Abstract
Permissiveness of monocytes and macrophages to human immunodeficiency virus (HIV) infection is modulated by various stimuli. In this study we demonstrate that stimulation of primary monocytes and monocyte-derived macrophages (MDM) through the receptors for the Fc portion of immunoglobulin G (IgG) (FcγR) inhibits HIV type 1 (HIV-1) replication. Viral p24 production was decreased by 1.5 to 3 log units in MDM infected with both R5 and X4 HIV-1 strains upon stimulation by immobilized IgG but not upon stimulation by soluble IgG or by F(ab′)2 IgG fragments. Although MDM activation by immobilized IgG induced high levels of macrophage-derived chemokine secretion as well as a sustained down-regulation of CD4 and a transient decrease in CCR5 expression, these factors did not appear to play a major role in the suppression of HIV-1 replication. Single-cycle infection of FcγR-stimulated MDM with HIV-1 virions pseudotyped with either HIV-1 R5 or vesicular stomatitis virus G envelopes was inhibited, suggesting a postentry restriction of viral replication. PCR analyses of HIV-1 DNA intermediate replication forms suggested that reverse transcription is not affected by stimulation with immobilized human IgG, at least during the first replication cycle. The accumulation of PCR products corresponding to nuclear unintegrated two-long-terminal-repeat circles and the relative decrease of integrated HIV-1 DNA signals suggest an inhibition of proviral integration. Our data, showing that FcγR-mediated activation of MDM is a potent mechanism of HIV-1 suppression, raise the possibility that FcγR cross-linking by immune complexes may contribute to the control of viral replication in macrophages.
Cells of the monocyte/macrophage lineage exert crucial functions in innate immune responses as well as in induction and regulation of cognate immunity against infectious agents. Macrophages can, however, be infected with any of several pathogens, including specific parasites, bacteria, and viruses, which replicate intracellularly, subverting one or more normal cellular functions. In particular, macrophages are a major target of human immunodeficiency virus type 1 (HIV-1). They are thought to play an important role in HIV-1 pathogenesis from early to late stages of the disease; according to several studies, macrophages participate in initial transmission of HIV-1 and in virus spreading and cell-to-cell transmission within lymphoid tissues, as well as in brain damage (references 28 and 41 and references therein). Because of the long life span of tissue macrophages and the lack of cytopathic effects of HIV infection, these cells are an important viral reservoir (16, 51). A study on a highly pathogenic simian model suggested that macrophages can even be the major reservoir of viral replication in late stages of AIDS (26). Consistent with the central role of macrophages in HIV dissemination and pathogenesis, a Vpx mutant of simian immunodeficiency virus which is unable to replicate in macrophages did not disseminate after intravenous or intrarectal inoculation (23). It is therefore reasonable to conclude that modulations of macrophage infection with HIV might have profound consequences for both host susceptibility to infection and control of viral dissemination in infected individuals.
Susceptibility of macrophages to HIV-1 infection varies among different donors and among tissue types within the same donor (15, 18, 37, 47, 50, 59) and is dependent on the stage of differentiation of blood monocytes into macrophages. Lower CCR5 expression and restriction of intracellular replication render monocytes less susceptible than macrophages to HIV-1 infection (17, 60, 69).
Macrophages are responsive to a wide variety of positive and negative stimuli, and their susceptibility to HIV infection is profoundly influenced by the type of stimulus. Anti-inflammatory cytokines modulate HIV-1 infection of monocytes/macrophages in opposite manners depending on the differentiation stage: interleukin-10 (IL-10), IL-4, and IL-13 enhance viral replication in monocytes but suppress HIV-1 infection in differentiated macrophages (31, 45, 49, 61). Proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), also induce dichotomous effects on HIV-1 replication in macrophages: treatment with TNF-α before infection increases β-chemokine production and decreases cell surface expression of CCR5, thus inhibiting HIV-1 R5 infection while stimulating HIV-1 replication in latently infected macrophages via NF-κB activation (22, 36). Cytokines which act by different mechanisms display synergistic effects in inhibiting a dualtropic HIV-1 strain (4). It has been suggested that various cytokines, including interferons (IFNs), and other inhibitory molecules present in the lung microenvironment can protect alveolar macrophages from HIV infection (1, 25). Exogenous stimuli such as bacterial lipopolysaccharides (LPS) are powerful inhibitors of macrophage infection with HIV R5 viruses, mainly by inducing a rapid down-regulation of CCR5 (6, 19, 33, 67, 71). Exposure of cells in the intestinal microenvironment to LPS might account for a lower expression of CCR5 on gut macrophages and consequently for their relative resistance to HIV-1 infection (59).
Monocytes/macrophages are readily activated upon interaction of certain environmental stimuli with receptors on the cell surface. Receptors for the Fc portion of immunoglobulin G (IgG) (FcγR) are constitutively expressed on both monocytes and macrophages. Three classes of FcγR are expressed on macrophages, i.e., the high-affinity FcγRI (CD64) and the low-affinity FcγRII (CD32) and FcγRIII (CD16), while monocytes express mainly FcγRI and II (FcγRIII is expressed on a subset of monocytes only). Mature macrophages express about six times more total FcγR than do monocytes (27). Binding of the Fc portion of IgG to the surface of monocytes and macrophages via FcγR triggers various functions, such as endocytosis and phagocytosis. It also activates diverse signaling pathways and induces cytokine secretion (14).
Immune complexes, consisting of antibody (Ab)-coated microorganisms, antigens, or cell debris, are a common stimulus for monocytes and macrophages. Circulating immune complexes are particularly abundant in a variety of chronic diseases, including hepatitis B and C, malaria, and tuberculosis, which are highly prevalent in developing countries, especially in Africa and Asia, where the HIV-1 epidemic prevails. In a context of persistent activation of the immune system by infectious agents, it is important to consider the potential impact of monocyte and macrophage activation on susceptibility to HIV infection. We therefore decided to investigate the effects of macrophage activation by FcγR cross-linking on HIV-1 infection.
We have established a simple and reproducible system for macrophage activation by human IgG (hIgG) immobilized on culture plates to cross-link FcγRs on the cell surface of monocyte-derived macrophages (MDM). Using this system, we found that FcγR activation strongly inhibits HIV-1 replication in MDM. Both R5 and X4 viruses were blocked, as well as HIV-1 particles pseudotyped by the vesicular stomatitis virus G (VSV-G) glycoprotein. Analysis of the viral cycle suggested that HIV-1 replication is restricted after reverse transcription and nuclear translocation of viral DNA.
MATERIALS AND METHODS
Monocytes and MDM.
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats of healthy seronegative donors (Centre de Transfusion Sanguine Ile-de-France, Rungis, France) by Ficoll-Hypaque (Pharmacia Biotech) density gradient centrifugation. PBMC were washed in ice-cold 0.3 mM EDTA-phosphate-buffered saline (PBS) without Ca2+ and Mg2+ (both from Life Technologies), incubated on ice for 10 min, and washed once with PBS alone at 4°C. Monocytes were isolated by adherence to plastic for 1 h at 37°C in RPMI 1640 medium supplemented with 200 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10 mM HEPES, 10 mM sodium pyruvate, 50 μM β-mercaptoethanol, 1% minimum essential medium vitamins, and 1% nonessential amino acids (all from Life Technologies) (MDM medium) containing 2% heat-inactivated human AB serum (Valbiotech, Paris, France). Nonadherent cells were removed by washing with PBS at 37°C, and adherent cells were subsequently cultured overnight in MDM medium supplemented with 10% human AB serum. Adherent cells were then detached by incubation at 4°C followed by shaking of the flasks on a vortex mixer and gentle scraping with a rubber policeman (27). For experiments with nondifferentiated monocytes, harvested cells were washed in PBS, incubated for 1 h at 37°C in MDM medium supplemented with 20% fetal calf serum (FCS) to remove human serum-derived Ig, and then seeded in MDM medium supplemented with 10% FCS plus 5% human AB serum. For differentiation into MDM, harvested monocytes (at a density of 5 × 105 cells/ml) were allowed to differentiate into macrophages for 7 days in hydrophobic Teflon bags in MDM medium supplemented with 15% human AB serum (27). MDM were then harvested, washed extensively in PBS, incubated in MDM medium with 20% FCS for 1 h at 37°C to remove serum hIg bound to the cell surface, and then resuspended in MDM medium containing 10% heat-inactivated FCS for experiments.
This technique allows for isolation of MDM activated at a basal level, as required for this work. More than 96% of the cells were macrophages (CD14+, CD11b+) as assessed by morphology and by fluorescence-activated cell sorter (FACS) analysis, and 7-day MDM expressed all three FcγR, i.e., FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16), as expected. FcγRI and FcγRII were expressed on both monocytes and differentiated macrophages, while FcγRIII was highly expressed on macrophages but not on monocytes.
The LPS contents in media and working solutions of reagents were below the threshold of detection, as assessed by using the QCL1000 Limulus amebocyte lysate (LAL) test (BioWhittaker) (test sensitivity, 0.015 endotoxin units/ml = 1.5 pg/ml). Tested solutions contained no LAL inhibitory activity, as assessed by spiking experiments performed according to the manufacturer's instructions.
Abs.
hIgG for therapeutic use (Iv-Ig) was from Tégéline, LFB (Courtaboeuf, France), or Baxter (Lessines, Belgium); hIgG F(ab′)2 fragments were generated by pepsin digestion of hIgG and depletion of Fc fragments by protein A affinity chromatography. Goat IgG was from Jackson ImmunoResearch (West Grove, Pa.), and goat IgG F(ab′)2 fragments were from Sigma Aldrich (St. Louis, Mo.). Anti-macrophage-derived chemokine (MDC) chicken IgY and irrelevant chicken IgY were from R&D Systems, Minneapolis, Minn.
