Abstract
Previously, our laboratory showed that interleukin-1β (IL-1β) secreted by lipopolysaccharide-activated monocytes induces promatrilysin expression in the prostate carcinoma cell line, LNCaP. We now demonstrate that IL-1β-induced promatrilysin expression is mediated by an indirect mechanism that requires nuclear factor Kappa B (NFκB)-dependent synthesis of IL-6. Inhibition of protein synthesis with cyclohexamide blocked IL-1β-mediated induction of matrilysin mRNA suggesting that synthesis of one or more additional factors is required for IL-1β-induced promatrilysin protein expression. Blockage of NFκB transactivation activity abrogated IL-1β-induced promatrilysin expression to baseline levels suggesting that NFκB transactivation activity is necessary. Inhibition of IL-6 activity attenuated IL-1β-induced promatrilysin, but not NFκB transactivation activity indicating that IL-6 acts downstream of NFκB in potentiation of IL-1β-mediated promatrilysin expression. Inhibition of protein synthesis with cyclohexamide did not alter IL-6-induced induction of matrilysin mRNA indicating that, contrary to the mechanism by which IL-1β regulates promatrilysin expression, IL-6-mediated matrilysin mRNA expression does not require new protein synthesis. Transient transfection with dominant negative STAT3 inhibited IL-1β- and IL-6-induced promatrilysin. These data provide evidence that NFκB-mediated IL-6 synthesis is required for IL-1β-induced promatrilysin expression, and IL-6 signaling through STAT3 plays a role in IL-1β-induced promatrilysin expression.
Keywords: matrilysin, prostate, matrix metalloproteinase, interleukin-6, interleukin-1
Introduction
The processes by which tumor cells can invade through the basal lamina as well as intravasate into the vasculature and extravasate into surrounding tissues are mediated, in part, by matrix metalloproteinases (MMPs) [1,2]. MMPs are a family of secreted zinc-dependent proteolytic enzymes responsible for degradation of the extracellular matrix [3–5]. MMP activity has been implicated in matrix degradation during both normal physiological processes, such as embryonic development, the cycling endometrium and wound healing, and pathologic conditions such as cancer. The MMP, matrilysin (MMP-7, PUMP-1), which, like other MMPs, is secreted in an inactive form (promatrilysin), is unique in that it is expressed primarily in glandular epithelial cells. Overexpression of promatrilysin has been associated with multiple types of malignancies including gastric, [6] esophageal, [7,8] colon [9], and prostate carcinomas [10,11]. In addition, high levels of promatrilysin expression have been observed by our laboratory in inflamed prostatic ducts and glands of the human acinar prostate [11], cycling endometrial tissues [12], and in the involuting rat prostate [13].
Previously, our laboratory demonstrated that physiologically relevant doses of the inflammatory cytokine, interleukin-1β (IL-1β), could induce promatrilysin protein expression up to 100-fold over baseline expression levels in the prostate carcinoma cell line, LNCaP [14]. IL-1β stimulates pleiotropic effects that regulate the acute immune response. Downstream effects of IL-1β include stimulation of T cell [15–17] and B cell [18] proliferation and expression of other signaling factors such as tumor necrosis factor-α, IL-6, and IL-8 [19]. Because of our in vitro finding that IL-1β can induce promatrilysin expression, we proposed that IL-1β signaling could also be responsible for the overexpression of promatrilysin in inflamed glandular epithelial tissues and prostatic carcinomas.
Several mechanisms of matrilysin regulation have been characterized. Induction of matrilysin gene expression by epidermal growth factor (EGF) and phorbol esters has previously been attributed to the activity of activator protein-1 (AP-1) and serum response protein transcription factors [20]. However, the molecular mechanism(s) involved in regulation of IL-1β-induced promatrilysin expression have not been elucidated.
IL-1β-induced activation of nuclear factor Kappa B (NFκB) has been shown to be involved in regulation of several other members of the MMP family including MMP-1 and -9 [21–25], as well as other MMPs [26–28]. Furthermore, bothMMP-1 [29] and -9 [30] are known to have NFκB binding elements residing within the 5′ flanking region of their respective genes. However, contrary to the MMPs that are known to be regulated by NFκB, the sequenced promoter region of the matrilysin gene does not contain a known binding element for NFκB. However, because IL-1β frequently elicits its downstream effects through NFκB transactivation activity, we conducted experiments to determine whether NFκB plays a role in IL-1β-induced expression of promatrilysin in LNCaP cells. We also examined whether the mechanism of promatrilysin induction by IL-1β is direct, whereby IL-1β induces NFκB-mediated transactivation of the matrilysin gene independent of synthesis of additional signaling factors or, indirect, whereby synthesis of one or more intermediate signaling factors is required. Because there has been no NFκB binding element identified within the sequenced region of the human matrilysin promoter, we hypothesized that IL-1β-induced NFκB-mediated transcription of an intermediate signaling factor(s) is required for potentiation of promatrilysin expression.
Our laboratory has previously demonstrated that overexpression of promatrilysin increases invasive capacity of the prostatic carcinoma cell line, DU-145 [9]. In addition, Hashimoto et al. [31] showed a significant correlation between the level of promatrilysin expression and the grade and stage of prostatic carcinoma, indicating that matrilysin may play an important role in tumor invasion and metastasis. These data suggested that control of matrilysin expression may be a key to preventing prostatic carcinoma progression; and upstream regulatory factors, including the cytokine, IL-1β, may be useful targets in developing a therapeutic strategy for treatment of prostate cancer at early stage, to block invasion and metastases; or at later stage, to prevent further progression.
Our data have characterized a signal transduction pathway by which IL-1β regulates promatrilysin expression through an indirect mechanism requiring NFκB-dependent synthesis of IL-6. This pathway demonstrates, for the first time, regulation of a MMP by a secreted signaling factor (IL-1β) through an indirect pathway that requires synthesis of an additional secreted protein (IL-6). In addition, we show that STAT3 appears to play a role in the signal transduction pathway downstream of IL-6.
Materials and Methods
Cell Culture
Lymph node carcinoma cells of the prostate (LNCaP) were acquired from the American Type Culture Collection (ATCC, Manasass, VA). LNCaP cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented supplemented with 10% fetal bovine serum, 100 εg/ml penicillin, and 100 εg/ml streptomycin, both from Gibco-BRL (Rockville, MD).
Cytokines
IL-1β and IL-6 were obtained from Calbiochem (La Jolla, CA). Cells were serum starved for approximately 16 hours before cytokine treatment in serum-free DMEM.
Plasmid Constructs
The plasmid construct used in the reporter assays to measure NFκB transactivation activity, NFκB-Luc, was generously provided by the laboratory of Zigang Dong (University of Minnesota, The Hormel Institute, Austin, MN). The construct encodes a 196-bp fragment of the HIV promoter containing 2X NFκB binding elements driving a luciferase reporter gene. The parent vector, PGL2-basic (Promega, Madison, WI) was used as a control. To generate the heterologous human matrilysin promoter (HMAT-luc) construct used in these studies, 1179 bp of the sequenced human matrilysin promoter (GenBank accession #L22525) located directly upstream of the TATA box were amplified by polymerase chain reaction and subcloned into pTAL-Luc (Promega). Polymerase chain reaction amplification of the human matrilysin promoter (kindly provided by the laboratory of Lynn Matrisian, Vanderbilt University) was carried out using the following heterologous primers, which contained either Nhe1 (upstream primer or Xho1 (downstream primer) restriction site sequences linked to matrilysin promoterspecific sequences (matrilysin-specific sequences are underlined): upstream primer 5′-CGTCTTGTCATTGGCGAATTC-3′, and the antisense downstream primer 5′-CCCCAGTGCAAGTGCAGGTGC-3′. The resultant 1217-bp amplification product was digested with Nhe1/Xho1, gel purified, and directionally cloned into Nhe1/Xho1-digested pTAL-Luc vector directly upstream of the thymidine kinase minimal promoter. The resultant plasmid construct was confirmed with DNA sequencing. The STAT3 dominant negative plasmid construct was generously provided by the laboratory of Ralph A. Bradshaw (University of California Medical School, Irvine, CA). The double mutant STAT3 contains both Tyr→Phe and Ser→Ala mutations preventing phosphorylation at sites critical for STAT3 activity [32]. The parent vector into which the STAT3 double mutant was cloned, pCMV-1, was used as a control plasmid.
