Abstract
The oxygen requirement for chromophore formation potentially limits the use of green fluorescent protein as a reporter under hypoxic conditions. In the light of this, the applicability of a hypoxia-responsive enhanced green fluorescent protein (EGFP)-based system to the measurement of tumor hypoxia was tested in human HT 1080 fibrosarcoma cells stably transfected with a destabilized EGFP vector containing the hypoxia-responsive 5HRE-hCMVmp promoter or, as a positive control, the strong constitutive CMV promoter. After various schedules of hypoxia and reoxygenation, EGFP fluorescence of live cells was assessed by flow cytometry, and protein levels were analyzed by Western blot. Fluorescence of CMV promoter positive control cells dropped to 38±5% of aerobic levels after 12 hours at <0.02% oxygen, but was unaffected by higher oxygen concentrations. Following 12 hours at <0.02% oxygen, cells transfected with the hypoxia-responsive vector exhibited maximum fluorescence after 4 hours of subsequent reoxygenation, reaching 68±2% of the levels in CMV promoter controls under aerobic conditions. With such reoxygenation, these cells exhibited a constant increase in fluorescence between 2% and <0.02% oxygen. EGFP chromophore formation is only affected by near-anoxic oxygen concentrations. The correlation of fluorescence and oxygen concentration is restored by a 4-hour reoxygenation period due to oxidation of pre-synthesized EGFP and a delayed increase in EGFP protein synthesis.
Keywords: green fluorescent protein, hypoxia, transfection, reoxygenation, fibrosarcoma
Introduction
The cloning of the green fluorescent protein (GFP) gene from Aequorea victoria and the subsequent development of mutants with characteristics optimized for various applications have made GFP a powerful reporter for a wide range of experimental designs [1–3]. The only fundamental limitation to the widespread applicability of GFP has been the requirement for molecular oxygen to dehydrogenate the α,β bond of residue 66, which is necessary for fluorescence [3]. This oxidation step is also the slowest step in GFP chromophore formation with a time constant of 2 to 4 hours in vivo for wild-type GFP [4,5]. While it is assumed that GFP cannot be expressed in obligate anaerobes [3], it is unclear as to what extent low oxygen concentrations affect chromophore formation in mammalian cells.
The assessment of hypoxia and, potentially, the selection of live cells based on oxygenation status are of great interest for several fields of biomedical research, including organ transplantation [6] and, in particular, oncology: In recent years, tumor hypoxia has been shown to be associated with poor clinical outcome in several tumor sites after radiotherapy and chemotherapy [7–9]. Whereas bioreductive markers such as NITP, EF5, and pimonidazole have been used extensively in tissue sections and single cell suspensions to estimate the oxygenation status of experimental and human tumors [10–14], these methods require fixation of the respective tissue or cells and make further analysis of live cells impossible. An experimental system using GFP as a marker of hypoxia would permit selection and further analysis of live aerobic and hypoxic cells, e.g., in clonogenic assays after in situ treatment. Here we describe studies to examine the extent to which the requirement for molecular oxygen by GFP interferes with accurate assessment of hypoxia in cells transfected with a vector containing a hypoxia-responsive promoter, and ways in which the limitations may be overcome. We show that GFP chromophore formation is only impaired at near-anoxic conditions (<0.06%), and even this limitation can be overcome by a 4-hour reoxygenation period after hypoxia. With such reoxygenation, the hypoxia-responsive system is shown to exhibit a constant increase in fluorescence with decreasing O2 concentrations below 2%.
Materials and Methods
Plasmid Construction
The methods for construction of a 5HRE-hCMVmp promoter, consisting of five copies of a 35-bp fragment from the hypoxia-responsive element (HRE) of the human VEGF gene and a human cytomegalovirus minimal promoter (hCMVmp), have been previously described for the construction of a luciferase reporter vector [15]. In the present study, the d2EGFP fragment from a Clontech (Palo Alto, CA) vector, encoding a destabilized red- shifted variant of wild-type GFP with an excitation maximum of 488 nm, an emission maximum of 507 nm, and a half-life of approximately 2 hours, was obtained by PCR using elongase enzyme mixture (Gibco BRL, Gaithersburg, MD) to create NcoI-NotI sites at the ends. The cDNA for the d2EGFP fragment was digested and subcloned at NcoI-NotI sites of a pEF/myc/cyto vector (Invitrogen, Carlsbad, CA) to make a C-terminal fusion with a c-myc epitope and checked by sequencing. To generate a hypoxia- inducible expression of the d2EGFP gene, the enhancer/promoter sequence digested by KpnI and NcoI from a 5HRE-hCMVmp vector was inserted into the pEF/myc/cyto vector containing d2EGFP. A positive control vector was constructed by insertion of the strong constitutive CMV promoter into the d2EGFP vector.
