Abstract
Retroviral DNA synthesized prior to integration, termed unintegrated viral DNA, is classically believed to be transcriptionally inert and to serve only as a precursor to the transcriptionally active integrated proviral DNA form. However, it has recently been found to be expressed under some circumstances during human immunodeficiency virus type 1 (HIV-1) replication and may play a significant role in HIV-1 pathogenesis. HIV-1 Vpr is a virion-associated accessory protein that is critical for HIV-1 replication in nondividing cells and induces cell cycle arrest and apoptosis. We find that Vpr, either expressed de novo or released from virions following viral entry, is essential for unintegrated viral DNA expression. HIV-1 mutants defective for integration in either the integrase catalytic domain or the cis-acting att sites can express unintegrated viral DNA at levels similar to that of wild-type HIV-1, but only in the presence of Vpr. In the absence of Vpr, the expression of unintegrated viral DNA decreases 10- to 20-fold. Vpr does not affect the efficiency of integration from integrase-defective HIV-1. Vpr-mediated enhancement of expression from integrase-defective HIV-1 requires that the viral DNA be generated in cells through infection and is mediated via a template that declines over time. Vpr activation of expression does not require exclusive nuclear localization of Vpr nor does it correlate with Vpr-mediated cell cycle arrest. These results attribute a new function to HIV-1 Vpr and implicate Vpr as a critical component in expression from unintegrated HIV-1 DNA.
Human immunodeficiency virus type 1 (HIV-1) Vpr is a 14-kDa basic molecule that plays an important role in HIV-1 replication and pathogenesis. Vpr is highly conserved among primate lentiviruses, including HIV-1, HIV-2, and simian immunodeficiency virus (SIV). Deletion of vpr alone or vpr and a homologous SIV gene, vpx, can ameliorate disease progression in SIV-infected macaques (24, 29, 33). A retrospective study has shown that in nonhuman primates and a laboratory worker initially infected with vpr strains, reversion resulting in a functional vpr gene occurs over the course of infection (25). Thus, there appears to be a positive selection for Vpr function in vivo.
Vpr is expressed from infected cells and is present in significant quantities in virions (13, 58). Vpr facilitates HIV-1 infection of nondividing cells, such as macrophages, by assisting in transport of preintegration complexes (PICs) into the nucleus (14, 28, 43). Vpr causes nuclear envelope herniations, thus possibly allowing the PICs to bypass the size restrictions of the nuclear pore complexes (15).
In addition to its role in nuclear transport and establishment of viral infection in macrophages, Vpr also contributes to HIV-1 replication in dividing cells. In transient transfection experiments, Vpr possesses weak transcriptional activity, no more than severalfold in magnitude, and transactivates the HIV-1 long terminal repeat (LTR) and other heterologous promoters (13). Vpr transactivation of HIV-1 is mediated through cis-acting elements within the viral LTR, including NF-κB, Sp1, and GRE enhancer sequences, and Vpr binds to Sp1, TF-IIB, and cyclin T1 in vitro (2, 47, 53, 54). Both de novo-expressed and virion-associated Vpr proteins induce cell cycle arrest of mammalian cells at the G2/M phase (27, 31, 44, 45). The Vpr-induced G2 arrest is characterized by inactivation of the Cdc2 kinase and requires the activity of protein phosphatases PP2A and Cdc25 (4, 17, 42, 44). Induction of G2 arrest by Vpr may also contribute to increased HIV-1 expression. The HIV-1 LTR is more transcriptionally active in the G2 phase, resulting in a threefold enhancement of viral replication (25).
During retroviral infection, the RNA genome is reverse transcribed into DNA intermediates which, after transport to the nucleus, are integrated into the host chromosome. Several forms of retroviral DNA have been identified in infected cells (12). Retroviral DNA synthesized in the cytoplasm is linear, while after nuclear transport, linear and closed circular DNA forms are found within the nucleus. For all retroviruses, the nuclear linear DNA molecule is the primary direct precursor to the integrated provirus. In contrast, the one- and two-LTR circular forms are generally incompetent for integration and are believed to be the dead-end products of reverse transcription formed by homologous recombination and self-ligation, respectively. For HIV-1, integration is usually required for the production of infectious virus and efficient viral gene expression (36, 46). Classically, the unintegrated viral DNA is thought to serve only as an intermediate for formation of the provirus or integrated form of viral DNA and does not express viral proteins. Nevertheless, recent studies with HIV-1 indicate that in some instances, infection of quiescent T cells and CD4+ cell lines with integrase-defective viruses can produce a self-limiting infection, allowing detection of multiple viral transcripts and proteins (9, 38, 56). Thus, unintegrated HIV-1 DNA can be transcriptionally active. During the asymptomatic phase of HIV-1 infection and in patients on highly active antiretroviral therapy, the prevalent form of HIV-1 found is the unintegrated circular DNA form (6, 11, 48). Thus, expression from unintegrated HIV-1 DNA and the ensuing viral products may contribute to ongoing viral replication.
In this study, we show that HIV-1 Vpr significantly upregulates expression from unintegrated DNA. In the presence of Vpr, either de novo or virion associated, there is a 10- to 20-fold increase in gene expression from HIV-1 that is defective for integration. The Vpr-enhanced expression from integrase-defective HIV-1 is mediated via a template that declines over time and requires a functional HIV-1 LTR. The effect of Vpr on unintegrated DNA is not dependent on Vpr's karyophilic properties or on the induction of G2 arrest. Therefore, Vpr specifically transactivates unintegrated HIV-1 DNA and may contribute to expression from unintegrated HIV-1 DNA.
MATERIALS AND METHODS
DNA constructs.
The nucleotide position numbers used to describe vector construction start at the 5′ end of HIVNL4-3 (1). NLlucΔBgl VprX, NLlucΔBgl VprX D64E, and NLlucΔBgl VprX U3-U5 del10 were derived by inserting the truncated vpr gene from NLthyΔBglVprX (41) into HIV-1NL4-3Luc env(−), NLlucΔBgl D64E, and NLlucΔBgl U3&U5 del10 (35, 36). The integrase-defective packaging plasmid pCMVΔR8.2DVpr D64E was constructed by inserting the pol gene from the BclI (nucleotide [nt] 2430) to SalI (nt 5786) sites from NLlucΔBglD64E into pCMVΔR8.2Dvpr (50). The lentiviral vectors pHR′CMV-EGFP and pHR′CMV-Vpr have been previously described (3, 50). Mutant vpr from BSVprE25K and BSVprR80A (23) was cloned into pHR′CMV-Vpr, producing pHR′CMV-VprE25K and pHR′CMV-Vpr80A, respectively. The lentiviral vectors NLlucDA, pCS-CMV-luc, and pCS-Rh-MLV-luc were derived from DAEGFP (3), CSC-G, and CS-Rh-MLV-E (32), respectively, by substituting luciferase in place of eukaryotic green fluorescent protein (EGFP).
