Abstract
The trafficking of varicella-zoster virus (VZV) gH was investigated under both infection and transfection conditions. In initial endocytosis assays performed in infected cells, the three glycoproteins gE, gI, and gB served as positive controls for internalization from the plasma membrane. Subsequently, we discovered that gH in VZV-infected cells was also internalized and followed a similar trafficking pattern. This observation was unexpected because all herpesvirus gH homologues have short endodomains not known to contain trafficking motifs. Further investigation demonstrated that VZV gH, when expressed alone with its chaperone gL, was capable of endocytosis in a clathrin-dependent manner, independent of gE, gI, or gB. Upon inspection of the short gH cytoplasmic tail, we discovered a putative tyrosine-based endocytosis motif (YNKI). When the tyrosine was replaced with an alanine, endocytosis of gH was blocked. Utilizing an endocytosis assay dependent on biotin labeling, we further documented that endocytosis of VZV gH was antibody independent. In control experiments, we showed that gE, gI, and gB also internalized in an antibody-independent manner. Alignment analysis of the VZV gH cytoplasmic tail to other herpesvirus gH homologues revealed two important findings: (i) herpes simplex virus type 1 and 2 homologues lacked an endocytosis motif, while all other alphaherpesvirus gH homologues contained a potential motif, and (ii) the VZV gH and simian varicella virus gH cytoplasmic tails were likely longer in length (18 amino acids) than predicted in the original sequence analyses (12 and 16 amino acids, respectively). The longer tails provided the proper context for a functional endocytosis motif.
Varicella-zoster virus (VZV) glycoprotein H (gH) is one of seven recognized glycoproteins in VZV (16). The product of open reading frame 37, gH is a 118-kDa type I transmembrane protein with a large ectodomain of 812 residues and a cytoplasmic tail that has been estimated at between 12 and 14 amino acids. VZV gH contains an immunodominant complement-independent neutralization epitope (67). Monoclonal antibodies against gH are able to block entry, egress, and cell-to-cell spread of the virus in cell culture (67, 83). These results demonstrate a role for gH in both entry and cell-to-cell spread. In addition, VZV gH, like herpes simplex virus type 1 (HSV-1), requires the formation of a heterodimeric complex with gL for complete maturation and cell surface expression (22, 46).
Among the human herpesviruses, gH is highly conserved, and many of its properties are common throughout the herpesvirus family. This glycoprotein is essential for penetration and cell-to-cell spread in pseudorabies virus (5, 78), HSV-1 (26), and Epstein-Barr virus (37, 66). The functional importance of the gH-gL complex formation is echoed in other herpesviruses, including HSV-1 (46), pseudorabies virus (53), Epstein-Barr virus (102), human cytomegalovirus (52, 88), human herpesvirus 6 (56), and human herpesvirus 7 (71).
VZV gH is considered the major VZV fusogen (19). While the gH biosynthetic pathway to the plasma membrane is well characterized, no research has investigated the trafficking of gH once it has reached the surface of the infected cell. In contrast, other herpesvirus glycoproteins have been demonstrated to undergo endocytosis in transient expression systems, including gE of VZV (2, 77), HSV-1 (3), and pseudorabies virus (91, 92); gB of VZV (42), pseudorabies virus (92), and human cytomegalovirus (81); and as a complex, gE-gI of VZV (1, 76, 94) and pseudorabies virus (92).
Internalization of membrane-integrated proteins is mediated by specific amino acid sequences located in the cytoplasmic tail. The most common motifs are tyrosine-based (YXXφ) (reviewed in reference 7) with a critical tyrosine residue (48). The tetrapeptide of the tyrosine-based motif is recognized by the μ2 subunit of AP-2, a clathrin-associated complex localized to the plasma membrane (6, 74). AP-2 is the driving force behind the formation of clathrin-coated vesicles by acting as the adaptor between the membrane protein and clathrin. In general, the internalization motif of type I transmembrane glycoproteins is located within cytoplasmic tails usually greater than 35 residues in length.
In this study, we report that VZV gH undergoes endocytosis in both infected and transfected cells via a functional endocytosis motif in the gH cytoplasmic tail. We provide a realignment of the VZV gH amino acid sequence which suggests that the cytoplasmic tail is longer than previously predicted. In addition, we present evidence for the first time that the four major VZV glycoproteins, gE, gI, gB, and gH, undergo endocytosis in a non-antibody-mediated manner.
MATERIALS AND METHODS
Cells, plasmids, and viruses.
HeLa cells (ATCC CCL-2) were maintained as previously reported (99). A human melanoma cell line (Mewo) highly permissive for VZV infection was similarly maintained (33, 35). Culture of VZV-32 (GenBank accession no. AH010537) was performed as previously described (33, 85). Monolayers infected with VZV were processed 24 h postinfection. Recombinant vaccinia virus and pTM1, a T7-driven vector, were utilized for individual and combined glycoprotein expression in HeLa cells, respectively. Construction and characterization of recombinant vaccinia virus expressing both gH and gL (VV-gH+gL) have been described previously (58). Vaccinia virus expressing the T7 polymerase (VV-T7) was obtained from the Bernard Moss laboratory (70). Construction of pTM1 vectors has been previously described: pTM1-gE and pTM1-gI (99), pTM1-gE-tailless (100), pTM1-gB (60), and pTM1-gH and pTM1-gL (19, 22). Monolayers infected with recombinant vaccinia virus or transfected with pTM1 were processed 18 h postinfection.
Antibodies and fluoroprobes.
Monoclonal antibody (MAb) 3B3 recognizes a well-characterized linear epitope in the ectodomain of gE (34, 39, 85). MAb 258 is a conformation-dependent anti-gH monoclonal specific for mature and immature glycosylated gH but not the nonglycosylated immature form (18, 19). MAb 6B5 and MAb 158 are directed against gI and gB, respectively (68, 98). Human zoster serum has been described previously (58). Human monoclonal antibody V1 is directed against gB (89). All fluorescent probes were purchased from Molecular Probes, Eugene, Oreg., and included the Alexa 488-fluoroconjugated secondary antibody goat anti-mouse immunoglobulin G (heavy and light chain), TOTO-3 for DNA staining, and WGA-Texas-Red-X to label the trans-Golgi network and trans-Golgi network-derived vesicles. For the fluid-phase endocytosis assay, dextran-Texas Red (70,000 molecular weight, neutral) was used along with rabbit anti-Texas Red.
Transfection protocols.
HeLa cells were transfected with pTM1 vectors or infected with recombinant vaccinia virus expressing VZV glycoproteins. Transfection with pTM1 vectors has been described previously (19, 70). Infection with recombinant vaccinia viruses performed on HeLa cells has also been described (58, 59).
Construction of VZV gH mutants in pTM1.
Recombination PCR site-directed mutagenesis was performed to construct pTM1-gH-Y835A and pTM1-gH-S830stop from pTM1-gH-wt (50, 70, 100, 101). The primers for gH mutagenesis are listed in Table 1. One set of primers contained a mismatch and overlapped the sequence in the gH cytoplasmic tail to be mutated. The second set was nonmutagenizing and bound to overlapping sites in the pTM1 ampicillin resistance gene (101). The PCR fragments, which contained homologous ends for recombination, were combined and used to transform Maximum Efficiency Escherichia coli DH5α cells (Life Technologies, Gaithersburg, Md.). Incorporation of the desired mutation was confirmed by fluorescent DNA sequencing at the University of Iowa DNA Core Facility.
TABLE 1.
Primers for recombination PCR site-directed mutagenesisa
| Primer | Sequence |
|---|---|
| Y835A (s) | 5′-TCCCGCCTTCGAGAAgccAATAAAATACCTCTGACATAAA |
| Y835A (as) | 5′-CAGAGGTATTTTATTggcTTCTCGAAGGCGGGAAT |
| S830stop (s) | 5′-GGATGTTATGTGGAAATtagCGCCTTCGAGAATATAATAA |
| S830stop (as) | 5′-TTATATTCTCGAAGGCGctaATTTCCACATAACATCCATC |
| Ampicillin (s) | 5′-ATTCTCAGAATGACTTGGTTGAATACTCACCAG |
| Ampicillin (as) | 5′-TTTCTGTGACTGGTGAGTATTCAACCAAGTC |
Mutated nucleotides are indicated in lowercase bold letters. s, sense primer; as, antisense primer. Primers were used to amplify from pTM1-gH-wt.
Standard confocal assay via laser scanning confocal microscopy.
The steady-state subcellular glycoprotein location was investigated with a standard confocal microscopy assay (19). For this assay, infected or transfected cells were fixed with 2% paraformaldehyde containing 0.05% Triton X-100 for 60 min. After a phosphate-buffered saline (PBS) wash, samples were incubated with the indicated primary antibodies in PBS for 30 min at room temperature. After PBS washing, the samples were incubated with the appropriate fluoroconjugated secondary antibody (diluted 1:1,250) in PBS for 1 h at room temperature under light-protected conditions. Some samples also contained TOTO-3 for nucleic acid staining. After a final PBS wash, the sample coverslips were mounted with Mowiol containing 10% 1,4-diazabicyclo(2, 2,2)octane on glass slides and viewed with a Zeiss 510 laser scanning confocal microscope (Carl Zeiss, Gottingen, Germany).
