Abstract
Unlike the pp65 protein of human cytomegalovirus (CMV), which has an immunodominant peptide, pp65495-503, recognized by human CD8+ cells in the context of HLA A∗0201, the fine peptide specificity for CMV IE1 has shown no such immunodominance. With the use of transgenic HLA A∗0201/Kb and HHD II mice, a selected pool of IE1 peptides, including IE1p256-264, IE1p297-304, and IE1p316-324, were shown to stimulate cytolytic T-lymphocyte lysis in the context of HLA A∗0201. Based on an intracellular gamma interferon response, IE1p297-304, a previously unrecognized CD8 epitope, triggered a prominent response to CMV IE1 in HLA A∗0201 subjects.
Murine models to predict human cytomegalovirus (CMV) vaccine immunogenicity are available to detect the presence of a specific immune response, such as cytolytic T-lymphocyte (CTL) function (2, 5, 28), CD4+ gamma interferon (IFN-γ) helper response (15, 22), or antibody response to specific CMV proteins (18, 20, 21). Of interest is the use of the transgenic A2Kb mouse containing the human HLA A∗0201with the murine α3 chain (Kb) (9, 19, 29). The murine model HHD II is also a transgenic HLA A∗0201 mouse that contains a disrupted murine major histocompatibility complex (MHC) molecule, forcing the mouse to present all its immunological epitopes through the HLA pathway (10). Both have become useful models to investigate peptide recognition of specific proteins that can be extrapolated to human subjects.
The immunodominant HLA A∗0201-restricted peptide (pp65495-503) of CMV pp65 has been well studied (3, 6, 12, 30), whereas there is no immunodominant peptide recognized for the CMV IE1 protein, despite the prevalence of an IE1-specific CTL response (11, 14, 16, 17). Three reports have described the stimulatory effect of peptides IE1p315-323, IE1p316-324, and IE1p354-362 from CMV IE1 in the context of HLA A∗0201 (11, 17, 24) in a cytokine flow cytometry or CTL assay. However, not all CMV-seropositive subjects respond to these peptides (17). This report describes the use of HLA A2 transgenic mice to identify a novel IE1 peptide, IE1-297, that is recognized by murine CTLs as well as human CD8 cells by cytokine flow cytometry.
T2 stabilization.
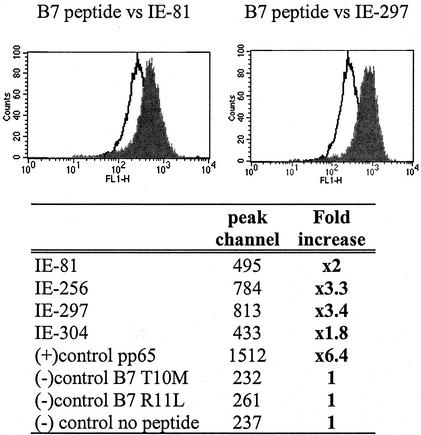
T2 cells are defective for endogenous class I presentation, but the presence of peptide binding to the MHC molecule will stabilize its expression on the cell surface. The stabilized MHC molecule can be detected by flow cytometry with a monoclonal antibody to the HLA A∗0201 molecule (13). The peptide sequences used in this study were collected from two algorithms for HLA peptide predicted motifs: SYFPEITHI (23) and BIMAS. The first five peptides with the highest scores and common to both databases were synthesized at the Beckman Research Institute of City of Hope: IE1p81-89 (IE1-81) (LLSEFCRVL), IE1p256-264 (IE1-256) (ILDEERDKV), IE1p297-304 (IE1-297) (TMYGGISLL), IE1p304-312 (IE1-304) (LLSEFCRVL), and IE1p316-324 (IE1-316) (VLEETSVML). The control peptides were CMV A2pp65495-503 (NLVPMVATV) (positive) and HIV468-476 (ILKEPVHGV), B7 pp65417-426 (TPRVTGGGAM), and B7 pp65265-275 (RPHERNGFTVL) (negative). Four IE1-derived peptides (IE1-81, IE1-256, IE1-297, and IE1-304) were tested individually for binding and stabilizing effect of the MHC molecule on T2 cells. Figure 1, upper panel, shows the displacement of the peak fluorescence to the right in cells incubated with representative IE1-81 (left) and IE1-297 (right) peptides compared to the background signal on T2 cells treated with mismatch (B7-restricted) peptide. All four IE1-derived peptides stabilized the HLA A2 molecule with varied binding affinities as tabulated in Fig. 1 where the peak values of IE1-297 (3.4-fold) and IE1-256 (3.3-fold) were highest and yet still lower than that for the positive control, pp65495-503 (6.4-fold). Therefore, all four peptides (IE1-81, IE1-256, IE1-297, and IE1-304) and later IE1-316 were used together, each at a 25 μM concentration (IE1 mix) to bind to autologous blast cells for in vitro stimulation. The IE1 mix was also used to sensitize target T2 cells for cytotoxicity recognition.
