Abstract
The creation of an improved vaccine for global measles control will require an understanding of the immune mechanisms of measles virus containment. To assess the role of CD8+ cytotoxic T lymphocytes in measles virus clearance, rhesus monkeys were depleted of CD8+ lymphocytes by monoclonal anti-CD8 antibody infusion and challenged with wild-type measles virus. The CD8+ lymphocyte-depleted animals exhibited a more extensive rash, higher viral loads at the peak of virus replication, and a longer duration of viremia than did the control antibody-treated animals. These findings indicate a central role for CD8+ lymphocytes in the control of measles virus infections and the importance of eliciting a cell-mediated immune response in new measles vaccine strategies.
Measles remains an important cause of morbidity and mortality in young children. Although a preventative live attenuated measles virus (MV) vaccine has long been available, its efficacy is incomplete. The immunogenicity of this vaccine has been limited in young infants by maternal antibody interference and immune system immaturity (6), leaving substantial numbers of vaccinated infants susceptible to disease. In fact, approximately 1 million deaths per year are still attributable to measles, with the majority of these deaths occurring in sub-Saharan Africa (1).
Defining the immune mechanisms critical for the control of MV replication will be important in developing new measles vaccination strategies. It is well established that anti-MV antibodies play a central role in protection against MV infection. However, accruing data have implicated cell-mediated immune responses in the control of MV replication (10, 16, 17, 23, 34). MV-specific, CD8+ cytotoxic T lymphocytes are known to be activated and expanded in the peripheral blood in temporal association with the onset of the measles rash (16, 17, 21, 30). Soluble CD8 and β2 microglobulin are increased in the plasma during acute measles infection in children (12).
A number of clinical observations have also implicated MV-specific cellular immune responses in the clearance of this virus. Children with cellular immune deficiencies have more severe clinical disease after MV infection than children who are hypogammaglobulinemic or who are immunologically intact (8, 24), and the potency of the cell-mediated immune response has been correlated with the ability of infected individuals to recover from MV infection (3). Human immunodeficiency virus-infected children are more likely to have prolonged shedding of MV than those who are human immunodeficiency virus negative (26), presumably because of impaired MV-specific cellular immune responses. In a transgenic mouse model of MV infection (25), the T-lymphocyte-mediated immune response is required for clearance of neuronal infection (22). Nevertheless, these observations have all been correlative. The importance of cell-mediated immunity in MV clearance has not been directly demonstrated.
Nonhuman primates can be infected with MV experimentally and provide the only available animal model of MV pathogenesis. MV-infected small laboratory animals do not develop systemic viral replication or clinical disease. MV-infected rhesus monkeys, on the other hand, show evidence of systemic viral replication, MV-induced immunosuppression and clinical signs of disease, including maculopapular rash and conjunctivitis (2). Because of this, rhesus monkeys have recently been used to study the efficacy of novel MV vaccine strategies (27).
In the present study, we directly investigated the role of CD8+ lymphocytes in the control of MV replication by eliminating CD8+ lymphocytes from rhesus monkeys and assessing the sequelae of MV infection. All monkeys in the study were between 7 and 11 years of age. Four normal rhesus monkeys, shown to be MV naive by negative MV-specific enzyme-linked immunosorbent assay and neutralizing-antibody assays, were infused by the intravenous route with a monoclonal anti-CD8 antibody (the mouse-human chimeric cM-T807; Centocor, Malvern, Pa.) at a dose of 5 mg/kg on days −3, 0, and 4 relative to the MV infection. Two normal rhesus monkeys, also shown to be MV naive, were similarly infused with the same quantities of a control monoclonal anti-RSV antibody (Synagis, MedImmune, Inc., Gaithersburg, Md.) according to the same schedule. Monkeys were inoculated intratracheally with a 50% tissue culture infective dose (104) of Bilthoven strain MV on day 0. All animals were maintained in accordance with the guidelines of the Committee on Animals for the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals.