The following fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated Abs were used: CD11b-PE (clone Bear1) and CD4-PE (clone 13B8.2) (both from Beckman Coulter), CD14-FITC (clone Leu M3) and CD3-FITC (clone Leu3) (both from Becton Dickinson, San Jose, Calif.), CXCR4-PE (clone 12G5) (Pharmingen, San Diego, Calif.), and CCR5-FITC (clone 182F) (R&D). Unconjugated monoclonal Abs (MAbs) were CD64 (clone 10.1) (Becton Dickinson) and CD32 (clone 2E1) and CD16 (clone 3G8) (both from Immunotech, Marseille, France). Isotype-matched uncoupled or FITC- or PE-coupled irrelevant control MAbs were from Beckman Coulter.
Flow cytometry and FACS analysis.
Cells were stained either with FITC- or PE-coupled MAbs or with uncoupled MAbs followed by secondary F(ab′)2 FITC-goat anti-mouse Ab (Immunotech) and analyzed with a FACSCalibur (Beckman Coulter).
Cytokine analysis. (i) ELISA.
The β-chemokines MIP-1α, MIP-1β, RANTES, and MDC, as well as secretion of transforming growth factor β1 (TGF-β1), IL-12, TNF-α, and macrophage colony-stimulating factor (M-CSF), were quantified in culture supernatants by using commercial enzyme-linked immunosorbent assay (ELISA) kits from R&D. The IL-10 ELISA kit was from DIACLONE (Besançon, France), and the IFN-α and IFN-β ELISA kits were from Research Diagnostics Inc. (Flanders, N.J.).
(ii) IFN-α/β functional test.
IFN-α/β was also detected by using a functional test. The IFN-α/β reporter cell line HL116 was incubated with unstimulated and hIgG- or LPS-stimulated MDM culture supernatants for 6 h. LH116 cells are derived from the human HT1080 cell line and stably express the luciferase reporter gene controlled by the immediate-early IFN-inducible 6-16 promoter (p6-16luci) (52) (a kind gift of Elisabetta Dondi, Pasteur Institute, Paris, France).
Activation of MDM.
For activation of MDM through FcγR cross-linking, culture plates were coated overnight with hIgG in PBS (33) at concentrations of 1 mg/ml (100 μl/well in 96-well microtiter plates or 500 μl/well in 12-well plates) or threefold dilutions thereof. The wells were then washed with PBS, and MDM were seeded. The plastic-bound fraction of hIgG was evaluated with a micro-bicinchoninic acid colorimetric assay (Pierce, Rockford, Ill.) (sensitivity, ≥0.37 μg/well).
The LPS content in working dilutions of hIgG solution was below the detection limit of the QCL1000 LAL test. Some IgG batches contained traces of LPS (<3 pg/ml) and were treated on a polymyxin B column prior to use. LPS levels were undetectable after treatment.
For activation with LPS, MDM were cultured in medium containing 1 μg or 100 ng of Escherichia coli LPS (Sigma Aldrich) per ml.
HIV-1 infection.
The following HIV-1 strains were used: the laboratory-adapted strains HIV-1BaL (R5) and HIV-1NL4-3 (X4) and the clinical isolates HIV-1BX08 (R5) (provided by H. Fleury, Bordeaux 2 University), HIV-1132, and HIV-1J34 (both X4 viruses given by G. Scarlatti, San Raffaele Scientific Institute, Milan, Italy). All HIV-1 strains were propagated in phytohemagglutinin (PHA)-activated PBMC and collected from cell supernatant at peak times of p24 production. Viral stocks were titrated on untreated MDM, except for HIV-1NL4-3, which was titrated on PHA-activated PBMC. Optimal concentrations of viral supernatants were determined in preliminary infectivity assays on MDM from different donors. For MDM infection experiments, 105 cells per well of untreated or IgG-coated microtiter plates were infected with HIV-1BaL (50 50% tissue culture infective doses [TCID50/106 cells), HIV-1BX08 (40 TCID50/106 cells), HIV-1NL4-3 (2.103 TCID50/106 cells), or HIV-1J34 (50 TCID50/106 cells) for 1 h at 37°C. For infection experiments in the presence of soluble hIgG, 105 cells per well were seeded, left to adhere to plastic for 1 h, and then infected with HIV-1BaL (50 TCID50/106 cells) in medium containing soluble hIgG (100 μg/well or subsequent threefold dilutions thereof). The cells were then washed extensively, and fresh medium was added. Culture supernatants were harvested twice a week and stored at −20°C. p24 antigen in cell-free supernatants was measured with an ELISA kit (Beckman Coulter) according to the manufacturer's instructions.
To test the inhibitory activity of MDM supernatants, untreated MDM were preincubated for 15 min with pure cell culture supernatants from hIgG-stimulated MDM or from control MDM, and infection and culture were carried out in the presence of 50% supernatants. For MDC neutralization, anti-MDC chicken IgY (50% neutralizing dose for 50 ng of human MDC per ml = 1.5 μg/ml) was used. Since cell mortality was observed with 30 μg of anti-MDC Ab per ml, concentrations of up to 10 μg/ml were used for MDC neutralization in infected MDM cultures. Either anti-MDC Ab or irrelevant Ab was added to hIgG-stimulated MDM immediately after HIV-1 infection. Half of the supernatant was replaced with medium containing neutralizing or control Abs each 2 days.
Single-round infectivity assay.
HIV-1/YU2 and HIV-1/VSV-G pseudotypes were produced as described previously (42) by transiently cotransfecting HEK293T cells with the proviral pNL4-3Nef−E−Luc DNA and either the HIV-1YU2 envelope (R5) or VSV-G expression vectors. The reporter virus DNA pNL4-3Nef−E−Luc was a kind gift of T. Dragic and N. Landau (Aaron Diamond AIDS Research Center, New York, N.Y.), and the HIV-1YU2 env and the VSV-G glycoprotein plasmids were kindly provided by J. A. Levy (University of California, San Francisco) and by M.-C. Dokhelar (Institut Cochin de Génétique Moléculaire, Paris, France), respectively. Supernatants containing pseudotyped viral particles were harvested at 48 h posttransfection and stored at −80°C. HIV-1 p24 antigen was quantified with a commercial ELISA kit (Beckman Coulter) and normalized to luciferase activity (relative light units per minute) by titration of viral supernatants on MDM.
For single-round infectivity assays, 105 MDM were seeded in untreated or hIgG-coated microtiter plate wells and incubated overnight with 100 μl of HIV-1/YU2 or HIV-1/VSV-G pseudotypes at a concentration of 30 or 10 ng of Gag p24 per ml, respectively. The MDM were then washed extensively, and the medium was replenished. After 48 h, cells were washed and lysed (the luciferase lysis buffer was from Promega, Madison, Wis.), and the luciferase activities in cell lysates were measured with a luminometer (Berthold LB 9501).
Detection of HIV-1-specific DNA by PCR.
For detection of HIV-specific DNA, 106 MDM were seeded in untreated or hIgG-coated 12-well plates and then exposed for 1 h at 37°C to HIV-1BaL (5 × 102 TCID50/106 cells) previously treated with DNase I (Roche Diagnostics GmbH, Mannheim, Germany). The cells were then washed extensively, and fresh MDM medium was added. At different time points after infection, cells were washed in PBS and lysed in a mix containing 1× PCR buffer (Hot Star; Qiagen GmbH, Hilden, Germany), 0.45% Tween 20, 0.45% NP-40, and 500 μg of proteinase K per ml.
For each PCR replicate, 5 μl of cell lysate (4,000 cells/μl) was subjected to PCR amplification in a total volume of 50 μl containing 5 μl of 10× PCR buffer (Hot Star; Qiagen), 1 μl of 10 nM deoxynucleoside triphosphates (dNTP) (GIBCO-BRL), 1 μl each of sense and antisense primers (from a 10 μM solution), and 0.1 μl of Hot Star Taq DNA polymerase (Qiagen). For the U3/gag PCR, 1 μl of MgCl2 (25 mM) was also added. For all PCR amplifications, samples were subjected to a 15-min denaturation step (72°C) followed by 40 cycles of PCR amplification and a terminal 7-min extension step. The following primers were used for pol: 5′-GCTGTCCCTGTAATAAACCCG-3′ and 5′-CCCTACAATCCCCAAAGTCAAGG-3′ (nucleotides 4481 to 4501 and 4235 to 4257 from the HIV-1BRU DNA sequence, respectively). PCR conditions included a 30-s denaturation step (92°C), a 1-min annealing step (50°C), and a 1-min extension step (72°C). For detection and quantitation of U3/gag and two-long-terminal-repeat (2LTR) circles, we used primers previously described (57). PCR conditions for U3/gag included a 30-s denaturation step (94°C), a 30-s annealing step (55°C), and a 1-min extension step (72°C), and those for 2LTR DNA included a 30-s denaturation step (94°C), a 30-s annealing step (57°C), and a 1-min extension step (72°C). Integrated viral DNA forms were detected by an Alu-LTR PCR followed by a nested PCR amplifying LTR (adapted from the procedure described in reference 10). The Alu-LTR PCR mixture contained 5 μl of lysate, 5 μl of 10× PCR buffer (Roche Diagnostics GmbH), 1.75 μl of 10 nM dNTP, 1.5 μl each of sense and antisense primers (from a 10 μM solution), and 0.75 μl of Long Expand Template Taq DNA polymerase (Roche Diagnostics GmbH). PCR samples were subjected to a 2-min denaturation step (92°C) followed by 30 cycles of PCR amplification comprising a 10-s denaturation step (94°C), a 30-s annealing step (65°C), and a 5-min extension step (68°C). The nested PCR mix contained 5 μl of a 1/40 dilution of Alu-LTR PCR product, 5 μl of 10× PCR buffer (Hot Star; Qiagen), 1 μl of 10 nM dNTP, 1 μl each of Ni-2 5 and Ni-2 3 primers (10) (from a 10 μM solution), and 0.5 μl of Hot Star Taq DNA polymerase. Samples were denatured for 15 min at 92°C and subjected to 5 cycles consisting of a 30-s denaturation step (92°C), a 30-s annealing step (68°C), and a 1-min extension step (72°C), followed by 25 cycles consisting of a 30-s denaturation step (92°C), a 30-s annealing step (63°C), and a 1-min extension step (72°C). As a control, DNA samples that had not been subjected to the first round of PCR were also amplified by using the nested PCR primers Ni-2 5 and Ni-2 3. PCR experiments were controlled by using parallel amplifications of serial dilutions of 8E5 cells containing one integrated copy of HIV-1LAI (8E5/LAI) or, in the case of 2LTR PCRs, of CEM cells infected with HIV-1LAI (CEM/LAI cells).
