Abstract
Bromelain is an aqueous extract from pineapple stem that contains proteinases and exhibits pleiotropic therapeutic effects, i.e., antiedematous, antiinflammatory, antimetastatic, antithrombotic, and fibrinolytic activities. In this study, we tested bromelain's effects on glioma cells to assess whether bromelain could be a potential contributor to new antiinvasive strategies for gliomas. Several complementary assays demonstrated that bromelain significantly and reversibly reduced glioma cell adhesion, migration, and invasion without affecting cell viability, even after treatment periods extending over several months. Immunohistochemistry and immunoblotting experiments demonstrated that α3 and β1 integrin subunits and hyaluronan receptor CD44 protein levels were reduced within 24 hours of bromelain treatment. These effects were not reflected at the RNA level because RNA profiling did not show any significant effects on gene expression. Interestingly, metabolic labelling with 35-S methionine demonstrated that de novo protein synthesis was greatly attenuated by bromelain, in a reversible manner. By using a trans-activating signaling assay, we found that CRE-mediated signaling processes were suppressed. These results indicate that bromelain exerts its antiinvasive effects by proteolysis, signaling cascades, and translational attenuation.
Keywords: bromelain, glioma cells, invasion, integrin, protein translation
Introduction
Primary brain tumors (gliomas) arise and typically remain within the central nervous system. They are characterized by diffuse and extensive infiltration into the brain parenchyma, rendering surgical removal and local radiotherapy ineffective. The pattern of invasion appears to follow the path of blood vessels and myelinated fiber tracts, and distant spread may thus occur through the corpus callosum, resulting in bilateral growth of the tumor [1]. Tumor cell invasion is defined as an active translocation of neoplastic cells through the host cellular and extracellular matrix (ECM) barriers [2], where ECM may be defined as the naturally occurring extracellular substrate upon and through which cells migrate, proliferate, and differentiate, respectively [3]. The distribution of the classical ECM proteins, such as laminin, collagen type IV, fibronectin, and vitronectin, in the brain is limited to vascular basement membranes and the glia limitans externa. During tumor progression, the ECM and its cell surface receptors may change. Changes in cell adhesion, proteolytic degradation of the ECM, and cell migration are common hallmarks of tumor cell invasion. The dissemination of glioma cells is a function of two cellular properties characterized by migration and invasion. Although the two terms are used interchangeably in the context of tumor metastasis, there are functional features that distinguish them. Classically, migration refers to cell locomotion. In contrast, the process of invasion involves migration, and the additional feature of a degradative function, typically reflecting the tumor cells' ability to translocate through ECM barriers by the release of proteases [4].
Integrins represent a large class of at least 20 transmembrane heterodimer receptor proteins consisting of various combinations of α and β subunits. Integrins mediate cell-cell or cell-matrix adhesion, and it is thus not surprising that gliomas utilize integrins for mobility along ECM components. Interestingly, an upregulation of various integrins in gliomas, compared to the normal brain, has been observed [5]. The α3β1 integrin complex has been identified as one of the main integrin receptors expressed on glioma cells [6]. This integrin complex binds to several ECM components such as laminin, fibronectin, and collagen type IV. Studies have demonstrated that blocking the α3 and β1 integrin subunits with neutralizing antibodies inhibits glioma cell migration and invasion [7–9].
Hyaluronic acid is a major component of the brain ECM. It has been suggested that infiltration of glioma cells requires interactions with hyaluronan receptors. Evidence for this has been provided by Gunia et al. [10] and Breyer et al. [11], who prevented glioma cell invasion by using specific monoclonal antibodies against CD44 in different in vivo glioma models.
Over the past two decades, bromelain, an extract from pineapple stem (Ananas comosus), has been used clinically for a wide variety of maladies including edema, thrombophlebitis, sinusitis, inflammation, rheumatic arthritis, and as adjuvant cancer treatment [12]. Although poorly understood, the pleiotropic effects of bromelain are considered to be due to the complex mixture of closely related cysteine proteinases, proteinase inhibitors, phosphatases, glucosidases, peroxidases, and other undefined compounds [13,14]. In addition, bromelain has shown both antiproliferative and antimetastatic effects in tumor models in vitro and in vivo [15–19]. Despite this knowledge, no data exist on bromelain's effects on glioma cells.
Because bromelain contains a mixture of proteinases, we hypothesized that cleavage of integrins could prevent their function as receptors and thereby also inhibit the invasive capacity of the glioma cells. To test this hypothesis, we analyzed the effects of a crude aqueous bromelain extract on glioma cell adhesion, migration, and invasion using several representative glioma cell lines in various in vitro assays. We studied the effects of bromelain on the expression of receptors known to be important in glioma cell invasion, namely α3β1 integrin and the hyaluronan receptor CD44 (HCAM). Finally, we assessed the effects of bromelain treatment on gene expression and cellular homeostasis. Our results show that bromelain can reversibly inhibit glioma cell migration and invasion by proteolytically affecting integrin expression. In addition, bromelain effects are transduced into the intracellular milieu of the cell to affect signaling cascades and protein translation processes.
Materials and Methods
Bromelain
Crude bromelain extract called base powder from pineapple stem (A. comosus) was purchased from CPC W. Mühlbauer, Hamburg, Germany. Its proteinase activity was 0.35 U/mg, using l-pyroglutamyl-l-phenylalanyl-l-leucine-p-nitroanilide as substrate [20].
Cell Lines and Cell Cultures
The human glioma cell lines AN1/lacZ, U-251/lacZ, U-251/GFP, A-172, and U-87/GFP were used in this study [7,21,22]. The cells were cultured in complete growth medium (CGM) consisting of: Dulbecco's modified essential medium (Sigma, St. Louis, MO) supplemented with 10% heat-inactivated newborn calf serum, four times the prescribed concentration of nonessential amino acids, 2% glutamine, penicillin (100 IU/ml), and streptomycin (100 µg/ml). For the lacZ stable cell lines, Geneticin (1 mg/ml, G-418; Sigma) was added to maintain the lacZ-positive cells under selection pressure. The cells were maintained in a standard tissue culture incubator at 37°C with 100% relative humidity, 95% air, and 5% CO2. Multicellular spheroids were initiated by seeding 5x106 cells into 80 cm2 tissue culture flasks base-coated with 0.75% agar in CGM [23]. The spheroids were used between 5 and 7 days of culture.