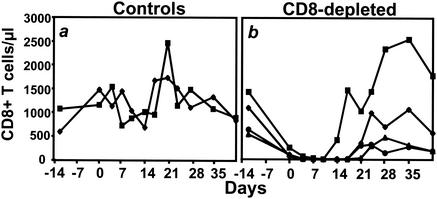
CD8+ T lymphocytes were assessed in the peripheral blood of the monkeys by cell staining and flow cytometric analysis with anti-CD8-PE (SK1; Becton Dickinson, San Jose, Calif.) as previously described (28). CD8+ T lymphocytes were undetectable in the peripheral blood of the four monoclonal anti-CD8 antibody-treated animals for at least 14 days after infection (Fig. 1). CD8+ T lymphocytes were subsequently detected in the peripheral blood of all of these animals by day 24 after infection. Near-total depletion of CD8+ lymphocytes and a slight reduction in CD3+ lymphocytes was achieved in the lymph nodes sampled on day 7 from the anti-CD8 antibody-treated animals (Fig. 2A and C). The CD8+ T-lymphocyte counts (Fig. 1) and the lymph node CD8+ and CD3+ lymphocyte populations (Fig. 2B and D) in the control animals were not affected by the administration of the control monoclonal antibody.
FIG. 1.
CD8+ lymphocytes are depleted in the peripheral blood of rhesus monkeys as a result of intravenous injection of the mouse-human chimeric monoclonal anti-CD8 antibody cM-T807. The anti-CD8 antibody (four monkeys) or a control antibody (two monkeys) was administered intravenously on days −3, 0, and 4 of an MV infection. CD8+ T lymphocytes were quantitated by using a phycoerythrin-coupled monoclonal antibody that was able to bind to CD8 in the presence of cM-T807, as previously reported (28). When CD8+ T lymphocytes were not detectable, >95% of the remaining lymphocytes were CD20+ B cells or CD4+ T lymphocytes. (a) CD8+ T-lymphocyte counts in control antibody-treated monkeys. (b) CD8+ T-lymphocyte counts in monkeys treated with anti-CD8 antibody.
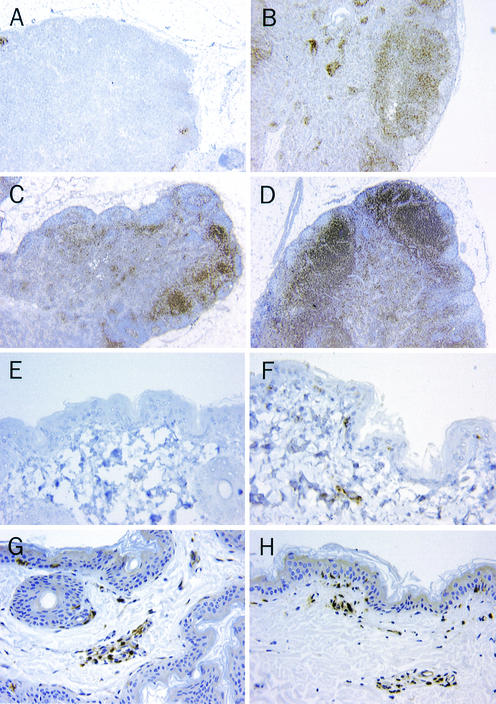
FIG. 2.