Virus production.
Vesicular stomatitis virus G protein (VSV-G) pseudotyped viruses were produced by calcium phosphate-mediated transfection of 293T cells as described previously (41). Cells (2 × 107) were transfected with 5 μg of pHCMVG and 12.5 μg of full-length HIV plasmids or, for the production of lentiviral vectors, 5 μg of pHCMVG, 12.5 μg of packaging plasmid, and 12.5 μg of the appropriate lentiviral vector. Virus stocks of a strain with a functional integrase (IN+), a strain with a mutation of the integrase protein (D64E), a strain with a functional integrase and a truncated vpr gene (VprX IN+), a strain with the D64E mutation and a truncated vpr gene (VprX D64E), and a strain with a deletion of the retroviral attachment sites and a truncated vpr gene (VprX att) were generated using NLlucΔBgl, NLlucΔBgl D64E, NLlucΔBgl VprX, NLlucΔBgl VprX D64E, and NLlucΔBgl VprX U3-U5 del10, respectively. Lentiviruses HR-EGFP, HR-Vpr, HR-E25K, and HR-R80A were produced using pCMVDR8.2DVpr and pHR′CMV-EGFP, pHR′CMV-Vpr, pHR′CMV-E25K, or pHR′CMV-R80A, respectively. The integration-defective lentiviral vectors were produced using pCMVΔR8.2DVpr D64E and NLlucDA, CS-CMV-luc, or pCS-Rh-MLV-luc. Vpr was incorporated into HIV-1 luciferase-expressing virions using NLlucΔBgl VprX or NLlucΔBgl VprX D64E and BSXCVpr. Virions devoid of viral RNA were produced by cotransfection of pHCMVG and pCMVΔR8.2 or pCMVΔR8.2DVpr, resulting in viruses with the phenotypes RNA− Vpr+ and RNA− Vpr−, respectively.
Culture supernatants were collected and concentrated as previously described (41). The multiplicity of infection (MOI) was determined by infecting 2 × 104 HeLa cells with the HR-EGFP vector and analyzing them by flow cytometry at day 2 postinfection for EGFP positivity. Using 20 and 200 ng of VSV-G pseudotyped virus p24 antigen resulted in MOIs of 0.1 and 1.0, respectively.
Cell culture.
293T cells and HeLa cells were maintained in Dulbecco's modified Eagle medium with 10% calf serum. CEMx174 cells were maintained in RPMI with 10% fetal calf serum. Cells were infected with VSV-G pseudotyped viruses for 4 h at 37°C in the presence of 10 μg of Polybrene per ml. Luciferase-expressing viruses were infected at low multiplicities (25 ng of p24 antigen per 2 × 104 cells), and HR-EGFP, HR-Vpr, and HR-Vpr mutant vectors were infected at high multiplicities (200 ng of p24 antigen). HeLa cells were transfected using Lipofectin Plus (Invitrogen) according to the manufacturer's instructions.
Cell synchronization of HeLa cells by thymidine blocking was initiated by incubating cells in the presence of 2 mM thymidine for 15 h. Cells were washed with phosphate-buffered saline, and fresh medium without thymidine was added. Cells were infected at 7 h post-thymidine release, when by flow cytometric analysis cells were estimated to be at the S/G2 border. After infection, cells were washed with phosphate-buffered saline, and either medium alone or medium with thymidine was added. Cells either were harvested at 40 h postinfection and analyzed for cell cycle or EGFP expression by flow cytometry or were lysed in 50 to 100 μl of 1× passive lysis buffer (Promega) and analyzed for luciferase activity.
Luciferase activity was measured using a Monolight 2010 luminometer and normalized to relative luciferase units (RLU) per microgram after protein levels were determined using the Bradford assay. Each sample was analyzed in duplicate or triplicate, and the average deviation was calculated. The background luciferase detection level was 0.3 × 102 RLU/μg.
Infected cells were analyzed by flow cytometry for EGFP percentages and cell cycle content by resuspending cells in fluorescence-activated cell sorting buffer or hypotonic propidium iodide (PI) buffer, respectively. All stained cells were analyzed on a FACScan II (Becton Dickinson) apparatus by the CELL QUEST software package.
Western blot analysis.
Concentrated virus stocks (10 ng of p24) were lysed, and Western blot analysis was performed as previously described (41).
Southern blot analysis.
HeLa cells were infected with VprX IN+ or VprX D64E and RNA− Vpr+ or RNA− Vpr− virus so that the viral DNA detected by Southern blot analysis was derived only from the VprX IN+ or VprX D64E virus. At 40 h postinfection, cells were harvested and DNA was purified by use of the Qiagen blood and cell culture DNA mini kit according to the manufacturer's instructions. The percentages of total, linear, and circular HIV DNA were analyzed as previously described (59). The relative intensities of bands corresponding to total viral DNA, linear DNA, and one- and two-LTR circular DNA were analyzed on a Molecular Dynamics Phosphorimager.
RESULTS
Vpr increases expression from integration-defective HIV-1.
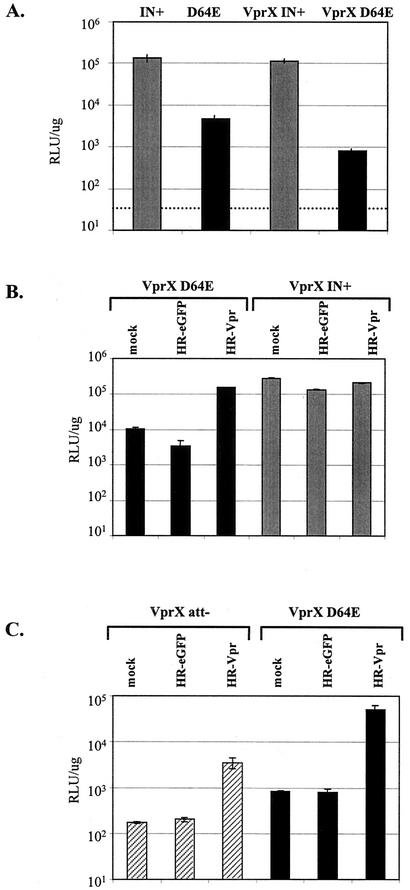
The level of expression, if any, from unintegrated viral DNA has been controversial, although recent studies indicate that unintegrated HIV-1 DNA is capable of viral gene expression (38, 56). We confirmed those observations by measuring expression from integration-defective HIV-1 using VSV-G pseudotyped HIV-1 expressing luciferase in place of Nef during a single-round infection of HeLa cells. The ability of HIV-1 to integrate was abolished by a point mutation of D64 to E in the core catalytic domain of the integrase protein which results in a 3- to 4-log decrease in viral infectivity and, depending on the experimental system, an 8- to 300-fold decrease in gene expression (34, 36, 52, 55). We also observed a 28-fold decrease in viral expression from the D64E virus relative to HIV-1 containing a functional integrase (IN+) (Fig. 1A). Nevertheless, the levels of luciferase expression from the D64E mutant virus were still significant, consistent with the recent reports of expression from unintegrated HIV-1. Luciferase activity from the D64E mutant virus was abolished in the presence of zidovudine (data not shown); therefore, viral expression was not due to pseudo-transduction of cells by VSV-G.