RESULTS
HIV-1 replication is suppressed in MDM stimulated by hIgG.
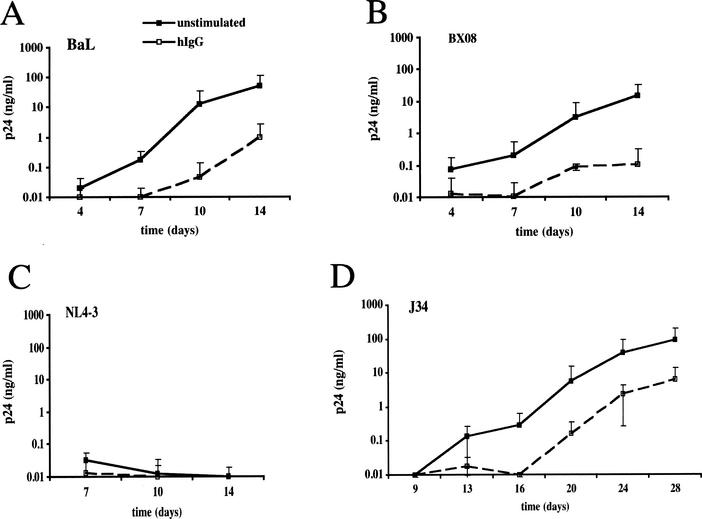
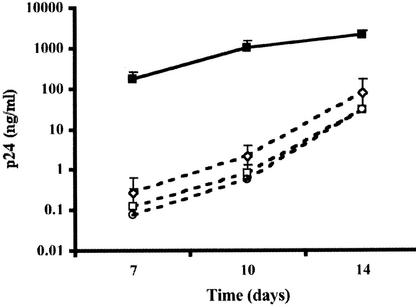
To address the effects of FcγR stimulation on HIV-1 infection of macrophages, MDM were activated by cross-linking the FcγRs expressed at their cell surface with hIgG immobilized on culture plates (39). MDM were seeded in wells coated with hIgG or left untreated and infected with the R5 isolate HIV-1BaL. Concentrations of viral p24 in culture supernatants of nonstimulated MDM progressively increased, reaching high levels on day 14 postinfection (consistently over 100 ng/ml) (Fig. 1A). In contrast, replication was strongly inhibited in MDM exposed to immobilized hIgG (2 to 3 log units lower than in control cultures at 14 days postinfection, depending on donor cells), as shown in Fig. 1A. Suppression of HIV-1BaL replication was consistently found with MDM from each of over 20 different donors and with hIgG preparations from different batches and suppliers. In addition, a higher viral inoculum was required to infect MDM upon hIgG exposure: HIV-1BaL titers were decreased by 1 log unit in hIgG-treated MDM compared to untreated control MDM (103.8 versus 104.8 TCID50/ml, respectively, on day 10 postinfection). HIV-1BaL replication was also inhibited in MDM exposed to immune complexes prepared with horse ferritin and rabbit anti-horse ferritin Abs (not shown).
FIG. 1.
Inhibition of HIV-1 replication in hIgG-activated MDM. Open symbols, MDM (105 cells/well) were seeded in microtiter plates coated with 100 μl of a 1-mg/ml hIgG solution, resulting in ≈2 μg of bound hIgG/well. Closed symbols, uncoated control plates. For infection experiments, stimulated or control MDM were inoculated with R5 isolate HIV-1BaL (50 TCID50/106 cells) (A) or HIV-1BX08 (40 TCID50/106 cells) (B) or with X4 isolate HIV-1NL4-3 (2.103 TCID50/106 cells) (C) or HIV-1J34 (50 TCID50/106 cells) (D). HIV-1 replication was monitored by measuring p24 antigen in culture supernatants at days 4, 7, 10, and 14 or until day 28 for HIV-1J34. Results are presented as the means of results from four independent replicates ± standard deviations.
Figure 1B shows that replication of an R5 primary isolate (HIV-1BX08) was also strongly suppressed in immobilized-hIgG-treated MDM. When MDM were infected with the laboratory-adapted X4 strain HIV-1NL4-3 or with the primary X4 isolate HIV-1132 (not shown), low or undetectable levels of p24 were found in both unexposed and hIgG-exposed MDM (Fig. 1C). Infection with the primary isolate HIV-1J34, which resulted in productive replication in control MDM, was delayed by 1 week and remained consistently lower (1 to 1.5 log units) in hIgG-exposed MDM (Fig. 1D). These results indicate that exposure to immobilized hIgG inhibits replication of both laboratory-adapted and primary HIV-1 strains in MDM independently of viral tropism. Because of the low efficacy of MDM infection with X4 viruses, subsequent analyses of IgG-induced inhibition were performed with HIV-1BaL.
hIgG-mediated suppression of HIV-1 replication in macrophages does not depend on the activation kinetics.
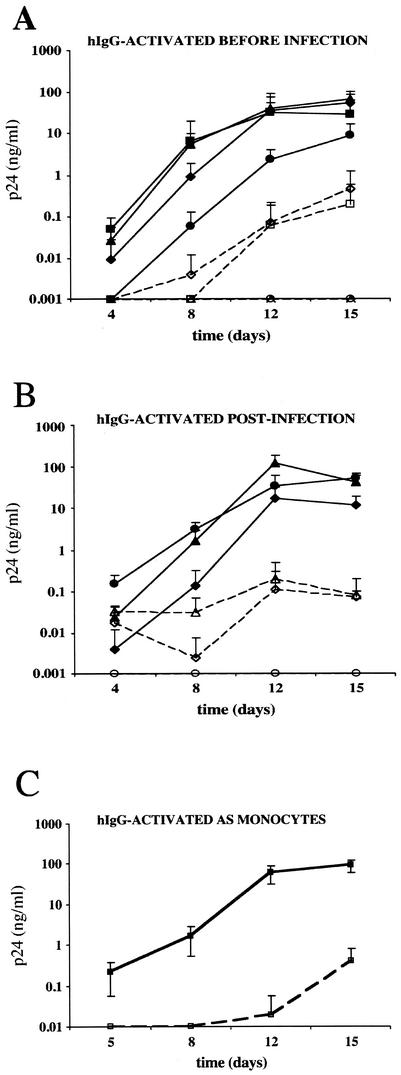
In order to determine the effects of varying the times of MDM exposure to immobilized hIgG on HIV infection, we infected MDM at various time points before or after exposure to immobilized hIgG. First, MDM were exposed to immobilized hIgG for 48, 24, or 6 h prior to infection with HIV-1BaL. Under all conditions tested, p24 production in hIgG-exposed MDM was inhibited by over 2 log units compared to that in control MDM (Fig. 2A). It is noteworthy that when control MDM were infected 5 to 6 h after seeding, p24 production was consistently lower than in MDM infected immediately or 24 to 48 h after seeding, perhaps because of a transient activation of cells following adhesion to plates (48). In a similar experiment, MDM in suspension were infected with HIV-1BaL at 6, 24, or 48 h prior to incubation with immobilized hIgG. Again, HIV-1 replication was suppressed by over 2 log units in each case (Fig. 2B). These results indicate that exposure of MDM to immobilized hIgG can inhibit HIV-1 replication even after the establishment of infection.
FIG. 2.
Kinetics of HIV replication in MDM following exposure of cells to hIgG at various times. (A and B) MDM (105 cells/well) were exposed to HIV-1BaL (50 TCID50/106 cells) and either stimulated with immobilized hIgG (open symbols) or exposed to medium alone (closed symbols) at various times prior to, during, and after infection. Activation times ranged from 48 h prior to infection (−48 h [diamonds], −24 h [triangles], −6 h [circles], and 0 h [squares]) (A) to 48 h postinfection (+6 h [circles], +24 h [triangles], and +48 h [diamonds]) (B). Virus production was measured by quantifying p24 antigen in culture supernatants at days 4, 8, 12, and 15 postinfection. Results are presented as the means of results from four independent wells ± standard deviations. (C) Monocytes (105 cells/well) were either activated with 100 μg of hIgG per well (open squares) or unstimulated (closed squares) and then infected with HIV-1BaL (50 TCID50/106 cells) for 1 h at 37°C. The cells were then washed extensively and cultured for 15 days in medium supplemented with 10% FCS plus 5% human AB serum. p24 antigen production in culture supernatants was quantified at days 5, 8, 12, and 15. Results are reported as the mean p24 production in four independent replicates ± standard deviations. Results from one representative experiment of two, performed with monocytes from different donors, are presented.
We then addressed the question of whether the differentiation stage of cells at the time of activation and infection would modify the effect of immobilized hIgG on HIV-1 infection. Monocytes harvested 24 h after isolation from PBMC were activated by immobilized hIgG and infected with HIV-1BaL. Monocytes were then cultured either in 10% FCS (data not shown) or in 10% FCS plus 5% human AB serum to allow full differentiation into macrophages. Under both culture conditions, HIV-1 replication was suppressed in hIgG-exposed MDM (Fig. 2C). These data suggest that exposure of monocytes to immobilized hIgG is able to inhibit HIV-1 replication in monocytes/macrophages even at early stages of maturation into macrophages.
HIV-1 suppression is specifically mediated by FcγR cross-linking.
