Abstract
TNF-related apoptosis-inducing ligand (TRAIL/APO-2L) is a member of the TNF family that promotes apoptosis by binding to the transmembrane receptors TRAIL-R1/DR4 and TRAIL-R2/DR5. Its cytotoxic activity is relatively selective to the human tumor cell lines without much effect on the normal cells. Hence, it exerts an antitumor activity without causing toxicity, as apparent by studies with several xenograft models. This review discusses the intracellular mechanisms by which TRAIL induces apoptosis. The major pathway of its action proceeds through the formation of DISC and activation of caspase-8. The apoptotic processes, therefore, follow two signaling pathways, namely the mitochondrial-independent activation of caspase-3, and mitochondrial-dependent apoptosis due to cleavage of BID by caspase-8, the formation of apoptosomes, and activation of caspase-9 and the downstream caspases. Bcl-2 and Bcl-XL have no effect on TRAIL-induced apoptosis in lymphoid cells, whereas these genes block or delay apoptosis in nonlymphoid cancer cells. TRAIL participates in cytotoxicity mediated by activated NK cells, monocytes, and some cytotoxic T cells. Hence, TRAIL may prove to be an effective antitumor agent. In addition, it may enhance the effectiveness of treatment with chemotherapeutic drugs and irradiation. Nontagged Apo-2L/TRAIL does not cause hepatotoxicity in monkeys and chimpanzees and in normal human hepatocytes. Thus, nontagged Apo-2L/TRAIL appears to be a promising new candidate for use in the treatment of cancer.
Keywords: TRAIL, death receptor, mitochondria, apoptosis, IAP, caspase
Introduction
Programmed cell death or apoptosis is a genetically controlled mechanism that is essential for the maintenance of tissue homeostasis involving development and the elimination of unwanted cells. There are a number of ligand-receptor families that are involved in this phenomenon. Some of the members of this family are TNF-α, CD95L/FasL/Apo-1L, and TRAIL/APO-2L. They regulate many biologic functions including cell metabolism, proliferation, cytokine production, and apoptosis [68,96,138]. TRAIL/APO-2L specifically kills transformed and cancer cells through binding with death receptors (DR4 and DR5). Most normal cells appear to be resistant to TRAIL activation, suggesting a higher activity of TRAIL with its receptors on tumor cells. Binding of DR4 or DR5 with TRAIL results in a caspase-activating signal leading to apoptosis [68,94]). Recent studies have shown that systemic administration of TRAIL is physiologically safe in mice and is effective in killing human breast or colon xenografted tumors [31]. It also prolongs survival of tumor-bearing mice [31]. TRAIL participates in cytotoxicity mediated by activated NK cells [60], monocytes [41], and some cytotoxic T cells [59,124]. The objective of this paper is to review the intracellular mechanisms by which TRAIL induces apoptosis and to assess its clinical use in cancer therapy.
TRAIL Receptors
Recent studies have identified four distinct cell surface TRAIL receptors: (a) TRAIL-R1 (DR4) [91,93]; (b) TRAIL-R2 (DR5/TRICK2/KILLER) [93,102,104,107,133,140]; (c) TRAIL-R3 (DcR1/TRID/LIT) [18,102]; and (d) TRAIL-R4 (DcR2/TRUNDD)17,80,92] (Figure 1). All these receptors have high sequence homology in their extracellular domains. The fifth receptor is the soluble osteoprotegerin, which may act as a decoy receptor and does not induce apoptosis [29]. Although TRAIL binds with all the receptors, the function of their intracellular domains is not uniform. The intracellular domains of DR4 and DR5 have been found to be essential for induction of apoptosis following receptor ligation [34,93,102,133,140]. TRAIL-R3/DcR1 and TRAIL-R4/DcR2/TRUNDD lack a functional cytoplasmic domain [16–18,29,80,91,107]. TRAIL-R3 and TRAIL-R4 may serve as “decoys” that compete with TRAIL-R1/TRAIL-R2 for binding to the TRAIL. Overexpression of either DcR1 or DcR2 protein confers protection against TRAIL-induced apoptosis [91,107].
Figure 1.
Schematic representation of TRAIL receptors and the components of TRAIL-DISC (death-inducing signaling complex). Trimerization of TRAIL receptors (TRAIL-R1/DR4 and TRAIL-R2/DR5) initiates recruitment of adaptor protein FADD. It appears that a GTP-binding adaptor protein DAP3 couples TRAIL receptors to FADD. FADD contains a death effector domain (DD) that promotes recruitment of procaspase-8 to the DISC, by homotypic interactions between the DD present on FADD and procaspase-8. The induced proximity of procaspase-8 molecules is postulated to cause their activation by autocatalytic processes. RIP may also bind to these DR4 and DR5, initiating a cascade causing cell necrosis. Decoy receptors DcR1 and DcR2 lack death domains and do not induce apoptosis.
The signal transduction machinery that couples these receptors to the initiation of the cell death cascade is not well understood. Overexpression of DR4 or DR5 can induce apoptosis independent of ligand binding in vitro, suggesting that the initiation of apoptosis could bypass ligand binding. The signal can be directly transduced to downstream sites that activate caspases. The cytoplasmic sequence of death receptors contains a shared 80-amino-acid death domain that, upon ligand binding, associates with a similar domain found in adaptor proteins such as FAS-associated with death domain (FADD) [34,39,103]. In Fas/FasL signaling, it has been shown that receptor preassociation is required for ligand attachment [10,109]. Association of death receptors with their cognate ligands results in receptor trimerization, and recruitment of adaptor protein [2]. The adaptor proteins also contain an effector domain that constitutively binds to cysteine proteases (caspases) that cleave specific proteins. The two caspases predominantly bound to these adaptor molecules are caspase-8 and caspase-10 [2,103]. Once recruited by association of adaptor proteins with death receptors, these caspases are trans-proteolyzed resulting in their activation. Caspase-8 activation initiates two pathways (mitochondrial-dependent and-independent pathways), which result in activation of caspase-3 [6,117]. This ultimately results in an irreversible commitment of cells to undergo apoptosis [2,103]. Recently, it has been reported that a GTP binding protein DAP3 binds directly to the death domain of DR4 and DR5 and causes TRAIL-induced apoptosis by associating with FADD [81]. In addition, recruitment of receptor-interacting protein (RIP) to the receptor complex has been shown to cause a caspase-8-independent form of cell death with necrotic morphology [46]. It is therefore, important to assess TRAIL-induced cell death employing assays for both apoptosis and necrosis.