FIG. 1.
Vpr increases expression from integration-defective HIV-1. (A) HeLa cells (2 × 104 cells) were infected with virus stocks (25 ng of p24 antigen) of NLlucΔBgl (IN+), NLlucΔBglD64E (D64E), NLlucΔBglVprX (VprX IN+), or NLlucΔBglVprXD64E (VprX D64E). (B and C) HeLa cells (2 × 104) were coinfected with 25 ng of p24 antigen from virus VprX IN+, VprX D64E, or VprX att and 200 ng of p24 antigen from vector HR-EGFP or HR-Vpr. Luciferase activity was analyzed at day 2 postinfection and is expressed as RLU per microgram of total protein. The dashed line indicates the background luciferase activity from an equivalent number of uninfected HeLa cells.
We next determined the viral factors that were involved in mediating gene expression from unintegrated HIV-1. Since HIV-1 Vpr is an immediate-early protein that has pleiotropic effects prior to integration, we tested the effect that deletion of vpr would have on expression from integrase-defective HIV-1. In the context of the D64E mutation, truncation of vpr (VprX D64E) caused a further decrease in luciferase expression relative to a vpr-containing HIV-1 (D64E) (8 × 102 versus 5 × 103 RLU, respectively) (Fig. 1A). In contrast, deletion of vpr had little effect on expression from integrase-competent viruses. Therefore, the presence of an intact vpr gene within the viral genome contributes to efficient viral expression from integrase-defective HIV.
We confirmed that Vpr mediates the enhanced expression from the D64E relative to the VprX D64E mutant virus by supplying Vpr to infected cells via trans-complementation. Using HIV-1 viruses containing truncated vpr and a functional or inactive integrase (VprX IN+ and VprX D64E, respectively), exogenous Vpr was provided by coinfection with a lentiviral vector expressing Vpr (HR-Vpr). As observed above, VprX D64E mutant virus expression was low and unchanged by coinfection with a lentiviral vector devoid of Vpr and expressing EGFP (HR-EGFP); thus, we could discount any potential effect of the wild-type integrase brought in by the lentiviral vector virions. The addition of Vpr to integrase-defective HIV-1 by coinfection with HR-Vpr vector led to a 14-fold increase in luciferase expression over that of the VprX D64E mutant virus alone (Fig. 1B). It is noteworthy that in some experiments such as the one described here, the level of expression from the VprX D64E mutant virus in the presence of Vpr approaches the level of expression from the VprX IN+ virus (1.5 × 105 versus 2.8 × 105 RLU, respectively). Coinfection with the HR-EGFP or HR-Vpr vector had no effect on expression from integration-competent VprX IN+ virus. Similar results were seen upon infection with lower MOIs of VprX IN+ virus, resulting in 104 RLU. Coinfection with HR-Vpr vector still did not increase expression from integration-competent HIV-1 (data not shown), excluding saturation of the luciferase assay. Thus, provision of Vpr in trans to cells infected with integrase-defective HIV-1 can rescue viral expression.
We also examined the effect of Vpr on HIV-1 rendered integration incompetent by deletion of the retroviral attachment (att) sites. In addition to integrase, retroviral integration requires specific sequences in the U3 region of the viral LTRs, termed att sites (12). The att sites serve as cis-acting recognition sequences for integrase and are crucial for the end joining and strand transfer processes. Deletion of the att sites (VprX att−) resulted in a substantial decrease in HIV-1 gene expression, similar to the effect of the integrase catalytic mutant (Fig. 1C). Provision of Vpr in trans by coinfection with HR-Vpr vector resulted in a 17-fold increase in luciferase activity over that from the VprX att− virus alone. Vpr is therefore capable of increasing expression from HIV-1 rendered defective for integration by either inactivation of the integrase active site or deletion of the retroviral attachment sites.
Vpr does not affect integration of integrase-defective HIV-1.
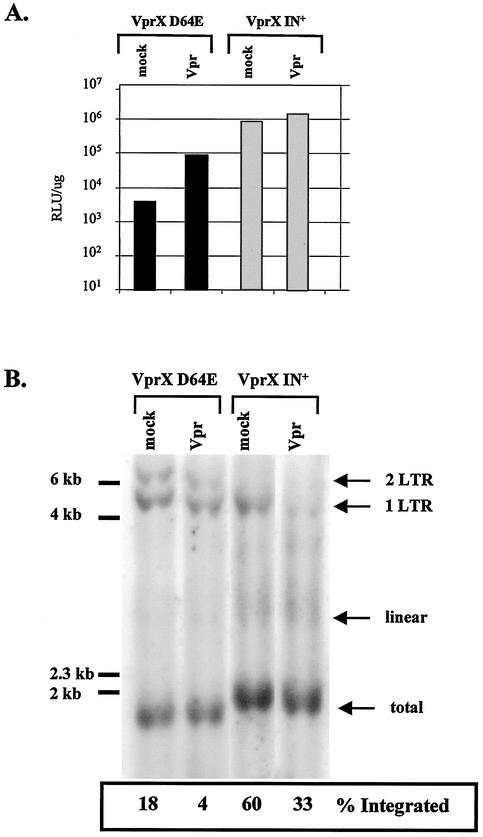
Our results are consistent with an effect of Vpr on enhancing expression of unintegrated HIV-1 DNA. However, it is formally a possibility that Vpr could be enhancing the low level of residual illegitimate integration by the catalytically inactive VprX D64E integrase mutant, resulting in increased viral expression from greater levels of integrated proviruses. Cells were infected with VprX IN+ or VprX D64E virus, and the level of integration in the presence or absence of Vpr was measured by Southern blot analysis (Fig. 2). Using selective restriction enzymes and probes, the relative integration efficiency can be calculated by subtracting the relative amounts of linear HIV-1 DNA and one- and two-LTR circles from the total amount of viral DNA (59). By day 2 postinfection and in the absence of Vpr, 60% of the viral DNA from the VprX IN+ virus had been integrated, compared to 18% of the DNA from the VprX D64E mutant virus. In agreement with previous reports (7, 18), the majority of the DNA from the VprX D64E mutant virus is in the form of one- and two-LTR circles. In the presence of Vpr, there is a decrease in the amount of integrated DNA in both VprX IN+ and VprX D64E virus infections to 33 and 4%, respectively, despite an 18-fold increase in expression from VprX D64E virus induced by Vpr. Therefore, we conclude that Vpr is not mediating increased expression by affecting the efficiency of integration from VprX D64E mutant virus.
