Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) ORF45 is encoded by an immediate-early gene in the KSHV genome. This protein was recently shown to interact with interferon regulatory factor 7 and inhibit virus-mediated alpha/beta interferon induction (Zhu et al., Proc. Natl. Acad. Sci. USA 99:5573-5578, 2002). ORF45 was characterized as a phosphorylated protein, and it is localized in the cytoplasm of infected cells. In this report, we provide evidence that ORF45 is associated with KSHV virions. (i) ORF45 was detected in gradient-purified virions by Western blotting along with known structural proteins of KSHV including gB, K8.1, and major capsid protein. In contrast, ORF50/Rta, K8α, and ORF59/PF8 were not detected in the same virion preparation. (ii) ORF45 comigrates with KSHV virions in sucrose gradient ultracentrifugation. (iii) Virion-associated ORF45 was resistant to trypsin digestion but became sensitive after the virions were treated with detergent which destroys the viral envelope. (iv) ORF45 remained associated with tegument-nucleocapsid complex when virion-specific glycoproteins were removed after detergent treatment. (v) An ORF45 protein band was visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of extensively purified KSHV virions and identified by mass spectrometry. (vi) By immunoelectron microscopy, virus-like structures were specifically stained by anti-ORF45 antibody. Based on the evidence, we conclude that ORF45 is associated with purified KSHV virions and appears to be a tegument protein. The presence of ORF45 in KSHV virions raised the possibility that this protein may be delivered to host cells at the start of infection and therefore have the opportunity to act at the very early stage of the infection, suggesting an important role of ORF45 in KSHV primary infection.
Kaposi's sarcoma-associated herpesvirus (KSHV), also referred to as human herpesvirus 8, is a human DNA tumor virus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman disease (7, 20). Based on phylogenetic analysis, this virus has been classified as a member of the Gammaherpesvirinea family and Rhadinovirus genus and is closely related to herpesvirus saimiri of squirrel monkeys and Epstein-Barr virus (EBV) (31). As a gammaherpesvirus, KSHV has two modes of replication, i.e., latent and lytic replication. In latent replication, circular viral episomes replicate in tandem with host cell DNA by using the host cell DNA replication machinery and no infectious virus is produced. In the lytic life cycle, viruses express most of their genes and viral DNAs are replicated by virus-encoded polymerases and factors and encapsidated into infectious virions (20). The latency can be disrupted by chemical induction or cytokine stimulation, resulting in lytic replication (6, 19, 27). The process of KSHV switching from latency to lytic replication is called reactivation.
During reactivation, viral gene expression is temporally regulated in a similar fashion as during primary infection. A few genes are expressed independently of de novo protein synthesis in the very early phase and are classified as immediate-early genes. In general, immediate-early genes encode regulatory proteins, which either regulate downstream viral gene expression or modulate the host cell physiological state to support viral replication. In addition, some immediate-early gene products were found to be involved in evasion of host antiviral immune defenses.
We have been interested in KSHV reactivation from latency to lytic replication. Initially, four immediate-early genes in the KSHV genome which are believed to be important in initiating and controlling the reactivation process were identified and characterized (41). Recently, we reported that one of the immediate-early gene products, namely ORF45, interacts with interferon (IFN) regulatory factor 7 (IRF-7) and inhibits the translocation of IRF-7 from the cytoplasm to the nucleus (42). IRF-7 is a transcription regulator which plays a critical role in virus-mediated induction of IFN-α/β gene expression. By blocking the nuclear translocation of IRF-7, ORF45 efficiently inhibits the activation of IFN-α/β genes during viral infection (42). IFNs constitute the primary innate immune response against virus infection (32, 35). It was shown that IFN-mediated responses were activated in host cells in response to KSHV, and many IFN-related genes, including IRF-7, are up-regulated during KSHV infection and reactivation (26). Thus, a successful KSHV infection or reactivation relies on the ability of the virus to overcome the IFN-related host immune defenses. Our results suggest that ORF45 is a protein that KSHV makes and uses to target components of the host antiviral defenses.
A typical herpesvirus particle (or virion) consists of four morphologically distinct components: a core which contains a linear double-stranded viral DNA, an icosadelahedral capsid that encloses the viral DNA core, an outer envelope with viral glycoproteins appearing as spikes on the surface, and electron-dense material defined as the tegument, which is located between capsid and envelope (28). Although little is known about the structure and function of the herpesvirus tegument, some predominant tegument proteins were found to be regulatory proteins and enzymes. They include the VP16 of herpes simplex virus type 1 (HSV-1), which is a transcription activator for viral immediate-early genes (reviewed in references 28 and 29), virion host shutoff protein (VHS or UL41), which is an RNase responsible for the degradation of mRNA and inhibition of host cell translation (10). Interestingly, among the herpesvirus tegument proteins are some viral immediate-early proteins such as the ICP0 and ICP4 of HSV-1 (39, 40), IE62 (ORF62), ORF4 and ORF63 of varicella-zoster virus (VZV) (13, 14), and pIRS1 and pTRS1 of cytomegalovirus (CMV) (30). Besides tegument proteins, certain mRNAs were also found in the CMV and HSV virions (5, 33). These virion-associated regulatory proteins and RNAs are thought to be important for the viral life cycle.
In the further characterization of the KSHV ORF45 protein, we demonstrated that this immediate-early protein is a phosphorylated and cytoplasmic protein. More interestingly, we found that ORF45 is associated with purified virions of KSHV. The data presented in this report strongly suggest that the virus-associated ORF45 is located within the virion and is likely present in the tegument region.
MATERIALS AND METHODS
Cells and viruses.
