Abstract
High expression of circulating plasma vascular endothelial growth factor (VEGF) in patients with cancer is an indicator of poor treatment response. Similarly, hypoxia in tumors, as measured by oxygen needle electrodes, has been found to predict for tumor-treatment failure. These two predictors may be related because hypoxia is a potent stimulator of VEGF expression in vitro. However, the demonstration of a relationship between hypoxia and VEGF in human tumors has, to date, been indirect or even negative. The purpose of this study was to test whether this unexpected result was caused by factors unique to human tumors, or whether the prior results could have been influenced by the known complexities of VEGF regulation. Therefore, we undertook a direct assessment of VEGF induction in human tumors using in situ hybridization and compared its distribution with that of hypoxia, as measured by the distribution of adducts of the hypoxia marker EF5. The distribution of both markers was assessed in relationship to the distribution of blood vessels, as measured by antibodies to CD31. Our hypothesis was that VEGF mRNA and hypoxia would colocalize, assuming that detectability of the former was not limiting. Four squamous cell carcinomas, three sarcomas and one glioblastoma multiforme were studied. When VEGF mRNA signal was detectable, its maxima colocalized with regional maxima of EF5 binding. The strongest levels of both signals were sometimes adjacent to regions of tissue necrosis. However, we were unable to predict absolute levels of EF5 binding based on absolute levels of VEGF mRNA. Conversely, for all tumors studied, regions with relatively low levels of EF5 binding had relatively low or undetectable VEGF mRNA. We found moderate EF5 binding in some keratinized cells but VEGF mRNA was not expressed by these differentiated cells. The paradigm that hypoxia and VEGF expression are linked in human tumors is supported by the data presented herein. A better understanding of the biology behind VEGF expression, including its modulation by hypoxia, is important for optimizing its use as a prognostic indicator and/or modulating its presence with biologic therapies.
Keywords: hypoxia, VEGF, EF5, tumor, human
Introduction
The relationship between hypoxia and treatment outcome in human cancer has been the subject of intensive investigation in recent years. Studies using polarographic needle electrodes to measure tumor oxygenation have shown correlations between low intratumoral pO2 and poor response to treatment in several tumor types [1–4]. Although the dependence of radiation killing on cellular oxygenation is well studied [5], the association between tumor oxygenation and metastasis or recurrence is less clear. Two paradigms to explain these observations have been proposed. The first is that hypoxia causes genetic instability [6], which allows selection for a more aggressive tumor phenotype [7]. The second is that hypoxia modulates specific cytokines, which are involved in tumor progression. The best-studied cytokines are those involved in tumor angiogenesis because tumor growth and metastasis are dependent on this process [8]. Several of these cytokines are modulated by tissue oxygenation and are referred to as oxygen-regulated proteins (ORPs) [9]. In human tumors, vascular endothelial growth factor (VEGF) is the most commonly expressed cytokine of this group. It is a potent endothelial mitogen and angiogenic growth factor in vivo [10]. VEGF also increases capillary leakiness and was first identified as vascular permeability factor [11]. Despite the problems of antigen stability, receptor-complex formation, and sample processing, several studies have shown that higher level of circulating VEGF protein is an indicator of poor patient prognosis (see Jelkman for review [12]). Infact, high levels of intratumoral VEGF have already been shown to correlate with poor prognosis in many human tumor types including squamous cell carcinoma (SCC) [13,14], node-negative breast carcinoma [15], pancreatic cancer [16], gastric carcinoma [17], and colon cancer [18]. However, such conclusions are not universally applicable [19].
Based on in vitro studies, hypoxia is a potent stimulator of VEGF expression in both human and rodent cell lines [11,20,21]. In multicellular spheroids, the colocalization of VEGF mRNA and binding of the hypoxia marker, EF5, has been demonstrated [22], providing support for the predicted relationship between VEGF expression and hypoxia. However, there are minimal in vivo data on this subject. Most available in vivo data are based on studies of animals exposed to a hypoxic environment with VEGF measurements in normal or sometimes tumor tissues (for example see Ref. [23]). Rosmorduc et al. [24] reported the colocalization of VEGF and binding of the hypoxia marker pimonidazole in experimental liver fibrogenesis. In situ hybridization (ISH) experiments performed on human tumors have shown that VEGF mRNA is localized to the cells adjacent to areas of necrosis, which are presumed to contain the most hypoxic cells [25]. In contrast, Raleigh et al. [26] found no correlation between the colocalization or levels of pimonidazole binding and VEGF protein staining in human SCCs. Similarly, Parliament et al. [27] found that regions of severe hypoxia in glioma xenografts (as measured by binding of another hypoxia marker, [3H]misonidazole) did not correspond to areas of VEGF protein or mRNA expression. Danielson and Rofstad [28] have concluded that endogenous expression levels of VEGF are more biologically important than induction by hypoxia. These studies brought the role of hypoxia regulation of VEGF expression in human cancers into question. To further elucidate this matter, we studied binding of EF5 (using immunohistochemical [IHC] detection of drug-tissue adducts) as a surrogate for hypoxia and VEGF mRNA (using ISH) in human tumors.
The tumors described herein were from a group of patients participating in a phase I study to determine whether EF5 could be used safely as a hypoxia marker [29,30]. In the phase I study design, it was possible to determine the maximum possible EF5 binding by the tumor tissue. EF5 binding is maximal under severely hypoxic conditions (<0.005% oxygen) and decreases with increasing oxygen concentration over the entire physiological range [31]. Our results show that in tumor tissues where the VEGF mRNA signal is easily detectable, there is colocalization with regional maxima of EF5 binding. This occurred in all tumors where EF5 binding was at least 25% of the maximum possible binding. Conversely, no VEGF mRNA signal was found in regions of EF5 binding less than 5% of maximum. Based on in vitro calibration studies, the oxygen levels corresponding to these two thresholds are roughly <0.5% and >3% oxygen, respectively. At intermediate EF5 binding levels, there was much more heterogeneity of VEGF mRNA signal. Thus, considering all tumors, absolute levels of VEGF signal were only roughly correlated with absolute levels of EF5 binding.
