Abstract
The initial interaction of murine polyomavirus (Py) with host cells occurs through direct binding of the major capsid protein VP1 with cell membrane molecules containing terminal sialic acids; however, these Py receptor molecules have not yet been identified. Analysis of the capsid protein primary sequences of all murine strains revealed the presence of integrin ligand motifs in the DE and EF loops of VP1 (LDV and DLXXL, respectively) and at the N terminus of VP2 (DGE). We show that infectivity of the Py A2 strain in mouse Swiss 3T3 fibroblasts is significantly reduced only in the presence of natural integrin ligands carrying an LDV motif or antibodies directed against the α4 and β1 integrin subunits. Furthermore, we demonstrate that expression of the α4 subunit in the α4-deficient BALB/c 3T3 cells increases viral infectivity. Addition of α4 function-blocking antibodies, prior to or after virus adsorption, blocks this increased infectivity without affecting virus binding to cells. Taken together, these data indicate that expression of α4 integrin enhances permissivity to Py, probably by acting as one of the postattachment receptors.
The first step of the life cycle of nonenveloped viruses involves the recognition and attachment of the viral capsid to an appropriate host cell membrane receptor, followed by virus entry. The events that occur during this initial virus-host cell interaction are not yet clearly understood, since each virus may have developed different entry strategies. However, this step is considered a major determinant of virus host range and tissue tropism.
Polyomaviruses are double-stranded DNA tumor viruses with an icosahedral capsid composed of 72 pentamers of the major capsid protein VP1, each associated with one copy of the minor coat protein VP2 or VP3 (10, 52). Polyomaviruses infect different species but are largely species specific, with a fairly narrow host range (10). Among these viruses, murine polyomavirus (Py) is one of the best characterized. In its natural host, it displays wide tissue tropism that has been correlated, in part, to the ability of the protein VP1 to attach to host cell membrane molecules containing terminal sialic acids (SA), which are broadly expressed in the mouse (49, 50). The VP1 protein is formed by β-strands, with “jellyroll” topology, which are connected by surface loops called BC, DE, EF, and HI (49). Extensive X-ray crystallography studies of the VP1-SA complex have predicted that the receptor binding site, involving residues of the outfacing BC and HI loops, is formed by three pockets that accommodate the terminal SA, the galactoside, and, for some strains, second branched SA residues (49, 50). The observation that single amino acid substitutions in the VP1 receptor binding site produce nonviable mutants indicates that VP1-SA interaction is critical for Py infectivity (3). However, attempts to isolate and identify a receptor molecule have been unsuccessful so far, and it is not known whether a single or multiple receptor species exist. The minor coat protein VP2 has also been proposed to participate in viral entry and, in particular, in membrane penetration, via its myristylated N terminus (7, 45).
Among the identified virus receptor molecules, integrins are receptors and essential entry molecules for several icosahedral viruses, such as adenoviruses, coxsackieviruses, foot-and-mouth disease viruses (FMDV), rotaviruses, and papillomaviruses (11, 26, 27, 31, 53). This family of α/β heterodimers mediates cell adhesion to extracellular matrix proteins and to other cells by specific recognition of ligand consensus sequence motifs, such as RGD, LDV, or DGE (29, 33, 41, 48). Similarly, the interaction of viruses with integrins occurs through specific recognition by integrins of these consensus sequences exposed on viral capsid proteins. For instance, coxackievirus A9, FMDV, and adenoviruses bind to integrin αvβ3 through RGD motifs displayed by their capsid proteins, whereas rotaviruses interact with α4β1 and α2β1 integrins by means of LDV and DGE motifs exposed on two of the coat proteins (9, 26, 27, 31, 53). Of relevant interest is the fact that for some viruses, such as FMDV and rotaviruses, integrin recognition has been demonstrated to correlate with cell permissivity (8, 31). For the murine Py, considerable evidence indicates that host range phenotype and tissue specificity depend, in part, on postentry events, regulated by enhancer-promoter elements and early regions of the viral genome (6, 38). In addition, interaction of the virus, through particular VP1 residues, with straight- or branched-SA-containing molecules has been correlated with different pathogenicity and tumorigenicity patterns (14, 15); however, so far there has been no direct demonstration that recognition of specific receptors or the presence of certain cell surface molecules can also affect cell susceptibility to Py.
In this work, we have identified integrin ligand consensus binding sites in Py VP1 and VP2 capsid proteins and have studied their potential role in Py infectivity. Our results show that α4β1 integrin is involved in Py infectivity and that α4 cell surface expression increases cell susceptibility to Py at a postattachment level. They also suggest the involvement of other, uncharacterized cell receptors.
MATERIALS AND METHODS
Cells.
Mouse Swiss 3T3, BALB/c 3T3, and 3T6 fibroblasts were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (GIBCO) in a 5% CO2 atmosphere, at 37°C. BALB/c 3T3 cells were transfected with empty pRK5 or pRK5-α4 vector, carrying the entire cDNA of the murine α4 integrin subunit (43) (kindly provided by B. Holzmann, Technische Universitat, Munich, Germany), using the Lipofectamine Plus reagent (Invitrogen). For stable transfections, we used as a selection marker the plasmid pBabe-Puro, containing the puromycin resistance gene (40). Two days after transfection, cells were split 1:5 in DMEM supplemented with puromycin at 1.3 μg/ml. The medium was changed every 2 to 3 days, and after 5 to 10 days, surviving colonies were isolated and amplified separately.
Viruses.
Py strain A2 was propagated at a low multiplicity of infection in 3T6 fibroblasts as described previously (17). Briefly, at 10 days postinfection, the cell lysate was collected by repeated freeze-thawing and centrifuged for 15 min at 8,000 × g. The resulting supernatant was collected as the viral lysate.
The GenBank accession number for the genome sequence of the Py A2 strain used is J02288.1; however, the VP1-coding sequence is identical to the GenBank M34958 sequence, except for an amino acid substitution at position 92 (Gly changed to Glu).
For binding experiments, viral particles were purified as follows. The viral lysate was first pelleted by centrifugation through a 20% sucrose cushion in buffer B (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 0.01 mM CaCl2). The pellet was resuspended in buffer B and purified by banding on a CsCl gradient and subsequently on a sucrose gradient (10 to 40% [wt/wt] in buffer B). Finally, viral particles were sedimented onto a 20% sucrose cushion in buffer B. The protein concentration was measured with the Bio-Rad protein assay. Biotinylation of purified viral particles was carried out as follows. Fifteen micrograms of purified virus was incubated with 1 mM biotin (Pierce) in a buffer containing 50 mM Na2CO3 (pH 8.5) for 30 min at room temperature. Labeled particles were then extensively dialyzed against buffer B.
Western blots.
