Abstract
Purpose
To examine different protocols for handling incidental findings on brain research MRIs, and provide a platform for establishing formal discussions of related ethical and policy issues.
Materials and Methods
Corresponding authors identified from a database of peer-reviewed publications in 1991–2002 involving functional MRI (fMRI), alone or in combination with other imaging modalities, were invited to participate in this web-based survey. The survey asked questions regarding knowledge and handling of incidental findings, as well as characteristics of the scanning environment, training required, IRB protocol requirements, and neuroradiologist involvement.
Results
Seventy-four investigators who conduct MRI studies in the United States and abroad responded. Eighty-two percent (54/66) reported discovering incidental findings in their studies, such as arteriovenous malformations, brain tumors, and developmental abnormalities. Substantial variability was found in the procedures for handling and communicating findings to subjects, neuroradiologist involvement, personnel permitted to operate equipment, and training.
Conclusion
Guidelines for minimum and optimum standards for detecting and communicating incidental findings on brain MRI research are needed.
Keywords: incidental findings, neuroethics, brain MRI, neuroimaging, fMRI, neuroscience and ethics
THE DISCOVERY OF incidental findings on brain MRI scans of participants recruited as healthy controls in research studies has garnered significant attention in the published literature over the past several years. Suspicious brain abnormalities have been reported to occur in as many as 40% of research participants, with clinically significant findings occurring in 2–8% of children and adults (1-4). Most recently, an age-related effect characterized by infrequent but clinically significant findings, including aneurysms and other vascular malformations in younger participants (18–59 years), and frequent but relatively routine findings such as chronic small-vessel ischemic disease in older (≥60 years) participants (4) has been reported.
In the light of such findings, and given the rapidly increasing number of research MRI examinations conducted each year (5-7), significant ethical questions about the procedures involved in detecting incidental findings, and the responsibility of disclosing such findings, have been raised. For example, what is the overall burden on a researcher who discovers an abnormality in a volunteer? Should all studies involving brain MRIs or other forms of neuroimaging involve a physician who is specifically trained and qualified to review images for abnormal findings and provide clinical referral? What standardized procedures are needed to facilitate the transfer of information from the laboratory to a clinical care setting? What is the obligation of an investigator in reporting findings that may have implications for a third party, such as non-accidental head trauma or a neurogenetic disorder?
Our goal was to examine different protocols and procedures presently in place for handling incidental findings on brain research MRIs, and to provide a platform for establishing formal discussions of related ethical and policy issues.
MATERIALS AND METHODS
Corresponding authors identified from a database of peer-reviewed publications in 1991–2002 involving functional MRI (fMRI), alone or in combination with other imaging modalities, were invited to participate in this web-based survey. A total of 843 e-mail invitations were sent to authors in 27 countries. The survey initially consisted of two parts, with a set of questions about procedures for detection and disclosure of incidental findings, followed by a set of questions related to details about the findings themselves. The survey also requested consent forms for characterizing language related to incidental findings. We sent all prospective responders an initial e-mail followed by a reminder several weeks later. Descriptive statistics were generated to characterize the survey samples.
A preliminary inspection of results from the first round of survey data suggested that many respondents were unable to either accurately report or describe findings specifically; therefore, the second part of the survey was eliminated in the reminder round. This also had the desirable effect of reducing the response burden to a few minutes, thereby increasing response rate.
The study was conducted under an Institutional Review Board (IRB)-approved protocol. Informed consent, provided by clicking an “Accept” box, was required prior to accessing the survey. All surveys were completed anonymously with an option to self-identify the institution with which the responder was affiliated.
RESULTS
The current results are reported according to three general subsections of the survey: the background characteristics of the responding cohort; training, safety, and subject incentives; and detection and management of incidental findings.
Background Characteristics of the Responding Cohort
Correcting for 15% undeliverable e-mails, the overall response rate was 11% (81/717; 81 accesses; 74 surveys completed in part or in full). The responding cohort was comprised of investigators who conduct both structural MRI and fMRI studies in the United States (58%; 15/26), France, Japan, Italy, The Netherlands, Scotland, United Kingdom, and Sweden (non-U.S.: 42% [11/26]). Of the responders, 71% (40/56) conduct fewer than 500 scans per year; 29% (16/56) conduct 500 or more per year. We found that 52% (25/48) of the responding investigators have been conducting imaging studies for less than five years; 48% (23/48) for five years or longer.