The extracellular domains of TNF receptor family members contain multiple disulfide bridges, which stabilize their configuration [5]. However, antiparallel β-sheets configuration of the receptor facilitates binding of the ligand [26,56]. TRAIL possesses a free Cys residue (Cys230). It has been shown that the Cys230 residue of TRAIL is an essential moiety of its proapoptotic activity. This is apparent from the loss of such activity if trimers of TRAIL formed by disulfide cross-linking are used. Data also suggest that the unoxidized (nondisulfide) TRAIL fits better into the receptor than the TRAIL modified by establishment of disulfide bridges. The higher activity of the TRAIL containing free Cys-SH is also apparent from the enhancement of its effect in the presence of certain trace metals such as Zn2+. Such ions are well known to stabilize the sulfhydryl group by coordination. Experiments involving the use of TRAIL in combination with these reagents in promoting its biologic effectiveness have not been apparently done.
FADD Is Involved in DR4-and DR5-Mediated Apoptosis
The onward cascade of apoptosis triggered by the binding of TRAIL to DRs was initially thought to be propagated further by the binding of FADD to the cytoplasmically located death domain of DRs [11,47,102,131]. However, some studies have shown that propagation of the process takes place also in the FADD-/- mice, suggesting the possibility of the involvement of other adaptor molecules capable of binding to DRs, in addition to FADD [136,141]. However, more recent studies have demonstrated that FADD-/- mouse embryonic fibroblast cells stably transfected with TRAIL receptors are resistant to TRAIL-mediated cell death [70]. TRAIL receptors stably transfected into heterozygous FADD+/- cells or FADD-/- cells reconstituted with a FADD retroviral construct are sensitive to the cytotoxic effects of TRAIL [70]. Therefore, it is highly likely that FADD is required for DR4-and DR5-mediated apoptosis.
We, and others, have shown that the cell lines deficient in FADD or caspase-8 are resistant to Fas-induced cell signals [52,53,117]. Incorporation of caspase-8 into TRAIL-receptor DISC is defective in cells lacking caspase-8 and FADD [6,66,110,117]). In addition, FADD cannot be substituted by other FADD-like proteins, and caspase-10 cannot replace caspase-8 for DISC formation [6,117]. TRAIL also fails to activate caspase-3 in FADD-deficient Jurkat cells [6,117]. Wild-type Jurkat cells are sensitive to TRAIL-induced apoptosis, whereas FADD-deficient and caspase-8-deficient Jurkat cells are resistant to TRAIL-induced apoptosis [6,117]. This resistance is attributable to a deficiency in caspase-8 or in FADD expression, rather than a reduced expression of caspase-3 or DR5. Therefore, FADD recruitment to DISC and caspase-8 activation are considered necessary events during TRAIL-DR5 mediated apoptosis.
Caspase Cascade in TRAIL-Induced Apoptosis
Caspases are a group of cysteine proteases requiring specifically the presence of aspartate at the cleavage site. The caspase gene family has at least 14 mammalian members [1]. These are initially expressed as single-chain zymogens, which upon apoptotic signaling are activated by proteolytic processing, either by autoactivation, transactivation, or by cleavage by other caspases [36,139]. Once activated, they proteolytically cleave a multitude of cellular proteins, leading to apoptosis. Therefore, the caspase activation is a key regulatory point in the commitment of the cell to apoptosis.
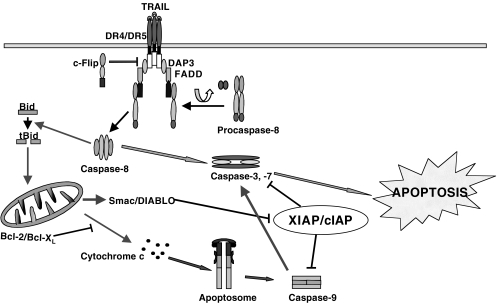
TRAIL signaling pathways involve two caspase cascades [67,68,90,117]. After initial activation of caspase-8 by TRAIL-DISC, divergence of signal occurs in two directions: (1) direct activation of caspase-3 without the involvement of mitochondria, and (2) formation of apoptosomes (mitochondrial proteins, dATP and Apaf-1), which lead to activation of caspase-9 (describe below) [42,43,74,79]. These two pathways appear to converge on caspase-3. In a mechanism not entirely understood, cytochrome c and dATP/ATP act as cofactors and stimulate Apaf-1 self-oligomerization. Once activated, caspase-9 can activate effector caspase-3 and-7 that finally dismantle the cell [76,111,145,] (Figure 2). The decline in mitochondrial membrane potential (ΔΨm) can be blocked by caspase-8 inhibitor, but not by caspase-9 inhibitor [62,64,117]. Thus, caspase-8 links the apoptotic signal from the activated TRAIL-DR to mitochondria leading to dissipation of ΔΨm and directly to the downstream apoptosis-executing caspases. Cleavage of the downstream protease, caspase-3, seems to occur due to activation of either pathway (Figure 2). Knowledge on the relative contributions of these two alternative pathways in TRAIL-induced apoptosis is yet incomplete.
Figure 2.
Intracellular mechanism of TRAIL-induced apoptosis. Apoptosis pathways activated by TRAIL and mitochondria are depicted. Ligation of death receptors by TRAIL leads to formation of DISC which in turn initiates two pathways: (a) activation of caspase-8 leading to apoptosis, and (b) activation of Bid to truncated Bid which in turn regulates mitochondrial functions. Cytochrome c along with Apaf-1 and dATP forms apoptosomes which activate caspase-9. Active forms of caspase-8 and -9 initiate a cascade of effector caspases such as caspase-3 and -7. Activated caspases cleaved several substrates leading to apoptosis. Bcl-2 and Bcl-XL seem to delay or have no affect on apoptosis-related mitochondrial events. IAPs inhibit caspase-3, -7, and -9. Smac/DIABLO inhibits XIAP/cIAP.
The mechanisms controlling the release of mitochondrial proteins are currently under intensive investigation. They may include opening of a mitochondrial permeability transition pore (PTP), the presence of a specific channel for cytochrome c in the outer mitochondrial membrane or mitochondrial swelling and rupture of the outer membrane without a loss in membrane potential [37]. Involvement of the PTP is supported by many reports that the ΔΨm collapses before activation of caspases and apoptosis [69]. The outer mitochondrial membrane becomes permeable to apoptogenic factors such as cytochrome c, Smac/DIABLO (direct inhibitor of apoptosis binding protein [IAP] with low pI) [23,127] and apoptosis-inducing factor (AIF) [120]. Furthermore, the proapoptotic activity of Smac/DIABLO is probably independent of the binding to IAPs. This can mediate the cleavage of DNA during caspase-independent cell death. TRAIL cleaves several substrates including DNA repair enzyme poly(ADP-ribose) polymerase (PARP) [117].