Biotinylated viral particles were lysed in Laemmli buffer, subjected to sodium dodecyl sulfate-13% polyacrylamide gel electrophoresis (SDS-13% PAGE), and transferred to nitrocellulose filters (Schleicher & Schuell). Western blot analysis was carried out by using horseradish peroxidase (HRP)-conjugated streptavidin (Pierce) after blocking nonspecific reactivity with 4% nonfat dry milk in Tris-buffered saline-0.05% Tween 20. Bands were detected by the enhanced chemiluminescence reaction (Pierce).
Sequence analysis.
The search for the presence in Py A2 VP1 and VP2 sequences of the integrin recognition sites LDV, DLXXL, DGE, RGD, NGR, RRETAWA, REDV, SDGR, YIGSR, YIGSE, RGES, RSGIY, RSGD, DRDE, and SRYD (28, 30) and sequence alignments were carried out with the CLUSTAL W multiple-sequence alignment program.
Ligands and Abs.
Fibronectin and fibronectin α-chymotryptic 40,000-molecular-weight (Fn40) and 120,000-molecular-weight (Fn120) fragments were purchased from Roche Molecular Biochemicals and GIBCO, respectively. Type I collagen and vitronectin were purchased from Sigma, and tenascin was purchased from Chemicon. Function-blocking monoclonal antibodies (MAbs) directed to mouse integrin subunits α4 (CD49d, clone R1-2), α2 (CD49b, clone HMα2), β1 (CD29, clone HMβ1-1), and β7 (clone FIB27) and an α4-isotype control Ab (A95-1) were purchased from BD PharMingen, whereas MAbs to αvβ6 (MAb 2077Z) and human αvβ3 (MAb 1976Z) were obtained from Chemicon. Polyclonal large T (LT) antiserum was obtained from Brown Norway rats inoculated with syngeneic Py-transformed cells. R-phycoerythrin (R-PE)-conjugated anti-rat and anti-mouse immunoglobulin G (IgG) (heavy plus light chain) Abs were purchased from ICN Biomedicals, and R-PE-conjugated anti-hamster IgG was purchased from BD PharMingen. Rat fluorescein isothiocyanate-conjugated IgG fraction Ab was obtained from Cappel.
Virus infectivity assays.
Cells (1.2 × 105) were washed twice with DMEM and incubated with or without natural integrin ligands or function-blocking Abs in DMEM at room temperature for 1 h, unless otherwise stated. Afterwards, cells were infected for 1 h with the virus (same virus inoculum added to Swiss 3T3 or BALB/c 3T3 cells) to have 1 to 10% LT-positive cells. The virus inoculum was removed, and cultures were maintained for 20 h at 37°C in DMEM-10% serum. Cells were then fixed with methanol-acetone (3:7, vol/vol) and immunostained for virus (LT expression) as previously described (17). Total nuclei were stained with 1 μg of DAPI (4′,6′-diamidino-2-phenylindole) solution in phosphate-buffered saline (PBS) per ml. Cells were washed with PBS and mounted in 70% glycerol. Infectivity was measured as the number of LT-positive cells among the total nuclei. A minimum of 1,000 nuclei were counted for each single plate.
Cell surface expression of integrin subunits.
Cell monolayers were detached with PBS-5 mM EDTA. The cells (4.5 × 105) were incubated for 30 min on ice with optimal dilutions of MAbs to integrin subunits in buffer A (PBS, 0.1 mM CaCl2, and 0.1 mM MgCl2 plus 1% bovine serum albumin), washed, and incubated for a further 30 min with the suitable R-PE-conjugated secondary Ab. After two washes, cellular fluorescence was analyzed on a FACSCalibur flow cytometer (Becton Dickinson). A positive relative linear median fluorescence intensity (RMFI) was defined as >1.2 and was calculated as the median fluorescence intensity of cells incubated with primary and secondary Abs divided by the median fluorescence intensity of cells incubated with secondary Ab alone.
Virus binding assays.
Cell monolayers were detached by incubation in PBS-5 mM EDTA, washed in buffer A, and incubated with or without function-blocking anti-α4 or its isotype Ab as a control (30 μg/ml) in binding buffer (buffer A) for 1 h on ice. Alternatively, cells were pretreated with 200 mU of Clostridium perfringens neuraminidase (Sigma) for 1 h at 37°C and then washed twice with buffer A. Afterwards, biotinylated virus particles (preincubated or not with 160 mM N-acetylneuraminic acid [NANA] [Sigma] for 1 h in ice) were added to cells and left for 1 h on ice. The cells were washed and incubated with PE-conjugated streptavidin (BD PharMingen) in binding buffer for 30 min on ice. After being washed twice, cells were fixed in paraformaldehyde and their fluorescence intensity was analyzed by fluorescence-activated cell sorting (FACS).
RESULTS
Murine Py VP1 and VP2 capsid proteins contain integrin ligand motifs.
The Py capsid proteins VP1 and, possibly, VP2 are involved in the initial interaction with host cells (3, 7, 45, 49, 50). We examined the amino acid sequences of the VP1 and VP2 proteins from the murine large-plaque strain A2 for the presence of known integrin ligand binding sites. Of the 15 motifs studied (see Materials and Methods), three consensus sequences were found to be conserved, two in VP1 and one in VP2.
An LDV motif was identified in the DE loop (amino acids [aa] 137 to 139) of the VP1 protein. Such a sequence, present in the CS1 fragment of fibronectin, is the minimal essential sequence for adhesion of fibronectin to the α4β1 and α4β7 integrins, which are expressed mainly on lymphoid cells (24, 33). Alignment of the VP1 DE loop with the region flanking the LDV motif of the CS1 fragment revealed 23.8% identity within a stretch of 20 aa (Fig. 1A). Interestingly, the LDV sequence was found to be totally conserved among murine Py strains (n = 7), with the exception of the Kilham strain, which is characterized by stringent host and cell specificities (55). In contrast, none of the nonmurine strains analyzed (n = 26) contained this motif in the DE loop (Fig. 1A).
FIG. 1.
Murine Py VP1 and VP2 capsid proteins contain integrin ligand motifs. (A) Alignment of the Py A2° VP1 LDV motif with the CS1 fragment of fibronectin (Fn CS1) (GenBank accession number AAD11500) and related Py sequences from aa 128 to 147. (B) Alignment of the Py A2° VP1 DLXXL motif with tenascin (Tn) (GenBank accession number P24821) and related Py sequences from aa 166 to 185. (C) Alignment of the Py A2° VP2 DGE motif with type I collagen (Cl) (GenBank accession number NP_000079) and related VP2 sequences from aa 31 to 50. *, :, and ., identical aa, strongly conserved, and weakly conserved amino acids, respectively, as defined by the CLUSTAL W program. The consensus motifs and the murine Py strains are in boldface. GenBank accession numbers of the analyzed viral proteins are indicated. °, VP1 and VP2 sequences of the Py A2 strain used in this work. Cerco. and Budger., Cercopitheci and Budgerigar fledgling disease viral strains, respectively.