CD8+ lymphocytes are nearly eliminated in inguinal lymph nodes from CD8+ lymphocyte-depleted monkeys on day 7 after infection. CD8+ lymphocytes do not appear in the CD3+ lymphocytic infiltrate of the axillary measles rash on day 14 after infection. The formalin-fixed tissues were embedded in paraffin, sectioned at 5 μm, and immunostained either with polyclonal anti-CD3 antibodies (Dako, Carpinteria, Calif.) or with monoclonal anti-CD8 antibodies (1A5; Vector Laboratories, Burlingame, Calif.). Tissues stained for CD3 were preheated in a microwave oven for 20 min by using antigen unmasking solution (Vector Laboratories); those stained for CD8 were preheated in an electric pressure cooker for 15 min with Trilogy solution (Cell Marque Corp., Hot Springs, Ark.). For each primary antibody, an appropriate negative control was used at the same concentration: a rabbit immunoglobulin fraction for CD3 and mouse IgG1 for CD8. Sections were counterstained with hematoxylin. Lymph nodes from a monoclonal anti-CD8 antibody-treated monkey (A) and a control antibody-treated monkey (B) stained for expression of CD8 (magnification, ×20), lymph nodes from a monoclonal anti-CD8 antibody-treated monkey (C) and a control monkey (D) stained for expression of CD3 (magnification, ×20), axillary skin from a monoclonal anti-CD8 antibody-treated monkey (E) and a control antibody-treated monkey (F) stained for expression of CD8 (magnification, ×400), and axillary skin from a monoclonal anti-CD8 antibody-treated monkey (G) and a control antibody-treated monkey (H) stained for expression of CD3 (magnification, ×400) are shown.
The course of clinical disease after MV infection differed in the monoclonal anti-CD8 antibody-treated and the control antibody-treated groups of monkeys. While all of the monkeys developed maculopapular skin rashes between days 7 and 10 after infection, the rashes were more prominent and extensive in the monoclonal anti-CD8 antibody-treated animals. Moreover, they persisted 2 to 4 days longer in these CD8+ lymphocyte-depleted monkeys. Interestingly, conjunctivitis, commonly seen in MV-infected rhesus monkeys, was observed in both control animals on day 10 but in none of the CD8+ lymphocyte-depleted monkeys.
Global immunologic consequences of MV infection were comparable in both groups of monkeys. Both groups of animals developed characteristic MV-induced cell-mediated immunosuppression, measured by peripheral blood mononuclear cell (PBMC) proliferation after stimulation with phytohemagglutinin (PHA; 2.5 μg/ml; Sigma, St. Louis, Mo.), as previously described (9) (data not shown). Differences were also not detected in mitogen-stimulated cytokine production by T lymphocytes (interleukin-4 [IL-4], IL-6, IL-12, gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) (data not shown). For these assays, Staphylococcus aureus Cowan strain (SAC; Sigma)-stimulated cells and associated culture supernatants were harvested at 24 h and PHA-stimulated cells and culture supernatants were harvested at 48 h. IL-12, and TNF-α were measured in the supernatants of the SAC-stimulated cells and IFN-γ, IL-4, and IL-6 were measured in the supernatants of the PHA-stimulated cells by immunoassays for each cytokine (Biosource, Camarillo, Calif.) according to the manufacturer's instructions.
Histologic evaluation of the skin biopsies taken from axillary areas of erythema on day 14 after infection demonstrated the expected absence of CD8+ T lymphocytes in the CD3+ lymphocytic infiltration of the dermis and epidermis of CD8+ T-lymphocyte-depleted animals (Fig. 2E). CD8+ T lymphocytes were present in the epithelial and perivascular regions of the skin specimens from control animals (Fig. 2F). Otherwise, however, the histologic appearance of these skin biopsies were comparable, since CD3+ intraepithelial and perivascular lymphocytes (Fig. 2G and H) were detectable in the skin of both CD8+ lymphocyte-depleted and control animals.
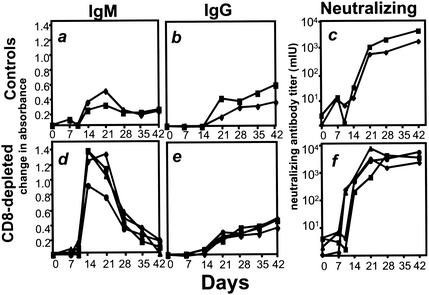
MV-specific immunoglobulin M (IgM) antibody was detectable in both groups of animals on day 14, but the IgM responses in the depleted animals reached higher titers (Fig. 3a and d). The neutralizing antibody response was detectable in two of the CD8+ lymphocyte-depleted animals by day 7 and in all of them by day 10; the control animals did not develop significant titers of neutralizing antibody until day 21 (Fig. 3c and f). However, CD8+ lymphocyte-depleted and control monkeys generated comparable MV-specific IgG antibody responses (Fig. 3b and e).