Materials and Methods
Human Subjects
In February 1998 EF5 was granted IND status for use in human patients with cancer by the FDA and the phase I protocol was approved by the University of Pennsylvania Institutional Review Board and the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI). Written informed consent was obtained from all patients entered. Eligible patients were those of all ethnic and gender groups undergoing incisional or excisional tumor biopsies for other medical indications. Exclusion criteria emphasized a history of grade III or IV peripheral neuropathy as defined by the NCI/DCTDC because neurotoxicity has previously been a problem in the studies of 2-nitroimidazole compounds used in high, multiple doses as hypoxic cell sensitizers [32]. Pregnant patients, patients less than 18 years of age and those who were HIV positive were excluded. A subgroup of the patients on the phase I study was selected for the current study based on tissue availability, varying histologic tissue types and tumor locations, and with the goal of studying tumors of both high and low EF5 binding [29,30]. Our goal was to determine whether ISH for VEGF would be observed in sections from these tumors. This staining needed to be in a region with identifiable morphologic landmarks to compare with IHC staining for EF5 and CD31 (to mark blood vessels) in adjacent or nearly adjacent sections. This was necessary because the procedures for ISH are not compatible with those for IHC staining.
Drug Administration
EF5 was supplied by the NCI Division of Cancer Treatment (NCI/DCT) in 100-ml vials containing 3 mg/ml EF5 with solvent composed of water containing 5% dextrose and 2.5% alcohol. EF5 was administered intravenously with a peripheral intravenous catheter at a rate no greater than 350 ml/hour. The intravenous infusion was over 1 to 2 hours with time of administration determined by the dose of EF5 being studied. Pharmacokinetic studies of EF5's plasma clearance indicated an average biologic half-life of about 12 hours [33]. Binding of EF5 is expected to vary inversely with tissue oxygen concentration and directly with tissue drug exposure [34]. Thus, measured binding (see below) was corrected for total drug exposure or area under the curve (AUC).
Tissue Acquisition and Storage, ISH, IHC Staining for EF5 Binding, and Other Markers
Surgical excisions often involve tissue devitalization (and, therefore, hypoxia induction) for variable amounts of time. Thus, it was considered important to have surgical biopsy or excision after most circulating drug was cleared and we chose a time of roughly 48 hours (range 40 to 55) following commencement of drug administration when the circulating drug would have decreased 16-fold, based on its 12-hour plasma half-life. The procedures for tissue storage, EF5 immunohistochemistry, fluorescence microscopy, and analysis of binding have been previously reported [29]. Briefly, tissues were placed on moist filter paper, coated with a thin layer of Tissue Tek (OCT compound, Sakura Finetek USA Inc., Torrance, CA), frozen on dry ice, and held at -65°C before sectioning.
In situ hybridization Single-stranded RNA probes were generated using a 517-bp human VEGF121 cDNA cloned into a pBluescript KS vector (kindly provided by Dr. H. Marti). The plasmid DNA was purified with a Maxiprep kit (Promega, Madison, WI). The construct was linearized with BamHI or EcoRI and transcribed in vitro from the T7 or T3 polymerase to yield the antisense or sense (control) probes, respectively. The base sequence of the plasmid DNA was confirmed by sequence analysis (DNA Sequencing Facility, Cancer Center, University of Pennsylvania). Transcription reactions were performed using the Maxiscript in vitro RNA transcription kit (Ambion, Austin, TX). The transcription reaction was carried out in the presence of 4 mM digoxigenin-UTP (Boehringer Mannheim, Indianapolis, IN) to yield the labeled probe.
Section pretreatment was performed as previously described [35]. Briefly, tissue was brought from -65° to -20°, cut in 10-µm sections and placed onto poly-l-lysine-coated slides. Frozen sections were fixed in 4% paraformaldehyde, rinsed, dehydrated through graded ethanols, and stored at -65° until use before hybridization, sections were brought to room temperature, rehydrated, permeabilized with Pronase (120 µg/ml) for 10 minutes at room temperature, placed in 0.2% glycine for 30 seconds at room temperature, placed in acetic anhydride (1:400 dilution in triethanolamine) for 10 minutes, and dehydrated through graded ethanols. Hybridization and color development were carried out as previously described [36] with minor modifications. Slides were prehybridized in hybridization buffer for 1 hour at 60° in a humidified chamber. Hybridization was carried out for 12 to 24 hours at 60° in a humidified chamber using 4 µg/ml of probe in hybridization buffer. Sections were then digested with RNAse A (20 µg/ml) and washed two times for 15 minutes in 2x SSC, 0.1% SDS (1x SSC is 0.3 M NaCl plus 0.03 M sodium citrate, pH 7.0) followed by two washes for 30 minutes in 0.1x SSC and 0.1% SDS at 55°C.
The signal was detected with an alkaline phosphatase color reaction using anti-digoxigenin antibody coupled to alkaline phosphatase (Boehringer Mannheim). Slides were pretreated in buffer 1 (0.1 M maleic acid, 0.1 M NaCl, pH 7.5) then blocked in buffer 2 (2% blocking reagent (Boehringer Mannheim) with 100 µg/ml tRNA, 50 µg/ml heparin) for 30 minutes. Antibody was applied in a 1:500 dilution in buffer 2 for 30 minutes at room temperature. Excess antibody was removed by two 20-minute rinses in buffer 1 and the color developed by incubating slides in buffer 3 (100 mM Tris-HCl pH 9.5, 50 mM MgCl2, 2 mM levamisole) containing 0.338 mg/ml 4-nitroblue tetrazolium chloride (NBT) and 0.173 mg/ml 5-bromo 4-chloro 3-indolyl-phosphate (BCIP) for 16 to 18 hours in the dark. The reaction was stopped in TE buffer (10 mM Tris-HCl, 1 mM EDTA) for 5 minutes.