A DLXXL motif was found in the EF loop of VP1 (aa 174 to 178). Such a sequence has been identified in tenascin as a ligand for the αvβ6 heterodimer, a rare integrin expressed in epithelial cells only and induced during repair processes (5, 34). Alignment of VP1 with tenascin extended over the DLXXL motif, showing 30% identity within a stretch of 20 aa (Fig. 1B). The DLXXL motif was present in all of the murine strains, including the Kilham strain (n = 8), as well as in the VP1 hamster sequences analyzed but was not present in the other members of the Py family (n = 24) (Fig. 1B). Of interest is that D174 is a residue involved in the calcium binding site of VP1, which is absolutely crucial for maintaining structural integrity of the viral capsid (16).
Finally, the VP2 protein displays a DGE motif (aa 39 to 41) in its N terminus. The DGE motif is present in the type I collagen sequence and serves as a recognition site for the α2β1 integrin, a heterodimer expressed on colon adenocarcinoma cells, platelets, and fibroblasts (23, 25, 48). Identity between VP2 and type I collagen reached 30% within a stretch of 17 aa flanking the consensus sequence (Fig. 1C). All murine strains except Kilham displayed this motif (n = 6), whereas it was not present in any of the nonmurine strains (n = 19).
Cell surface expression of integrins on Swiss 3T3 fibroblasts.
The potential involvement of integrins in Py infectivity was investigated with murine Swiss 3T3 fibroblasts, a permissive cell line normally used for studying Py infection. Initially, cell surface expression of the α4, β1, β7, α2, and αvβ6 integrins was analyzed by flow cytometry. The β1 subunit was found to be abundantly expressed on these cells (RMFI of 90.9). The α4 subunit and αvβ6 integrin were also detected at low but significant levels (RMFIs of 1.55 and 1.79, respectively), whereas the α2 and the β7 subunits were not expressed at detectable levels (RMFIs of 0.75 and 0.95, respectively).
Natural integrin ligands reduce Py infectivity.
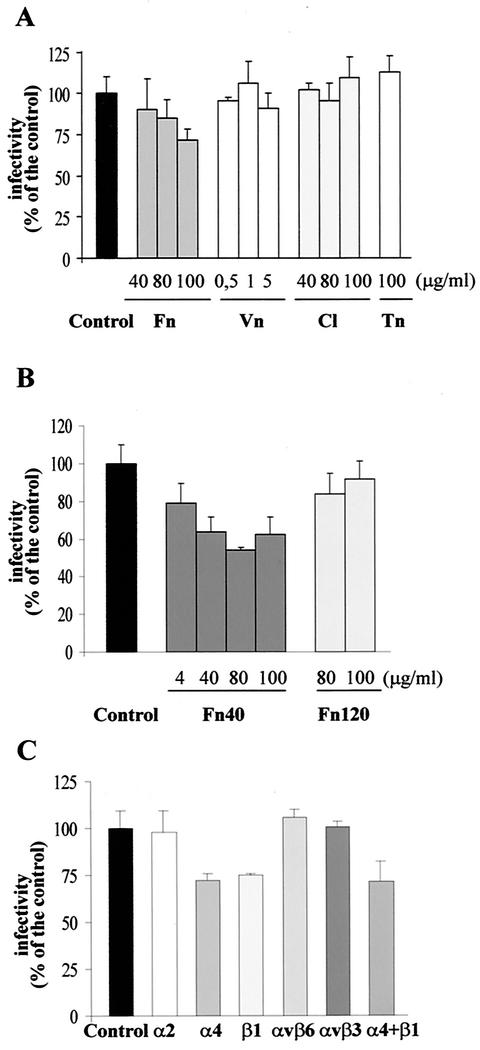
To determine whether integrins play a role in Py infection, virus infectivity assays were performed in the presence of natural integrin ligands. Swiss 3T3 cells were incubated with increasing concentrations of different extracellular matrix proteins (fibronectin, vitronectin, type I collagen, or tenascin) before virus adsorption. Their competitor effect on Py infectivity was measured by indirect immunofluorescence. A significant dose-dependent reduction of infectivity was observed in presence of fibronectin (up to 28% ± 6% [mean ± standard deviation]) when 100 μg/ml was used, but no reduction was observed in the presence of vitronectin, collagen, or tenascin (Fig. 2A).
FIG. 2.
Integrin ligands and function-blocking Abs reduce Py infectivity. (A and B) Py infectivity is inhibited by integrin ligands in a dose-dependent manner. Swiss 3T3 cells were pretreated for 1 h at room temperature with various concentrations of integrin specific ligands, i.e., fibronectin (Fn), vitronectin (Vn), collagen (Cl), or tenascin (Tn) (A) or α-chymotryptic fibronectin fragments (Fn40 or Fn120) (B), before infection with virus particles for 1 h at 4°C. The virus inoculum was removed, and cells were further incubated for 20 h at 37°C before fixation. Infected cells were immunostained for LT (see Materials and Methods). Results are presented as the percentage of virus infectivity obtained when cells were preincubated with DMEM without competitors (control). One hundred percent of virus infectivity corresponded to an average of 7 to 10% LT positive cells. Data correspond to the means and standard deviations from two or three independent experiments, each performed in duplicate or triplicate. (C) Inhibition of Py infectivity by integrin-specific Abs. Swiss 3T3 cells were pretreated for 1 h at room temperature with a 30-μg/ml concentration of Abs to specific integrins before infection with Py for 1 h. After virus particles were removed, cells were further incubated for 20 h at 37°C before fixation. Infected cells were stained for LT by indirect immunofluorescence. Results are presented as described for panels A and B. Data correspond to the means and standard deviations from two or three independent experiments, each performed in duplicate or triplicate.
Two α-chymotryptic fibronectin fragments have been previously described as showing different integrin binding abilities: the Fn40 fragment, which displays an LDV motif, is the region necessary to bind to α4 integrins, while the 120K (Fn120) fragment has an RGD motif recognized by α5β1, αvβ3, and αvβ6 integrins (5, 34, 41, 54). In order to determine which integrin ligand motif of fibronectin was competing with Py, cells were pretreated with increasing concentrations of fragment Fn40 or Fn120 before infection. Fragment Fn40 significantly reduced Py infectivity, even when used at low concentrations. A 21% ± 10% reduction was observed when Fn40 was used at 4 μg/ml and a 46% ± 2% reduction was reached when Fn40 was used at 80 μg/ml, whereas no significant reduction was observed with the Fn120 fragment, even when cells were incubated with 100 μg/ml (Fig. 2B). These data indicate that only specific cell matrix proteins carrying the LDV motif reduce Py infectivity, implying a role for α4 integrins in viral infection.
