Abstract
It has been shown previously (S. Wadd, H. Bryant, O. Filhol, J. E. Scott, T.-T. Hsieh, R. D. Everett, and J. B. Clements, J. Biol. Chem. 274:28991-28998, 2000) that ICP27, an essential and multifunctional herpes simplex virus type 1 (HSV-1) protein, interacts with CK2 and with heterogeneous ribonucleoprotein K (hnRNP K). CK2 is a pleiotropic and ubiquitous protein kinase, and the tetrameric holoenzyme consists of two catalytic α or α′ subunits and two regulatory β subunits. We show here that HSV-1 infection stimulates CK2 activity. CK2 stimulation occurs at early times after infection and correlates with redistribution of the holoenzyme from the nucleus to the cytoplasm. Both CK2 stimulation and redistribution require expression and cytoplasmic accumulation of ICP27. In HSV-1-infected cells, CK2 phosphorylates ICP27 and affects its cytoplasmic accumulation while it also phosphorylates hnRNP K, which is not ordinarily phosphorylated by this kinase, suggesting an alteration of hnRNP K activities. This is the first example of CK2 stimulation by a viral protein in vivo, and we propose that it might facilitate the HSV-1 lytic cycle by, for example, regulating trafficking of ICP27 protein and/or viral RNAs.
ICP27 is a herpes simplex virus type 1 (HSV-1) phosphoprotein of 63 kDa which is essential for viral replication and expression of certain early and late viral genes (reviewed in reference 35) and is the only HSV-1 immediate-early regulatory gene with homologues in every mammalian and avian herpesvirus sequenced so far. Many studies have highlighted the multifunctional nature of this protein. ICP27 influences and associates with the cellular RNA polymerase II (17, 60) and affects transcription of certain late genes (16). Acting posttranscriptionally, ICP27 enhances pre-mRNA 3′ processing of early and late viral genes with inherently weak poly(A) sites (20, 21), inhibits host cell splicing, causes redistribution of splicing components (15, 37, 39, 45), uses an RGG motif for RNA binding to bind intronless viral transcripts (23, 44), and shuttles between the nucleus and the cytoplasm (22, 38, 44, 50). More recently, Koffa et al. showed that ICP27 exports viral intronless RNAs, which form the majority of HSV-1 transcripts, by interacting with the REF proteins to recruit the TAP/NXF1 factor involved in cellular mRNA export (19).
Using the yeast two-hybrid system, coimmunoprecipitation, and in vitro binding assays, Wadd et al. have shown that ICP27 interacts with heterogeneous ribonucleoprotein K (hnRNP K) and CK2 (53). Like ICP27, hnRNP K is a multifunctional protein capable of shuttling from the nucleus to the cytoplasm, with a possible role in the processing and transport of pre-mRNA (32). HnRNP K has both RNA and DNA binding properties, interacts with proteins of cellular and viral origin, acts as a transcriptional regulator (26), and affects translation (31). It also interacts with inducible kinases (47, 52) and, via phosphorylation, regulates its interactions with protein and RNA partners (34, 35).
Protein kinase CK2 (previously known as casein kinase II) is a pleiotropic and ubiquitous protein kinase (27) with specificity for serine/threonine residues. The tetrameric holoenzyme, consisting of two catalytic subunits (α or α′) and two regulatory β subunits, can be found as an α2β2, αα′β2, or α′2β2 combination. No specific role for the different α and α′ subunits has been shown (12), although there is a difference in autophosphorylation activity between the α2β2 and α′2β2 holoenzymes (6). CK2 is known to phosphorylate more than 200 proteins and is involved in processes such as signal transduction, transcriptional control, apoptosis, cell cycle regulation, and cancer growth (6, 12).
Moreover, CK2 has been implicated in cell cycle-dependent phosphorylation of the carboxy-terminal domain of RNA polymerase II, which alters transcription efficiency (5). Interestingly, changes in the phosphorylation of RNA polymerase II correlate with enhanced transcription of the HSV-1 genome (17). Many viral proteins have been described previously as substrates for CK2 (18), including the HSV-1 structural proteins VP22 and VP16, the latter being required for formation of a complex with cellular components Oct-1 and HCF (30). Although CK2 has been considered to be constitutively active, stimulation of its activity by stress signaling agents (46) and heat shock (10) can occur, while other agents inhibit its activity (11, 13).
As ICP27 interacts with CK2 (53) and serine residues at positions 16 and 18 are likely targets for the detected CK2 phosphorylation of ICP27 in infected cells (59), we examined the role of this phosphorylation and whether it is regulated by the virus. We found that in HSV-1-infected cells, CK2 activity is stimulated at early times postinfection and the kinase relocalizes from the nucleus to the cytoplasm. ICP27 viral protein expression and cytoplasmic localization are required for both CK2 activation and redistribution. In turn, phosphorylation by CK2 is required for the cytoplasmic accumulation of ICP27. We propose that HSV-1 infection induces CK2 activation by controlling ICP27 protein and/or viral RNA trafficking to facilitate viral replication.
MATERIALS AND METHODS
Cells viruses and labeling.