FIG. 2.
Vpr does not increase integration from integrase-defective HIV-1. HeLa cells (5 × 105) were coinfected with 800 ng of p24 antigen from virus VprX IN+ or VprX D64E and 2.5 μg of p24 antigen from virus with the phenotype RNA− Vpr− or RNA− Vpr+ (mock or Vpr, respectively). At day 2 postinfection, cells were harvested and subjected to luciferase analysis (A) or Southern blot analysis (B). The amount of integrated proviral DNA was calculated by subtracting the signals of unintegrated linear and circular viral DNA from the total amount of viral DNA.
Effect of Vpr on unintegrated DNA requires HIV-1 infection.
Previous studies have assessed the transactivation activity of Vpr by cotransfection of HIV-1 LTR-reporter constructs and Vpr expression constructs. The effect previously observed is generally small, only severalfold in magnitude. In our hands, we have consistently observed a 15- to 20-fold increase of HIV-1 expression with Vpr, and this effect occurs with integration-defective but not integration-competent HIV-1. We next investigated whether the method of delivery of viral DNA template affects the observed Vpr-mediated upregulation of expression from VprX D64E mutant virus. We compared the effect of Vpr on the VprX D64E mutant virus during DNA transfection with that during viral infection. Cells were either transfected with HIV-1 DNA or infected with virus and then were infected with HR-Vpr vector. When the viral DNA template was introduced by transient transfection (Fig. 3A), there was no observable effect of Vpr on expression from either VprX IN+ or VprX D64E virus. Similar results were observed when smaller amounts of DNA were used for transfection (data not shown), excluding saturation of the assay. In contrast, there was a 39-fold increase in the presence of Vpr when the viral template was provided by infection with VprX D64E virus. Thus, the effect of Vpr on VprX D64E virus expression is more dramatic during a viral infection, indicating that Vpr is specifically acting on the unintegrated DNA intermediates present in PICs and not on integrated DNA or transfected DNA.
FIG. 3.
Vpr-induced expression from integrase-defective HIV-1 requires a viral template that forms only during a viral infection and declines over time. (A) HeLa cells (5 × 104) were transfected (0.05 μg of DNA) with NLlucΔBglVprX or NLlucΔBglVprXD64E or were infected (50 ng of p24 antigen) with VprX IN+ or VprX D64E virus, followed by infection with 250 ng of p24 antigen from HR-EGFP or HR-Vpr vector. Luciferase activity was analyzed at 2 days posttransfection or -infection. (B) HeLa cells were infected with VprX D64E virus (250 ng of p24 antigen per 2 × 105 cells), and 5 × 104 cells were either coinfected (Vpr d0-2), infected 24 h later (Vpr d1-3), or infected 48 h later (Vpr d2-4) with vector HR-Vpr (500 ng of p24 antigen), as shown schematically. Luciferase activity was analyzed 2 days post-HR-Vpr vector infection and is expressed as the fold induction relative to luciferase activity from cells that were coinfected with VprX D64E and HR-EGFP viruses.
Vpr stimulates expression from an unintegrated viral template.
We have shown that the increased Vpr-mediated expression from unintegrated HIV-1 DNA is not due to an effect of Vpr on the integration process. We next investigated whether Vpr may be specifically transactivating an unintegrated DNA template. In contrast to integrated provirus DNA which is stable and propagated during cell division, the levels of HIV-1 unintegrated one- and two-LTR episomal circles in dividing cells decline over time, with an estimated half-life of 24 to 48 h (8, 39, 40, 48). The loss of unintegrated HIV-1 DNA has been attributed to either decay due to instability of the episomal circles or dilution during cell division. We thus predicted that if Vpr is affecting expression from unintegrated DNA, the effect of Vpr should be muted if cells are infected with Vpr several days post-HIV-1 infection due to the progressive decline in the amount of the unintegrated DNA products over time. To test our prediction, at various times post-VprX D64E virus infection, cells were infected with the lentiviral vector expressing Vpr (Fig. 3B). When cells were simultaneously infected with HR-Vpr vector and VprX D64E virus, there was a 17-fold increase in luciferase activity. In contrast, when Vpr was introduced into VprX D64E virus-infected cells at 24 or 48 h post-HIV-1 infection, we observed a 4- and 1.5-fold increase, respectively. Therefore, the ability of Vpr to increase expression requires that Vpr be present soon after VprX D64E virus infection. When Vpr is delivered to cells 24 to 48 h post-VprX D64E virus infection, there is a minimal effect on expression. This result lends support to the theory that Vpr preferentially transactivates an HIV-1 template that is not stably integrated and thus declines over time, namely unintegrated DNA.
Virion-associated Vpr increases integrase-defective HIV-1 expression.
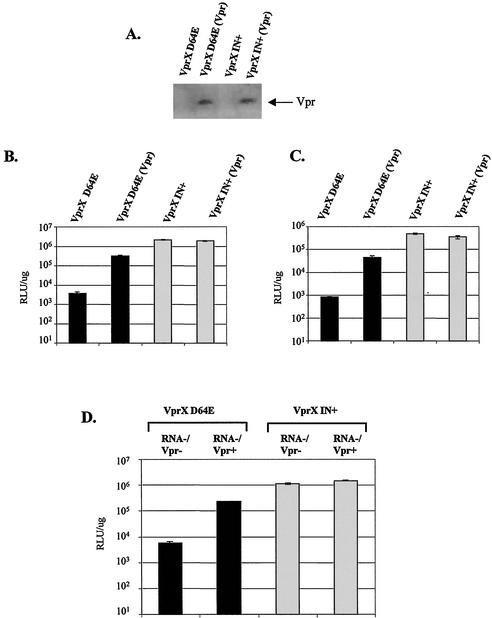
In the experiment shown in Fig. 1B, we observed an increase in viral gene expression when Vpr was provided in trans by de novo expression of Vpr from a lentiviral vector. In addition to being expressed from infected cells, Vpr is also incorporated into HIV-1 virions and virion-associated Vpr can mediate cell cycle arrest, apoptosis, and transactivation of transfected HIV-1 LTR reporter constructs (30, 41, 50). We next investigated whether Vpr incorporated into virions, in the absence of de novo Vpr expression, is sufficient to mediate upregulation of gene expression from integration-defective HIV-1. Vpr was introduced into the same virions as the template genome by cotransfecting a Vpr expression construct during virus production of VprX IN+ or VprX D64E virus. This resulted in packaging of Vpr within virions that are incapable of de novo synthesis of Vpr (Fig. 4A). Infection of HeLa cells with integrase-defective virions that contain Vpr but do not express Vpr in the genome [VprX D64E (Vpr)] resulted in an 83-fold increase in luciferase expression compared to infection with integrase-defective virions that do not contain Vpr (VprX D64E) (Fig. 4B).
FIG. 4.