FIG. 3.
Anti-MV antibody responses after MV infection in the CD8+ lymphocyte-depleted and control monkeys. Neutralizing antibody was measured in a plaque reduction assay using the Chicago-1 strain of MV and Vero cells as previously described (2). MV-specific IgM and IgG were measured in the MV IgG indirect enzyme immunoassay kit (Sigma) by substituting a horseradish-peroxidase-conjugated goat anti-monkey IgM (Nordic, Capistrano Beach, Calif.) and an alkaline phosphatase-conjugated rabbit anti-monkey IgG (Biomakor; Accurate Chemicals, Westbury, N.J.), respectively, for the secondary antibody. Sera were diluted 1:100, and secondary antibodies were diluted 1:2,000 in 1% normal rabbit serum and 0.05% Tween 20 in phosphate-buffered saline for all assays. Fast pNPP (Tab set-n2770; Sigma) was used as a substrate for the alkaline phosphatase-conjugated rabbit anti-monkey IgG, and plates were read by determining the optical density. Samples were tested in duplicate, and displayed values represent the mean change in absorbance from preinfected serum obtained from the same animal. (a) MV-specific IgM in control monkeys; (b) MV-specific IgG in control monkeys; (c) MV-neutralizing antibody in control monkeys; (d) MV-specific IgM in CD8-depleted monkeys; (e) MV-specific IgG in CD8-depleted monkeys; (f) MV-neutralizing antibodies in CD8-depleted monkeys.
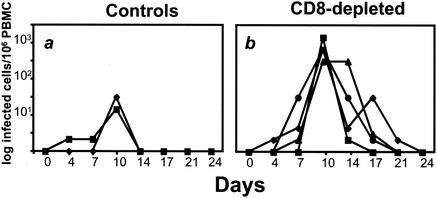
The most compelling differences between the CD8+ lymphocyte-depleted and control monkeys after infection was the extent of MV replication observed in these animals. Infectious virus, measured by cocultivation techniques with B95-8 cells (20), was 1.5 logs higher at the peak of viremia in animals that received the anti-CD8 monoclonal antibody (Fig. 4). At 14 days after infection, the control animals had undetectable infectious virus, whereas infectious virus remained demonstrable in the peripheral blood lymphocytes (PBL) of all of the CD8+ lymphocyte-depleted monkeys.
FIG. 4.
Titers of infectious MV in the blood are greater in the CD8+ lymphocyte-depleted animals than in the control monkeys during early infection. Infectious virus was measured by cocultivation of PBL with B95-8 cells. (a) MV viremia in control monkeys; (b) MV viremia in CD8+ lymphocyte-depleted monkeys.
In order to quantitate MV load in these monkeys, an MV-specific reverse transcription-PCR (RT-PCR) assay was developed (the sensitivity of the assay was 15 copies of MV RNA). A pBluescript SK plasmid containing the MV hemagglutinin gene was linearized with PvuII (New England Biolabs, Beverly, Mass.) and used to create an RNA standard, as previously described (15). RNA was extracted from 106 PBMCs stored in 1 ml of RNA-Stat 60 (Teltest, Friendswood, Tex.) according to the manufacturer's protocol and then frozen at −70°C until analysis. One-step RT-PCR was performed by using the TaqMan One-Step RT-PCR kit (PE Applied Biosystems, Foster City, Calif.) and MV H gene-specific primers (5′-CAATCGAGCATCAGGTCAAGG-3′ and 5′-GTCCTCAGGCCCACTTCATC-3′) and labeled probe (5′-FAM-CGTGCTACACCACTCTTCAAAATCATCGG-TAMRA-3′) (PE Applied Biosystems). The 50-μl reactions included 25 μl of TaqMan One-Step RT-PCR Master Mix, 2.5 μl of 10 μM concentrations of each primer, 1.5 μl of 10 μM fluorogenic probe, 1 μl of ×40 MultiScribe and RNase inhibitor mix (PE Applied Biosystems), and 18 μl of RNA. Quantitative RT-PCR was performed on an ABI Prism 7700 under the following conditions: hold for 30 min at 48°C, hold for 10 min at 95°C, and 45 cycles of 15 s at 95°C and 1 min at 60°C. For each sample, the CT value, defined as the minimum number of cycles necessary to exceed threshold values, was measured and applied to the standardization curve created from the RNA transcript dilution series.