Cationic Lipid Transfection
LNCaP cells were seeded into 12.5 cm2 flasks with 1x106 cells per flask, 24 hours before transfection. The cationic lipid DMRIE-DOPE (Vical Inc., La Jolla, CA) was used as per standard procedures. Briefly, plasmid DNA (2.5 εg/flask) and DMRIE-DOPE (10 εg/flasks) were incubated in a polystyrene tube for 30 minutes. The transfection complex was then added to cells in serum-free DMEM(1.5 ml/flask). Cells remained in transfection medium for approximately 16 hours at which time the cells were removed from the transfection medium and stimulated with cytokine under serum-free conditions. For co-transfection experiments, 1.25 βg of each plasmid was used per flask such that the total DNA content per flask was 2.5 βg. To ensure consistency of transient transfection efficiency, LNCaP cells were transfected with pIRES-EGFP (Promega) and viewed under a fluorescent light microscope to visualize enhanced green fluorescent protein. Transient transfection efficiency was consistently reproducible at 20% using these conditions.
NFκB Inhibitors
Sulfasalazine and pyrrolidine dithiocarbamate (PDTC) were obtained from Sigma Chemical Co. (St. Louis, MI). LNCaP cells were treated with 0.5 to 1 mM sulfasalazine in serum-free DMEM, 2 hours before IL-1β stimulation. NFκB SN50 cell-permeable inhibitor and NFκB SN50M inactive control peptide were obtained from Calbiochem (La Jolla, CA). LNCaP cells were treated with 18 µM inhibitory or control peptide in serum-free DMEM, 1 hour before stimulation with IL-1µ.
Quantification of Promatrilysin Expression by ELISA
Sandwich ELISA analyses were performed as described [14]. The capture antibody (10D2), a mouse monoclonal antibody, and detection antibody (RB2), a rabbit polyclonal antibody, were produced by and obtained from the laboratory of Raymond Nagle (Arizona Cancer Center, Tucson, AZ). Horseradish peroxidase-conjugated goat anti-rabbit antibody (Pierce, Rockford, IL) was used to detect RB2. Purified promatrilysin was used to generate a standard curve within the linear range of 0.2 to 12.5 ng/ml.
Quantification of IL-6 Expression by ELISA
Sandwich ELISA analyses were performed by the UMAB Cytokine Core Laboratory in Baltimore, MD. Conditioned media samples were diluted and shipped overnight on dry ice. Human IL-6 was measured by two antibody ELISA using biotin-streptavidin-peroxidase detection. Cytokine concentration in each sample was calculated from the standard curve equation. The range of the assay is 1.562 to 200 pg/ml. A standard curve was generated using concentrations within this range and an internal control containing 50 pg/ml was analyzed in parallel.
Measurement of Luciferase
Total protein concentration of each sample was determined using the DC Bio-Rad Protein Assay (Bio-Rad, Cambridge, MA). Ten to 30 µg of total protein of each sample was used for the luciferase assays. A TD 20/20 luminometer (Turner Designs, Pharmingen, San Diego, CA) was used to quantify the emitted light, which is directly proportional to the amount of luciferase present in the sample.
Northern Analyses
Total RNA was extracted using RNEasy (Qiagen, Valencia, CA) 8 to 10 hours following IL-1β or 2 hours following IL-6 stimulation. Twenty micrograms of total RNA was subjected to electrophoresis on a 1% agarose, 3-{N-morpholino} propane-sulfonic acid/formaldehyde gel. The RNA was transblotted to a nylon membrane (GeneScreen, NEN Research Products, Boston, MA) and crosslinked with ultraviolet light (GS Genelinker, Biorad, Hercules, CA). The membranes were then hybridized with a 32P-labeled random primed probe generated from cDNA for the mRNA of interest using the TS RadPrime DNA labeling system (Gibco-BRL, Gaithersburg, MD) and washed according to the membrane manufacturer instructions. The membranes were then exposed to a storage phosphorscreen (Molecular Dynamics, Sunnyvale, CA). A Molecular Dynamics phosphorimager equipped with ImageQuant package was used for obtaining and analyzing digital images. As a control for loading and transfer of RNA, membranes were stripped by boiling on 0.1% SDS for 30 minutes and reprobed for glyceraldehyde-3′-phosphate-dehydrogenase (GAPDH) as described with a probe generated from an 800-bp Xba1-Pst1 fragment from pHcGAP (ATCC, Rockville, MD).
Results
NFκB Mediates IL-1β-Induced Promatrilysin Expression
NFκB is known to mediate transcription of IL-1β-induced genes including the cytokines IL-6 and IL-8 [33]. To determine whether NFκB transactivation activity is necessary for potentiation of IL-1β-induced promatrilysin expression in LNCaP cells, we used a variety of inhibitors of NFκB activation or nuclear localization to block NFκB transactivation activity in IL-1β-treated cells. These inhibitors, which are both known to inhibit activation of NFκB, included the antioxidant and metal chelator, PDTC, as well as sulfasalazine, a drug commonly prescribed for inflammatory disorders including ulcerative colitis and rheumatoid arthritis [34,35]. We also used the NFκB SN50 cell-permeable inhibitor peptide (Calbiochem, San Diego, CA), which specifically binds to and blocks the nuclear localization site on the p50 subunit of NFκB, thereby inhibiting nuclear translocation of the active NFκB heterodimer following degradation of IκB. The NFκB SN50M cell-permeable inactive control peptide is scrambled and does not have the ability to bind p50 [36].
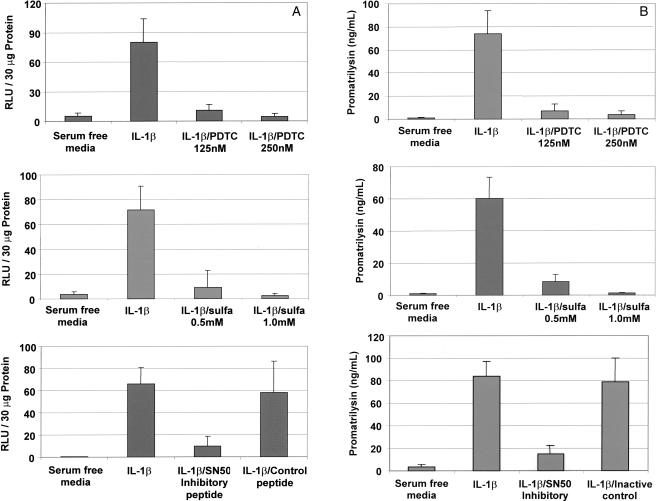
We first demonstrated that the inhibitors were effective in blocking NFκB transactivation activity in LNCaP cells using a luciferase reporter assay. LNCaP cells were transfected with a plasmid construct encoding a minimal HIV promoter containing 2X NFκB cis elements driving a luciferase reporter gene. Transfected cells were pretreated with inhibitor for 1 hour before stimulation with 200 pg/ml IL-1β. Inhibitor concentration remained constant throughout the duration of IL-1β stimulation. Transcription through NFκB cis elements was determined by quantification of luciferase expression in whole cell lysates. IL-1β-induced transcription through NFκB cis elements was significantly abrogated by pretreatment with NFκB inhibitors PDTC (Figure 1A, top), sulfasalazine (center), and NFκB SN50 cell-permeable inhibitor peptide (bottom, column 3), but not by NFκB SN50M cell-permeable inactive control peptide (bottom, column 4).