Treatment of cells with TRAIL results in caspase-8 activation, followed by BID (Bcl-2 inhibitory BH3-domain-containing protein) cleavage at its amino terminus [42,43,74,79,117]. The truncated BID (tBID) translocates and gets inserted into the mitochondrial membrane [74]. The presence of tBID into the mitochondria appears to be a stress signal and triggers Bax oligomerization and cytochrome c release [37]. Furthermore, BID-deficient mice are resistant to Fas-induced hepatocellular apoptosis [142]. Immunodepletion of tBID from subcellular fractions argues that tBID is required for cytochrome c release from the mitochondria. Treatment of cells with TRAIL resulted in BID cleavage, suggesting that BID is a substrate for caspase-8 [62,117].
Another form of regulation of caspase activation has recently come to light by the finding that Akt, a serine/threonine kinase involved in some cell survival pathways, and p21-Ras, an activator of Akt, induce phosphorylation of procaspase-9 [9]. In cytosolic extracts prepared from cells expressing either active Ras or Akt, the cytochrome c-dependent activation of caspase-9 is abrogated, suggesting that phosphorylation of procaspase-9 inhibits its processing and activation. Although it is unclear how phosphorylation inhibits its processing, it is suggested that it may facilitate enzyme dimerization through an allosteric mechanism.
Effects of IAPs on TRAIL-Induced Apoptosis
IAPs were discovered by Crook et al. as baculoviral products [15]. They could suppress apoptosis of cells infected with a p35-deleted baculoviral strain [15]. Subsequent genetic and sequence-based experiments identified a group of cellular IAP homologs in yeasts, C. elegans, Drosophila, and vertebrates [14,25,45,71,78,100,101,126]. The precise mechanism of how IAPs inhibit apoptosis is unclear. The mammalian IAPs, XIAP (MIHA, hILP), c-IAP1 (MIHB, HIAP2), c-IAP2 (MIHC, HIAP1), NAIP, and survivin can bind to and inhibit caspases. Early genetic evidence indicated that baculoviral inhibitor of apoptosis repeat (BIR) domains might be essential for the caspase-inhibitory function of XIAP, as they bind directly to distinct caspases (Figure 3). Human IAPs, such as XIAP, c-IAP1, and c-IAP2 inhibit both the initiator caspase-9 and the effector caspase-3 and -7 [21,27,101,108]. In vitro kinetic studies revealed that XIAP is the most potent caspase inhibitor in the IAP family [21,27,101,108]. New structural data also support the concept that XIAP might inhibit initiator and effector caspases by different means (Table 1). Thus, a limited number of IAPs can inhibit caspases bound to the apoptosome and, consequently, might prevent the possibility of an amplification loop outside the complex. Because TRAIL activates primarily caspase-8, -9, and -3 (and possibly caspase-7), it appears that XIAP can inhibit TRAIL-induced apoptosis [117].
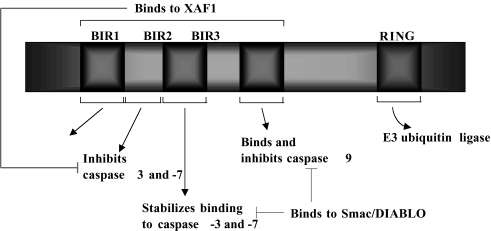
Figure 3.
Structure and function of XIAP. Functional domains of the XIAP protein. Baculoviral inhibitor of apoptosis repeat (BIR) domains is essential for the caspase-inhibitory function of XIAP. XIAP inhibits both the initiator caspase-9 and the effector caspase-3 and -7. XAF1, XIAP-associated factor 1. The function of the BIR1 domain of XIAP is still unclear.
Table 1.
Caspase Inhibition by IAP.
| IAP gene | BIR | RING | CARD | Caspase Inhibition | Ki (nm) | References |
| XIAP | 3 | 1 | 0 | Caspase-3 | 0.7 | [73] |
| Caspase-7 | 0.2 | [73] | ||||
| Caspase-9 | ND | |||||
| cIAP1 | 3 | 1 | 1 | Caspase-3 | 108 | [73] |
| Caspase-7 | 42 | [73] | ||||
| cIAP2 | 3 | 1 | 1 | Caspase-3 | 35 | [73] |
| Caspase-7 | 29 | [73] | ||||
| NAIP | 3 | 0 | 0 | Caspase-3 | ND | |
| Caspase-7 | ND | |||||
| Livin | 1 | 1 | 0 | Caspase-3 | ND | |
| Caspase-7 | ND | |||||
| Caspase-9 | ND | |||||
| Survivin | 1 | 0 | 0 | Caspase-3 | 20.9 | [74] |
| Caspase-7 | 11.5 | [74] | ||||
The type and number of functional domains are indicated for each IAP gene. The inhibitory constants (Ki) for caspase inhibition are shown next to the target caspase.
ND, not determined; BIR, baculoviral inhibitor of apoptosis repeat; CARD, caspase recruitment domain; IAP, inhibitor of apoptosis; RING, really interesting new gene.
Overexpression of XIAP protects cells from divergent apoptotic signals, including ultraviolet irradiation, γ-irradiation, and chemotherapeutic drugs. But mice with a targeted deletion of XIAP are normal and show no signs of misregulated apoptosis [44]. The loss of XIAP, however, is accompanied by an increase in the levels of c-IAP1 and c-IAP2, suggesting that IAP activity is critical for cell survival and that the loss of XIAP needs to be compensated for.
The Bcl-2 proteins can block only the mitochondrial branch of apoptosis by preventing the release of cytochrome c, whereas IAPs block both the mitochondrial- and death-receptor-mediated pathways of apoptosis by directly binding to and inhibiting both the initiator and effector caspases. The discovery of cellular proteins that interact with XIAP and modulate its antiapoptotic activity points to the critical role of XIAP in cellular homeostasis. Overexpression of IAP family proteins inhibits apoptosis induced by Bax and other proapoptotic Bcl-2 family proteins, which are known for their ability to target mitochondria and induce cytochrome c release [8,21,54]. The IAPs, however, do not interfere with Bax-mediated release of cytochrome c from mitochondria in vitro, as well as in intact cells [54], an observation that is consistent with other data indicating that the human IAPs block caspase activation and apoptosis downstream of Bax, Bik, Bak, and cytochrome c [20,21,24,101,121]. The failure of IAPs to prevent cell death stimuli from triggering cytochrome c release has important implications for determining whether cell death will be prevented in the long term or is merely delayed. These diverse apoptotic inhibitors from mammals, insects, and their associated viral pathogens are providing important insight into the regulatory mechanisms of TRAIL-induced caspase activation and apoptosis.