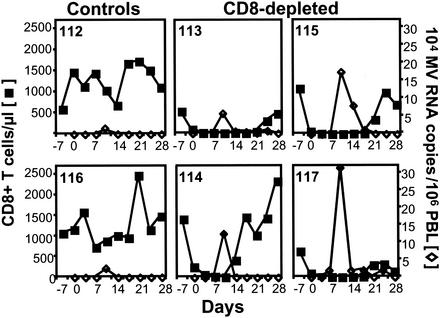
The CD8+ lymphocyte-depleted animals had substantially higher MV RNA levels in their PBMCs at the peak of the infection compared to control monkeys (Fig. 5). Moreover, the clearance of virus RNA in PBMCs occurred just prior to or concurrently with the repopulation of CD8+ T lymphocytes in the peripheral blood of the monoclonal anti-CD8 antibody-treated monkeys (Fig. 5).
FIG. 5.
PBL MV RNA levels are higher in CD8+ lymphocyte-depleted than in control monkeys, and clearance coincides temporally with the repopulation of CD8+ lymphocytes. The CD8+ lymphocyte count (▪) and MV RNA level in PBL (⋄), measured by quantitative MV-specific RT-PCR, were assessed prospectively after MV infection in two control monkeys (animals 112 and 116) and four CD8+ lymphocyte-depleted monkeys (animals 113, 114, 115, and 117).
The depletion of CD8+ lymphocytes resulted in changes in two clinical sequelae of MV infection in these monkeys: the skin rash and conjunctivitis. It has long been appreciated that the skin rash in MV infection is a manifestation of the cellular immune response to the virus (19). The histologic appearance of the typical MV-associated maculopapular rash includes focal keratosis and edema with a perivascular mononuclear infiltrate (32). In fact, children with deficiencies in cell-mediated immune function develop either atypical skin rashes or no rashes at all in association with MV infections (7, 18). The observation in the present study that the CD8+ lymphocyte-depleted monkeys develop rashes indicates that this immunopathogenic process does not require CD8+ lymphocytes. The more pronounced and longer duration of rashes in the CD8+ lymphocyte-depleted monkeys compared to control monkeys may simply reflect the particularly high and prolonged viral replication in the experimental monkeys. The severity of the rash is also consistent with the possibility that CD8+ lymphocytes play a regulatory role in this dermatopathologic process. The finding in the present study of conjunctivitis in control monkeys with no evidence of conjunctivitis in CD8+ lymphocyte-depleted monkeys suggests that CD8+ lymphocytes play a central role in the etiology of this inflammatory response. Alternatively, the increased viral load in the CD8+ lymphocyte-depleted monkeys could have resulted in a more profound immunosuppression that prevented conjunctival inflammation. However, this possibility is unlikely since mitogen-induced proliferation and cytokine production studies did not detect an effect of CD8+ lymphocyte depletion on MV-induced immunosuppression.