Cell Culture and Transfection
The human HT 1080 human fibrosarcoma line was obtained from the American Type Culture Collection (Rockville, MD). Cells were cultured on glass Petri dishes in α-MEM with 10% FCS at 37°C with 5% CO2 in a well-humidified incubator. The 5HRE-hCMVmp-d2EGFP and CMV-d2EGFP plasmids, respectively, were stably transfected into tumor cells using a 3-hour incubation with Superfect reagent (Qiagen, Valencia, CA) and subsequent clonal selection in G-418 (400 µg/ml) as described previously [16]. To obtain a sufficient number of cells for the hypoxia experiments, wild type and both types of transfected cells were maintained in culture under non- selective conditions for 3 weeks. Portions of 2x106 cells in 1 ml DMSO/α-MEM (1:10) per vial were frozen at -80°C.
Hypoxia Treatment
All experiments were performed on exponentially growing cells from frozen stock with one further passage after plating. Before each hypoxia treatment, the medium was changed. The hypoxic marker EF5 (a generous gift of C. Koch, University of Pennsylvania, Philadelphia, PA) was added at a final concentration of 100 µM if additional labeling was desired. Hypoxia was achieved by a series of gas exchanges with 95% N2/5% CO2 under standardized conditions [17], producing oxygen concentrations of 20%, 6%, 2%, 0.6%, 0.2%, 0.06%, and <0.02%, respectively. After the end of the treatment, cells were either immediately detached with trypsin/EDTA, washed in α-MEM, and suspended in PBS for flow cytometry or returned to the incubator for a reoxygenation period and then processed.
Immunocytochemistry
Detection of the hypoxic marker EF5 was performed according to published methods for flow cytometry [18]. Immediately after hypoxic treatment, cells were trypsinized, washed in PBS, and fixed in 4% paraformaldehyde/PBS for 60 minutes at 4°C. After three rinses in PBS, 106 cells were blocked overnight at 4°C in PBS containing 0.3% Tween 20, 1.5% albumin, 20% fresh skim milk, and 5% normal mouse serum. Cells were then washed in PBS and incubated at 4°C for 6 hours in 100 µl of PBS containing a 1:30 dilution of the ELK3-51 biotin antibody (generous gift of C. Koch, University of Pennsylvania), a monoclonal mouse antibody raised against EF5. After three 40-minute washes in PBS containing 0.3% Tween 20 and 1.5% albumin, cells were resuspended in a 1:100 dilution of streptavidin R-phycoerythrin (PE) conjugate (Molecular Probes, Eugene, OR) and incubated at 4°C for 1 hour. Cells were washed twice in PBS containing 0.3% Tween 20 and once in PBS.
Flow Cytometry
Cells were analyzed on a Becton Dickinson FacsCalibur flow cytometer equipped with a 15-mW argon laser. Data were analyzed using CellQuest software (Becton Dickinson, San Jose, CA). Enhanced green fluorescent protein (EGFP) fluorescence data were collected in the FL1 channel (530/30) using log amplification. Twenty-thousand events were collected for each sample. Dead cells and debris were excluded based on forward-angle and side scatter. A clearly distinguishable cell population with the background fluorescence level of non-transfected cells (<10% in each experiment) was considered to have lost the transfection and was also excluded from analysis. The percentage of such nontransfected cells was not significantly affected by the hypoxic treatment (6.8±0.9% in cells transfected with 5HRE-hCMVmp-d2EGFP vector after 12 hours at <0.02% oxygen and 8.6±0.2% in the same cells under aerobic conditions; two-sided P>.05, Mann-Whitney U test). When the antibody against the hypoxic marker EF5 was simultaneously detected using the streptavidin R-PE conjugate, PE fluorescence was detected in FL2 (585/42) and electronic compensation was used (FL1 — 30% FL2; FL2 — 1.5% FL1). Consistency of instrument settings was established using the Linear Flow Green Flow Cytometry Intensity Calibration Kit (Molecular Probes). Only minimal adjustments of FL1 amplification were necessary between experiments.