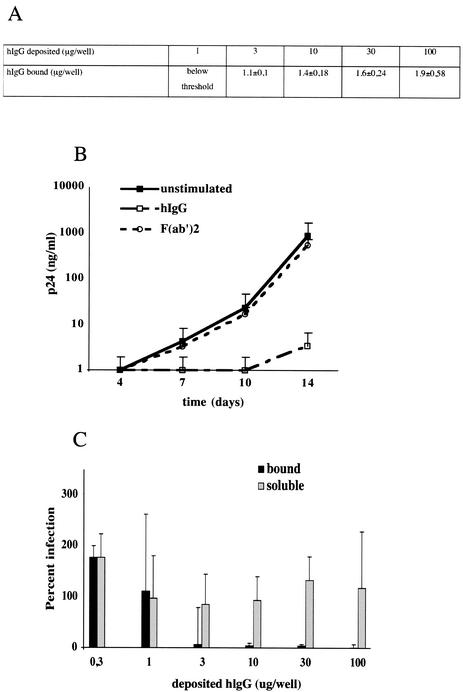
In order to assess whether hIgG-induced inhibition of HIV-1 replication in MDM was through FcγR cross-linking, MDM were incubated with immobilized hIgG, with immobilized hIgG F(ab′)2 fragments, or with soluble monomeric hIgG and then infected with HIV-1BaL. Figure 3A shows the relationship between soluble hIgG concentrations deposited in wells and the plastic-bound fraction of hIgG. Similar concentrations of hIgG and of hIgG F(ab′)2 fragments were found to bind to wells (data not shown). Therefore, MDM incubated with soluble hIgG were actually exposed to higher concentrations of hIgG than MDM incubated with immobilized hIgG. As shown in Fig. 3B, treatment of MDM with immobilized hIgG at ≈2 μg/well (corresponding to 100 μg of deposited hIgG per well) strongly suppressed p24 production (2.5 log units at day 14 postinfection), whereas immobilized hIgG F(ab′)2 fragments at the same concentration did not affect viral replication. When tested at decreasing concentrations, immobilized hIgG at concentrations down to ≈1.1 μg/well efficiently suppressed HIV-1 replication (>90% p24 reduction), whereas 100-fold-higher concentrations of soluble hIgG failed to affect viral production (Fig. 3C). HIV-1BaL replication was also inhibited upon stimulation of MDM with goat IgG, because of cross-species reactivity between IgG and FcγR, while F(ab′)2 fragments of goat IgG failed to affect viral replication (data not shown). Taken together, these data indicate that the inhibitory effect of IgG on HIV-1 replication is mediated by cross-linking of the FcγR through the Fc portion of IgG.
FIG. 3.
Immobilized hIgG, but not immobilized hIgG F(ab′)2 fragments or soluble hIgG, mediates viral suppression in MDM. (A) Plastic-bound hIgG corresponding to deposited amounts of hIgG was measured by using a bicinchoninic acid colorimetric assay. Soluble hIgG was added to cultured MDM, and cells were immediately infected with HIV-1BaL. A nonlinear correlation between deposited and bound hIgG concentrations in the measurable range was observed. (B) MDM (105 cells/well) were seeded in wells coated with whole hIgG or hIgG F(ab′)2 fragments (100 μg of deposited protein per well). Cells were infected with HIV-1BaL, and infection was monitored by measuring p24 antigen in the culture supernatants. The results presented here correspond to the means of results from four independent wells ± standard deviations and are representative of those from two experiments performed with MDM from each of two different donors. (C) MDM (105 cells/well) were exposed to increasing concentrations of soluble hIgG or to the corresponding bound hIgG (ranging from 0.3 to 100 μg of hIgG/well) and infected with HIV-1BaL. HIV-1 replication in hIgG-exposed MDM is expressed as the percentage of p24 production by control MDM on day 12 postinfection. Results are presented as the means of results from four independent replicates ± standard deviations. Results from one representative experiment of two performed with cells from different donors are shown.
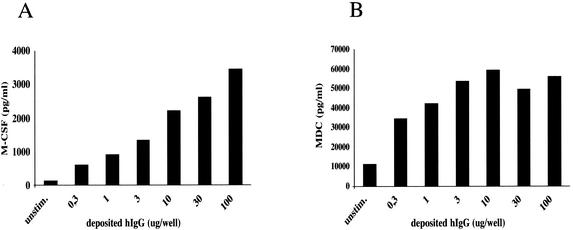
Because FcγR cross-linking on human monocytes has been reported to induce M-CSF secretion (40), we monitored FcγR-mediated activation by measuring M-CSF secretion in cell culture supernatants. Exposure of MDM to immobilized hIgG induced M-CSF production in a dose-dependent manner (Fig. 4A). Moreover, immobilized-hIgG-stimulated MDM secreted large amounts of MDC. MDC release reached saturating levels at lower immobilized-hIgG concentrations than did M-CSF release. Secretion of both cytokines by MDM was strictly dependent on the Fc portion of hIgG, as hIgG F(ab′)2 fragments did not induce M-CSF secretion above background levels (data not shown). Traces of MDC, but no M-CSF, were detected in supernatants from MDM exposed to the highest hIgG concentration (100 μg of soluble hIgG per well), probably due to the presence of hIgG aggregates (data not shown), confirming that cytokine secretion required FcγR cross-linking.
FIG. 4.
M-CSF and MDC secretion profiles with respect to plate-bound hIgG. MDM (105 cells/well of a microtiter plate) were exposed to increasing amounts of bound hIgG (corresponding to 0.3 to 100 μg of deposited hIgG/well), and cell culture supernatants were harvested at 48 h postactivation. Supernatants from duplicate wells were pooled, and M-CSF (A) and MDC (B) production was quantified by using a commercial ELISA kit. The data presented are representative of those from two experiments performed with MDM from different donors.
Since LPS stimulation inhibits HIV-1 R5 isolates (references 19, 33, 67, and 71 and data not shown), MDM infectivity assays were also performed in the presence of polymyxin B as a further control. Polymyxin B inhibits LPS from different origins by binding to the lipid A portion of LPS (9). hIgG-dependent suppression of HIV-1BaL replication was not reversed by the presence of polymyxin B (4 μg/ml) in the culture medium (data not shown). In contrast, this dose of polymyxin B reversed LPS-mediated viral suppression (data not shown).
To exclude the possibility that natural Abs in hIgG preparations directed against HIV-1 cell receptors, including CCR5, may contribute to the hIgG-mediated inhibition of HIV-1 infection (7), PHA-activated T CD4+ lymphocytes (which do not express FcγR) were incubated with hIgG (100 μg/well) and infected with HIV-1BaL. The level of HIV replication was not affected in hIgG-treated T CD4+ cells in comparison to untreated control cells (not shown).
The cytokine secretion profiles induced upon stimulation of MDM by immobilized hIgG and by LPS are different.
The release of virus-inhibitory molecules induced by FcγR activation may contribute to the suppression of HIV-1 replication and/or spreading. We therefore quantified the levels of cytokines and chemokines reported to affect HIV-1 infection in supernatants from immobilized-hIgG-stimulated and control MDM. For comparison, we also quantified the same soluble factors secreted upon activation of MDM by LPS. As reported in Table 1, we found that, as opposed to immobilized hIgG, LPS did not induce M-CSF or MDC secretion. Secretion of the β-chemokines MIP-1α and MIP-1β was also induced by hIgG stimulation, although to a lesser extent than by LPS, whereas RANTES remained at background levels. TNF-α was detected in both immobilized-hIgG- and LPS-stimulated MDM supernatants. IL-10 was slightly induced by LPS but not by FcγR cross-linking, as expected (62). IL-12 and TGF-β1 remained undetectable, as did IFN-α and -β (Table 1), which were measured both by ELISA and by a functional assay based on the reporter cell line LH116 (Table 1). Cytokine secretion profiles were similar in MDM supernatants from four different donors, although amounts varied depending on the donor cells.
TABLE 1.
Cytokine secretion in cell culture supernatants of unstimulated, hIgG-activated, and LPS-activated MDMa
| Cytokine | Cell stimulation | Concn (pg/ml)b at:
|
|
|---|---|---|---|
| 24 h | 72 h | ||
| M-CSF | Unstimulated | 91 | 1,209 |
| hIgG | 23,268 | 16,827 | |
| LPS | 1,142 | 283 | |
| MDC | Unstimulated | 6,444 | 30,222 |
| hIgG | 35,437 | 294,542 | |
| LPS | 6,407 | 13,435 | |
| MIP-1α | Unstimulated | BTc | BT |
| hIgG | 3,118 | 252 | |
| LPS | 17,838 | 2,267 | |
| MIP-1β | Unstimulated | 66 | BT |
| hIgG | 3,476 | 275 | |
| LPS | >20,000 | 3,222 | |
| RANTES | Unstimulated | BT | BT |
| hIgG | 53 | BT | |
| LPS | 1,304 | BT | |
| TNF-α | Unstimulated | 16 | 5 |
| hIgG | 509 | 93 | |
| LPS | 683 | 212 | |
| IL-10 | Unstimulated | BT | BT |
| hIgG | 38.7 | BT | |
| LPS | 1,197 | 229 | |
| IL-12 | Unstimulated | BT | BT |
| hIgG | BT | BT | |
| LPS | BT | BT | |
| TGF-β1 | Unstimulated | BT | BT |
| hIgG | BT | BT | |
| LPS | BT | BT | |
| IFN-α + IFN-βe | Unstimulated | NDd | BT |
| hIgG | ND | BT | |
| LPS | ND | BT | |
MDM (106 cells/well) were cultured in 12-well plates preadsorbed with 500 μg of hIgG per well or in medium containing 100 ng of LPS per ml or were left untreated, 20% supernatant from each well was harvested and replaced with fresh medium at 24 hours postactivation, and supernatants were harvested again at 72 hours postactivation. Harvested supernatants were pooled and centrifuged to remove any cellular debris, and aliquots were stored at −80°C. M-CSF, MDC, MIP-1α, MIP-1β, RANTES, TNF-α, IL-10, IL-12, TGF-β1, IFN-α, and IFN-β secretion in culture supernatants was quantified by using commercial ELISA kits. IFN-α and -β were also monitored by using a functional assay based on the HL116 IFN-α/β reporter cell line.
Results for cytokines induced upon FcγR stimulation are in bold. Data are reported as cytokine concentration in culture supernatants. Similar secretion profiles induced by FcγR cross-linking were observed in four experiments performed with MDM from four different donors.