Recently, a XIAP-interacting protein named XIAP-associated factor1 (XAF1) has been identified [77]. XAF1 antagonizes the ability of XIAP to suppress caspase activity and cell death in vitro. It is not known how XAF1 interacts with XIAP to inhibit its activity. In contrast to Smac/DIABLO, XAF1 does not need to be processed, and it seems to be constitutively able to interact with and inhibit XIAP. Unlike XIAP, however, which is found primarily in the cytoplasm, endogenous XAF1 is localized to the nucleus. The different compartmentalization of XIAP and XAF1 raises the question of how these two proteins can interact — does XAF1 translocate into the cytoplasm where it inhibits XIAP, or does XIAP enter the nucleus where it is sequestered by XAF1? It is conceivable that XIAP could be transported into the nucleus within the caspase-3 protein complex, where it would continue to inhibit caspase-3 activity. This caspase-inhibiting activity could then be relieved by nuclear XAF1, in a fashion similar to the cytoplasmic inhibition of XIAP by Smac/DIABLO.
There are several outstanding issues that need to be addressed experimentally and will probably provide vital clues about the regulation of XIAP. Understanding the biology of XIAP not only provides the intellectual satisfaction of untangling the complex regulatory networks that control life and death, but it might also supply us with powerful therapeutic approaches for the treatment of several human diseases.
Effects of Bcl-2 or Bcl-XL on TRAIL-Induced Apoptosis and Mitochondrial Dysfunctions
Apoptosis can be induced by both mitochondrial-dependent and -independent pathways [64,68,69,95,117,134]. Membrane depolarization and subsequent loss of cytochrome c and other cofactors from the mitochondrial intermembrane space appear to be the early event in the mitochondrial dependent pathway [37,69]. Permeabilization of the outer mitochondrial membrane is controlled by members of the Bcl-2 family [19]. The antiapoptotic members such as Bcl-2 or Bcl-XL inhibit the release of mitochondrial apoptogenic factors, whereas the proapoptotic members (e.g., Bax, and Bak) trigger the release. It is not clear how Bcl-2 family members modulate permeabilization of the outer mitochondrial membrane and also preserve mitochondrial function. Overexpression of Bcl-2 or Bcl-XL does not block [62,64,117] TRAIL-induced apoptosis in lymphoid cells, suggesting tumor cells that have already acquired resistance to chemotherapeutic drugs by Bcl-2 or Bcl-XL can be killed by TRAIL. Thus, TRAIL may be a promising candidate for the treatment of patients carrying drug-resistant tumors. Treatment of CEM, Jurkat, U937, MDA-MD231, and MCF-7 cells with TRAIL causes a steady decline in ΔΨm [62,64,117]. The time-dependent loss of ΔΨm in cells due to TRAIL treatment is delayed, but not prevented, by the overexpression of Bcl-2 or Bcl-XL [64,117]. In contrast, overexpression of Bcl-2 blocks TRAIL-induced apoptosis in human lung [119] and prostate cancer cells [83,86,99]. Thus, TRAIL can induce apoptosis through both mitochondrial-dependent and -independent pathways, whereas most anticancer drug-induced apoptosis requires mitochondrial events and is inhibited by overexpression of Bcl-2 or Bcl-XL [62,64,113,115,132].
Role of NFκB in TRAIL-Induced Apoptosis
NFκB is widely known for its ubiquitous roles in inflammation, immune responses, cell division, and apoptosis [4,128]. NFκB is composed of members of the Rel family that share a 300-amino-acid region, known as the Rel homology domain, which mediates dimerization, nuclear translocation, DNA binding, and interaction with NFκB inhibitors [55,128]. Activation of NFκB is controlled by a family of inhibitors, or IκBs, that bind to NFκB dimmers and mask the nuclear localization sequence of NFκB, thus retaining the entire complex in the cytoplasm (Figure 4). Activation of NFκB is achieved by the phosphorylation and activation of the IκB kinase (IKK) complex. The activated IKK complex specifically phosphorylates the IκBs, which are then rapidly polyubiquitinated, targeting them for degradation by the proteosome. This results in release and translocation of NFκB dimmers from the cytoplasm to the nucleus where they bind target genes and stimulate transcription (Figure 4). NFκB activates a variety of target genes relevant to the human diseases such as ischemic stroke, Alzheimer's disease and Parkinson's disease, as well as genes that regulate cell proliferation and mediate cell survival. NFκB also activates the IκBα gene, thus replenishing the cytoplasmic pool of its own inhibitor.
Figure 4.
Involvement of NFκB in TRAIL-induced apoptosis. Binding of death receptors DR4 and DR5 with ligand TRAIL results in the activation of NFκB. p50 and p65 subunits of NFκB are maintained in the cytoplasm by binding to IκB inhibitory proteins. In response to cellular stimulation, IκB proteins are phosphorylated (by IKK), ubiquitinated, and degraded. Removal of IκB from NFκB allows NFκB to translocate into the nucleus where it transactivates numerous apoptosis related genes such as c-IAPs, DR4, DR5, and Bcl-XL.
The involvement of NFκB in TRAIL-induced apoptosis is not well understood [2]. It seems that TRAIL does not activate the immune system in vivo, at least in mouse and monkey. No knockout mice lacking TRAIL or its receptors have been reported, and so the physiological function of this death factor system remains elusive. TRAIL activates NFκB in vitro [32,47]. Constitutively active NFκB prevents TRAIL-induced apoptosis in renal cancer cells [89]. However, NFκB activation is not sufficient for protecting cells from TRAIL-induced apoptosis [47]. It is possible that various subunits of NFκB play different roles in regulating cellular response of TRAIL. In a recent report, NFκB-c-Rel subunit enhances TRAIL-induced apoptosis by enhancing the expression of death receptors DR4 and DR5, whereas NFκB-Rel-A inhibits TRAIL-induced apoptosis by increasing the expression of Bcl-XL [98]. Further studies are needed to confirm the role of specific subunits of NFκB in TRAIL signaling.
Factors Influencing TRAIL Sensitivity in Normal and Cancer Cells
A critical feature of any approach to cancer treatment is the ability of selectively interfering with growth or viability of cancer cells while avoiding or minimizing toxicity to normal noncancer cells. Several factors have been proposed to be involved in imposing TRAIL sensitivity, such as the relative numbers of death and decoy receptors, temperature, and the relative activities of FLICE-inhibitory protein (FLIP), caspase-8 and -10, and the constitutively active AKT/PKB. Delineation of the factors involved in the apoptotic response of TRAIL is complex. The response also varies with the species.