Inhibition of Py infectivity by function-blocking anti-integrin Abs.
Similar virus infectivity experiments were performed with Abs with function-blocking activity directed against specific integrin subunits as competitors. We observed a reduction of infectivity of 28% ± 4% when the Ab specific for the α4 subunit was used as a competitor (Fig. 2C). An analogous level of reduction was observed in the presence of the Ab specific for the β1 subunit (25% ± 1%). The reduction of infectivity was dose dependent with both Abs (data not shown). Moreover, the combination of the anti-α4 and anti-β1 MAbs did not increase infectivity blocking (28% ± 10%). Since both MAbs function to block the ligand binding site of the integrin, this observation means that anti-α4 and anti-β1 Abs recognize and compete with the same ligand binding site on the same integrin heterodimer. In contrast, no significant reduction was observed when anti-α2, anti-αvβ6, or control anti-human αvβ3 Abs were used as competitors. Taken together with the results obtained with integrin ligands, these data suggest that the integrin involved in Py infectivity is the α4β1 heterodimer. Incomplete inhibition of Py infectivity by anti-α4 and anti-β1 Abs could be attributed to the only partial function-blocking activity of these MAbs and/or could be due to additional cell surface molecules recognized by Py.
Expression of the α4 integrin subunit enhances Py infectivity in α4-negative BALB/c 3T3 cells.
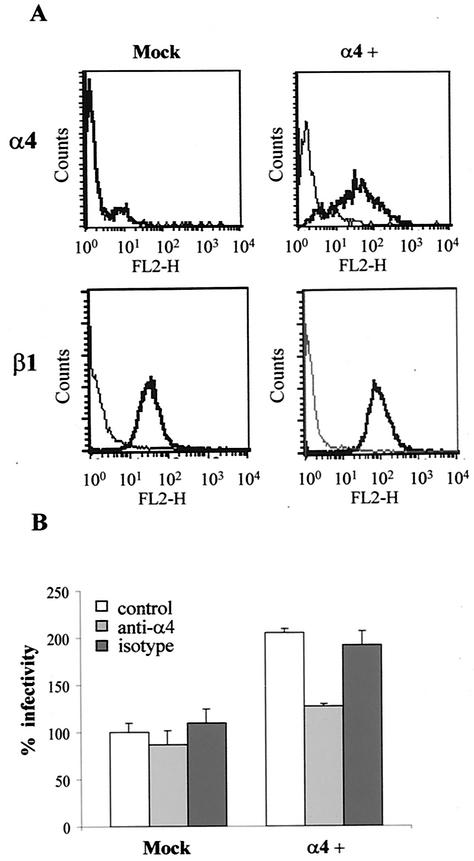
Even though Py shows broad tissue tropism in its natural host, it replicates with different efficiencies depending on the cell type (4). To test whether cell surface expression of a particular integrin heterodimer, i.e., α4β1, could be correlated with a different replication ability, we selected the murine fibroblast cell line BALB/c 3T3, which was previously described as α4 negative (54) and determined to be less permissive for Py infection than Swiss 3T3 cells (our unpublished results). First, we verified by flow cytometry that BALB/c 3T3 cells did not express α4 on their surface (RMFI of 0.95), whereas β1 expression was high (RMFI of 87.6). Next, to determine whether expression of the integrin α4 subunit could enhance Py infectivity, BALB/c 3T3 cells were transiently transfected with α4 and then infected with Py. In these preliminary infectivity assays, we observed that α4-transfected cells were approximately 50% more permissive to Py than those transfected with an empty vector (data not shown). Py infectivity was then studied in BALB/c 3T3 fibroblasts stably transfected with the α4 subunit. Cells were stably transfected with either the vector pRK5-α4 or the empty vector and with the selection marker construct. Ten puromycin-resistant clones were isolated, individually amplified, and initially tested for the presence of the α4 subunit by Western blotting to identify those with the highest expression levels. Clone 8, designated the α4-BALB/c 3T3 cell line, was selected for further studies, as it showed a good level of α4 expression and had growth properties similar to those of the mock-transfected clone (BALB/c 3T3 cells transfected with the empty vector). As shown in Fig. 3A, cell surface expression of α4 in the α4-BALB/c 3T3 cell line was confirmed by FACS, whereas the mock-transfected clone was α4 negative. In contrast, both cell lines expressed high levels of the β1 subunit (Fig. 3A).
FIG. 3.
Expression of the α4 integrin subunit enhances Py infectivity in the α4-negative BALB/c 3T3 fibroblasts. (A) Flow cytometric analysis of α4 and β1 subunit surface expression on mock-transfected and α4-BALB/c 3T3 (α4+) cells. Cells were either preincubated (heavy line) or not preincubated with anti-α4 or anti-β1 Abs for 30 min on ice as described in Materials and Methods. After washes, the cells were incubated with PE-conjugated secondary Abs. The histograms display relative cell numbers as a function of relative fluorescence intensities. (B) The α4-expressing BALB/c 3T3 cell line is more susceptible to Py infection than the mock-transfected clone. Mock-transfected and α4-BALB/c 3T3 cells were either pretreated or not pretreated for 1 h at room temperature with 30 μg of anti-α4 or its isotype Ab per ml before infection with Py for 1 h at 37°C. After the virus particles were removed, cells were further incubated for 20 h at 37°C before fixation. Infected cells were stained for LT by indirect immunofluorescence. Results are reported with the value obtained when mock-transfected cells were preincubated with DMEM without competitors (control) considered to be 100% virus infectivity (corresponding to an average of 3% LT positive cells). Data correspond to the means and standard deviations from two independent experiments.
Virus infectivity assays were then performed with mock-transfected and α4-BALB/c 3T3 cells. We observed that α4 expression rendered α4-BALB/c 3T3 cells more susceptible to Py infection (twofold increase) than the α4-negative mock-transfected cells (Fig. 3B). In order to verify that the enhanced infectivity observed in α4-BALB/c 3T3 cells was specifically dependent on the cell surface expression of the α4 integrin, virus infectivity assays were performed in the presence of function-blocking Abs. As shown in Fig. 3B, the presence of MAb directed to the α4 integrin subunit, but not that of its control isotype Ab, blocked the increase of Py infectivity conferred by α4 expression in α4-BALB/c 3T3 cells, whereas neither the MAb directed to the α4 integrin subunit nor its control isotype Ab had any effect on mock-transfected cells. These findings demonstrate that although they are not absolutely essential for Py infectivity, α4 integrins are consistently involved in cellular permissivity for Py.
α4β1 integrin is involved in Py infectivity and cell susceptibility to Py at a postattachment level.