Figure 1.
NFκB mediates promatrilysin expression in LNCaP cells. LNCaP cells were transiently transfected with a plasmid construct encoding a minimal HIV promoter containing 2X NFκB binding elements driving a luciferase reporter gene. Transfected cells were treated with either PDTC (125 or 250 nM), sulfasalazine (0.5 or 1.0 mM), SN50 NFκB inhibitory peptide, or SN50B inactive control peptide (18 µM) 1 hour before stimulation with IL-1β. Twenty-four hours following IL-1β stimulation, (A) whole cell lysates were collected and analyzed for luciferase expression and (B) promatrilysin expression in conditioned media was quantified using ELISA analyses. The results shown represent the means and standard deviations of three experiments each performed in triplicate.
Inhibition of NFκB transactivation activity also blocked IL-1β-induced promatrilysin expression (Figure 1B). Incubation and treatment times were identical to the preceding experiment measuring NFκB transactivation activity. Twenty-four hours following IL-1β stimulation, promatrilysin expression in conditioned media was quantified using ELISA analyses. In agreement with the pattern observed for inhibition of transcription through NFκB cis elements, IL-1β-induced promatrilysin expression was blocked by NFκB inhibitors, PDTC (Figure 1B, top), sulfasalazine (center), and NFκB SN50 cell-permeable inhibitor peptide (bottom, column 3), but not by NFκB SN50M cell-permeable inactive control peptide (bottom, column 4).
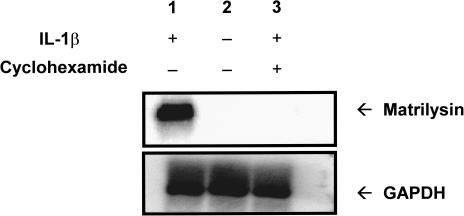
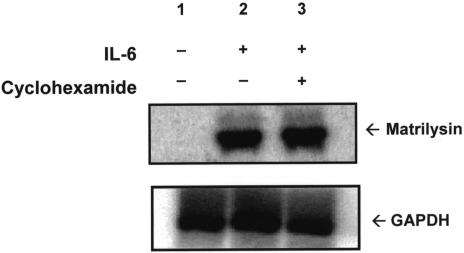
Cyclohexamide Blocks IL-1β-Induced Matrilysin mRNA
The next step was to determine whether the induction of promatrilysin expression by IL-1β is indirect and dependent on synthesis of one or more intermediate signaling factors, or whether IL-1β acts directly to upregulate promatrilysin expression in LNCaP cells without synthesis of one or more intermediate signaling factors. LNCaP cells were concurrently treated with cyclohexamide (10 µg/ml) and stimulated with IL-1β. Northern analyses for matrilysin messenger RNA were performed 8 hours after IL-1β stimulation. The 8-hour time point was used because that is when peak matrilysin message is observed (data not shown). A strong induction of matrilysin message was apparent following treatment with IL-1β alone (Figure 2, lane 1) compared with cells not stimulated with IL-1β (Figure 2, lane 2). It is of interest that, although basal promatrilysin expression can be detected at low levels in LNCaP conditioned media, matrilysin mRNA is difficult to detect using Northern analyses. Concurrent treatment with cyclohexamide blocked IL-1β-induced expression of matrilysin mRNA (Figure 2, lane 3) indicating a requirement for new protein synthesis for the induction of promatrilysin expression by IL-1β. Inhibition of protein synthesis was confirmed by quantification of 35S-methionine uptake with the dose of cyclohexamide used (data not shown). Reprobing of the stripped blots for GAPDH mRNA demonstrated equal loading of RNA samples (Figure 2, lower panel).
Figure 2.
Cyclohexamide blocks IL-1β-induced matrilysin mRNA. LNCaP cells were serum starved for 16 hours then were treated with either 200 pg/ml IL-1β (lane 1), serum-free DMEM (lane 2), or IL-1β (200 pg/ml) and cyclohexamide (10 µg/ml) simultaneously (lane 3). Total RNA was collected 8 hours following IL-1β stimulation. RNA samples were subjected to 1% agarose gel electrophoresis and transferred and crosslinked to a nylon membrane. The membrane was probed for matrilysin mRNA. Blots were stripped and reprobed for GAPDH (lower panel). The blot shown is representative of five repeats of this experiment.
IL-1β Induces Expression of IL-6 by LNCaP Cells
IL-1β has been shown to induce synthesis of the cytokine IL-6 through NFκB transactivation activity in numerous cell types [19,37]. In addition, multiple IL-6 responsive consensus elements (NFIL6) have been identified within the published region of the human matrilysin promoter sequence [12]. It had previously been established that LNCaP cells do not constitutively express IL-6 [38]; however, the ability of IL-1β to induce IL-6 expression in LNCaP cells had not been tested.
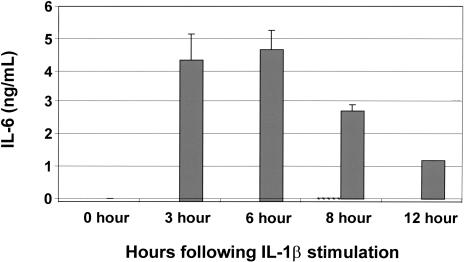
We determined that IL-1β does stimulate high levels of expression of IL-6 in LNCaP cells. Cells were stimulated with IL-1β, then ELISA analyses were used to quantify IL-1β-induced IL-6 expression at various time points. IL-1β-induced IL-6 secretion was substantially increased within 3 hours following stimulation with IL-1β, and peaked at approximately 5 ng/ml (Figure 3). The secretion of IL-6 protein induced by IL-1β precedes the IL-1β-induced increase in matrilysin mRNA expression that begins approximately 4 hours after stimulation and peaks at approximately 8 hours. Inhibition of NFκB with sulfasalazine completely blocked IL-1β-induced expression of IL-6. In addition, p50-specific peptide also completely abrogated IL-1β expression of IL-6, and IL-6 levels were not detected at any time point in cells not stimulated with IL-1β (data not shown).
Figure 3.
IL-1β induces expression of IL-6 in LNCaP cells. LNCaP cells were serum starved for 16 hours then they either remained in serum-free DMEM or were stimulated with IL-1β in serum-free DMEM. Conditioned media samples were collected at the indicated time points after IL-1β stimulation and analyzed for IL-6 concentration using ELISA analyses. Cells stimulated with IL-1β that were pretreated with sulfasalazine (1 mM), to inhibit NFκB transactivation activity, did not secrete a measurable amount of IL-6 (data not shown). IL-1β-stimulated cells (solid bar) demonstrate induction of IL-6 expression. The results shown represent the means and standard deviations of three experiments each performed in triplicate.
These data agree with published literature demonstrating that IL-6 has an inhibitory effect on LNCaP cell growth [39].
