Abstract
PDZ domains are protein modules that mediate protein-protein interactions. Here, we present the identification and characterization of a protein similar to the recently identified PDZ-containing protein TACIP18, which we have named SITAC (similar to TACIP18). SITAC is preferentially expressed in cells of the digestive tract, associated with intracellular membranes. Despite the high degree of sequence identity between the PDZ domains of TACIP18 and those of SITAC, none of the known ligands of the former shows interaction with the latter, as judged by two-hybrid analysis. SITAC interacts with peptides containing bulky hydrophobic amino acids two positions upstream of the C-terminal residue. Surprisingly, SITAC also shows interaction with peptides ending in C, a previously unacknowledged ability of PDZ domains. The sequence -Y-X-C-COOH, bound in vitro by SITAC, is present in the member of the tetraspanin superfamily, the L6 antigen. Coimmunoprecipitation experiments show that SITAC interacts with L6A, but not with an L6A C-terminal mutant, confirming the capacity of SITAC to interact with proteins ending in C. Confocal analysis shows that the interaction between L6A and SITAC is necessary for the precise colocalization of both molecules in the same subcellular compartment. In summary, the characterization of the protein SITAC has unveiled novel sequences recognized by PDZ domains, and it suggests that L6A is a natural ligand of this PDZ protein.
INTRODUCTION
Regulated protein-protein associations govern critical aspects of the cell biology such as signal transduction pathways or intracellular protein targeting. Several specialized modules, responsible for specific protein-protein interactions, have been found in a wide variety of proteins. These protein modules recognize short sequences, usually variations of a basic motif, and include, for example, the well characterized src-homology domains (SH2 and SH3) or the recently described PDZ domains (for review see (Pawson and Scott, 1997)).
PDZ is the acronym of the first three PDZ-containing proteins described: PSD95/SAP90, Dlg-A, and ZO-1. To date, nearly 100 different proteins with diverse structure and function have been shown to contain PDZ domains (for recent reviews see Fanning and Anderson, 1999). In general, PDZ domains bind to the very C-terminus of the cytoplasmic domain of transmembrane proteins,and it has been well documented that PDZ-containing proteins act as scaffolds in the organization of protein complexes near the plasma membrane (Fanning and Anderson, 1999 and references therein). In addition, several reports indicate that PDZ proteins may have a dynamic role in different compartments of the secretory pathway. For example, mutations in the cytoplasmic tail of the ligand of the epidermal growth factor receptor, proTGF-α, that disrupt its interaction with the PDZ domains of TACIP18/Syntenin/mda-9 (hereafter referred to as TACIP18), also cause the retention of the former in early compartments of the secretory pathway (Fernández-Larrea et al., 1999). The PDZ protein EBP50 has been implicated in the endocytic sorting of the β2-adrenergic receptor (Cao et al., 1999). LIN-10, a PDZ protein primarily located near to or at the trans-Golgi in Caenorhabditis elegans cells, is necessary for the correct basolateral localization of the epidermal growth factor receptor in epithelial vulval cells (Whitfield et al., 1999). Also, LIN-10 has recently been shown to participate in a network of PDZ-protein interactions that connect the kinesin superfamily motor protein KIF17 with the NMDA receptor, containing vesicles that are transported along microtubules in mammalian neurons (Setou et al., 2000).
PDZ domains have been classified according to the primary sequence of the C-termini they bind (Songyang et al., 1997). In general, all PDZs show strong preferences for peptides with a hydrophobic amino acid (usually V) at the C-terminus (frequently referred to as position 0). PDZs belonging to type I, such as those of PSD95/SAP90 or Dlg-A, bind peptides with S or T in position -2 (Cho et al., 1992). Type II PDZs, such as those of human LIN-2 or AF-6 (Hoskins et al., 1995, Prasad et al., 1993), bind to peptides with a bulky hydrophobic amino acid in position -2 (usually Y or F) (Songyang et al., 1997). Existence of a third type of PDZ has also been proposed, one which would include that of neuronal nitric oxide synthase (nNOS). Type III PDZs would interact with peptides containing D or E in position -2 (Tochio et al., 1999). Thus the identity of the residues in position 0 and -2 of the target peptide clearly determines its interaction with PDZ domains; however, additional residues probably influence this interaction, as mere introduction of -S/T-X-V-COOH, -F/Y-X-V-COOH at the C-terminus of any protein does not guarantee its interaction with type I or type II PDZs, respectively. Confirming this notion, it has been shown that the identity of the amino acid in position -1 of some target proteins determines their interaction with certain PDZs (Setou et al., 2000) and that an acidic amino acid is frequently found in position -3 of type I PDZ-binding proteins (Songyang et al., 1997). Also, the recently described protein TACIP18, which contains two PDZ domains, has an unusually relaxed specificity. TACIP18 interacts with the targets of PDZs of type I (such as proTGF-α) and type II (such as the cell adhesion molecule L-Selectin (Grootjans et al., 1997), or the ligands of the Eph receptors Ephrin Bs (Brückner et al., 1999, Lin et al., 1999, Torres et al., 1998)). In addition, TACIP18 interacts with peptides with acidic amino acids in position -2, the targets of type III PDZs (Fernández-Larrea et al., 1999). Therefore, although the structural basis of the interaction between known PDZ domains and their targets is reasonably well understood, new aspects of this interaction are being uncovered as new PDZ proteins are identified.