Endocytosis assay via laser scanning confocal microscopy.
Glycoprotein endocytosis was investigated with immunofluorescence labeling as previously described with some modifications (75). At the appropriate times postinfection, cell monolayers were incubated with the indicated primary antibodies in cold Hanks' balanced salt solution for 30 min at 4°C. After washing with cold Hanks' balanced salt solution, the 0-min time point monolayers were fixed and permeabilized as above. The remaining monolayers were washed and returned to 37°C with warmed medium to allow internalization of glycoprotein-primary antibody complexes. After incubation at 37°C for the specified amount of time, the monolayers were fixed and permeabilized, labeled with fluoroprobes, and mounted as above.
Inhibition of clathrin-coated pit-mediated glycoprotein endocytosis.
Clathrin-coated pit formation was inhibited with either hypertonic medium or monodansylcadaverine (MDC) (Sigma, St. Louis, Mo.). Cells were processed as for the endocytosis assay with the following alterations. To test the specificity of sucrose and MDC, endocytosis of dextran-Texas Red was assayed in the presence of the inhibitors. For hypertonic medium inhibition, warmed 10% minimal essential medium containing 0.3 M sucrose was added during the 37°C internalization step, along with 1 mg of dextran-Texas Red per ml. For chemical inhibition with MDC, samples were pretreated with medium containing 100 μM MDC for 30 min at 37°C. The incubation steps for antibody binding at 4°C and endocytosis at 37°C also contained 100 μM MDC in the medium. Immediately following the 37°C incubation, instead of paraformaldehyde fixation, the samples were returned to 4°C and incubated for 30 min with 20 μl of rabbit anti-Texas Red antibodies per ml to quench any Texas Red dye remaining at the cell surface. Following this incubation, the samples were briefly washed, then fixed and permeabilized. The samples were then labeled with goat anti-mouse immunoglobulin-Alexa 488 to label glycoprotein, mounted, and viewed as described above.
Endocytosis of VZV gH assayed by biotinylation.
To determine whether VZV glycoprotein endocytosis was antibody mediated, internalization of biotinylated surface glycoproteins was assayed. To this end, HeLa cells were transfected with pTM1-gE, pTM1-gE-tailless, pTM1-gH-wt + pTM1-gL, pTM1-gH-Y835A + gL, pTM1-gH-S830stop + gL, pTM1-gI, or pTM1-gB. For VZV gE and gI, one 35-mm well per sample was used, while one 25-cm2 monolayer per sample was used for each gH + gL and gB transfection. At 18 h after transfection, cultures were washed three times with 10 mM borate buffer (pH 8.8) containing 0.1 M NaCl and then overlaid with Sulfo-NHS-SS-biotin (Pierce, Rockford, Ill.) (10, 29, 81) in borate buffer as follows. To a 35-mm well, 7.5 μl of biotin in 1 ml of borate buffer was added, and to a T-25 monolayer, 22.5 μl of biotin in 3 ml of borate buffer was added. After a 20-min incubation at 4°C, the solution was replaced with an identical fresh solution. The reaction was continued for 15 min at 4°C and then washed once with borate buffer. The biotinylation reaction was stopped by addition of 10 mM NH4Cl solution for 20 min at 4°C. The cells were washed four times with 50 mM Tris-HCl (pH 7.2)-0.1 M NaCl, once with cold Hanks' balanced salt solution, and then two times with warm culture medium. Samples were then incubated for 0, 15, 30, or 60 min at 37°C to allow endocytosis of the biotinylated proteins.
To document inhibition of clathrin-mediated endocytosis, one 60-min sample with gE contained 0.3 M sucrose during the 37°C incubation. Internalization was stopped by rapid cooling on ice, and then the cells were washed twice with PBS++ (PBS containing 0.9 mM CaCl2 and 0.5 mM MgCl2, pH 7.4) and incubated twice for 90 min with freshly prepared glutathione buffer (60 mg/ml glutathione [pH 8.0], 83 mM NaCl, 1.1 mM CaCl2, 1.1 mM MgCl2) to remove any remaining biotin label from proteins present at the cell surface. After several washes with PBS++, the cells were harvested; immunoprecipitation was performed with human herpes zoster serum to detect gH, MAb 3B3 for detection of gE, MAb 6B5 for gI, or MAb V1 for gB. After polyacrylamide gel electrophoresis (PAGE) under nonreducing conditions, the internalized biotinylated glycoproteins were detected with streptavidin-horseradish peroxidase by Western blot.
RESULTS
Endocytosis of VZV glycoproteins in VZV-infected cells.
To date, endocytosis of VZV glycoproteins has not been investigated in as much detail in infected cells as under transfection conditions. To characterize endocytosis in infected cells, we applied the endocytosis assay in a manner described previously (77). In the course of these studies, we investigated endocytosis of VZV gE, gB, gI, and gH as it occurred in VZV-infected Mewo cells. To this end, infected monolayers were processed for the immunofluorescence endocytosis assay at 24 h postinfection. At this point, syncytium formation was not heavily advanced; therefore, most syncytia consisted of fewer than 20 fused cells. VZV glycoproteins present on the cell surface were labeled with a monoclonal antibody specific for the glycoprotein of interest.
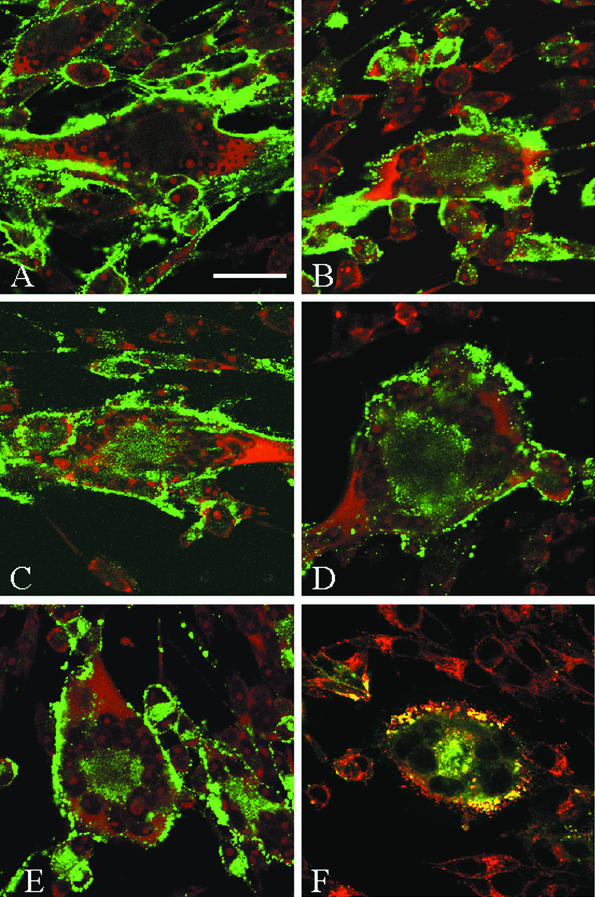
At the beginning of the endocytosis assay, only the glycoproteins on the cell surface were labeled by primary antibody. Samples never returned to 37°C, referred to as 0-min time points, had no glycoprotein visible inside the cell (micrograph representative for all glycoproteins shown in Fig. 1A). After 60 min at 37°C, the glycoproteins found at the surface in the 0-min time point had moved from the plasma membrane to the interior of the syncytia. Shown in Fig. 1 are representative 60-min time points for gE, gB, gI, and gH (Fig. 1B, C, D, and E, respectively). The center of each micrograph had a representative syncytium induced by infection with VZV, surrounded by singly infected cells not yet fused into the syncytium. For each glycoprotein investigated, a green signal was detected at the center of each syncytium, representing glycoprotein that had internalized from the cell surface. A ring of nuclei was visible with the TOTO-3 counterstain. All VZV glycoproteins investigated were internalized in both syncytia and single infected cells.
FIG. 1.
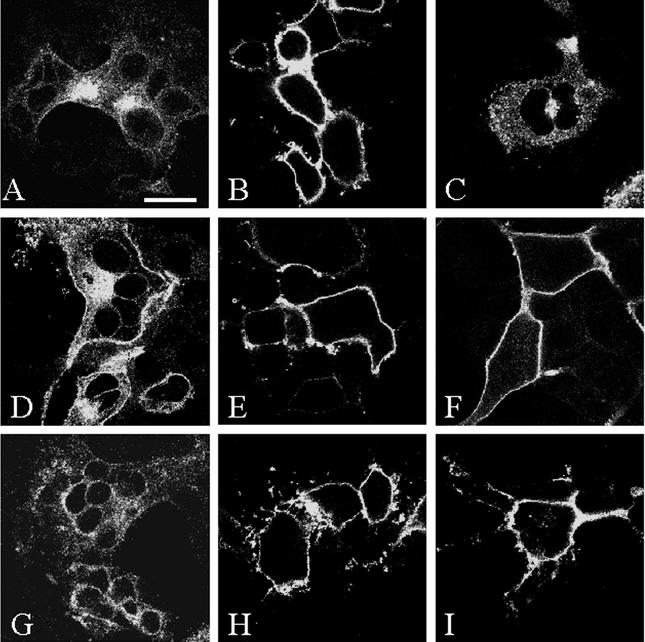
Confocal microscopy analysis of VZV glycoprotein endocytosis in VZV-infected cells. VZV-32-infected Mewo cells were processed for the confocal endocytosis assay as described in the text. A representative micrograph of infected cells was labeled for glycoprotein at 0 min of endocytosis (A). Infected cells were subsequently examined at the 60-min endocytosis time point after labeling with monoclonal antibodies specific for gE (B), gB (C), gI (D), or gH (E and F). The postendocytosis localization of VZV gH was investigated by dual staining with WGA and Texas Red (Fig. 1F). In each of the 60-min samples, labeled glycoprotein was located at the plasma membrane of the syncytia and in a vesicular pattern in the cytoplasm. In panels A, B, C, D, and E, TOTO-3 (red) was used as a counterstain. (A) Bar, 25 μm.