Cell Adhesion Assay
Cell adhesion was evaluated according to Schulz et al. [24]. Briefly, near-confluent monolayers were treated with 1 mM EDTA in phosphate-buffered saline (PBS), rinsed, and suspended in CGM. The cells were seeded into 96-well plates (3x104 cells/well in 200 µl). After 30 and 60/80 minutes of incubation, under standard culture conditions, the attached cells were fixed in 1% glutaraldehyde in PBS. Fifteen minutes after fixation, the supernatant was aspirated and 0.1% amido black (Merck, Darmstadt, Germany) in sodium acetate buffer (pH 3.5) was added to the plate for 30 minutes. The wells were rinsed with acidic water (acidified with HCl to pH 3.5) and protein-bound dye was eluted with 50 mM NaOH. The optical densities of the wells were recorded using a photometric microplate reader (Titertek Multiscan Plus, Heidenreich, Oslo, Norway) at 595 nm (maximum absorbance). The data, which were corrected for background absorbance (CGM without cells), were analyzed by one-way analysis of variance (ANOVA), and Scheffé's test was used for post-hoc comparisons of mean values.
Tumor Cell Migration
Cell migration was determined by seeding multicellular spheroids (between 175 and 300 µm in diameter) individually into 96-well culture plates, and then the cell outgrowth from the spheroids was evaluated [25,26]. Time lapse studies were performed using a Leica DM IRBE inverted microscope (Leica Lasertechnik GmbH, Heidelberg, Germany) with an incubator (Edmund Bühler GmbH & Co., Bodelshausen, Germany), which maintained a temperature of 37°C and an atmosphere of 95% O2 and 5% CO2. Digitized images were obtained using a Photonic Science analog camera, which was computer-controlled by Improvision Open Lab 1.7.0 software (Improvision, Image Processing and Vision, Coventry, UK). This apparatus enabled the automatic recording of pictures of the migrating cells as a function of time. The images were obtained using a x10 Hoffman objective. The assay was performed over 116 hours.
Invasion Assay
Tumor cell invasion was evaluated using an in vitro co-culture model, where normal fetal rat brain cell aggregates were confronted with multicellular tumor spheroids from the green fluorescence protein (GFP)-transfected U-87 cell line. The procedure for producing brain cell aggregates has been described in detail previously [27,28]. Briefly, the brain aggregates were prepared by mincing brains from 18-day-old inbred BD-IX rat fetuses [29]. The brain tissue was serially trypsinized and the resulting single cell suspension was allowed to reaggregate by seeding 3x106 cells into 16-mm multiwell plates, base-coated with agar in CGM. The aggregates were transferred to an 80-cm2 tissue culture flask after 48 hours and cultured for 18 days prior to confrontation with tumor spheroids.
U-87/GFP tumor spheroids (between 200 and 313 µm in diameter) were transferred into 96-well plates base-coated with agar in CGM. Single brain cell aggregates of approximately the same size as the tumor spheroids were added. The tumor spheroids and the brain cell aggregates were placed in close contact with each other. The cocultures were incubated in CGM in the presence and absence of 50 µg/ml bromelain. The optimal bromelain concentration was determined by dose-response experiments, using the cell adhesion and migration assays. After 72 hours, the co-cultures were examined using a Leica TCS NT confocal laser scanning microscope (Leica) attached to an inverted (Leica DM IRBE) microscope. Thirty-two optical sections were recorded over a distance of 100 µm into the co-cultures, with a resolution of 512x512 pixels per channel. In order to improve the quality of the slow scan mode, each section was recorded as an average of four pictures. GFP was excited with the 488 nm line of an Ar-Kr laser and fluorescence was obtained through a long pass filter (590 nm). The pinhole setting was optimized to gain maximum confocal quality. Visualization of the co-cultures was performed by the fluorescence detector and the transmission detector, which visualized the spheroids and the brain cell aggregates, respectively. The serial image files were transferred to a Silicon Graphics O2 workstation for three-dimensional visualization, using AVS/Express (Advanced Visual Systems, Waltham, MA) as described elsewhere [30]. The invasion was quantified by analyzing the 32nd section in each co-culture by the native Leica software. Invasion was defined as the penetration of the malignant green fluorescent cells into the brain cell aggregates. Tumor cell invasion was quantified by assessing the tissue area representing the original spherical shape of the brain cell aggregate. Thereafter, the area representing the remaining brain cell aggregate was measured. The replacement of brain tissue by tumor cells was calculated as the percent reduction of the brain cell aggregate area [26]. The data were analyzed by Mann-Whitney rank test.
Tumor Spheroid Growth
Spheroids (between 225 and 362 µm in diameter), prepared from the AN1/lacZ and U-87/GFP cell lines, were cultured individually in 16-mm multiwell dishes base-coated with agar in CGM in the presence and absence of 50 µg/ml bromelain. Two orthogonal diameters of the spheroids were recorded over a 20-day period. The relative increase in spheroid volume was calculated using the formula for the volume of a sphere.
Immunohistochemistry
Tumor spheroids, prepared from the AN1/lacZ glioma cell line, were incubated for 4 days in 50 µg/ml bromelain-supplemented CGM or in CGM alone, rinsed in PBS, embedded in Tissue-Tec (Miles, Elkhart, IN), and frozen in thawing isopentane. Ten-micrometer sections were cut using a Reichert cryomicrotome (Leica Instruments, Heidelberg, Germany) and transferred to poly-l-lysine-coated slides. The specimens were fixed in acetone for 3 minutes prior to incubation for 1 hour at 25°C with a panel of monoclonal antibodies (α3 integrin subunit (1:50 dilution in PBS) (Becton Dickinson, San Jose, CA), β1 integrin subunit (1:100) (P4C10; Gibco BRL, Gaithersburg, MD), and CD44 (1:50) (Becton Dickinson). Following 3x5-minute wash cycles in PBS, FITC-conjugated sheep anti-mouse IgG (1:20) (Dako, Glostrup, Denmark) was added for 1 hour at 25°C. Sections were then washed 3x5 minutes in PBS, digested with RNAse (0.5 mg/ml), and nuclear-stained with 10 µg/ml propidium iodide. The sections were examined using a Leica TCS NT confocal laser scanning microscope (Leica) attached to an upright (Leica DMRXA) microscope.
Protein Extracts
Protein extracts were prepared by scraping cells in 1 ml of boiling extraction buffer [20 mM Tris, pH 8.0, 1 mM EDTA, 65 mM DTT, 0.3% sodium dodecyl sulfate (SDS)]. The suspension was separated by centrifugation at 15,000g for 30 minutes at 4°C. The supernatant was collected and protein concentrations were determined by the Bio-Rad dye binding assay (Bio-Rad Laboratories, Munich, Germany) using bovine serum albumin as a standard. Twenty micrograms of protein extracts was heated at 95°C for 5 minutes in SDS sample buffer (0.125 M Tris/HCl, pH 6.8, 17.4% v/v glycerol, 2% w/v SDS, 0.001% bromophenol blue, 2% v/v β-mercaptoethanol) and samples were separated by SDS polyacrylamide gel electrophoresis (PAGE) using the Criterion system (Bio-Rad).