The different sensitivities of TRAIL in regulating apoptosis was proposed due to the higher expression of decoy receptors in normal cells, but the absence of or lower expression of these receptors in transformed cells [2,13,73]. As more cell lines were examined, the expression level of DR5, DcR1, and DcR2 did not correlate with TRAIL sensitivity [39,40,65]. It may be possible that TRAIL sensitivity is primarily regulated at the intracellular level rather than at the receptor level [40,112]. Further studies are needed to confirm the involvement of decoy receptors in TRAIL sensitivity.
Various versions of TRAIL have been found to exert differential effects on normal human hepatocytes [35]. Several soluble TRAIL receptor agonists have been used to test toxicity on normal human hepatocytes in vitro. They are (i) nontagged, soluble and native-sequence TRAIL (amino acids 114–281) [3], (ii) polyhistidine-tagged recombinant soluble TRAIL (amino acids 114–281) [51], (iii) recombinant soluble TRAIL fused to a trimerizing leucine zipper (amino acids 95–281) [135], and (iv) agonist antibody [40,48]. Nontagged, soluble, and native-sequence TRAIL, and leucine zipper TRAIL did not cause hepatotoxicity in rat, mouse, cynomolgus monkeys, and chimpanzees [3,72,135]. In contrast, polyhistidine-tagged TRAIL induced some apoptosis in cultured human hepatocytes [51]. Nontagged soluble TRAIL was nontoxic to normal keratinocytes, whereas histidine-tagged and leucine zipper-fused TRAIL were cytotoxic [97]. In addition, histidine-tagged TRAIL acted as a negative regulator of normal erythropoiesis [144] and induced apoptosis in lymphocytes [88]. In another study FLAG-tagged TRAIL induced apoptosis in human brain [87]. The differences in cytotoxicity are due to the aberrant biochemical and structural properties of tagged TRAIL. In particular, native TRAIL contains an internal zinc atom bound by cysteine residues at position 230 of each subunit, which is crucial for trimer stability and biologic activity [7]. By comparison, the polyhistidine-tagged recombinant soluble form of TRAIL does not contain as much zinc as native TRAIL [85]. Thus, limited studies on primate models indicate that systemic administration of nontagged TRAIL to cancer patients is unlikely to be toxic.
Temperature also changes the affinity of TRAIL to its receptor as examined in in vitro studies [125]. Death receptors (TRAIL-R1, TRAIL-R2, TRAIL-R3, and OPG) have similar affinities for TRAIL at 4°C, but their affinities significantly differ at 37°C, with TRAIL-R2 having the highest and OPG having the weakest. Thus, the physiological temperature could be an important determinant for TRAIL signaling in pathophysiological situations. Further studies are needed to evaluate the significance TRAIL binding to its receptors in vivo.
Analysis of the DR-DISC components provides evidence for the importance of caspase-8 in TRAIL-induced apoptosis. Such analysis failed to identify a similar function for caspase-10, which was suggested by overexpression studies [91,136]. The dendritic cells expressing a mutant caspase-10 are resistant to TRAIL signaling [136]. Alternatively, a catalytically inactive caspase-10 mutant may act as a nonreleasable substrate trap for caspase-8, thereby inactivating the caspase, as has been proposed for FLIP [49]. TRAIL signaling pathway may therefore, differ in cells from different origins. However, identification of components present in DISC provides a reliable method of identifying the initial events of TRAIL-induced apoptosis.
Examination of TRAIL-induced apoptosis in vitro has demonstrated that there are both TRAIL-sensitive and TRAIL-resistant human melanoma and colon carcinoma cell lines [6,38,63,122,133]. The reason for the differential sensitivity remains unknown, but it is not regulated solely by the differential expression of the known TRAIL receptors [38]. Instead, it appears that intracellular inhibitor (s) acting downstream of the TRAIL receptors renders some transformed cells insensitive to TRAIL [38]. Addition of protein synthesis inhibitors to TRAIL-resistant melanomas renders them sensitive to TRAIL, indicating that the presence of intracellular apoptosis inhibitors may mediate resistance to TRAIL-mediated apoptosis [38]. Expression of FLIP is highest in the TRAIL-resistant melanomas, being low or undetectable in the TRAIL-sensitive melanomas [39]. Furthermore, addition of actinomycin D to TRAIL-resistant melanomas resulted in decreased intracellular concentrations of FLIP, which correlated with their effectiveness of TRAIL sensitivity [38]. In HeLa cells, apoptosis induction by TRAIL is dependent on the presence of cycloheximide [130]. Interestingly, cycloheximide downregulates cFLIP, and overexpression of cFLIP inhibited death receptor-induced NFκB activation [130]. This suggests a novel functional role of cFLIP as a negative regulator of gene induction and apoptosis. Furthermore, treatment of resistant cells with some anticancer drugs renders cells sensitive to TRAIL. For example, cisplatin and carboplatin enhance the sensitivity of bladder cancer cells to TRAIL [82].
Some prostate cancer cells express constitutively active Akt/protein kinase B due to a complete loss of lipid phosphatase PTEN gene [50,75,129,137], a negative regulator of PI-3 kinase pathway. Constitutively active Akt/PKB promotes cellular survival and resistance to chemotherapy and radiation [22,105,116]. We and others have shown that some human prostate cancer cells are resistant to TRAIL [12,84]. Cell line expressing the highest level of constitutively active Akt was most resistant to undergo apoptosis by TRAIL than those expressing the lowest level [12,84]. Downregulation of constitutively active Akt by PI-3 kinase inhibitors, wortmannin and LY294002, reverses cellular resistance to TRAIL [12,84,123]. Treatment of resistant cells with cycloheximide (a protein synthesis inhibitor) renders cells sensitive to TRAIL. Transfecting dominant negative Akt decreased Akt activity and increased TRAIL-induced apoptosis in cells with high Akt activity. Conversely, transfecting constitutively active Akt into cells with low Akt activity increased Akt activity and attenuated TRAIL-induced apoptosis. Inhibition of TRAIL sensitivity occurs at the level of BID cleavage, as caspase-8 activity was not affected. Enforced expression of antiapoptotic protein Bcl-2 or Bcl-XL inhibits TRAIL-induced mitochondrial dysfunction and apoptosis [12,84,123]. We therefore, identify Akt as a constitutively active kinase that promotes survival of prostate cancer cells and demonstrate that modulation of Akt activity, by pharmacological or genetic approaches, alters the cellular responsiveness to TRAIL. Thus, TRAIL, in combination with agents that downregulate Akt activity, can be used to treat prostate cancer.