Western Blot Analysis
Cultured HT 1080 cells transfected with the 5HRE-hCMVmp-d2EGFP vector were lysed in 1% Triton X-100 lysis buffer at different timepoints during reoxygenation after 12 hours of hypoxia at <0.02% O2 or immediately after a 12-hour treatment at different oxygen concentrations. Total protein was determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA). For immunoblotting, whole cell lysates (30 µg/lane) were separated by SDS-PAGE (10% polyacrylamide gel; Invitrogen) and transferred to a nitrocellulose membrane (Invitrogen) using a Novex Xcell II semi-dry blotter (Invitrogen). Membranes were blocked overnight with 5% non- fat dry milk, 0.2% Tween-20 in PBS. For EGFP detection, membranes were incubated with JL-8 monoclonal anti-GFP antibody (Clontech), recognizing all GFP variants, for 2 hours at 1:1000 dilution in blocking buffer and subsequently with secondary goat anti-mouse IgG (H+L) AP antibody (Zymed, San Francisco, CA) for 1 hour at 1:5000 in blocking buffer. Detection was performed with enhanced chemofluorescence (ECF; Amersham, Piscataway, NJ).
Statistical Analysis
Data on the different schedules of hypoxia and reoxygenation are given as mean±SEM from n=4 independent experiments. Data are calculated as percentage of fluorescence of aerobic positive control cells transfected with the CMV-d2EGFP vector. However, absolute fluorescence in these cells was very reproducible (see aerobic cells in Figure 3).
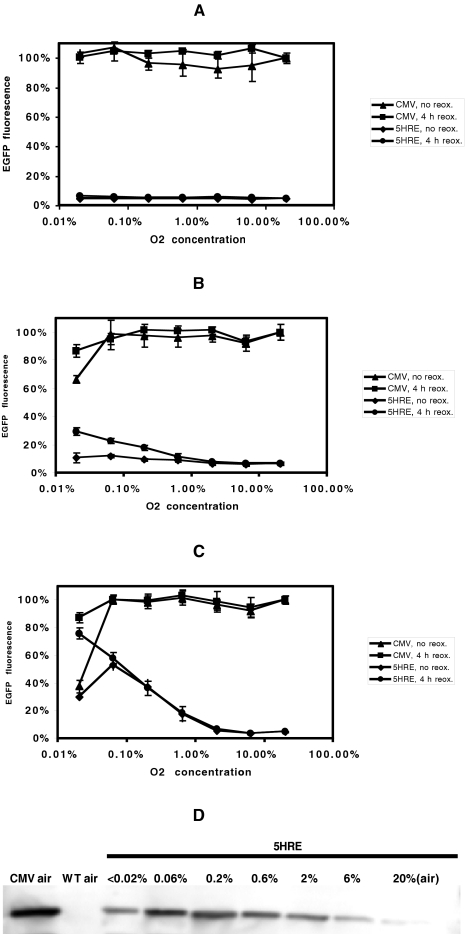
Figure 3.
EGFP fluorescence in HT 1080 human fibrosarcoma cells transfected with the hypoxia-responsive 5HRE-hCMVmp-d2EGFP vector (destabilized EGFP protein, half-life 2 hours), after treatment at oxygen concentrations between 20% and <0.02% for 1 hour (A), 4 hours (B) and 12 hours (C), respectively, with or without a 4-hour reoxygenation period. Fluorescence is given as the percentage of fluorescence in positive control cells transfected with CMV-d2EGFP vector (mean±SEM, n= 4). Western blot of EGFP protein in cells transfected with 5HRE-hCMVmp-d2EGPF vector (5HRE), immediately after 12 hours at different O2 concentrations, with aerobic CMV-transfected and wild-type (WT) cells for comparison (D). At <0.02% O2, the EGFP protein level in 5HRE-hCMVmp-d2EGFP-transfected cells is lower than at 0.06% or 0.2%, suggesting reduced EGFP protein synthesis under near-anoxic conditions, and not only unoxidated chromophore, as an explanation for the drop of fluorescence at the lowest oxygen concentration in (C).