Virion-associated Vpr is sufficient to induce expression from VprX D64E virus. (A) Western blot analysis for the presence of virion Vpr within wild-type or integrase-defective HIV-1 virions defective for de novo Vpr synthesis [VprX IN+ (Vpr) or VprX D64E (Vpr)]. The arrow indicates the protein band corresponding to Vpr. (B and C) HeLa cells (B) or CEMx174 cells (C) were infected with 25 ng of p24 antigen from wild-type or integrase-defective HIV-1 lacking (VprX IN+ and VprX D64E) or containing [VprX IN+ (Vpr) and VprX D64E (Vpr)] Vpr within the virions. (D) HeLa cells were coinfected with VprX IN+ or VprX D64E virus and virions lacking a viral genome and lacking or containing Vpr (RNA− Vpr− or RNA− Vpr+), respectively. For panels B, C, and D, luciferase activity was analyzed at 2 days postinfection.
It has been suggested that the level of transient gene expression from unintegrated DNA may be dependent on target cells and/or the indicator assay (38). Similar to the results in HeLa cells, infection of a T-cell line, CEMx174 cells, with VprX D64E (Vpr) virus also resulted in a substantial increase (53-fold) relative to non-virion Vpr-containing VprX D64E virus (Fig. 4C). We also saw a Vpr-mediated increase of gene expression from an integrase-defective HIV-1 expressing the mouse thy1.2 gene as measured by an increase in both the percentage of Thy1.2 positivity and fluorescence intensity (data not shown). We therefore conclude that Vpr in virions introduced during early events in infection can enhance expression of unintegrated HIV-1 DNA.
We next examined whether Vpr protein alone, in the absence of a viral genome, could induce expression from integrase-defective HIV-1. We have previously used Vpr packaged into “genome-less” virions as a model delivery system for Vpr protein (50). Infection with virions devoid of HIV-1 RNA but containing Vpr can induce cell cycle arrest and apoptosis as efficiently as de novo-expressed Vpr. Delivery of Vpr protein by genome-less virions resulted in increased luciferase activity from integration-defective HIV-1 (Fig. 4D, compare coinfection of VprX D64E and RNA− Vpr+ virus with coinfection of VprX D64E and RNA− Vpr− virus). Again, there was no effect of Vpr on integration-competent VprX IN+ virus. Thus, Vpr protein delivered by virions lacking a viral genome is sufficient to increase expression from unintegrated HIV-1 DNA.
Vpr-mediated expression from integrase-defective HIV-1 does not require exclusive nuclear localization of Vpr.
Vpr is a nucleophilic protein that is localized primarily at the perinuclear membrane. The ability of Vpr to localize to the nucleus is critical for HIV-1 infection of nondividing cells by participating, in conjunction with matrix and integrase, in nuclear translocation of PICs. However, nuclear localization of Vpr appears to be dispensable for its transcriptional activity since Vpr mutants that localized primarily in the cytoplasm were still capable of transactivating HIV-1 LTR expression constructs (22). We explored whether the increased expression from integrase-defective HIV-1 by Vpr required wild-type subcellular localization of Vpr by utilizing two well-characterized mutants of Vpr with differing phenotypes in regards to their subcellular distribution. The E25K mutant consists of a point mutation within the first α-helical domain of Vpr that prevents exclusive localization to the nucleus and is found predominantly in the cytoplasm. The R80A mutant consists of a point mutation in the basic amino acid-rich C-terminal domain that localizes to the nucleus in a pattern similar to that for wild-type Vpr (23). Cells were coinfected with VprX D64E virus and lentiviral vectors expressing either wild-type Vpr or Vpr with mutation E25K or R80A (Fig. 5A). Despite the primarily cytoplasmic localization of the E25K mutant, coinfection with HR-E25K vector increased VprX D64E virus expression to the same extent as wild-type Vpr. The R80A mutant had a nuclear staining pattern similar to that of wild-type Vpr yet had no effect on luciferase activity from VprX D64E virus. Therefore, the ability of Vpr to mediate increased expression from integrase-defective HIV-1 does not require Vpr to be exclusively localized to the nucleus.
FIG. 5.
Induction of expression from VprX D64E virus does not require exclusive Vpr nuclear localization or G2 arrest. (A) HeLa cells (2 × 104) were coinfected with VprX IN+ or VprX D64E virus and HR-Vpr, HR-R80A, or HR-E25K vector. Luciferase activity was analyzed at 2 days postinfection. (B) Cells were coinfected with VprX D64E and HR-Vpr viruses, and cultures were maintained in medium or medium with thymidine (Vpr and Vpr plus thymidine, respectively). Luciferase activity is expressed as the fold induction relative to cells that were infected with VprX D64E and HR-EGFP viruses. In medium alone, cells infected with VprX D64E and HR-EGFP viruses had 62 and 37% of the cells in the G1 and G2/M phases, respectively, and a luciferase activity of 8.5 × 103 RLU/μg. In medium with thymidine, infection with VprX D64E and HR-EGFP viruses resulted in 94 and 6% of the cells being in the G1 and G2/M phases, respectively, and a luciferase activity of 2.7 × 103 RLU/ug.
Increased expression from VprX D64E virus does not require G2 arrest by Vpr.
We next determined whether the effect of Vpr on integrase-defective HIV-1 was dependent on the induction of G2 arrest. Earlier reports concluded that cell cycle arrest and transcriptional activation by Vpr were linked because mutational loss of G2 arrest also resulted in loss of transcriptional upregulation of the HIV-1 LTR (22, 57). We have shown that the R80A mutant, which is defective for cell cycle arrest, was also unable to increase VprX D64E virus expression. This suggests that Vpr-induced G2 arrest may be important for the induction of expression from integrase-defective HIV-1. To further define the necessity of Vpr-induced arrest for integration-defective HIV-1 transactivation, we prevented the Vpr-induced G2 arrest by using experimental conditions that maintain cells in the G1 phase. Thymidine, which affects synthesis of deoxynucleoside triphosphates and results in decreased intracellular nucleoside pools, was used to synchronize cells in the G1/S phase. Cells were subsequently coinfected with VprX D64E virus and lentiviral vectors expressing EGFP or Vpr, after which either medium alone or medium containing thymidine was added. At 40 h postinfection, cells were analyzed for EGFP expression, cell cycle profile, and luciferase expression. The presence of thymidine did not overtly affect viral expression, as similar levels of EGFP-positive cells were detected in the presence or absence of thymidine (57 and 55%, respectively) (data not shown). However, thymidine did affect the ability of Vpr to arrest cells in G2. In medium alone, infection with Vpr resulted in cell cycle arrest with 80% of the cells in the G2 phase (Fig. 5B). In contrast, when Vpr-infected cells were maintained in thymidine-containing medium, only 7% of the cells were in the G2/M phase, with the majority of cells (85%) being in the G1/S phase. Therefore, the addition of thymidine in the medium prevented cells from becoming arrested in G2 by Vpr. Despite the absence of G2 arrest in Vpr-infected cells maintained in thymidine, there was a comparable increase of expression from VprX D64E virus relative to Vpr-arrested cells in the absence of thymidine (11- versus 14-fold, respectively). Thus, Vpr-mediated G2 arrest is not required for induction of VprX D64E virus expression.