Metabolic Labelling
Cells were cultured to 70% confluence in CGM prior to the addition of 50 µg/ml bromelain. Following up to 7 days of bromelain treatment, cells were incubated in 1 mCi/ml 35-S methionine in Dulbecco's modified Eagle medium without methionine (Gibco BRL). In some instances, bromelain-treated cells were allowed to recover for up to 3 days in CGM prior to radioactive methionine pulsing. After pulse labelling for 30 minutes, cells were washed with CGM prior to extract preparation. Proteins were fractionated by SDS-PAGE, stained with Coomassie blue, dried, and exposed to film until the desired signal-to-noise ratio was obtained.
Western Blotting
Fractioned proteins were transferred to nitrocellulose membranes by electroblotting using the Criterion system (Bio-Rad). Immunoblot analysis was performed in a Western processor robot (Amersham, Buckinghamshire, UK). Briefly, blots were placed in the processor and treated with the following layers at 4°C: 1) TBST (10 mM Tris, pH 7.4, 154 mM NaCl, 0.5% Tween 20) and 5% dry milk for 1 to 2 hours; 2) primary antibody diluted 1:1000; integrin α3 (I-19, sc-6592; Santa Cruz Biotechnology, Santa Cruz, CA); integrin β1 (M-106, sc-8978; Santa Cruz Biotechnology), HCAM (CD44) (H-300, sc-7946; Santa Cruz Biotechnology), in TBST for 4 to 8 hours; 3) three 1-hour washes in TBST; 4) secondary antibody diluted in TBST for 2 hours; and 5) three 1-hour washes in TBST. The blots were then reacted with a chemiluminescence reagent (Pierce Chemical, Rockford, IL) and exposed to film until the desired signal-to-noise ratio was obtained. Primary antibodies were detected by anti-rabbit, anti-goat, or anti-mouse IgG (dilution 1/10,000; Amersham).
Gene Arrays
The expression of over 40 genes known to be important for metastasis and apoptosis was profiled on membranes from a GEArray Kit (SuperArray, Bethesda, MD). Briefly, RNA was isolated (Trizol; Gibco BRL) from cells that had been cultured either in CGM or treated with 50 µg/ml bromelain in CGM for 1 or 24 hours. Radioactive probes were prepared using α-32-P dCTP and a RT-PCR protocol and primers from the GEArray Kit (SuperArray). Membranes containing DNA spots were hybridized at 68°C in hybridization buffer containing radiolabeled DNA probes. Following hybridization, membranes were washed extensively. The most stringent wash was with 0.1x SSC (15 mM NaCl and 1.5 mM Na-citrate), 0.5% SDS at 68°C. Membranes were then wrapped in plastic and exposed in a Phosphorimager (Fujifilm FLA-2000, Stockholm, Sweden) for a time course to determine linear exposure times. Visual inspection was used to analyze the results. Each membrane contained 21 or 23 genes that were spotted in duplicate. In addition, each membrane contained multiple spots of the housekeeping genes (actin and GAPDH) and a negative control (pUC18, bacterial plasmid).
Transactivation Signaling Assay
Cells (AN1/lacZ) were seeded into 96-well plates (104 cells/well). After 24 hours, the cells were transfected with expression constructs containing promoter elements 5′ to the luciferase reporter gene. Three different promoter configurations were used: 1) a weak promoter and a cAMP response element (CRE); 2) a weak promoter with an AP-1 cis-acting element; or 3) a weak promoter alone. These constructs were obtained from Clontech (Clontech Mercury signaling systems; Clontech Laboratories, Palo Alto, CA). Fugene (Roche Molecular Biochemicals, Roche, Oslo, Norway) was used according to the manufacturer's instructions for cell transfection. Three days after the transfection, cells were treated with 50 µg/ml bromelain in CGM for 1 and 24 hours. Control cells were maintained in CGM. Extracts were prepared using a luciferase reagent (Promega, Madison, WI) and measured in a luminometer (Anthos Lucy 1; Anthos Labtec Instruments GmbH, Salzburg, Austria). Controls consisted of untransfected extract and yielded no signal over the background. The data were entered into Kaleidagraph (V3.5, Synergy) and box plots were generated.
Results
Bromelain Reversibly Disrupts Glioma Cell Adhesion and Migration
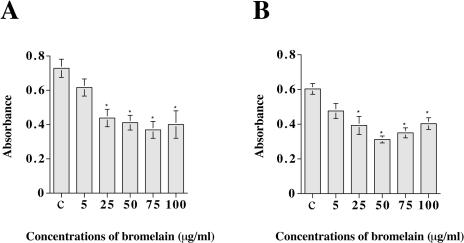
We added bromelain to monolayer cultures of AN1/lacZ and U-251/lacZ glioma cells and observed after 24 hours that the cells were detached from the plastic surface and floated in suspension as rounded and aggregated cells. Dose-response experiments were performed to determine the concentration of bromelain required to cause effects on cell adhesion. There were small differences in the monolayer cell adhesion to the plastic surface after 30 minutes of incubation with the addition of different doses of bromelain. But the addition of 25 to 100 µg/ml bromelain to the medium for 60 (U-251/lacZ) and 80 minutes (AN1/lacZ) caused a significant decrease in optical density, and the cells started to lose contact with the substrate (Figure 1). We chose 50 µg/ml bromelain for use in further experiments. This concentration of bromelain was comparable to those used in other experimental systems [19].
Figure 1.
Bromelain inhibits glioma cell adhesion. The diagrams show adhesion of AN1/lacZ (A) and U-251/lacZ (B) human glioma cells exposed to increasing concentrations of bromelain. The number of cells adhering to the plastic was quantified using photometry (y-axis). Data expressed as mean±SEM; n=8 in each group. Asterisks indicate significant differences from control (ANOVA and Sheffé's test). c=control (complete growth medium).