Controls for endogenous alkaline phosphatase activity were performed by treating the samples with chromogen alone. Sense strand probes were used as a control for nonspecific binding. Samples known to express VEGF were used for positive controls.
Two independent observers reviewed the ISH sense and antisense images and graded the signal of the antisense images on a scale of 0 to 4: 0 (no staining), 1 (light brown staining observable in scattered cells using microscope), 2 (regionally defined light brown staining observable by microscope), 3 (regionally defined medium brown staining, with intensity gradients observable, visible by eye), 4 (dense brown or purplish-brown, color and intensity gradients, easily visible by eye).
Digital microscopy The ISH (and hematoxylin and eosin) slides were photographed using an RGB-ELE liquid crystal color filter (Cambridge Research and Instrumentation, Cambridge, MA) in conjunction with the digital camera (described below). Because of the large images required for color photography, the regions imaged were identified by best contrast of the ISH stain and with tissue landmarks (holes, necrotic areas, edges) that could be applied to find the same areas for EF5 and CD31 stains in adjacent or nearly adjacent sections. It was necessary to compare adjacent tissue sections because the processing for ISH and immunohistochemistry were incompatible.
Immunohistochemistry Frozen tissue sections were made at 10 µm, fixed, blocked, and stained with ELK3-51 antibody (75 µg/ml) conjugated with Cy3 dye. Epifluorescence measurements were made using a Nikon LabPhot microscope (Melville, NY) with 100-Whigh-pressure mercury arc lamp attached to a cooled (-25°) CCD digital camera (Photometrics Quantix, KF1400, Photometrics, Tucson, AZ). The manual stage of the microscope was replaced by a Ludl Electronic Products 99S000 (Hawthorne, NY) automatic stage with 0.1-µm step size. The camera and stage were controlled by a Macintosh 9600 Power PC computer (Apple Computer Inc., Cupertino, CA). Software was Scanalytics “IP Lab” (Scanalytics, Inc., Fairfax, VA) a general-purpose image analysis program with modular support of the hardware components. Manual or semiautomatic scanning of the section, setting of camera exposure and focus, and automatic collection of images over a defined area with known positional coordinates were performed using software written in IP Lab macro code. Known positional coordinates allowed reimaging of the same portion of a section after additional staining (e.g., CD31, hematoxylin and eosin). The regions were imaged using a 10x microscope objective (field size set electronically at 1.08x0.72 mm2) and typically 3x3 contiguous fields were examined for each section, overlapping a 2x2 field imaged for ISH. The resulting images were then aligned (sometimes with rotation) and cropped using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
IHC staining for CD31/PECAM: Some tissue sections were stained first for CD31 and subsequently for EF5 to determine the spatial relationship between hypoxia and blood vessel distribution. A protocol allowing primary staining by a mouse monoclonal to human CD31 followed by labeled secondary rat anti-mouse antibody was used. The secondary antibody labeling allowed detection by peroxidase stain or fluorescence. Because subsequent staining with mouse monoclonal antibodies against EF5 adducts would disrupt the rat anti-mouse secondary labeling for CD31, a short fixation step was added (4% paraformaldehyde, 20 min. at room temperature) before standard rinsing, blocking and staining for EF5 adducts.
Quantitation of EF5 Staining Intensities
Our previous papers have described the quantitative aspects of measuring total EF5 adducts by a calibrated fluorescence detection system [37,38]. In brief, the optical aspects of the fluorescence microscopy are calibrated with a reference concentration of fluorescent dye. EF5 binding depends directly on tissue drug exposure, so this is accounted for by measuring the drug pharmacokinetics. The maximum possible binding rate for the specific tissue of interest is determined by “cube-reference” binding, wherein small tumor tissue pieces are incubated with EF5 or a closely related analogue (EF3) under conditions of severe hypoxia in vitro [29,37,38]. This allows EF5 binding to be monitored as an absolute value (dependent on the calibrated fluorescence) or as a percentage of cube-reference binding. To determine the oxygen dependence of binding, we assume the average oxygen dependence of adduct formation determined from multiple cell lines in tissue culture [38]. A rule of thumb estimate for the oxygen dependence of EF5 binding is that it is maximal for pO2 below 0.1%, decreases inversely with increasing oxygen pO2 (by a factor of 30 to 100) between 0.1% and 10% oxygen and then remains constant above 10% oxygen.
Nonspecific Staining
Because tumor tissue cannot be collected before treatment with EF5, we have devised a method to test for the specificity of observed EF5 binding to allow a measurement of the “background” signal that would result from (minor) non-specific binding by the detecting antibodies. This procedure is termed “competed stain” and is accomplished by mixing EF5 with the antibody before application to the tissue section. The EF5 blocks all specific binding sites of the antibody. In animal and tissue culture studies, we have shown that this method provides an accurate measure of what the antibody signal would have been for control conditions (i.e., without administration of EF5) [38].
Results
The goal of our studies was to evaluate patterns and intensities of VEGF mRNA and to compare these with EF5 binding at the same spatial location in a sampling of tumors, using pattern and semiquantitative analyses. Eight tumors were examined with ISH for VEGF mRNA and immunohistochemistry of EF5 binding for hypoxia (Table 1). These tumors were selected based on varying histology (soft tissue sarcomas [STS], SCCs, and glioblastoma multiforme [GBM]); site (cervix and head and neck for SCC; trunkal, gastrointestinal and extremity for STS); and EF5 binding level (a range of values). For the SCC tumors, both moderate and well-differentiated tumors were selected. A nondifferentiated SCC was not available for study.
Table 1.