Results
Time Course of EGFP Fluorescence and Protein Levels in Cells Transfected with Hypoxia-Responsive Vector
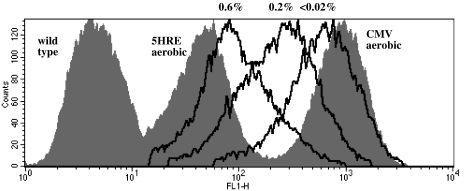
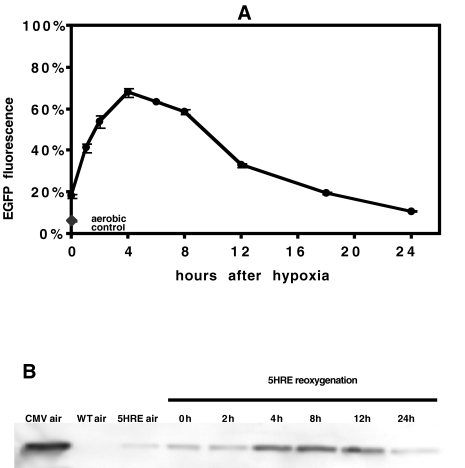
The fluorescence in aerobic cells transfected with the 5HRE-hCMVmp vector was 5.4±0.4% of the aerobic positive control CMV-d2EGFP cells, compared to 0.44±0.05% in non- transfected cells (see Figure 1), indicating some responsiveness of the 5HRE-hCMVmp promoter under aerobic conditions. The time course of EGFP fluorescence in HT 1080 cells transfected with this vector after 12 hours at the lowest achievable oxygen concentration (<0.02%) and with 0 to 24 hours of reoxygenation is shown in Figure 2A. Fluorescence immediately after the 12-hour hypoxic treatment was higher than in aerobic controls of the same cell type and increased during the first 4 hours to reach a maximum of 67.8±2.1% of positive control aerobic CMV-d2EGFP cells before decreasing to 10.8±2.4% after 24 hours. EGFP protein levels peaked between 4 and 12 hours of reoxygenation, but remained low during the first 2 hours after the hypoxic treatment (Figure 2B), in contrast to the rapid increase of fluorescence at the 1- and 2-hour timepoints in Figure 2A. These results indicate that the increase in EGFP fluorescence following reoxygenation after 12 hours at nearanoxic conditions (<0.02% oxygen) is the result of an early increase in chromophore oxidation (0 to 2 hours) as well as a delayed increase in EGFP protein levels (2 to 4 hours).
Figure 1.
EGFP fluorescence in HT 1080 human fibrosarcoma cells transfected with the hypoxia-responsive 5HRE-hCMVmp-d2EGFP vector (destabilized EGFP protein, half-life 2 hours), after treatment at oxygen levels between 20% (“5HRE aerobic,” grey area) or the concentrations indicated (black lines) for 12 hours and a subsequent 4-hour reoxygenation period. Fluorescence of positive control cells transfected with CMV-d2EGFP vector (“CMV aerobic,” grey area) and of wild-type HT 1080 cells (grey area) is shown for comparison. Gating for scatter and presence of transfection was performed. Not all oxygen concentrations are shown for clarity. Flow cytometry data are from a representative experiment.
Figure 2.
Time course of EGFP fluorescence in HT 1080 human fibrosarcoma cells transfected with the hypoxia-responsive 5HRE-hCMVmp-d2EGFP vector (destabilized EGFP protein, half-life 2 hours), after reoxygenation following a 12-hour hypoxia treatment at <0.02% oxygen (A). Fluorescence in these cells is given as the percentage of fluorescence in positive control cells transfected with CMV-d2EGFP vector (mean±SEM, n=4). Fluorescence of aerobic 5HRE-hCMVmp-d2EGFP-transfected cells is indicated for comparison. Corresponding Western blot showing EGFP protein levels in whole cell lysates (B). Cells transfected with the CMV-d2EGFP vector serve as a positive control. Under aerobic conditions, cells transfected with the 5HRE-hCMVmp-d2EGFP vector (5HRE) exhibit background fluorescence as compared to wild-type (WT) cells. After 12 hours of hypoxia at < 0.02%, EGFP protein levels remain low at 0 and 2 hours of reoxygenation, not accounting for the rapid increase in fluorescence shown in (A) and thus suggesting an early chromophore oxidation of non-fluorescent EGFP present at the end of the hypoxia treatment.