FIG. 1.
Stabilization of HLA A2 expression by IE1-derived peptides. T2 cells incubated overnight with each peptide separately (100 μM) were labeled with an HLA A∗0201 monoclonal antibody. Plots show HLA A2 stabilization by the IE1-81 peptide and IE1-297. The clear area is the background signal detected by a mismatched HLA B7 peptide. Tabulated results are peak channel values derived for each peptide and control peptide.
DNA immunization with IE1, pp65mII, and GM-CSF combinations.
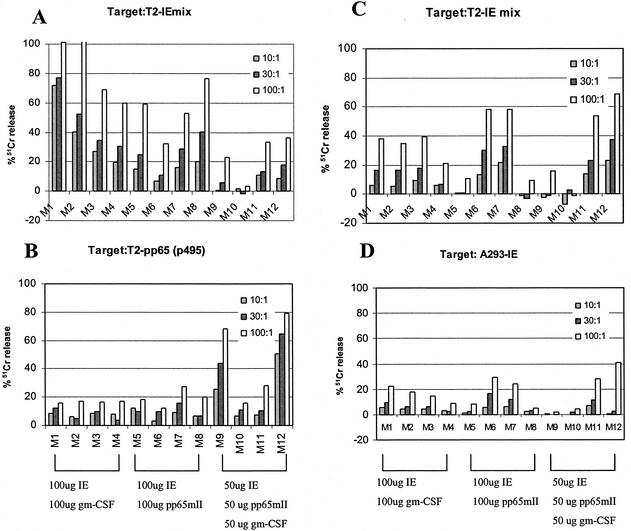
Six- to eight-week-old A2Kb mice (1) were immunized three times at 4-week intervals with various combinations of DNA expressing CMV pp65mII, CMV IE1, and granulocyte-macrophage colony-stimulating factor (GM-CSF). The genes for pp65mII, IE1, and murine GM-CSF were inserted into the mammalian expression vector pcDNA3.1+ (Invitrogen, San Diego, Calif.) containing the intron A as described previously (12). Mice M1 to M4 were inoculated intramuscularly with 100 μg of pcDNA-IE1 and 100 μg of pcDNAGM-CSF, M5 to M8 were inoculated with 100 μg of pcDNA-IE1 and 100 μg of pcDNApp65mII, and M9 to M12 were inoculated with 50 μg of pcDNA-IE1, 50 μg of pcDNApp65mII, and 50 μg of pcDNAGM-CSF. Figure 2 shows the results of CTL killing mediated by splenocytes collected 10 days after the last immunization (Fig. 2A and B) or collected 60 days after the last immunization (C and D). The specific targets used in this experiment were T2 cells incubated with IE1 mix or pp65495-503 or A293 cells constitutively expressing the IE1 gene. The control target T2 cells (no peptides) or nontransfected A293 cells were not lysed by the effector cells (data not shown). The pp65mII DNA was considered a positive control that resulted in an immune response in 50% of immunized mice (12). The GM-CSF DNA was added as a cytokine adjuvant to increase the immune response to DNA immunization in mice (25, 27).
FIG. 2.
Cytotoxicity on transgenic A2Kb mice immunized with 100 μg of pcDNA-IE1 and 100 μg of pcDNAGM-CSF (M1 to M4); 100 μg of pcDNA-IE1 and 100 μg of pcDNApp65mII (M5 to M8); and 50 μg of pcDNA-IE1, 50 μg of pcDNApp65mII, and 50 μg of pcDNAGM-CSF (M9 to M12). (A and B) Responses in spleen cells collected at day 10 after the fourth immunization. (C and D) Long-term memory responses in spleen cells collected at day 60 after the third immunization. The percent chromium release is reported at an effector/target ratio of 10:1, 30:1, or 100:1. Blasts and target cells were incubated with a mixture of five nonapeptides (25 μM each) derived from the IE1 protein for all panels except panel B, in which pp65p495 was used.