BCBL-1, a latent-KSHV-infected primary effusion lymphoma cell line, BJAB, a KSHV-free Burkitt lymphoma cell line, and HR-1, a latent-EBV-infected Burkitt lymphoma cell line, were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). BC-1, a primary effusion cell line carrying both KSHV and EBV, was cultured in RPMI 1640 with 15% FBS. Human 293 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS. Sf9 cells were propagated by using SF900II serum-free medium (Invitrogen, Carlsbad, Calif.) at 27°C. For virus induction, BC-1 cells were treated with 3 mM sodium butyrate (Sigma, St. Louis, Mo.). BCBL-1 cells were stimulated with 20 ng of 12-O-tetradecanoylphorbol-13-acetate (TPA) (Sigma)/ml.
Overexpression and purification of ORF45 from recombinant baculovirus-infected Sf9 cells.
Recombinant baculovirus was constructed according to the method of Whitbeck et al. (37). In brief, the full-length coding sequence of KSHV ORF45 with a polyhistidine tag sequence at the carboxyl terminus was generated by PCR with the oligonucleotides ORF45-BAC-N (5′-AT TAG GAT CCA AAT ATG GCG ATG TTT GTG AGG ACC-3′) and ORF45-BAC-C (5′-AAT AGC GGC CGC TTA TCA ATG ATG ATG ATG ATG ATG GTC CAG CCA CGG CCA GTT ATA-3′) as primers. The PCR fragment was cloned into baculovirus transfer vector pAcPAK8 (Clontech, Palo Alto, Calif.), and recombinant plasmids verified by DNA sequencing were cotransfected with BaculoGold-linearized baculovirus DNA (Pharmingen, San Diego, Calif.) into Sf9 cells. Two rounds of plaque purification were performed, and individual plaques were picked and screened by Western blotting with an anti-His antibody as well as an anti-ORF45 peptide antibody. A large stock of positive recombinant virus was prepared, and virus titers were determined. Two liters of Sf9 cells (2 × 106 per ml) was infected with recombinant virus at a multiplicity of infection of 5 for 3 days or until cell viability dropped to 70%. Then the cells were collected and lysed in the lysis buffer (1× phosphate-buffered saline [PBS], 300 mM NaCl, 1 mM NaF, 1 mM sodium pyrophosphate, 1% Triton X-100, and protease inhibitor tabulate [Roche Applied Science, Indianapolis, Ind.]). After sonication, the cell extract was cleared by centrifugation and incubated with nickel resin (Qiagen, Valencia, Calif.). The resin was washed, and ORF45 protein was eluted with imidazole.
Antibodies and Western blotting.
Polyclonal antibodies against ORF45 were generated in two rabbits by using the baculovirus-synthesized ORF45 as an antigen. Immunoglobulin G's (IgGs) were purified through affinity chromatography on a Protein-G Hitrap column (Amersham, Piscataway, N.J.). Polyclonal antibody to ORF50/Rta was provided by Don Ganem at the University of California—San Francisco. The polyclonal antibodies to K8 and K8.1 were obtained from Jae Jung at the New England Regional Primate Research Center. The antibody to LANA was from Rolf Renne at Case Western Reserve University. The antibody to the major capsid protein (MCP) was from Dean Kedes at the University of Virginia. The antibody to PF8/ORF59 was from Robert Riccardi at the University of Pennsylvania. Purified polyclonal rabbit IgGs against gB and monoclonal hybridoma supernatant against K8.1A/B 4A4 were obtained from Bala Chandran at the University of Kansas Medical Center.
About 25 μg of cell extract and 5 μg of purified virions were resolved on sodium dodecyl sulfate (SDS)-4 to 15% or 4 to 20% polyacrylamide gel electrophoresis (PAGE) and transferred to Hybond enhanced chemiluminescence nitrocellulose membranes (Amersham). The membranes were blocked in 5% dried milk in PBS plus 0.2% Tween 20 (PBST) buffer and then incubated with diluted primary antibody against ORF45 (1:2,000 to 1:5,000), K8 (1:2,000), ORF50/Rta (1:2,000), K8.1A/B (rabbit polyclonal, 1:1,000; mouse monoclonal 4A4, 1:1), gB (1:20), LANA (1:2,000), PF8/ORF59 (1:1,000), or MCP (1:200) for 2 h at room temperature or 4°C overnight. Anti-rabbit or anti-mouse IgG antibody conjugated to horseradish peroxidase (Amersham) was used as the secondary antibody. The enhanced chemiluminescence system (Amersham) was used for detection.
Immunofluorescence assay.
TPA-induced BCBL cells were applied on slides, air dried, and fixed with cold 50% methanol-acetone for 15 min. The fixed cells were blocked in 1× PBS plus 1% bovine serum albumin (BSA) for 30 min, then incubated with primary antibody (against ORF45) at a dilution of 1:200 in PBS plus 1% BSA at room temperature for 30 min. The slides were then washed 3 times with 1× PBS plus 1% BSA and further incubated with secondary antibody conjugated with fluorescein isothiocyanate (1:100) for 30 min at room temperature. After washing with 1× PBS, the slides were mounted with Vectoshield (Vector, Burlingame, Calif.) and examined by confocal microscopy as previously described (42).
Alkaline phosphatase treatment of ORF45 protein.
Whole-cell extracts were prepared from BCBL-1 cells that were induced with TPA for 48 h or left untreated. About 500 μg of protein was incubated with 5 μg of purified rabbit IgG against KSHV ORF45 at 4°C for 2 h. The antibody-antigen complexes were recovered with protein A-agarose resin (Invitrogen). The precipitates were washed 5 times with TBS buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl) and resuspended in 100 μl of TBS buffer. Ten microliters of the slurry was treated with calf intestinal alkaline phosphatase (Amersham) at 37°C for 1 h in the absence and presence of phosphatase inhibitor cocktail (Sigma). The reactions were stopped by adding 2× SDS loading buffer, and then the reaction mixtures were boiled for 10 min. The samples were resolved on SDS-PAGE and followed by Western blotting with anti-ORF45 antibody.
Labeling of phosphorylated proteins with [32P]orthophosphate.