Effect of Duration of Hypoxia, Oxygen Concentration, and Reoxygenation Period on EGFP Fluorescence and Protein Levels
Fluorescence after hypoxic treatments of varying duration and oxygen concentration, with or without a subsequent 4-hour reoxygenation period (chosen based on the results of Figure 2A), was compared for cells transfected with the hypoxia-responsive 5HRE-hCMVmp-d2EGFP vector and with the positive control CMV-d2EGFP vector (Figure 3). A 1-hour treatment, even at the lowest oxygen concentration, had no effect on EGFP fluorescence (Figure 3A). Four hours of hypoxia at the lowest oxygen level reduced fluorescence in positive control cells to 66±3%, an effect that was largely recovered by the 4-hour reoxygenation period (Figure 3B). A similar increase in fluorescence was observed with the 4-hour reoxygenation period in the cells transfected with the 5HRE-hCMVmp-d2EGFP vector. Twelve hours of hypoxia produced a sharp decrease in EGFP fluorescence at <0.02% oxygen for both positive control cells (CMV promoter) and for cells transfected with the hypoxia-responsive promoter (Figure 3C). Again, the 4-hour reoxygenation period restored fluorescence in the CMV-transfected cells to near 100% levels, and markedly increased fluorescence in cells transfected with the 5HRE-hCMVmp-d2EGFP vector. Significantly, there was little or no difference between treatments with or without reoxygenation at oxygen concentrations of 0.06% or greater. With reoxygenation, there was a constant increase in fluorescence between 2% and <0.02% oxygen. After 12 hours at different oxygen concentrations, cells transfected with the 5HRE-hCMVmp-d2EGFP vector exhibited maximal EGFP protein levels at 0.06% O2, but reduced levels after near-anoxic conditions (Figure 3D), corresponding to the pattern of fluorescence seen after hypoxia without reoxygenation in Figure 3C.
Labeling with the Hypoxic Marker EF5
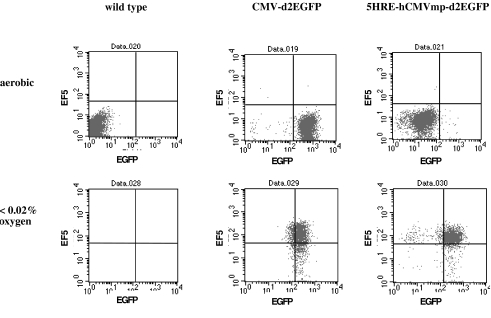
To investigate the feasibility of detecting a second signal simultaneously with EGFP in this system and to correlate EGFP fluorescence with a widely used hypoxic marker, EF5 was added to the medium before hypoxic treatment, and a two-step reaction with the ELK3-51 biotin monoclonal mouse antibody and subsequent incubation with a streptavidin R-PE conjugate was performed immediately after hypoxic treatment (Figure 4). In cells transfected with the CMV-d2EGFP vector, fluorescence after 12 hours at <0.02% O2 was lower than under aerobic conditions due to immediate processing of cells for EF5 detection without reoxygenation. Cells transfected with the 5HRE-hCMVmp-d2EGFP vector exhibited some background fluorescence as compared to wild-type negative controls under aerobic conditions and showed a marked increase in both EGFP and PE fluorescence after 12 hours of hypoxia at <0.02%. These experiments show that the hypoxia marker EF5 and EGFP driven by the hypoxia-responsive promoter label essentially the same population of hypoxic cells and have a similar dynamic range.
Figure 4.
Human HT 1080 fibrosarcoma cells, wild type or transfected with the hypoxia-responsive 5HRE-hCMVmp-d2EGFP vector or the positive control CMV-d2EGFP vector under aerobic (20% oxygen) or hypoxic (<0.02% oxygen for 12 hours) conditions. EGFP fluorescence versus streptavidin-PE fluorescence after labeling with ELK3-51 biotin, an antibody against the hypoxic marker EF5. Cells were processed for EF5 detection immediately after hypoxic treatment. The populations were gated for scatter, and electronic compensation was applied. The data are from a representative experiment.