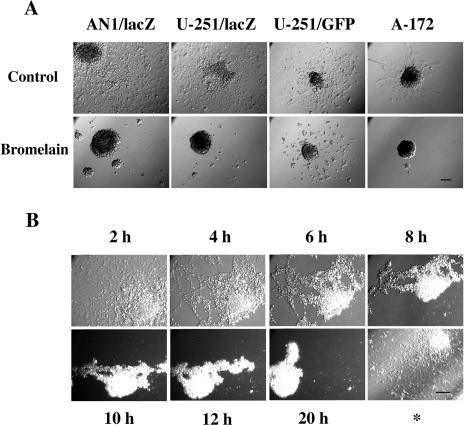
In order to study glioma cell migration following bromelain treatment, we generated spheroids and performed cell migration assays in CGM (Figure 2A, upper panel) and in CGM containing bromelain (lower panel). Spheroids are stable multicellular configurations that occur when cells are grown in dishes, which have been base-coated with agar; when placed in plastic tissue culture dishes with CGM, the spheroids attach to the bottom of the dish and the cells migrate outwards. All four cell lines tested (AN1/lacZ, U-251/lacZ, U-251/GFP, A-172) showed a reduction in cell migration after exposure to 50 µg/ml bromelain (Figure 2A). Treatment with heat-treated bromelain (1 hour at 70°C) or trypsin (50 µg/ml) did not induce cell detachment in monolayer cultures. We also observed that spheroids prepared from different human glioblastoma biopsies were unable to attach or migrate in bromelain-supplemented medium, showing that the effects of bromelain were not limited to glioma cell lines (data not shown).
Figure 2.
Bromelain inhibits glioma cell migration. (A) Cell migration (48 hours) from multicellular spheroids from different human glioma cell lines in 96-wells with and without bromelain (50 µg/ml). Bar, 100 µm. (B) Time lapse study of cells from an AN1/lacZ spheroid, which had been allowed 48 hours of migration in DMEM supplemented with 10% serum, before the medium was changed to a medium containing 50 µg/ml bromelain. The time points refer to the time after bromelain was added to the medium. *This picture was taken 96 hours after the bromelain medium was replaced with ordinary serum supplemented medium. Bar, 100 µm.
Time lapse studies were performed to identify the temporal characteristics of bromelain action. We observed that in bromelain-supplemented media, the migrating cells lost their adherence to the plastic surface and retracted, during a 20-hour period, back to spheroids (Figure 2B). The spheroid lost contact with the bottom of the dish after 8 to 10 hours. Some single cells were observed, but most were in the spheroid configuration. To demonstrate that this was a reversible process, bromelain was removed and replaced with fresh CGM. The spheroids then reattached to the substrate and the cells started to migrate out again, demonstrating that the cells were able to recover from the bromelain treatment (Figure 2B*).
Bromelain Treatment Reduces Tumor Cell Invasion
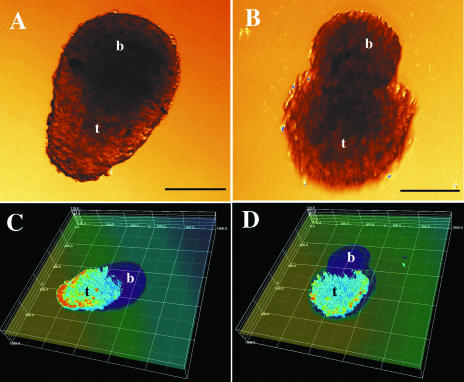
Because bromelain inhibited cell migration from multicellular spheroids, we hypothesized that bromelain would inhibit the tumors' invasive properties as well. To examine this possibility, we made co-cultures of brain cell aggregates with U-87 glioma cells that had been stably transfected with GFP. This model enabled us to evaluate tumor cell invasion in living cultures by monitoring GFP expression over the course of 3 days. Tumor cells characteristically invaded the brain aggregates in the untreated co-cultures (Figure 3A and C). Less invasion was observed following treatment with bromelain, along with a more defined border between the tumor spheroid and the brain aggregate (Figure 3B and D). Quantification of the results showed significant differences. In the control group (n=4), there was a 68% reduction in the amount of brain tissue after 3 days of co-culture, whereas in the bromelain-treated group (n=4), there was only a 25% reduction (P<0.02, Mann-Whitney statistical test for unpaired nonparametric comparison).
Figure 3.
Bromelain reduces glioma cell invasion into normal brain cell aggregates. Tumor cell invasion in 72-hour co-cultures of spheroids prepared from the U-87/GFP human glioma cell line and normal fetal rat brain cell aggregates. Transmission light microscopy pictures of a control co-culture (A) and a bromelain-treated co-culture (B). Scale bars, 100 µm. t=tumor, b=brain. Three-dimensional reconstructions of confocal microscopy sections of the same tumor spheroids combined with transmission pictures of the brain aggregate (C, control; D, bromelain-treated). A linear color map ranging from zero (dark blue, no transmission) to 255 (red, highest fluorescence intensity) was used. The distance between the lines is 200 µm along the x-and y-axes, and 27 µm along the z-axis.
Cell Surface Protein Expression Is Affected by Bromelain Treatment
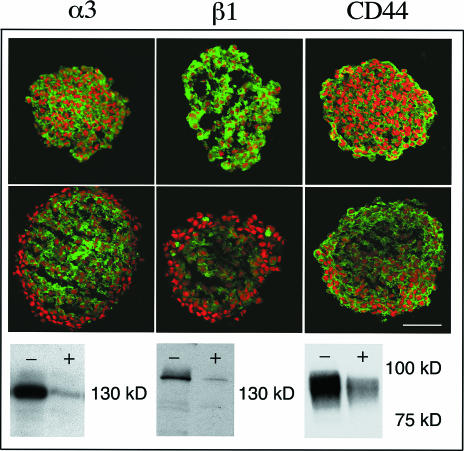
We postulated that the reduced cell adhesion, migration, and invasion were due, at least in part, to the proteolytic effects of bromelain on the cell surface. To address this assumption, we assayed for expression of α3 and β1 integrin subunits; these represent two cell surface proteins that have been implicated in glioma cell migration and invasion. In addition, we assayed for another cell surface receptor associated with invading glioma cells, CD44 (HCAM). Immunohistochemical experiments, on control spheroids from the AN1/lacZ cell line, showed that all of these proteins were expressed throughout the spheroid (Figure 4, upper panel). After 4 days of bromelain exposure, integrin and CD44 expression was diminished and the expression of the α3 and β1 subunits was completely lost in the outermost three to four cell layers of the spheroids (Figure 4], middle panel). We confirmed and extended these results by performing Western blotting experiments on monolayers that had been treated with bromelain for 2 and 24 hours. Bromelain treatment for 2 hours did not alter α3 and β1 integrin and HCAM expression (data not shown). In contrast, a decrease in α3 integrin, β1 integrin, and HCAM levels was observed after 24 hours of bromelain treatment (Figure 4, lower panel). Immunoblots, using different glioma cell lines and human glioblastoma biopsy extracts, also showed a reduced expression of cell surface protein after bromelain treatment (data not shown).
