Abstract
The human immunodeficiency virus type 1 (HIV-1) virulence factor Nef enhances viral infectivity in single-cycle infection assays and accelerates HIV-1 replication in vitro. It has been reported that the effects of Nef are mediated early after viral entry and before the completion of reverse transcription, as viral DNA synthesis is strongly attenuated during infection by Nef-defective virions. Our previous work has demonstrated that Nef is associated with mature HIV-1 cores, implicating Nef in the regulation of HIV-1 core stability. Here we report a comparative analysis of HIV-1 cores isolated from wild-type and Nef-defective particles. We observed no effect of Nef on HIV-1 core structure or stability; however, Nef cosedimented with a subviral ribonucleoprotein complex following dissociation of CA. These results indicate that Nef interacts tightly with an internal component of the HIV-1 core. They further suggest that virion-associated Nef may facilitate an early step in HIV-1 infection following dissociation of the viral capsid in the target cell.
Primate lentiviruses, such as human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus, encode several accessory proteins. One of these, Nef, functions as a virulence factor of AIDS pathogenesis. The requirement for Nef in vivo has been demonstrated most clearly in adult rhesus macaques, in which infection by Nef-defective simian immunodeficiency virus results in a markedly delayed onset of AIDS (16). In humans, infection by Nef-defective HIV-1 is also correlated with long-term nonprogression to AIDS (18, 20, 27, 28). In tissue culture systems, Nef downregulates CD4 and major histocompatibility complex class I molecules from the cell surface, stimulates T-cell activation, and enhances the infectivity of viral particles (for reviews, see references 5, 11, 25, and 26). Although the contribution of each individual function of Nef to AIDS pathogenesis is unknown, the mechanism by which Nef stimulates HIV-1 infectivity is of clear interest.
In single-cycle infectivity assays, Nef-defective particles are approximately 10-fold less infectious than wild-type HIV-1 (9, 22), but the mechanism of infectivity enhancement by Nef remains incompletely defined. Enhancement of infectivity by Nef appears to depend on the route of viral entry, since pseudotyping of HIV-1 virions by vesicular stomatitis virus G envelope glycoprotein, which redirects viral entry to the endocytic pathway, suppresses the requirement for Nef (3). However, Nef has been thought to have no effect on the overall efficiency of viral entry. Rather, several studies point to a postentry action of Nef (4, 17, 29). Nef-defective viruses are attenuated at an early stage of reverse transcription, as viral DNA synthesis in target cells is significantly reduced compared to wild type (4, 29). However, the impaired DNA synthesis is apparently not due to defects inherent in the particle, as permeabilized Nef-defective virions undergo reverse transcription in vitro as extensively as wild type (17, 29). Furthermore, Nef-defective particles were restored to wild-type infectivity by stimulation of intravirion reverse transcription prior to infection (17). Although Nef clearly acts in the virus producer cell to modify the virion, no Nef-dependent structural or biochemical alterations of HIV-1 virions have been reported. Instead, Nef itself is packaged into virions (6, 24, 30, 32), associated with the viral core (19), and cleaved by the viral protease (6, 7, 14, 15, 21, 23). These studies suggest that Nef plays a role in an early postentry process of HIV infection and are consistent with the hypothesis that Nef regulates the disassembly of the viral core. In this study, we specifically examined the stability of wild-type and Nef− cores in vitro by using sensitive and quantitative assays of HIV-1 core stability.
Isolation of wild-type and Nef-defective HIV-1 cores.