Discussion
The two major limitations of GFPs as markers of gene expression are the requirement of molecular oxygen for formation of the fluorescent chromophore and a low temporal resolution resulting from the slow oxidation rate [3–5]. Although GFP has occasionally been used as a marker of gene expression under hypoxic conditions [19], no quantitative data on the effect of low oxygen concentrations on fluorescence at the single cell level have been published. Reid and Flynn [5] described chromophore formation as a three-step intramolecular reaction, consisting of protein folding, cyclization of the chromophore tripeptide, and the slowest phase, oxidation of the cyclized chromophore. In a report comparing the properties of wild type and mutant GFPs detected in anaerobically grown Escherichia coli cultures, fluorescence was found to increase after admission of air with time constants of 2 and 0.45 hour, respectively, in wild-type GFP and its S65T mutant [20]. This finding may have led investigators to choose reoxygenation periods of about 3 hours for detection of maximum fluorescence [19].
Here we used a strong hypoxia-responsive promoter driving a destabilized EGFP vector, with a protein half-life of 2 hours, to test whether a GFP-based system can be applied to measure hypoxia. The promoter used has been shown to be extremely hypoxia-responsive, exhibiting up to 500- fold gene expression in transient transfection reporter gene assays [15], thereby exceeding the hypoxia responsiveness of other published HRE constructs [21], and was therefore expected to provide a sufficient dynamic range of EGFP fluorescence. Application of the short half-life EGFP, as compared to a half-life of over 24 hours for non- destabilized EGFP [21], was expected to improve the temporal resolution though at the expense of the dynamic range of fluorescence. Cells transfected with the same vector containing a constitutive CMV promoter were studied in parallel to obtain a reference level of EGFP fluorescence and to determine how hypoxic conditions would effect EGFP fluorescence per se, irrespective of hypoxia-responsive regulation of gene expression.
As a first step, the time course of EGFP fluorescence after maximum hypoxia (<0.02% oxygen) for 12 hours was investigated. Whereas the cells transfected with the hypoxia-responsive 5HRE-hCMVmp-d2EGFP vector exhibited some response of the HREs under aerobic conditions (12- fold increase of fluorescence compared to wild-type cells), their fluorescence was only 5% of that observed in cells transfected with the non-hypoxia-dependent CMV-d2EGFP vector (Figure 1). Such background fluorescence could be caused by an induction of the transcription factor subunit HIF-1α by serum growth factors under aerobic conditions, which has been demonstrated in vascular smooth muscle cells [22] or a constitutive expression of HIF-1α due to a mutation of a tumor suppressor gene [23]. Following 12 hours of hypoxia at <0.02% O2, fluorescence in cells with the hypoxia-responsive vector reached a maximum of 68% of the positive control after 4 hours of reoxygenation, thus giving a dynamic range of approximately 13- fold. A reoxygenation period of 4 hours was therefore used in experiments with different hypoxia durations and oxygen concentrations.
One-hour hypoxia treatments neither increased EGFP fluorescence in cells with the hypoxia-responsive vector nor did they affect fluorescence in positive control cells, suggesting that only chronically hypoxic cells are recognized by the system. After 4 or 12 hours at <0.02% oxygen, but not at higher concentrations, a marked drop in fluorescence occurred in the cells with the CMV promoter, indicating that very low oxygen levels, but not anoxia, allow for unimpaired formation of the fluorescent chromophore. This is underlined by the nearly identical curves of fluorescence versus oxygen concentration with and without 4 hours of reoxygenation after 12-hour treatment, in cells with the hypoxia-responsive vector, above 0.06% oxygen (Figure 3C). With prolonged hypoxia (12 hours) and subsequent reoxygenation, fluorescence in the hypoxia-responsive system increased constantly with decreasing oxygen concentrations between 2% and <0.02%.
