and D. J. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graeve, L., C. Garreis-Wabnitz, M. Zauke, M. Breindl, and J. Kruppa. 1986. The soluble glycoprotein of vesicular stomatitis virus is formed during or shortly after the translation process. J. Virol. 57:968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guirakhoo, F., F. X. Heinz, and C. Kunz. 1989. Epitope model of tick-borne encephalitis virus envelope glycoprotein E: analysis of structural properties, role of carbohydrate side chain, and conformational changes occurring at acidic pH. Virology 169:90-99. [DOI] [PubMed] [Google Scholar]
- 24.Hallenberger, S., S. P. Tucker, R. J. Owens, H. B. Bernstein, and R. W. Compans. 1993. Secretion of a truncated form of the human immunodeficiency virus type 1 envelope glycoprotein. Virology 193:510-514. [DOI] [PubMed] [Google Scholar]
- 25.Hammond, C., I. Braakman, and A. Helenius. 1994. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc. Natl. Acad. Sci. USA 91:913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond, C., and A. Helenius. 1994. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J. Cell Biol. 126:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinz, F. X., and S. L. Allison. 2000. Structures and mechanisms in flavivirus fusion. Adv. Virus Res. 55:231-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinz, F. X., G. Auer, K. Stiasny, H. Holzmann, C. Mandl, F. Guirakhoo, and C. Kunz. 1994. The interactions of the flavivirus envelope proteins: implications for virus entry and release. Arch. Virol. Suppl. 9:339-348. [DOI] [PubMed] [Google Scholar]
- 29.Heinz, F. X., and C. Kunz. 1981. Homogeneity of the structural glycoprotein from European isolates of tick-borne encephalitis virus: comparison with other flaviviruses. J. Gen. Virol. 57:263-274. [DOI] [PubMed] [Google Scholar]
- 30.Herscovics, A. 1999. Importance of glycosidases in mammalian glycoprotein biosynthesis. Biochim. Biophys. Acta 1473:96-107. [DOI] [PubMed] [Google Scholar]
- 31.Iacono-Connors, L. C., J. F. Smith, T. G. Ksiazek, C. L. Kelley, and C. S. Schmaljohn. 1996. Characterization of Langat virus antigenic determinants defined by monoclonal antibodies to E, NS1 and preM and identification of a protective, non-neutralizing preM-specific monoclonal antibody. Virus Res. 43:125-136. [DOI] [PubMed] [Google Scholar]
- 32.Ishak, R., D. G. Tovey, and C. R. Howard. 1988. Morphogenesis of yellow fever virus 17D in infected cell cultures. J. Gen. Virol. 69:325-335. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson, G. B., T. D. Butters, R. A. Dwek, and F. M. Platt. 1993. Effects of the imino sugar N-butyldeoxynojirimycin on the N-glycosylation of recombinant gp120. J. Biol. Chem. 268:570-576. [PubMed] [Google Scholar]
- 34.Konishi, E., and P. W. Mason. 1993. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J. Virol. 67:1672-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 37.Linstedt, A. D., and H. P. Hauri. 1993. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 4:679-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenz, I. C., S. L. Allison, F. X. Heinz, and A. Helenius. 2002. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J. Virol. 76:5480-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackenzie, J. M., and E. G. Westaway. 2001. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 75:10787-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandl, C. W., F. X. Heinz, and C. Kunz. 1988. Sequence of the structural proteins of tick-borne encephalitis virus (western subtype) and comparative analysis with other flaviviruses. Virology 166:197-205. [DOI] [PubMed] [Google Scholar]
- 41.Mason, P. W., S. Pincus, M. J. Fournier, T. L. Mason, R. E. Shope, and E. Paoletti. 1991. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology 180:294-305. [DOI] [PubMed] [Google Scholar]
- 42.Molinari, M., and A. Helenius. 2000. Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science 288:331-333. [DOI] [PubMed] [Google Scholar]
- 43.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 44.Rodan, A. R., J. F. Simons, E. S. Trombetta, and A. Helenius. 1996. N-linked oligosaccharides are necessary and sufficient for association of glycosylated forms of bovine RNase with calnexin and calreticulin. EMBO J. 15:6921-6930. [PMC free article] [PubMed] [Google Scholar]
- 45.Russell, P. K., W. E. Brandt, and J. M. Dalrymple. 1980. Chemical and antigenic structure of flaviviruses. In R. W. Schlesinger (ed.), The togaviruses. Academic Press, New York, N.Y.
- 46.Saito, T., and I. Yamaguchi. 2000. Effect of glycosylation and glucose trimming inhibitors on the influenza A virus glycoproteins. J. Vet. Med. Sci. 62:575-581. [DOI] [PubMed] [Google Scholar]
- 47.Saraste, J., and E. Kuismanen. 1984. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell 38:535-549. [DOI] [PubMed] [Google Scholar]
- 48.Schalich, J., S. L. Allison, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1996. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J. Virol. 70:4549-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweizer, A., J. A. Fransen, T. Bachi, L. Ginsel, and H. P. Hauri. 1988. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 107:1643-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh, I., R. W. Doms, K. R. Wagner, and A. Helenius. 1990. Intracellular transport of soluble and membrane-bound glycoproteins: folding, assembly and secretion of anchor-free influenza hemagglutinin. EMBO J. 9:631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stadler, K., S. L. Allison, J. Schalich, and F. X. Heinz. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 71:8475-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stiasny, K., S. L. Allison, C. W. Mandl, and F. X. Heinz. 2001. Role of metastability and acidic pH in membrane fusion by tick-borne encephalitis virus. J. Virol. 75:7392-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stiasny, K., S. L. Allison, J. Schalich, and F. X. Heinz. 2002. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J. Virol. 76:3784-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teasdale, R. D., and M. R. Jackson. 1996. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus. Annu. Rev. Cell. Dev. Biol. 12:27-54. [DOI] [PubMed] [Google Scholar]
- 55.Wada, I., D. Rindress, P. H. Cameron, W. J. Ou, J. J. Doherty II, D. Louvard, A. W. Bell, D. Dignard, D. Y. Thomas, and J. J. Bergeron. 1991. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 266:19599-19610. [PubMed] [Google Scholar]
- 56.Wang, J. J., C. L. Liao, Y. W. Chiou, C. T. Chiou, Y. L. Huang, and L. K. Chen. 1997. Ultrastructure and localization of E proteins in cultured neuron cells infected with Japanese encephalitis virus. Virology 238:30-39. [DOI] [PubMed] [Google Scholar]
- 57.Winkler, G., F. X. Heinz, and C. Kunz. 1987. Studies on the glycosylation of flavivirus E proteins and the role of carbohydrate in antigenic structure. Virology 159:237-243. [DOI] [PubMed] [Google Scholar]
- 58.Wu, S. F., C. J. Lee, C. L. Liao, R. A. Dwek, N. Zitzmann, and Y. L. Lin. 2002. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 76:3596-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zitzmann, N., A. S. Mehta, S. Carrouee, T. D. Butters, F. M. Platt, J. McCauley, B. S. Blumberg, R. A. Dwek, and T. M. Block. 1999. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. USA 96:11878-11882. [DOI] [PMC free article] [PubMed] [Google Scholar]