Tumor Information.
| Patient # | Tumor Site/Histology | Stage/Grade | EF5 Binding,* Regional Maximum | EF5 Binding,* % Cube Reference | VEGF mRNA† | Keratinization? | Colocalization‡ |
| 1 | Cervix/SCC | T1BNOMO | 47.3 | 13 | 3.5 | Yes, but not the same areas as EF5 binding | + |
| 5 | Retromolar triangle/SCC | T2N2M0 | 76.9 | 26 | 2 | Yes, but not the same areas as EF5 binding | + |
| 7 | Retromolar triangle/SCC | T4N1M0 | 75.6 | 15 | 0 | Yes, same area as maximal EF5 binding | 0 |
| 10 | Extremity/leiomyosarcoma | High grade | 190.2 | 91 | 4 | No | + |
| 12 | Large bowel/spindle cell SA | High grade | 36.5 | 15 | 1 | No | IND |
| 16 | Trunkal/synovial cell SA | High grade | 132.6 | 25 | 4 | No | + |
| 26 | Retromolar triangle/SCC | T4N0M0 | 41.1 | 6 | 0 | Yes, same area as maximal EF5 binding | 0 |
| 27 | Brain/GBM | Recurrent | 81.9 | 17 | 1.5 | No | IND |
SCC, Squamous cell carcinoma, GBM, gliobastoma multiforme, SA, sarcoma.
EF5 binding is measured on an absolute fluorescence scale, which can then be compared to the maximum possible binding using the cube-reference technique (see Materials and Methods section).
Approximate scale of VEGF mRNA staining: 0 (no staining), 1 (light brown staining observable in scattered cells using microscope), 2 (regionally defined light brown staining observable by microscope), 3 (regionally defined medium brown staining, with intensity gradients observable, visible by eye), 4 (dense brown or purplish-brown, color and intensity gradients, easily visible by eye).
There is an interaction between the level of VEGF staining, and the ability to determine how closely the two stains colocalize. Thus, a simple (+) is used to indicate that the patterns of observed binding were the same, (0) no VEGF signal, (IND) not possible to determine due to technical limitations, and (-) a negative correlation (not observed to date).
The independent observers agreed on the level of VEGF mRNA signal in six of the eight samples, and in the other two samples (patients #1 and #27) they disagreed by one level. For these two samples, the average of the two scores was accepted. Four tumors were SCCs, three arising in the retromolar triangle (patients #5, #7, #26) and one arising in the uterine cervix (patient #1). VEGF mRNA staining was strongly positive in sections from two of these tumors (patients #1, #5); For sections from the cervical cancer (patient #1), VEGF staining was quite widespread, with local maxima corresponding to local regional maxima of EF5 (Figure 1). In contrast, VEGF binding in sections from patient #5 was localized exclusively to regions containing regional maxima of EF5 binding (Figure 2). Tissues from patients #7 and #26 had moderate to well-differentiated SCC and showed no detectable VEGF mRNA signal. Because the fluorescence signal for IHC stain of EF5 has a large dynamic range (factor of ∼100) it was possible to discern gradients of EF5 binding in virtually all tumors. For patient #26, the highest regional maxima of EF5 binding (though only 6% of cube-reference binding) was present in terminally differentiated keratinized tissue (Figure 3). This does not involve a staining artifact, because the competed-stain controls were negative. Additionally, counterstaining of these tissues with antibodies to CD31/PECAM demonstrated an inverse localization between EF5 binding in keratinized cells and blood vessels (Figure 3). This relationship has been a general observation for human tumors previously studied (i.e., that EF5 binding almost always increases as a function of distance from nearest blood vessel [29,30]).
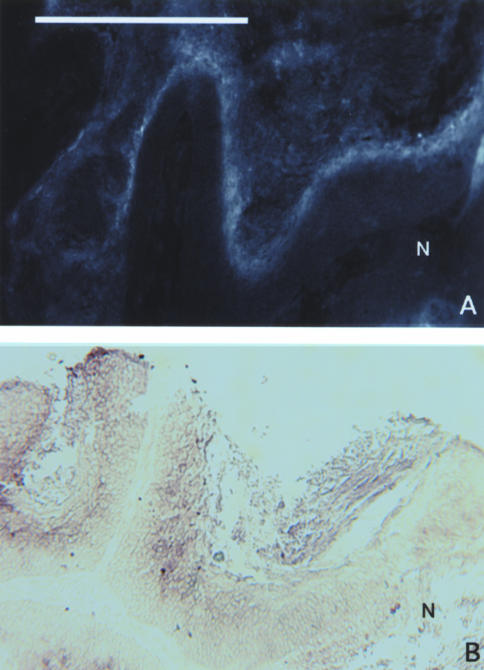
Figure 1.
EF5 IHC and VEGF mRNA antisense staining patterns in a cervical SCC tumor from patient #1. EF5 staining and ISH techniques were performed as described in the text. The two sections were significantly displaced from each other (approximately 200 µm). Thus, the patterns and location of the highly invaginated and corded structures are similar but not identical. (A) Regions of high EF5 fluorescent intensity are seen adjacent to regions of necrosis (N). (B) Strong VEGF mRNA intensity is seen in the cellular regions that correspond to the brightest areas of EF5 binding. Bar=500 µ.
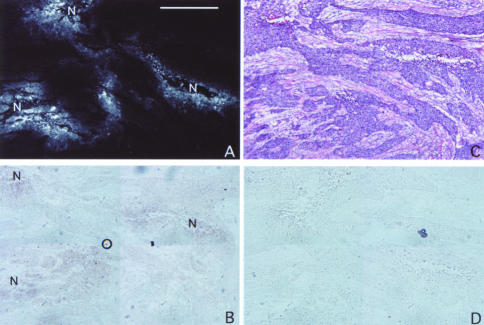
Figure 2.