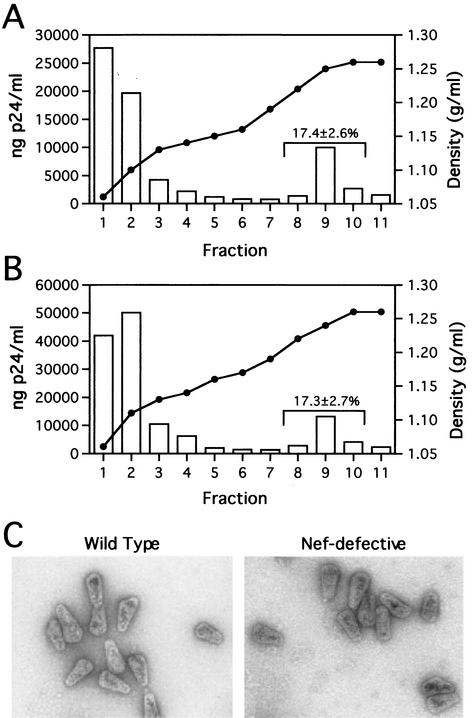
To analyze the stability of wild-type and Nef− HIV-1 cores in vitro, we isolated mature cores from virions, as previously described (12, 19). Wild-type (R9) and Nef-defective (R9ΔXhoI) HIV-1 stocks were produced by transfection of 293T cells with proviral DNA plasmids. As previously reported (4), Nef-defective particles exhibit a 10-fold decrease in single-cycle infectivity relative to wild type (data not shown). Intact cores sedimented to an equilibrium density of approximately 1.25 g/ml. For wild-type HIV-1, approximately 17% of virion-associated CA was detected in the denser fractions upon detergent treatment, while the majority of the CA remained at the top of the gradient (Fig. 1A). Upon ultracentrifugation through the detergent layers, cores from Nef-defective virions were detected at the same density as wild-type cores and in equal quantities (approximately 17% of input virions [Fig. 1B]), suggesting that Nef-defective HIV-1 cores are similar in stability to wild-type cores.
FIG. 1.
Analysis of wild-type and Nef− core yields and morphology. (A and B) Quantitation of wild-type and Nef-defective HIV-1 core yields. Concentrated virions were layered onto 30-to-70% (wt/vol) linear sucrose gradients atop a layer of Triton X-100. Following ultracentrifugation at 100,000 × g for 16 h at 4°C, fractions were collected from the top of the gradient and were analyzed by p24 ELISA. The density of each fraction was determined by refractometry. Panels A and B show the recovery of cores from wild-type and Nef-defective HIV-1 particles, respectively. Values shown represent the mean percentages (and standard deviations) of p24 found in the core-containing fractions for a minimum of five independent determinations. (C) Ultrastructural analysis of wild-type and Nef− cores. Gradient fractions containing intact cores were pelleted at 100,000 × g for 30 min and resuspended in 15 μl of STE buffer. Samples were loaded onto carbon-coated grids and stained with 1% uranyl acetate. Samples were visualized at a magnification of ×40,000 in a Philips CM12 transmission electron microscope.
Wild-type and Nef-defective HIV-1 cores are morphologically indistinguishable.
To determine if deletion of Nef from the virus led to altered viral morphogenesis, we analyzed purified cores from wild-type and Nef-defective virions. Concentrated samples from the core-containing fractions were loaded onto carbon-coated grids and visualized by negative staining with 1% uranyl acetate. At least 100 particles were examined for both wild-type (n = 104) and Nef− (n = 107) viruses. Considerable heterogeneity, both in size and shape, was observed for the purified HIV-1 cores, consistent with previous reports (1, 19, 31). Although cone-shaped particles predominated (Fig. 1C), cylindrical and other aberrantly shaped particles were also observed. The dimensions (width and length) were similar for wild-type and Nef-defective cores. Particles from wild-type virions averaged 105 nm in length and 53 nm in width, while those from Nef-defective virions averaged 103 nm in length and 52 nm in width. The distribution of these dimensions was similar for both wild-type and Nef-defective viruses (data not shown). These measurements are also consistent with the results of a previously reported analysis of purified wild-type HIV-1 cores (31).
Isolated wild-type and Nef-defective HIV-1 cores are equally stable under a variety of experimental conditions.