IL-6 Induces Promatrilysin Expression in LNCaP Cells
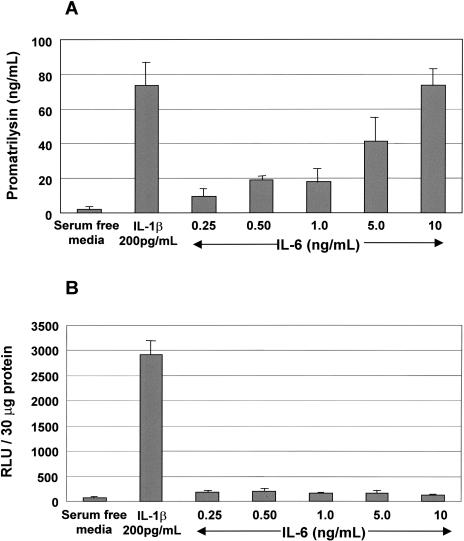
Previous studies have shown that IL-6 induces expression of several MMPs including MMP-2 and-9 [40]. To determine whether IL-6 is an intermediate required for potentiation of IL-1β-induced promatrilysin expression, we first measured promatrilysin expression in LNCaP cells treated with escalating dose levels of recombinant IL-6. Promatrilysin expression in conditioned media were quantified using ELISA analyses 24 hours following IL-6 stimulation. Our data demonstrate that IL-6 induces promatrilysin expression in a dose-dependent manner (Figure 4A). The induction of promatrilysin with 10 ng/ml recombinant IL-6 was equivalent to the induction observed with 200 pg/ml IL-1β stimulation. Induction of promatrilysin observed using the concentration of IL-6 present in medium from IL-1β-treated cells (5 ng/ml) was approximately 35% less than the level achieved with IL-1β stimulation.
Figure 4.
IL-6 induces promatrilysin expression in LNCaP cells. LNCaP cells were transiently transfected with a plasmid construct encoding a minimal HIV promoter containing 2X NFκB binding elements driving a luciferase reporter gene. Transfected cells were treated with escalating doses of recombinant IL-6. Twenty-four hours following cytokine stimulation, (A) conditioned media were analyzed for matrilysin expression using ELISA analyses and (B) luciferase expression in whole cell lysates was quantified. The results shown represent the means and standard deviations of at least three experiments each performed in triplicate.
To determine whether NFκB plays a role in IL-6-induced promatrilysin expression, we quantified NFκB transactivation activity in IL-6-stimulated cells. LNCaP cells were transiently transfected with a plasmid construct encoding a minimal HIV promoter with 2X NFκB cis elements. Transfected cells were stimulated with increasing doses of IL-6. Luciferase activity in whole cell lysates was quantified 8 hours following cytokine stimulation. IL-6 did not significantly induce NFκB transactivation activity (Figure 4B), indicating that IL-6-induced promatrilysin expression is not mediated by NFκB transactivation activity.
IL-6 Mediates IL-1β-Induced Promatrilysin Expression
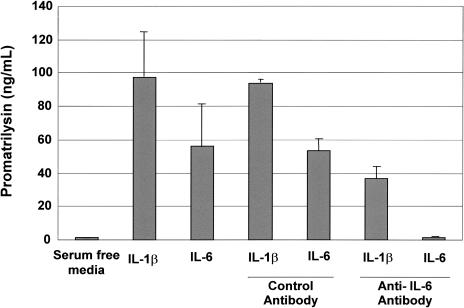
We then conducted studies to test whether inhibition of IL-6 activity could block IL-1β-induced promatrilysin expression. LNCaP cells were treated with recombinant IL-1β alone or IL-1β plus either an IL-6 neutralizing antibody directed against the IL-6 ligand or, to control for a nonspecific antibody effect, an antibody directed against an unrelated human protein (Nm23). Cells were pretreated with 10 µg/ml antibody for 1 hour then, in the presence of antibody, stimulated with recombinant IL-1β (200 pg/ml) or IL-6 (5 ng/ml) in serum-free conditions. An additional 10 µg/ml of each respective antibody was added to the media 4 hours subsequent to cytokine stimulation. The dose of 5 ng/ml recombinant IL-6 was chosen to simulate the concentration of IL-6 produced by LNCaP cells in response to treatment with 200 pg/ml IL-1β. Using ELISA analyses, secreted promatrilysin expression in conditioned media was measured 14 hours following stimulation with either IL-1β or IL-6 (Figure 5). The first column shows the basal level of promatrilysin expression in the absence of cytokine stimulation. The second and third columns demonstrate the induction of promatrilysin with 200 pg/ml of IL-1β and 5 ng/ml IL-6, respectively. As anticipated, the rabbit polyclonal antibody directed against an unrelated human protein had no effect on IL-1β- or IL-6-induced promatrilysin expression (columns 4 and 5). As shown in column 6, IL-6 neutralizing antibody significantly blocked IL-1β-induced promatrilysin expression (approximately 60%). Inhibition of IL-6 activity by the neutralizing antibody was confirmed by demonstrating virtually complete inhibition of IL-6-induced promatrilysin expression by the IL-6 neutralizing antibody (column 7). Higher concentration of IL-6 neutralizing antibody did not enhance the inhibitory effect (data not shown).
Figure 5.
IL-6 mediates IL-1β-induced promatrilysin expression. LNCaP cells were serum starved for 16 hours then stimulated with either IL-1β or IL-6 in the presence of 10 µg/ml of an antibody against either IL-6 or an antibody against an unrelated human protein. An additional 10 µg/ml of the respective antibody was added to the medium 4 hours following the first antibody treatment and cytokine stimulation. Using ELISA analyses, promatrilysin expression was measured 10 hours following IL-6 stimulation. The results shown represent the means and standard deviations of at least three experiments each performed in triplicate.
Cyclohexamide Does Not Block IL-6-Induced Matrilysin mRNA
Similar to the approach taken to characterize the pathway by which IL-1β induces promatrilysin expression, the next step was to determine whether the induction of promatrilysin by IL-6 is indirect, and dependent on synthesis of one or more intermediate signaling factors, or whether IL-6 acts directly to upregulate promatrilysin expression in LNCaP cells without synthesis of one or more intermediate signaling factors. LNCaP cells were concurrently treated with cyclohexamide (10 µg/ml) and stimulated with IL-6. Northern analyses for matrilysin messenger RNA were performed 2 hours after IL-6 stimulation. The 2-hour time point was chosen because we observed that matrilysin message peaked 2 hours following stimulation with IL-6 (data not shown). A significant induction of matrilysin message was apparent following treatment with IL-6 alone (Figure 6, lane 2) compared with unstimulated cells. It is of interest that concurrent treatment of cyclohexamide with IL-6 stimulation did not block IL-6-mediated transcription of matrilysin mRNA (lane 3) indicating that the mechanism by which IL-6 induces promatrilysin expression is direct and does not require synthesis of new proteins. Inhibition of protein synthesis was confirmed by quantification of 35S-methionine uptake with the dose of cyclohexamide used (data not shown). Reprobing of the stripped blots for GAPDH mRNA demonstrated equal loading of RNA samples (Figure 6, lower panel).
Figure 6.
Cyclohexamide does not block IL-6-induced matrilysin mRNA. LNCaP cells were serum starved for 16 hours then were treated with either serum free DMEM (lane 1), 5 ng/ml IL-6 (lane 2), or IL-6 (5 ng/ml) and cyclohexamide (10 µg/ml) simultaneously (lane 3). Total RNA was collected 2 hours following IL-1β stimulation. RNA samples were subjected to 1% agarose gel electrophoresis and transferred and crosslinked to a nylon membrane. The membrane was probed for matrilysin mRNA. Blots were stripped and reprobed for GAPDH (lower panel). The blot shown is representative of at least five repeats of this experiment.