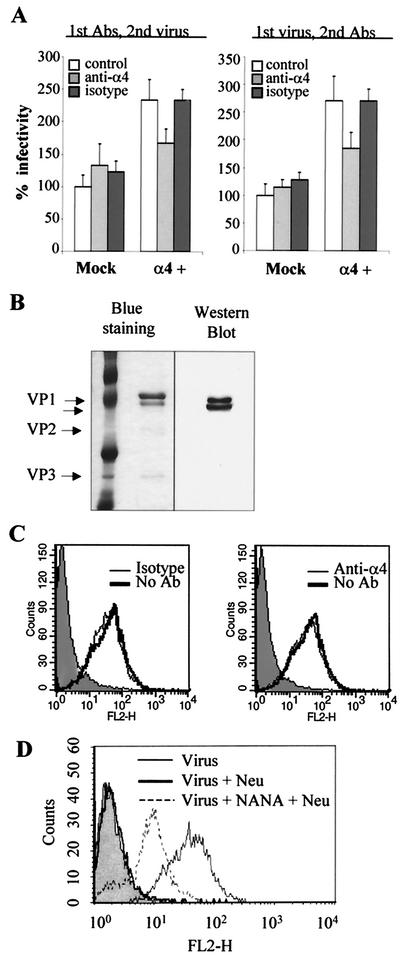
To investigate whether Py interaction with cell surface α4β1 integrin occurs during attachment or at a postattachment step, virus infectivity assays similar to those described above were performed with mock-transfected and α4-BALB/c 3T3 cells, but infection was carried out at 4°C, under conditions where viruses attach to but do not penetrate the cell membrane. Function-blocking anti-α4 or control isotype Abs were added to cells either before or after virus binding to cells. As shown in Fig. 4A, addition of anti-α4 Abs after virus adsorption (right panel) still reduced Py infectivity in α4-BALB/c 3T3 cells to levels similar to those observed when they were added before virus adsorption (left panel), whereas isotype Abs had no effect on Py infectivity in these cells. These data suggested that α4β1 integrin was probably involved in Py infectivity at the postattachment stage.
FIG. 4.
Expression of the α4 integrin subunit enhances cell permissivity to Py at a postattachment step. (A) Addition of α4 function-blocking Abs prior to or after virus adsorption blocks the increased infectivity of Py in α4-BALB/c 3T3 cells. Mock-transfected and α4-BALB/c 3T3 cells were either pretreated or not pretreated for 1 h at 4°C with 30 μg of anti-α4 or its isotype Ab per ml before infection with Py for 1 h at 4°C (left panel) or after infection with Py for 1 h at 4°C (right panel). After the virus particles were removed, cells were further incubated for 20 h at 37°C before fixation and immunostaining for LT. Results are reported with the value obtained when mock-transfected cells were preincubated with DMEM without competitors (control) considered to be 100% virus infectivity (average of 1% LT positive cells). Results of a representative experiment performed in duplicate are reported as described for Fig. 3B. (B) Characterization of the purity and labeling efficiency of biotinylated Py viral particles. Left panel, SDS-PAGE of purified viral particles (500 ng) stained with GelCode Blue staining reagent. Right panel, Western blot analysis of biotinylated viral particles with HRP-conjugated streptavidin. (C) Function-blocking MAb directed to α4 does not affect the binding of biotinylated Py particles to α4-BALB/c 3T3 cells. α4-BALB/c 3T3 cells were pretreated for 30 min on ice with a 30-μg/ml concentration of Ab directed to α4 integrin (right panel) or its isotype (left panel) before treatment with 500 ng of biotinylated viral particles for 1 h at 4°C. Cells incubated with the binding buffer alone are shown as negative control (shaded area). After two washes, cells were incubated for 30 min with PE-conjugated streptavidin. Fixed cells were analyzed by FACS. The histograms display relative cell numbers as a function of relative fluorescence intensities. (D) SA-containing receptors are involved at the attachment level. One microgram of biotinylated viral particles was incubated for 1 h at 4°C with or without NANA and then added to α4-BALB/c 3T3 cells pretreated with 200 mU of neuraminidase (Neu) and left for 1 h at 4°C. Binding of virus on untreated cells is also shown. Cells incubated with the binding buffer alone as a negative control are shown (shaded area). After two washes, cells were incubated for 30 min with PE-conjugated streptavidin. Fixed cells were analyzed by FACS. The histograms display relative cell numbers as a function of relative fluorescence intensities.
To verify this hypothesis, virus binding experiments were then performed with virus particles purified and labeled with biotin as described in Materials and Methods. The purity of the resulting particles and the efficiency of labeling were analyzed by staining proteins separated by SDS-PAGE and Western blotting with HRP-conjugated streptavidin (Fig. 4B). Labeled viral particles showed a correct morphology, as determined by negative-staining electron microscopy and by the fact that they were still able to hemagglutinate sheep red cells (data not shown), indicating that the biotinylation reaction had not altered the biological and physical properties of the viral particles. The ability of these particles to bind to the α4-BALB/c 3T3 cells was then measured by FACS by detecting bound viruses with PE-conjugated streptavidin. The binding of labeled viruses appeared to be dependent on the viral input added to the cells (data not shown). When binding experiments were performed with α4-BALB/c 3T3 cells pretreated with anti-integrin Abs, we observed that neither anti-α4 nor isotype Abs inhibited Py binding to the target cells (Fig. 4C). Therefore, taken together with data from the virus infectivity assays, these results indicate that α4β1 integrin is a cell receptor for Py, likely to be involved in a postattachment event.
SA-containing receptors are involved at the attachment level.
SA are cell receptors for Py virus (49, 50). To study their role in Py cell binding, the binding of labeled viruses preincubated with or without NANA was analyzed by FACS in α4-BALB/c 3T3 cells pretreated with neuraminidase. As shown in Fig. 4D, pretreatment of cells with neuraminidase reduced virus binding to background levels, confirming that SA are critical for Py cell binding. However, preincubation of virus with NANA allowed the virus to bind to cells pretreated with neuraminidase (Fig. 4D). These results lead us to hypothesize that exposure of Py to SA may allow the virus to bind to SA-independent receptors.
DISCUSSION
Murine Py is one of the best characterized among the Py family. However, important information regarding the initial step of the life cycle of the virus and, especially, the route of entry into cells is still lacking. In particular, a specific receptor molecule has not yet been identified. In this work, we identified integrin ligand consensus sequences in two of the Py capsid proteins, VP1 and VP2, and investigated their putative role in the initial interaction of Py with fibroblast cells. We report for the first time the identification of a candidate receptor molecule for Py.