Vpr-induced transactivation of unintegrated HIV-1 DNA requires a functional HIV LTR.
In addition to the HIV-1 LTR, Vpr has been reported to be capable of transactivating various other viral and cellular promoters in transfection assays (13). We next examined whether the ability of Vpr to increase unintegrated DNA expression is specific for the HIV-1 LTR by comparing expression from the HIV-1 LTR with expression directed by either the CMV promoter or a murine leukemia virus LTR (Rh-MLV) (32). Lentiviral vector virions were produced that were integration incompetent by using a packaging plasmid that expresses catalytically inactive integrase. The CMV or Rh-MLV promoter directing luciferase expression was introduced as an internal promoter in self-inactivating lentiviral vectors with deletions in the HIV-1 LTRs that abolish HIV-1 LTR-driven transcription (Fig. 6A). Similar to the effect on full-length VprX D64E virus, Vpr also increased expression (29-fold) of an integration-defective HIV-1-based vector in which luciferase was transcribed from the HIV-1 LTR. A smaller increase was observed in the presence of Vpr when luciferase expression was under the control of the CMV or RH-MLV promoter (10- and 5-fold, respectively). We conclude that Vpr mediates increased expression from unintegrated DNA through transactivation of the HIV-1 LTR.
FIG. 6.
Vpr requires an intact HIV-1 LTR to induce expression from integration-defective HIV-1. (A) Schematic diagram of lentiviral vectors expressing luciferase under the control of various promoters. For the CMV- and Rh-MLV-driven luciferase lentivirus vectors, there is a 133-bp deletion in the U3 region of the HIV-1 LTR from −145 to −8, encompassing the TATA start site. (B) HeLa cells (2 × 104) were infected with integrase-defective lentiviral vectors expressing luciferase under the control of the HIV-1 LTR, the CMV promoter, or the Rh-MLV LTR as indicated (25 ng of p24 antigen) and were coinfected with either HR-EGFP or HR-Vpr vector (200 ng of p24 antigen). The fold induction was calculated as the level of luciferase activity (mean ± standard deviation of three independent experiments) in the HR-Vpr vector-infected cultures relative to the HR-EGFP vector-infected cells.
DISCUSSION
We present evidence demonstrating a significant enhancement of gene expression from integrase-defective HIV-1 that is mediated by Vpr. While Vpr has been previously shown to be a mild transactivator of the HIV-1 LTR, the effect of Vpr on HIV-1 expression is much more prominent when HIV-1 is unable to integrate due to either loss of the viral integrase activity or deletion of the viral DNA attachment sites. Vpr, either expressed de novo from a lentiviral vector or packaged into virions, can increase expression from integrase-defective HIV-1 in both HeLa cells and the T-cell line CEMx174. The 15- to 18-fold increase in expression induced by Vpr was seen only with integrase-defective HIV-1 as a template and required that the viral DNA be generated in cells through infection.
Mechanism of Vpr-induced upregulation.
We explored several possible mechanisms by which Vpr activates expression from integrase-defective HIV-1. It was conceivable that Vpr may be indirectly affecting the integration process mediated by the HIV-1 integrase, resulting in increased illegitimate integration. However, when the integration efficiency of integrase-defective virus was examined in the presence of Vpr, no increase in integrated viral DNA was detected. Therefore, the Vpr-induced increase in expression from integrase-defective HIV-1 is not due to increased levels of integration.
Another possibility is that Vpr has an effect on the half-life of the unintegrated viral DNA species that are preferentially generated during infection with integrase-defective HIV-1. Unintegrated HIV-1 DNA in dividing cells has an estimated half-life of 1 to 2 days, due to either molecular decay or dilution of the episomal circles by cell division (8, 40, 48). The effect of Vpr appears to be mediated via a DNA template that is lost over time, as we observed a decrease in Vpr-induced expression when Vpr was supplied several days post-HIV-1 infection. The decrease in Vpr-mediated expression over time may be due to stability of the viral DNA itself or to changes in unintegrated DNA-associated proteins. Upon examination of the levels of the one- and two-LTR circles, we found that, similar to cells infected with non-Vpr-containing virus, cells coinfected with integrase-defective HIV-1 and Vpr also showed a progressive loss of the circular forms of HIV-1 over time (data not shown). Thus, it is unlikely that Vpr is effecting increased expression by increasing the levels of the unintegrated viral DNA intermediates.
Another possibility we considered was whether Vpr was increasing expression by facilitating nuclear import of integrase-defective HIV-1. Vpr is critical in the infection of nondividing cells but not of dividing cells, and all cells used for this study (except those for Fig. 5B) were exponentially dividing cells in which Vpr has no effect on nuclear import. In addition, our results suggest that the ability of Vpr to exclusively localize to the nucleus is not critical for Vpr-mediated transactivation of HIV-1 unintegrated DNA. The Vpr mutant E25K that localized primarily to the cytoplasm was still capable of enhanced expression from integrase-defective HIV-1, whereas the R80A mutant that retained its karyophilic property was unable to induce expression. Another argument against Vpr-enhanced nuclear import as an explanation for the increased expression from integrase-defective HIV-1 comes from the analysis of the integrated and unintegrated forms of HIV-1 DNA generated after infection (Fig. 2). Since one- and two-LTR circles arise only after the viral DNA is transported to the nucleus, one would expect increased levels of DNA circles in the presence of Vpr if Vpr was affecting nuclear import. Southern blot analysis revealed no notable increase in the levels of one- and two-LTR circles from integrase-defective HIV-1 in the presence of Vpr. Finally, infection with the lentiviral vectors with different promoters controlling luciferase is dependent on the same HIV-1 nuclear import pathway. If Vpr was facilitating nuclear import of unintegrated DNA, one would expect to see a similar fold enhancement from each vector in the presence of Vpr. Rather we observe differences that are dependent on the context of the promoters regulating luciferase expression. Therefore, we propose that the increase of expression by Vpr is the result of transcriptional activation of the HIV-1 LTR in the context of unintegrated viral DNA.
Vpr and transcription.