BCBL-1 cells and BJAB cells were induced with TPA for 48 h or left untreated. The cells were washed once with phosphate-free DMEM and incubated in 1 ml of phosphate-free DMEM supplemented 10% dialyzed FBS (Invitrogen) and 0.5 mCi of [32P]orthophosphate (ICN, Costa Mesa, Calif.) for 4 h at 37°C in 5% CO2. The labeled cells were washed twice with 1× PBS, and cell lysates were subjected to immunoprecipitation as described above. The precipitates were extensively washed and analyzed by SDS-PAGE. The gel was dried and exposed to an X-ray film at −70°C.
Purification of KSHV virions.
BCBL cells (0.5 × 106/ml) were induced with 20 ng of TPA/ml and 0.5 μM ionomycin (Sigma) for 7 days. The media was then collected and cleared by centrifugation at 4,000 × g for 30 min and then at 8,000 × g for 15 min to remove cells and cell debris. Then the medium was filtered through 0.45-μm-pore-size filters. Virions were pelleted at 27,000 rpm for 1 h through 5 ml of 5% sucrose cushion in a Beckman SW28 rotor and resuspended in 1× PBS plus 0.1% bacitracin (Sigma) buffer in 1/100 of the original volume. The concentrated virus particles were centrifuged through 20 to 35% nycodenz (Sigma) step gradient at 24,000 rpm for 2 h or 30 to 60% sucrose step gradient at 17,000 rpm for 4 h. The virus band at the gradient junction was collected. The virions were then purified in a second round of gradient centrifugation. In some experiments, an additional continuous 20 to 35% nycodenz gradient was performed to further purify virions.
Detergent treatment of purified virions.
Double-gradient-purified virions were treated with 1% Triton X-100 or 1% Triton X-100 plus 0.5% deoxycholate (DOC) for 30 min at 37°C. The reaction mixture was separated in two fractions, supernatant and pelleted tegument nucleocapsid, by centrifugation at 100,000 × g for 1 h. Equal amounts of protein from the supernatant and pellet were analyzed by Western blotting.
Trypsin treatment of purified virions.
Purified virions (10 μg of total protein) were treated with trypsin (4 μg/ml) (Promega, Madison, Wis.) in 100 μl of buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM CaCl2) at 37°C for 1 h. Trypsin digestions were terminated by adding phenylmethylsulfonyl fluoride to a 0.5 mM concentration and a 1/100 volume of protease inhibitors (Sigma). In parallel experiments, Triton X-100 was added to a final of concentration of 1% to remove the viral envelope and expose the tegument nucleocapsid to the protease. The samples were analyzed by Western blotting.
Mass spectrometric analysis.
Purified virions were resolved on a 4 to 12% Bis-Tris NuPAGE gel with morpholinepropanesulfonic acid (MOPS) running buffer (Invitrogen) and stained with a colloidal Coomassie G-250 staining kit (Invitrogen). The desired bands were excised and subjected to trypsin digestion. A portion of the peptide digest was injected onto a nanocapillary reverse-phase high-performance liquid chromatograph coupled to a nanoelectrospray ionization source of an ion trap mass spectrometer (ThermoFinnigan LCQ). This mass spectrometer measures peptide masses and then fragments individual peptides to produce mass spectrometry/mass spectrometry (MS/MS) spectra of fragments that reflect the peptide sequence. The MS/MS spectra are run against a sequence database by using the program SEQUEST. The mass spectromtery was carried out in the protein microchemistry mass spectrometry facility at the Wistar Institute.
Immunoelectron microscopy.
BCBL-l cells induced with TPA for 96 h were fixed with 0.1% paraformaldehyde, washed, and incubated with anti-ORF45 antibodies at 37°C for 30 min. Cells were washed and incubated with anti-rabbit secondary antibodies conjugated with 10-nm-diameter gold particles at 37°C for 30 min. These cells were fixed with glutaraldehyde and embedded in resins. Thin sections were made, stained, and examined under an electron microscope.
RESULTS
Kinetics of KSHV ORF45 expression.
To characterize the ORF45 protein, we expressed and produced recombinant ORF45 protein in a baculovirus system. The baculovirus expression system was chosen for two reasons. First, baculovirus retains genuine posttranslational modification mechanisms as eukaryotic cells. Secondly, the toxicity of a foreign gene product to the host cells is minimized because it is expressed in the very late phase of viral life cycle. A histidine tag was introduced to the carboxyl end of ORF45 to facilitate the screening of the recombinant virus and purification of the recombinant protein. Recombinant baculovirus was generated, and the His-tagged ORF45 protein was purified to homogeneity. The baculovirus-expressed ORF45 protein was seen as a polypeptide of 78 kDa on SDS-PAGE (data not shown).
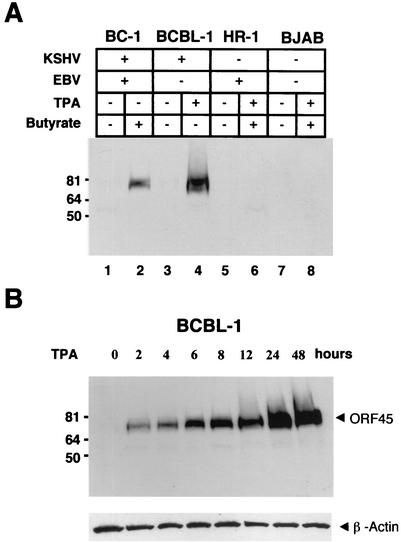
The purified His-tagged ORF45 protein was used to generate rabbit polyclonal antibody. The antibody reacted with in vitro-translated ORF45 but not with luciferase on Western blots or by immunoprecipitation (data not shown). To further test the specificity of this antibody, various cell lines that do or do not carry KSHV were examined by Western blot analysis. A 78-kDa protein was detected in TPA-induced BCBL-1 cells (KSHV positive) and butyrate-induced BC-1 cells (KSHV and EBV positive) but not in uninduced BCBL-1 and BC-1 cells or any KSHV-free cells, including BJAB cells (KSHV negative, EBV negative) and HR-1 cells (KSHV negative, EBV positive) (Fig. 1A).