TRAIL as a Potential Chemotherapeutic Agent
Solid tumors such as breast and prostate cancer are currently controlled through surgery and/or radiotherapy protocols and frequently supported by adjuvant chemotherapy. Unfortunately, there are limited treatment options available for the disease because chemo- and radiotherapies are largely ineffective, and metastatic disease frequently redevelops even after surgery. Therefore, there is an urgent need for novel and effective therapies against cancer. Ligation of death receptors to induce apoptosis in tumor cells has been proposed as a viable method of treating epithelial cell derived cancers; unfortunately, the administration of FasL and TNF has toxic effects [31,135]. The discovery of TRAIL may offer less toxic effects on noncancerous cells.
Ligation of Fas receptors with FasL can also play a role in chemotherapeutic drug-induced apoptosis in epithelial cells, because FAS and/or FasL are upregulated following exposure of cells to anticancer drugs such as etoposide [57] and microtubule-damaging drugs [114]. Interestingly, CD40 (a member of the TNF receptor superfamily) ligation with its receptors can also induce functional FasL, TRAIL, and TNF in apoptosis-susceptible carcinoma cells and upregulate expression of Fas [28]. The expression of DR4 and/or DR5 is more highly regulated than Fas. In addition to chemotherapeutic drugs, ionizing radiation can also induce DR4 and/or DR5 [13,32,65,140]. Thus, upregulation of DR4 and DR5 by anticancer drugs or irradiation may further enhance the efficacy of TRAIL.
Combination of TRAIL with chemotherapeutic drugs (e.g., etoposide, camptothecin, doxorubicin, and 5-fluorouracil) or irradiation results in a synergistic apoptotic response [3,13,32,33,61]. In addition, TRAIL synergizes with a synthetic retinoid (CD437) in inducing apoptosis of human lung cancer cells by upregulating DR5 [118]. Recent studies have demonstrated that systemic TRAIL inhibits the growth of breast and colon cancer xenografts in mice [3,135]. In some cell types, DR5 expression is regulated in a p53-dependent manner, whereas in other cells increased DR5 expression seems p53 independent [106]. p53 acts as a tumor suppressor by inducing cell cycle arrest and apoptosis. It is well known that p53-mediated cell cycle arrest involves in the activation of p21 WAF1/CIP1, a cyclin-CDK inhibitor, whereas the mechanism of p53-mediated apoptosis appears to involve multiple downstream effector target genes. TRAIL synergizes with radiation in inducing apoptosis in p53 wild-type breast carcinoma cells, but not in p53 negative cell lines [13]. This suggests that p53 may mediate synergistic interaction between radiation and TRAIL. One of the mechanisms of this synergy is that p53 upregulates mRNA expression of the DR5 receptors [106]. Radiation also upregulates expression of the DR5 protein, but not the DR4 protein [13]. By expressing more of the death receptor, cells may then become sensitive to TRAIL. TRAIL and radiation may also activate distinct apoptotic pathways, which may converge and further amplify treatment response.
TRAIL is expressed on IFN-α-stimulated peripheral blood T cells [58] and IFN-stimulated human monocytes and dendritic cells [30,41]. Cytokines IL2, IFNs or IL-15 can induce expression of TRAIL on CD3-NK1.1+ NK cells [60,143]. TRAIL-expressing CD4+ T cells and mouse NK cells can mediate apoptosis in a TRAIL-specific fashion [59,60]. It has recently been shown that TRAIL contributes to IFN-γ-dependent NK cell protection from tumor metastasis. Thus, TRAIL may play a role in the cytolytic effector function of monocytes, CD4+ T lymphocytes, dendritic cells, and NK cells. This would be important in viral clearance and suppression of autoimmunity, as well as tumor immunity.
The several strategies that have now been described for treating cancer by targeting death receptor expression are likely to provide useful insights into the role of TRAIL function in apoptosis. TRAIL appears to be a cancer-specific cytotoxic agent, and it offers several additional advantages for the cancer therapy. Furthermore, it may enhance the antitumor potential of traditional chemotherapeutic drugs or irradiation. Recent studies suggest the potential use of TRAIL in gene therapy against cancer. Testing of the therapeutic potential of these strategies in treating tumors now awaits the translation of these approaches in relevant in vivo systems where both antitumor effectiveness and complicating side effects on normal cell function can be evaluated.
Acknowledgements
I acknowledge my colleagues at the University of Maryland at Baltimore for their support and many valuable discussions.
Footnotes
This work was supported by grants from Susan G. Komen Breast Cancer Foundation, Charlette Geyer Foundation, and United States Department of Defense.