It is well established that MV infection in humans is associated with a global immune suppression that can last a number of weeks. Individuals recently infected with MV demonstrate decreased responsiveness to cutaneous antigen challenge (31, 33) and the PBL of these individuals do not proliferate normally in vitro in response to mitogen stimulation (14, 31). The etiology of this immune suppression remains uncertain. However, the observation in the present study that lymphocytes of the control and CD8+ lymphocyte-depleted MV-infected monkeys had comparable cytokine production and proliferative responses to mitogen stimulation suggests that CD8+ lymphocytes do not play an important role in this immune dysregulation.
After infection, the CD8+ lymphocyte-depleted monkeys developed higher-titer MV-specific IgM and earlier neutralizing antibody responses than did the control monkeys. However, the experimental and control monkeys developed comparable MV-specific IgG responses. Since previous studies have shown that CD8+ lymphocyte depletion of rhesus monkeys does not affect the ability of the monkeys to generate antibody responses (29) and MV-induced immunosuppression has not been shown to affect class-switching of B lymphocytes, this difference is not likely to represent an immunologic change in the ability of the monkeys to mount these immune responses. Rather, this difference probably reflects immune responses in the CD8+ lymphocyte-depleted monkeys to higher levels of viral antigen. The similarity of the IgG antibody titers in the two groups of monkeys is consistent with the likelihood that the exposure of the immune system to viral antigen in the two groups of monkeys was comparable after CD8+ lymphocytes returned to normal levels in the experimentally treated animals.
The particularly high MV load in the CD8+ lymphocyte-depleted monkeys is most likely a result of absent CD8+ cytotoxic T lymphocytes, cells which have been shown to be important in the control of many other viruses. However, the CD8+ lymphocyte population of the monkey includes both cytotoxic T cells (CD8+ CD3+) and natural killer (NK) cells (CD8+ CD3−). Both cell subpopulations are eliminated from the peripheral blood in the course of CD8+ lymphocyte depletion. It is, therefore, formally possible that the differences in viral replication between the two groups of animals could be due to the absence of NK cell immunity in the CD8+ lymphocyte-depleted monkeys. However, this is unlikely given that NK cell activity is reduced during the acute phase of MV infection (11).
The clinical, virologic, and immunologic differences between these groups of monkeys provide convincing evidence that CD8+ lymphocytes play a role in the containment of MV replication. However, the present study also demonstrates that other immunologic mechanisms contribute to this immune containment. The CD8+ lymphocyte-depleted monkeys never developed uncontrolled viremia with an associated encephalitis and pneumonitis that might have been seen if CD8+ lymphocytes were the sole effective mediators of anti-MV immunity. Moreover, viremia began to decline during the period of primary infection in the experimentally treated monkeys before CD8+ lymphocytes were detected in peripheral blood. This containment of MV replication during the period of CD8+ lymphocyte depletion could have been facilitated by residual or repopulating CD8+ lymphocytes in the secondary lymphoid organs of the monkeys, since CD8+ lymphocytes were certainly present in lymph nodes on day 7 after infection. However, it is likely that humoral responses also contributed to the containment of MV replication. In support of this contention, neutralizing antibody titers correlate with protection from MV infection (4) and antibody-dependent cell cytotoxicity has been implicated in the control of MV replication (5). In addition, it should be recalled that an inactivated MV immunogen showed efficacy as a vaccine in preventing MV infection in humans, until neutralizing antibody titers waned (13). Since such a vaccine cannot elicit CD8+ virus-specific cytotoxic T lymphocytes, the protection must have been antibody mediated.
Acknowledgments
This work was supported by NIH grant AI-20729 (N.L.L.), grant P51 RR00163 (ORPC), grant RR-00168 (NERPC), grant AI-23047 (D.E.G.), and by Center for AIDS Research grant A128691. The monoclonal anti-CD8 antibody (cMT807) was produced by the National Cell Culture Center with funds provided by NCRR award RR16001, and the hybridoma was provided by Centocor.
We are grateful to Alex Valsamakis (JHSM) for the gift of the MV hemagglutinin plasmid and to V. Petkova, D. Pauley, M. O'Connell, K. Marano, R. Khunkhun, P. F. McKay, and C. Lord for assistance and advice.