Baby hamster kidney (BHK) C13 cells were infected with HSV-1 wild-type (wt) strain KOS or the following virus mutants as described previously (53): the ICP27 insertion mutant 27LacZ (48), the ICP0 deletion mutant dl1403 (51), or the VP16 insertion mutant in1814 (1). The following viral mutants from mutations in the HSV-1 ICP27 gene were a gift from S. J. Silverstein (Columbia University): vBSLG4 (tsR480H), a temperature-sensitive mutant at the KH3 homology domain (14, 50); vBS3-3, an intragenic revertant of vBSLG4 (49); vBSD448A, with a point mutation in the Sm homology domain; vBSL387N, with a point mutation in the KH2 homology domain; and vBSR3A, which contains a triple mutation in the RGG box (49). The ICP27 viral mutant M15, carrying two point mutations at amino acids 465 and 466, and deletion mutants d1-2, d2-3, d3-4, d4-5, and d5-6 (2, 23, 24, 41, 42, 43) have been described previously. Subconfluent BHK cell monolayers were infected with either wt or mutant HSV-1 viruses at a multiplicity of 10 (except the monolayer infection with in1814, which was at a multiplicity of 100), for the times indicated. Infected cells were grown at 37°C in 5% CO2 before harvesting; in experiments with the vBSLG4 virus, infected cells were grown at 39.2°C (nonpermissive temperature) or at 32°C (permissive temperature). Following virus absorption for 1 h, cells were changed into the appropriate medium and labeled with [35S]methionine (40 μCi/ml) or [32P]orthophosphate (150 μCi/ml) for 6 h.
Immunoprecipitation and Western blot analysis.
For immunoprecipitations, infected or mock-infected BHK cells harvested from a 60-mm-diameter plate were incubated for 30 min on ice in 250 μl of binding buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.05% Nonidet P-40) containing a protease inhibitor mixture (Roche Molecular Biochemicals) and passed five times through a syringe with a 26-gauge needle. Cell debris was removed by centrifugation at 13,000 × g. The cell lysate was mixed with the primary antibodies for 2 h in binding buffer, and protein A/G Sepharose was added for another 1 h. For Western blotting, equal amounts of protein from infected or mock-infected BHK cell extracts (as determined by Bradford assays) from a 30-mm-diameter plate were incubated for 30 min on ice in 30 μl of buffer W (20 mM HEPES, [pH 7.6], 0.4 M KCl, 10 mM EGTA, 5 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 0.4% Triton X-100) and treated as described above. Preimmune serum or the polyclonal antibody 54 was used for immunoprecipitation of hnRNP K (52). Other antibodies used were the monoclonal antibodies 1113 and 1119 against ICP27 (Goodwin Institute, Plantation, Fla.), the polyclonal β-c antibody against the CK2 β subunit, and Rα403 against the α and α′ subunits (9), obtained from O. Filhol-Cochet. The rabbit anti-hnRNP K antibody 54 was used at a 1:10,000 dilution, the mouse H1113 was used at a 1:3,000 dilution, the mouse anti-PKCδ was used at a 1:500 dilution, and the mouse anti-phosphotyrosine was used at a 1:1,000 dilution. The β-c antibody against the β subunit and the Rα403 antibody against the α and α′ subunits were used at a 1:1,000 dilution.
CK2 assays in situ.
BHK cells from a 30-mm-diameter plate were incubated on ice for 30 min in 30 μl of buffer W containing 1 mM NaVO3, 5 mM NaF, and protease inhibitors, and cell lysates were prepared as outlined in the previous section. Cell lysates were electrophoresed on SDS-12% polyacrylamide gels containing dephosphorylated casein at a concentration of 0.5 mg/ml. Following denaturation and renaturation, in situ kinase reactions were carried out as described previously (18). Control reactions performed in the absence of casein substrate in the gel showed no phosphorylation of the CK2 bands, ruling out autophosphorylation of CK2.
Immunofluorescence staining.
BHK cells were grown on glass coverslips for 16 h and then infected with wt HSV-1 or mutant viruses. DRB (the ATP analogue 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole), a cell-permeative inhibitor of CK2 that acts in vitro and in vivo (4, 53, 58), was added at the time of infection at a 5 μM concentration. Cells were fixed in 5% formaldehyde and 2% sucrose in PBS for 10 min and then permeabilized in phosphate-buffered saline containing 0.5% NP-40 and 10% sucrose. Primary antibodies used included the monoclonal antibodies 1113 and 1119 against ICP27 at a dilution of 1:500, 9H10 against hnRNP A1 (kindly provided by G. Dreyfuss, University of Philadelphia) at a dilution of 1:500, the polyclonal antibody β-c against the CK2 β subunit at a dilution of 1:100, and the goat polyclonal antibody sc-6481 against the CK2 α′ subunit (Santa Cruz Biotechnology) at a dilution of 1:100.
RESULTS
HSV-1 infection up-regulates CK2 activity.
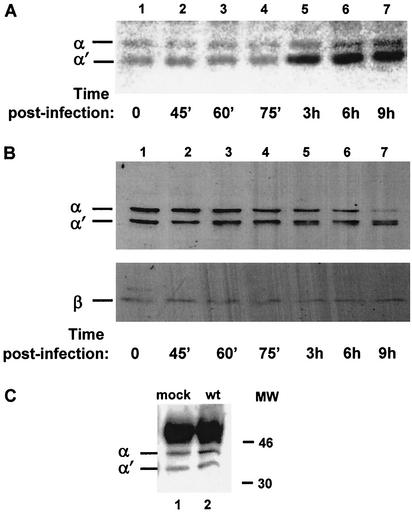
Although CK2 has been considered to be constitutively active, enzyme activity can be stimulated by the stress pathway (46). To examine whether CK2 activity is regulated in infected cells, BHK cells were infected with wt HSV-1 KOS and extracts made at various times postinfection were tested, using a casein-containing in situ gel kinase assay, for CK2 activity (Fig. 1A). Band quantification of the CK2 α′ subunit identified a 3-fold increase in casein phosphorylation by 3 h postinfection (Fig. 1A; compare lanes 1 and 5), reaching a maximum 3.5-fold increase by 6 h (Fig. 1A; compare lanes 1 and 6), with a moderate increase in α activity. No significant change in CK2 activity was detected at later times postinfection, and similar results were obtained using wt HSV-1 strain 17+ (data not shown).
FIG. 1.