FIG. 1.
Kinetics of KSHV ORF45 protein expression. (A) A polyclonal antibody generated with baculovirus-expressed ORF45 recombinant protein reacted with uninduced or n-butyrate-induced BC-1 cells (lanes 1 and 2), uninduced and TPA-induced BCBL-1 cells (lanes 3 and 4), and uninduced and TPA-plus-n-butyrate-induced HR-1 cells (lanes 5 and 6) and BJAB cells (lanes 7 and 8). +, present, −, absent. (B) Time course of KSHV ORF45 protein expression. Whole-cell extracts prepared from BCBL-1 cells that were induced with TPA for various times as indicated were resolved by SDS-PAGE followed by Western blotting with anti-ORF45 antibody. Reactions of these samples with an antibody specific to β-actin proved the presence of approximately equal amounts of β-actin protein. Sizes of protein markers are shown at the left on each gel.
ORF45 has been identified as an immediate-early gene. Its mRNA can be detected during the immediate-early stage of viral reactivation by Northern analysis, and the transcription was resistant to cycloheximide treatment (41). Recently, a systematic survey of KSHV transcription patterns with a DNA microarray technique showed that ORF45 is among the first class of genes transcribed after induction (11). Using the anti-ORF45 antibody, we studied the kinetics of ORF45 protein expression during KSHV reactivation. BCBL-1 cells were induced with TPA, and the levels of ORF45 expression were analyzed at different time points by Western blotting. As shown in Fig. 1B, ORF45 can be detected as early as 2 h after the induction. This result indicated that the ORF45 protein was expressed in the very early stage during KSHV reactivation.
ORF45 is a phosphorylated protein.
Based on the analysis of the mRNA sequence, ORF45 is expected to encode a protein of 407 amino acids with a molecular mass of about 50 kDa (41). However, ORF45 was displayed as a diffuse band at the position of 78 kDa on a Western blot after SDS-PAGE. This observation implied that ORF45 might be highly posttranslationally modified, resulting in its reduced mobility in denaturing SDS-PAGE. A survey of the primary amino acid sequence of ORF45 revealed multiple potential sites of phosphorylation, including a serine-rich region and other consensus sites for phosphorylation by the serine/threonine kinase, casein kinase II, and protein kinase C. To determine whether ORF45 is phosphorylated, ORF45 was immunoprecipitated from TPA-induced BCBL-1 cell extracts and treated with calf intestinal alkaline phosphatase (CIAP) in the presence or absence of phosphatase inhibitors. As illustrated in Fig. 2A, CIAP treatment resulted in a sharper and faster-moving band under denaturing electrophoresis conditions (Fig. 2A, lane 5). In contrast, the mobility remains unchanged when phosphatase inhibitor was present in the reaction mixture (Fig. 2A, lane 6), suggesting that the reduced mobility of ORF45 in SDS-PAGE is, at least in part, attributed to protein phosphorylation.
FIG. 2.
ORF45 is a phosphorylated protein. (A) Uninduced and TPA-induced BCBL-1 cell extracts were immunoprecipitated with an anti-ORF45 antibody. The precipitates were either mock treated or treated with CIAP in the absence (−) or presence (+) of phosphatase inhibitor. The positions of ORF45 and IgG heavy chain (IgG HC) are indicated at the right. (B) [32P]orthophosphate-labeled uninduced and TPA-induced BJAB (lanes 1 and 2) and BCBL-1 (lanes 3 and 4) cell lysates were immunoprecipitated with an anti-ORF45 antibody and resolved by SDS-PAGE. The sizes of protein markers are shown at the left.
Next, we tested whether ORF45 can be labeled with [32P]orthophosphate in vivo. Uninduced and TPA-induced BCBL-1 cells along with BJAB cells were incubated in vivo with [32P]orthophosphate for 4 h. Whole-cell extracts were made and immunoprecipitated with anti-ORF45 antibody. A specific 78-kDa signal, which is the expected size of ORF45, was detected in the TPA-induced BCBL-1 cells (Fig. 2B, lane 4) but not in uninduced BCBL-1 cells (Fig. 2B, lane 3) and BJAB cells (Fig. 2B, lanes 1 and 2). Therefore, we conclude that ORF45 is a phosphorylated protein.
ORF45 is localized in the cytoplasm.
TPA-induced and uninduced BCBL-l cells were examined by immunofluorescence staining with the anti-ORF45 antibody. Less than 1% of uninduced cells were positively stained, representing spontaneously reactivated cells (27). The percentage of cells that were positively stained by anti-ORF45 antibody increased up to 25% when the cells were induced with TPA for 48 h (data not shown). ORF45 was found mostly localized in the cytoplasm (Fig. 3A). To test whether the cytoplasmic localization of KSHV ORF45 is determined by ORF45 itself or is affected by other viral proteins, pCR3.1-ORF45 was used to transfect 293 cells and the transfected cells were then stained with the anti-ORF45 antibody. ORF45 was only detected in the cytoplasm (data not shown). In addition, ORF45-green fluorescent protein fusion protein was also expressed in the cytoplasm (Fig. 3B). Thus, we are convinced that ORF45 is mainly localized in the cytoplasm.
FIG. 3.
ORF45 is localized in the cytoplasm. (A) TPA-induced BCBL-1 cells were stained with an anti-ORF45 antibody and fluorescein isothiocyanate-labeled secondary antibody. (B) 293 cells were transfected with the expression vector of the ORF45-GFP fusion protein. The living cells were observed by confocal microscopy.
ORF45 is associated with KSHV virions.