BT, below threshold.
ND, not done.
IFN-α and -β in 24-h supernatants were quantified in similar experiments performed with MDM from different donors and remained undetectable.
Of the cytokines known to inhibit HIV-1 infection in MDM, FcγR cross-linking induced high levels of MDC, which has been reported to affect a post-reverse transcription step of the viral replication cycle in macrophages (12). In order to determine whether MDC was involved in the observed suppression of HIV-1 replication, hIgG-stimulated and control MDM were infected with HIV-1BaL in the presence of MDC-neutralizing Ab. Anti-MDC Ab at neutralizing concentrations (10 μg/ml) was not able to reverse FcγR-mediated viral inhibition in MDM from two different donors (Fig. 5). To further assess the modulation of MDM infection by factors present in FcγR-activated MDM supernatants, we infected untreated MDM in the presence of supernatants from hIgG-stimulated or control MDM (at a 1:1 ratio with standard cell culture medium). Cell culture supernatants from stimulated MDM partially inhibited HIV-1BaL infection of MDM (60% p24 inhibition compared to infection in the presence of untreated MDM supernatants) in one out of three experiments (not shown). We then carried out inhibition experiments with three supernatants containing different concentrations of MDC (294, 128, and 60 ng/ml). Inhibition of HIV-1BaL infection (50% p24 reduction) was found in two out of four experiments and was not correlated with MDC levels (Table 2). It is noteworthy that a supernatant (supernatant 3) that was used with MDM from two different donors inhibited infection in only one of the two MDM cultures (Table 2). These data suggest that soluble factors alone may only partially account for the strong suppression of viral replication described above and that the inhibition can vary depending on the cell donor.
FIG. 5.
MDM infection in the presence of MDC-neutralizing Ab. hIgG-stimulated (dashed lines) or unstimulated (solid line) MDM (105 cells/well of a microtiter plate) were infected with HIV-1BaL for 1 h and then cultured in the presence of 1 μg of either MDC-neutralizing Ab (diamonds) or a control Ab (circles) or no Ab (open squares). Every 2 days half of the supernatant (100 μl) was replaced with fresh medium containing the same Ab concentration. Virus production was measured by quantifying p24 antigen in culture supernatants at days 7, 10, and 14 postinfection. Results are presented as the means of three independent replicates ± standard deviations. Similar results were obtained with MDM from a different donor.
TABLE 2.
MDM infection in the presence of supernatants from hIgG-stimulated MDM
| Supernatant | Concn (ng/ml)a
|
HIV-1 inhibition (%)b | |||
|---|---|---|---|---|---|
| MDC | M-CSF | MIP-1β | TNF-α | ||
| 1 | 294 | 17 | 0.3 | 0.5 | 0 |
| 2 | 128 | 43 | 1.8 | 0.14 | 50 |
| 3 | 60 | 43 | 32 | 2.4 | 50, 0 |
MDC, M-CSF, MIP-1β, and TNF-α concentrations in supernatants from hIgG-stimulated MDM collected at 72 h (supernatants 1 and 2) or 48 h (supernatant 3) after stimulation were quantified by ELISA. Untreated MDM were incubated with supernatants from hIgG-stimulated MDM or from control MDM and infected with HIV-1BaL. Cultures were carried out in the presence of 50% supernatants and viral production was evaluated by p24 quantification.
HIV-1 inhibition is expressed as the decrease of p24 in the presence of supernatants from hIgG-stimulated MDM in comparison with that in controls. Supernatant 3 was used with MDM from two different donors.
CD4 and CCR5 are down-modulated in FcγR-stimulated MDM.
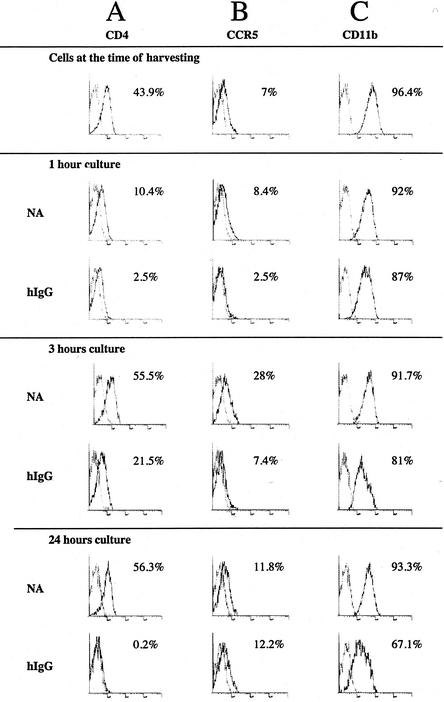
FcγR cross-linking has been reported to modulate protein expression on the cell surface of macrophages (8). We thus decided to analyze whether the expression of the HIV-1 CD4 receptor and coreceptors CCR5 and CXCR4 was modified upon hIgG stimulation of MDM. FACS analysis was performed on MDM and revealed that CD4 expression was down-regulated within 1 h of cell seeding on culture plates in both control and hIgG-stimulated MDM and remained low during the first hours. CD4 expression, however, was fully recovered in control MDM by 3 h but was not recovered in hIgG-activated MDM, even after 24 h (Fig. 6A). CCR5 expression was down-regulated 1 h after activation by hIgG but progressively increased, reaching control levels by 24 h (Fig. 6B). CXCR4 cell surface expression was very low on differentiated MDM, as previously described (48), and was not significantly modified in hIgG-stimulated or unstimulated MDM after seeding (data not shown). FcγR cross-linking induced only weak variations in expression of CD11b (Fig. 6C), also known as complement receptor 3 (CR3), consistent with the reportedly independent modulation of complement and Fc receptors in monocytes/macrophages (43).
FIG. 6.
Modulation of CD4 and CCR5 cell surface expression in FcγR-stimulated MDM. MDM (106 cells/well) were seeded in 12-well plates previously coated with 500 μg of hIgG or left untreated (NA) and were harvested after 1, 3, or 24 h for FACS analysis. Cells were stained with PE-labeled anti-CD4, anti-CCR5, and anti-CD11b MAbs or with matched isotype controls. Data representative of those from two different experiments performed with MDM from two different donors are shown.
Infection with HIV-1 Env and VSV-G-pseudotyped HIV-1 particles (HIV-1/YU2 and HIV-1/VSV-G) is inhibited in FcγR-stimulated MDM.
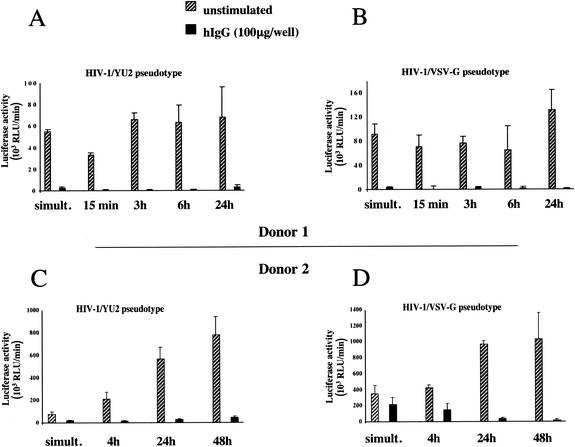
The down-modulation of CD4 and CCR5 induced by FcγR stimulation, particularly the sustained reduction in CD4, could be one of the mechanisms responsible for the inhibition of HIV-1 infection of MDM (48, 54). To test whether HIV-1 entry in MDM was inhibited by immobilized-hIgG stimulation, we performed single-round infection assays with pseudotyped viral particles. We used an envelope-defective luciferase reporter HIV-1 provirus complemented in trans with the R5 HIV-1 envelope of strain YU2 or BaL or with the VSV-G glycoprotein. In this system, luciferase expression serves as a surrogate marker for the expression of early HIV-1 genes, requiring only completion of virus replication steps from entry to translation of early viral proteins. MDM were seeded in hIgG-coated or uncoated wells and infected with the pseudotyped viral particles immediately or at various intervals after activation. Results from experiments performed with MDM from two different donors are presented in Fig. 7. Luciferase activity was suppressed in FcγR-activated MDM infected with viruses pseudotyped with HIV-1 YU2 (Fig. 7A and C) and BaL (not shown) envelopes and, importantly, with VSV-G (Fig. 7B and D). Inhibition of HIV-1 replication following MDM activation through FcγR, which was consistently detected in productive infection experiments, showed some variability in pseudotype infection assays. Inhibition of luciferase activity in hIgG-activated MDM after pseudotype infection was observed in 11 out of 14 experiments and ranged from 35 to over 95%, depending on the donor cells (Fig. 7A and B versus C and D). It is likely that variations due to primary monocyte heterogeneity and differences between donors are more evident when the effect of FcγR stimulation of MDM is examined over only the first infection cycle.
FIG. 7.
Inhibition of infection with HIV-1/YU2 and HIV-1/VSV-G pseudotypes in FcγR-stimulated MDM. MDM (105 cells/well) were seeded in 96-well plates previously coated with 100 μg of hIgG or left untreated and were infected immediately or after various times. MDM were infected overnight with HIV-1YU2 (A and C) and HIV-1VSV-G (B and D) pseudotyped viral particles, used at 33 ng of p24 per ml and 10 ng of p24 per ml, respectively. Forty-eight hours later, cells were washed with PBS and lysed, and luciferase activities in activated and control MDM cell lysates at different times were measured (means of results from four independent wells ± standard deviations are shown). Results obtained with MDM from two donors are shown (donor 1, panels A and B; donor 2, panels C and D).
Overall, VSV-G and HIV-1 Env pseudotypes were inhibited to similar degrees. Since VSV-G-mediated entry is not dependent on HIV-1 receptors, this result implies a restriction by hIgG at a postentry, envelope-independent step of the HIV-1 replication cycle.
Reverse transcription and nuclear import of viral DNA are not the limiting steps of HIV replication in FcγR-stimulated MDM during the first replication cycle.