HSV-1 infection activates CK2 by 3 h postinfection. (A) Extracts of cells at different times postinfection with wt HSV-1 (KOS strain) were applied to a polyacrylamide gel containing dephosphorylated casein and subjected to electrophoresis. The gel was denatured, renatured, and then incubated with kinase reaction buffer in the presence of [γ-32P]ATP and, after extensive washes, was dried and autoradiographed. (B) Western blot analysis, using the polyclonal Rα403 antibody against CK2 α and α′ subunits (upper panel) and the polyclonal β-c antibody against the β subunit (lower panel), of the CK2 subunits at different times postinfection with wt HSV-1. (C) Using extracts from mock- or wt-infected cells and the polyclonal antibody β-c against the CK2 β subunit, immunoprecipitations were performed at 4 h postinfection. Western blot analysis was performed using the Rα405 polyclonal antibody against the CK2 α and α′ subunits. The intense ∼50-kDa band corresponds to the heavy chain of the antibody used for immunoprecipitation.
To see whether the stimulation was due to elevated CK2 protein levels, aliquots of the extracts used for the in situ gel kinase assay were examined by Western blot analysis to determine the levels of the three CK2 subunits. Interestingly, the levels of the α subunit showed a decline by 6 h postinfection (Fig. 1B; compare lanes 1 and 5). The levels of the α′ subunit were slightly affected, and the band at this region formed a doublet which was apparent at 6 h (Fig. 1B, lane 6). The levels of the β subunit remained unchanged throughout the time period (Fig. 1B). Since both catalytic subunits of CK2 undergo appreciable autophosphorylation at tyrosine residues (7), the doublet at the α′ location might represent differentially phosphorylated forms of α or α′. Thus, we examined the tyrosine phosphorylation of CK2 α and α′ during HSV-1 infection; anti-phosphotyrosine antibodies immunoprecipitated both subunits from mock- and HSV-1-infected cells at similar levels (data not shown), implying that the doublet results from another modification, although we cannot exclude a limited proteolysis.
HSV infection led to increased specific activity of both catalytic subunits, but mainly of α′, beginning at early times postinfection (3 h), although no increase in the protein level was observed. In fact, a decline in the levels of the α catalytic subunits was apparent at later times (6 to 9 h). To examine whether the composition of the CK2 holoenzyme was altered in infected cells, immunoprecipitations were performed using a polyclonal antibody (β-c) against the β subunit. At 4 h postinfection (when stimulation of CK2 activity had occurred), both the α and α′ subunits were immunoprecipitated from infected extracts at levels similar to those seen with the mock-infected cells (Fig. 1C). Further, when in situ gel kinase assays were performed using calmodulin (25) as a specific substrate for free α or α′ subunits (3), there was no difference in calmodulin phosphorylation between mock-infected or infected cell extracts (data not shown), indicating that the stimulated α and α′ subunits in HSV-1-infected cells are part of the CK2 holoenzyme.
These data suggest that HSV-1 infection up-regulates the CK2 kinase activity without changing the composition of the holoenzyme.
Expression and nucleocytoplasmic shuttling of HSV-1 ICP27 are necessary for CK2 up-regulation.
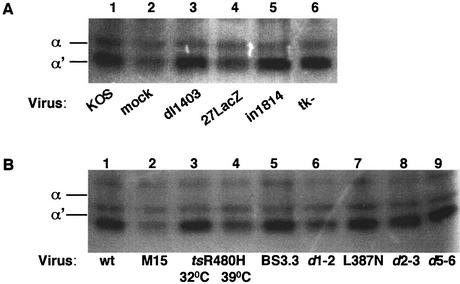
The CK2 stimulation at 3 h postinfection coincided with the accumulation of viral IE proteins, raising the question of whether they are implicated in this effect. Cells were infected with a panel of different mutant viruses which fail to express functional forms of immediate-early, early, and late viral proteins, and in situ gel kinase assays were performed using extracts from cells at 6 h postinfection. Strikingly, infection with virus 27lacZ, which lacks functional ICP27 (Fig. 2A, lane 4), did not up-regulate CK2 activity, indicating that ICP27 expression was required for stimulation, whereas, for example, infection of cells with the immediate-early ICP0 null mutant dl1403 or infection with a mutant that lacks the early gene thymidine kinase (tk−) stimulated CK2 activity to a level similar to wt levels (Fig. 2A, lanes 3 and 6). Infection with the mutant in1814 at a high multiplicity (i.e., at a level at which the virus replicates normally but contains an inactive form of the virion transactivator VP16) (1) stimulated CK2 activity to a level similar to that stimulated by wt infection (Fig. 2A, lane 5).
FIG. 2.
ICP27 is required for CK2 activation. (A) Equal amounts of mock-infected cell extracts (lane 2) or cell extracts infected with different mutant viruses were subjected to the casein-containing in situ kinase assay as follows: lane 1, wt KOS; lane 3, dl1403; lane 4, 27LacZ; lane 5, in1814; and lane 6, tk−. (B) Equal amounts of cell extracts infected with the wt or with mutants with different mutations in the ICP27 gene and collected at 6 h postinfection were subjected to the in situ gel kinase assay. Infection with tsR480H at the permissive temperature (32°C) activated CK2 (lane 3), but infection at the restrictive temperature (39.2°C; lane 4), at which ICP27 is deficient in nucleocytoplasmic shuttling, did not. Infection with the M15 (lane 2) or the d1-2 (lane 6) deletion mutant, both of which also disrupt ICP27 shuttling, failed to activate the CK2 α′ subunit. The ICP27 viral mutants vBS3.3 (lane 5), vBSL387N (lane 7), d2-3 (lane 8), and d5-6 (lane 9) activate CK2 to levels similar to that activated by the wt (lane 1).