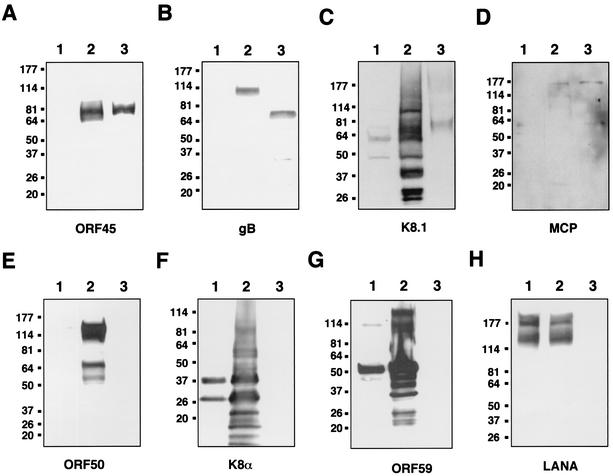
Since some of the immediate-early gene products of herpesviruses were found to be virion components located in the tegument region, we decided to examine whether ORF45 is associated with KSHV virions. Extracellular KSHV virions were purified from induced BCBL-1 cells through a double-gradient ultracentrifugation (see Materials and Methods). The virus purification scheme was monitored by the loss of the precursors to KSHV glycoproteins gB and K8.1, which are known to be associated with cell membrane (1, 2, 45). Only the mature forms of these two proteins are found in virions. Five micrograms of proteins from purified virions was resolved on SDS-PAGE, transferred to nitrocellulose paper, and immunoblotted with anti-ORF45 antibody as well as control antibodies against various KSHV proteins (Fig. 4). ORF45 was easily detected in TPA-induced BCBL-1 cells and in the purified virion lysate (Fig. 4A, lanes 2 and 3), but not in uninduced BCBL-1 cells (Fig. 4A, lane 1). Some KSHV structural proteins, including gB (Fig. 4B), K8.1 (Fig. 4C), and the MCP (Fig. 4D), were also detected in the virion preparation as expected. As previously reported, gB was initially synthesized as a 110-kDa precursor molecule and was then processed to 75- and 56-kDa mature forms which are present in virions. As shown in Fig. 4B, the 110-kDa precursor was only detected in the induced BCBL-1 cell lysate but not in purified virions, indicating that the purified virions were free of cellular membrane contamination. In addition, it was reported previously that an anti-K8.1 monoclonal antibody (4A4) recognizes multiple forms of K8.1 A/B ranging from 34 to 72 kDa in the cell extracts from TPA-induced BCBL-1 cells and 68- to 72-kDa proteins from the virion particle (45). These multiple proteins represent the precursors and glycosylated form of K8.1. The mature form of K8.1 in virions had undergone extensive posttranslational modification with both N- and O-glycosylation (45). With a monoclonal (4A4) or a polyclonal antibody (17) against K8.1 protein, a broad band around 68 to 72 kDa was detected in the virion lysate by Western analysis (Fig. 4C and data not shown). In contrast, immediate-early proteins ORF50/Rta (Fig. 4E) and K8α (Fig. 4F), delayed-early protein ORF59 (Fig. 4G), and latent protein LANA (Fig. 4H) were not detected in the virion preparation. In order to rule out the possibility that the association of ORF45 with purified virion is an artifact due to the specific purification method, the experiments were repeated with various virion preparations. They include virions purified with different approaches, such as (i) using polyethylene glycol to precipitate the virus instead of ultracentrifugation, (ii) virions banded in different gradient media (sucrose and nycodenz), and (iii) whole-virus lysate made from induced KS-1 cells and obtained from Advanced Biotechnologies, Inc. (Columbia, Md.). In all cases, ORF45 was always readily detected along with K8.1 and MCP while ORF50/RTA, K8α, ORF59, and LANA were never found (data not shown). Glycoprotein gB was detected in nycodenz-purified virions but barely detectable in sucrose-purified virions, suggesting that gB might be stripped from the envelope during sucrose gradient centrifugation.
FIG. 4.
Association of KSHV ORF45 with purified virions. Uninduced and TPA-induced BCBL-1 cell extracts (lanes 1 and 2) and gradient-purified virions (lanes 3) were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibodies against ORF45 (A), gB (B), K8.1A/B (C), MCP (D), ORF50/Rta (E), K8α (F), ORF59/PF8 (G), and LANA (H). The precursor to gB of 110 kDa was detected in the TPA-induced BCBL-1 cells, and the mature form of gB (75 kDa) was detected in the purified virion (B). Multiple forms of K8.1A/B (with a major form of 37-kDa) were detected in TPA-induced BCBL-1 cells, and the virion-associated K8.1A/B forms, as a broad band of 68 to 75 kDa, were detected in purified virions (C). The sizes of protein markers are shown at the left on each gel.
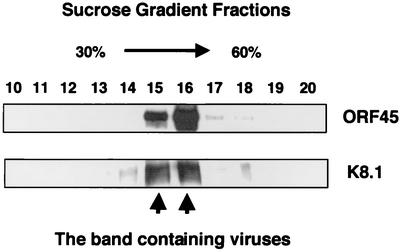
To further confirm the association of ORF45 with purified virus particles, the double-gradient-purified virions were centrifuged through a 30 to 60% sucrose gradient. Fractions from top to bottom were collected and resolved on SDS-PAGE followed by Western blotting with anti-ORF45 antibody. The samples were also probed with an antibody to K8.1 as a control for the presence of virions. A distinct virus band can be seen around the 40% sucrose gradient corresponding to fractions 15 and 16. These two fractions were subjected to electron microscopic examination, and herpesvirus-like structures were seen in the sample (data not shown). The Western blots showed that both ORF45 and K8.1 peaked in the same fractions as virions, demonstrating that ORF45 comigrated with the KSHV particles (Fig. 5).
FIG. 5.
KSHV ORF45 cosediments with virions in sucrose gradient ultracentrifugation. The gradient-banded virions were again loaded on a 30 to 60% sucrose gradient. After centrifugation, a distinct virus band was seen in the position corresponding to fractions 15 and 16. Fractions of 0.5 ml were taken from top to bottom and resolved by SDS-PAGE followed by Western blot analysis with anti-ORF45 antibody and anti-K8.1A/B monoclonal antibody. The positions of ORF45 and K8.1 on the Western blots were indicated at the right.