EF5 IHC, VEGF mRNA antisense, hematoxylin and eosin, and VEGF mRNA sense staining patterns in a retromolar triangle SCC from patient #5. EF5 staining and ISH techniques were performed as described in the text. The sections for EF5, VEGF mRNA sense and antisense were nearly adjacent to each other. The section for ISH was counterstained for hematoxylin and eosin. (A) Moderately differentiated head and neck SCC stained for EF5. Regions of bright binding are seen adjacent to regions of necrosis (N). (B) Moderate signal for VEGF mRNA antisense are seen in cellular regions that correspond to the brightest areas of EF5 binding. The circle in the center of the figure is a bubble artifact. (C) Hematoxylin and eosin demonstrates that the tissues that stain for EF5 and VEGF mRNA antisense are viable tumor tissue. The regions that are more eosinophilic (pink) are stromal tumor tissue. (D) Staining for VEGF mRNA with the sense probe reveals no signal. Black region at 3 o'clock is an artifact. Bar=500 µ.
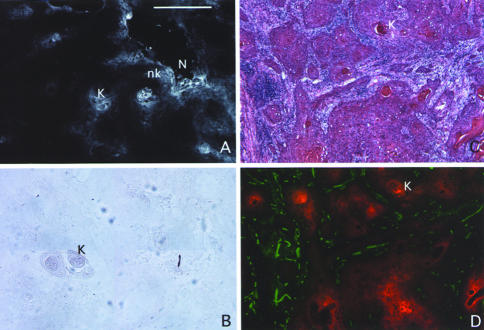
Figure 3.
EF5 IHC, VEGF mRNA antisense, hematoxylin and eosin, and CD31/PECAM versus EF5 patterns in a retromolar triangle SCC from patient #26. EF5, CD31 staining, and ISH techniques were performed as described in the text. Sections probed for EF5 and antisense VEGF mRNA were adjacent to each other. Sections for CD31/EF5 were from the same tissue mass and counterstained with hematoxylin and eosin. (A) Regions of bright EF5 binding are seen in keratinized (K) as well as nonkeratinized (nk) tumor tissues. A region of necrosis (N) is seen adjacent to regions of high EF5 binding. (B) VEGF mRNA signal is not seen in this tissue. Keratin pearls (K) are easily identified. (C) Hematoxylin and eosin staining demonstrates complex pathologic patterns in these SCC tumors. Keratin pearls (K) and nonkeratinized tumor tissue and tumor stroma are all identified. (D) Staining for both EF5 (red) and CD31 (green) demonstrates the inverse anatomic relationship between keratinized tumor regions that bind EF5 and blood vessels. This relationship supports the hypothesis that these keratinized tumor tissues are hypoxic. Bar=500 µ.
All three sarcoma tumors (patients #10, #12, #16) were positive for VEGF mRNA. For the two tumors with strong VEGF staining (patients #10 and #16) colocalization with regional maxima in EF5 binding was found (Figure 4). For sections from the third patient of the sarcoma group (patient #12) VEGF binding was too weak to adequately capture digitally. Thus it was not possible to determine colocalization patterns with EF5.
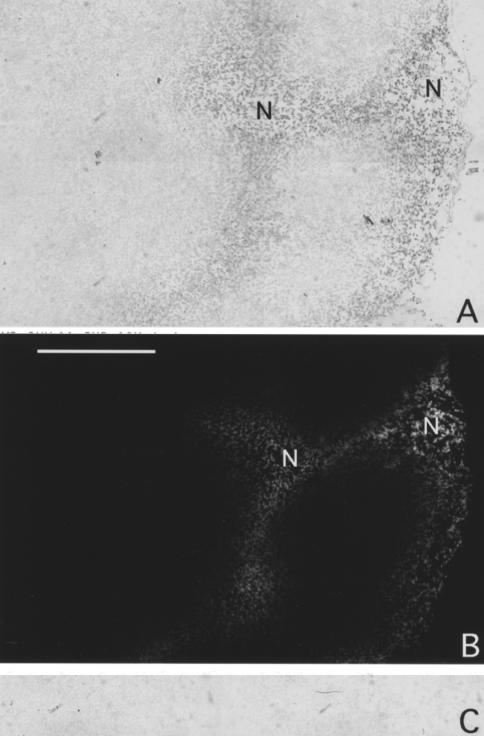
Figure 4.
EF5 IHC, VEGF mRNA antisense and sense staining patterns in a high-grade synovial cell sarcoma from patient #16. EF5 staining and ISH techniques were performed as described in the text. The two sections were adjacent to each other. (A) High VEGF mRNA signal is seen in an “H”-shaped region adjacent to areas of necrosis. (B) Regions of bright EF5 binding are seen, representing hypoxia and these correspond to the VEGF mRNA signal seen in Figure 3C. In the middle of both areas of EF5 binding, acellular regions are seen, representing microscopic areas of necrosis (N). (C) VEGF mRNA sense staining pattern corresponding to region of strongest VEGF antisense signal. Peroxidase staining is not seen, providing a negative control for comparison. Bar=500 µ.
The recurrent GBM (patient #27) had extensive necrosis, moderate EF5 binding, and moderate levels of VEGF mRNA staining. However, both types of binding were scattered throughout the tumor, often confined to individual cells or small groups of cells. Because this patient had been extensively pretreated, this pattern was most likely secondary to the radiation-induced changes present (vascular hyalinization; necrosis). Due to the scattered nature of the binding, it was not possible to determine whether the same cells stained for both VEGF and EF5 (data not shown).