To further investigate the regulatory influence of ICP27 on CK2, cells were infected with viral mutants created by mutations in different functional regions of this gene. Infection with ICP27 point or deletion mutants that show deficiencies in ICP27 shuttling and viral late gene expression, such as the M15 mutant (22), tsR480H (at the restrictive temperature of 39.2°C) (49), and d1-2 (22), failed to up-regulate CK2 activity (Fig. 2B, lanes 2, 4, and 6, respectively). By contrast, infection at the permissive temperature with the tsR480H virus stimulated CK2 to levels of activity similar those stimulated by infection with the wt (Fig. 2B, lane 3). ICP27 viral mutants that affect other properties of the protein (49), such as vBSR3A (triple mutation at the RGG box), vBSL387N (point mutation at the KH2 homology domain), and vBSD448A (point mutation at the Sm homology domain), stimulated CK2 activity to levels of activity similar those stimulated by infection with the wt. Moreover, the ICP27 deletion mutants d2-3, d3-4, d4-5, and d5-6, which remove regions of the protein involved in nuclear localization (24) and RNA binding (23), still activated CK2 (representative mutants are shown in Fig. 2B, lanes 8 and 9).
CK2 activation coincides with its redistribution from the nucleus to the cytoplasm.
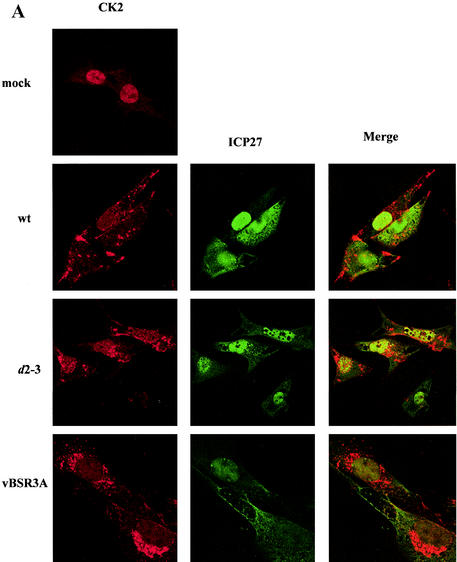
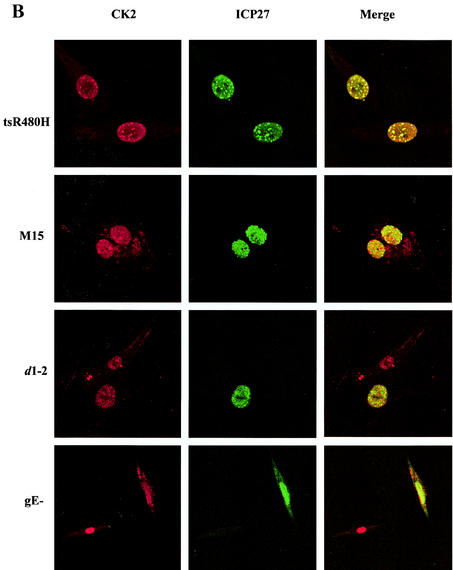
To explore the CK2 stimulation, the subcellular localization of CK2 α, α′, and β subunits was examined. Cells were infected with wt HSV-1 or with ICP27 viral mutants, and immunofluorescence was performed at 6 h postinfection. In mock-infected cells, the CK2 distribution was almost exclusively nuclear (Fig. 3A). Strikingly, following infection with wt virus there was a redistribution of the β subunit, with most of the subunits now present throughout the cytoplasm in a speckled pattern (Fig. 3A), and a similar effect was seen with the CK2 α′ subunit (data not shown). The CK2 relocalization was evident from 3 h and was pronounced by 6 h postinfection. Infection with tsR480H (at a nonpermissive temperature), M15, and d1-2 (Fig. 3B), all mutants that exhibited reduced ICP27 shuttling and failed to stimulate CK2 activity, caused no redistribution of CK2 from the nucleus to the cytoplasm. In cells infected with these mutant viruses, there was colocalization of CK2 and ICP27 within the nucleus; in some cases, such as infection with tsR480H at a nonpermissive temperature, the two proteins colocalized in speckles. In marked contrast, ICP27 viral mutants that do not significantly affect its cytoplasmic accumulation (such as d2-3 and vBSR3A [Fig. 3A], as well as d3-4 and vBSD448A [data not shown]) showed redistribution of CK2 from the nucleus to the cytoplasm and no colocalization with CK2. Thus, in HSV-infected cells there is a correlation between ICP27 cytoplasmic accumulation, CK2 kinase stimulation, and redistribution of CK2 from the nucleus to the cytoplasm.
FIG. 3.
CK2 redistribution to the cytoplasm correlates with ICP27 cytoplasmic accumulation. Immunofluorescence staining was performed 6 h postinfection on mock-infected BHK cells or cells infected with the viruses indicated, and antibodies were used to detect ICP27 and the CK2 β subunit. Infection with wt HSV, or with ICP27 viral mutants that do not significantly affect its cytoplasmic accumulation (VBSR3A and d2-3), resulted in a dramatic redistribution of CK2 from the nucleus to the cytoplasm (A), whereas infection with ICP27 viral mutants that inhibit or decrease ICP27 shuttling (tsR480H, M15, and d1-2) prevented the redistribution (B). Infection with HSV-1 gE− was used to exclude the possibility that redistribution of CK2 is due to cross-reaction between the rabbit polyclonal CK2 β subunit antibody and the HSV-1 glycoprotein E, which can act as a low-affinity Fc receptor.
The CK2 inhibitor DRB reduces ICP27 phosphorylation, cytoplasmic accumulation, and interaction with hnRNP K.