STAT3 Plays a Role in IL-6-Induced Promatrilysin Expression
We previously showed that transcriptional enhancer elements present in the human matrilysin promoter region are responsive to IL-1² [14]. We next demonstrated that transcriptional enhancer elements present in the human matrilysin promoter region are also responsive to IL-6 stimulation. To study the downstream transcription factor(s) involved in the induction of matrilysin transcription by IL-1β and IL-6, STAT3 was examined for several reasons. First, many studies have shown that IL-6 signals directly through STAT3. For example, Villavicencio et al. [41] demonstrated that autocrine and paracrine interactions of IL-6 family cytokines cause STAT3 activation in the angiotensin II pathway in rat hepatocytes. STAT3 activation has also been shown to be responsible for IL-6-dependent T cell proliferation through preventing apoptosis [42]. Furthermore, Chung et al. [39] and Spiotto and Chung [43,44] have shown that IL-6 directly activates STAT3, which then regulates growth inhibition and differentiation in LNCaP cells. In addition, the published human matrilysin promoter sequence is known to contain numerous cis elements including NF-IL6 elements to which STAT3 may be capable of binding.
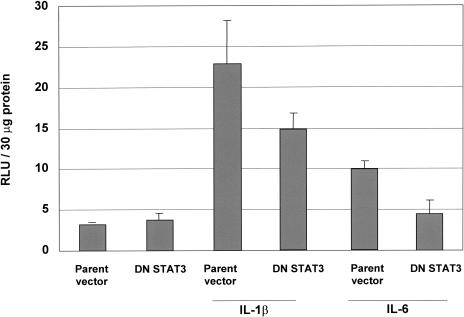
LNCaP cells were transiently co-transfected with a plasmid construct encoding 1.2 kb of the human matrilysin promoter driving a luciferase reporter gene and, either a plasmid construct encoding a dominant negative STAT3 containing point mutations at critical phosphorylation sites, or pCMV-1, the parent vector into which the STAT3 double mutant was cloned. The ratio of promoter construct to dominant negative or parent vector was 1:1. Columns 1 and 2 (Figure 7) show baseline luciferase activity in unstimulated cells. Both IL-1β and IL-6 induced activity of the 1.2-kb human matrilysin promoter as demonstrated by induction of luciferase activity (columns 3 and 4). It is of interest that the fold induction of promoter activity achieved with IL-1β versus IL-6 agrees with the promatrilysin protein expression levels observed with the respective cytokines as measured by ELISA analyses shown in Figure 5. Co-transfection with the STAT3 dominant negative abrogated the fold increase of IL-1β-induced promoter activity by approximately 45%. In addition, dominant negative STAT3 inhibited IL-6-induced promoter activity to baseline levels. These results also concur with the results presented in Figure 5, where neutralization of IL-6 inhibited IL-1β-induced promatrilysin expression by approximately 60%. A higher ratio of dominant negative STAT3 plasmid to promoter construct (2:1) did not cause further inhibition of IL-1β-induced matrilysin promoter activity (data not shown).
Figure 7.
STAT3 mediates IL-6-induced promoter activity. LNCaP cells were transiently co-transfected with a plasmid construct encoding 1.1 kb of the human matrilysin promoter driving a luciferase reporter gene and, either a plasmid construct encoding a dominant negative STAT3 containing point mutations at critical phosphorylation sites, or pCMV-1, the parent vector into which the STAT3 double mutant was cloned. Transfected cells were treated with either IL-1β or IL-6, then luciferase activity in whole cell lysates was quantified 24 hours following cytokine stimulation. The results shown represent the means and standard deviations of at least three experiments.
Discussion
It has been suggested that the inflammatory response may play a key role in the development and progression of prostatic carcinoma. Okada et al. [45] examined inflammation in needle biopsy specimens in both normal and carcinoma prostatic tissue to reveal the possible contribution of histologic inflammation within the prostate to the abnormal elevation of serum prostate-specific antigen (PSA) levels. The presence of histologic inflammation within the prostate was shown to correlate significantly with serum PSA levels [45]. Histologically defined acute inflammation within the prostate was found to be a significant contributor to elevated serum PSA levels. Because of these results, it is now hypothesized that the assessment of inflammation in needle biopsy specimens, in conjunction with PSA levels, may be a useful diagnostic or prognostic indicator for prostatic carcinoma. Other studies have concurred with these data [45,46].
Our data demonstrate that IL-1β-induced promatrilysin expression in LNCaP cells is dependent on NFκB-mediated synthesis of IL-6. In addition, we demonstrate that STAT3 plays a role in the pathway downstream of IL-6. Previously, using immunohistochemical staining of paraffin-embedded serial sections of primary prostate tumor tissue, our laboratory demonstrated that high levels of promatrilysin were detected adjacent to dilated ducts or atrophic glands that were surrounded by inflammatory cell infiltrates, which presumably contain a high concentration of cytokines, including IL-1β and IL-6 [11]. Subsequently, we showed that LNCaP cells secrete a high level (70- to 100-fold increase over baseline expression levels) of promatrilysin in response to stimulation with recombinant IL-1β. We now reveal that IL-1β-induced promatrilysin expression is potentiated through a multi-step pathway that requires NFκB-mediated IL-6 synthesis and downstream signaling by STAT3.
IL-6 is particularly relevant to prostatic carcinoma for several reasons. Adler et al. [47] have shown that patients with metastatic and hormone refractory prostatic carcinoma have a high level of IL-6 circulating in their peripheral blood. Furthermore, both IL-6 [48,49] and matrilysin [50] are known to play a role in differentiation of bone, the tissue to which prostatic carcinoma characteristically metastasizes, and circulating levels of IL-6 have been associated with bone metastasis in patients with prostate carcinoma [47]. In addition, other studies have demonstrated that the more progressed and less differentiated hormone-independent prostatic carcinoma cell lines, PC3 and DU-145, express a constitutive level of secreted IL-6; however, those data also showed that the less-progressed and hormone-responsive LNCaP cells did not secrete any detectable IL-6 [39].
IL-6 has been shown to regulate the expression of several MMP family members [51,52]. Kossakowska et al. [40,53] demonstrated that elevated IL-6 expression correlated with upregulation of MMP-2 and -9 in tumor biopsy specimens from patients with non-Hodgkin's lymphoma (NHL). In addition, IL-6 induced expression of MMP-2 and-9, and significantly upregulated transmigration in the Matrigel invasion assay by the lymphoid cell lines Raji, Jurkat, and NC-37 [40,53]. Those data suggested that IL-6 may play a role in determining aggressiveness of NHL by regulation of MMP production. In addition, our laboratory demonstrated that stable transfection of the prostatic carcinoma cell line, DU-145, with the full-length cDNA of the human matrilysin gene enhanced invasive capacity of DU-145 cells both in vitro and in vivo [9].
In the initial studies conducted with LNCaP cells, a dose of 200 pg/ml of IL-1β was sufficient to elicit a measurable induction of promatrilysin [14]. However, this dose was insufficient for induction of a measurable level of promatrilysin by the other cytokines tested, including IL-6. It has since been determined that IL-6 requires a much higher concentration (ng/ml range) to elicit its observed downstream effects. Interestingly, elevated levels (ng/ml range) of IL-6 have been shown to be clinically relevant in the peripheral blood of patients with hormone refractory prostatic carcinoma [54]. For the first time, we demonstrate that LNCaP cells have the capacity to secrete IL-6 in response to IL-1β through a NFκB-dependent mechanism.