Here, we present the identification and characterization of a novel PDZ protein, similar to TACIP18, that we have named SITAC (SImilar to TACIP18). SITAC is preferentially expressed in association with intracellular membranes in cells of the digestive system. The two PDZ domains of SITAC and those of TACIP18 are similar and, like TACIP18, both PDZ domains of SITAC are needed to detect interaction with its targets. However, SITAC does not interact with any of the known ligands of TACIP18, arguing that the binding specificity of both proteins is different. SITAC has the specificity of a type II PDZ because it binds to peptides with Y/W/F in position -2. An unusual feature of SITAC is its ability to bind peptides with C at the C-terminus. Sequences found to interact with SITAC are present in several human transmembrane proteins, and one of them containing the sequence -Y-X-C-COOH, the L6 antigen (L6A), was found to interact in vitro and in cultured cells with SITAC. Furthermore, we found that the binding between SITAC and L6A depends on the C-terminal C of the latter and that this binding is required for the precise colocalization of both protein in the same subcellular compartment. These results indicate that SITAC exemplifies a new type of PDZ/target interaction.
Experimental Procedures
Cloning of SITAC and L6 Antigen
The sequence of the following oligonucleotides was deduced from expressed sequence tag (EST) clones whose amino acid sequence was found 50% identical to TACIP18:
5′–GGATCCGGAATTCAGCCATGTCATCCCTGTACCC-ATC–3′
5′–TCTAGACTCGAGCCCCACAAGGGATGCAGG–3′
Using these oligonucleotides and an aliquot of a human fetal brain library (Clontech, Hampshire, United Kingdom), we amplified the expected 465 bp cDNA fragment, which was confirmed by sequencing and subsequently used as a probe to screen ∼300.000 clones of the same library. The longest cDNA clone identified, containing a 1.4 kb insert, was sequenced and used to make all the constructs of this study. The cDNA encoding human L6 antigen was amplified from a human liver cDNA library, using appropriate oligonucleotides. All the SITAC- or L6A-based constructs were made by polymerase chain reaction (PCR) and standard molecular biology techniques.
Northern and Dot Blot Analysis
The cDNAs encoding SITAC and TACIP18, were labeled with α32P-dCTP (Amersham, Buckinghamshire, United Kingdom) and with the Random Primed DNA Labeling Kit from Boehringer Mannheim (Mannheim, Germany) and used to probe the Human Multiple Tissue Northern Blot and the Human Multiple Tissue Expression Array from Clontech under high stringency conditions, following the instructions of the manufacturer. Washed filters were exposed to autoradiographic films for different periods of time, as indicated.
Production of Polyclonal Antibodies Against SITAC
SITAC cDNA tagged at the C-terminus with six histidines was produced by subcloning the full-length cDNA in the bacterial expression vector pET-21b (Novagen, Madison, WI) and transformed into Escheria coli BL21(DE3) cells for expression of the recombinant protein. His-tagged SITAC protein was purified by immobilized-Ni2+ affinity chromatography using Ni-NTA agarose from QIAGEN (Valencia, CA) and instructions of the manufacturer. The protocol used to obtain anti-SITAC antibodies have been previously described (Fernández-Larrea et al., 1999).