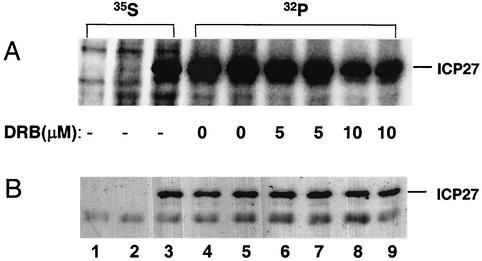
Low, nontoxic doses of DRB, a cell-permeative inhibitor of CK2, were applied to living cells to examine effects on ICP27 phosphorylation. Uninfected BHK cells or cells infected with wt HSV-1 or 27lacZ virus were labeled with either [35S]methionine or [32P]orthophosphate for 6 h in the presence or absence of DRB, and then immunoprecipitations using the ICP27 specific monoclonal antibodies 1113 and 1119 were performed on cell extracts. Phosphorylation of ICP27 (Fig. 4A) was reduced (to 65%) in the presence of 5 μM DRB; the inhibition was greater with 10 μM DRB (down to 30%) but was not abolished, suggesting the activity of other kinases. Western blot analysis of the same samples showed that the protein accumulation of ICP27 was not affected by the presence of DRB (Fig. 4B), thus excluding the possibility that the reduction of phosphorylation was due to DRB inhibition of ICP27 transcription.
FIG. 4.
DRB reduces phosphorylation of ICP27 by CK2. (A) Uninfected or infected cells were labeled with either [35S]methionine (lanes 1 to 3) or [32P]orthophosphate (lanes 4 to 9) for 6 h in the presence or absence of DRB. Total extracts from uninfected cells (lane 1), cells infected with the ICP27 null mutant 27LacZ (lane 2), or wt-infected cells (lanes 3 to 9), in the absence (lanes 1 to 5) or presence of 5 μM (lanes 6 and 7) or 10 μM (lanes 8 and 9) of DRB, were used for immunoprecipitation of ICP27. A mixture of the monoclonal antibodies 1113 and 1119 against ICP27 was used, followed by SDS-polyacrylamide gel electrophoresis and autoradiography. A band migrating at 63 kDa, corresponding to full-length ICP27, is shown. (B) Western blot analysis was performed subsequently on the same membrane, using the ICP27 antibody H1113.
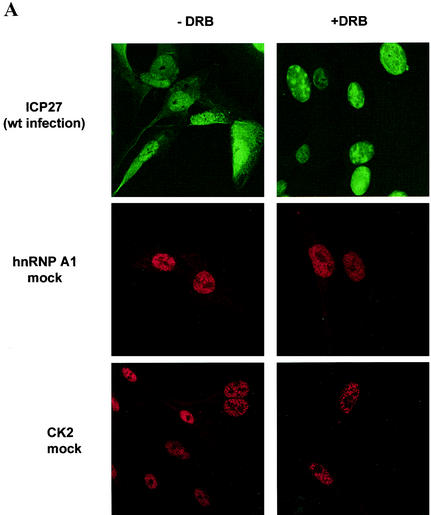
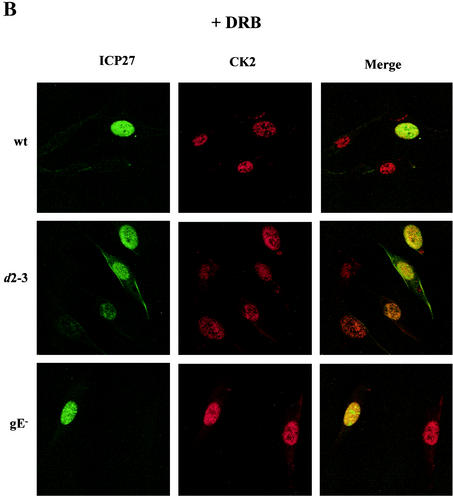
Immunofluorescence was performed on cells infected with wt HSV-1 in the presence or absence of DRB, and as a control, levels of the cellular protein hnRNP A1 were monitored. ICP27 appeared in the cytoplasm of 50 to 80% of cells at 7 h postinfection with the wt, whereas infected cells incubated with 5 μM DRB showed only nuclear localization (Fig. 5A), indicating that shuttling of ICP27 was affected. In the presence of this low concentration of DRB, hnRNP A1 remained nuclear (Fig. 5A) and showed no accumulation in the cytoplasm, a characteristic of transcriptional inhibition of rapidly shuttling proteins (40). CK2 in mock-infected cells was also not affected by DRB treatment and remained nuclear (Fig. 5A). Interestingly, in wt-infected cells, the CK2 that was ordinarily redistributed to the cytoplasm (Fig. 3) showed nuclear localization in the presence of DRB (Fig. 5B). DRB treatment of cells infected with viral mutants like d2-3, which redistributed CK2 to the cytoplasm and showed ICP27 shuttling, resulted in nuclear accumulation of both ICP27 and CK2 (Fig. 5B), and ICP27 and CK2 showed partial colocalization within the nucleus.
FIG. 5.
Cytoplasmic accumulation of ICP27 is inhibited by DRB. (A) BHK cells were mock infected or infected with wt HSV-1 and were fixed 7 h after infection. Cells remained untreated, or DRB (5 μM) was added with virus. Immunofluorescence staining was performed with the 1113 monoclonal antibody for ICP27, with a monoclonal antibody for the cellular protein hnRNPA1, and with the polyclonal β-c antibody for CK2. (B) HSV-induced cytoplasmic redistribution of CK2 is inhibited by DRB. BHK cells were infected with wt HSV-1 or with different ICP27 viral mutants. The example shown is that of the d2-3 virus, which redistributes CK2 to the cytoplasm in the absence of DRB.
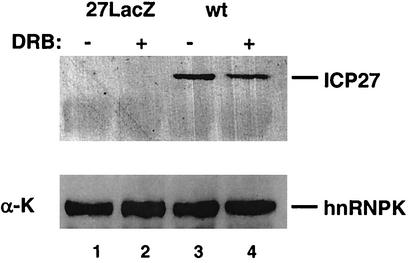
Finally, we examined the effect of the presence of DRB on the interaction of ICP27 with its partner protein hnRNP K. Extracts from cells infected with wt or 27LacZ virus were immunoprecipitated with polyclonal antibody against hnRNP K and were then subjected to SDS-polyacrylamide gel electrophoresis and Western blot analysis. In the presence of DRB, the amount of ICP27 protein interacting with hnRNP K was reduced reproducibly (this reduction was quantified as a twofold decrease [Fig. 6, upper panel; compare lanes 3 and 4]) while hnRNP K was immunoprecipitated in approximately equal amounts from the extracts (Fig. 6, lower panel). This suggests that CK2 phosphorylation regulates binding of ICP27 to hnRNP K in vivo.