References
- 1.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 5.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 7.Bodmer JL, Meier P, Tschopp J, Schneider P. Cysteine 230 is essential for the structure and activity of the cytotoxic ligand TRAIL. J Biol Chem. 2000;275:20632–20637. doi: 10.1074/jbc.M909721199. [DOI] [PubMed] [Google Scholar]
- 8.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 10.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5 a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Constitutively active Akt in prostate cancer cells directly correlates with cellular survival and resistance to TRAIL. Oncogene. 2001 doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- 13.Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy [In Process Citation] Proc Natl Acad Sci USA. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clem RJ, Miller LK. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degli-Esposti M. To die or not to die — the quest of the TRAIL receptors. J Leukocyte Biol. 1999;65:535–542. doi: 10.1002/jlb.65.5.535. [DOI] [PubMed] [Google Scholar]
- 17.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 18.Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3 a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 20.Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 22.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 23.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 24.Duckett CS, Li F, Wang Y, Tomaselli KJ, Thompson CB, Armstrong RC. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol Cell Biol. 1998;18:608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duckett CS, Nava VE, Gedrich RW, Clem RJ, Van Dongen JL, Gilfillan MC, Shiels H, Hardwick JM, Thompson CB. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 26.Eck KM, Yuan L, Duffy L, Ram PT, Ayettey S, Chen I, Cohn CS, Reed JC, Hill SM. A sequential treatment regimen with melatonin and all-trans retinoic acid induces apoptosis in MCF-7 tumour cells. Br J Cancer. 1998;77:2129–2137. doi: 10.1038/bjc.1998.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- 28.Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH, Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol. 2000;20:5503–5515. doi: 10.1128/mcb.20.15.5503-5515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 30.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.French LE, Tschopp J. The TRAIL to selective tumor death [news; comment] Nat Med. 1999;5:146–147. doi: 10.1038/5505. [DOI] [PubMed] [Google Scholar]
- 32.Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL. Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol. 2000;20:205–212. doi: 10.1128/mcb.20.1.205-212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999;59:6153–6158. [PubMed] [Google Scholar]
- 34.Golstein P. Cell death: TRAIL and its receptors. Curr Biol. 1997;7:R750–R753. doi: 10.1016/s0960-9822(06)90000-1. [DOI] [PubMed] [Google Scholar]
- 35.Gores GJ, Kaufmann SH. Is TRAIL hepatotoxic? Hepatology. 2001;34:3–6. doi: 10.1053/jhep.2001.25173. [DOI] [PubMed] [Google Scholar]
- 36.Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 37.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 38.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 39.Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 40.Griffith TS, Rauch CT, Smolak PJ, Waugh JY, Boiani N, Lynch DH, Smith CA, Goodwin RG, Kubin MZ. Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol. 1999;162:2597–2605. [PubMed] [Google Scholar]
- 41.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while Bcl-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 43.Han Z, Bhalla K, Pantazis P, Hendrickson EA, Wyche JH. Cif (cytochrome c efflux-inducing factor) activity is regulated by Bcl-2 and caspases and correlates with the activation of Bid. Mol Cell Biol. 1999;19:1381–1389. doi: 10.1128/mcb.19.2.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 46.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 47.Hu WH, Johnson H, Shu HB. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem. 1999;274:30603–30610. doi: 10.1074/jbc.274.43.30603. [DOI] [PubMed] [Google Scholar]
- 48.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP, Zhou T. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 49.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 50.Ittmann MM. Chromosome 10 alterations in prostate adenocarcinoma (review) Oncol Rep. 1998;5:1329–1335. doi: 10.3892/or.5.6.1329. [DOI] [PubMed] [Google Scholar]
- 51.Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 52.Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol. 1998;8:1001–1008. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- 53.Juo P, Woo MS, Kuo CJ, Signorelli P, Biemann HP, Hannun YA, Blenis J. FADD is required for multiple signaling events downstream of the receptor Fas. Cell Growth Differ. 1999;10:797–804. [PubMed] [Google Scholar]
- 54.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 56.Karpusas M, Hsu YM, Wang JH, Thompson J, Lederman S, Chess L, Thomas D. 2 A crystal structure of an extracellular fragment of human CD40 ligand [published erratum appears in Structure 1995 Dec 15;3 (12):1426] Structure. 1995;3:1031–1039. [PubMed] [Google Scholar]
- 57.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR. DNA damaging agents induceexpression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 58.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- 60.Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, Okamura H, Nakanishi K, Okumura K, Yagita H. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163:1906–1913. [PubMed] [Google Scholar]
- 61.Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- 62.Keogh SA, Walczak H, Bouchier-Hayes L, Martin SJ. Failure of Bcl-2 to block cytochrome c redistribution during TRAIL-induced apoptosis. FEBS Lett. 2000;471:93–98. doi: 10.1016/s0014-5793(00)01375-2. [DOI] [PubMed] [Google Scholar]
- 63.Kim CH, Gupta S. Expression of TRAIL (Apo2L), DR4 (TRAIL receptor 1), DR5 (TRAIL receptor 2) and TRID (TRAIL receptor 3) genes in multidrug resistant human acute myeloid leukemia cell lines that overexpress MDR 1 (HL60/Tax) or MRP (HL60/AR) Int J Oncol. 2000;16:1137–1139. doi: 10.3892/ijo.16.6.1137. [DOI] [PubMed] [Google Scholar]
- 64.Kim EJ, Suliman A, Lam A, Srivastava RK. Failure of bcl-2 to block mitochondrial dysfunction during TRAIL-induced apoptosis. Int J Oncol. 2001;18:187–194. [PubMed] [Google Scholar]
- 65.Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 66.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 67.Krammer PH. The CD95 (Apo-1/Fas)/CD95L system. Toxicol Lett. 1998;102–103:131–137. doi: 10.1016/s0378-4274(98)00297-5. [DOI] [PubMed] [Google Scholar]
- 68.Krammer PH. CD95(Apo-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 69.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 70.Kuang AA, Diehl GE, Zhang J, Winoto A. FADD is required for DR4-and DR5-mediated apoptosis: lack of trail-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J Biol Chem. 2000;275:25065–25068. doi: 10.1074/jbc.C000284200. [DOI] [PubMed] [Google Scholar]
- 71.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 72.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 73.Leverkus M, Neumann M, Mengling T, Rauch CT, Brocker EB, Krammer PH, Walczak H. Regulation of tumor necrosis factor-related apoptosis-inducing ligand sensitivity in primary and transformed human keratinocytes. Cancer Res. 2000;60:553–559. [PubMed] [Google Scholar]
- 74.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittman M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 76.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 77.Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW, Korneluk RG. Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat Cell Biol. 2001;3:128–133. doi: 10.1038/35055027. [DOI] [PubMed] [Google Scholar]
- 78.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda JE, MacKenzie A, Korneluk RG. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 79.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 80.Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- 81.Miyazaki T, Reed JC. A GTP-binding adapter protein couples TRAIL receptors to apoptosis-inducing proteins. Nat Immunol. 2001;2:493–500. doi: 10.1038/88684. [DOI] [PubMed] [Google Scholar]
- 82.Mizutani Y, Nakao M, Ogawa O, Yoshida O, Bonavida B, Miki T. Enhanced sensitivity of bladder cancer cells to tumor necrosis factor related apoptosis inducing ligand mediated apoptosis by cisplatin and carboplatin. J Urol. 2001;165:263–270. doi: 10.1097/00005392-200101000-00076. [DOI] [PubMed] [Google Scholar]