Based on the above-described results indicating that HIV infection in MDM exposed to hIgG was blocked at a point between viral entry and translation of early gene products, we analyzed more precisely the step at which the HIV-1 cycle was affected during the first round of infection. We measured postinfection viral DNA products by PCR with primers specific for intermediate (pol) and late (U3-gag) reverse transcription products; primers specific for 2LTR circles (2LTR), which are present exclusively within the nucleus; and primers specific for integrated provirus (Alu-LTR). MDM were harvested at different times postinfection, and PCR amplifications were carried out on cell lysates. Intermediate and late reverse transcription products were detected in both hIgG-stimulated and control MDM (Fig. 8), confirming that entry into the host cell is not affected in the first cycle of infection and indicating that reverse transcription is not blocked upon FcγR activation. In the experiment shown in Fig. 8, 2LTR circle amplification products were strongly increased, whereas Alu-LTR signals were detected only in control MDM and not in hIgG-stimulated MDM. Variations in the intensity and kinetics of PCR signals corresponding to nuclear viral DNA forms were observed with MDM from different donors (data not shown). Overall, analyses of MDM from four different donors showed higher levels of nuclear 2LTR circle products and decreased Alu-LTR products in hIgG-activated MDM, suggesting a restriction of viral DNA integration within the host genome. At 7 days postinfection, all viral DNA forms were strongly reduced in FcγR-activated MDM compared to those in control MDM (Fig. 8). Despite a consistent reduction in PCR signals in hIgG-stimulated MDM lysates compared to those in control MDM lysates on day 7, the intensity of PCR signals varied according to the donor cells, as seen also by p24 quantification. These variations may be related to differences in efficiency of replication inhibition and of viral spreading over time in culture.
FIG. 8.
Analysis of transcripts and nuclear HIV-1 DNA in FcγR-stimulated or control MDM. MDM (106 cells/well of a 12-well plate) were activated with hIgG and immediately infected with HIV-1BaL (5 × 102 TCID50/106 cells) for 1 h at 37°C. Cells were lysed at the indicated time points after infection, and 5 μl of cell lysate (corresponding to approximately 20,000 cells) was analyzed by PCR. Primers specific for intermediate (pol) and late (U3/gag) reverse transcripts, nuclear 2LTR circles, or integrated provirus (Alu-LTR) were used. PCR products were analyzed by gel electrophoresis. Four independent experiments performed with MDM from different donors showed similar PCR amplification profiles despite signal intensity variations. NI, noninfected; −, absence of hIgG; +, presence of hIgG.
DISCUSSION
In this study, we show that exposure to immobilized hIgG inhibits HIV-1 replication in primary human macrophages through FcγR-mediated stimulation. FcγR-mediated stimulation consistently suppresses HIV-1 productive infection with macrophages from different donors and with primary or laboratory-adapted HIV-1 strains. Viral suppression is independent of viral tropism, since both R5 and X4 viruses are affected. Viral suppression in MDM involves hIgG-mediated FcγR cross-linking, as immobilized hIgG is responsible for a strong suppression of HIV-1 replication, whereas immobilized IgG F(ab′)2 fragments, which do not bind FcγR, and soluble monomeric hIgG, which does not cross-link FcγR, do not affect HIV-1 replication (Fig. 3B and C). Furthermore, only immobilized hIgG, and neither equivalent amounts of immobilized hIgG F(ab′)2 fragments nor higher concentrations of soluble monomeric hIgG, triggers MDM activation, as revealed by cytokine release and particularly by M-CSF and MDC production (Fig. 4).
Suppression of luciferase activity after infection of FcγR-activated MDM with pseudotyped HIV-1 viruses (Fig. 7) indicates that HIV-1 replication is restricted at a step located between virus entry and translation of early viral genes. HIV-1/VSV-G pseudotypes are capable of infecting cells through an endocytic pathway independent of both CD4 and chemokine receptor CCR5 or CXCR4 (2), suggesting that FcγR-mediated HIV-1 suppression in MDM involves a postentry level of restriction. HIV-1 entry into macrophages can occur either by a classical fusion process at the cell membrane or by macropinocytosis (38). This alternative route of HIV-1 entry might be increased in FcγR-stimulated MDM, thereby directing part of the internalized virus to endosomal compartments and to lysosomes, resulting in an overall decrease of HIV replicative forms within the cell. This hypothesis is unlikely, however, since the PCR signals corresponding to 2LTR circles, a marker that the nuclear preintegration steps have been achieved, were actually increased in FcγR-activated MDM compared to those in untreated MDM controls (Fig. 8). Whereas reverse transcription does not appear to be affected by immobilized-hIgG stimulation, at least in the first cycle of infection, the decreased signal observed with Alu-LTR PCR indicates a restriction of proviral HIV-1 DNA integration in MDM upon hIgG stimulation. Accordingly, the relative increase of 2LTR DNA PCR forms may reflect an accumulation of unintegrated DNA in the nucleus (29) related to the block of proviral DNA integration. The decrease in HIV-1 integration observed in FcγR-stimulated MDM does not by itself, however, suffice to explain the strong and prolonged inhibitory effect of FcγR cross-linking on viral infection. Additional restriction points may be involved, particularly in productive infection, which require further studies.
In productive infection, which involves multiple replication cycles, other mechanisms, such as the down-regulation of CD4 expression on the cell surface, may contribute to suppressing HIV replication. The sustained down-modulation of CD4 receptor induced by FcγR cross-linking might affect the susceptibility of MDM to both R5 and X4 strains of HIV-1 (3, 54), although macrophage infection by laboratory adapted strains, such as HIV-1BaL, can occur at low levels of CD4 expression (18).
Inhibitory monokines secreted by activated MDM might contribute to the control of HIV-1 replication, as has been observed, for example, following CD40 ligand or LPS stimulation (13, 32, 67). Whereas the concentrations of β-chemokines MIP-1α, MIP-1β, and RANTES in FcγR-activated MDM supernatants were much lower than those reported to suppress HIV-1 infection (30), high levels of MDC were secreted (Table 1). MDC has been reported to block a yet-unidentified post-reverse transcription step of viral replication in macrophages (12). However, our results are not in favor of a major role of MDC in the suppression of HIV-1 replication under our experimental conditions: supernatants from hIgG-stimulated MDM containing elevated concentrations of MDC did not inhibit HIV-1 infection of unstimulated MDM (Table 2); MDC neutralization did not reverse HIV-1 suppression in hIgG-stimulated MDM cultures (Fig. 5); and the kinetics of MDC secretion induced by hIgG stimulation was slow and reached high levels only 2 to 3 days after stimulation (Fig. 3), whereas the decrease of viral integration was detected already at 24 h postinfection (Fig. 8). In addition, HIV-1 suppression was also observed in one-cycle infections (Fig. 7).
FcγR stimulation of macrophages also induces the secretion of cytokines which could enhance HIV-1 replication, such as TNF-α and M-CSF (5, 22, 34, 40, 68). It is noteworthy that in our system, the secretion of TNF-α and the high levels of M-CSF were unable to reverse FcγR-mediated HIV-1 suppression in MDM, suggesting that the inhibitory mechanisms induced by FcγR stimulation prevail over a potential enhancement by these cytokines. It is possible that the net effect of activated MDM supernatants on HIV-1 infection represents a balance of contrasting inhibitory and enhancing effects.
The inhibitory effect on HIV-1 replication in MDM mediated through FcγR-mediated activation is broader than that induced by other stimuli, which mainly affect R5 viruses (12, 21, 35, 44). In particular, infection of LPS- or IFN-γ-activated macrophages with R5 HIV-1 isolates is inhibited, whereas infection with X4 HIV-1 viruses is increased (46, 70). Inhibition of HIV-1 R5 viruses by LPS has been linked to the prolonged internalization of CCR5 from the cell surface and to the secretion of high levels of β-chemokines, which contribute to inhibit virus entry (19, 67). In contrast, hIgG stimulation leads to a restriction of later steps of the viral replication cycle. It is noteworthy that the pattern of cytokine secretion induced by immobilized hIgG differs markedly from that induced by LPS, suggesting that distinct signaling pathways are triggered by the two distinct stimuli.
Our results may appear to conflict with data reported by Tsitsikov, who observed an enhancement of HIV-1 LTR-driven transcription in monocytes upon FcγR cross-linking with immobilized hIgG (66). Nevertheless, the infection model used in that study is very different from ours, since it was based on monocytic cell lines (THP-1 and the THP-1-derived BF24 line). Tsitsikov suggested that the increased transcriptional activity induced by FcγR stimulation in BF24 cells, which carry an HIV-1 LTR-chloramphenicol acetyltransferase reporter construct, was mediated through activation of the transcription factor NF-κB. In our studies with primary macrophages, NF-κB was only slightly activated in differentiated MDM upon exposure to immobilized hIgG compared to that in untreated MDM (data not shown). Because transcription factors can be differently induced according to the maturation stage of monocytes/macrophages (6, 69), the HIV-1 LTR activity may be differently modulated by FcγR stimulation. Our results showing that hIgG-mediated activation suppresses HIV-1 replication in preinfected MDM (Fig. 2) suggest that any potential activation of the HIV-1 LTR is overwhelmed by FcγR-mediated mechanisms of inhibition of viral replication. HIV-1 suppression in MDM infected 48 h before IgG stimulation, when HIV-1 integration has already occurred in initially infected cells, is probably due to inhibition of further viral replication in neighboring cells, thus blocking viral spreading.
Several studies have shown that in vitro infection of monocytes and macrophages with HIV-1 can be enhanced in the presence of anti-HIV Abs (Ab-dependent enhancement [ADE]) (24, 56, 64). ADE has been associated with virus-Ab complexes binding to the Fc or the complement receptors (24, 55, 63, 65) and may be observed or not depending on various parameters, among which are monocyte/macrophage differentiation and activation (20, 58). ADE is observed at low antiviral Ab concentrations (53, 55, 64). It is conceivable that low levels of Abs, although not sufficient to induce FcR cross-linking, enhance virus binding to the cell surface and favor infection. In contrast, immune complexes mediated inhibition rather than enhancement of infection upon binding activating FcγR on primary monocytes and macrophages (11), which is consistent with our results.