FIG. 6.
Inhibition of CK2 phosphorylation reduces the binding of ICP27 to hnRNP K. (Upper panel) Cells were infected with 27LacZ (lanes 1 and 2) or with the wt (lanes 3 and 4) in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of 5 μM DRB. Immunoprecipitates of cell extracts were made using the 54 polyclonal antibody against hnRNP K, followed by SDS-polyacrylamide gel electrophoresis and Western blot analysis. Using the 1113 antibody, ICP27 is immunoprecipitated only from the wt-infected extracts (lanes 3 and 4), and DRB reduces this binding twofold. (Lower panel) Blotting with the anti-hnRNP K antibody demonstrated equal loading of the gel.
DISCUSSION
We show here that wt HSV-1 infection up-regulates CK2 activity, and the essential viral ICP27 protein is required for this stimulation. This is the first example of CK2 stimulation by a virus in vivo, although recombinant HIV-1 Rev protein is capable of stimulating CK2 activity in vitro (29). The CK2 stimulation occurs at early times and remains until late times after infection and coincides with the onset of viral DNA replication, expression of structural proteins, and cytoplasmic accumulation of ICP27. Interestingly, viruses that show defects in ICP27 nucleocytoplasmic shuttling and fail to activate CK2, such as d1-2 and M15, are severely defective for growth and are apoptotic in HEp-2 cells (2), and virus tsR480H has a lethal phenotype at the restrictive temperature (50). In contrast, viruses (such as d2-3, d3-4, d4-5, and d5-6) that activate CK2 at levels comparable to that activated by the wt virus show significant DNA replication and are nonapoptotic (2), while vBSD448A and vBSL387N show moderate growth defects (49).
In addition, ICP27 is phosphorylated by CK2, and this phosphorylation seems to be important for the localization of the protein as well as the interaction with its protein partners. DRB, an inhibitor of CK2, reduces ICP27 phosphorylation and induces nuclear accumulation of both ICP27 and CK2 at nontoxic concentrations. Phosphorylation of ICP27 by CK2 also regulates its binding to hnRNPK, and interestingly, ICP27 expression represses hnRNPK tyrosine phosphorylation in response to stress treatment (our unpublished observations). Tyrosine phosphorylation of hnRNPK can activate translation of silenced mRNAs in vivo (33); thus, the regulation of ICP27 binding to hnRNPK by CK2 might affect translation of cell and/or viral mRNAs.
Phosphopeptide mapping indicated that serine residues at positions 16 and 18 of ICP27 are likely targets for CK2 phosphorylation in vivo (59). However, no dramatic defect in ICP27 activity was observed by complementation assays using the CK2 consensus site mutant Δ16-18aa (59). This might be due either to the lack of sensitivity of the assay or to alternative phosphorylation sites, since, based on findings of experiments using the CK2 phosphorylation site motif S*XX(D/E) (36), there are additional putative sites in ICP27 protein.
CK2 stimulation correlates with its redistribution from the nucleus to the cytoplasm. Viral mutants that fail to activate CK2 do not cause this redistribution of CK2 protein. The subcellular location of CK2 appears to be a key to its function (8). CK2 has been proposed to play a role during cell division by shifting its location between the cytoplasm and nucleus, with the CK2 α′ subunit present in the nucleus during G1 phase and in the cytoplasm during S phase (55). As CK2 is a multifunctional protein kinase, changes in its subcellular location following HSV-1 infection might serve to facilitate the viral lytic cycle.
CK2 activity is also induced by stress, mediated via a direct interaction with the p38 mitogen-activated protein kinase (p38 MAPK) through what appears to be an allosteric mechanism (46). HSV-1 infection also stimulates p38 MAPK via the virion tegument protein VP16, enhancing transcription of specific viral gene promoters and increasing viral yield (56, 57). However, since the 27LacZ virus that doesn't express functional ICP27 and fails to stimulate CK2 still activates p38 MAPK (57), the connection between viral CK2 stimulation and/or redistribution and p38 activation remains unclear.
The functional consequence of stimulating CK2 is not yet clear, but it might regulate ICP27 protein trafficking and/or export of viral RNAs. PHAX, the export adaptor of U snRNA export, is phosphorylated in the nucleus and then exported with RNA to the cytoplasm, and its phosphorylation is essential for assembly of the export complex (28). ICP27 is produced very early after infection but accumulates in the cytoplasm later (by 4 to 5 h), when the activation and redistribution of CK2 occurs. Thus, it is tempting to speculate that phosphorylation by activated CK2 is required either for RNA-independent ICP27 protein transport or for transport of ICP27 with its viral RNA cargo. Since treatment with a CK2 inhibitor reduces cytoplasmic accumulation of ICP27, our results indicate a role for CK2 in phosphorylation-dependent ICP27 protein export, which is necessary for its ability to export viral RNAs.
Since RNA-dependent ICP27 protein export from the nucleus requires binding of REF and TAP proteins in the area adjacent to the nuclear localization signal of the protein (19), the presence of CK2 in the cytoplasm (and subsequent phosphorylation) might affect the release of these factors and reimport of ICP27; an effect of CK2 on importin α binding to NLS and import rate has been reported for the simian virus 40 large T antigen (54). Thus, the compartmentalization of CK2 might contribute to the directionality of protein or RNA nuclear export. Further work with viral ICP27 point mutants in potential CK2 phosphoacceptor sites is ongoing to examine effects on cytoplasmic accumulation of ICP27 and CK2 and on viral RNA export.