Of the responders who provided information about their systems, 47% (28/59) operate GE scanners, 31% (18/59) operate Siemens scanners, and 12% (7/59) operate both; 10% (6/59) reported using other systems. Three percent (2/61) operate scanners at a field strength of <1.5 T, 33% (20/61) at 1.5 T, 36% (22/61) at both 1.5 T and higher, and 28% (17/61) at only >1.5 T. All scans are acquired with the use of one or a combination of high-resolution T1-weighted, three-dimensional spoiled gradient-recalled echo (SPGR) images, short-echo time (TE) images, proton density-weighted spin-echo or fast spin-echo images with long repetition time (TR) and short TE, and fluid attenuated inversion recovery (FLAIR) images.
Personnel Training and Safety Requirements
Figure 1 shows the distribution of people at different levels of professional training who are permitted to conduct scans. Forty-one percent (25/61) of the responders reported that students (graduate and undergraduate) may have primary responsibility for imaging; 21% (13/61) allow only postdoctoral and professional personnel (including MRI technologists, principal investigators, and M.D./Ph.D. fellows) to scan, and 38% (23/61) allow only professional personnel to conduct scans. Training also varies substantially, with formal course work or MRI licensure required in 56% (32/57) of the laboratories. Only hands-on and/or safety training is required in 44% (25/57) of the responding laboratories.
Figure 1.
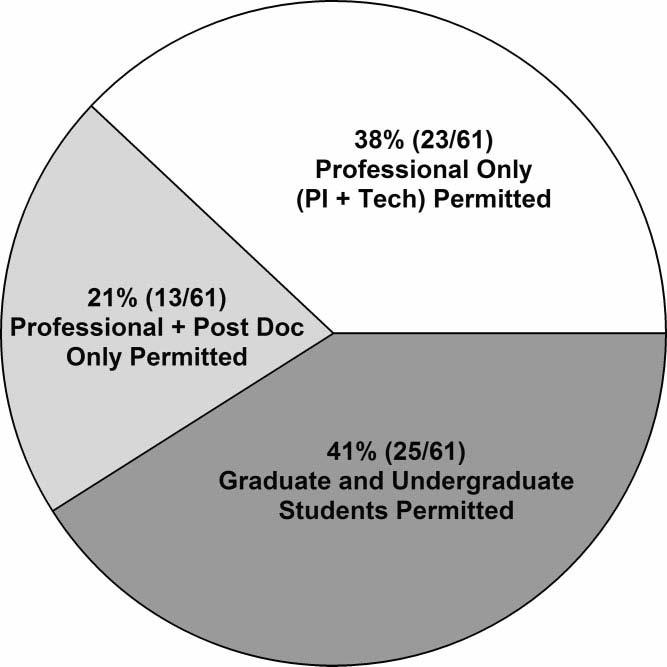
Research personnel at different career levels who are permitted to conduct scans.
In consideration of subject privacy and confidentiality, we asked about personnel permitted in the scanning environment. In 34% (20/59) of responding laboratories, nonresearch personnel are permitted as observers; of these laboratories, 35% (7/20) allow non-research personnel in the scanning environment only when they are family members accompanying a minor undergoing a study.
In laboratories from which we received responses to our question, about incentives, we learned that a printout of a subject's brain scan is regularly or occasionally given in appreciation for subject participation 75% (44/59) of the time, and not at all in the other 25% (15/59).