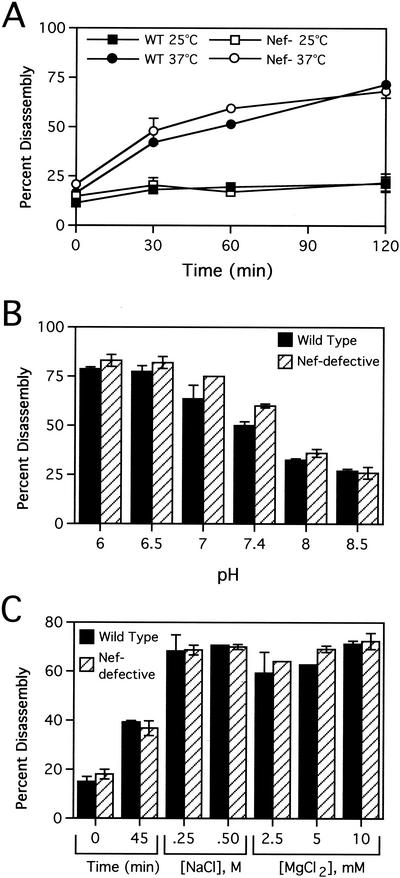
To further compare the stability of wild-type and Nef-defective HIV-1 cores, we employed a kinetic assay of core disassembly, according to a recently reported procedure (12). Purified cores were diluted in STE buffer (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA) and incubated under various conditions (typically pH 7.4 and 37°C). Following incubation, samples were subjected to ultracentrifugation (at 100,000 × g for 20 min); under these conditions, intact cores pellet to the bottom of the tube, while solubilized CA remains in the supernatant (12). To quantify the extent of core disassembly, CA release was monitored by p24-specific enzyme-linked immunosorbent assay (ELISA) analysis of both the supernatant and the pellet following incubation. Wild-type HIV-1 cores disassembled rapidly at 37°C but remained intact at 25°C (Fig. 2A) and 4°C (data not shown). Nef-defective cores exhibited similar disassembly kinetics. To evaluate the effects of buffer conditions on the stability of HIV-1 cores, we performed disassembly reactions for 45 min at 37°C in buffers of various NaCl and MgCl2 concentrations or various pH values. The disassembly of both wild-type and Nef− cores was strongly dependent on pH (Fig. 2B). The results indicated that the HIV-1 capsid was destabilized at low pH and stabilized at high pH, as previously reported (12). Both wild-type and Nef− cores were also sensitive to changes in ionic strength, as increases in MgCl2 and NaCl concentrations stimulated core disassembly (Fig. 2C). Under this series of conditions, Nef− cores exhibited identical stability to wild type.
FIG. 2.
Nef does not detectably affect HIV-1 core disassembly in vitro. Purified wild-type (WT) and Nef-defective (Nef−) cores were incubated in STE buffer at the indicated temperatures for the times shown (A) or at 37°C for 45 min at the indicated pH values (B) or NaCl or MgCl2 concentrations (C). Following incubation, the samples were subjected to ultracentrifugation at 100,000 × g for 15 min at 4°C. The extent of disassembly was determined as the percentage of the total CA protein in the reaction mixture present in the supernatant. Shown are mean values of duplicate determinations, with error bars representing the range of values.
Isolated viral ribonucleoprotein complexes contain Nef.
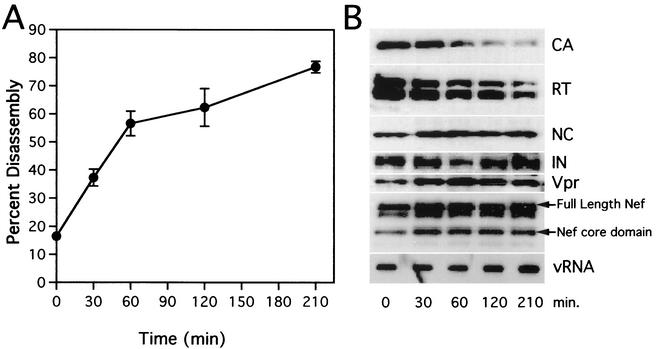
Our laboratory has previously demonstrated the association of Nef with intact HIV-1 cores, both in 293T-derived virions (19) and in virions harvested from infected CEM T cells (unpublished results). To define more precisely the location of Nef within the virion, we analyzed internal viral ribonucleoprotein complexes (vRNP complexes) released during HIV-1 core disassembly. Following incubation for various lengths of time at 37°C, the pellets from core disassembly reactions were analyzed for virion protein and RNA content. As shown by Western blot analysis, CA was steadily released from the cores (Fig. 3). However, NC, Vpr, IN, and viral RNA remained as a pelletable complex throughout the 3.5-h time course. Nef was also stably associated with this vRNP complex. Previous reports have demonstrated that Nef is cleaved within the virion by the viral protease. Cleavage occurs between residues 57 and 58, releasing a 20-kDa “core” domain from the 7-kDa amino-terminal “linker” domain (6, 7, 14, 15, 21, 23, 24). Both intact and 20-kDa cleaved forms remained in pelletable form consistently throughout the incubation time course. A band with a slightly higher mobility than full-length Nef was also detected by the Nef antiserum. Although we have not definitively identified this band, we suspect that this protein corresponds to a previously reported nonmyristoylated form of Nef produced by translation initiated from an internal methionine codon (2).