Acknowledgments
This work was supported by Medical Research Council funding (G9826324).
We are grateful to J. McLauchlan, D. Blackbourn, and P. Malik for comments on the manuscript. Thanks to O. Filhol-Cochet, Laboratoire de Biochimie des Regulations Cellulaires Endocrines, Grenoble, France, for the polyclonal β-c and Rα403 antibodies; S. J. Silverstein, Department of Microbiology, Columbia University, New York, N.Y., for kindly providing ICP27 viral mutants; C. M. Preston, MRC Virology Unit, Glasgow, Scotland, for the VP16 viral mutant; R. D. Everett, MRC Virology Unit, Glasgow, Scotland, for the ICP0 viral mutant; G. Dreyfuss, University of Pennsylvania School of Medicine, Philadelphia, Pa., for the antibody against hnRNPA1; and K. Bomsztyk, University of Washington, Seattle, Wash., for polyclonal antibody 54 against hnRNP K.
REFERENCES
- 1.Ace, C. I., T. A. McKee, J. M. Ryan, J. M. Cameron, and C. M. Preston. 1989. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J. Virol. 63:2260-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 75:1013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benitez, M. J., C. Cochet, and J. S. Jimenez. 2001. A surface plasmon resonance study of the interactions between the component subunits of protein kinase CK2 and two protein substrates, casein and calmodulin. Mol. Cell. Biochem. 227:31-36. [PubMed] [Google Scholar]
- 4.Blaydes, J. P., and T. R. Hupp. 1998. DNA damage triggers DRB-resistant phosphorylation of human p53 at the CK2 site. Oncogene 17:1045-1052. [DOI] [PubMed] [Google Scholar]
- 5.Bregman, D. B., R. G. Pestell, and V. J. Kidd. 2000. Cell cycle regulation and RNA polymerase II. Front. Biosci. 1:244-257. [DOI] [PubMed] [Google Scholar]
- 6.Dobrowolska, G., F. J. Lozeman, D. Li, and E. G. Krebs. 1999. CK2, a protein kinase of the next millennium. Mol. Cell. Biochem. 191:3-12. [PubMed] [Google Scholar]
- 7.Donella-Deana, A., L. Cesaro, S. Sarno, A. M. Brunati, M. Ruzzene, and L. A. Pinna. 2001. Autocatalytic tyrosine-phosphorylation of protein kinase CK2 alpha and alpha′ subunits: implication of Tyr182. Biochem. J. 357:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faust, M., and M. Montenarh. 2000. Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res. 301:329-340. [DOI] [PubMed] [Google Scholar]
- 9.Filhol, O., C. Cochet, P. Loue-Mackenbach, and E. M. Chambaz. 1994. Oligomeric casein kinase II isoforms are expressed in bovine tissues and adrenocortical cells in culture. Biochem. Biophys. Res. Commun. 198:660-665. [DOI] [PubMed] [Google Scholar]
- 10.Gerber, D. A., S. Souquere-Besse, F. Puvion, M. F. Dubois, O. Bensaude, and C. Cochet. 2000. Heat-induced relocalization of protein kinase CK2. Implication of CK2 in the context of cellular stress J. Biol. Chem. 275:23919-23936. [DOI] [PubMed] [Google Scholar]
- 11.Gotz, C., P. Wagner, O. G. Issinger, and M. Montenarh. 1996. p21WAF1/CIP1 interacts with protein kinase CK2. Oncogene 13:391-398. [PubMed] [Google Scholar]
- 12.Guerra, B., C. Gotz, P. Wagner, M. Montenarh, and O. G. Issinger. 1997. The carboxy terminus of p53 mimics the polylysine effect of protein kinase CK2-catalyzed MDM2 phosphorylation. Oncogene 14:2683-2688. [DOI] [PubMed] [Google Scholar]
- 13.Guerra, B., and O. G. Issinger. 1999. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 20:391-408. [DOI] [PubMed] [Google Scholar]
- 14.Hardwicke, M. A., P. J. Vaughan, R. E. Sekulovich, R. O'Conner, and R. M. Sandri-Goldin. 1989. The regions important for the activator and repressor functions of herpes simplex virus type 1 α protein ICP27 map to the C-terminal half of the molecule. J. Virol. 63:4590-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (γ2) genes in infected cells. Virology 283:273-284. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins, H. L., and C. A. Spencer. 2001. RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells. J. Virol. 75:9872-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, S. J., and C. R. Kahn. 1997. Insulin regulation of mitogen-activated protein kinase kinase (MEK), mitogen-activated protein kinase and casein kinase in the cell nucleus: a possible role in the regulation of gene expression. Biochem. J. 323:621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGregor, F., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J. Virol. 70:1931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLauchlan, J., S. Simpson, and J. B. Clements. 1989. Herpes simplex virus induces a processing factor that stimulates poly(A) site usage. Cell 59:1093-1105. [DOI] [PubMed] [Google Scholar]
- 22.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 23.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mears, W. E., V. Lam, and S. A. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meggio, F., B. Boldyreff, O. G. Issinger, and L. A. Pinna. 1994. Casein kinase 2 down-regulation and activation by polybasic peptides are mediated by acidic residues in the 55-64 region of the beta-subunit. A study with calmodulin as phosphorylatable substrate. Biochemistry 33:4336-4342. [DOI] [PubMed] [Google Scholar]
- 26.Michelotti, E. F., G. A. Michelotti, A. I. Aronsohn, and D. Levens. 1996. Heterogeneous nuclear ribonucleoprotein K is a transcription factor. Mol. Cell. Biol. 16:2350-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niefind, K., B. Guerra, L. A. Pinna, O. G. Issinger, and D. Schomburg. 1998. Crystal structure of the catalytic subunit of protein kinase CK2 from Zea mays at 2.1 A resolution. EMBO J. 17:2451-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohno, M., A. Segref, A. Bachi, M. Wilm, and I. W. Mattaj. 2000. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 101:187-198. [DOI] [PubMed] [Google Scholar]