Cell Culture, Transfections, and Biochemical Techniques
CHO or HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (GIBCO BRL, Grand Island, NY). CaCo cells were cultured in Minimum Essential Medium with Eagle's salts and l-glutamine (GIBCO BRL) supplemented with nonessential amino acids, sodium piruvate, and 20% fetal bovine serum (GIBCO BRL). Transient transfections were performed using the DEAE Dextran method and analyzed by confocal microscopy, western blotting, or metabolic labeling and immunoprecipitation 48 h after transfection, as previously described (Fernández-Larrea et al., 1999, Ureña et al., 1999).
Immunohistochemical Staining
For immunohistochemical staining, human normal intestinal wall tissue was fixed for 12 h in 10% neutral formalin, dehydrated, and embedded in paraffin. Tissue sections, of a thickness of 4 μm, were placed on poly-lysine-coated glass slides, deparaffined in xylene, and rehydrated in graded alcohols. Endogenous peroxidase was blocked by immersion in 0.03% hydrogen peroxide for 15 min. After incubation with a 1:600 dilution of anti-SITAC antibody for 2 h, peroxidase-labeled polymer conjugated to goat anti-rabbit antibodies (DAKO En Vision+ System, Carpinteria, CA) was applied to slides. Then, sections were visualized using 3,3′-diaminobenzidine as a chromogen and lightly counterstained with Mayer's hematoxylin.
Yeast Two-hybrid Assays
cDNAs coding for the cytoplasmic tail of wild-type or various carboxy-terminal proTGF-α or Syndecan constructs were synthesized by PCR amplification, as previously described (Fernández-Larrea et al., 1999). The cDNAs coding for the nine carboxy terminal amino acids of Ephrin B, Liprin-α, L6 antigen, and Jagged 1 were codified by appropiate oligos fused to the GAL4-AD in vector pGAD GH (Clontech). Two-hybrid analysis was performed as described in (Fernández-Larrea et al., 1999)
RESULTS
Molecular Cloning and Expression Pattern of SITAC
Looking for novel PDZ proteins, we searched the dbest (expressed sequence tags) database using the blast (basic local alignment search tool) program. Several EST clones were found to encode for a protein fragment of 130 amino acids 50% identical to the C-terminus of TACIP18 (our unpublished results). Using appropriate oligonucleotides, a cDNA fragment was amplified by PCR from a human fetal brain cDNA library and used as probe to screen the same library. The largest clone isolated contained a ∼1.4-kb fragment encoding an open reading frame of 292 amino acids, which is 60% identical to that of TACIP18, which we named SITAC (Similar to TACIP18) (Figure 1A). Like TACIP18, SITAC contains two contiguous PDZ domains flanked by N-terminal and short C-terminal regions with no homology with known proteins (Figure 1A).
Figure 1.
Predicted structure and expression pattern of SITAC and TACIP18. (A) Deduced amino acid sequence of SITAC and alignment with that of TACIP18. Numbers denote amino acid positions—identical amino acids are boxed. The first and second PDZ domains are underlined and double underlined, respectively. (B) Human Multiple Tissue Northern Blot or (C) Human Multiple Tissue Expression Array from Clontech were probed under high stringency conditions with radiolabeled probes corresponding to SITAC or TACIP18 cDNAs. The Human Multiple Tissue Northern Blots corresponding to SITAC and TACIP18 were exposed for 8 days and 20 h, respectively. The Tissue Expression Array were equally exposed.
Next, we analyzed the expression pattern of SITAC mRNA in different tissues and compared it with that of TACIP18. In Northern blots of polyA+ RNA, unique transcripts of 1.6 kb and 2.4 kb, corresponding to SITAC and TACIP18, respectively, were detected (Figure 1B). The lengths of these transcripts and that of the longest cDNA clones isolated are in agreement: 1418 for SITAC and 2560 bp for TACIP18 (this report and Fernández-Larrea et al., 1999). Using probes of similar specific activities (not shown), we found that the relative level of expression of SITAC was lower than that of TACIP18 in the tissues analyzed (Figure 1B; note that the blots corresponding to SITAC and TACIP18 were exposed for 8 days and 20 h, respectively). In addition, while TACIP18 seems to be ubiquitously expressed, SITAC is barely detectable in skeletal muscle and kidney (Figure 1B). To determine whether SITAC is predominantly expressed in tissues not included in the Northern blot shown in Figure 1B, we used a dot blot including a wide variety of human tissues. As shown in Figure 1C, SITAC is mainly expressed in the digestive tract from esophagus to rectum, where TACIP18 is not particularly abundant. Interestingly, a panel of diverse cell lines, including a cell line derived from a colon adeno-carcinoma, do not express detectable amounts of SITAC transcripts (Figure 1C, A10-H10).