FIG. 3.
Kinetic and biochemical analyses of HIV-1 core disassembly in vitro. Purified HIV-1 cores were incubated at 37°C for the indicated times, followed by separation of soluble and core-associated CA by ultracentrifugation. (A) Dissociation of CA from pelletable cores during incubation at 37°C. Supernatants and pellets were analyzed by p24 ELISA. The extent of disassembly was determined as the percentage of the total CA protein in the reaction mixture detected in the supernatant. Values represent means of triplicate determinations with error bars representing one standard deviation. (B) Biochemical analysis of particles remaining following disassembly of HIV-1 cores. Pellets from disassembly reactions shown in panel A were subjected to Western blotting and RNA slot blot analyses. The Western blot membrane was probed sequentially with rabbit anti-CA, anti-RT, anti-NC, anti-IN, anti-Vpr, and anti-Nef antibodies. RNA was extracted from pellets, denatured, and immobilized by vacuum filtration. Relative HIV-1 RNA levels were determined by hybridization with a 32P-labeled HIV-1 probe, followed by detection with autoradiography.
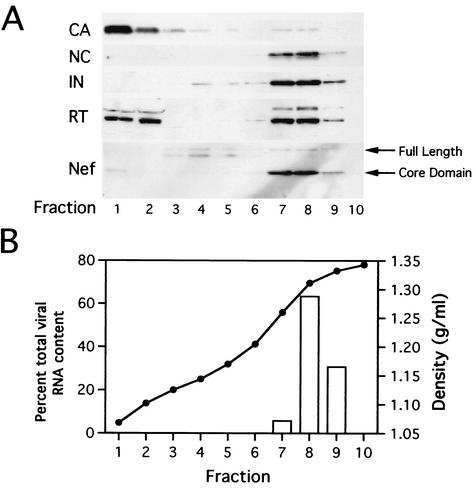
As an additional approach to determine the intravirion localization of Nef, vRNP complexes were isolated directly from wild-type HIV-1 virions and purified by equilibrium density ultracentrifugation. When vRNP complexes were applied to the same type of sucrose gradients used for the isolation of HIV-1 cores (30 to 70% sucrose [wt/vol]), they consistently sedimented to the bottom of the tube, at a density distinct from that of HIV-1 cores. To achieve better resolution of the complex, we employed a denser gradient (20 to 70% sucrose [wt/wt]). Concentrated virions were treated with 1% Triton X-100 to remove the lipid bilayer, and the internal capsid was subsequently disassembled at 37°C for 3 h. Following incubation, particles were loaded onto a 20-to-70% (wt/wt) sucrose gradient and subjected to ultracentrifugation at 100,000 × g for 16 h. Western blot analysis revealed components of the internal viral complex, including NC, IN, reverse transcriptase (RT), and viral RNA, at an equilibrium density of approximately 1.31 g/ml (Fig. 4). In contrast, CA was detected almost exclusively in fractions near the top of the gradient (greater than 95% of the total CA content), presumably due to its dissolution during incubation at 37°C. A fraction of RT was also released during the incubation period and was therefore detected near the top of the gradient. In contrast, Nef, both full-length and cleaved forms, cosedimented with NC, IN, and viral RNA, lending further support to the hypothesis that Nef associates with the vRNP complex.
FIG. 4.