Macrophages express activating FcγR, namely FcγRI, FcγRIIA and C, and FcγRIII, whose cytoplasmic domains bear an immunoreceptor tyrosine-based activating motif (ITAM), together with the inhibitory FcγRIIB, which bears an immunoreceptor tyrosine-based inhibitory motif. Coaggregation of FcγRIIB with ITAM-bearing FcγR interferes with the signal transduction cascade induced by ITAM-bearing FcγR. We found that immobilized-hIgG-mediated stimulation of MDM induces the transcription factor AP-1 (data not shown) and high levels of cytokine release (Table 1 and Fig. 4B and C), consistent with a highly activated phenotype. Therefore, a direct role of FcγRIIB in the observed viral suppression seems unlikely. FcγR cross-linking triggers a complex pattern of signaling pathways among which are the phosphatidylinositol 3-kinase, the protein kinase C, and the mitogen-activated protein kinase pathways (14), which might interfere with HIV-1. FcγR-mediated inhibition of HIV-1 replication in macrophages represents a useful model to identify new antiviral cell pathways and molecules.
FcγR-mediated inhibition of HIV-1 replication in macrophages could affect different aspects of HIV-1 infection, including viral dissemination and persistence, in particular among individuals who are exposed to macrophage activation by immune complexes. This phenomenon may occur, for example, among HIV-1-exposed populations, such as prostitutes, intravenous-drug users, or partners of seropositive individuals, particularly in African or Asian countries where both HIV-1 infection and other chronic infections coexist at high prevalence rates. It is tempting to speculate that macrophages activated through FcγR cross-linking, alone or in synergy with other stimuli such as LPS or cytokines, could contribute to natural protection against HIV-1 infection in some exposed uninfected individuals by inhibiting viral transmission and/or controlling viral spread after contact with the virus. Further in vitro studies and ex vivo functional analyses of primary monocytes derived from HIV-1-exposed individuals in Africa or Asia are needed to address this hypothesis.
Acknowledgments
We are grateful to T. Jungi for transmitting technology for isolation and culture of MDM in Teflon bags, as well as for valuable advice and for critically reviewing the manuscript. We also thank R. H. Bassin for further criticism and revision of the English and M. Daëron for valuable advice. In addition, we gratefully acknowledge the technical help provided by Pierre Versmisse. We thank Elisabetta Dondi for help in quantifying IFN-α/β by functional tests.
This work was supported by ANRS grant 2001/004. Fellowships for D.P.-B. were provided in France by the Ministère de l'Education Nationale, de la Recherche et de la Technologie and by Ensemble contre le SIDA (ECS).
REFERENCES
- 1.Agostini, C., R. Sancetta, A. Cerutti, and G. Semenzato. 1995. Alveolar macrophages as a cell source of cytokine hyperproduction in HIV-related interstitial lung disease. J. Leukoc. Biol. 58:495-500. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asjo, B., I. Ivhed, M. Gidlund, S. Fuerstenberg, E. M. Fenyo, K. Nilsson, and H. Wigzell. 1987. Susceptibility to infection by the human immunodeficiency virus (HIV) correlates with T4 expression in a parental monocytoid cell line and its subclones. Virology 157:359-365. [DOI] [PubMed] [Google Scholar]
- 4.Bailer, R. T., B. Lee, and L. J. Montaner. 2000. IL-13 and TNF-alpha inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. Eur. J. Immunol. 30:1340-1349. [DOI] [PubMed] [Google Scholar]
- 5.Bergamini, A., C. F. Perno, L. Dini, M. Capozzi, C. D. Pesce, L. Ventura, L. Cappannoli, L. Falasca, G. Milanese, R. Calio, et al. 1994. Macrophage colony-stimulating factor enhances the susceptibility of macrophages to infection by human immunodeficiency virus and reduces the activity of compounds that inhibit virus binding. Blood 84:3405-3412. [PubMed] [Google Scholar]
- 6.Bernstein, M. S., S. E. Tong-Starksen, and R. M. Locksley. 1991. Activation of human monocyte-derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J. Clin. Investig. 88:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouhlal, H., H. Hocini, C. Quillent-Grégoire, V. Donkova, S. Rose, A. Amara, R. Longhi, N. Haeffner-Cavaillon, A. Beretta, S. V. Kaveri, and M. D. Kazatchkine. 2001. Antibodies to C-C chemokine receptor 5 in normal human IgG block infection of macrophages and lymphocytes with primary R5-tropic strains of HIV-1. J. Immunol. 166:7606-7611. [DOI] [PubMed] [Google Scholar]
- 8.Buchi, D., and W. de Souza. 1993. Internalization of surface components during Fc-receptor mediated phagocytosis by macrophages. Cell Struct. Funct. 18:399-407. [DOI] [PubMed] [Google Scholar]
- 9.Cavaillon, J. M., and N. Haeffner-Cavaillon. 1986. Polymyxin-B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Mol. Immunol. 23:965-969. [DOI] [PubMed] [Google Scholar]
- 10.Chun, T.-W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. M. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor, R. I., N. B. Dinces, A. L. Howell, J. L. Romet-Lemonne, J. L. Pasquali, and M. W. Fanger. 1991. Fc receptors for IgG (Fc gamma Rs) on human monocytes and macrophages are not infectivity receptors for human immunodeficiency virus type 1 (HIV-1): studies using bispecific antibodies to target HIV-1 to various myeloid cell surface molecules, including the Fc gamma R. Proc. Natl. Acad. Sci. USA 88:9593-9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cota, M., M. Mengozzi, E. Vicenzi, P. Panina-Bordignon, F. Sinigaglia, P. Transidico, S. Sozzani, A. Mantovani, and G. Poli. 2000. Selective inhibition of HIV replication in primary macrophages but not T lymphocytes by macrophage-derived chemokine. Proc. Natl. Acad. Sci. USA 97:9162-9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter, R. L., J. Zheng, M. Che, D. Niemann, Y. Liu, J. He, E. Thomas, and H. E. Gendelman. 2001. Regulation of human immunodeficiency virus type 1 infection, β-chemokine production, and CCR5 expression in CD40L-stimulated macrophages: immune control of viral entry. J. Virol. 75:4308-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daëron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203-225. [DOI] [PubMed] [Google Scholar]
- 15.Eisert, V., M. Kreutz, K. Becker, C. Königs, U. Alex, H. Rübsamen-Waigmann, R. Andreesen, and H. von Briesen. 2001. Analysis of cellular factors influencing the replication of human immunodeficiency virus type I in human macrophages derived from blood of different healthy donors. Virology 286:31-44. [DOI] [PubMed] [Google Scholar]
- 16.Embretson, J, M. Zupancic, J. L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and a. T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362:359-362. [DOI] [PubMed] [Google Scholar]
- 17.Fantuzzi, L., L. Conti, M. C. Gauzzi, P. Eid, M. Del Corno, B. Varano, I. Canini, F. Belardelli, and S. Gessani. 2000. Regulation of chemokine/cytokine network during in vitro differentiation and HIV-1 infection of human monocytes: possible importance in the pathogenesis of AIDS. J. Leukoc. Biol. 68:391-399. [PubMed] [Google Scholar]
- 18.Fear, W. R., A. M. Kesson, H. Naif, G. W. Lynch, and A. L. Cunningham. 1998. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J. Virol. 72:1334-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchin, G., G. Zybarth, W. W. Dai, L. Bubrovsky, N. Reiling, H. Schmidtmayerova, M. Bukrinsky, and B. Sherry. 2000. Lipopolysaccharide inhibits HIV-1 infection of monocyte-derived macrophages through direct and sustained down-regulation of CC chemokine receptor 5. J. Immunol. 164:2592-2601. [DOI] [PubMed] [Google Scholar]
- 20.Halstead, S. B. 1994. Antibody-dependent enhancement of infection: a mechanism for indirect virus entry into cells, p. 493-516. In Cellular receptors for animal viruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Herbein, G., and S. Gordon. 1997. Fifty-five- and 75-kilodalton tumor necrosis factor receptors mediate distinct actions in regard to human immunodeficiency virus type 1 replication in primary human macrophages. J. Virol. 71:4150-4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbein, G., L. J. Montaner, and S. Gordon. 1996. Tumor necrosis factor alpha inhibits entry of human immunodeficiency virus type 1 into primary human macrophages: a selective role for a 75-kilodalton receptor. J. Virol. 70:7388-7397. (Erratum, 71:1581, 1997.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch, V. M. 1998. Vpx is required for dissemination and pathogenesis of SIV (SM) pBj: evidence of macrophage-dependent viral amplification. Nat. Med. 4:1401-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homsy, J., M. Meyer, M. Rateno, S. Clarkson, and J. A. Levy. 1989. The Fc and not CD4 receptor mediated antibody enhancement of HIV infection in human cells. Science 244:1357-1360. [DOI] [PubMed] [Google Scholar]
- 25.Honda, Y., L. Rogers, K. Nakata, B. Y. Zhao, R. Pine, Y. Nakai, K. Kurosu, W. N. Rom, and M. Weiden. 1998. Type I interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J. Exp. Med. 188:1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jungi, T. W., and S. Hafner. 1986. Quantitative assessment of Fc receptor expression and function during in vitro differentiation of human monocytes to macrophages. Immunology 58:131-137. [PMC free article] [PubMed] [Google Scholar]
- 28.Kaul, M., G. A. Garden, and S. A. Lipton. 2001. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988-994. [DOI] [PubMed] [Google Scholar]
- 29.Kazazi, F., J. M. Mathijs, P. Foley, and A. L. Cunningham. 1989. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J. Gen. Virol. 70:2661-2672. [DOI] [PubMed] [Google Scholar]