Virus infectivity assays performed with mouse fibroblast Swiss 3T3 cells in the presence of fibronectin or function-blocking Abs directed against the α4 or β1 integrin subunits showed a consistent and specific reduction of Py infectivity (30 to 50%). A similar level of reduction of viral infectivity has been previously observed for other viruses, such as rotaviruses, which use integrins as cell receptors (9, 21, 26, 37). The specific competitor effect observed with the Fn40 fibronectin fragment, but not with the Fn120 fragment, supports the hypothesis that integrin α4β1 is involved in infectivity of the Py A2 strain, probably by recognizing the LDV motif present on the DE loop of the VP1 protein of the viral capsid.
We observed that (i) ligands and Abs to the α4β1 integrin used as competitors in virus infectivity assays did not completely block Py infectivity in Swiss 3T3 cells and that (ii) although α4 deficient, mouse BALB/c 3T3 cells can sustain viral infection. Taken together, these data suggest that α4β1 integrin is not the only receptor molecule utilized for Py entry and infection in fibroblast cells and that additional cellular membrane components or coreceptors are involved in the cell entry process.
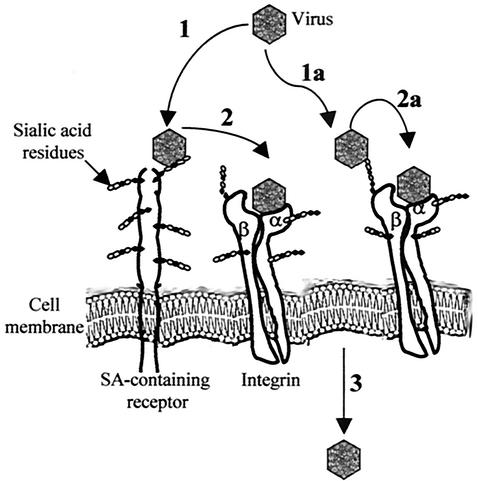
The question of whether integrins are used for attachment and/or entry into host cells has been studied for several viruses that use integrins as cell receptors. For example, integrin αvβ6 is used by FMDV for binding to cells (32), whereas integrins are required only in a postattachment step for adenoviruses and rotaviruses (8, 53). In the case of murine Py, SA-containing glycoproteins appear to be the major cell attachment receptors, since neuraminidase treatment of cells inhibited virus cell binding (Fig. 4). Such observations have led to the hypothesis that interaction of the Py capsid with α4β1 integrin occurs after SA-VP1 interaction. Our present results are in accordance with such a model, as anti-α4 Abs reduce viral infectivity even when added after virus adsorption but do not decrease virus cell binding (Fig. 4). We also observed that exposure of viral particles to SA restores to a large extent virus binding to cells pretreated with neuraminidase (Fig. 4). This suggests that interaction of the viral capsid with α4β1 integrin involves a two-step mechanism, in which initial binding of VP1 to SA is a prerequisite for subsequent proper recognition of the α4β1 integrin, either to promote a conformational change of the Py capsid, rendering the LDV motif more accessible to the integrin molecule, or to bring it in closer proximity to the integrin. Such an event may be necessary, as the LDV motif is not located in the tip of the loop, which is the most cell surface exposed part of the loop. A diagram of this possible virus-integrin interaction is presented in Fig. 5. Our model may also explain the differences in pathogenicity and replication ability displayed by murine Py strains; the interaction of VP1 with only straight SA or with straight and branched SA could trigger different conformational changes of the viral capsid that may affect subsequent interaction of VP1 with α4β1 integrin. It is noteworthy that a similar two-step entry model has already been demonstrated for viruses such as adenoviruses or rotaviruses that recognize integrins as secondary receptors necessary for the entry process (8, 53). The fact that both α4 and β1 integrin subunits are heavily glycosylated, carrying, in particular, terminal SA residues (23), has led to the suggestion that integrins can themselves be part of the SA-containing molecules for rotaviruses (26). For Py, future work aimed at studying its binding to cells expressing glycosylated or underglycosylated α4β1 integrin should help to verify the two-step entry model.
FIG. 5.
Proposed model for the early interaction of Py with host cells. First, Py interacts with SA-containing cell receptors (step 1). This initial interaction is a prerequisite for subsequent proper recognition of the α4β1 integrin (step 2), either to promote a conformational change of the Py capsid, rendering the LDV motif more accessible to the integrin molecule, or to bring it in closer proximity to the integrin. This second interaction may facilitate the entry of the virus into the cells (step 3). Considering that both α4 and β1 integrin subunits are heavily glycosylated, carrying, in particular, terminal SA residues, integrins may themselves be a component of the SA-containing receptor molecules for Py (steps 1a and 2a) (see text).
Virus-receptor interactions in general play a major role in determining virus tropism and tissue-specific pathology associated with virus infection. In particular, recognition of integrins as cellular receptors has been demonstrated to determine, at least in part, cellular susceptibility to FMDV and rotaviruses (8, 31). With regard to murine Py strains, host range and cellular permissivity have mostly been correlated to specific intranuclear mechanisms required for virus replication and to the activity of enhancer elements and regulatory regions. In this work, we show that stable transfection of the poorly susceptible, α4-negative, murine fibroblast BALB/c 3T3 cell line with the cDNA encoding the α4 subunit significantly enhances Py infectivity (twofold increase). This is the first direct demonstration that receptor usage, such as α4β1 integrin recognition, is also a critical determinant for cell permissivity to Py. In this regard, it is known that α4β1 integrin expression is differentially regulated during myogenesis, with α4β1 integrin being expressed only in differentiated myoblasts and myotubes (44). In vitro, undifferentiated myoblasts are poorly permissive or nonpermissive to Py, whereas differentiated myoblasts sustain efficient Py replication (12). This observation is consistent with the notion that the differentiation stage-dependent permissivity to Py may correlate with α4β1 integrin expression. Moreover, the α4β1 heterodimer is expressed mainly on leukocytes and plays an important role both in hematopoiesis and in the immune response (23, 46). The possible implication of VP1-α4β1 interactions in tissue tropism, pathogenicity, tumorigenicity, and host immune response should be studied by analyzing the in vivo properties of Py LDV motif mutants. Interestingly, the Kilham strain does not display an LDV motif and exhibits stringent host and cell specificities during primary infection, in comparison to the broad tissue tropism of other murine Py strains. A possible determinant of this different tropism could be the enhancer element, but it is possible that adsorption and uptake processes are involved (55). In fact, the receptor for this virus has not been identified and might be different from that used by other murine Py viruses.
Recognition of integrins has been shown to mediate viral uptake into either clathrin-coated vesicles, as seen for adenovirus, or caveola-associated vesicles, as recently reported for echovirus 1 (36, 39). For Py, the nature of the vesicles mediating virus entry into cells still remains a subject of debate (19, 20, 42). Future studies aimed at determining whether Py VP1-integrin interactions can also affect the internalization route of viral particles, depending on the cell type, should provide greater insight into this controversial question.