To determine whether the effect of reoxygenation after near-anoxic treatment on EGFP fluorescence was solely due to chromophore oxidation in pre-synthesized EGFP, Western blot analysis of 5HRE-hCMV-d2EGFP-transfected cells was performed in whole cell lysates obtained at different timepoints after 12 hours at <0.02%. Although higher than under aerobic conditions, EGFP protein levels were found to be similarly low at 0 and 2 hours post-hypoxia with maximum levels at 4, 8, and 12 hours (Figure 2B). In contrast, there was a rapid increase of fluorescence during the first 2 hours of reoxygenation (Figure 2A). Considering the time course of GFP (S65T mutant, as present in EGFP) fluorescence restoration after reoxygenation from anoxia in a cell-free system, where approximately 85% of maximum fluorescence was reached within 1 hour after readmission of air [20], the oxidation of existing EGFP protein is likely to be the main mechanism explaining the initial fluorescence increase during reoxygenation. The lagging increase in EGFP protein synthesis observed between 4 and 12 hours of reoxygenation can well account for the slow decrease in fluorescence (Figure 2A), which could not otherwise be explained in light of the short half-life of the EGFP protein used in the present study. The time course of EGFP protein synthesis, as observed on Western blot analysis, explains the effect of a reoxygenation period after the shorter 4-hour treatment even at intermediate oxygen concentrations (Figure 3B): Activation of the HREs, transcription, and translation of EGFP are not complete during the 4-hour treatment, and continue, regardless of oxygenation, during the additional 4 hours. Corresponding to the fluorescence data (Figure 3C), EGFP protein levels immediately after 12-hour hypoxia increased with decreased oxygen concentrations, except for lower protein expression under near-anoxic conditions (Figure 3D).
After sufficient reoxygenation, the system presented makes possible the sorting of live chronically hypoxic cells from a tumor cell suspension based on EGFP fluorescence by fluorescence-activated cell sorting (FACS). Potential applications include the isolation of such cells after treatment of experimental tumors in vivo with cytotoxic substances or ionizing radiation for subsequent separate analysis by survival assays in vitro. Furthermore, sorted cells could be analyzed for mutation status or changes in response to cytotoxic agents during reoxygenation. Obviously, a need to reoxygenate for several hours would preclude the assessment of any hypoxia-associated short-term changes in gene expression. However, reoxygenation can be omitted if anoxic cells do not need to be analyzed.
The range in which the hypoxia-responsive system exhibits a correlation between EGFP fluorescence and O2 concentrations, i.e., between 2% and anoxia, is relevant for studies of tumor hypoxia. Firstly, the fact that the transcription factor subunit HIF-1α, which has been found to be mainly responsible for the regulation of the hypoxic response, including the expression of angiogenic and glycolytic enzymes [24,25], directly activates the HREs in the system now studied underscores the biological relevance of EGFP fluorescence. HIF-1α protein levels increased modestly between 6% and 2%, but drastically below 2% O2 in HeLa S3 cells [26]. Whereas EGFP protein levels in 5HRE-hCMVmp-d2EGFP-transfected cells after 12 hours at <0.02% O2 are in good agreement with published HIF-1α protein levels [26], the detection of EGFP fluorescence seems to be less sensitive, not detecting increased EGFP levels at 6% or 2% oxygen.
The effectiveness of the hypoxia-responsive system in more severe hypoxia makes it particularly applicable to studies of radiation resistance in hypoxic cells. Early in vitro data indicated that the half-maximal effect of radiation sensitivity enhancement by oxygen is around 0.5% O2 [27,28]. Multiple studies of invasive intratumoral oxygen measurements using the Eppendorf oxygen electrode have identified hypoxia as an adverse prognostic factor, particularly in cervix and head and neck cancer, based on the percentage of readings below 5 mm Hg [9] or below 2.5 mm Hg [7] or a median of below 10 mm Hg [29], the equivalent of about 0.7%, 0.3%, and 1.4% O2 concentrations, respectively. Finally, much of the experience gained with hypoxia in experimental systems is based on the use of injectable nitroimidazole derivatives such as pimonidazole and EF5, which form intracellular adducts at below 10 mm Hg [11,12], corresponding to about 1.4% O2, and have a similar pattern of O2 concentration-dependent fluorescence on flow cytometry as the EGFP-based system [18].
In conclusion, EGFP can serve as a reporter protein of tumor hypoxia, in spite of the oxygen requirement for chromophore formation, if sufficient time is allowed for reoxygenation. Anoxia lasting 1 hour or less and prolonged hypoxia at O2 concentrations of at least 0.06% have no detrimental effect on EGFP fluorescence in the system investigated. The present data show that live cells can be sorted based on their oxygenation status for further analysis. As indicated by the successful labeling of the hypoxic marker EF5, the EGFP signal can be simultaneously measured with antibody-conjugated fluorochromes after 4% paraformaldehyde fixation. Whereas the hypoxic response of the EGFP-based system may be cell type-specific, the availability of other GFP mutants with different half-lives and spectral characteristics leaves room for adjusting the system for specific applications.