REFERENCES
- 1.Anonymous. 1999. Global measles control and regional elimination, 1998-1999. Morb. Mortal. Wkly. Rep. 48:1124-1130. [PubMed] [Google Scholar]
- 2.Auwaerter, P. G., P. A. Rota, W. R. Elkins, R. J. Adams, T. DeLozier, Y. Shi, W. J. Bellini, B. R. Murphy, and D. E. Griffin. 1999. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J. Infect. Dis. 180:950-958. [DOI] [PubMed] [Google Scholar]
- 3.Burnet, F. M. 1968. Measles as an index of immunological function. Lancet ii:610-613. [DOI] [PubMed] [Google Scholar]
- 4.Chen, R. T., L. E. Markowitz, P. Albrecht, J. A. Stewart, L. M. Mofenson, S. R. Preblud, and W. A. Orenstein. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036-1042. [DOI] [PubMed] [Google Scholar]
- 5.Forthal, D. N., G. Landucci, A. Habis, M. Zartarian, J. Katz, and J. G. Tilles. 1994. Measles virus-specific functional antibody responses and viremia during acute measles. J. Infect. Dis. 169:1377-1380. [DOI] [PubMed] [Google Scholar]
- 6.Gans, H. A., A. M. Arvin, J. Galinus, L. Logan, R. DeHovitz, and Y. Maldonado. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527-532. [DOI] [PubMed] [Google Scholar]
- 7.Good, R. A. 1977. Use of a radioimmune assay in detection of measles antibodies in cerebrospinal fluid and serum. Acta Neurol. Scand. Suppl. 63:199-206. [PubMed] [Google Scholar]
- 8.Good, R. A., and S. J. Zak. 1956. Disturbance in gammaglobulin synthesis as “experiments of nature.” Pediatrics 18:109-149. [PubMed] [Google Scholar]
- 9.Griffin, D. E., B. J. Ward, E. Jauregui, R. T. Johnson, and A. Vaisberg. 1990. Immune activation during measles: interferon-gamma and neopterin in plasma and cerebrospinal fluid in complicated and uncomplicated disease. J. Infect. Dis. 161:449-453. [DOI] [PubMed] [Google Scholar]
- 10.Griffin, D. E., B. J. Ward, E. Jauregui, R. T. Johnson, and A. Vaisberg. 1989. Immune activation in measles. N. Engl. J. Med. 320:1667-1672. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, D. E., B. J. Ward, E. Jauregui, R. T. Johnson, and A. Vaisberg. 1990. Natural killer cell activity during measles. Clin. Exp. Immunol. 81:218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin, D. E., B. J. Ward, E. Juaregui, R. T. Johnson, and A. Vaisberg. 1992. Immune activation during measles: β2-microglobulin in plasma and cerebrospinal fluid in complicated and uncomplicated disease. J. Infect. Dis. 166:1170-1173. [DOI] [PubMed] [Google Scholar]
- 13.Guinee, V. F., D. A. Henderson, H. L. Casey, S. T. Wingo, D. W. Ruthig, T. A. Cockburn, T. O. Vinson, D. C. Calafiore, W. Winkelstein, Jr., D. T. Karzon, M. L. Rathbun, E. R. Alexander, and D. R. Peterson. 1966. Cooperative measles vaccine field trial. I. Clinical efficacy. Pediatrics 37:649-665. [PubMed] [Google Scholar]
- 14.Hirsch, R. L., D. E. Griffin, R. T. Johnson, S. J. Cooper, I. Lindo de Soriano, S. Roedenbeck, and A. Vaisberg. 1984. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin. Immunol. Immunopathol. 31:1-12. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 16.Jaye, A., A. F. Magnusen, A. D. Sadiq, T. Corrah, and H. C. Whittle. 1998. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J. Clin. Investig. 102:1969-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaye, A., A. F. Magnusen, and H. C. Whittle. 1998. Human leukocyte antigen class I- and class II-restricted cytotoxic T lymphocyte responses to measles antigens in immune adults. J. Infect. Dis. 177:1282-1289. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan, L. J., R. S. Daum, M. Smaron, and C. A. McCarthy. 1992. Severe measles in immunocompromised patients. JAMA 267:1237-1241. [PubMed] [Google Scholar]