For Fig. 2A, immunized A2Kb mice were boosted 10 days prior to splenocyte collection. The splenocytes were incubated with IE1 mix-blasts during a 6-day in vitro stimulation and then subjected to a chromium release assay in the presence of T2 target cells labeled with IE1 mix peptides. Of 12 mice, 11 showed various levels of CTL activity for the mixture of IE1 peptides ranging from 20 to 100% 51Cr release, demonstrating a strong CTL immune response to the CMV IE1 immunization. Figure 2B represents the same spleen cells, stimulated with the peptide specific to CMV pp65mII (p495-503) instead of IE1 mix. Only the mice immunized with the pp65mII DNA (M5 to M12) showed some CTL activity in the presence of T2-p495, demonstrating specificity of response with the respective peptides. Mice M9 to M12, receiving lower doses of the combination regimen, showed a higher lytic response to pp65 than to IE mix stimulation. Without knowing the relative expression of pp65 and IE1 after DNA immunization, it is difficult to interpret differences in percent lysis between these two CMV proteins.
In Fig. 2C, the presence of memory T cells directed to the CMV IE1 gene was detected in immunized splenocytes collected at day 60 after the last injection, stimulated with IE1 mix as described above, and incubated in the presence of target T2-IE1 mix cells. In Fig. 2D, memory effector cells can also recognize endogenously processed IE1 in human A293 targets (A293-IE1), implying that the pool of IE1 peptides may include peptides that are naturally processed through the proteasome of the cell. These data indicate, therefore, that stimulation with the IE1 mixture of peptides generates CTLs that can recognize T2 cells labeled with the IE1 mix and that the CTL can also recognize endogenously processed IE1 CMV gene.
CTL response to individual peptides.
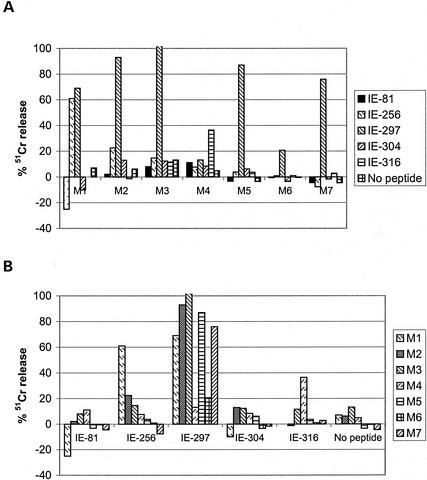
The DNA immunizations for individual peptide analysis consisted of one Sensorcaine-MPF (bivucaine HCl [USP], 0.05%) injection into the thigh followed 5 days later with one injection of 50 μg of pcDNA-IE1 and 50 μg of pcDNAGM-CSF (8, 26). The splenocytes were collected at day 20 postimmunization and stimulated for 6 days with IE1 mix-blasts. The splenocytes from responsive mice, as determined by lysis of IE1 mix-loaded T2 cells, were then tested with T2 cells loaded with individual peptides. The results of target cell lysis by splenocytes from seven IE1-immunized mice are shown in Fig. 3. Mouse M1 recognized T2 targets loaded with IE1-256 or IE1-297. M2, M3, M5, M6, and M7 splenocytes recognized IE1-297, and M4 splenocytes recognized IE1-316. M1 spleen cells were stimulated in vitro with the pool of IE1 peptides six times whereas the other splenocytes were stimulated only once. Figure 3B shows that the peptide most frequently recognized by A2Kb spleen cells was IE1-297, though IE1-256 and IE1-316 were also present. In this group of effector cells, no response to IE1-81 or IE1-304 was detected.
FIG. 3.
Immunodominant IE1 epitopes in A2Kb mice. Splenocytes from mice immunized with IE1 and GM-CSF DNA were stimulated in vitro with a peptide mixture and used as effector cells. (A) For preferential recognition of peptide in individual mice (M1 to M7), the specificity of the stimulated population was determined by Cr release with T2 cells sensitized with each individual peptide (100 μM) as targets (effector/target ratio of 100). (B) For preferential evaluation of each peptide, the IE1-297 peptide is shown as the most frequently recognized epitope in the mixture.