Identification of ORF45 protein in purified virion by using mass spectrometry.
The KSHV virions, which were purified twice through a nycodenz step gradient followed by an additional continuous gradient ultracentrifugation, were resolved on a 4 to 12% NuPAGE gel (Invitrogen) and stained by colloidal Coomassie staining. About 30 protein bands were seen in the gel (Fig. 6A). A weak band corresponding to the expected mass of ORF45 was excised and subjected to a mass spectrometric analysis to see whether ORF45 is indeed present in the purified virions. The excised gel was digested with trypsin, and three proteolytic fragments were identified and found to match the KSHV ORF45 sequence when the sequences were used to search the nonredundant protein database (Fig. 6B and C). An additional band above the ORF45 band was also excised from the gel and analyzed by mass spectrometry. No ORF45 fragment was found in this band. The mass spectra result confirmed that ORF45 is a component of purified KSHV virions.
FIG. 6.
Mass spectrometric analysis of purified KSHV virion proteins. (A) Purified virion lysate was revolved on a 4 to 12% Bis-Tris NuPAGE gel with MOPS running buffer and stained by using a silver staining kit (Amersham). The band which was suspected to be ORF45 is indicated. The numbers on the right indicate molecular masses (in kilodaltons) of protein markers. (B) The purified virions were precipitated with TCA, and the proteins were resolved on a NuPAGE gel as in panel A. The gel was stained with colloidal Coomassie G-250. The suspected ORF45 band and the band above were excised and subjected to mass spectrometry analysis. The resultant MS/MS spectra were run against a sequence database by using the SEQUEST program. Three peptide sequences (among them one was a partially digested peptide and shares overlap sequence with another peptide) were identified which matched KSHV ORF45 (C). The identified proteolytic peptide fragments in the ORF45 sequence are shown in boldface type in panel B.
ORF45 is localized inside KSHV virions.
There are two possibilities for the association of ORF45 with purified KSHV virions, i.e., ORF45 nonspecifically adheres to the outside of virions or it is actually incorporated into the virus particle. To resolve this issue, we first tried to determine the fate of ORF45 when the virion envelope glycoproteins were solubilized with detergents. The double-gradient-purified virions were treated with 1% Triton X-100 or 1% Triton plus 0.5% DOC and then were subjected to high-speed centrifugation. Both the supernatant and the pellet were analyzed by immunoblotting (Fig. 7). The result showed that no ORF45 was released into the supernatant fraction if the virions were treated with Triton X-100 alone (Fig. 7A, lane 1). If the virions were treated with a harsher condition (Triton X-100 plus DOC), a small amount of ORF45 was found in the supernatant (Fig. 7A, lane 3). As a control, the glycoprotein K8.1 was released from the virions into the supernatant after the detergent treatments (Fig. 7B, lanes 1 and 3). These observations suggest that ORF45 is neither a membrane protein nor a protein adhering to the surface of virions. Instead, ORF45 remained associated with the pelleted tegument-capsid structure. However, its association with viral capsid was breakable by harsher detergent treatment. A similar behavior was seen with tegument proteins of HSV-1 (IE ICP0, ICP4) (39, 40) and VZV (IE62, ORF4, and ORF63) (13, 14).
FIG. 7.
Effect of detergent treatment on the association of ORF45 with purified virions. Double-gradient-purified virions were treated with 1% Triton X-100 (lanes 1 and 2) or 1% Triton plus 0.5% DOC (lanes 3 and 4) for 30 min and then centrifuged at 100,000 × g for 1 h. The resultant supernatant (S) and pelleted tegument-nucleocapsid complexes (P) of equal amounts of proteins were analyzed by SDS-PAGE and Western blotting with anti-ORF45 antibody (A) and anti-K8.1A/B monoclonal antibody 4A4 (B). The numbers on the left indicate molecular masses (in kilodaltons) of protein markers.
If ORF45 is present within the virion, then ORF45 should be protected from trypsin digestion by the virion envelope. Contaminating proteins that are nonspecifically copurified with KSHV virions, as well as viral envelope glycoproteins, should be degraded by protease treatment. To test this, purified virions were treated with trypsin in the absence and presence of Triton X-100. After 1 h of proteolysis, the samples were analyzed by Western blotting. As shown in Fig. 8, glycoprotein K8.1 was sensitive to trypsin treatment both in the presence (Fig. 8B, lane 2) and absence (Fig. 8B, lane 3) of detergent. However, ORF45 can be degraded by trypsin only when Triton X-100 was present (Fig. 8A, lane 2), suggesting that ORF45 is located within virions, either as a capsid or tegument protein.
FIG. 8.
Effect of trypsin digestion on the association of ORF45 with the purified virions. The purified virions were treated with trypsin either in the presence (+) (lanes 2) or absence (−) (lanes 3) of 1% Triton X-100 for 1 h at 37°C. The proteolysis reactions were terminated by the addition of 0.5 mM phenylmethylsulfonyl fluoride and 1/100 of a volume of protease inhibitor. The samples were analyzed by Western blotting with anti-ORF45 (A) and anti-K8.1A/B (B) antibodies. Lane 1 contains mock-treated purified virions. The numbers on the left indicate molecular masses (in kilodaltons) of protein markers.
KSHV capsid components have been recently studied (24), and the capsid structure has been solved by cryomicroscopy. ORF45 was not among the capsid components (36, 38). Furthermore, herpesvirus capsids are assembled in the nucleus (28). If ORF45 is a capsid protein, one would expect it to be in the nucleus. However, ORF45 is exclusively localized in the cytoplasm (Fig. 3). Thus, it is most likely that ORF45 is a tegument protein which is acquired by the virion in the cytoplasm during viral particle assembly (18, 28).
Immunoelectron microscopic study.