Detection of Incidental Findings and Management
Of the investigators who responded to the question of whether they had knowledge of incidental findings in their studies, 82% (54/66) responded affirmatively. The survey did not specifically ask for investigators to report the diagnoses of the incidental findings; however, several respondents included narrative descriptions of findings in the comments section. Of those investigators who included the specific diagnoses of the incidental findings, the reported findings and their frequencies were as follows: tumors (N = 3; specific histological types unknown), arachnoid cysts of varying sizes (N = 2), arteriovenous malformation (N = 1), extensive falx calcification (N = 1), severe global atrophy (N = 1), Arnold Chiari malformations with and without syrinx (N = 1 and N = 4, respectively), agenesis of the corpus callosum (N = 1), and radiological changes consistent with early multiple sclerosis (N = 2). Additionally, a few respondents noted that neither the subject nor the treating physician reported back to the investigator with confirmation of the medical diagnosis or treatment outcome.
Of the responders who provided information about their specific protocols, 53% (28/53) reported that they have standardized procedures in place for handling incidental findings and communicating with research participants in whom findings are discovered. All others proceed on an ad hoc, case-by-case basis.
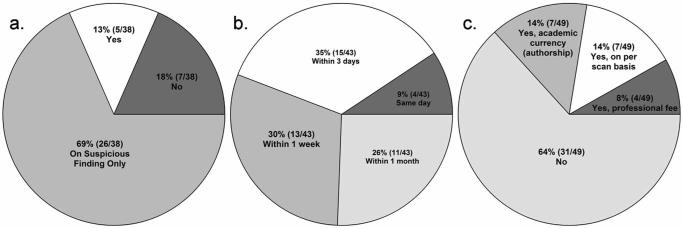
Neuroradiologist involvement is an IRB requirement for 22% (11/49) of laboratories reporting on this question. In laboratories in which neuroradiologist involvement is not required by the IRB, scans are nonetheless always read 13% (5/38) of the time, upon suspicious finding 69% (26/38) of the time, and not at all 18% (7/38) of the time (Fig. 2a). Lag time between data acquisition and reading generally occurs within one week in 74% (32/43), and within one month in 26% (11/43) of the laboratories. Same-day (4/43) or within three-day readings (15/43) were reported in 44% of the laboratories (Fig. 2b). Neuroradiologists are compensated for their services in 36% (18/49) of responding laboratories; 14% (7/49) are financially compensated on a per scan basis, 8% (4/49) by a professional fee, and 14% (7/49) in academic currency, such as authorship of publications, in lieu of professional fees (Fig. 2c). Neuroradiologists are consulted during the development of study protocols 24% of the time (14/59).
Figure 2.
Neuroradiologist involvement. a: Frequency of neuroradiological review of research scans when neuroradiology involvement is not an IRB requirement. b: Lag time for neuroradiological readings of research scans. c: Frequency and type of neuroradiologist compensation.
Only six consent forms were submitted, which limited our ability to conduct a quantitative analysis of language related to incidental findings. However, we observed that four forms did not contain any language specifically related to incidental findings. One center that allows only fully trained radiographers to operate the scanners sends reports of incidental findings directly to the subject's primary care provider identified on the consent form. Qualitatively, the most comprehensive relevant text submitted was as follows:
The MRI scan being done is designed to answer research questions, not examine your brain medically. This MRI scan is not a substitute for one a doctor would order. It may not show problems that would be picked up by a medical MRI scan. However, if we believe that we have found a medical problem in your MRI scan, we will ask a doctor who is trained in the reading of MRI scans, a radiologist, to help us review the scan. If the radiologist thinks that there may be an abnormality in your MRI scan, we will contact you and will help you get medical follow-up for the problem. If you have a primary care doctor, we can contact your doctor, with your permission, and help him or her get the right follow-up for you. No information generated in this study will become part of a hospital record routinely. However, if the study detects an abnormality in your MRI scan, then this information may become part of the hospital record. It is possible that you could be unnecessarily worried if a problem were suspected, but not actually found.
DISCUSSION
Considerable enthusiasm for the growing knowledge about human neurocognitive function available from neuroimaging is paralleled by growing empirical evidence about individual brain pathology that is unexpected and accidentally discovered during such studies. Incidental findings have implications for research participants and researchers alike. The data here suggest substantial procedural variability across research units for engaging participants, protecting participants in the research environment, and detecting and communicating abnormal findings to them. Moreover, the data point to the need for researchers to take such findings into account when designing studies and creating research teams.