Comparison of CTL responses between HHD II and A2Kb mice.
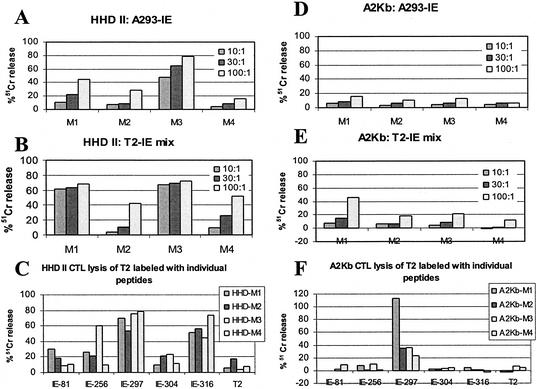
The immune response to full-length IE1 induced by recombinant adeno-associated virus (recAAV-IE1) was characterized for preferential peptide presentation in HHD II and A2Kb mice. The HHD II mice (HLA-A-0201 α1-α2, H-2Db α3-transmembrane and intracytoplasmic domains) in which the H-2Db and mouse β2m genes have been disrupted by homologous recombination (10) were obtained from F. Lemonnier. The recAAV CWRSP plasmid was a gift from S. Chatterjee (4). The internal cassette was removed from the CWRSP plasmid backbone, leaving the internal transcription region from AAV2 intact, and replaced by the CMV promoter, intron A, multiple cloning site, and BGHpA cassette from pcDNA3.1+. The IE1 gene was then placed in the multiple cloning site at the EcoRI/XbaI site (CwCMV-IE1). The AAV Helper-Free system (Stratagene, Cedar Creek, Tex.) was used to encapsidate the AAV. The viral lysate was cleared by centrifugation, and the titer of the supernatant was determined on HT1080 cells. The viral vector recAAV containing the IE1 gene was used as another mode of immunization to check for preferential peptide presentation. Four HHD II and four A2Kb mice were immunized intramuscularly with a single dose of 1.5 × 108 IU of recAAV-IE1 per mouse. The splenocytes were collected 30 days after immunization, stimulated with autologous blast cells loaded with the IE1 mix peptides for 6 days, and assayed for IE1 CTL response. In Fig. 4, panels A to C show the results for HHD II mice and panels D to F show results for the A2Kb mice. After one IE1 mix stimulation, three out of four HHD II mice generated CTLs recognizing endogenously processed IE1 peptides (A293-IE) (Fig. 4A) whereas four out of four HHD II mice responded to the IE1 immunization with IE1 mix T2 target cells (Fig. 4B). When the same splenocytes were incubated with T2 cells labeled with individual peptides, IE1-297 and IE1-316 were the most frequently recognized peptides (four mice), followed by IE1-256 (one mouse) (Fig. 4C).
FIG. 4.
Efficiency of IE1 epitope recognition in A2Kb and HHD II transgenic mice after recAAV vector immunization. HHD II mice (A to C) and A2Kb (D to F) mice were injected with 1.5 × 108 IU of recAAV-IE1. Splenocytes, collected 30 days postimmunization, were stimulated in vitro with a mixture of IE1 peptides. (A and D) Recognition of endogenously processed IE1; (B and E) cytotoxicity against T2 cells sensitized with the same peptide-IE1 mix; (C and F) recognition of individual peptides is shown for HHD II mice and A2Kb mice, respectively.
However, for the A2Kb mice the response to HLA A2-IE1 target cells was low (Fig. 4D), probably due to the fact that the murine CTLs can respond to either the murine MHC or HLA A2 presentation of the antigen. One out of four mice showed a substantial CTL recognition with IE1 mix-T2 targets after IE1 mix stimulation (Fig. 4E). However, the CTL response was specific for the IE1-297 peptide in all four A2Kb mice (Fig. 4F). This response probably reflects a preferential recognition of the peptide IE1-297 in the targeting of CTLs generated by CMV IE1 immunization.
Intracellular cytokine response to IE1-297 in human HLA A∗0201 lymphocytes.