Gradient analysis of HIV-1 vRNPs isolated directly from virions. Concentrated virions were incubated in the presence of 0.5% Triton X-100 for 3 h at 37°C. Following incubation, the sample was loaded onto a 20-to-70% (wt/wt) sucrose gradient and subjected to ultracentrifugation at 100,000 × g for 16 h. Fractions were collected from the top of the gradient. (A) Proteins were precipitated with trichloroacetic acid and analyzed by Western blotting. (B) RNA was immobilized on nitrocellulose and viral RNA content was determined by hybridization with a 32P-labeled HIV-1 probe, followed by phosphorimager quantification. Fraction densities were determined by refractometric analysis of sucrose concentrations.
Nef does not alter HIV-1 core stability in vitro.
In this study, we probed the potential role of Nef in HIV-1 core disassembly. Purified wild-type and Nef-defective cores were isolated in identical yields and were structurally indistinguishable by electron microscopic analysis. Assays of capsid release from purified cores under a range of conditions revealed no detectable differences in the stability of wild-type and Nef-defective viral cores. Our laboratory's previous analysis of HIV-1 CA mutants supports the validity of the in vitro approach for studying HIV-1 core stability (12). In that study, we identified CA mutations that either increased or decreased HIV-1 core stability without detectably altering HIV-1 core structure. We observed a strong correlation between altered core stability and impaired HIV-1 infectivity, supporting the relevance of the in vitro core stability phenotype. Taken together, our results imply that Nef does not influence the inherent stability of the HIV-1 core. We have also observed that T-cell cytoplasmic extracts alter the stability of both wild-type and Nef− cores in an equivalent manner (unpublished results), further suggesting that Nef does not affect the process of HIV-1 core disassembly following entry into the target cell. Nevertheless, it remains possible that Nef could regulate HIV-1 CA release from the core following delivery into the target cell through a mechanism that is not revealed in our in vitro experiments.
Nef associates with the vRNP complex.
We previously reported that Nef is present in highly purified preparations of HIV-1 core particles (19). In this study, we provide evidence for the association of Nef with the internal vRNP complex. Purification of vRNP complexes from disassembled cores, as well as from detergent-treated virions, demonstrated that Nef cosediments with NC, IN, Vpr, and viral RNA, despite the loss of the majority of CA. The component that mediates the association of Nef with the vRNP complex is unclear, but Nef has been shown to bind viral RNA (10) and to associate with purified HIV-1 RT in vitro (13).
The association of Nef with the internal vRNP complex suggests that Nef may directly facilitate a step in HIV-1 infection following dissociation of the viral capsid in the target cell. Nef-defective virions exhibit attenuated viral DNA synthesis in target cells (4, 8, 29). In vitro evidence suggests that Nef does not directly influence the process of reverse transcription, since intravirion stimulation of reverse transcription resulted in similar levels of DNA synthesis and infectivity for Nef+ and Nef− viruses (17, 29). However, the possibility that Nef directly regulates reverse transcription within the target cell cannot be excluded. For instance, Nef may directly modulate the stability of the reverse transcription complex within the cell, or Nef may interact with a cellular component necessary for intracellular reverse transcription. Nef might also facilitate reverse transcription indirectly by targeting the reverse transcription complex RTC to a specific intracellular compartment necessary for productive viral DNA synthesis.
Acknowledgments
We thank members of the Aiken laboratory for helpful suggestions and Howard Wynder for assistance with electron microscopy. We also thank Lou Henderson for rabbit antiserum to HIV-1 NC and Didier Trono for rabbit antisera to HIV-1 CA and Nef. The following reagents were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: antisera to HIV-1 RT (catalog no. 634), HIV-1 Vpr (catalog no. 3252) from Velpandi Ayyavoo, and HIV-1 IN (catalog no. 758) from Duane P. Grandgenett.
This work was supported by NIH grant AI40364 and a Discovery Grant from Vanderbilt University School of Medicine to C.A. B.M.F. was supported by an NIH institutional training grant.