Research conducted by Okamoto et al. [38] demonstrated that IL-6 is constitutively expressed by the prostatic carcinoma cell lines, DU-145 and PC3, but not by the prostatic carcinoma cell line, LNCaP. It is of interest that IL-1κ and IL-6 have been reported to inhibit the growth of LNCaP cells, but not DU-145 or PC-3 cells. It is possible that the inhibitory effect of IL-1κ on the growth of LNCaP cells is mediated by the induction of IL-6 expression in these cells. Spiotto and Chung [43] have recently determined that the inhibition of growth in LNCaP cells caused by IL-6 is due to activation of the STAT3 pathway. Furthermore, they determined that this pathway is not functional in DU-145 and PC-3 cells. Is it also of interest that IL-1 has been shown to inhibit growth and induce neuroendocrine differentiation in LNCaP cells [55,56].
IL-1 family members and IL-6 may be relevant to cancers other than prostate carcinoma. Tomimatsu et al. [57] have shown that the presence of IL-1α and IL-6 in gastric carcinoma tissues correlated with a more differentiated cell type within the tumor and with occurrence and recurrence of liver metastases. In addition, Singh et al. [58] have demonstrated that, of nine melanoma samples examined, only the most invasive cell line, originally established from a lymph node metastases, expressed both IL-1κ and IL-6, in addition to other inflammatory cytokines.
We previously determined that IL-1κ does not induce promatrilysin in DU-145 or PC-3 cells (data not shown), and these cells, which are considered to be more invasive than LNCaP cells, have no basal expression of promatrilysin, although they are known to secrete IL-6 constitutively [38]. There is, therefore, a correlation between the ability of IL-6 to induce promatrilysin and the presence of an intact STAT3 pathway. In addition, new protein synthesis is not required for transcription of matrilysin mRNA by IL-6, and dominant negative STAT3 inhibited IL-1κ-and IL-6-induced matrilysin promoter activity. Thus, STAT3 is strongly implicated in the transcriptional regulation of promatrilysin expression by IL-6. This suggests that IL-6 downstream signaling through STAT3 initiates transcription of the matrilysin gene.
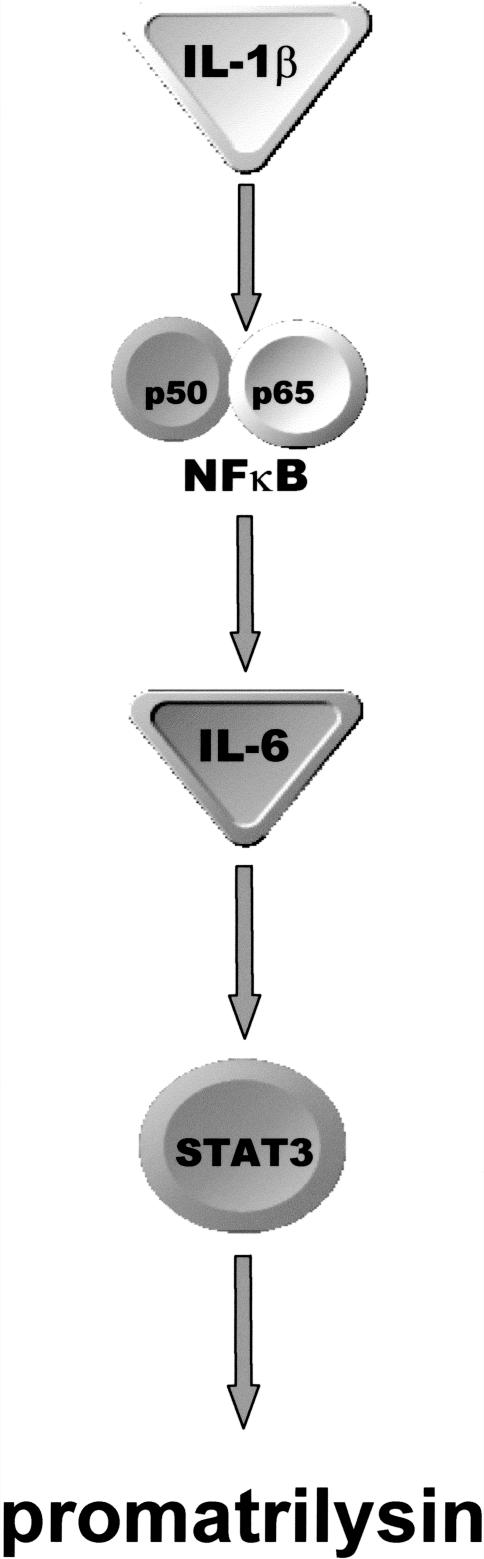
In our proposed model (Figure 8), NFκB is activated in response to IL-1κ ligand binding to its receptor. NFκB subsequently translocates to the nucleus and stimulates transcription of the IL-6 gene. Once secreted, IL-6 binds to its receptor, which, in turn, results in activation of STAT3. We hypothesize that IL-1β-induced expression of promatrilysin may be regulated by both autocrine and paracrine pathways of IL-6 ligand binding. Prostatic carcinoma tumors are heterogenous in nature; therefore, paracrine signaling mechanisms may be the most relevant in vivo, and secretion of a cytokine, like IL-6, or a MMP, like matrilysin, could potentially elicit multiple effects on the different types of cells that are present within a tumor. Degradation of the extracellular matrix by promatrilysin secreted by an epithelial cell may enhance invasive capacity of neighboring cells.
Figure 8.
Proposed IL-1β-induced promatrilysin expression signaling model. IL-1β-induced promatrilysin expression is mediated by synthesis of IL-6 through a NFκB-dependent pathway. In this model, IL-1β ligand binding to its receptor initiates the signaling cascade that results in degradation of IκB allowing nuclear translocation of the active NFκB heterodimer. Once in the nucleus, NFκB binds cis elements and induces transcription of IL-6 and other downstream effectors, which, in turn, result in transcription of the matrilysin gene. IL-6-mediated promatrilysin expression may be mediated through both autocrine and paracrine pathways depending on whether the IL-6 binds to the IL-6 receptor on the cell from which it was secreted or binds the IL-6 receptor on a neighboring cell that could be of a different type. STAT3 is activated in response to IL-6, and dominant negative STAT3 inhibits IL-6-mediated promatrilysin expression to baseline levels, which strongly indicates that STAT3 is involved in transcriptional regulation of IL-1β- and IL-6-induced promatrilysin expression.
It is of interest that, although IL-6 appears to be the major factor responsible for promatrilysin expression in response to IL-1β, these data suggest that an additional signaling factor(s) may be involved. In our system, approximately 5 ng/ml IL-6 is secreted by LNCaP cells following stimulation with 200 pg/ml IL-1β, and promatrilysin expression is increased up to 100-fold over baseline expression levels. However, stimulation with 5 ng/ml of exogenously added recombinant IL-6 induces only a 50- to 60-fold induction of promatrilysin. Furthermore, concurrent treatment of LNCaP cells with IL-1β and IL-6 neutralizing antibody reduces IL-1β-induced promatrilysin by 50% to 60%, not to baseline expression levels. Inhibition of IL-1β- and IL-6-induced matrilysin promoter activity by dominant negative STAT3 agreed with the levels of inhibition observed with the IL-6 neutralizing antibody. Thus, IL-1β may induce one or more factors in addition to IL-6 that upregulate promatrilysin expression, and the total induction of promatrilysin observed on stimulation with IL-1β could be the result of an additive or synergistic effect of multiple factors. However, because inhibition of NFκB transactivation activity completely abrogated IL-1β-induced promatrilysin expression, the other factor(s) involved must also be regulated by NFκ.