Figure 4.
Bromelain affects cell surface protein expression. Scanning confocal microscopy pictures of α3 and β1 integrin subunits and CD44 immunostained spheroid sections (green signal) from the AN1/lacZ human glioma cell line. Nuclei are stained with propidium iodide (red). Upper panel: control spheroids; middle panel: bromelain-treated spheroids. Scale bar, 100 µm. Lower panel: corresponding Western blot of monolayer cultures; control cells marked with (-) and bromelain-treated cells marked with (+).
Transcriptional Profiling Is Unchanged Following Bromelain Treatment
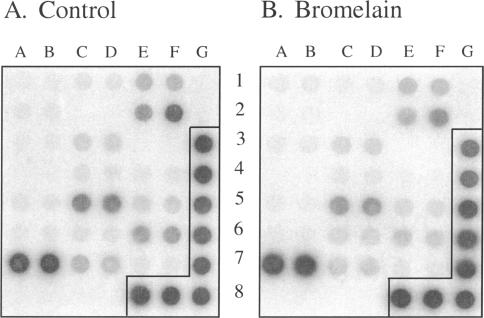
To identify if these effects on protein expression were linked to a reduction in mRNA expression, we conducted experiments using a gene array hybridization strategy. We selected arrays that contained DNA encoding for genes regulated during tumor metastasis and apoptosis pathways. The α3 integrin and the CD44 genes were both included in these 44 genes studied. Interestingly, no major changes were observed in the gene expression profile following bromelain treatment for 1 to 2 hours or 24 hours (Figure 5). These results supported the notion that bromelain exerts its effect through proteolytic cleavage.
Figure 5.
Transcriptional profiling following bromelain treatment. Gene expression profiles of control (A) and bromelain-treated (24 hours) AN1/lacZ human glioma cells (B) in a cancer/metastasis GEArray. Probes were prepared using RT-PCR techniques and hybridized to membrane genes involved in tumor cell adhesion and genes for proteolysis of ECM during tumor cell invasion. Following extensive washing, membranes were exposed to a phosphoimager to capture an image. No large variations in gene expression were detected following the bromelain treatment. The α3 integrin and CD44 are located, respectively, at 5C, 5D and 1E, 1F. The two housekeeping genes actin and GAPDH are located, respectively, at 3G, 4G and 5G, 6G, 7G, 8E, 8F, 8G, whereas the negative control (pUC18, bacterial plasmid) is located at 1G, 2G. The signals were found to be within the linear range of exposure.
Bromelain Treatment Does Not Influence Cell Survival
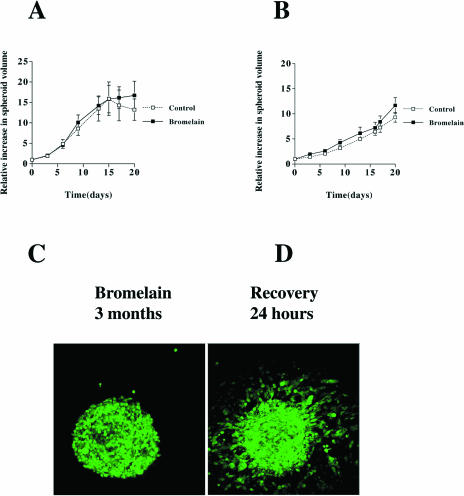
We subjected spheroids prepared from AN1/lacZ and U-87/GFP cells to bromelain treatment and analyzed the spheroid volume for 20 days. Bromelain treatment had no significant effect on spheroid growth for the two cell lines studied (Figure 6A and B). In addition, we did not observe any increase in dead cells as determined by propidium iodide staining, suggesting no cytotoxic effects resulting from bromelain treatment. In a separate set of experiments, we assessed cellular proliferation in bromelain-treated cells. We cultured cells in bromelain for 3 days prior to addition of tritium-labelled thymidine. Analysis for radioactive incorporation indicated that bromelain-treated cells incorporated the radiolabeled thymidine, suggesting that bromelain-treated cells continued to divide during and following bromelain treatment (data not shown). Based on the results above, we allowed spheroids to grow in bromelain for longer periods of time, up to 3 months, prior to replacement with CGM. After bromelain removal, cells once again attached and began to migrate out of the spheroid (Figure 6C and D).
Figure 6.
Glioma spheroid growth and reversal of inhibited migration from spheroids. Cell growth of AN1/lacZ (A) and U-87/GFP (B) human glioma spheroids with and without bromelain (50 µg/ml). The relative increase in volume is expressed as mean±SEM; control, n=4 (AN1/lacZ), n=5 (U-87/GFP); bromelain-treated group, n=5. (C) U-87/GFP human glioma spheroid grown in bromelain-containing medium for 3 months. The spheroid is floating in the medium. (D) Twenty-four hours after bromelain removal. The spheroid is now attached to the culture dish and cells are migrating out from the spheroid.
Bromelain Attenuates De Novo Protein Synthesis
We treated cells with bromelain for 24 hours up to 7 days and then pulsed them with 35-S methionine for 30 minutes. Cellular extracts were prepared and proteins fractionated by SDS-PAGE. This assay showed that following bromelain treatment protein synthesis was decreased compared to untreated cells (Figure 7). Interestingly, following 3 days of recovery in CGM, we observed normal levels of protein translation. This was our first indication that bromelain treatment could affect intracellular mechanisms. There was no indication that bromelain gained access to the intracellular milieu because the GFP-expressing cells remained GFP-positive (Figures 3C and D and 6C and D).
Figure 7.