- 83.Munshi A, Pappas G, Honda T, McDonnell TJ, Younes A, Li Y, Meyn RE. TRAIL (APO-2L) induces apoptosis in human prostate cancer cells that is inhibitable by Bcl-2. Oncogene. 2001;20:3757–3765. doi: 10.1038/sj.onc.1204504. [DOI] [PubMed] [Google Scholar]
- 84.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 85.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 86.Nimmanapalli R, Perkins CL, Orlando M, O'Bryan E, Nguyen D, Bhalla KN. Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res. 2001;61:759–763. [PubMed] [Google Scholar]
- 87.Nitsch R, Bechmann I, Deisz RA, Haas D, Lehmann TN, Wendling U, Zipp F. Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL) Lancet. 2000;356:827–828. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 88.Okada H, Kobune F, Sato TA, Kohama T, Takeuchi Y, Abe T, Takayama N, Tsuchiya T, Tashiro M. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch Virol. 2000;145:905–920. doi: 10.1007/s007050050683. [DOI] [PubMed] [Google Scholar]
- 89.Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M. Constitutive activation of nuclear factor-kappaB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene. 2001;20:3888–3896. doi: 10.1038/sj.onc.1204525. [DOI] [PubMed] [Google Scholar]
- 90.Ozoren N, Kim K, Burns TF, Dicker DT, Moscioni AD, El-Deiry WS. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000;60:6259–6265. [PubMed] [Google Scholar]
- 91.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 92.Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998;424:41–45. doi: 10.1016/s0014-5793(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 93.Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 94.Peter ME, Krammer PH. Mechanisms of CD95 (Apo-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 95.Peter ME, Scaffidi C, Medema JP, Kischkel F, Krammer PH. The death receptors. Results Probl Cell Differ. 1999;23:25–63. doi: 10.1007/978-3-540-69184-6_3. [DOI] [PubMed] [Google Scholar]
- 96.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 97.Qin J, Chaturvedi V, Bonish B, Nickoloff BJ. Avoiding premature apoptosis of normal epidermal cells. Nat Med. 2001;7:385–386. doi: 10.1038/86401. [DOI] [PubMed] [Google Scholar]
- 98.Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ, Bedi A. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol. 2001;3:409–416. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- 99.Rokhlin OW, Guseva N, Tagiyev A, Knudson CM, Cohen MB. Bcl-2 oncoprotein protects the human prostatic carcinoma cell line PC3 from TRAIL-mediated apoptosis. Oncogene. 2001;20:2836–2843. doi: 10.1038/sj.onc.1204410. [DOI] [PubMed] [Google Scholar]
- 100.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 101.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- 103.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 104.Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ, Bell JI. TRICK2 a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol. 1997;7:693–696. doi: 10.1016/s0960-9822(06)00297-1. [DOI] [PubMed] [Google Scholar]
- 105.Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 106.Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr, el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998;58:1593–1598. [PubMed] [Google Scholar]
- 107.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 108.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and-7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 109.Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 110.Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 111.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 112.Srivastava RK. Intracellular mechanisms of TRAIL and its role in cancer therapy. Mol Cell Biol Res Commun. 2000;4:67–75. doi: 10.1006/mcbr.2001.0265. [DOI] [PubMed] [Google Scholar]
- 113.Srivastava RK, Mi QS, Hardwick JM, Longo DL. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA. 1999;96:3775–3780. doi: 10.1073/pnas.96.7.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Srivastava RK, Sasaki CY, Hardwick JM, Longo DL. Bcl-2-mediated drug resistance: inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J Exp Med. 1999;190:253–265. doi: 10.1084/jem.190.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Srivastava RK, Sollott SJ, Khan L, Hansford R, Lakatta EG, Longo DL. Bcl-2 and Bcl-X(L) block thapsigargin-induced nitric oxide generation, c-Jun NH(2)-terminal kinase activity, and apoptosis. Mol Cell Biol. 1999;19:5659–5674. doi: 10.1128/mcb.19.8.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 117.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 118.Sun SY, Yue P, Hong WK, Lotan R. Augmentation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by the synthetic retinoid 6. Cancer Res. 2000;60:7149–7155. [PubMed] [Google Scholar]
- 119.Sun SY, Yue P, Zhou JY, Wang Y, Choi Kim HR, Lotan R, Wu GS. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Commun. 2001;280:788–797. doi: 10.1006/bbrc.2000.4218. [DOI] [PubMed] [Google Scholar]
- 120.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 121.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 122.Tanaka M, Suda T, Yatomi T, Nakamura N, Nagata S. Lethal effect of recombinant human Fas ligand in mice pretreated with Propionibacterium acnes. J Immunol. 1997;158:2303–2309. [PubMed] [Google Scholar]
- 123.Thakkar H, Chen X, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Pro-survival function of Akt/PKB in prostate cancer cells: relationship with TRAIL resistance. J Biol Chem. 2001;276:38361–38369. doi: 10.1074/jbc.M103321200. [DOI] [PubMed] [Google Scholar]
- 124.Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195–2200. [PubMed] [Google Scholar]
- 125.Truneh A, Sharma S, Silverman C, Khandekar S, Reddy MP, Deen KC, McLaughlin MM, Srinivasula SM, Livi GP, Marshall LA, Alnemri ES, Williams WV, Doyle ML. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem. 2000;275:23319–23325. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- 126.Uren AG, Coulson EJ, Vaux DL. Conservation of baculovirus inhibitor of apoptosis repeat proteins (BIRPs) in viruses, nematodes, vertebrates and yeasts. Trends Biochem Sci. 1998;23:159–162. doi: 10.1016/s0968-0004(98)01198-0. [DOI] [PubMed] [Google Scholar]
- 127.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 128.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 129.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 130.Wajant H, Haas E, Schwenzer R, Muhlenbeck F, Kreuz S, Schubert G, Grell M, Smith C, Scheurich P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- 131.Wajant H, Johannes FJ, Haas E, Siemienski K, Schwenzer R, Schubert G, Weiss T, Grell M, Scheurich P. Dominant-negative FADD inhibits TNFR60-, Fas/Apo1- and TRAIL-R/Apo2-mediated cell death but not gene induction. Curr Biol. 1998;8:113–116. doi: 10.1016/s0960-9822(98)70042-9. [DOI] [PubMed] [Google Scholar]
- 132.Walczak H, Bouchon A, Stahl H, Krammer PH. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2-or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer Res. 2000;60:3051–3057. [PubMed] [Google Scholar]
- 133.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walczak H, Krammer PH. The CD95 (Apo-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 135.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 136.Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, Yao X, Puck JM, Straus SE, Lenardo MJ. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 137.Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 139.Wolf BB, Goldstein JC, Stennicke HR, Beere H, Amarante-Mendes GP, Salvesen GS, Green DR. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood. 1999;94:1683–1692. [PubMed] [Google Scholar]
- 140.Wu GS, Burns TF, McDonald ER, III, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, el-Deiry WS. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene [letter] Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 141.Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry WS, Lowe SW, Goeddel DV, Mak TW. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 142.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 143.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zamai L, Secchiero P, Pierpaoli S, Bassini A, Papa S, Alnemri ES, Guidotti L, Vitale M, Zauli G. TNF-related apoptosis-inducing ligand (TRAIL) as a negative regulator of normal human erythropoiesis. Blood. 2000;95:3716–3724. [PubMed] [Google Scholar]
- 145.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]