Induction of matrilysin gene expression by factors including EGF and phorbol esters is attributable to AP-1 and other serum response factor-regulated transcription factors [20]. However, until now, the pathway by which the inflammatory cytokine, IL-1κ, induces promatrilysin expression has been uncharacterized. Our data show a novel pathway by which NFκB-mediated synthesis of IL-6 is required for potentiation of IL-1κ-mediated transcription of the matrilysin gene through STAT3 transactivation activity downstream of IL-6. These data reveal a novel pathway for MMP regulation. Examination of paracrine interactions between different cells types and MMP expression may provide a valuable model to help elucidate invasive and metastatic mechanisms that occur in vivo in cancer progression that could lead to identification of novel therapeutic targets including signaling factors upstream of MMPs, including IL-1κ and IL-6.
Acknowledgement
We thank Steven P. Stratton, PhD for technical assistance and critical review of this manuscript.
Abbreviations
- GAPDH
glyceraldehyde-3′-phosphate-dehydrogenase
- IL
interleukin
- MMP
matrix metalloproteinase
- NFκB
nuclear factor Kappa B
- NHL
non-Hodgkin's lymphoma
- PDTC
pyrrolidine dithiocarbamate
- STAT
signal transducer and activator of transcription
Footnotes
This work was supported in part by NCI CA56666.
E-mail: msuzannestratton@aol.com.
References
- 1.Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Thomas GT, Lewis MP, Speight PM. Matrix metalloproteinases and oral cancer. Oral Oncol. 1999;35:227–233. doi: 10.1016/s1368-8375(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 3.Baramova E, Foidart JM. Matrix metalloproteinase family. Cell Biol Int. 1995;19:239–242. [PubMed] [Google Scholar]
- 4.Cockett MI, Birch ML, Murphy G, Hart IR, Docherty AJ. Metalloproteinase domain structure, cellular invasion and metastasis. Biochem Soc Trans. 1994;22:55–57. doi: 10.1042/bst0220055. [DOI] [PubMed] [Google Scholar]
- 5.Matrisian LM, Gaire M, Rodgers WH, Osteen KG. Metalloproteinase expression and hormonal regulation during tissue remodeling in the cycling human endometrium. Contrib Nephrol. 1994;107:94–100. doi: 10.1159/000422966. [DOI] [PubMed] [Google Scholar]
- 6.Adachi Y, Itoh F, Yamamoto H, Matsuno K, Arimura Y, Kusano M, Endoh T, Hinoda Y, Oohara M, Hosokawa M, Imai K. Matrix metalloproteinase matrilysin (MMP-7) participates in the progression of human gastric and esophageal cancers. Int J Oncol. 1998;13:1031–1035. doi: 10.3892/ijo.13.5.1031. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa S, Koshikawa N, Momiyama N, Moriyama K, Ichikawa Y, Ishikawa T, Mitsuhashi M, Shimada H, Miyazaki K. Matrilysin-specific antisense oligonucleotide inhibits liver metastasis of human colon cancer cells in a nude mouse model. Int J Cancer. 1998;76:812–816. doi: 10.1002/(sici)1097-0215(19980610)76:6<812::aid-ijc8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Itoh F, Hinoda Y, Imai K. Suppression of matrilysin inhibits colon cancer cell invasion in vitro. Int J Cancer. 1995;61:218–222. doi: 10.1002/ijc.2910610213. [DOI] [PubMed] [Google Scholar]
- 9.Powell WC, Knox JD, Navre M, Grogan TM, Kittelson J, Nagle RB, Bowden GT. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993;53:417–422. [PubMed] [Google Scholar]
- 10.Hashimoto K, Yano A, Tanabe T, Usui T, Kihira Y, Matsuo Y. Localization and expression of matrix metalloproteinase-7 in human prostate. Nippon Hinyokika Gakkai Zasshi. 1997;88:852–857. doi: 10.5980/jpnjurol1989.88.852. [DOI] [PubMed] [Google Scholar]
- 11.Knox JD, Wolf C, McDaniel K, Clark V, Loriot M, Bowden GT, Nagle RB. Matrilysin expression in human prostate carcinoma. Mol Carcinog. 1996;15:57–63. doi: 10.1002/(SICI)1098-2744(199601)15:1<57::AID-MC8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Gaire M, Magbanua Z, McDonnell S, McNeil L, Lovett DH, Matrisian LM. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. J Biol Chem. 1994;269:2032–2040. [PubMed] [Google Scholar]
- 13.Powell WC, Domann FE, Jr, Mitchen JM, Matrisian LM, Nagle RB, Bowden GT. Matrilysin expression in the involuting rat ventral prostate. Prostate. 1996;29:159–168. doi: 10.1002/1097-0045(199609)29:3<159::aid-pros2990290304>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Klein RD, Borchers AH, Sundareshan P, Bougelet C, Berkman MR, Nagle RB, Bowden GT. Interleukin-1 beta secreted from monocytic cells induces the expression of matrilysin in the prostatic cell line LNCaP. J Biol Chem. 1997;272:14188–14192. doi: 10.1074/jbc.272.22.14188. [DOI] [PubMed] [Google Scholar]
- 15.Bismuth G, Duphot M, Theze J. LPS and specific T cell responses: interleukin 1 (IL 1)-independent amplification of antigen-specific T helper (TH) cell proliferation. J Immunol. 1985;134:1415–1421. [PubMed] [Google Scholar]
- 16.Siese A, Jaros PP, Willig A. Analysis of interleukin (IL)-1 beta and transforming growth factor (TGF)-beta-induced signal transduction pathways in IL-2 and TGF-beta secretion and proliferation in the thymoma cell line EL4. OB-1. Scand J Immunol. 1999;49:139–148. doi: 10.1046/j.1365-3083.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 17.Zieleniewski W, Zieleniewski J, Stepien H. Interleukin-1 beta, but not IL-1 alpha, stimulates cell proliferation in the adrenal cortex. Cytobios. 1995;84:199–204. [PubMed] [Google Scholar]
- 18.Greenbaum LA, Horowitz JB, Woods A, Pasqualini T, Reich EP, Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988;140:1555–1560. [PubMed] [Google Scholar]
- 19.Kitamura H, Okamoto S, Shimamoto Y, Morimatsu M, Terao A, Saito M. Central IL-1 differentially regulates peripheral IL-6 and TNF synthesis. Cell Mol Life Sci. 1998;54:282–287. doi: 10.1007/s000180050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundareshan P, Nagle RB, Bowden GT. EGF induces the expression of matrilysin in the human prostate adenocarcinoma cell line, LNCaP. Prostate. 1999;40:159–166. doi: 10.1002/(sici)1097-0045(19990801)40:3<159::aid-pros3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi H, Shimizu R, Fujii K, Itoh S, Yang D, Onozaki K. Resistance to IL-1 anti-proliferative effect, accompanied by characteristics of advanced melanoma, permits invasion of human melanoma cells in vitro, but not metastasis in the nude mouse. Int J Cancer. 1997;71:416–421. doi: 10.1002/(sici)1097-0215(19970502)71:3<416::aid-ijc19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Oleksyszyn J, Augustine AJ. Plasminogen modulation of IL-1-stimulated degradation in bovine and human articular cartilage explants. The role of the endogenous inhibitors: PAI-1, alpha 2-antiplasmin, alpha 1-PI, alpha 2-macroglobulin and TIMP. Inflammation Res. 1996;45:464–472. doi: 10.1007/BF02252318. [DOI] [PubMed] [Google Scholar]
- 23.Origuchi T, Migita K, Nakashima T, Tominaga M, Nakamura H, Nakashima M, Aoyagi T, Kawakami A, Kawabe Y, Eguchi K. IL-1-mediated expression of membrane type matrix-metalloproteinase in rheumatoid osteoblasts. Clin Exp Rheumatol. 2000;18:333–339. [In Process Citation] [PubMed] [Google Scholar]