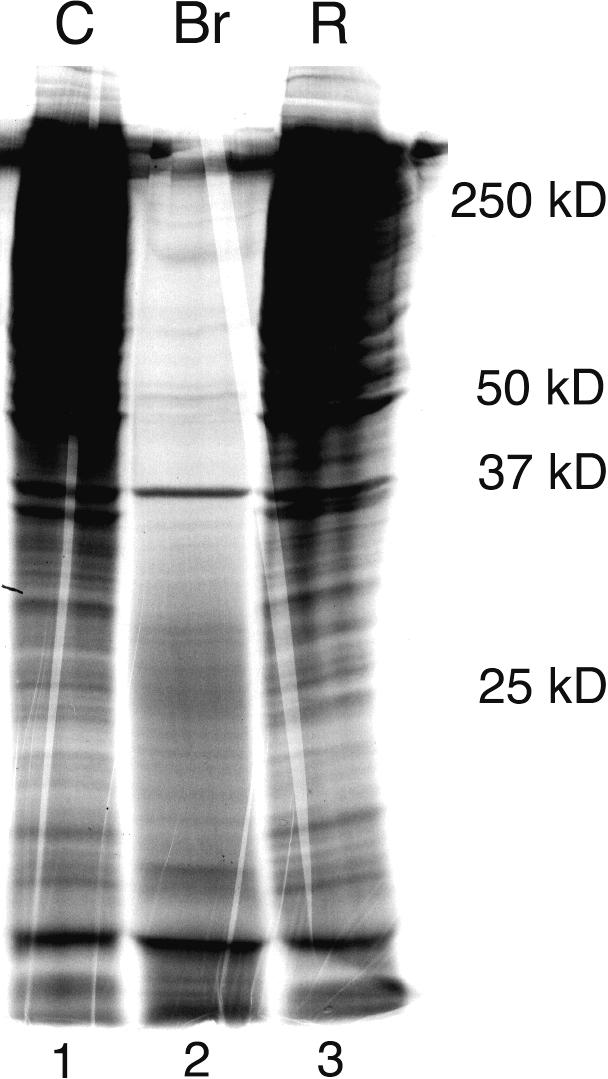
Bromelain attenuates de novo protein synthesis in glioma cells. SDS-PAGE showing newly synthesized proteins from 35-S methionine-labelled U-87/GFP- transfected human glioma control cells (Lane 1), cells treated with bromelain for 7 days (Lane 2), and cells treated with bromelain for 4 days and then allowed a 3-day recovery period in complete growth medium (Lane 3). Molecular size markers are indicated to the right.
Bromelain Alters Intracellular Signaling
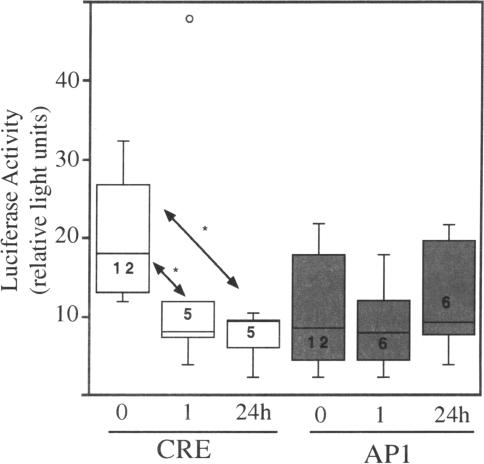
Because protein synthesis was attenuated, we next addressed if any signaling processes were interrupted following bromelain treatment. Luciferase reporter constructs bearing cis-acting elements coupled to weak promoters were transfected into equal numbers of cells for 3 days. This was followed by bromelain treatment for 1 and 24 hours. The expression of luciferase was examined in three different promoter paradigms: 1) a weak promoter with a CRE element; 2) a weak promoter with an AP-1 element; and 3) a weak promoter. Phosphorylation of the trans-acting factor (CREB or AP-1) was expected to facilitate binding to the respective element and to activate the promoter sequences to drive expression of the luciferase enzyme. The data presented in the box plot (Figure 8) were obtained following extract preparation and processing for the luciferase assay. Several observations were notable. Basal levels of both CRE-and AP-1-mediated luciferase activity could be measured, and the CRE element appeared to activate luciferase expression to higher levels. CRE activation was significantly decreased, following 1 hour of bromelain treatment, and this effect was also observed at the 24-hour timepoint. It should be emphasized that the level of CRE-activated luciferase was reduced, but not totally blocked. In contrast, AP-1-activated sequences were not significantly affected by bromelain at any of the time points studied. Control experiments were performed with transfected constructs that contained only the weak promoter and the luciferase gene, and luciferase activity was never detected over the background levels.
Figure 8.
Bromelain affects nuclear signaling. AN1/lacZ cells were transfected with luciferase reporter constructs containing CRE or AP-1 cis-acting elements prior to bromelain treatment for 0, 1, or 24 hours. Cells were extracted and luciferase was measured. The data, shown in the form of a box plot, indicate that CRE signaling was significantly affected following bromelain treatment. The rectangle represents 50% of the data, and the mean is indicated by the horizontal line dissecting the box. The lines above and below the box establish the range of the data; a small open circle represents an outlier. Numbers in the rectangle represent trial numbers. Trials were done in duplicates. Asterisks denote significance [CRE 1 hour: P<.002; CRE 24 hours: P<.01, Kaleidagraph (V3.5, Synergy)].
Discussion
A fundamental management challenge presented by glioma patients is to control the invasion of malignant cells into the normal brain. The highly infiltrative behavior of gliomas causes severe difficulties in achieving complete surgical resections. To hinder the migration of the invasive glioma cells without negative impact on neighboring bystander cells represents an attractive treatment approach. In this study, we have examined effects of the pineapple extract bromelain on several glioma cell lines and glioblastoma biopsy spheroids. Glioma cell lines with different characteristics were chosen based on results from previous studies, assay suitability (e.g., stably transfected with GFP), and cell availability. Our salient findings are: 1) bromelain treatment of glioma cells reduces their ability to migrate and invade; 2) cells undergoing treatment are viable and can divide; 3) bromelain affects cell surface proteins, protein translation, and intracellular signaling pathways; and 4) the effects of bromelain are reversible.
Intrinsically, glioma cells are programmed to migrate and invade brain tissue. All the glioma cells studied displayed reduced adhesive, migratory, and invasive properties following bromelain treatment. The cells were able to survive for long periods of time in bromelain, there were no cellular signs of destruction, and the effects were reversible, indicating the potential also for neighboring non-cancer cells to recover. Bromelain is thus a drug which, by local delivery to the tumor, could localize the tumor and restrict glioma migration for extended periods. These favorable antiinvasive drug attributes may provide a complementary treatment strategy in combination with surgical removal. Bromelain was found to affect cell surface receptor systems important for glioma cell migration and invasion, namely the α3 and β1 integrin subunits and CD44. Reduced expression of CD44 caused by bromelain has also been shown by others on leukemia and melanoma cells [19,31]. The reduction of cell surface proteins is best explained by proteolysis because transcriptional profiling revealed no obvious alterations. It is likely that the mixture of proteases in bromelain cleaved many, if not all, cell surface markers. This assumption was supported by the fact that molecules important for cell-cell adhesion, as well as epidermal growth factor receptor expression, also were decreased after bromelain treatment.