Characterization of Anti-SITAC Antiserum and Subcellular Localization of SITAC
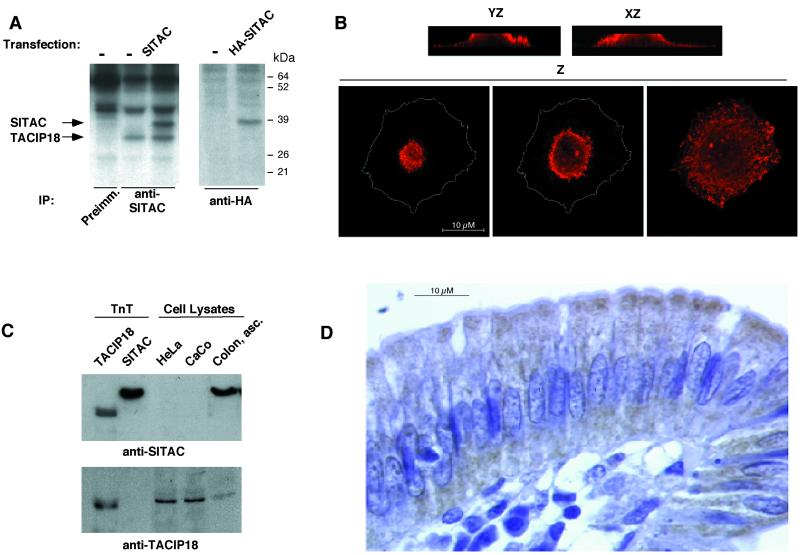
To analyze its subcellular localization, we raised polyclonal antibodies against bacterially expressed SITAC. Although the anti-SITAC antiserum readily reacted with recombinant SITAC, it did not recognize any specific endogenous protein in a panel of cell lines including HeLa, CHO, CaCo, or SW480 as judged by immunoprecipitation or western blotting (our unpublished results). In contrast, immunoprecipitation of lysates from transiently transfected HeLa cells allowed the detection of a specific band of ∼38 kDa (Figure 2A). The estimated molecular weight of SITAC is 31.5 kDa; however, the 38 kDa band was identified as SITAC because it comigrates with in vitro transcribed and translated and bacterially expressed SITAC (Figure 2C and our unpublished results). In addition, anti-SITAC immunoprecipitates, from HeLa but not from CHO cells, a polypeptide of 32 kDa that comigrates with in vitro transcribed and translated TACIP18 and is not immunoprecipitated by preimmune serum (Figure 2A and our unpublished results), indicating that the anti-SITAC polyclonal antibody cross-reacts with human TACIP18. This cross-reaction was confirmed by immunoprecipitation (our unpublished results) and western blotting (see Figure 2C). Thus, to specifically analyze its subcellular distribution, we used SITAC tagged at the C-terminus with the HA epitope and anti-HA monoclonal antibodies. As expected, a unique, specific, ∼39 kDa protein can be immunoprecipitated from metabolically labeled extracts prepared from transiently transfected HeLa or CHO cells (Figure 2A and our unpublished results). The images obtained by stacking single planes of transfected CHO cells through the YZ and XZ axes obtained by confocal microscopy indicate that SITAC is located near the apical region of the cell and also in intracellular compartments (Figure 2B, YZ, XZ). Evaluation of serial single planes through the Z axis are in agreement with this conclusion (Figure 2B, Z). Similar results were obtained in transfected HeLa cells (our unpublished results). The same distribution was observed when CHO cells transfected with nontagged SITAC were stained with anti-SITAC antisera (our unpublished results), arguing that the epitope does not alter the subcellular distribution of HA/SITAC (our unpublished results).
Figure 2.