This effect of Vpr on integrase-defective HIV-1 expression is distinct from prior transactivation studies using HIV-1 LTR expression constructs introduced into cells by transient transfection. In those instances, Vpr weakly increased reporter gene levels only a few fold. Similarly, the effect of Vpr on HIV-1 replication utilized integration-competent virus, and the increase in viral replication was also only a few fold. In contrast, we observe the substantial Vpr-mediated increase in expression only with integration-incompetent viral DNA generated during an active infection process. The specific effect of Vpr on the viral DNA template generated during infection may be explained by the structural differences of retroviral unintegrated DNA generated during an infection compared to both naked DNA introduced into cells by transfection as well as HIV-1 DNA integrated into the chromosome. After virus entry and reverse transcription, the DNA present in HIV-1 PICs exists as a high-molecular-weight nucleoprotein complex that consists of the viral DNA substrate and various viral and host proteins (5, 19, 20, 37). Protein-DNA footprinting revealed different patterns between plasmid DNA and DNA contained within PICs and even between the integration-competent and circular forms of HIV-1 DNA in PICs (5, 10, 37). These differences in DNA configuration and protein association may permit Vpr to differentially access and transcribe unintegrated DNA from HIV-1 PICs more efficiently than plasmid or integrated DNA.
In addition to its transcriptional activity, Vpr is also capable of inducing G2 cell cycle arrest. Previous mutational studies of Vpr suggested a functional link between Vpr-mediated G2 arrest and transactivation, as the loss of one function also resulted in loss of the other. One prevailing theory of the way that Vpr effects increased viral production is through the creation of a cellular environment in which the HIV-1 LTR is more transcriptionally active. In support of an indirect role for Vpr-mediated arrest, elutriation to obtain a pure population of cells in G2 also resulted in a threefold increase in HIV-1 LTR expression (26). A Cdc2 transdominant protein that arrests cells in G2 also increased HIV-1 LTR-driven expression to levels similar to that seen with Vpr (25). Vpr-induced G2 arrest, which results in dephosphorylation and inactivation of the Cdc2 kinase, may be modulating the transcriptional coactivator p300, which is involved in NF-κB and cyclin/Cdc2 interaction (21). Thus, the G2 arrest induced by Vpr may be indirectly affecting the basal transcriptional machinery through the availability and/or activity of cellular transcription factors that act upon the HIV-1 LTR. However, there is evidence that Vpr may have a more direct effect on transcription by specifically interacting with cellular transcription factors. Studies have reported an in vitro association between Vpr and transcription factors including Sp1, TFIIB, and cyclin T1 (2, 47, 54). Vpr was also capable of up-regulating HIV-1 viral transcription in macrophages (16, 51), which are refractive to G2 arrest. Our results show that Vpr induction of expression from integrase-defective HIV-1 also does not appear to rely on cell cycle arrest. Vpr is still capable of increasing HIV-1-mediated luciferase activity when cells are prevented from undergoing Vpr-mediated G2 arrest by thymidine arrest in G1/S. We therefore propose that the increased expression from unintegrated DNA is not an indirect effect due to the cells being in G2 but rather a direct effect of Vpr on enhancing transcription of the HIV-1 DNA.
Potential consequence of Vpr-mediated expression of unintegrated HIV-1 DNA.
Expression from integration-defective HIV-1, as determined by Gag p24 production, has been previously described (6, 9, 38, 49, 56). Recently, in primary quiescent CD4+ T cells, nef mRNA and Nef protein were evident upon infection with integrase-defective HIV-1 (56). tat transcripts, but not Tat protein, were also detected. Transactivation of the integrated HIV-1 LTR-β-galactosidase in Magi cells upon infection with integrase-defective HIV-1 has been attributed to Tat expressed from unintegrated DNA (18, 55). Yet it is unclear how the unintegrated DNA template would have been capable of expressing Tat. Initiation of transcription from unintegrated DNA would require an immediate-early protein that is expressed prior to integration and most likely contained within the virion. Tat has yet to be detected within HIV-1 virions. In contrast, Vpr is found in significant quantities within HIV-1 virions and we have shown that virion-associated Vpr protein alone can induce expression from unintegrated HIV-1. We propose that virion-incorporated Vpr transactivates HIV-1 DNA prior to integration and potentiates expression of other viral proteins, including Tat. Transcription of tat and nef in the absence of a productive infection can lead to increased T-cell activation and viral replication in vitro (56). In the case of integration-competent virus, this would create a cascade effect whereby Tat synthesized prior to integration would be available to immediately activate transcription of the newly integrated provirus, thus increasing the levels of infectious virus. The immediate-early function of Vpr to mediate transcription from unintegrated HIV-1 may also contribute to the ongoing viral replication in vivo at sites of HIV-1 infection where high levels of unintegrated circular HIV-1 intermediates are detected.
Acknowledgments
We thank members of the Chen lab, J. A. Zack, and S. A. Chow for their critical readings of the manuscript and L. Duarte for preparation of the manuscript.
This work was supported by the National Institutes of Health grants CA70018 and AI43190, UCLA Center For AIDS Research grant AI28697, and the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry and Virology core facilities.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agostini, I., J. M. Navarro, F. Rey, M. Bouhamdan, B. Spire, R. Vigne, and J. Sire. 1996. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J. Mol. Biol. 261:599-606. [DOI] [PubMed] [Google Scholar]
- 3.An, D. S., K. Morizono, Q. X. Li, S. H. Mao, S. Lu, and I. S. Chen. 1999. An inducible human immunodeficiency virus type 1 (HIV-1) vector which effectively suppresses HIV-1 replication. J. Virol. 73:7671-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartz, S. R., M. E. Rogel, and M. Emerman. 1996. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky, M., N. Sharova, and M. Stevenson. 1993. Human immunodeficiency virus type 1 2-LTR circles reside in a nucleoprotein complex which is different from the preintegration complex. J. Virol. 67:6863-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky, M. I., T. L. Stanwick, M. P. Dempsey, and M. Stevenson. 1991. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254:423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. [DOI] [PubMed] [Google Scholar]
- 8.Butler, S. L., E. P. Johnson, and F. D. Bushman. 2002. Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J. Virol. 76:3739-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cara, A., F. Guarnaccia, M. S. Reitz, R. C. Gallo, and F. Lori. 1995. Self-limiting, cell type-dependent replication of an integrase-defective human immunodeficiency virus type 1 in human primary macrophages but not T lymphocytes. Virology 208:242-248. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H., S. Q. Wei, and A. Engelman. 1999. Multiple integrase functions are required to form the native structure of the human immunodeficiency virus type I intasome. J. Biol. Chem. 274:17358-17364. [DOI] [PubMed] [Google Scholar]
- 11.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffin, J. M. 1996. Retroviridae: the viruses and their replication, p. 1767-1847. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott Raven, Philadelphia, Pa.