Of considerable interest is the observation that bromelain inhibited protein translation. This is a novel finding that has not been associated with bromelain treatment previously. It is important to note that the translation machinery was functional because we detected the expression of some proteins. It is unknown how bromelain affects protein translation. We have no evidence that bromelain has gained intracellular access; if so, a reduced GFP expression in the cell lines following bromelain treatment would have been expected. If bromelain were present intracellularly, we should also expect detrimental consequences to the cells due to the proteolytic capacity of this compound. We therefore favor the most simplistic model whereby bromelain proteolytically cleaves the cell surface receptors resulting in, among other phenomena, reduced cell migration. In addition, the cleavage of transmembrane proteins can disrupt intracellular signaling cascades and thereby disturb the translational machinery.
The specific signaling mechanisms affected by bromelain are unknown, but integrins are, in addition to promoting cell adhesion and migration, known to contribute to intracellular signaling processes [32]. Integrins are considered to influence several pathways by regulating the activity of transcription factors [33,34]. Downstream effects of bromelain have been reported by Mynott et al. [35], who found that bromelain inhibited ERK-2 phosphorylation and signaling in T cells. The ERKs belong to the family of mitogen-activated kinases (MAPK), which act as a key element in the signaling pathways involved in transducing receptor-initiated signals to the nucleus [36,37]. There is also some evidence that links integrin engagement together with the activation of the MAPK pathway [38]. Cell adhesion to the ECM has also been suggested to induce a rapid increase in the translation of preexisting messenger RNA [39,40] and translational control at the site of integrin binding [41]. Bromelain, by its effect on integrins, may interfere with such mechanisms. The inhibition of cell adhesion by bromelain may also result in an inability of the cells to transmit the signals normally generated by attached cells to the nucleus, thus resulting in reduced protein synthesis. The CRE-mediated luciferase activity was significantly reduced after bromelain treatment. Whether a bromelain-mediated relationship exists between CRE signaling and protein translation is unknown. We suggest that the decrease in CRE-mediated activation was not due to a general decrease in intracellular signaling because bromelain did not affect AP-1-mediated luciferase activity. To address these issues will require further studies, but the results indicate that intracellular pathways are differentially affected during bromelain treatment.
Our results suggest that the antiinvasive property of bromelain is dependent on its proteolytic activity. However, in 1988, Batkin et al. [16] showed that heat inactivation of bromelain did not abolish its antimetastatic capacity in the Lewis lung tumor model on C57B1/6 mice. Grabowska [42] found, by evaluating tumor cell invasion through an ECM layer in vitro, that heat inactivation of bromelain did not reduce its antiinvasive capacity on mammary tumor and melanoma cells. These observations, in contrast to the here reported findings, may only be settled when the responsible components of the crude bromelain extract have been isolated and characterized by biochemical and pharmacological criteria, the latter by means of animal experiments.
Bromelain consists of a mixed group of proteins with different fractions and activities. Future challenges will be to isolate and characterize, using biochemical methods, the component(s) of bromelain responsible for the various effects observed at the cellular level, and to put these findings into the complex context of invading glioma cells both in vitro and in vivo. The striking effects of bromelain on glioma cell adhesion, migration, and invasion in vitro provide valuable insight for further work with this compound.
Acknowledgements
The technical assistance of Tove Johansen, Bodil Berger Hansen, and Olav Bjørkelund is greatly appreciated. We thank Alison Reith for critical reading of the manuscript. The present work was supported by the Norwegian Cancer Society, Familien Brynildsens Legat, Frank Mohn A/S, Inger Margrethe and Per Jæger, The Norwegian Research Council, Innovest, and the Norwegian Ministry of Health.
Abbreviations
- ECM
extracellular matrix
- CGM
complete growth medium
- CRE
cAMP response element
- ERK
extracellular signal-regulated kinase
- GFP
green fluorescence protein
References
- 1.Russell DS, Rubinstein LJ. Pathology of Tumours of the Nervous System. 5th ed. London: Williams and Wilkins; 1989. Tumours of Central Neuroepithelial Origin; pp. 83–350. [Google Scholar]
- 2.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Rutka JT, Apodaca G, Stern R, Rosenblum M. The extracellular matrix of the central and peripheral nervous systems: structure and function. J Neurosurg. 1988;69:155–170. doi: 10.3171/jns.1988.69.2.0155. [DOI] [PubMed] [Google Scholar]
- 4.Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. doi: 10.2741/uhm. [DOI] [PubMed] [Google Scholar]
- 5.Paulus W, Baur I, Schuppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol. 1993;143:154–163. [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus W. Brain Extracellular Matrix, Adhesion Molecules, and Glioma Invasion. In: Mikkelsen T, Bjerkvig R, Laerum OD, Rosenblum ML, editors. Brain Tumor Invasion: Biological, Clinical, and Therapeutic Considerations. New York: Wiley-Liss; 1998. pp. 301–322. [Google Scholar]
- 7.Tysnes BB, Larsen LF, Ness GO, Mahesparan R, Edvardsen K, Garcia-Cabrera I, Bjerkvig R. Stimulation of glioma cell migration by laminin and inhibition by anti-alpha3 and anti-beta1 integrin antibodies. Int J Cancer. 1996;67:777–784. doi: 10.1002/(SICI)1097-0215(19960917)67:6<777::AID-IJC5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Tysnes BB, Haugland HK, Bjerkvig R. Epidermal growth factor and laminin receptors contribute to migratory and invasive properties of gliomas. Invasion Metastasis. 1997;17:270–280. [PubMed] [Google Scholar]
- 9.Fukushima Y, Ohnishi T, Arita N, Hayakawa T, Sekiguchi K. Integrin alpha3beta1-mediated interaction with laminin-5 stimulates adhesion, migration and invasion of malignant glioma cells. Int J Cancer. 1998;76:63–72. doi: 10.1002/(sici)1097-0215(19980330)76:1<63::aid-ijc11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Gunia S, Hussein S, Radu DL, Putz KM, Breyer R, Hecker H, Samii M, Walter GF, Stan AC. CD44s-targeted treatment with monoclonal antibody blocks intracerebral invasion and growth of 9L gliosarcoma. Clin Exp Metastasis. 1999;17:221–230. doi: 10.1023/a:1006699203287. [DOI] [PubMed] [Google Scholar]