- 24.Shingu M, Miyauchi S, Nagai Y, Yasutake C, Horie K. The role of IL-4 and IL-6 in IL-1-dependent cartilage matrix degradation. Br J Rheumatol. 1995;34:101–106. doi: 10.1093/rheumatology/34.2.101. [DOI] [PubMed] [Google Scholar]
- 25.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 26.Dias S, Boyd R, Balkwill F. IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int J Cancer. 1998;78:361–365. doi: 10.1002/(SICI)1097-0215(19981029)78:3<361::AID-IJC17>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999;5:189–196. [PubMed] [Google Scholar]
- 28.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincenti MP, Coon CI, Brinckerhoff CE. Nuclear factor kappa B/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1 beta-stimulated synovial fibroblasts. Arthritis Rheum. 1998;41:1987–1994. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappa B elements. Cell Growth Differ. 1999;10:353–367. [PubMed] [Google Scholar]
- 31.Hashimoto K, Kihira Y, Matuo Y, Usui T. Expression of matrix metalloproteinase-7 and tissue inhibitor of metalloproteinase-1 in human prostate. J Urol. 1998;160:1872–1876. [PubMed] [Google Scholar]
- 32.Wu YY, Bradshaw RA. Activation of the Stat3 signaling pathway is required for differentiation by interleukin-6 in PC12-E2 cells. J Biol Chem. 2000;275:2147–2156. doi: 10.1074/jbc.275.3.2147. [DOI] [PubMed] [Google Scholar]
- 33.Yoon JH, Kim KS, Kim HU, Linton JA, Lee JG. Effects of TNF-alpha and IL-1 beta on mucin, lysozyme, IL-6 and IL-8 in passage-2 normal human nasal epithelial cells. Acta Oto-Laryngol. 1999;119:905–910. doi: 10.1080/00016489950180261. [DOI] [PubMed] [Google Scholar]
- 34.Ferran C, Millan MT, Csizmadia V, Cooper JT, Brostjan C, Bach FH, Winkler H. Inhibition of NF-kappa B by pyrrolidine dithiocarbamate blocks endothelial cell activation. Biochem Biophys Res Commun. 1995;214:212–223. doi: 10.1006/bbrc.1995.2277. [DOI] [PubMed] [Google Scholar]
- 35.Liptay S, Bachem M, Hacker G, Adler G, Debatin KM, Schmid RM. Inhibition of nuclear factor kappa B and induction of apoptosis in T-lymphocytes by sulfasalazine. Br J Pharmacol. 1999;128:1361–1369. doi: 10.1038/sj.bjp.0702937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 37.Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, Dejana E, Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989;142:549–553. [PubMed] [Google Scholar]
- 38.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 39.Chung TD, Yu JJ, Spiotto MT, Bartkowski M, Simons JW. Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999;38:199–207. doi: 10.1002/(sici)1097-0045(19990215)38:3<199::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA, Janowska-Wieczorek A. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin's lymphomas. Blood. 1999;94:2080–2089. [PubMed] [Google Scholar]
- 41.Villavicencio RT, Liu S, Kibbe MR, Williams DL, Ganster RW, Dyer KF, Tweardy DJ, Billiar TR, Pitt BR. Induced nitric oxide inhibits IL-6-induced stat3 activation and type II acute phase mRNA expression. Shock. 2000;13:441–445. doi: 10.1097/00024382-200006000-00004. [In Process Citation] [DOI] [PubMed] [Google Scholar]
- 42.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 43.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced growth inhibition in the human prostate cancer cell line LNCaP. Prostate. 2000;42:88–98. doi: 10.1002/(sici)1097-0045(20000201)42:2<88::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 44.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–195. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 45.Okada K, Kojima M, Naya Y, Kamoi K, Yokoyama K, Takamatsu T, Miki T. Correlation of histological inflammation in needle biopsy specimens with serum prostate-specific antigen levels in men with negative biopsy for prostate cancer. Urology. 2000;55:892–898. doi: 10.1016/s0090-4295(00)00519-7. [DOI] [PubMed] [Google Scholar]
- 46.Irani J, Goujon JM, Ragni E, Peyrat L, Hubert J, Saint F, Mottet N. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Pathologist Multi Center Study Group. Urology. 1999;54:467–472. doi: 10.1016/s0090-4295(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 47.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta 1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 48.Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Modulation of osteoclast differentiation by local factors. Bone. 1995;17:87S–91S. doi: 10.1016/8756-3282(95)00185-g. [DOI] [PubMed] [Google Scholar]
- 49.Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, Martin TJ, Hirota H, Taga T, Kishimoto T, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. 1995;182:1461–1468. doi: 10.1084/jem.182.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busiek DF, Ross FP, McDonnell S, Murphy G, Matrisian LM, Welgus HG. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992;267:9087–9092. [PubMed] [Google Scholar]
- 51.Louis E, Ribbens C, Godon A, Franchimont D, De Groote D, Hardy N, Boniver J, Belaiche J, Malaise M. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol. 2000;120:241–246. doi: 10.1046/j.1365-2249.2000.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solis-Herruzo JA, Rippe RA, Schrum LW, de La Torre P, Garcia I, Jeffrey JJ, Munoz-Yague T, Brenner DA. Interleukin-6 increases rat metalloproteinase-13 gene expression through stimulation of activator protein 1 transcription factor in cultured fibroblasts. J Biol Chem. 1999;274:30919–30926. doi: 10.1074/jbc.274.43.30919. [DOI] [PubMed] [Google Scholar]
- 53.Kossakowska AE, Hinek A, Edwards DR, Lim MS, Zhang CL, Breitman DR, Prusinkiewicz C, Stabbler AL, Urbanski LS, Urbanski SJ. Proteolytic activity of human non-Hodgkin's lymphomas. Am J Pathol. 1998;152:565–576. [PMC free article] [PubMed] [Google Scholar]
- 54.Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999;41:127–133. doi: 10.1002/(sici)1097-0045(19991001)41:2<127::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 55.Chiao JW, Hsieh TC, Xu W, Sklarew RJ, Kancherla R. Development of human prostate cancer cells to neuroendocrine-like cells by interleukin-1. Int J Oncol. 1999;15:1033–1037. doi: 10.3892/ijo.15.5.1033. [DOI] [PubMed] [Google Scholar]
- 56.Kawada M, Ishizuka M, Takeuchi T. Enhancement of antiproliferative effects of interleukin-1 beta and tumor necrosis factor-alpha on human prostate cancer LNCaP cells by coculture with normal fibroblasts through secreted interleukin-6. Jpn J Cancer Res. 1999;90:546–554. doi: 10.1111/j.1349-7006.1999.tb00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomimatsu S, Ichikura T, Mochizuki H. Significant correlation between expression of interleukin-1 alpha and liver metastasis in gastric carcinoma. Cancer. 2001;91:1272–1276. doi: 10.1002/1097-0142(20010401)91:7<1272::aid-cncr1128>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 58.Singh RK, Gutman M, Radinsky R. Heterogeneity of cytokine and growth factor gene expression in human melanoma cells with different metastatic potentials. J Interferon Cytokine Res. 1995;15:81–87. doi: 10.1089/jir.1995.15.81. [DOI] [PubMed] [Google Scholar]