This study has a number of limitations that must be recognized in interpreting the data. First, the 11% response rate limits the generalizability of the results to the larger MRI community. Second, the uniformity of sampling, and characteristics of the responding laboratories are unknown. Therefore, the impact that affiliation with or independence from a medical center or radiology department has on scanning protocols and procedures for consenting research participants, especially insofar as incidental findings are concerned, will require future study.
Nonetheless, a number of important results emerge from this study that, juxtaposed with other recent reports about incidental findings, are especially noteworthy. First, more than 80% of the respondents reported findings of unexpected pathology in their studies. Second, despite this large effect, only a fraction of research scans are reviewed professionally within a time frame of one week or less. This raises particular concerns in light of a recent study by Illes et al (4) showing that while the number of abnormalities detected in college-age subjects (one of the largest cohorts used in MRI research today) is low, the majority of such findings are highly significant. These data complement clinical reports of incidental findings in the central nervous system (CNS) of asymptomatic choroid plexus cysts in the lateral ventricles on diffusion-weighted MRI (8), non-functioning pituitary adenomas (9,10), and abnormal MR signal intensity indicating a subclinical stage of primary progressive multiple sclerosis (11).
Also, in a subset of the responders for whom neuroradiologist involvement is not an IRB requirement, almost 20% do not send scans out to be read at all. This may be a problem that is compounded by the fact that a substantial proportion of personnel who conduct research using imaging protocols such as MRI (including undergraduate and graduate students, post-docs, and many PIs) are not medically qualified to review scans for incidental findings. It is often the case that PIs and other senior researchers only view processed functional data presented on a standardized brain, and never view the original scans themselves. However, even senior researchers are often not M.D.s, let alone trained radiologists certified in image interpretation who, in nearly every other setting, would be the ones interpreting those same images. Third, given higher rates than expected of incidental findings, the presence of nonre-search personnel in the scanning environment during adult studies may be inadvisable given institutional and Health Insurance Portability and Accountability Act of 1996 (HIPPA) requirements for medical privacy.
We predict that although subjects sign consent forms acknowledging no clinical benefit and an understanding that the scans conducted are of research (not clinical) quality, they will nonetheless presume that if pathology exists, it will be detected and reported to them. We are currently testing this hypothesis in a study of subject expectations in both medical and nonmedical research settings.
On one hand, despite the high degree of variability observed, current procedures for handling incidental findings may be adequate. To our knowledge, neither the legal nor the medical literature has yet documented an adverse outcome due to a missed finding, and while brain MRI may represent an ideal modality for neurologic and neuroradiologic screening prior to subject enrollment, this would be difficult to implement in terms of both practicality and cost. Moreover, even though neuroimaging research may present an ideal opportunity for expanding collaborations among clinicians and researchers, and especially for providing hands-on research experience to clinical trainees in neuroradiology and other specialists who are familiar with reading brain MRIs (e.g., neurologists and certain psychiatrists), the sheer volume of scans requiring review would be daunting.
The counterpoint, on the other hand, bears on the potential imprudence of waiting for a catastrophic event or significant legal action before taking steps to recognize and manage any abnormalities that are discovered. The number of research participants scanned today is staggering—no doubt in the tens of thousands. The data reported here provide for the first time a perspective on incidental findings from the purview of the investigator, and further support our urgings in previous publications on this subject: before scientific progress is forestalled, and for the sake of both scientific integrity and public trust, we must revisit the relative trade-offs among all procedural alternatives and identify minimum and optimum standards of practice. We believe that the duty of disclosure is transparent to all professionals in the medical setting—clinical or research—regardless of professional degree (12,13).
Ethics research, quite apart from ethical conduct, has not been a focus of the medical imaging community in the past. However, the new field of neuroethics has gained substantial visibility and credibility in imaging, neurosciences, and other areas (including law, public policy, and the humanities) over the past few years (14). The study of neuroethics has shown that dialogue and methodologies drawn from multidisciplinary areas are not only desirable but are a powerful means of achieving scientific solutions to difficult research dilemmas. In the present case, we believe that processes formulated with the benefit of a wide range of disciplinary input will have greater acceptability and relevance, and will be easier to implement than those that may be imposed through a later regulatory process. Like other guidelines for research conduct that are embodied in our professional code of ethics, such as those for protecting the confidentiality of human subjects and authorship, guidelines regarding unexpected findings on brain scans are vital to our continuing research.