Integrins are pivotal signaling receptors involved in a variety of cellular processes (18). In particular, they contribute to cell cycle progression from the G1 to the S phase (2). The interaction of Py with the cell membrane promotes, through the VP1 protein, cell cycle progression of quiescent cells by transcriptional stimulation of early-response genes (56). Studies to determine whether this stimulation depends on integrin-associated signaling pathways are in progress.
The relevance of the other two integrin ligand motifs, DLXXL and DGE, found in the VP1 and VP2 sequences of all reported murine Py strains still has to be established. Although they did not appear to be implicated in Py infectivity in the Swiss 3T3 cell line, it is possible that their interaction with integrins α2β1 and αvβ6 could be relevant in other cell types. We therefore believe that the ability of Py to recognize not only different integrin heterodimers but probably also other receptor molecules that display different tissue distribution explains, at least in part, its broad tissue tropism.
The combined abilities of the Py VP1 protein to self-assemble into virus-like particles (VLPs) and to nonspecifically interact with homologous or heterologous DNA has led to VP1 VLPs being proposed as potential vectors for gene therapy (1, 35, 51). VLPs efficiently transfer DNA and allow its expression in mammalian cells in vitro and in vivo with a wide tissue distribution (13, 22, 47). By demonstrating that the initial interaction of Py with host cells is certainly a far more complex mechanism than predicted up to now, probably involving the use of different receptors depending on the cell type, the present work highlights the necessity to identify natural Py receptors and their tissue distribution in order to design genetically engineered Py VP1 VLPs with efficient tissue retargeting.
Acknowledgments
We thank Michaela Cavaldesi for assistance in some experiments, Bernhard Holzmann for providing the pRK5-α4 vector, and Tiziana Moretti and Graziella Bernardini for preliminary FACS analysis. We are grateful to Peter Altevogt, Guido Tarone, and Rossella Maione for useful suggestions and critical discussions.
This work was supported by an EC grant (BIO4-CT97-2147) and by MIUR and CNR funds.
REFERENCES
- 1.An, K., E. T. Gillock, J. A. Sweat, W. M. Reeves, and R. A. Consigli. 1999. Use of the baculovirus system to assemble polyomavirus capsid-like particles with different polyomavirus structural proteins: analysis of the recombinant assembled capsid-like particles. J. Gen. Virol. 80:1009-1016. [DOI] [PubMed] [Google Scholar]
- 2.Assoian, R. K., and M. A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48-53. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, P. H., C. Cui, T. Stehle, S. C. Harrison, J. A. De Caprio, and T. L. Benjamin. 1999. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J. Virol. 73:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin, T. L. 2001. Polyoma virus: old findings and new challenges. Virology 289:167-173. [DOI] [PubMed] [Google Scholar]
- 5.Breuss, J. M., N. Gillett, L. Lu, D. Sheppard, and R. Pytela. 1993. Restricted distribution of integrin beta6 mRNA in primate epithelial tissues. J. Histochem. Cytochem. 41:1521-1527. [DOI] [PubMed] [Google Scholar]
- 6.Caruso, M., C. Iacobini, C. Passananti, A. Felsani, and P. Amati. 1990. Protein recognition sites in polyomavirus enhancer: formation of a novel site for NF-1 factor in an enhancer mutant and characterization of a site in the enhancer D domain. EMBO J. 9:947-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, X. S., T. Stehle, and S. C. Harrison. 1998. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J. 17:3233-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciarlet, M., S. E. Crawford, E. Cheng, S. E. Blutt, D. A. Rice, J. M. Bergelson, and M. K. Estes. 2002. VLA-2 (α2β1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J. Virol. 76:1109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulson, B. S., S. L. Londrigan, and D. J. Lee. 1997. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA 94:5389-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckhart, W. 1990. Polyomavirinae and their replication, p. 1593-1607. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 11.Evander, M., I. H. Frazer, E. Payne, Y. M. Qi, K. Hengst, and N. A. McMillan. 1997. Identification of the α6 integrin as a candidate receptor for papillomaviruses. J. Virol. 71:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsani, A., R. Maione, L. Ricci, and P. Amati. 1985. Coordinate expression of myogenic functions and polyoma virus replication. Cold Spring Harbor Symp. Quant. Biol. 50:753-757. [DOI] [PubMed] [Google Scholar]
- 13.Forstovà, J., N. Krauzewicz, V. Sandig, J. Elliott, Z. Palkovà, M. Strauss, and B. E. Griffin. 1995. Polyoma virus pseudocapsids as efficient carriers of heterologous DNA into mammalian cells. Hum. Gene Ther. 6:297-306. [DOI] [PubMed] [Google Scholar]
- 14.Freund, R., R. L. Garcea, R. Sahli, and T. L. Benjamin. 1991. A single-amino-acid substitution in polyomavirus VP1 correlates with plaque size and hemagglutination behavior. J. Virol. 65:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund, R., A. Calderone, C. J. Dawe, and T. L. Benjamin. 1991. Polyomavirus tumor induction in mice: effects of polymorphisms of VP1 and large T antigen. J. Virol. 65:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcea, R. L., and R. C. Liddington. 1997. Structural biology of polyomaviruses, p. 187-208 In W. Chiu, R. M. Burnett, and R. L Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 17.Garcia, M. I., M. Perez, M. Caruso, O. Sthandier, R. Ferreira, M. Cermola, C. Macchia, and P. Amati. 2000. A mutation in the DE loop of the VP1 protein that prevents polyomavirus transcription and replication. Virology 272:293-301. [DOI] [PubMed] [Google Scholar]
- 18.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert, J. M., and T. L. Benjamin. 2000. Early steps of polyomavirus entry into cells. J. Virol. 74:8582-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith, G. R., S. J. Marriott, D. A. Rintoul, and R. A. Consigli. 1988. Early events in polyomavirus infection: fusion of monopinocytotic vesicles containing virions with mouse kidney cell nuclei. Virus Res. 10:41-51. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero, C. A., E. Mendez, S. Zarate, P. Isa, S. Lopez, and C. F. Arias. 2000. Integrin alpha(v)beta3 mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 97:14644-14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidari, S., N. Krauzewicz, M. Kalantari, A. Vlastos, B. E. Griffin, and T. Dalianis. 2000. Persistence and tissue distribution of DNA in normal and immunodeficient mice inoculated with polyomavirus VP1 pseudocapsid complexes or polyomavirus. J. Virol. 74:11963-11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemler, M. E., J. G. Jacobson, and J. L. Strominger. 1985. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J. Biol. Chem. 260:15246-15252. [PubMed] [Google Scholar]