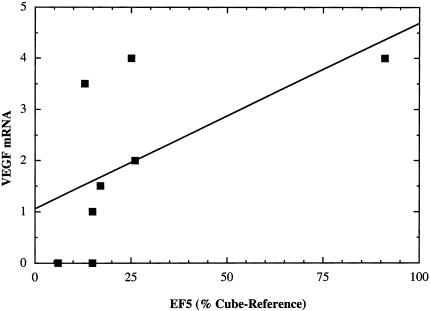
For all of the tumors studied, background EF5 staining was not observed in slides treated by the competed-stain method or without antibody (data not shown). Similarly, background labeling was not observed in slides stained without VEGF probe and very minimal background staining was seen with the sense probe (Figures 2 and 4). Thus, it is important to note that all tumor specimens contained regions with low EF5 binding and no VEGF staining. This prompted the consideration of whether there was an overall relationship between observable VEGF staining and EF5 binding. The two measures of EF5 binding (both of which sample the maximum binding, presumably reflecting minimum oxygen levels) were absolute binding and percent cube-reference binding (see Table 1). A plot of VEGF stain intensity versus percent cube-reference EF5 binding results in a reasonable correlation with r value of ∼0.6 (Figure 5).
Figure 5.
Relationship between observed degree of VEGF mRNA signal (measured by ISH) and EF5 binding (measured by IHC staining, and assessed as a percentage of cube-reference binding).
Discussion
Studies exploring the relationship between pro-angiogenic cytokines such as VEGF and hypoxic tumor regions have generally relied on indirect measures of tumor hypoxia, i.e., VEGF signal in cellular regions distant from blood vessels and/or close to necrosis have been assumed to be hypoxic (for example see Ref. [21]). However, hypoxia is only one of several pathophysiologic processes that may cause cell death or regions of high vascularity. For example, necrosis may be due to the absence of nutrients other than oxygen, such as glucose [39]. Regions of high vascularity may be hypoxic if the blood in the vessels is not well oxygenated [40] although it is generally assumed that high blood vessel densities imply a high degree of oxygenation. The recent availability of hypoxia markers that are safe for administration to humans [26,29] has allowed more specific and detailed analysis of the relationship between hypoxia and VEGF signal. The work reported herein has employed the 2-nitroimidazole EF5 for hypoxia detection based partially on previous studies of VEGF mRNA and EF5 colocalization in HT29 and EMT6 spheroids [22]. In that study, as well as herein, expression of VEGF mRNA colocalized with regional maxima in EF5 binding.
Studies measuring VEGF protein by immunohistochemistry have been more controversial. Mouse liver has been found to bind pimonidazole and produce VEGF protein [24]. However, studies by Parliament et al. [27] suggest a lack of correlation between VEGF protein expression and the hypoxia marker, misonidazole, in human tumor xenografts. Similarly, Raleigh et al. [26] reported that in human SCC, pimonidazole labeling was adjacent to areas of necrosis, whereas VEGF protein staining tended to be distant from regions of necrosis. The differences between these studies and our findings may relate to differences in the hypoxiadetection agent used (Pimonidazole or misonidazole vs. EF5), detection modalities, the endpoint measured for VEGF (protein vs. mRNA), analysis methods or the patient population studied. Although similar in the overall mechanism of their bioreduction and binding processes, EF5 and pimonidazole differ in substantive ways. For example, because of the equilibrium of different physical-chemical states of pimonidazole, its biodistribution may depend strongly on local pH and it has several types of non-oxygen dependent metabolism [33,41]. However, the former is unlikely to be the explanation for the lack of correlation seen in the SCC patients analyzed for binding of pimonidazole because VEGF has been reported to be upregulated by acidic microenvironments [42].
EF5 is the only hypoxia marker in current use where absolute levels of binding are being assessed. Although this adds complexity to the analysis, it is intriguing that the highest levels of VEGF RNA expression were only found to be associated with the highest levels of EF5 binding (i.e., >25% of cube reference) (Table 1). To our knowledge, this is the first time that in situ methods for VEGF detection have been combined with a hypoxia marker in tumors from human patients. We chose to study VEGF mRNA, versus protein, because two of the four VEGF isoforms (VEGF121 and VEGF165) are secreted and freely diffusible [43]. Thus, we hypothesized that VEGF mRNA, rather than protein, would more likely be colocalized with hypoxia. Support of this hypothesis would therefore confirm, in human tumors, the upregulation of VEGF by hypoxia established previously in model systems.