Since IE1-297 peptide had been identified as an important epitope in the process of CTL response to CMV IE1 immunization in A2 transgenic mice, it was then used to determine if it was recognized by CD8 cells from CMV-seropositive human subjects. Cytokine flow cytometry was performed essentially as described in the work of Dunn et al. (7) with 200 μl of fresh blood, stimulated with 100 μM CMV-specific peptides for pp65 (p495-503) or IE1 (p297-305) for 6 h. Fixed cells were stained for intracellular IFN-γ with an anti-IFN-γ-antigen-presenting cell conjugate and analyzed on a FACSCalibur (Becton Dickinson, San Jose, Calif.) flow cytometer. Fresh whole-blood samples collected at days 40, 120, 150, and 180 post-stem cell transplantation from three subjects susceptible to CMV reactivation were stimulated with pp65495-503, IE1-297, human immunodeficiency virus peptide as negative control, and phytohemagglutinin. Two out of four patient samples showed a cytokine response to the IE1-297 peptide whereas four out of four responded to the pp65495-503 peptide (Table 1). These data indicate that HLA A∗0201 CD8+ lymphocytes can respond to IE1-297 stimulation and that this peptide can be used to characterize the status of a CMV cellular response in human subjects.
TABLE 1.
Cytokine flow cytometry with fresh blood from CMV-seropositive stem cell transplantation subjects stimulated with either the pp65 epitope or the IE1-297 epitope
| UPNa | Day posttransplant | pp65p495
|
IE1-297
|
PHA,d % CD8+/IFN-γ | ||
|---|---|---|---|---|---|---|
| % CD8+/IFN-γ | Total no. of cells/literb | % CD8+/IFN-γ | Total no. of cells/liter | |||
| 100 | 40 | 0.11 | 4.08 × 105 | 0 | 0 | 2.09 |
| 93 | 120 | 3.62 | 4.77 × 107 | 0.16 | 2.11 × 106 | 37.6 |
| 70 | 150 | 0.47 | NAc | 0.20 | NA | 33.15 |
| 70 | 180 | 1.26 | 6.97 × 106 | 0.01 | 5.53 × 104 | 19.2 |
Unique patient number.
Total number of IFN-γ-positive cells was calculated taking into account the percentage of total lymphocytes in the blood and the percent CD8+ cells from that fraction and expressed as number of cells per liter of blood. A total of 50,000 events were counted for each sample. The human immunodeficiency virus-negative controls (range, 0.03 to 0.31%) were subtracted from the reported numbers.
NA, total leukocyte count and percent lymphocyte count not available.
PHA, phytohemagglutinin.
In conclusion, a novel IE1 epitope, IE1-297 (IE1p297-304), has been found to be a relatively strong stimulator for IE1 immune CTL response in human CD8+ lymphocytes. Moreover, the use of transgenic mice is an important tool to define immune responses to specific epitopes in the context of HLA. The report here shows that it is possible to extrapolate the results to human application. The information derived from this murine model for elucidating CMV IE1 epitopes will be useful in more fully characterizing the CMV immune response in subjects at risk for CMV disease.
Acknowledgments
We are especially grateful to Simon Lacey, Susan Markel, and Don J. Diamond (D.J.D.) for constructing and providing the IE1 cDNA and for the gift of the BB7 antibody, to Josh Ellenhorn for providing the murine GM-CSF cDNA, and to S. Chatterjee for providing the CWRSC AAV construct and the HEK293 cells.
This study was supported in part by United States Public Health Service program grant PO1 CA 30206 (S. J. Forman [S.J.F.]; project 1 in support of J.A.Z. and project 3 in support of D.J.D.), by grants AI43267, and CA77544 (D.J.D. in support of M.C.V.), by grants 6122-01 (S.J.F.) and 6116-98 (D.J.D.) from the Leukemia and Lymphoma Society, and by grant MO1 RR-43 from the GCRC branch of the National Center for Research Resources, NIH.