- 13.Cohen, E. A., G. Dehni, J. G. Sodroski, and W. A. Haseltine. 1990. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J. Virol. 64:3097-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 15.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein, D. A., M. P. Sherman, M. L. Penn, P. S. Chin, C. M. de Noronha, W. C. Greene, and M. A. Goldsmith. 2001. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J. Exp. Med. 194:1407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder, R. T., M. Yu, M. Chen, X. Zhu, M. Yanagida, and Y. Zhao. 2001. HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology 287:359-370. [DOI] [PubMed] [Google Scholar]
- 18.Engelman, A., G. Englund, J. M. Orenstein, M. A. Martin, and R. Craigie. 1995. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 69:2729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farnet, C. M., and F. D. Bushman. 1997. HIV-1 cDNA integration: requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell 88:483-492. [DOI] [PubMed] [Google Scholar]
- 20.Farnet, C. M., and W. A. Haseltine. 1991. Circularization of human immunodeficiency virus type 1 DNA in vitro. J. Virol. 65:6942-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felzien, L. K., C. Woffendin, M. O. Hottiger, R. A. Subbramanian, E. A. Cohen, and G. J. Nabel. 1998. HIV transcriptional activation by the accessory protein, Vpr, is mediated by the p300 co-activator. Proc. Natl. Acad. Sci. USA 95:5281-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forget, J., X. J. Yao, J. Mercier, and E. A. Cohen. 1998. Human immunodeficiency virus type 1 vpr protein transactivation function: mechanism and identification of domains involved. J. Mol. Biol. 284:915-923. [DOI] [PubMed] [Google Scholar]
- 23.Gaynor, E. M., and I. S. Y. Chen. 2001. Analysis of apoptosis induced by HIV-1 Vpr and examination of the possible role of the hHR23A protein. Exp. Cell Res. 267:243-257. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for Vpr or Vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 26.Gummuluru, S., and M. Emerman. 1999. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J. Virol. 73:5422-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoch, J., S. M. Lang, M. Weeger, C. Stahl-Hennig, C. Coulibaly, U. Dittmer, G. Hunsmann, D. Fuchs, J. Muller, and S. Sopper. 1995. vpr deletion mutant of simian immunodeficiency virus induces AIDS in rhesus monkeys. J. Virol. 69:4807-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hrimech, M., X. J. Yao, F. Bachand, N. Rougeau, and E. A. Cohen. 1999. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 73:4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kung, S., D. S. An, and I. S. Y. Chen. 2000. A murine leukemia virus LTR derived from rhesus macaques in the context of a lentiviral vector results in high-level gene expression in human T lymphocytes. J. Virol. 74:3668-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang, S. M., M. Weeger, C. Stahl-Hennig, C. Coulibaly, G. Hunsmann, J. Muller, H. Muller-Hermelink, D. Fuchs, H. Wachter, and M. M. Daniel. 1993. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 67:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leavitt, A. D., G. Robles, N. Alesandro, and H. E. Varmus. 1996. Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J. Virol. 70:721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda, T., M. J. Kuroda, and S. Harada. 1998. Specific and independent recognition of U3 and U5 att sites by human immunodeficiency virus type 1 integrase in vivo. J. Virol. 72:8396-8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda, T., V. Planelles, P. Krogstad, and I. S. Chen. 1995. Genetic analysis of human immunodeficiency virus type 1 integrase and the U3 att site: unusual phenotype of mutants in the zinc finger-like domain. J. Virol. 69:6687-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, M. D., C. M. Farnet, and F. D. Bushman. 1997. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J. Virol. 71:5382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima, N., R. Lu, and A. Engelman. 2001. Human immunodeficiency virus type 1 replication in the absence of integrase-mediated DNA recombination: definition of permissive and nonpermissive T-cell lines. J. Virol. 75:7944-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauza, C. D., P. Trivedi, T. S. McKechnie, D. D. Richman, and F. M. Graziano. 1994. 2-LTR circular viral DNA as a marker for human immunodeficiency virus type 1 infection in vivo. Virology 205:470-478. [DOI] [PubMed] [Google Scholar]
- 40.Pierson, T. C., T. L. Kieffer, C. T. Ruff, C. Buck, S. J. Gange, and R. F. Siliciano. 2002. Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. J. Virol. 76:4138-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Y. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266-269. [DOI] [PubMed] [Google Scholar]
- 42.Poon, B., J. B. Jowett, S. A. Stewart, R. W. Armstrong, G. M. Rishton, and I. S. Chen. 1997. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent nitrogen mustard. J. Virol. 71:3961-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popov, S., M. Rexach, L. Ratner, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 273:13347-13352. [DOI] [PubMed] [Google Scholar]
- 44.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogel, M. E., L. I. Wu, and M. Emerman. 1995. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol. 69:882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakai, H., M. Kawamura, J. Sakuragi, S. Sakuragi, R. Shibata, A. Ishimoto, N. Ono, S. Ueda, and A. Adachi. 1993. Integration is essential for efficient gene expression of human immunodeficiency virus type 1. J. Virol. 67:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawaya, B. E., K. Khalili, J. Gordon, R. Taube, and S. Amini. 2000. Cooperative interaction between HIV-1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J. Biol. Chem. 275:35209-35214. [DOI] [PubMed] [Google Scholar]
- 48.Sharkey, M. E., I. Teo, T. Greenough, N. Sharova, K. Luzuriaga, J. L. Sullivan, R. P. Bucy, L. G. Kostrikis, A. Haase, C. Veryard, R. E. Davaro, S. H. Cheeseman, J. S. Daly, C. Bova, R. T. Ellison, B. Mady, K. K. Lai, G. Moyle, M. Nelson, B. Gazzard, S. Shaunak, and M. Stevenson. 2000. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 6:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson, M., S. Haggerty, C. A. Lamonica, C. M. Meier, S. K. Welch, and A. J. Wasiak. 1990. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J. Virol. 64:2421-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Y. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. USA 96:12039-12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subbramanian, R. A., A. Kessous-Elbaz, R. Lodge, J. Forget, X. J. Yao, D. Bergeron, and E. A. Cohen. 1998. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J. Exp. Med. 187:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taddeo, B., W. A. Haseltine, and C. M. Farnet. 1994. Integrase mutants of human immunodeficiency virus type 1 with a specific defect in integration. J. Virol. 68:8401-8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanitharani, R., S. Mahalingam, Y. Rafaeli, S. P. Singh, A. Srinivasan, D. B. Weiner, and V. Ayyavoo. 2001. HIV-1 Vpr transactivates LTR-directed expression through sequences present within −278 to −176 and increases virus replication in vitro. Virology 289:334-342. [DOI] [PubMed] [Google Scholar]
- 54.Wang, L., S. Mukherjee, F. Jia, O. Narayan, and L. J. Zhao. 1995. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J. Biol. Chem. 270:25564-25569. [DOI] [PubMed] [Google Scholar]
- 55.Wiskerchen, M., and M. A. Muesing. 1995. Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J. Virol. 69:376-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503-1506. [DOI] [PubMed] [Google Scholar]
- 57.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan, X., Z. Matsuda, M. Matsuda, M. Essex, and T. H. Lee. 1990. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res. Hum. Retrovir. 6:1265-1271. [DOI] [PubMed] [Google Scholar]
- 59.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]