Further inspection of cells revealed that while some glycoprotein-laden vesicles remained near the plasma membrane, most of the internalized glycoproteins were found at the syncytial centers, inside a ring of nuclei formed during syncytium formation. We determined via immunofluorescence microscopy that this region of syncytia contained both endosomal and trans-Golgi network components (data not shown). The postendocytosis localization of VZV gH was characterized by dual labeling with the cellular probe wheat germ agglutinin (WGA), which preferentially labels the trans-Golgi network, trans-Golgi network-derived vesicles, and proteins carrying trans-Golgi network-derived sugar modifications. At the center of the syncytium shown in Fig. 1F, VZV gH (green) and WGA (red) colocalized after 60 min of gH endocytosis, as shown by the yellow signal at the center of the syncytium. Thus, in VZV-infected cells, gH internalized, and a portion of this population of gH trafficked to the trans-Golgi network.
Of importance, the positive result with gH was completely unexpected, the presumption having been that most gH would remain on the cell surface during the endocytosis assay. The pattern of internalized gH was strikingly similar to that of the three VZV glycoproteins with known internalization motifs in their cytoplasmic tails. The apparent endocytosis of gH was a novel finding that we chose to study in further detail.
Time course of VZV gH internalization in infected cells.
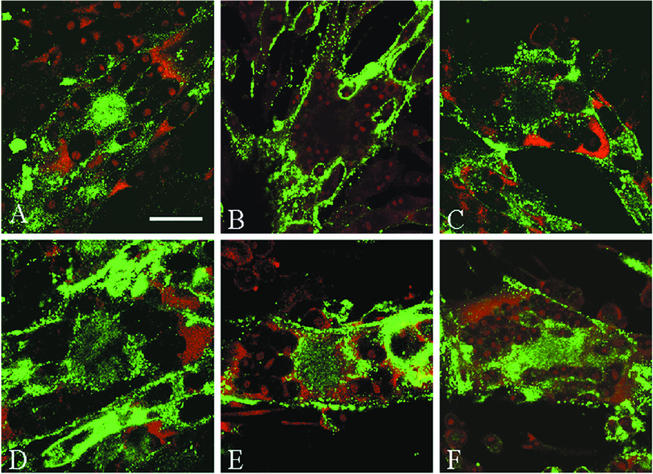
Because gH has not been reported to undergo endocytosis in any herpesvirus system, we considered the hypothesis that the gH protein contained previously unrecognized internalization signals. To initiate this investigation, we first performed a trafficking experiment to determine if the kinetics of gH endocytosis resembled that of the extensively investigated gE protein (32). To this end, gH endocytosis was analyzed after 0, 15, 30, 45, and 60 min at 37°C in VZV-infected cells. The steady-state localization of VZV gH is shown in Fig. 2A. When studied in an endocytosis assay, the 0-min time point had a halo pattern of gH staining (Fig. 2B). However, after 15 min, the accumulation of numerous vesicles was visible inside the cells (Fig. 2C). The number of these vesicles increased until 30 min at 37°C, after which gH appeared to reach a steady state of trafficking. An abundant amount of internalized gH was seen in the 30-, 45-, and 60-min samples (Fig. 2D, E, and F, respectively) in vesicles clustered in the syncytial centers and beneath the plasma membrane. Thus, in VZV-infected cells, gH underwent endocytosis in an efficient and continuous process.
FIG. 2.
Confocal microscopy analysis of VZV gH endocytosis in a time course assay. Mewo cells were infected with VZV-32 and processed as described in the text. VZV gH was labeled with MAb 258 and Alexa 488-fluoroconjugated goat anti-mouse immunoglobulin secondary antibody. The steady-state subcellular location of gH was determined with the standard steady-state confocal assay (A). To plot the time course of gH trafficking in infected cells, micrographs were examined after 0 min (B), 15 min (C), 30 min (D), 45 min (E), or 60 min (F) at 37°C. TOTO-3 (red) was used as a counterstain. (A) Bar, 25 μm.
Endocytosis of gH coexpressed with gL in transfected cells.
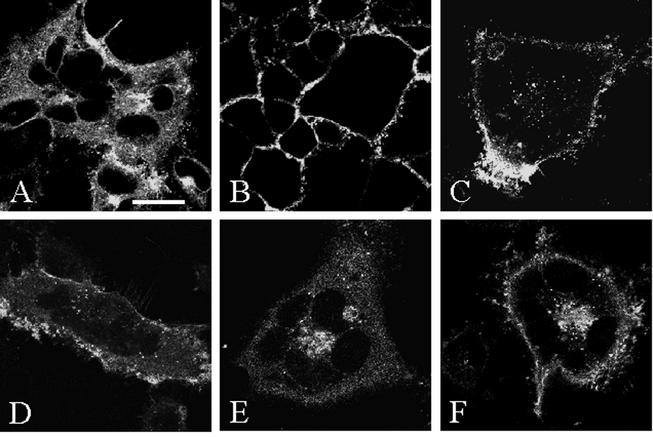
Having confirmed that gH was internalized in an infected cell culture, we postulated that gH endocytosis was not dependent on the endocytosis of gE, gI, or gB. To test this hypothesis, we switched to assays performed with recombinant vaccinia viruses containing the genes of interest. Previous studies have demonstrated that VZV gH requires the presence of gL to traffic to the plasma membrane (22). To determine if gH underwent endocytosis when coexpressed with its chaperone gL, we infected HeLa cells with a recombinant vaccinia virus expressing both gH and gL (VV-gH+gL) to guarantee simultaneous expression of both glycoproteins. In a steady-state assay, VZV gH was found at the cell surface, throughout the cytoplasm, and in the biosynthetic pathway (Fig. 3A). Examination of the 0-min endocytosis time point revealed a halo pattern of staining in which only the glycoproteins at the plasma membrane of infected cells were labeled (Fig. 3B). However, after 15 min at 37°C, vesicles representing gH internalized from the cell surface were visible (Fig. 3C). As the incubation at 37°C progressed, the number of gH vesicles increased, as judged by visual examination of multiple representative micrographs. After 30 min of endocytosis, numerous vesicles were visible throughout the cytoplasm (Fig. 3D). The accumulation of vesicles continued after 45 and 60 min (Fig. 3E and 3F). In short, the confocal micrographs established that once gH reached the plasma membrane, it was internalized in the absence of VZV glycoproteins gE, gI, and gB.
FIG. 3.
Confocal microscopy analysis of gH in VV-gH+gL-infected cells. HeLa cells were infected with VV-gH+gL and processed for the confocal endocytosis assay as described in the text. VZV gH was labeled as for Fig. 2. The steady-state localization of gH was determined with the standard steady-state confocal assay (A). Micrographs are representative of time points after 0 min (B), 15 min (C), 30 min (D), 45 min (E), or 60 min (F) at 37°C. (A) Bar, 25 μm.
VZV gH internalization via a clathrin-coated vesicle-dependent mechanism.
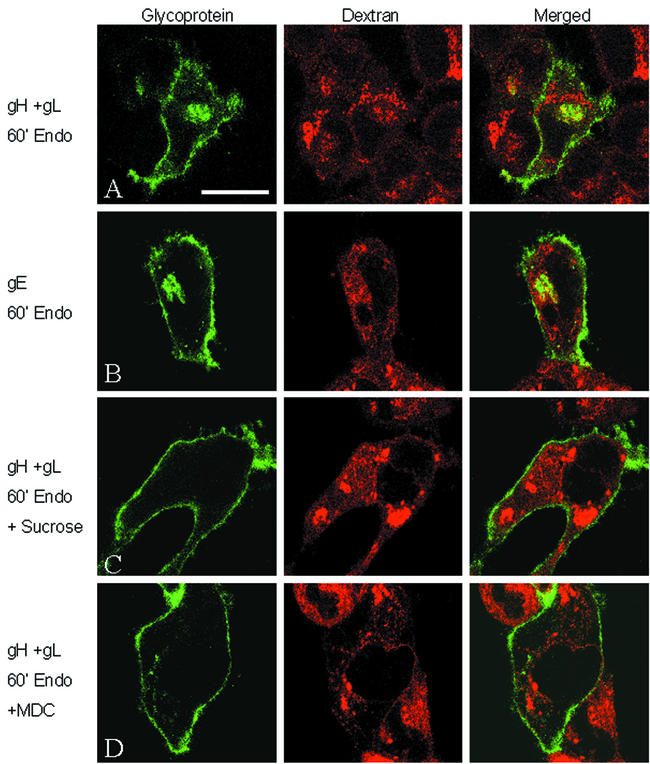
Thus far in our studies, VZV gH trafficked similarly to gE, a glycoprotein that is dependent on clathrin-coated pit formation for endocytosis (77). We therefore postulated that gH requires a similar mechanism. To determine if endocytosis of VZV gH was dependent on a clathrin-mediated step, the endocytosis assay was carried out in the presence of 0.3 M sucrose, an inhibitor of clathrin-mediated endocytosis (14, 43). Clathrin-mediated endocytosis can also be inhibited chemically; MDC inhibits a transglutaminase required for clathrin-mediated endocytosis (15). To test the specificity of the sucrose medium and MDC to inhibit only clathrin-mediated endocytosis, internalization of both VZV gH and dextran-Texas Red was assayed simultaneously. Dextran is internalized via fluid-phase endocytosis, a non-clathrin-mediated mechanism.