REFERENCES
- 1.BenMohamed, L., R. Krishnan, J. Longmate, C. Auge, L. Low, J. Primus, and D. J. Diamond. 2000. Induction of CTL response by a minimal epitope vaccine in HLA A∗0201/DR1 transgenic mice: dependence on HLA class II restricted T(H) response. Hum. Immunol. 61:764-779. [DOI] [PubMed] [Google Scholar]
- 2.Berencsi, K., R. F. Rando, C. deTaisne, E. Paoletti, S. A. Plotkin, and E. Gonczol. 1993. Murine cytotoxic T cell response specific for human cytomegalovirus glycoprotein B (gB) induced by adenovirus and vaccinia virus recombinants expressing gB. J. Gen. Virol. 74:2507-2512. [DOI] [PubMed] [Google Scholar]
- 3.Boppana, S. B., and W. J. Britt. 1996. Recognition of human cytomegalovirus gene products by HCMV-specific cytotoxic T cells. Virology 222:293-296. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee, S., P. R. Johnson, and K. K. Wong, Jr. 1992. Dual-target inhibition of HIV-1 in vitro by means of an adeno-associated virus antisense vector. Science 258:1485-1488. [DOI] [PubMed] [Google Scholar]
- 5.Del Val, M., H. J. Schlicht, H. Volkmer, M. Messerle, M. J. Reddehase, and U. H. Koszinowski. 1991. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J. Virol. 65:3641-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond, D. J., J. York, J. Y. Sun, C. L. Wright, and S. J. Forman. 1997. Development of a candidate HLA A∗0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood 90:1751-1767. [PubMed] [Google Scholar]
- 7.Dunn, H. S., D. J. Haney, S. A. Ghanekar, P. Stepick-Biek, D. B. Lewis, and H. T. Maecker. 2002. Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. J. Infect. Dis. 186:15-22. [DOI] [PubMed] [Google Scholar]
- 8.Endresz, V., K. Burian, K. Berencsi, Z. Gyulai, L. Kari, H. Horton, D. Virok, C. Meric, S. A. Plotkin, and E. Gonczol. 2001. Optimization of DNA immunization against human cytomegalovirus. Vaccine 19:3972-3980. [DOI] [PubMed] [Google Scholar]
- 9.Engelhard, V. H., E. Lacy, and J. P. Ridge. 1991. Influenza A-specific, HLA-A2.1-restricted cytotoxic T lymphocytes from HLA-A2.1 transgenic mice recognize fragments of the M1 protein. J. Immunol. 146:1226-1232. [PubMed] [Google Scholar]
- 10.Firat, H., F. Garcia-Pons, S. Tourdot, S. Pascolo, A. Scardino, Z. Garcia, M. L. Michel, R. W. Jack, G. Jung, K. Kosmatopoulos, L. Mateo, A. Suhrbier, F. A. Lemonnier, and P. Langlade-Demoyen. 1999. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur. J. Immunol. 29:3112-3121. [DOI] [PubMed] [Google Scholar]
- 11.Frankenberg, N., S. Pepperl-Klindworth, R. G. Meyer, and B. Plachter. 2002. Identification of a conserved HLA-A2-restricted decapeptide from the IE1 protein (pUL123) of human cytomegalovirus. Virology 295:208-216. [DOI] [PubMed] [Google Scholar]
- 12.Gallez-Hawkins, G., N. A. Lomeli, X. L. Li, Z. Q. Yao, C. La Rosa, D. J. Diamond, and J. A. Zaia. 2002. Kinase-deficient CMVpp65 triggers a CMVpp65 specific T-cell immune response in HLA-A∗0201.Kb transgenic mice after DNA immunization. Scand. J. Immunol. 55:592-598. [DOI] [PubMed] [Google Scholar]
- 13.Gricks, C. S., E. Rawlings, L. Foroni, J. A. Madrigal, and P. L. Amlot. 2001. Somatically mutated regions of immunoglobulin on human B-cell lymphomas code for peptides that bind to autologous major histocompatibility complex class I, providing a potential target for cytotoxic T cells. Cancer Res. 61:5145-5152. [PubMed] [Google Scholar]
- 14.Gyulai, Z., V. Endresz, K. Burian, S. Pincus, J. Toldy, W. I. Cox, C. Meric, S. Plotkin, E. Gonczol, and K. Berencsi. 2000. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181:1537-1546. [DOI] [PubMed] [Google Scholar]
- 15.Kern, F., N. Faulhaber, C. Frommel, E. Khatamzas, S. Prosch, C. Schonemann, I. Kretzschmar, R. Volkmer-Engert, H. D. Volk, and P. Reinke. 2000. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur. J. Immunol. 30:1676-1682. [DOI] [PubMed] [Google Scholar]