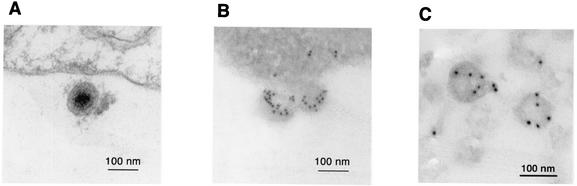
Finally, electron microscopy was employed to examine the ORF45 in KSHV virions. BCBL-1 cells were induced with TPA for 96 h. The cells were fixed and stained with the IgG of anti-ORF45 antibody and then a secondary antibody conjugated with 10-nm-diameter gold particles. Gold particles were seen on mature viruses surrounding the cytoplasmic membrane (Fig. 9B). Staining with IgG from preimmune serum showed no reactivity to viral particles (data not shown). Figure 9A is a transmission electron micrograph showing a clearer structure of mature KSHV virions. In addition, gradient-purified KSHV virions were also immunogold stained with anti-ORF45 antibody IgG and 10-nm-diameter gold-conjugated secondary antibody and electron microscopic images showed specific staining of the purified KSHV virions (Fig. 9C).
FIG. 9.
Transmission electron microscopy and immunoelectron microscopy of KSHV particles. (A) At 4 days postinduction with TPA, BCBL-1 cells were fixed, thin sectioned, and examined by transmission electron microscopy. A mature virion is shown with transmission electron microscopy. (B) The induced BCBL-1 cells were then processed by immunogold staining with anti-ORF45 antibody IgG and 10 nM gold-conjugated secondary antibody. (C) Gradient-purified KSHV virions were immunogold stained with anti-ORF45 antibody IgG and 10 nM gold-conjugated secondary antibody and examined by electron microscopy.
DISCUSSION
KSHV ORF45 is associated with KSHV virions and appears to be a tegument protein.
In this report, we demonstrated that the KSHV immediate-early gene product ORF45 is a phosphorylated protein and localized in the cytoplasm of infected cells. More interestingly, we found that ORF45 is associated with purified virions of KSHV. The conclusion that ORF45 is a virion protein rather than a contaminant copurified with virions was drawn based on the following observations. (i) ORF45 was detected in extensively purified virions by using various purification procedures. The elimination of the precursors to two glycoproteins, gB and K8.1, during purification and failure to detect other nonstructural protein such as LANA, ORF50/Rta, and K8 in the purified virions strengthened the argument that ORF45 was not a contaminant. (ii) A virion protein band on an SDS-PAGE gel was identified as ORF45 by mass spectrometric analysis. (iii) When virions were treated with trypsin, ORF45 was resistant to proteolysis but became sensitive after detergent treatment, suggesting that ORF45 is located within the virion. After treatment with detergents and ultracentrifugation, the majority of ORF45 was found in the pellet, which excluded the possibility that ORF45s are embedded in lipid vesicles which are copurified with KSHV virions.
Some tegument proteins can be associated with capsid. In alpha- and betaherpesviruses, tegument proteins are usually distinguished from capsid proteins by their absence in the capsid purified from the nuclei of infected cells (39, 40). However, purification of capsids from the nucleus has been unsuccessful with human gammaherpesviruses (24). Thus, we could not use this approach to demonstrate whether ORF45 is a tegument protein. However, the following facts argue that KSHV ORF45 is likely to be a tegument protein. (i) Herpesvirus capsids are assembled in the nucleus (18, 28), and ORF45 is not detected in the nucleus. Thus, the cytoplasmic localization of ORF45 diminishes the possibility of it being a capsid protein. (ii) The components of KSHV capsids have been recently determined, and the capsid structure has been solved by cryomicroscopy. ORF45 was not among the KSHV capsid components (36, 38). Therefore, ORF45 is likely to be a tegument protein and acquired when viruses egress from the nucleus to cytoplasm.
The presence of ORF45 in KSHV virions implicates its role in the KSHV life cycle.
The finding that ORF45 is associated with virions as a tegument protein is exciting but not surprising because it is conceivable that some functions of immediate-early genes are required in both reactivation and primary infection, such as immune evasion, gene activation, and cell cycle modulation. Some herpesvirus immediate-early proteins, for example ICP0 and ICP4 of HSV-1 (39, 40), IE62, ORF4, and ORF63 of VZV (13, 14) and pIRS1 and pTRS1 of CMV (30), were reported to be virion components. Tegument proteins are delivered to cells at the start of infection and, therefore, have the opportunity to act at very early times in infection. Therefore, our finding suggests that ORF45, as a virion protein and an immediate-early protein, plays roles in both primary infection of KSHV and reactivation from latent infection.
ORF45 has been found to interact with cellular IRF-7 and prohibit IRF-7 from being transported from the cytoplasm to the nucleus in response to viral infection (42). IRF-7 is a transcription regulator which plays a critical role in virus-mediated induction of IFN-α and IFN-β gene expression. By blocking the nuclear translocation of IRF-7, ORF45 efficiently inhibits the activation of IFN-α/β genes during viral infection (42). These results suggest that ORF45 offers a strategy for KSHV to target components of the host antiviral defenses.