The results of this assay are shown in Fig. 4. In the untreated samples, VZV gH (Fig. 4A) and VZV gE (Fig. 4B), both green in Fig. 4, were internalized after 60 min at 37°C, along with the dextran-Texas Red (red). In addition, only limited colocalization of either gH or gE and dextran was observed inside the cells, demonstrating that these proteins were internalized mainly into different organelles. In contrast to the untreated cells, the addition of sucrose inhibited both gH (Fig. 4C) and gE (data not shown [77]) endocytosis without affecting internalization of the dextran. Similarly, addition of MDC to the medium also inhibited both gH (Fig. 4D) and gE (data not shown) internalization without any effect on dextran. Thus, no endocytosis of either gH or gE was observed under conditions that suspended clathrin-mediated endocytosis, while at the same time dextran internalization was not inhibited. The results obtained by this assay were consistent with internalization of VZV gH and gE via a clathrin-mediated mechanism.
FIG. 4.
Role of clathrin in endocytosis of VZV gH. HeLa cells were infected with VV-T7 and then either cotransfected with pTM1-gH-wt and pTM1-gL (A, C, and D) or transfected with pTM1-gE alone (B). Samples were processed for clathrin inhibition and fluid-phase endocytosis with dextran-Texas Red as described in the text. Samples were left untreated (A and B) or treated with 0.3 M sucrose medium (C) or 100 μM MDC (D). In each panel, glycoprotein is labeled green, while the fluid-phase endocytosis tracer dextran-Texas Red is red. (A) Bar, 25 μm.
VZV gH tyrosine-based endocytosis motif.
The fact that gH was capable of endocytosis in both VZV-infected and VV-gH+gL-infected cells supported our hypothesis that the cytoplasmic tail of gH contained a previously unrecognized endocytosis motif. We reexamined the transmembrane (TM) and cytoplasmic domains of gH. First, we analyzed the gH amino acid sequence with the TM domain prediction program SOSUI (http://sosui.proteome.bio.tuat.ac.jp/sosuiframe0.html). SOSUI predicted the presence of the TM domain at amino acids 807 to 829 and an endodomain from 830 to 841 (SRLREYNKIPLT).
Inspection of the gH cytoplasmic tail revealed a putative tyrosine-based motif, YNKI, encompassing amino acids 835 to 838. This motif fit the YXXφ tyrosine-based motif identified in other endocytic proteins (6, 7, 40). To determine if this motif was functional and able to direct internalization of gH, we designed two mutant gH constructs in pTM1 plasmids, similar to gE experiments conducted in this laboratory (76, 77). The first construct, pTM1-gH-Y835A, contained a gH protein in which the tyrosine residue located at position 835 was converted to an alanine. The second construct, pTM1-gH-S830stop, contained a gH protein with a termination codon inserted at serine 830 to create a gH truncation mutant lacking the last 12 amino acids of the cytoplasmic tail. We constructed this second mutant to aid in determining if endocytosis of gH was dependent on an interaction at the ectodomain or if further cytoplasmic tail interactions besides the tyrosine motif occurred.
As shown in Fig. 5, VZV wild-type gH and both gH cytoplasmic tail mutants, when coexpressed with gL in a steady-state assay, demonstrated similar gH expression levels (Fig. 5A, D, and G). In the endocytosis assay at the 0-min time point, each gH protein was found in a halo pattern at the cell surface (Fig. 5B, E, and H). These figures also demonstrated that all forms of gH had similar levels of surface expression. As expected, wild-type gH was found in a vesicular pattern throughout the cytoplasm of transfected cells after 60 min at 37°C (Fig. 5C). In contrast, the results with the gH-Y835A tyrosine mutant (Fig. 5F) and the gH-S830stop truncation mutant (Fig. 5I) demonstrated a markedly diminished level of endocytosis after 60 min at 37°C. Both mutants closely resembled the 0-min time point samples, with only a halo pattern of gH staining and negligible gH endocytosis. These results documented that VZV gH contained a YXXφ endocytosis motif that directed internalization of gH from the plasma membrane.
FIG. 5.
Endocytosis analysis of gH cytoplasmic tail mutants. HeLa cells were infected with VV-T7 and then cotransfected with pTM1-gH-wt (A to C), pTM1-gH-Y835A (D to F), or pTM1-gH-S830stop (G to I), along with pTM1-gL. VZV gH was labeled as described above. Panels A, D, and G were processed with the standard confocal microscopy assay. Each form of gH was examined in the endocytosis assay at 0 min (B, E, and H) and at 60 min (C, F, and I). (A) Bar, 25 μm.
Endocytosis of VZV gH is not dependent on an antibody-mediated mechanism.
Some studies on herpesvirus glycoproteins have addressed the possibility that endocytosis is dependent on an antibody-mediated mechanism. It has been reported that pseudorabies virus gE and gC are not induced to undergo endocytosis by addition of antibodies, while pseudorabies virus gB and gD endocytosis is antibody dependent (20, 21, 92). Human cytomegalovirus gB endocytosis has been demonstrated to be antibody independent (81). In our earlier studies of VZV gE and gI endocytosis, we had not eliminated the possibility that internalization was dependent on the monoclonal antibody used as a glycoprotein-specific probe (76, 77). To resolve the question about the role of antibody in VZV glycoprotein endocytosis, we decided to test VZV gH along with VZV gE, gI, and gB.
For these experiments, we used a biotin endocytosis assay that employed the disulfide-linked NHS-SS-biotin, which permits cleavage of any biotin from the cell surface after the 37°C endocytosis step with glutathione (8, 10, 29, 38, 81, 95). Internalization of biotinylated glycoprotein protects the biotin from cleavage. Thus, this assay provides an unequivocal answer to the question of whether antibody is required for VZV glycoprotein endocytosis. The results of the biotin endocytosis assay are shown in Fig. 6. The intervals for the 37°C incubation and cleavage with glutathione are indicated above the lanes. The 0-min and 60-min steps show total cell surface biotinylation of viral glycoproteins before the glutathione cleavage step. The 0-min+ lane shows a sample in which the monolayer was not returned to 37°C and was instead immediately treated with glutathione; the absence of a glycoprotein band in this sample demonstrated that the cleavage step removed all remaining cell surface biotin to an undetectable level. The 15 min+, 30-min+, and 60-min+ samples were returned to 37°C for the indicated time before the cleavage step. Thus, if the tested glycoproteins were internalized, a band would be detectable in these lanes.
FIG. 6.
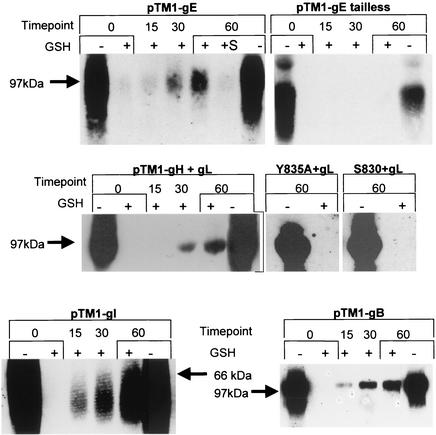
Endocytosis of VZV glycoproteins in a biotin endocytosis assay. HeLa cells were infected with VV-T7 and then transfected with pTM1-gE, pTM1-gE-tailless, pTM1-gH-wt + gL, pTM1-gH-Y835A + gL, pTM1-gH-S830stop + gL, pTM1-gI, or pTM1-gB, as indicated above each set of gels. The samples were processed for the biotinylation endocytosis assay as described in the text. Assays for gE and gI were performed with samples from 35-mm wells, while gH + gL and gB assays were performed with samples from 25-cm2 monolayers. The numbers above each gel indicate the endocytosis time points at 37°C, while the + or − below the numbers indicates glutathione (GSH) treatment. Samples gE, gH, and gB were loaded into 8% gels, while gI was loaded into a 10% gel. Each gel was cropped to show just glycoprotein bands, and the corresponding molecular size marker position is indicated. The experiment with each plasmid was analyzed in the same gel.