A synopsis of the four possible patterns of signal from EF5 binding versus VEGF mRNA induction is presented in Table 2. When VEGF mRNA staining was high, it typically localized to regional maxima in EF5 binding (tumors 5, 10, 16, with 27 possible). Conversely, in regions where EF5 binding was very low (<5% of cube reference) no VEGF signal was detected. Using the calibrated scale of EF5 binding, we can estimate that such low levels of binding would be found at partial pressures greater than ∼3% oxygen. Areas with such low levels of EF5 binding and lack of VEGF mRNA staining were present in all tumors examined and can be observed in all figures described herein. This result was not expected because factors other than hypoxia can upregulate VEGF signal [44]. The third possible pattern would be that of VEGF +ve and EF5 binding -ve. The cervix tumor studied (patient #1, Figure 1) provided an example that was closest to this possibility because it had relatively low EF5 binding, but high expression of VEGF mRNA. However, the highest levels of VEGF expression still corresponded to regional maxima in EF5 binding and these areas were localized adjacent to regions of necrosis (Figure 1). We have hypothesized that necrosis in tumors like this (i.e., without severe hypoxia) might be due to a lack of a nutrient other than oxygen, for example glucose [29]. Lack of glucose has been shown to induce VEGF in multicellular spheroids [45] and it is possible that hypoglycemic stress led to the induction of VEGF in the tumor of patient #1. Alternatively, the moderate hypoxia in this tumor may have been sufficient to cause induction of VEGF mRNA. The fourth possible pattern of staining is that regions of relatively high EF5 binding could occur without measurable upregulation of the VEGF signal; two well-differentiated SCCs showed this pattern (tumors #7 and #26). Of course, the whole point of defining a scale of absolute binding is to avoid terms such as “relatively high” and because the signal:noise ratio of EF5 adduct detection is very high, almost all tumors can be imaged with demonstrable gradients of EF5 binding. Thus, for both of these tumors the maximum EF5 fluorescence was much less than 25% of maximum, and furthermore the regional maximal binding occurred almost exclusively in keratinizing cells that were distant from blood vessels (Figure 3). We have demonstrated that EF5 binding in these terminally differentiated cells is due neither to autofluorescence nor to nonspecific staining by the antibody, as unstained sections and sections stained with competed stain (antibody mixed with authentic soluble antigen — see Materials and Methods section) show essentially no fluorescence in these areas [29]. The conclusion that this binding is hypoxia dependent is supported by the inverse localization of blood vessels and hypoxic keratinization (Figure 3). We suggest the possibility that hypoxic keratinized cells do not induce VEGF production because of their differentiated state. This is supported by studies from Eisma et al. [46] wherein poorly differentiated SCC cells stained more intensely for VEGF protein than did well-differentiated cells [46]. In terms of tumor therapy response, the role of hypoxic keratinized cells is unknown. It seems likely that they would have little influence. Thus, if hypoxia is considered a negative prognostic indictor, it may be necessary to ensure that the hypoxia signal is not emanating from terminally differentiated cells. An advantage of IHC studies is that they allow the investigation of such questions through counterstaining by standard hematoxylin and eosin techniques. This would not be possible for electrode measurements or noninvasive assays of binding by hypoxic markers. Although VEGF ISH signal was not found in the keratinizing, moderately hypoxic cells, we do not have an example where the EF5 staining was greater than 25% of cube-reference binding in these cells. One problem with VEGF ISH signal is its relative difficulty in detection. Not only is the ISH technique much more time consuming than routine IHC staining, but the dynamic range of the signal is quite small. The maximal values appear to vary in a tissue-specific way, undoubtedly due to other factors involved with its regulation. This may be a problem of probe specificity, assay sensitivity or RNA stability of the frozen tissue and we are presently investigating some of these possibilities.
The results reported herein demonstrate that in all tumors that were highly positive for VEGF, the VEGF mRNA signal pattern was coincident with the hypoxia signal from EF5 binding. There have been numerous previous studies evaluating the relationship among the factors of hypoxia, ORPs and outcome. Although these factors may be interrelated, it is not clear that their relationships are limited to a causal sequence, e.g., that hypoxia leads to upregulation of ORPs, which then results in a poor prognosis. In specific tumors one or the other factor may be important or they may be independent factors for prognosis. Future work will address more detailed studies of these possibilities in a larger number of patients. However, we have clearly demonstrated in this report that human tumors upregulate VEGF mRNA by hypoxia.
Table 2.
Summary of the Histologic Relationship between Patterns of EF5 Binding and VEGF mRNA Expression in Human Tumors.
| VEGF+ | VEGF- | |
| EF5+ | Viable hypoxic cells | Keratinized cells or VEGF signal too low to detect |
| EF5- | Not detected in this study | Cells oxic or dead |
Acknowledgements
The authors thank R. Canate-Solare for help in developing the in situ hybridization technique, H. Marti for providing the VEGF probe, A. Giaccia for helpful discussions, and D. Solomon for technical assistance.
Footnotes
This work was supported by grant NIH CA 75285 (S.M.E.).
References
- 1.Hockel M, Knoop C, Schlenger K, Vordran B, Baussmann E, Mitze M, Knapstein PG, Vaupel P. Intratumor pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 2.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tissue oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 3.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 4.Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Chapman W, Levin W, Manchul L, Keane TJ, Hill RP. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–156. doi: 10.1016/s0167-8140(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 5.Moulder JE, Rockwell SC. Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev. 1987;5:313–341. doi: 10.1007/BF00055376. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–5757. [PubMed] [Google Scholar]
- 7.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Molls M, Stadler P, Becker A, Feldmann H, Dunst J. Relevance of oxygen in radiation oncology. Mechanisms of action, correlation to low hemoglobin levels. Strahlen Therapie Und Onkologie. 1998;174(Suppl. 4):13–16. [PubMed] [Google Scholar]