- 30.Kelly, M. D., H. M. Naif, S. L. Adams, A. L. Cunningham, and A. R. Lloyd. 1998. Dichotomous effects of beta-chemokines on HIV replication in monocytes and monocyte-derived macrophages. J. Immunol. 160:3091-3095. [PubMed] [Google Scholar]
- 31.Kootstra, N. A., A. van 't Wout, H. G. Huisman, F. Miedema, and H. Schuitemaker. 1994. Interference of interleukin-10 with human immunodeficiency virus type 1 replication in primary monocyte-derived macrophages. J. Virol. 68:6967-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornbluth, R. S., K. Kee, and D. D. Richman. 1998. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1 suppressive beta-chemokines. Proc. Natl. Acad. Sci. USA 95:5205-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornbluth, R. S., P. S. Oh, J. R. Munis, P. H. Cleveland, and D. D. Richman. 1989. Interferons and bacterial lipopolysaccharides protect macrophages from productive infection by human immunodeficiency virus in vitro. J. Exp. Med. 169:1137-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kutza, J., L. Crim, S. Feldman, M. P. Hayes, M. Gruber, J. Beeler, and K. A. Clouse. 2000. Macrophage colony-stimulating factor antagonists inhibit replication in HIV-1 in human macrophages. J. Immunol. 164:4955-4960. [DOI] [PubMed] [Google Scholar]
- 35.Kutza, J., M. P. Hayes, and K. A. Clouse. 1998. Interleukin-2 inhibits HIV-1 replication in human macrophages by modulating expression of CD4 and CC-chemokine receptor-5. AIDS 12:F59-F64. [DOI] [PubMed] [Google Scholar]
- 36.Lane, B. R., D. M. Markovittz, N. L. Woodford, R. Rochford, R. M. Strieter, and M. J. Coffey. 1999. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J. Immunol. 163:3653-3661. [PubMed] [Google Scholar]
- 37.Li, L., G. K. Meng, M. F. Graham, G. M. Shaw, and P. D. Smith. 1999. Intestinal macrophages display reduced permissiveness to human immunodeficiency virus 1 and decreased surface CCR5. Gastroenterology 116:1043-1053. [DOI] [PubMed] [Google Scholar]
- 38.Maréchal, V., M.-C. Prevost, C. Petit, E. Perret, J.-M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh, C. B, J. E. Gadek, G. C. Kindt, S. A. Moore, and M. D. Wewers. 1995. Monocyte Fc gamma receptor cross-linking induces IL-8 production. J. Immunol. 155:3161-3167. [PubMed] [Google Scholar]
- 40.Marsh, C. B, R. P. Pomerantz, J. M. Parker, A. V. Winnard, e. L. Mazzaferri, Jr., N. Moldovan, T. W. Kelley, E. Beck, and M. D. Wewers. 1999. Regulation of monocyte survival in vitro by deposited IgG: role of macrophage colony-stimulating factor. J. Immunol. 162:6217-6225. [PubMed] [Google Scholar]
- 41.Martìn, J. C., and J. C. Banrés. 1999. Cells of the monocyte-macrophage lineage and pathogenesis of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 22:413-429. [DOI] [PubMed] [Google Scholar]
- 42.Merat, R., H. Raoul, T. Leste-Lasserre, P. Sonigo, and G. Pancino. 1999. Variable constraints on the principal immunodominant domain of the transmembrane glycoprotein of human immunodeficiency virus type 1. J. Virol. 73:5698-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michl, J., M. M. Pieczonka, J. C. Unkeless, and S. C. Silverstein. 1979. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J. Exp. Med. 150:607-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montaner, L. J. 1993. Interleukin 13 inhibits human immunodeficiency virus type 1 production in primary blood-derived human macrophages in vitro. J. Exp. Med. 178:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montaner, L. J., R. T. Bailer, and S. Gordon. 1997. IL-13 acts on macrophages to block the completion of reverse transcription, inhibit virus production, and reduce virus infectivity. J. Leukoc. Biol. 62:126-132. [DOI] [PubMed] [Google Scholar]
- 46.Moriuchi, M., H. Moriuchi, W. Turner, and A. S. Fauci. 1998. Exposure to bacterial products renders macrophages highly susceptible to T-tropic HIV-1. J. Clin. Investig. 102:1540-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naif, H. M., S. Li, M. Alali, J. Chang, C. Mayne, J. Sullivan, and A. L. Cunningham. 1999. Definition of the stage of host cell genetic restriction of replication of human immunodeficiency virus type 1 in monocytes and monocyte-derived macrophages by using twins. J. Virol. 73:4866-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naif, H. M., S. Li, M. Alali, A. Sloane, L. Wu, M. Kelly, G. Lynch, A. Lloyd, and A. L. Cunningham. 1997. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 72:830-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naif, H. M., S. Li, M. Ho-Shon, J.-M. Mathijs, P. Williamson, and A. L. Cunningham. 1997. The state of maturation of monocytes into macrophages determines the effects of IL-4 and IL-13 on HIV replication. J. Immunol. 158:501-511. [PubMed] [Google Scholar]
- 50.Nakata, K., M. Weiden, T. Harkin, D. Ho, and W. N. Rom. 1995. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1 infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol. Med. 1:745-757. [PMC free article] [PubMed] [Google Scholar]
- 51.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 276:1857-1861. [DOI] [PubMed] [Google Scholar]
- 52.Pellegini, S., J. John, M. Shearer, I. M. Kerr, and G. R. Stark. 1989. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol. Cell. Biol. 9:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perno, C. F., M. W. Baseler, S. Broder, and R. Yarchoan. 1990. Infection of monocytes by human immunodeficiency virus type 1 blocked by inhibitors of CD4-gp120 binding, even in the presence of enhancing antibodies. J. Exp. Med. 171:1043-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pesenti, E., C. Pastore, F. Lillo, A. G. Siccardi, D. Vercelli, and L. Lopalco. 1999. Role of CD4 and CCR5 levels in the susceptibility of primary macrophages to infection by CCR5-dependent HIV type 1 isolates. AIDS Res. Hum. Retroviruses 15:983-987. [DOI] [PubMed] [Google Scholar]
- 55.Robinson, W. E. J., D. C. Montefiore, D. H. Gillespie, and W. M. Mitchell. 1989. Complement-mediated, antibody-dependent enhancement of HIV-1 infection in vivo is characterized by increased protein and RNA synthesis and infectious virus release. J. Acquir. Immune Defic. Syndr. 2:33-42. [PubMed] [Google Scholar]
- 56.Robinson, W. E. J., D. C. Montefiore, and W. M. Mitchell. 1988. Antibody-dependent enhancement of human immunodeficiency virus type 1 infection. Lancet i:790-794. [DOI] [PubMed] [Google Scholar]
- 57.Schmidtmayerova, H., M. Alfano, G. Nuovo, and M. Bukrinsky. 1998. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J. Virol. 72:4633-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shadduck, P. P., B. J. Weinberg, A. F. Haney, J. A. Bartlett, A. Langlois, D. P. Bolognesi, and T. J. Matthews. 1991. Lack of enhancing effect of human anti-human immunodeficiency virus type 1 (HIV-1) antibody on HIV-1 infection of human blood monocytes and peritoneal macrophages. J. Virol. 65:4309-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith, P. D., G. Meng, G. M. Shaw, and L. Li. 1997. Infection of gastrointestinal tract macrophages by HIV-1. J. Leukoc. Biol. 62:72-77. [DOI] [PubMed] [Google Scholar]
- 60.Sonza, S., A. Maerz, N. Deacon, J. Meanger, J. Mills, and S. Crowe. 1996. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J. Virol. 70:3863-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sozzani, S., S. Ghezzi, G. Iannolo, W. Luini, A. Borsatti, N. Polentarotti, A. Sica, M. Locati, C. Mackay, T. N. Wells, P. Biswas, E. Vicenzi, G. Poli, and A. Mantovani. 1998. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J. Exp. Med. 187:439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutterwala, F. S., G. J. Noel, P. Salgame, and D. M. Mosser. 1998. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J. Exp. Med. 188:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeda, A., R. W. Sweet, and F. Ennis. 1990. Two receptors are required for antibody-dependent enhancement of human immunodeficiency virus type 1 infection: CD4 and FcgR. J. Virol. 64:5605-5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda, A., C. U. Tuazon, and F. A. Ennis. 1988. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science 242:580-583. [DOI] [PubMed] [Google Scholar]
- 65.Trischmann, H., D. Davis, and P. Lachmann. 1995. Lymphocytotropic strains of HIV type 1 when complexed with enhancing antibodies can infect macrophages via FcgRIII, independently of CD4. AIDS Res. Hum. Retroviruses 11:343-352. [DOI] [PubMed] [Google Scholar]
- 66.Tsitsikov, E. N., R. Fulinan, K. McIntosh, P. R. Scholl, and R. S. Geha. 1995. Cross-linking of Fc gamma receptors activates HIV-1 infection in human monocytes. Int. Immunol. 7:1665-1670. [DOI] [PubMed] [Google Scholar]
- 67.Verani, A., G. Scarlatti, M. Comar, E. Tresoldi, S. Polo, M. Giacca, P. Lusso, A. Siccardi, and D. Vercelli. 1997. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J. Exp. Med. 185:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, J., G. Roderiquez, T. Oravecz, and M. A. Norcross. 1998. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J. Virol. 72:7642-7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiden, M., N. Tanaka, Y. Qiao, B. Y. Zhao, Y. Honda, K. Nakata, A. Canova, D. E. Levy, W. N. Rom, and R. Pine. 2000. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J. Immunol. 165:2028-2039. [DOI] [PubMed] [Google Scholar]
- 70.Zaitseva, M., S. Lee, C. Lapham, R. Taffs, L. King, T. Romantseva, J. Manischevitz, and H. Golding. 2000. Interferon gamma and interleukin 6 modulate the susceptibility of macrophages to human immunodeficiency virus type 1 infection. Blood 96:3109-3117. [PubMed] [Google Scholar]
- 71.Zybarth, G., N. Reiling, H. Schimidtmayerova, B. Sherry, and M. Bukrinsky. 1999. Activation-induced resistance of human macrophages to HIV-1 infection in vitro. J. Immunol. 162:400-406. [PubMed] [Google Scholar]