Abbreviations
- EGFP
enhanced green fluorescent protein
- FCS
fetal calf serum
- GFP
green fluorescent protein
- hCMVmp
human cytomegalovirus minimal promoter
- HIF
hypoxia-inducible factor
- HRE
hypoxia-responsive element
- PE
phycoerythrin
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (to D. V.) and a grant from the US National Cancer Institute, P01-CA67166 (to J. M. B.).
References
- 1.Prasher DC, et al. Primary structure of the Aequorea victoria green fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 2.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 3.Tsien Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 4.Heim R, Prasher DC, Tsien RY. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA. 1994;91:12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid BG, Flynn GG. Chromophore formation in green fluorescent protein. Biochemistry. 1997;36:6786–6791. doi: 10.1021/bi970281w. [DOI] [PubMed] [Google Scholar]
- 6.Schemmer P, et al. Reperfusion injury in livers due to gentle in situ organ manipulation during harvest involves hypoxia and free radicals. J Pharmacol Exp Ther. 1999;290:235–240. [PubMed] [Google Scholar]
- 7.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–40. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 8.Brizel DM, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 9.Fyles AW, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–156. doi: 10.1016/s0167-8140(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkiss RJ, Jones G, Long A. Flow cytometric evaluation of hypoxic cells in solid experimental tumours using immunodetection. Br J Cancer. 1991;63:119–125. doi: 10.1038/bjc.1991.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide]: analysis of drug adducts by fluorescent antibodies vs. bound radioactivity. Br J Cancer. 1995;72:865–870. doi: 10.1038/bjc.1995.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raleigh JA, Chou SC, Arteel GE, Horsman MR. Comparisons among pimonidazole binding, oxygen electrode measurements and radiation response in C3H tumors. Radiat Res. 1999;151:580–589. [PubMed] [Google Scholar]
- 13.Haustermans K, et al. Diffusion limited hypoxia estimated by vascular image analysis: Comparison with pimonidazole staining in human tumors. Radiother Oncol. 2000;55:325–333. doi: 10.1016/s0167-8140(00)00206-1. [DOI] [PubMed] [Google Scholar]
- 14.Evans SM, et al. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–2024. [PubMed] [Google Scholar]
- 15.Shibata T, Giaccia AJ, Brown JM. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 2000;7:493–498. doi: 10.1038/sj.gt.3301124. [DOI] [PubMed] [Google Scholar]
- 16.Shibata T, Giaccia AJ, Brown JM. Hypoxia-inducible regulation of a prodrug activating enzyme for tumor-specific gene therapy. Neoplasia. doi: 10.1038/sj.neo.7900189. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JM. SR 4233 (tirapazamine): A new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer. 1993;67:1163–1170. doi: 10.1038/bjc.1993.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Siemann DW, Koch CJ, Lord EM. Direct relationship between radiobiological hypoxia in tumors and monoclonal antibody detection of EF5 cellular adducts. Int J Cancer. 1996;67:372–378. doi: 10.1002/(SICI)1097-0215(19960729)67:3<372::AID-IJC11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Koshikawa N, Takenage K, Tagawa M, Sakiyama S. Therapeutic efficacy of the suicide gene driven by the promoter of vascular endothelial growth factor gene against hypoxic tumor cells. Cancer Res. 2000;60:2936–2941. [PubMed] [Google Scholar]
- 20.Heim R, Cubbitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 21.Ruan H, et al. Killing of brain tumor cells by hypoxia-responsive element mediated expression of BAX. Neoplasia. 1999;1:431–437. doi: 10.1038/sj.neo.7900059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- 23.Wiesener M, et al. Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1α in clear cell renal carcinoma. Cancer Res. 2001;61:5215–5222. [PubMed] [Google Scholar]
- 24.Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 25.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 26.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 27.Whillans DW, Hunt JW. A rapid-mixing comparison of the mechanisms of radiosensitization by oxygen and misonidazole in CHO cells. Radiat Res. 1982;90:126–141. [PubMed] [Google Scholar]
- 28.Koch CJ, Stobbe CC, Bump EA. The effect on the Km for radiosensitization at 0 degrees C of thiol depletion by diethylmaleate pretreatment: Quantitative differences found using the radiation sensitizing agent misonidazole or oxygen. Radiat Res. 1984;98:141–153. [PubMed] [Google Scholar]
- 29.Hockel M, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]