We began our assay using gE and, as a control, gE-tailless, which lacks the entire gE cytoplasmic domain. Endocytosis of gE became evident in the 30-min+ lane and continued to accumulate through the 60-min+ time point. Longer exposures of the blot demonstrated that gE endocytosis was evident even at 15 min at 37°C (data not shown). In addition, we added 0.3 M sucrose medium to a 60-min+ gE sample; sucrose was shown in Fig. 4 to inhibit gE endocytosis. Inspection of this lane, labeled +S, revealed that gE was not internalized in the presence of sucrose, further supporting our results in Fig. 4. Repetition of the biotin endocytosis assay with gE-tailless demonstrated that removal of the gE cytoplasmic domain prevented endocytosis of gE, as evidenced by a complete absence of gE bands in the 15-min+, 30-min+, and 60-min+ lanes. With this assay, we confirmed that VZV gE endocytosis was not dependent on the addition of anti-gE antibody to the monolayer.
Once we determined that the biotin endocytosis assay could specifically detect glycoprotein endocytosis, we tested VZV gH in the same assay. When coexpressed with gL, internalized gH was detected at very low levels after 15 min at 37°C, and the levels of internalized gH increased through 30 min and 60 min at 37°C. The efficiency of gH endocytosis from the plasma membrane appeared consistent with levels reported for other surface proteins in similar assays (8, 38, 76, 77, 95). When the biotin endocytosis assay was repeated with gH-Y835A or gH-S830stop coexpressed with gL, no gH endocytosis was detected. In contrast to the results described above for wild-type gH, the endocytosis-deficient forms of gH gave no gH bands in the 60-min+ samples, demonstrating that mutation or removal of the YNKI motif effectively interrupted gH endocytosis. Most importantly, this assay established that gH endocytosis transpired in the absence of glycoprotein-specific antibodies.
To complete this study of VZV glycoprotein endocytosis, we also performed the assay on VZV gI and gB. As we found with gE and gH, VZV gI and gB bands were identified in the 30-min+ and 60-min+ samples, indicating that endocytosis of these glycoproteins was not dependent on an antibody-mediated mechanism. Furthermore, these results supported previous data regarding motif-dependent endocytosis of VZV gI in transfected HeLa cells (76) and VZV gB in transfected Mewo cells (42). In summary, we have provided evidence that the four major VZV glycoproteins internalized from the cell surface in a manner independent of antibody binding. Combining these results with those of previous studies that investigated the functionality of endocytosis motifs in the aforementioned VZV glycoproteins, we concluded that endocytosis of the VZV glycoproteins was not a side effect of antibody binding but rather a specific event in the viral life cycle.
Sequence alignment analysis of cytoplasmic tails of gH homologues.
The discovery of a functional endocytosis motif in the VZV gH cytoplasmic tail prompted us to prepare a compilation of cytoplasmic domain sequences for 16 gH homologues. In a comparison of gH homologues from HSV-1, VZV, cytomegalovirus, Epstein-Barr virus, and herpesvirus saimiri, Gompels et al. (30) reported that on the basis of greatest similarity in the C-terminal region, alphaherpesvirus homologues could be distinguished from those of the beta- and gammaherpesviruses. These authors also noted the short length of the gH cytoplasmic tail domains. In our study, we furthered this comparison by looking at 16 gH homologues and noting not only their unusual lengths but also the presence of trafficking motifs.
As shown in Table 2, our analysis revealed a number of trends. Not one of the homologues had a tail length exceeding 20 amino acids, and the alphaherpesvirus gH cytoplasmic tails were about twice the length of those of the beta- and gammaherpesviruses. Of interest, many gH homologues contained potential endocytosis motifs. Important exceptions were HSV-1 and HSV-2 gHs, the only alphaherpesvirus gHs lacking a putative tyrosine motif. It has been noted that gH of HSV-1 lacks homology in the entire C-terminal third to other gH homologues (30), a deviation that may explain their lack of an endocytosis motif. Except for Epstein-Barr virus, the beta- and gammaherpesviruses also appeared to have motifs. However, the ability of the tyrosine and leucine residues in these tails to interact with the adaptor proteins is questionable, since it has been demonstrated that both motifs require a minimal spanning distance between the TM domain and the critical motif residue. Collawn et al. (12) found that at least seven residues are required for internalization of the transferrin receptor, while Rohrer et al. (84) demonstrated that six residues are sufficient for endocytosis of Lamp-1. This minimal distance also applies for the dileucine motifs (27). Thus, the spanning distance of the beta- and gammaherpesvirus gH cytoplasmic tails may prevent interaction with the adaptor proteins that direct endocytosis. If so, internalization of these homologues would be inhibited. The length parameter would similarly limit recognition of the somewhat conserved ML dileucine-type sequence proximal to the TM domain in the alphaherpesviruses. It remains to be determined which, if any, of the putative motifs in the gH homologues are functional.
TABLE 2.
Comparison of herpesvirus gH homologues: prediction of potential endocytosis motifs in cytoplasmic tail domains and correction of predicted tail lengths for VZV and SVVa
| Virus | Tail length (aa) | Motif |
|---|---|---|
| Alphaherpesvirus subfamily 1 | ||
| HSV-1 | 14 | KVLRTSVPFFWRRE (none predicted) |
| HSV-2 | 14 | RVVRTCVPFLWRRE (none predicted) |
| Alphaherpesvirus subfamily 2 | ||
| VZV | 12 | SRLREYNKIPLT |
| SVV | 16 | LCGSPRNIEYTAVPLV |
| PrV | 19 | KMLCSFSSEGYSRLINARS |
| BHV-1 | 19 | KMLCSSVPLARGYSSVPVF |
| BHV-5 | 19 | KMLCSSVPIARGYSAVPAF |
| EHV-1 | 19 | KMLCGGVTNDGYKLLLSYE |
| EHV-4 | 19 | KMLCGGVINNDYSLLLNSE |
| FHV-1 | 17 | KMLCSFTPDVRYTLLNN |
| Betaherpesvirus family | ||
| HCMV | 6 | RMLKTC |
| HHV-6 | 6 | RLIRLC |
| HHV-7 | 10 | FGIYHVFRLF |
| Gammaherpesvirus family | ||
| EBV | 8 | HKIVMFFL (none predicted) |
| HHV-8 | 8 | YRLFSILY |
| HVS | 8 | YKLFVYLT |
| Corrected cytoplasmic tail domain lengths | ||
| VZV | 18 | WMLCGNSRLREYNKIPLT |
| SVV | 18 | WMLCGSPRNIEYTAVPLV |
Bold letters in tail sequences indicate potential tyrosine- and dileucine-based sequences in the gH homologues. Only the VZV gH endocytosis motif has been proven to be functional. See Results for further details. References: HSV-1 (61, 97); HSV-2 tail prediction based on GenBank sequence (accession no. CAB06746); pseudorabies virus (PrV) (9, 54) and SOSUI prediction; bovine herpesvirus 1 (BHV-1) tail prediction based on a previous sequence (64); BHV-5 (65); equine herpesvirus 1 (EHV-1) tail prediction based on a previous sequence (82); EHV-4 (72); feline herpesvirus 1 (FHV-1) tail prediction based on a previous sequence (57); SVV (80); human cytomegalovirus (HCMV) (13); human herpesvirus 6 (HHV-6) (4, 51); HHV-7 (87); Epstein-Barr virus (EBV) (41); HHV-8 tail prediction based on sequence from GenBank (accession no. AAB08395) (69); herpesvirus saimiri (HVS) (30).
Most notably, our compilation of herpesvirus gH homologues revealed the possibility that the cytoplasmic domains for VZV and simian varicella virus (SVV) are longer than previously predicted. During our comparisons, we observed two distinct differences between VZV and the other alpha-2 subfamily gH homologues. First, the cytoplasmic domain of VZV gH was predicted to be 12 amino acids in length, while the others were generally 19 amino acids long. Second, alpha-2 homologues contained a KMLC sequence immediately proximal to the TM domain; only VZV and SVV lacked this conserved sequence. When we entertained the possibility that the VZV gH tail was longer than previously described, we found that VZV gH contained a WMLC tetrapeptide towards the N terminus. If added to the previous predicted gH tail, the tail length of VZV gH became 18 amino acids in length. The longer tail attained remarkable sequence homology to the other alpha-2 gH tails. Furthermore, if the gH tail has only 12 amino acids, then only five spanning residues would be present, and gH presumably could not be endocytosed. Since we have found that VZV gH is internalized, the cytoplasmic tail most likely contains 18 residues and has a spanning length of 11 residues, a length that fits the requirement of at least 6 amino acids.
We were able to perform the same adjustment to the tail of SVV. Previously, SVV gH was predicted to have a tail length of 16 amino acids (80). However, immediately upstream of the published SVV tail is a WM sequence which, when included, gave SVV gH a tail length of 18 residues. As with VZV gH, addition of these amino acids made the SVV gH cytoplasmic domain remarkably homologous to the gH tails of other alpha-2 subfamily gH proteins. Thus, based on the above discussion, we postulate that both the VZV and SVV gH cytoplasmic domains are 18 amino acids in length.
DISCUSSION
VZV glycoprotein endocytosis.