- 16.Kern, F., I. P. Surel, N. Faulhaber, C. Frommel, J. Schneider-Mergener, C. Schonemann, P. Reinke, and H. D. Volk. 1999. Target structures of the CD8+ T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 73:8179-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan, N., M. Cobbold, R. Keenan, and P. A. Moss. 2002. Comparative analysis of CD8+ T cell responses against human cytomegalovirus proteins pp65 and immediate early 1 shows similarities in precursor frequency, oligoclonality, and phenotype. J. Infect. Dis. 185:1025-1034. [DOI] [PubMed] [Google Scholar]
- 18.Morello, C. S., M. Ye, and D. H. Spector. 2002. Development of a vaccine against murine cytomegalovirus (MCMV), consisting of plasmid DNA and formalin-inactivated MCMV, that provides long-term, complete protection against viral replication. J. Virol. 76:4822-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newberg, M. H., J. P. Ridge, D. R. Vining, R. D. Salter, and V. H. Engelhard. 1992. Species specificity in the interaction of CD8 with the alpha 3 domain of MHC class I molecules. J. Immunol. 149:136-142. [PubMed] [Google Scholar]
- 20.Pande, H., K. Campo, B. Tanamachi, S. J. Forman, and J. A. Zaia. 1995. Direct DNA immunization of mice with plasmid DNA encoding the tegument protein pp65 (ppUL83) of human cytomegalovirus induces high levels of circulating antibody to the encoded protein. Scand. J. Infect. Dis. Suppl. 99:117-120. [PubMed] [Google Scholar]
- 21.Pepperl, S., J. Munster, M. Mach, J. R. Harris, and B. Plachter. 2000. Dense bodies of human cytomegalovirus induce both humoral and cellular immune responses in the absence of viral gene expression. J. Virol. 74:6132-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price, P., S. D. Olver, A. E. Gibbons, and G. R. Shellam. 1993. B-cell activation following murine cytomegalovirus infection: implications for autoimmunity. Immunology 78:14-21. [PMC free article] [PubMed] [Google Scholar]
- 23.Rammensee, H., J. Bachmann, N. P. Emmerich, O. A. Bachor, and S. Stevanovic. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213-219. [DOI] [PubMed] [Google Scholar]
- 24.Retiere, C., V. Prod'homme, B. M. Imbert-Marcille, M. Bonneville, H. Vie, and M. M. Hallet. 2000. Generation of cytomegalovirus-specific human T-lymphocyte clones by using autologous B-lymphoblastoid cells with stable expression of pp65 or IE1 proteins: a tool to study the fine specificity of the antiviral response. J. Virol. 74:3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedegah, M., G. T. Brice, W. O. Rogers, D. L. Doolan, Y. Charoenvit, T. R. Jones, V. F. Majam, A. Belmonte, M. Lu, M. Belmonte, D. J. Carucci, and S. L. Hoffman. 2002. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8+ T-cell populations. Infect. Immun. 70:3493-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomason, D. B., and F. W. Booth. 1990. Stable incorporation of a bacterial gene into adult rat skeletal muscle in vivo. Am. J. Physiol. 258:C578-C581. [DOI] [PubMed] [Google Scholar]
- 27.Timmerman, J. M., G. Singh, G. Hermanson, P. Hobart, D. K. Czerwinski, B. Taidi, R. Rajapaksa, C. B. Caspar, A. Van Beckhoven, and R. Levy. 2002. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 62:5845-5852. [PubMed] [Google Scholar]
- 28.Villacres, M. C., J. Zuo, and C. C. Bergmann. 2000. Maintenance of CD8+ T-cell memory following infection with recombinant sindbis and vaccinia viruses. Virology 270:54-64. [DOI] [PubMed] [Google Scholar]
- 29.Vitiello, A., D. Marchesini, J. Furze, L. A. Sherman, and R. W. Chesnut. 1991. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J. Exp. Med. 173:1007-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills, M. R., A. J. Carmichael, K. Mynard, X. Jin, M. P. Weekes, B. Plachter, and J. G. Sissons. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70:7569-7579. [DOI] [PMC free article] [PubMed] [Google Scholar]