- 29.Ohtsuki, K., T. Maekawa, S. Harada, A. Karino, Y. Morikawa, and M. Ito. 1998. Biochemical characterization of HIV-1 Rev as a potent activator of casein kinase II in vitro. FEBS Lett. 428:235-240. [DOI] [PubMed] [Google Scholar]
- 30.O'Reilly, D., O. Hanscombe, and P. O'Hare. 1997. A single serine residue at position 375 of VP16 is critical for complex assembly with Oct-1 and HCF and is a target of phosphorylation by casein kinase II. EMBO J. 16:2420-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostareck, D. H., A. Ostareck-Lederer, M. Wilm, B. J. Thiele, M. Mann, and M. W. Hentze. 1997. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell 89:597-606. [DOI] [PubMed] [Google Scholar]
- 32.Ostareck-Lederer, A., D. H. Ostarek, and M. W. Hentze. 1998. Cytoplasmic regulatory functions of the KH-domain proteins hnRNPs K and E1/E2. Trends. Biochem. Sci. 23:409-411. [DOI] [PubMed] [Google Scholar]
- 33.Ostareck-Lederer, A., D. H. Ostareck, C. Cans, G. Neubauer, K. Bomsztyk, G. Superti-Furga, and M. W. Hentze. 2002. c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol. Cell. Biol. 22:4535-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostrowski, J., Y. Kawata, D. S. Schullery, O. N. Denisenko, Y. Higaki, C. K. Abrass, and K. Bomsztyk. 2001. Insulin alters heterogeneous nuclear ribonucleoprotein K protein binding to DNA and RNA. Proc. Natl. Acad. Sci. USA 98:9044-9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostrowski, J., D. S. Schullery, O. N. Denisenko, Y. Higaki, J. Watts, R. Aebersold, L. Stempka, M. Gschwendt, and K. Bomsztyk. 2000. Role of tyrosine phosphorylation in the regulation of the interaction of heterogenous nuclear ribonucleoprotein K protein with its protein and RNA partners. J. Biol. Chem. 275:3619-3628. [DOI] [PubMed] [Google Scholar]
- 36.Pearson, R. B., and B. E. Kemp. 1991. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 200:62-81. [DOI] [PubMed] [Google Scholar]
- 37.Phelan, A., and J. B. Clements. 1998. Posttranscriptional regulation in herpes simplex virus. Semin. Virol. 8:309-318. [Google Scholar]
- 38.Phelan, A., and J. B. Clements. 1997. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol. 78:3327-3331. [DOI] [PubMed] [Google Scholar]
- 39.Phelan, A., M. Carmo-Fonseca, J. McLauchlan, A. I. Lamond, and J. B. Clements. 1993. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc. Natl. Acad. Sci. USA 90:9056-9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinol-Roma, S., and G. Dreyfuss. 1992. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature 355:730-732. [DOI] [PubMed] [Google Scholar]
- 41.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus α protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice, S. A., V. Lam, and D. M. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 67:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandri-Goldin, R. M., M. K. Hibbard, and M. A. Hardwicke. 1995. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J. Virol. 69:6063-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sayed, M., S. O. Kim, B. S. Salh, O. G. Issinger, and S. L. Pelech. 2000. Stress-induced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. J. Biol. Chem. 275:16569-16573. [DOI] [PubMed] [Google Scholar]
- 47.Schullery, D. S., J. Ostrowski, O. N. Denisenko, L. Stempka, M. Shnyreva, H. Suzuki, M. Gschwendt, and K. Bomsztyk. 1999. Regulated interaction of protein kinase C delta with the heterogeneous nuclear ribonucleoprotein K protein. J. Biol. Chem. 274:15101-15109. [DOI] [PubMed] [Google Scholar]
- 48.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 49.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via Crm1-dependent and -independent pathways. J. Virol. 74:2814-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 52.Van Seuningen, I., J. Ostrowski, and K. Bomsztyk. 1995. Description of an IL-1-responsive kinase that phosphorylates the K protein. Enhancement of phosphorylation by selective DNA and RNA motifs. Biochemistry 34:5644-5650. [DOI] [PubMed] [Google Scholar]
- 53.Wadd, S., H. Bryant, O. Filhol, J. E. Scott, T.-T. Hsieh, R. D. Everett, and J. B. Clements. 1999. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Biol. Chem. 274:28991-28998. [DOI] [PubMed] [Google Scholar]
- 54.Xiao, C. Y., P. Jans, and D. A. Jans. 1998. Negative charge at the protein kinase CK2 site enhances recognition of the SV40 large T-antigen NLS by importin: effect of conformation. FEBS Lett. 440:297-301. [DOI] [PubMed] [Google Scholar]
- 55.Yu, I. J., D. L. Spector, Y.-S. Bae, and D. R. Marshak. 1991. Immunocytochemical localization of casein kinase II during interphase and mitosis. J. Cell Biol. 114:1217-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zachos, G., J. B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]
- 57.Zachos, G., M. Koffa, C. M. Preston, J. B. Clements, and J. Conner. 2001. Herpes simplex virus type 1 blocks the apoptotic host cell defense mechanisms that target Bcl-2 and manipulates activation of p38 mitogen-activated protein kinase to improve viral replication. J. Virol. 75:2710-2728. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Zandomeni, R. O. 1989. Kinetics of inhibition by 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole on calf thymus casein kinase II. Biochem. J. 262:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhi, Y., and R. M. Sandri-Goldin. 1999. Analysis of the phosphorylation sites of herpes simplex virus type 1 regulatory protein ICP27. J. Virol. 73:3246-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou, C., and M. D. Knipe. 2002. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]