Thus far, a majority of herpesvirus glycoprotein endocytosis investigations have utilized transfection systems expressing one or two viral proteins. While relevant, transfection systems lack the ability to delineate how the protein will behave when expressed in the infected cell, since all of the components in each viral glycoprotein complex have probably not yet been delineated. Therefore, a detailed investigation to characterize glycoprotein endocytosis is best performed with both transfected and infected cell systems. In this report, we demonstrated that VZV gE, gI, gB, and gH are all capable of undergoing endocytosis in VZV-infected cells. Previous research has demonstrated similar results for gE, gI, and gB only in transfected cell systems (2, 42, 76, 77). The observation that VZV gH underwent endocytosis was a novel and unexpected finding. Furthermore, we discovered that VZV gH, when expressed in a transfection system without the endocytosis-competent glycoproteins gE, gI, and gB (but with gL), was capable of rapid and continuous internalization from the cell surface. In addition, we demonstrated that gH internalized in a clathrin-dependent manner, similar to that of gE, gI, and gB (42, 76, 77). These discoveries prompted us to examine the cytoplasmic domain of VZV gH further.
Cytoplasmic domains of herpesvirus gH homologues contain potential motifs.
In prior cursory examinations of the VZV gH endodomain, we had not considered that a short cytoplasmic tail contained functional motifs. Our studies described herein demonstrated by mutational analysis that the VZV gH cytoplasmic domain contained a tyrosyl residue at position 835 that was critical for endocytosis. This discovery prompted us to evaluate gH homologues across the herpesvirus family and compare the gH cytoplasmic tail to those of well-characterized cellular and viral proteins. Our analysis of 16 gH homologue cytoplasmic tails suggested that VZV gH might not be the only homologue capable of endocytosis. As mentioned above in Results, many of the alphaherpesvirus gH homologues contained a potential tyrosine-based endocytosis motif (Table 2). In contrast, the beta- and gammaherpesvirus homologues contained potential motifs but with an inappropriate context for endocytosis. In turn, this observation raises a question about the precise length of the cytoplasmic domains for many gH homologues. With VZV gH as a critical example, we found that we could adjust the definition of the gH tail to 18 residues, WMLCGNSRLREYNKIPLT. It is also possible that the predicted cytoplasmic domains of some beta- and gammaherpesvirus gH homologues may be incorrect. For instance, human cytomegalovirus gH contains a YRML sequence positioned immediately at the predicted TM-cytoplasmic tail interface (13). When we looked at the hydrophobic residues in the N terminus of this human cytomegalovirus motif, we suggested the possibility that the TM alpha helix could be shifted by at least seven residues to allow a functional internalization motif C-terminal to the TM domain.
A review of the literature regarding cytoplasmic tails revealed that the length of the VZV gH cytoplasmic domain was indeed unusual. The vast majority of surface proteins that undergo endocytosis have cytoplasmic tails with at least 35 amino acids. The longest is the epidermal growth factor receptor, having 542 residues (93). “Short” cytoplasmic tails are generally regarded as less than 35 amino acids (7). The shortest of all are found in the lysosome-targeted proteins. They have endodomains of 10 to 11 residues, with a tyrosine-based YXXφ motif at the extreme end of the cytoplasmic domain, which augments AP interactions with the motif (17, 73). Thus, lysosomal proteins fit the TM domain-to-motif distance requirements discussed above. As shown in Table 3, VZV gH was similar to the lysosome-targeted proteins in that both contained short cytoplasmic tails and tyrosine-based endocytosis motifs. Also in Table 3, we compared the original and corrected versions of the VZV gH tail. As shown, the original predicted sequence did not have sufficient spanning residues, while the corrected version does. It must be noted, however, that even though we compared VZV gH to lysosomal proteins, we do not predict lysosomal targeting of gH, since lysosome proteins contain an additional glycine residue implicated in lysosome-specific sorting (45).
TABLE 3.
Comparison of short cytoplasmic domains and spanning distancesa
| Protein | Motif |
|---|---|
| Endolyn | TM -9- YHTL - COOH |
| Lamp-1 | TM -6- GYQTI - COOH |
| Lamp-2 | TM -6- GYEQF - COOH |
| LAP | TM -6- PGYRHV -7- COOH |
| Limp-I | TM -6- GYEVM - COOH |
| Limp-II (rat) | TM -12- DERAPLI -2- COOH |
| VZV gH | TM -5- YNKI -3- COOH |
| VZV gH (corrected) | TM -11- YNKI -3- COOH |
Numbers within the sequences indicate the number of amino acids between the motifs and the TM domain and between the motif and the C-terminus. Endocytosis motifs are indicated in bold. The non-VZV proteins are a lysosomally targeted (reviewed in reference 17). The endolyn, Lamp-1, Lamp-2, and Limp-1 motifs occur at the extreme end of the cytoplasmic tail. Limp-II requires the presence of acidic residues upstream of the dileucine motif for proper lysosomal targeting (90). References: endolyn (47); Lamp-1 (36, 96); Lamp-2 (11, 31); LAP (lysosomal acid phosphatase) (55, 79); Limp-I (44, 63); Limp-II (25, 90).
Role of viral glycoprotein endocytosis in herpesviruses.
Based on our results with VZV gH, we propose that most other alphaherpesvirus gH homologues are also endocytosis competent. HSV-1 and HSV-2 gHs were notable exceptions in our study of gH homologues, as they lacked both a YXXφ and a dileucine motif. We propose three possibilities for why HSV gH does not require this motif. First, HSV gH in an infected cell forms a complex with an endocytosis-competent HSV protein such as gB. Second, HSV gH contains an unconventional or as yet unrecognized endocytosis motif. And third, the population of HSV glycoprotein that reaches the plasma membrane is separate from the population incorporated into progeny virions.
Since we have demonstrated that antibody binding is not the sole mechanism by which VZV glycoproteins are internalized, we assume that there is a role for endocytosis in the viral life cycle. One possibility is that endocytosis regulates the amount of gH on the cell surface. VZV is a highly fusogenic virus, both in the infected human and in cell culture, more fusogenic than HSV-1, HSV-2, or pseudorabies virus. In the VZV system, gH is a major fusogen (19). Thus, increasing the surface density of gH on the cell surface would presumably lead to increased fusion. Increased surface density and cell-to-cell fusion by a retroviral glycoprotein fusogen has been demonstrated previously with simian immunodeficiency virus after mutation of the endocytosis motif in the transmembrane protein (86).
A second possibility is that endocytosis targets the glycoprotein to the trans-Golgi network, the proposed site of viral assembly (28). A recent report by Fraile-Ramos et al. found that human cytomegalovirus UL33, US27, and US28 trafficking most likely plays a role in targeting these proteins to the organelle of virion assembly (23, 24). For human cytomegalovirus gB, it has been demonstrated that surface-biotinylated human cytomegalovirus gB was internalized, targeted to the trans-Golgi network, and incorporated into progeny virions (81). However, inhibition of gB endocytosis had no effect on virus release or production of infectious virions (49, 81). These data, considered together with the observation that pseudorabies virus gE endocytosis is not required for incorporation (92) and the fact that HSV gH lacks an apparent motif, bring into question the role of viral glycoprotein endocytosis in the herpesvirus life cycle. Yet again, the point must be emphasized that VZV has the smallest genome and the fewest glycoproteins among the alphaherpesviruses studied (16). Thus, VZV may not have alternative pathways for viral glycoprotein egress and viral assembly.
Endocytosis of gH is antibody independent.
In this study, we found that all four major VZV glycoproteins were capable of endocytosis in an antibody-independent method, as shown by biotinylation assays. The results with VZV gE and gB are in agreement with those of pseudorabies virus gE and human cytomegalovirus gB (81, 92). However, our results with VZV gB were in conflict with the results reported for pseudorabies virus gB in monocytes (20, 21). The pseudorabies virus investigators proposed a role for antibody-mediated endocytosis in immune evasion. Binding of viral glycoprotein-specific antibodies to surface glycoproteins followed by internalization of the bound antibodies, in theory, would decrease the host's immune response against the infected cell. Another possibility, one that does not give specific advantage to the virus, is that cross-linking of surface glycoproteins forms aggregates that are internalized in a cellular mechanism that removes clustered membrane proteins from the cell surface. However, these mechanisms do not appear relevant to the VZV life cycle in a cultured cell system.
Acknowledgments
This research was supported by NIH grants AI22795 and AI53846. L.M. was supported by a fellowship award from the VZV Research Foundation, New York, N.Y.
Microscopy was performed at the University of Iowa CMRF.