- 19.Kimura, A., K. Tosaka, and T. Nakao. 1975. Measles rash. I. Light and electron microscopic study of skin eruptions. Arch. Virol. 47:295-307. [DOI] [PubMed] [Google Scholar]
- 20.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreth, H. W., V. ter Meulen, and G. Eckert. 1979. Demonstration of HLA restricted killer cells in patients with acute measles. Med. Microbiol. Immunol. 165:203-214. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence, D. M., M. M. Vaughn, A. R. Belman, J. S. Cole, and G. F. Rall. 1999. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J. Virol. 73:1795-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mongkolsapaya, J., A. Jaye, M. F. Callan, A. F. Magnusen, A. J. McMichael, and H. C. Whittle. 1999. Antigen-specific expansion of cytotoxic T lymphocytes in acute measles virus infection. J. Virol. 73:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahmias, A. J., D. Griffith, C. Salsbury, and K. Yoshida. 1967. Thymic aplasia with lymphopenia, plasma cells, and normal immunoglobulins: relation to measles virus infection. JAMA 201:729-734. [PubMed] [Google Scholar]
- 25.Oldstone, M. B., H. Lewicki, D. Thomas, A. Tishon, S. Dales, J. Patterson, M. Manchester, D. Homann, D. Naniche, and A. Holz. 1999. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell 98:629-640. [DOI] [PubMed] [Google Scholar]
- 26.Permar, S. R., W. J. Moss, J. J. Ryon, M. Monze, F. Cutts, T. C. Quinn, and D. E. Griffin. 2001. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J. Infect. Dis. 183:532-538. [DOI] [PubMed] [Google Scholar]
- 27.Polack, F. P., S. H. Lee, S. Permar, E. Manyara, H. G. Nousari, Y. Jeng, F. Mustafa, A. Valsamakis, R. J. Adams, H. L. Robinson, and D. E. Griffin. 2000. Successful DNA immunization against measles: neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nat. Med. 6:776-781. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz, J. E., M. A. Simon, M. J. Kuroda, M. A. Lifton, M. W. Ollert, C. W. Vogel, P. Racz, K. Tenner-Racz, B. J. Scallon, M. Dalesandro, J. Ghrayeb, E. P. Rieber, V. G. Sasseville, and K. A. Reimann. 1999. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am. J. Pathol. 154:1923-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sissons, J. G., S. D. Colby, W. O. Harrison, and M. B. Oldstone. 1985. Cytotoxic lymphocytes generated in vivo with acute measles virus infection. Clin. Immunol. Immunopathol. 34:60-68. [DOI] [PubMed] [Google Scholar]
- 31.Starr, S., and S. Berkovich. 1964. Effects of measles, gamma-globulin-modified measles and vaccine measles on tuberculin test. N. Engl. J. Med. 270:386-391. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi, H., Y. Umino, T. A. Sato, T. Kohama, Y. Ikeda, M. Iijima, and R. Fujisawa. 1996. Detection and comparison of viral antigens in measles and rubella rashes. Clin. Infect. Dis. 22:36-39. [DOI] [PubMed] [Google Scholar]
- 33.Tamashiro, V. G., H. H. Perez, and D. E. Griffin. 1987. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr. Infect. Dis. J. 6:451-454. [DOI] [PubMed] [Google Scholar]
- 34.van Binnendijk, R. S., M. C. Poelen, K. C. Kuijpers, A. D. Osterhaus, and F. G. Uytdehaag. 1990. The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles: clonal analyses of human CD8+ class I MHC-restricted CTL. J. Immunol. 144:2394-2399. [PubMed] [Google Scholar]