ACKNOWLEDGMENTS
We thank Drs. Pamela Schraedley-Desmond and Allyson Rosen.
Footnotes
Contract grant sponsor: The Greenwall Foundation; Contract grant sponsor: NIH/NINDS; Contract grant number: RO1 NS045831.
Presented at the Annual Meeting of the Society for Neurosciences, New Orleans, Nov. 8–12, 2003.
NOTE ADDED IN PROOF
On January 6–7, 2005, a trans-NIH workshop involving NINDS, NIDA, NIA, NHGRI, NIBIB, NICHD and NIMH is being held to achieve this goal.
REFERENCES
- 1.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282:36–39. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- 2.Kim BS, Illes J, Kaplan RT, Reiss A, Atlas SW. Incidental findings on pediatric MR images of the brain. AJNR Am J Neuroradiol. 2002;23:1674–1677. [PMC free article] [PubMed] [Google Scholar]
- 3.Illes J, Desmond JE, Huang LF, Raffin TA, Atlas SW. Ethical and practical considerations in managing incidental findings in functional magnetic resonance imaging. Brain Cogn. 2002;50:358–365. doi: 10.1016/s0278-2626(02)00532-8. [DOI] [PubMed] [Google Scholar]
- 4.Illes J, Rosen AC, Huang L, et al. Ethical consideration of incidental findings on adult brain MRI in research. Neurology. 2004;62:888–890. doi: 10.1212/01.wnl.0000118531.90418.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illes J, Kirschen MP, Gabrieli JD. From neuroimaging to neuroethics. Nat Neurosci. 2003;6:205. doi: 10.1038/nn0303-205. [DOI] [PubMed] [Google Scholar]
- 6.Illes J. Neuroethics in a new era of neuroimaging. AJNR Am J Neuroradiol. 2003;24:1739–1741. [PMC free article] [PubMed] [Google Scholar]
- 7.Illes J, Kirschen M. New prospects and ethical challenges for neuroimaging within and outside the health care system. AJNR Am J Neuroradiol. 2003;24:1932–1934. [PMC free article] [PubMed] [Google Scholar]
- 8.Cakir B, Karakas HM, Unlu E, Tuncbilek N. Asymptomatic choroid plexus cysts in the lateral ventricles: an incidental finding on diffusion-weighted MRI. Neuroradiology. 2002;44:830–833. doi: 10.1007/s00234-002-0803-1. [DOI] [PubMed] [Google Scholar]
- 9.Krsek M, Weiss V. Incidental findings of expansive processes in the sellar region. Cas Lek Cesk. 2003;142:14–18. [PubMed] [Google Scholar]
- 10.Molitch ME. Incidental pituitary adenomas. Am J Med Sci. 1993;306:262–264. doi: 10.1097/00000441-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell GV, Cabrera-Gomez J, Calne DB, Li DK, Oger J. Clinical presentation of primary progressive multiple sclerosis 10 years after the incidental finding of typical magnetic resonance imaging brain lesions: the subclinical stage of primary progressive multiple sclerosis may last 10 years. Mult Scler. 2003;9:204–209. doi: 10.1191/1352458503ms890cr. [DOI] [PubMed] [Google Scholar]
- 12.Reilly PR, Boshar MF, Holtzman SH. Ethical issues in genetic research: disclosure and informed consent. Nat Genet. 1997;15:16–20. doi: 10.1038/ng0197-16. [DOI] [PubMed] [Google Scholar]
- 13.Boendermaker PM, Pols J, Scherpbier AJ. Unexpected pathologic findings during skills training or assessment. Acad Med. 1998;73:1126. doi: 10.1097/00001888-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Farah MJ, Illes J, Cook-Deegan R, et al. Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci. 2004;5:421–425. doi: 10.1038/nrn1390. [DOI] [PubMed] [Google Scholar]