REFERENCES
- 1.Alconada, A., U. Bauer, L. Baudoux, J. Piette, and B. Hoflack. 1998. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J. Biol. Chem. 273:13430-13436. [DOI] [PubMed] [Google Scholar]
- 2.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 3.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, R. A., D. X. Liu, and U. A. Gompels. 1996. Definition of a human herpesvirus-6 betaherpesvirus-specific domain in glycoprotein gH that governs interaction with glycoprotein gL: substitution of human cytomegalovirus glycoproteins permits group-specific complex formation. Virology 217:517-526. [DOI] [PubMed] [Google Scholar]
- 5.Babic, N., B. G. Klupp, B. Makoschey, A. Karger, A. Flamand, and T. C. Mettenleiter. 1996. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J. Gen. Virol. 77:2277-2285. [DOI] [PubMed] [Google Scholar]
- 6.Boll, W., H. Ohno, Z. Songyang, I. Rapoport, L. C. Cantley, J. S. Bonifacino, and T. Kirchhausen. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15:5789-5795. [PMC free article] [PubMed] [Google Scholar]
- 7.Bonifacino, J. S., and E. C. Dell'Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bretscher, M. S., and R. Lutter. 1988. A new method for detecting endocytosed proteins. EMBO J. 7:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 10.Busch, G., D. Hoder, W. Reutter, and R. Tauber. 1989. Selective isolation of individual cell surface proteins from tissue culture cells by a cleavable biotin label. Eur. J. Cell Biol. 50:257-262. [PubMed] [Google Scholar]
- 11.Cha, Y., S. M. Holland, and J. T. August. 1990. The cDNA sequence of mouse LAMP-2. Evidence for two classes of lysosomal membrane glycoproteins. J. Biol. Chem. 265:5008-5013. [PubMed] [Google Scholar]
- 12.Collawn, J. F., M. Stangel, L. A. Kuhn, V. Esekogwu, S. Q. Jing, I. S. Trowbridge, and J. A. Tainer. 1990. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 63:1061-1072. [DOI] [PubMed] [Google Scholar]
- 13.Cranage, M. P., G. L. Smith, S. E. Bell, H. Hart, C. Brown, A. T. Bankier, P. Tomlinson, B. G. Barrell, and T. C. Minson. 1988. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J. Virol. 62:1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daukas, G., and S. H. Zigmond. 1985. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J. Cell Biol. 101:1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies, P. J., D. R. Davies, A. Levitzki, F. R. Maxfield, P. Milhaud, M. C. Willingham, and I. H. Pastan. 1980. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature 283:162-167. [DOI] [PubMed] [Google Scholar]
- 16.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 17.Dell'Angelica, E. C., and G. S. Payne. 2001. Intracellular cycling of lysosomal enzyme receptors: cytoplasmic tails' tales. Cell 106:395-398. [DOI] [PubMed] [Google Scholar]
- 18.Duus, K. M., and C. Grose. 1996. Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH. J. Virol. 70:8961-8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duus, K. M., C. Hatfield, and C. Grose. 1995. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology 210:429-440. [DOI] [PubMed] [Google Scholar]
- 20.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, and M. B. Pensaert. 2000. Role of anti-gB and -gD antibodies in antibody-induced endocytosis of viral and cellular cell surface glycoproteins expressed on pseudorabies virus-infected monocytes. Virology 267:151-158. [DOI] [PubMed] [Google Scholar]
- 21.Favoreel, H. W., G. Van Minnebruggen, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forghani, B., L. Ni, and C. Grose. 1994. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology 199:458-462. [DOI] [PubMed] [Google Scholar]
- 23.Fraile-Ramos, A., T. N. Kledal, A. Pelchen-Matthews, K. Bowers, T. W. Schwartz, and M. Marsh. 2001. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 12:1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraile-Ramos, A., A. Pelchen-Matthews, T. N. Kledal, H. Browne, T. W. Schwartz, and M. Marsh. 2002. Localization of human cytomegalovirus UL33 and US27 in endocytic compartments and viral membranes. Traffic 3:218-232. [DOI] [PubMed] [Google Scholar]
- 25.Fujita, H., M. Saeki, K. Yasunaga, T. Ueda, T. Imoto, and M. Himeno. 1999. In vitro binding study of adaptor protein complex (AP-1) to lysosomal targeting motif (LI-motif). Biochem. Biophys. Res. Commun. 255:54-58. [DOI] [PubMed] [Google Scholar]
- 26.Fuller, A. O., R. E. Santos, and P. G. Spear. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J. Virol. 63:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisler, C., J. Dietrich, B. L. Nielsen, J. Kastrup, J. P. Lauritsen, N. Odum, and M. D. Christensen. 1998. Leucine-based receptor sorting motifs are dependent on the spacing relative to the plasma membrane. J. Biol. Chem. 273:21316-21323. [DOI] [PubMed] [Google Scholar]
- 28.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghebrehiwet, B., S. Bossone, A. Erdei, and K. B. Reid. 1988. Reversible biotinylation of C1q with a cleavable biotinyl derivative. Application in C1q receptor (C1qR) purification. J. Immunol. Methods 110:251-260. [DOI] [PubMed] [Google Scholar]
- 30.Gompels, U. A., M. A. Craxton, and R. W. Honess. 1988. Conservation of glycoprotein H (gH) in herpesviruses: nucleotide sequence of the gH gene from herpesvirus saimiri. J. Gen. Virol. 69:2819-2829. [DOI] [PubMed] [Google Scholar]
- 31.Granger, B. L., S. A. Green, C. A. Gabel, C. L. Howe, I. Mellman, and A. Helenius. 1990. Characterization and cloning of lgp110, a lysosomal membrane glycoprotein from mouse and rat cells. J. Biol. Chem. 265:12036-12043. [PubMed] [Google Scholar]
- 32.Grose, C. 2001. The predominant varicella-zoster virus gE and gI glycoprotein complex, p. 183-211. In E. Bogner and A. Holzenburg (ed.), Structure-function relationships of human pathogenic viruses. Kluwer Academic Press, London, United Kingdom.
- 33.Grose, C., and P. A. Brunel. 1978. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32°C. Infect. Immun. 19:199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grose, C., D. P. Edwards, W. E. Friedrichs, K. A. Weigle, and W. L. McGuire. 1983. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect. Immun. 40:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grose, C., D. M. Perrotta, P. A. Brunell, and G. C. Smith. 1979. Cell-free varicella-zoster virus in cultured human melanoma cells. J. Gen. Virol. 43:15-27. [DOI] [PubMed] [Google Scholar]
- 36.Guarnieri, F. G., L. M. Arterburn, M. B. Penno, Y. Cha, and J. T. August. 1993. The motif Tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J. Biol. Chem. 268:1941-1946. [PubMed] [Google Scholar]
- 37.Haddad, R. S., and L. M. Hutt-Fletcher. 1989. Depletion of glycoprotein gp85 from virosomes made with Epstein-Barr virus proteins abolishes their ability to fuse with virus receptor-bearing cells. J. Virol. 63:4998-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harter, C., and I. Mellman. 1992. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J. Cell Biol. 117:311-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatfield, C., K. M. Duus, J. Chen, D. H. Jones, and C. Grose. 1997. Epitope mapping and tagging by recombination PCR mutagenesis. BioTechniques 22:332-337. [DOI] [PubMed] [Google Scholar]
- 40.Heilker, R., M. Spiess, and P. Crottet. 1999. Recognition of sorting signals by clathrin adaptors. Bioessays 21:558-567. [DOI] [PubMed] [Google Scholar]
- 41.Heineman, T., M. Gong, J. Sample, and E. Kieff. 1988. Identification of the Epstein-Barr virus gp85 gene. J. Virol. 62:1101-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heineman, T. C., and S. L. Hall. 2001. VZV gB endocytosis and Golgi localization are mediated by YXXΦ motifs in its cytoplasmic domain. Virology 285:42-49. [DOI] [PubMed] [Google Scholar]
- 43.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotta, H., A. H. Ross, K. Huebner, M. Isobe, S. Wendeborn, M. V. Chao, R. P. Ricciardi, Y. Tsujimoto, C. M. Croce, and H. Koprowski. 1988. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 48:2955-2962. [PubMed] [Google Scholar]
- 45.Hunziker, W., and H. J. Geuze. 1996. Intracellular trafficking of lysosomal membrane proteins. Bioessays 18:379-389. [DOI] [PubMed] [Google Scholar]
- 46.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihrke, G., S. R. Gray, and J. P. Luzio. 2000. Endolyn is a mucin-like type I membrane protein targeted to lysosomes by its cytoplasmic tail. Biochem. J. 345 Pt 2:287-296. [PMC free article] [PubMed] [Google Scholar]
- 48.Jadot, M., W. M. Canfield, W. Gregory, and S. Kornfeld. 1992. Characterization of the signal for rapid internalization of the bovine mannose 6-phosphate/insulin-like growth factor-II receptor. J. Biol. Chem. 267:11069-11077. [PubMed] [Google Scholar]
- 49.Jarvis, M. A., K. N. Fish, C. Soderberg-Naucler, D. N. Streblow, H. L. Meyers, G. Thomas, and J. A. Nelson. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones, D. H., and B. H. Howard. 1991. A rapid method for recombination and site-specific mutagenesis by placing homologous ends on DNA using polymerase chain reaction. BioTechniques 10:62-66. [PubMed] [Google Scholar]
- 51.Josephs, S. F., D. V. Ablashi, S. Z. Salahuddin, L. L. Jagodzinski, F. Wong-Staal, and R. C. Gallo. 1991. Identification of the human herpesvirus 6 glycoprotein H and putative large tegument protein genes. J. Virol. 65:5597-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (human cytomegalovirus) forms a stable complex with the human cytomegalovirus UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 53.Klupp, B. G., J. Baumeister, A. Karger, N. Visser, and T. C. Mettenleiter. 1994. Identification and characterization of a novel structural glycoprotein in pseudorabies virus, gL. J. Virol. 68:3868-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klupp, B. G., and T. C. Mettenleiter. 1991. Sequence and expression of the glycoprotein gH gene of pseudorabies virus. Virology 182:732-741. [DOI] [PubMed] [Google Scholar]
- 55.Lehmann, L. E., W. Eberle, S. Krull, V. Prill, B. Schmidt, C. Sander, K. von Figura, and C. Peters. 1992. The internalization signal in the cytoplasmic tail of lysosomal acid phosphatase consists of the hexapeptide PGYRHV. EMBO J. 11:4391-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu, D. X., U. A. Gompels, L. Foa-Tomasi, and G. Campadelli-Fiume. 1993. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology 197:12-22. [DOI] [PubMed] [Google Scholar]
- 57.Maeda, K., Y. Kawaguchi, N. Kamiya, M. Ono, Y. Tohya, C. Kai, and T. Mikami. 1993. Identification and nucleotide sequence of a gene in feline herpesvirus type 1 homologous to the herpes simplex virus gene encoding the glycoprotein H. Arch. Virol. 132:183-191. [DOI] [PubMed] [Google Scholar]
- 58.Maresova, L., L. Kutinova, V. Ludvikova, R. Zak, M. Mares, and S. Nemeckova. 2000. Characterization of interaction of gH and gL glycoproteins of varicella-zoster virus: their processing and trafficking. J. Gen. Virol. 81:1545-1552. [DOI] [PubMed] [Google Scholar]
- 59.Maresova, L., T. J. Pasieka, and C. Grose. 2001. Varicella-zoster virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 75:9483-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maresova, L., T. J. Pasieka, W. Jackson, and C. Grose. Identification of the authentic varicella-zoster virus gB (gene 31) initiating methionine overlapping the 3′ end of gene 30. J. Med. Virol., in press. [DOI] [PubMed]
- 61.McGeoch, D. J., and A. J. Davison. 1986. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 14:4281-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGeoch, D. J., and A. J. Davison. 1999. The Molecular Evolutionary History of the Herpesviruses, p. 441-466. In E. Domingo, R. G. Webster, and J. J. Holland (ed.), Origin and evolution of viruses. Academic Press, London, United Kingdom.