- 24.Hemler, M. E., C. Huang, and L. Schwarz. 1987. Characterization of the cell surface heterodimer VLA-4 and related peptides. J. Biol. Chem. 262:3300-3309. [PubMed] [Google Scholar]
- 25.Hemler, M. E., C. Crouse, Y. Takada, and A. Sonnenberg. 1988. Multiple very late antigen (VLA) heterodimers on platelets. Evidence for distinct VLA-2, VLA-5 (fibronectin receptor), and VLA-6 structures. J. Biol. Chem. 263:7660-7665. [PubMed] [Google Scholar]
- 26.Hewish, M. J., Y. Takada, and B. S. Coulson. 2000. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes, P. J., C. Horsnell, T. Hyypia, and G. Stanway. 1995. The coxsackievirus A9 RGD motif is not essential for virus viability. J. Virol. 69:8035-8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphries, M. J., S. K. Akiyama, K. Komoriya, and K. M. Yamada. 1986. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J. Cell Biol. 103:2637-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynes, R. O. 1999. Cell adhesion: old and new questions. Trends Cell Biol. 9:33-37. [PubMed] [Google Scholar]
- 30.Iwamoto, Y., F. A. Robey, J. Graf, M. Sasaki, H. K. Kleinman, Y. Yamada, and G. R. Martin. 1987. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science 238:1132-1134. [DOI] [PubMed] [Google Scholar]
- 31.Jackson, T., A. Sharma, R. A. Ghazaleh, W. E. Blakemore, F. M. Ellard, D. L. Simmons, J. W. Newman, D. I. Stuart, and A. M. King. 1997. Arginine-glycine-aspartic acid-specific binding by foot-and mouth disease viruses to the purified integrin α(v)β3 in vitro. J. Virol. 71:8357-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. Q. King. 2000. The epithelial integrin α(v)β6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komoriya, A., L. J. Green, M. Mervic, S. S. Yamada, K. M. Yamada, and M. J. Humphries. 1991. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J. Biol. Chem. 266:15075-15079. [PubMed] [Google Scholar]
- 34.Kraft, S., B. Diefenbach, R. Mehta, A. Jonczyk, G. A. Luckenbach, and S. L. Goodman. 1999. Definition of an unexpected ligand recognition motif for alpha(v)beta6 integrin. J. Biol. Chem. 274:1979-1985. [DOI] [PubMed] [Google Scholar]
- 35.Krauzewicz, N., and B. E. Griffin. 2000. Polyoma and papilloma virus vectors for cancer gene therapy. Adv. Exp. Med. Biol. 465:73-82. [DOI] [PubMed] [Google Scholar]
- 36.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via α(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 72:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Londrigan, S. L., M. J. Hewish, M. J. Thomson, G. M. Sanders, H. Mustafa, and B. S. Coulson. 2000. Growth of rotaviruses in continuous human and monkey cell lines that vary in their expression of integrins. J. Gen. Virol. 81:2203-2213. [DOI] [PubMed] [Google Scholar]
- 38.Maione, R., C. Passananti, V. De Simone, P. Delli-Bovi, G. Augusti-Tocco, and P. Amati. 1985. Selection of mouse neuroblastoma cell-specific polyoma virus mutants with stage differentiative advantages of replication. EMBO J. 4:3215-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plow, E. F., T. A. Haas, L. Zhang, J. Loftus, and J. W. Smith. 2000. Ligand binding to integrins. J. Biol. Chem. 275:21785-21788. [DOI] [PubMed] [Google Scholar]
- 42.Richterovà, Z., D. Liebl, M. Horak, Z. Palkovà, J. Stokrovà, P. Hozak, J. Korb, and J. Forstovà. 2001. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75:10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rietzler, M., M. Bittner, W. Kolanus, A. Schuster, and B. Holzmann. 1998. The human WD repeat protein WAIT-1 specifically interacts with the cytoplasmic tails of beta7-integrins. J. Biol. Chem. 273:27459-27466. [DOI] [PubMed] [Google Scholar]
- 44.Rosen, G. D., J. R. Sanes, R. LaChance, J. M. Cunningham, J. Roman, and D. C. Dean. 1992. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 69:1107-1119. [DOI] [PubMed] [Google Scholar]
- 45.Sahli, R., R. Freund, T. Dubensky, R. L. Garcea, R. Bronson, and T. L. Benjamin. 1993. Defect in entry and altered pathogenicity of a polyoma virus mutant blocked in VP2 myristylation. Virology 192:142-153. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu, Y., D. M. Rose, and M. H. Ginsberg. 1999. Integrins in the immune system. Adv. Immunol. 72:325-380. [DOI] [PubMed] [Google Scholar]
- 47.Soeda, E., N. Krauzewicz, C. Cox, J. Stokrovà, J. Forstovà, and B. E. Griffin. 1998. Enhancement by polylysine of transient, but not stable, expression of genes carried into cells by polyoma VP1 pseudocapsids. Gene Ther. 5:1410-1419. [DOI] [PubMed] [Google Scholar]
- 48.Staatz, W. D., K. F. Fok, M. M. Zutter, S. P. Adams, B. A. Rodriguez, and S. A. Santoro. 1991. Identification of a tetrapeptide recognition sequence for the alpha2beta1 integrin in collagen. J. Biol. Chem. 266:7363-7367. [PubMed] [Google Scholar]
- 49.Stehle, T., Y. Yan, T. L. Benjamin, and S. C. Harrison. 1994. Structure of polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369:160-163. [DOI] [PubMed] [Google Scholar]
- 50.Stehle, T., and S. C. Harrison. 1997. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: implications for assembly and receptor binding. EMBO J. 16:5139-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stokrovà, J., Z. Palkovà, L. Fischer, Z. Richterovà, J. Korb, B. E. Griffin, and J. Forstovà. 1999. Interactions of heterologous DNA with polyomavirus major structural protein VP1. FEBS Lett. 445:119-125. [DOI] [PubMed] [Google Scholar]
- 52.Tooze, J. 1981. Structure and genomic organization of SV40 and polyoma virus, p. 61-123. In J. Tooze (ed.), DNA tumor viruses. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, N.Y.
- 53.Wickhman, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha(v)beta3 and alpha(v)beta5 promote adenovirus internalization but not virus attachment. Cell 73:7663-7669. [DOI] [PubMed] [Google Scholar]
- 54.Zeller, Y., J. Lohr, M. Sammar, E. C. Butcher, and P. Altevogt. 1998. Asp-698 and Asp-811 of the integrin alpha4-subunit are critical for the formation of a functional heterodimer. J. Biol. Chem. 273:6786-6795. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, S., and G. Magnusson. 2001. Kilham polyomavirus: activation of gene expression and DNA replication in mouse fibroblast cells by an enhancer substitution. J. Virol. 75:10015-10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zullo, J., C. D. Stiles, and R. L. Garcea. 1987. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc. Natl. Acad. Sci. USA 84:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]