IFNs constitute the primary innate immunity and act as the first line of defense for the host in attempts to control viral infection (3, 32, 35). IFNs elicit antiviral states as well as multiple biological responses involved in cell growth regulation and immune activation. The importance of IFNs in controlling herpesvirus infection in vivo were illustrated in IFN receptor-null mice with either alphaherpesvirus (HSV-1) or gammaherpesvirus (MHV68) (8, 15). Studies of HSV-1 and CMV demonstrated that infectious or UV-inactivated virions could trigger the IFN response (21, 25, 43, 44). Furthermore, a soluble form of glycoprotein B of CMV could induce a full set of IFN responses (4, 34). However, in the case of HSV-1, virus entry appears to be required for induction of IFN (21). KSHV infection also triggers IFN-mediated responses and many IFN-related genes including IRF-7 were up-regulated during KSHV infection and reactivation (26). The viral glycoprotein K8.1 was found to induce IFN action (S. Perry and T. Compton, personal communication). Since the IFN-induced cellular antiviral response is the primary defense mechanism against viral infection, many viruses have evolved mechanisms to either preclude the synthesis of IFNs or evade downstream antiviral events (reviewed in references 3, 12, 16, and 32). It was reported that HSV-1 infection triggers an IFN-mediated host antiviral response which is then immediately disarmed by a viral gene product(s). The authors presented data showing that there must be an immediate-early gene product which is responsible for inhibiting IFN antiviral response (22, 23). Increasing evidence suggests that the ICP0 of herpesvirus saimiri-1 might exert this function even though the underlying mechanism has not been revealed (9, 21, 22, 23). It is conceivable that the viral function of immune evasion is critical for successful viral infection and reactivation. Since KSHV infection can activate IFN-mediated antiviral responses (26), we speculate that the virus is able to immediately disarm the antiviral defenses with strategies including inhibition of IRF-7 activation by ORF45. However, ORF45 may have multiple functions and may also involve cellular or viral processes other than blocking IRF-7 activation. Nevertheless, the presence of ORF45 in virions, together with its expression kinetics during reactivation as an immediate-early protein, suggest that ORF45 plays important roles in viral life cycle, in both primary infection and reactivation of KSHV.
Acknowledgments
We thank Gary Cohen at the University of Pennsylvania for critical reading of the manuscript. We are grateful to Charles Whitbeck for help in the generation of ORF45 recombinant baculovirus and to Manuel Ponce de Leon for excellent technical help in the purification of KSHV virions. We thank Don Ganem at the University of California—San Francisco for ORF50 antibody, Jae Jung at New England Regional Primate Research Center for K8 and K8.1 antibodies, Rolf Renne at Case Western Reserve University for LANA antibody, Dean Kedes at the University of Virginia for the antibody to the MCP, Robert Riccardi at the University of Pennsylvania for the PF8/ORF59 antibody, and Bala Chandran at the University of Kansas Medical Center for polyclonal antibody against gB and monoclonal hybridoma supernatant against K8.1A/B (4A4).
This work was supported by National Institutes of Health grant CA86839 (to Y.Y.)
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. [DOI] [PubMed] [Google Scholar]
- 2.Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. [DOI] [PubMed] [Google Scholar]
- 3.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 4.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bresnahan, W. A., and T. Shenk. 2000. A subset of viral transcripts packaged within human cytomegalovirus particles. Science 288:2373-2376. [DOI] [PubMed] [Google Scholar]
- 6.Chang, J., R. Renne, D. Dittme, and D. Ganem. 2000. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17-25. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 8.Dutia, B. M., D. J. Allen, H. Dyson, and A. A. Nash. 1999. Type I interferons and IRF-1 play a critical role in the control of a gammaherpesvirus infection. Virology 261:173-179. [DOI] [PubMed] [Google Scholar]
- 9.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenner, R. G., M. M. Alba, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katze, M. G., Y. He, and M. Gale. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 13.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinchington, P. R., D. Bookey, and S. E. Turse. 1995. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J. Virol. 69:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy, D. E., and A. Garcia-Sastre. 2001. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 12:143-156. [DOI] [PubMed] [Google Scholar]
- 17.Li, M., J. MacKey, S. C. Czajak, R. C. Desrosiers, A. A. Lackner, and J. U. Jung. 1999. Identification and characterization of Kaposi's sarcoma-associated herpesvirus K8.1 virion glycoprotein. J. Virol. 73:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 21.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nealon, K., W. W. Newcomb, T. R. Pray, C. S. Craik, J. C. Brown, and D. H. Kedes. 2001. Lytic replication of Kaposi's sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J. Virol. 75:2866-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholl, M. J., J. H. Robinson, and C. M. Preston. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J. Gen. Virol. 81:2215-2218. [DOI] [PubMed] [Google Scholar]
- 26.Poole, L. J., Y. Yu, P. S. Kim, Q. Z. Zheng, J. Pevsner, and G. S. Hayward. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 28.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 29.Roizman, B., and D. Spector. 1992. The induction of α genes by the α trans-inducing factor, p 17-28. In E. K. Wagner (ed.), Herpesvirus transcription and its replication. CRC Press, Boca Raton, Fla.
- 30.Romanowski, M. J., E. Garrido-Guerrero, and T. Shenk. 1997. pIRS1 and pTRS1 are present in human cytomegalovirus virions. J. Virol. 71:5703-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciortino, M. T., M. Suzuki, B. Taddeo, and B. Roizman. 2001. RNAs extracted from herpes simplex virus 1 virions: apparent selectivity of viral but not cellular RNAs packaged in virions. J. Virol. 75:8105-8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 36.Trus, B. L., J. B. Heymann, K. Nealon, N. Cheng, W. W. Newcomb, J. C. Brown, D. H. Kedes, and A. C. Steven. 2001. Capsid structure of Kaposi's sarcoma-associated herpesvirus, a gammaherpesvirus, compared to those of an alphaherpesvirus, herpes simplex virus type 1, and a betaherpesvirus, cytomegalovirus. J. Virol. 75:2879-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitbeck, J. C., C. Peng, H. Lou, R. Xu, S. H. Willis, M. Ponce de Leon, T. Peng, A. V. Nicola, R. I. Montgomery, M. S. Warner, A. M. Soulika, L. A. Spruce, W. T. Moore, J. D. Lambris, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J. Virol. 71:6083-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, L., P. Lo, X. Yu, J. K. Stoops, B. Forghani, and Z. H. Zhou. 2000. Three-dimensional structure of the human herpesvirus 8 capsid. J. Virol. 74:9646-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao, F., and R. J. Courtney. 1989. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J. Virol. 63:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao, F., and R. J. Courtney. 1992. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J. Virol. 66:2709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 99:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA 94:13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, L., V. Puri, and B. Chandran. 1999. Characterization of human herpesvirus-8 K8.1A/B glycoproteins by monoclonal antibodies. Virology 262:237-249. [DOI] [PubMed] [Google Scholar]