- 11.Breyer R, Hussein S, Radu DL, Putz KM, Gunia S, Hecker H, Samii M, Walter GF, Stan AC. Disruption of intracerebral progression of C6 rat glioblastoma by in vivo treatment with anti-CD44 monoclonal antibody. J Neurosurg. 2000;92:140–149. doi: 10.3171/jns.2000.92.1.0140. [DOI] [PubMed] [Google Scholar]
- 12.Taussig SJ, Batkin S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J Ethnopharmacol. 1988;22:191–203. doi: 10.1016/0378-8741(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 13.Harrach T, Eckert K, Schulze-Forster K, Nuck R, Grunow D, Maurer HR. Isolation and partial characterization of basic proteinases from stem bromelain. J Protein Chem. 1995;14:41–52. doi: 10.1007/BF01902843. [DOI] [PubMed] [Google Scholar]
- 14.Harrach T, Eckert K, Maurer HR, Machleidt I, Machleidt W, Nuck R. Isolation and characterization of two forms of an acidic bromelain stem proteinase. J Protein Chem. 1998;17:351–361. doi: 10.1023/a:1022507316434. [DOI] [PubMed] [Google Scholar]
- 15.Taussig SJ, Szekerczes J, Batkin S. Inhibition of tumour growth in vitro by bromelain, an extract of the pineapple plant (Ananas comosus) Planta Med. 1985;6:538–539. [PubMed] [Google Scholar]
- 16.Batkin S, Taussig SJ, Szekerezes J. Antimetastatic effect of bromelain with or without its proteolytic and anticoagulant activity. J Cancer Res Clin Oncol. 1988;114:507–508. doi: 10.1007/BF00391501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batkin S, Taussig S, Szekerczes J. Modulation of pulmonary metastasis (Lewis lung carcinoma) by bromelain, an extract of the pineapple stem (Ananas comosus) Cancer Invest. 1988;6:241–242. doi: 10.3109/07357908809077053. [DOI] [PubMed] [Google Scholar]
- 18.Garbin F, Harrach T, Eckert K, Maurer HR. Bromelain proteinase F9 augments human lymphocyte-mediated growth inhibition of various tumor cells in vitro. Int J Oncol. 1994;5:197–203. [PubMed] [Google Scholar]
- 19.Grabowska E, Eckert K, Fichtner I, Schulze-Forster K, Maurer HR. Bromelain proteases suppress growth, invasion and lung metastasis of B16F10 mouse melanoma cells. Int J Oncol. 1997;11:243–248. doi: 10.3892/ijo.11.2.243. [DOI] [PubMed] [Google Scholar]
- 20.Filippova I, Lysogorskaya EN, Oksenoit ES, Rudenskaya GN, Stepanov VM. L-pyroglutamyl-L-phenylalanyl-L-leucine-p-nitroanilide — a chromogenic substrate for thiol proteinase assay. Anal Biochem. 1984;143:293–297. doi: 10.1016/0003-2697(84)90665-1. [DOI] [PubMed] [Google Scholar]
- 21.Bigner DD, Bigner SH, Pontén J, Westermark B, Mahaley MS, Ruoslaht E, Herschmann H, Eng LF, Wickstrand CJ. Heterogeneity of genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981;40:201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen P-H, Edvardsen K, Garcia-Cabrera I, Mahesparan R, Thorsen J, Mathiesen B, Rosenblum M, Bjerkvig R. Migratory patterns of lac-z transfected human glioma cells in the rat brain. Int J Cancer. 1995;62:767–771. doi: 10.1002/ijc.2910620620. [DOI] [PubMed] [Google Scholar]
- 23.Yuhas JM, Li AP, Martinez AO, Ladman AJ. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977;37:3639–3643. [PubMed] [Google Scholar]
- 24.Schulz J, Dettlaff S, Fritzsche U, Harms U, Schiebel H, Derer W, Fusenig NE, Hulsen A, Bohm M. The amido black assay: a simple and quantitative multipurpose test of adhesion, proliferation, and cytotoxicity in microplate cultures of keratinocytes (HaCaT) and other cell types growing adherently or in suspension. J Immunol Methods. 1994;167:1–13. doi: 10.1016/0022-1759(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 25.Nederman T. Effects of vinblastine and 5-fluorouracil on human glioma and thyroid cancer cell monolayers and spheroids. Cancer Res. 1984;44:254–258. [PubMed] [Google Scholar]
- 26.Lund-Johansen M, Bjerkvig R, Humphrey PA, Bigner SH, Bigner DD, Laerum OD. Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 1990;50:6039–6044. [PubMed] [Google Scholar]
- 27.Bjerkvig R, Steinsvag SK, Laerum OD. Reaggregation of fetal rat brain cells in a stationary culture system: I. Methodology and cell identification. In Vitro Cell Dev Biol. 1986;22:180–192. doi: 10.1007/BF02623302. [DOI] [PubMed] [Google Scholar]
- 28.Bjerkvig R. Reaggregation of fetal rat brain cells in a stationary culture system: II. Ultrastructural characterization. In Vitro Cell Dev Biol. 1986;22:193–200. doi: 10.1007/BF02623303. [DOI] [PubMed] [Google Scholar]
- 29.Druckrey H. Genotypes and phenotypes of ten inbred strains of BD-rats. Arzneimittel-Forschung. 1971;21:1274–1278. [PubMed] [Google Scholar]
- 30.Porwol T, Strohmaier AR, Spiess E. Cytotomography. Methods Enzymol. 1999;307:108–118. doi: 10.1016/s0076-6879(99)07009-3. [DOI] [PubMed] [Google Scholar]
- 31.Harrach T, Gebauer F, Eckert K, Kunze R, Maurer HR. Bromelain proteinases modulate the CD44 expression on human Molt 4/8 leukemia and SK-Mel 28 melanoma cells in vitro. Int J Oncol. 1994;5:485–488. doi: 10.3892/ijo.5.3.485. [DOI] [PubMed] [Google Scholar]
- 32.Humphries MJ. Towards a structural model of an integrin. Biochem Soc Symp. 1999;65:63–78. [PubMed] [Google Scholar]
- 33.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 34.Troussard AA, Tan C, Yoganathan TN, Dedhar S. Cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol Cell Biol. 1999;19:7420–7427. doi: 10.1128/mcb.19.11.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mynott TL, Ladhams A, Scarmato P, Engwerda CR. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J Immunol. 1999;163:2568–2575. [PubMed] [Google Scholar]
- 36.Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- 37.Adachi M, Fukuda M, Nishida E. Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)- dependent active transport mechanism. J Cell Biol. 2000;148:849–856. doi: 10.1083/jcb.148.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benecke BJ, Ben-Ze'ev A, Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978;14:931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- 40.Farmer SR, Ben-Ze'av A, Benecke BJ, Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978;15:627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- 41.Chicurel ME, Singer RH, Meyer CJ, Ingber DE. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392:730–733. doi: 10.1038/33719. [DOI] [PubMed] [Google Scholar]
- 42.Grabowska E. Freie Universität Berlin. Berlin: Freie Universität Berlin; 2001. Dissertation. [Google Scholar]