- 63.Metzelaar, M. J., P. L. Wijngaard, P. J. Peters, J. J. Sixma, H. K. Nieuwenhuis, and H. C. Clevers. 1991. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J. Biol. Chem. 266:3239-3245. [PubMed] [Google Scholar]
- 64.Meyer, A. L., E. A. Petrovskis, W. P. Duffus, D. R. Thomsen, and L. E. Post. 1991. Cloning and sequence of an infectious bovine rhinotracheitis virus (BHV-1) gene homologous to glycoprotein H of herpes simplex virus. Biochim. Biophys. Acta 1090:267-269. [DOI] [PubMed] [Google Scholar]
- 65.Meyer, G., O. Bare, and E. Thiry. 1999. Identification and characterization of bovine herpesvirus type 5 glycoprotein H gene and gene products. J. Gen. Virol. 80:2849-2859. [DOI] [PubMed] [Google Scholar]
- 66.Molesworth, S. J., C. M. Lake, C. M. Borza, S. M. Turk, and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J. Virol. 74:6324-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montalvo, E. A., and C. Grose. 1986. Neutralization epitope of varicella zoster virus on native viral glycoprotein gp118 (VZV glycoprotein gpIII). Virology 149:230-241. [DOI] [PubMed] [Google Scholar]
- 68.Montalvo, E. A., R. T. Parmley, and C. Grose. 1985. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J. Virol. 53:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcoma. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. Product review. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 71.Mukai, T., A. Hata, Y. Isegawa, and K. Yamanishi. 1997. Characterization of glycoprotein H and L of human herpesvirus 7. Microbiol. Immunol. 41:43-50. [DOI] [PubMed] [Google Scholar]
- 72.Nicolson, L., A. A. Cullinane, and D. E. Onions. 1990. The nucleotide sequence of an equine herpesvirus 4 gene homologue of the herpes simplex virus 1 glycoprotein H gene. J. Gen. Virol. 71:1793-1800. [DOI] [PubMed] [Google Scholar]
- 73.Ohno, H., M. C. Fournier, G. Poy, and J. S. Bonifacino. 1996. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 271:29009-29015. [DOI] [PubMed] [Google Scholar]
- 74.Ohno, H., J. Stewart, M. C. Fournier, H. Bosshart, I. Rhee, S. Miyatake, T. Saito, A. Gallusser, T. Kirchhausen, and J. S. Bonifacino. 1995. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269:1872-1875. [DOI] [PubMed] [Google Scholar]
- 75.Olson, J. K., G. A. Bishop, and C. Grose. 1997. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J. Virol. 71:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olson, J. K., and C. Grose. 1998. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J. Virol. 72:1542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peeters, B., N. de Wind, R. Broer, A. Gielkens, and R. Moormann. 1992. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J. Virol. 66:3888-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters, C., M. Braun, B. Weber, M. Wendland, B. Schmidt, R. Pohlmann, A. Waheed, and K. von Figura. 1990. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 9:3497-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pumphrey, C. Y., and W. L. Gray. 1995. DNA sequence of the simian varicella virus (SVV) gH gene and analysis of the SVV and varicella zoster virus gH transcripts. Virus Res. 38:55-70. [DOI] [PubMed] [Google Scholar]
- 81.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hubinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 82.Robertson, G. R., N. A. Scott, J. M. Miller, M. Sabine, M. Zheng, C. W. Bell, and J. M. Whalley. 1991. Sequence characteristics of a gene in equine herpesvirus 1 homologous to glycoprotein H of herpes simplex virus. DNA Seq. 1:241-249. [DOI] [PubMed] [Google Scholar]
- 83.Rodriguez, J. E., T. Moninger, and C. Grose. 1993. Entry and egress of varicella virus blocked by same anti-gH monoclonal antibody. Virology 196:840-844. [DOI] [PubMed] [Google Scholar]
- 84.Rohrer, J., A. Schweizer, D. Russell, and S. Kornfeld. 1996. The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J. Cell Biol. 132:565-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santos, R. A., J. A. Padilla, C. Hatfield, and C. Grose. 1998. Antigenic variation of varicella zoster virus Fc receptor gE: loss of a major B cell epitope in the ectodomain. Virology 249:21-31. [DOI] [PubMed] [Google Scholar]
- 86.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Secchiero, P., Z. N. Berneman, D. Sun, J. Nicholas, and M. S. Reitz, Jr. 1997. Identification of envelope glycoproteins H and B homologues of human herpesvirus 7. Intervirology 40:22-32. [DOI] [PubMed] [Google Scholar]
- 88.Spaete, R. R., K. Perot, P. I. Scott, J. A. Nelson, M. F. Stinski, and C. Pachl. 1993. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology 193:853-861. [DOI] [PubMed] [Google Scholar]
- 89.Sugano, T., T. Tomiyama, Y. Matsumoto, S. Sasaki, T. Kimura, B. Forghani, and Y. Masuho. 1991. A human monoclonal antibody against varicella-zoster virus glycoprotein III. J. Gen. Virol. 72:2065-2073. [DOI] [PubMed] [Google Scholar]
- 90.Tabuchi, N., K. Akasaki, and H. Tsuji. 2000. Two acidic amino acid residues, Asp(470) and Glu(471), contained in the carboxyl cytoplasmic tail of a major lysosomal membrane protein, LGP85/LIMP II, are important for its accumulation in secondary lysosomes. Biochem. Biophys. Res. Commun. 270:557-563. [DOI] [PubMed] [Google Scholar]
- 91.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ullrich, A., L. Coussens, J. S. Hayflick, T. J. Dull, A. Gray, A. W. Tam, J. Lee, Y. Yarden, T. A. Libermann, J. Schlessinger, et al. 1984. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309:418-425. [DOI] [PubMed] [Google Scholar]
- 94.Wang, Z., M. D. Gershon, O. Lungu, C. A. Panagiotidis, Z. Zhu, Y. Hao, and A. A. Gershon. 1998. Intracellular transport of varicella-zoster glycoproteins. J Infect. Dis. 178(Suppl. 1):S7-S12. [DOI] [PubMed] [Google Scholar]
- 95.Weixel, K., and N. A. Bradbury. 2002. Analysis of CFTR endocytosis by cell surface biotinylation. Methods Mol. Med. 70:323-340. [DOI] [PubMed] [Google Scholar]
- 96.Williams, M. A., and M. Fukuda. 1990. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J. Cell Biol. 111:955-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson, D. W., N. Davis-Poynter, and A. C. Minson. 1994. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J. Virol. 68:6985-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yao, Z., and C. Grose. 1994. Unusual phosphorylation sequence in the gpIV (gI) component of the varicella-zoster virus gpI-gpIV glycoprotein complex (VZV gE-gI complex). J. Virol. 68:4204-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao, Z., W. Jackson, B. Forghani, and C. Grose. 1993. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J. Virol. 67:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao, Z., W. Jackson, and C. Grose. 1993. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J. Virol. 67:4464-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yao, Z., D. H. Jones, and C. Grose. 1992. Site-directed mutagenesis of herpesvirus glycoprotein phosphorylation sites by recombination polymerase chain reaction. PCR Methods Appl. 1:205-207. [DOI] [PubMed] [Google Scholar]
- 102.Yaswen, L. R., E. B. Stephens, L. C. Davenport, and L. M. Hutt-Fletcher. 1993. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology 195:387-396. [DOI] [PubMed] [Google Scholar]