REFERENCES
- 1.Accola, M. A., A. Ohagen, and H. G. Gottlinger. 2000. Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6gag. J. Virol. 74:6198-6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad, N., and S. Venkatesan. 1988. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science 241:1481-1485. [DOI] [PubMed] [Google Scholar]
- 3.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4:189-199. [DOI] [PubMed] [Google Scholar]
- 6.Bukovsky, A., T. Dorfman, A. Weimann, and H. G. Gottlinger. 1997. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J. Virol. 71:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y.-L., D. Trono, and D. Camaur. 1998. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J. Virol. 72:3178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 9.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. S. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echarri, A., M. E. Gonzalez, and L. Carrasco. 1996. Human immunodeficiency virus (HIV) Nef is an RNA binding protein in cell-free systems. J. Mol. Biol. 262:640-651. [DOI] [PubMed] [Google Scholar]
- 11.Emerman, M., and M. Malim. 1998. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280:1880-1884. [DOI] [PubMed] [Google Scholar]
- 12.Forshey, B. M., U. von Schwedler, W. I. Sundquist, and C. Aiken. 2002. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 76:5667-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier, C., J.-C. Cortay, C. Carbonnelle, C. Ehresmann, R. Marquet, and P. Boulanger. 2002. The HIV-1 Nef protein enhances the affinity of reverse transcriptase for RNA in vitro. Virus Genes 25:255-269. [DOI] [PubMed] [Google Scholar]
- 14.Freund, J., R. Kellner, J. Konvalinka, V. Wolber, H.-G. Krausslick, and H. R. Kalbitzer. 1994. A possible regulation of negative factor (Nef) activity of human immunodeficiency virus type 1 by the viral protease. Eur. J. Biochem. 223:589-593. [DOI] [PubMed] [Google Scholar]
- 15.Gaedigk-Nitschko, K., A. Schon, G. Wachinger, V. Erfle, and B. Kohleisen. 1995. Cleavage of recombinant and cell derived human immunodeficiency virus (HIV-1) Nef protein by HIV-1 protease. FEBS Lett. 357:275-278. [DOI] [PubMed] [Google Scholar]
- 16.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 17.Khan, M., M. Garcia-Barrio, and M. D. Powell. 2001. Restoration of wild-type infectivity to human immunodeficiency virus type 1 strains lacking nef by intravirion reverse transcription. J. Virol. 75:12081-12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 19.Kotov, A., J. Zhou, P. Flicker, and C. Aiken. 1999. Association of Nef with the human immunodeficiency virus type 1 core. J. Virol. 73:8824-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, M. D., M. T. Warmerdam, S. S. Ferrell, R. Benitez, and W. C. Greene. 1997. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology 234:215-225. [DOI] [PubMed] [Google Scholar]
- 22.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandori, M., H. Craig, L. Moutouh, J. Corbeil, and J. Guatelli. 1998. Virological importance of the protease-cleavage site in human immunodeficiency virus type 1 Nef is independent of both intravirion processing and CD4 down-regulation. Virology 251:302-316. [DOI] [PubMed] [Google Scholar]
- 24.Pandori, M. W., N. J. S. Fitch, H. M. Craig, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1996. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J. Virol. 70:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peter, F. 1998. HIV Nef: the mother of all evil? Immunity 9:433-437. [DOI] [PubMed] [Google Scholar]
- 26.Piguet, V., and D. Trono. 1999. The Nef protein of primate lentiviruses. Rev. Med. Virol. 9:111-120. [DOI] [PubMed] [Google Scholar]
- 27.Premkumar, D. R., X. Z. Ma, R. K. Maitra, B. K. Chakrabarti, J. Salkowitz, B. Yen-Lieberman, M. S. Hirsch, and H. W. Kestler. 1996. The nef gene from a long-term HIV type 1 nonprogressor. AIDS Res. Hum. Retrovir. 12:337-345. [DOI] [PubMed] [Google Scholar]
- 28.Salvi, R., A. R. Garbuglia, A. Di Caro, S. Pulciani, F. Montella, and A. Benedetto. 1998. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J. Virol. 72:3646-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz, O., V. Marechal, O. Danos, and J.-M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69:4053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welker, R., M. Harris, B. Cardel, and H.-G. Krausslich. 1998. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J. Virol. 72:8833-8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welker, R., H. Hohenberg, U. Tessmer, C. Huckhagel, and H. G. Krausslich. 2000. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J. Virol. 74:1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welker, R., H. Kottler, H. R. Kalbitzer, and H.-G. Krausslich. 1996. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology 219:228-236. [DOI] [PubMed] [Google Scholar]