- 10.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 11.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 12.Jelkman W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 13.Mineta H, Miura K, Ogino T, Takebayashi S, Misawa K, Ueda Y, Suzuki I, Dictor M, Borg A, Wennerberg J. Prognostic value of vascular endothelial growth factor (VEGF) in head and neck squamous cell carcinomas. Br J Cancer. 2000;83:775–781. doi: 10.1054/bjoc.2000.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda K, Chung Y-S, Takatsuka S, et al. Tumor angiogenesis and tumor cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer. 1995;72:319–323. doi: 10.1038/bjc.1995.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linderholm B, Tavelin B, Grankvist K, Henriksson R. Does vascular endothelial growth factor (VEGF) predict local relapse and survival in radiotherapy-treated node-negative breast cancer? Br J Cancer. 1999;81:727–732. doi: 10.1038/sj.bjc.6690755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Buchler MW, Korc M. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309–1316. [PubMed] [Google Scholar]
- 17.Maeda K, Kang SM, Onoda N, Ogawa M, Kato Y, Sawada T, Chung KH. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer. 1999;86:566–571. doi: 10.1002/(sici)1097-0142(19990815)86:4<566::aid-cncr4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 19.Salven P, Heikkila P, Anttonen A, Kajanti M, Joensuu H. Vascular endothelial growth factor in squamous cell head and neck carcinoma: expression and prognostic significance. Mod Pathol. 1997;10:1128–1133. [PubMed] [Google Scholar]
- 20.Rossler J, Breit S, Havers W, Schweigerer L. Vascular endothelial growth factor expression in human neuroblastoma: upregulation by hypoxia. Int J Cancer. 1999;81:113–117. doi: 10.1002/(sici)1097-0215(19990331)81:1<113::aid-ijc19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 22.Waleh NS, Brody MD, Knapp MA, Mendonca HL, Lord EM, Koch CJ, Laderoute KR, Sutherland RM. Mapping of the vascular endothelial growth factor-producing hypoxic cells in multicellular tumor spheroids using a hypoxia-specific marker. Cancer Res. 1995;55:6222–6226. [PubMed] [Google Scholar]
- 23.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95:15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol. 1999;155:1065–1073. doi: 10.1016/S0002-9440(10)65209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 26.Raleigh JA, Calkins-Adams DP, Rinker LH, Ballenger CA, Weissler MC, Fowler WC, Novottny DB, Varia MA. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinoma using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765–3768. [PubMed] [Google Scholar]
- 27.Parliament MB, Allalunis-Turner MJ, Franko AJ, Olive PL, Mandyam R, Santos C, Wolokoff B. Vascular endothelial growth factor expression is independent of hypoxia in human malignant glioma spheroids and tumours. Br J Cancer. 2000;82:635–641. doi: 10.1054/bjoc.1999.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danielson T, Rofstad EK. The constitutive level of vascular endothelial growth factor (VEGF) is more important than hypoxia-induced VEGF upregulation in the angiogenesis of human melanoma xenografts. Br J Cancer. 2000;82:1528–1524. doi: 10.1054/bjoc.2000.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans SM, Hahn S, Pook DR, Jenkins WT, Chalian AA, Zhang P, Stevens C, Weber R, Benjamin I, Mirza N, Morgan M, Rubin S, McKenna WG, Lord EM, Koch CJ. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–2024. [PubMed] [Google Scholar]
- 30.Evans SM, Hahn S, Pook DR, Zhang PJ, Jenkins WT, Fraker D, Hsi RA, McKenna WG, Koch CJ. Hypoxia in human intraperitoneal and extremity sarcomas. Int J Radiat Oncol Biol Phys. 2000;49:587–596. doi: 10.1016/s0360-3016(00)01494-2. [DOI] [PubMed] [Google Scholar]
- 31.Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoroproply)acetamide]: analysis of drug adducts by fluorescent antibodies versus bound radioactivity. Br J Cancer. 1995;72:869–874. doi: 10.1038/bjc.1995.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman CN, Wasserman TH, Urtasun RC, Halsey J, Noll L, Hancock S, Phillips TL. Final report of the phase I trial of the hypoxic cell radiosensitizer SR 2508 (etanidazole) Radiation Therapy Oncology Group 83-03. Int J Radiat Oncol Biol Phys. 1990;18:389–393. doi: 10.1016/0360-3016(90)90105-s. [DOI] [PubMed] [Google Scholar]
- 33.Koch CJ, Hahn SM, Rockwell KJ, Covey JM, McKenna WK, Evans SM. Pharmacokinetics of the 2-nitroimidazole EF5 [2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide] in human patients: implications for hypoxia measurements in vivo. Cancer Chemother Pharmacol. 2001;48:177–187. doi: 10.1007/s002800100324. [DOI] [PubMed] [Google Scholar]
- 34.Koch CJ. The reductive activation of nitroimidazoles; modification by oxygen and other redox-active molecules in cellular systems. Sel Act Drugs Redox Processes. NATO ASI Ser, Ser A. 1990;198:237–247. [Google Scholar]
- 35.Breier G. In situ hybridization with RNA probes. Methods Mol Biol. 1999;96:107–117. doi: 10.1385/1-59259-258-9:107. [DOI] [PubMed] [Google Scholar]
- 36.Canete Soler R, Gui YH, Linask KK, Muschel RJ. MMP-9 (gelatinase B) mRNA is expressed during mouse neurogenesis and may be associated with vascularization. Dev Brain Res. 1995;88:37–52. doi: 10.1016/0165-3806(95)00079-s. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin KM, Evans SM, Jenkins WT, Tracy M, Chan CY, Lord EM, Koch CJ. Biodistribution of the nitroimidazole EF5 [2-(2-nitro-1H-imidazole-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)-acetamide] in mice bearing subcutaneous EMT6 tumors. J Pharmacol Exp Ther. 1996;277:1049–1057. [PubMed] [Google Scholar]
- 38.Koch CJ. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and the 2-nitroimidazole EF5. Methods Enzymol — Antioxid Redox Cycling. 2001 doi: 10.1016/s0076-6879(02)52003-6. in press. [DOI] [PubMed] [Google Scholar]
- 39.Koch CJ, Giandomenico AR, Lee Iyenga CW. Bioreductive metabolism of AF-2 [2(2-furyl)-3-(5-nitro-2-furyl)acrylamide] combined with 2-nitroimidazole radiosensitizing agents. Biochem Pharmacol. 1993;46:1029–1036. doi: 10.1016/0006-2952(93)90667-l. [DOI] [PubMed] [Google Scholar]
- 40.Dewhirst MW. Concepts of oxygen transport at the microcirculatory level. Semin Radiat Oncol. 1998;8:143–150. doi: 10.1016/s1053-4296(98)80040-4. [DOI] [PubMed] [Google Scholar]
- 41.Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem. 1998;253:743–750. doi: 10.1046/j.1432-1327.1998.2530743.x. [DOI] [PubMed] [Google Scholar]
- 42.Constant JS, Feng JJ, Zabel DD, Yuan H, Suh DY, Scheuenstuhl H, Hunt TK, Hussain MZ. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair Regener. 2000;8:353–360. doi: 10.1111/j.1524-475x.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- 43.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 44.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 45.Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisma RJ, Spiro JD, Kreutzer DL. Vascular endothelial growth factor expression in head and neck squamous cell carcinoma. Am J Surg. 1997;174:513–517. doi: 10.1016/s0002-9610(97)00166-9. [DOI] [PubMed] [Google Scholar]