Expression and subcellular localization of transfected or endogenous SITAC. (A) HeLa cells transiently transfected with SITAC or HA/SITAC were metabolically labeled and immunoprecipitated with anti-SITAC or anti-HA antibodies as indicated. Immunoprecipitates were analyzed by SDS-PAGE and fluorography. (B) CHO transiently transfected with HA/SITAC were permeabilized and incubated with anti-HA antibodies, TRITC-labeled anti-mouse antibodies and analyzed by confocal microscopy. Several consecutive single confocal planes stacked through the YZ and XZ axis, respectively, and three consecutive single confocal planes through the Z axis are shown.The perimeter of the cell as seen in the right panel has been drawn on the left and middle lower panels to show the relative position of the fluorescence within the cell. (C) In vitro transcribed and traslated TACIP18 or SITAC or total cell lysates from HeLa or CaCo cells or from fresh human colon samples were analyzed by western blotting with anti-SITAC or anti-TACIP18 polyclonal antibodies as indicated. (D) Immunohistochemical staining of human intestinal wall with anti-SITAC polyclonal antibodies.
To determine the subcellular localization of SITAC in vivo, we immunostained with anti-SITAC antiserum paraffin embedded epithelial mucosa from human colon. Although, as mentioned above, anti-SITAC polyclonal antibodies cross-react with TACIP18, no cross-reaction can be detected in total cell lysates from human colon, as judged by western blot (Figure 2C). Even in overexposed Westerns of HeLa or CaCo cell lysates, where TACIP18 can be detected with anti-SITAC, no cross-reaction is detected in human colon (not shown); this is probably due to the lower level of expression of TACIP18 in this tissue (Figure 2C) and indicates that the anti-SITAC antiserum stains primarily SITAC in human colon. In this tissue, an enrichment is detectable at the apical region of epithelial cells (Figure 2D), also, anti-SITAC polyclonal antibodies stains the interior of these cells (Figure 2D). Because subcellular fractionation by centrifugation shows that SITAC is associated to membranes (not shown), we concluded that, as in transfected cells, SITAC is associated with intracellular membranes and is enriched in the apical region of the cell in vivo.
Specificity of the PDZ Domains of SITAC
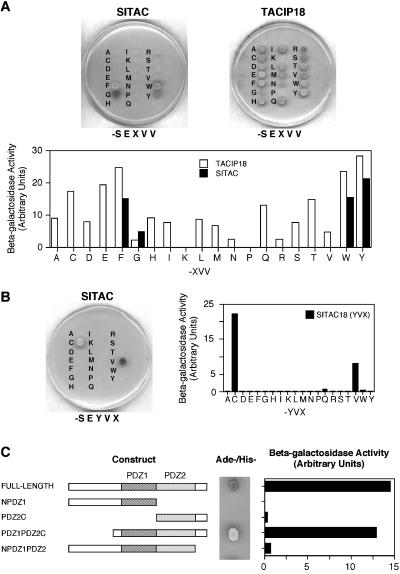
It has been established that the amino acids in positions 0 and -2 of the target protein determine its binding to PDZ domains. Therefore, to assess its specificity, we analyzed the binding of SITAC to a series of point mutants in positions 0 and -2. Since the PDZ domains of SITAC are most similar to those of TACIP18, as a backbone we initially used the cytoplasmic domain of proTGF-α, one of the targets of the latter PDZ protein. In contrast to TACIP18, which shows a very relaxed specificity in position -2 (Figure 3A; see also Fernández-Larrea et al., 1999), SITAC shows a restricted specificity, typical of type II PDZs, because it binds preferentially to the C-terminus of proteins that contain a hydrophobic amino acid in position -2 (Figure 3A). To characterize more fully the binding specificity of SITAC, we constructed a new series of mutants containing Y in position -2 (an amino acid frequently found in that position of PDZ-binding proteins [Songyang et al., 1997]) and all possible amino acids in position 0. Surprisingly, in addition to baits containing V, baits containing C in position 0 showed interaction with SITAC (Figure 3B).
Figure 3.
Analysis of the interaction of SITAC and TACIP18 with different proTGF-α C-terminal mutants. Yeast coexpressing the different proTGF-α cytoplasmic domain mutants in positions -2 (-E-X-V-V mutants) or in position 0 (-E-Y-V-X mutants) fused to the GAL4 DBD and SITAC, TACIP18 or different SITAC deletion constructs fused to GAL4 AD were seeded in -His/-Ade plates and scored for growth or assayed for β-galactosidase activity. The histograms are the averages of duplicate determinations.
Because SITAC contains two PDZ domains, the baits bound by the full-length molecule could be interacting with the first, the second or both PDZs. To distinguish between these possibilities, several deletion constructs were assayed against a bait with Y and C in positions -2 and 0, respectively. Unfortunately, as shown in Figure 3C, the constructs containing only one PDZ domain scored negative in the two-hybrid assay preventing the analysis of the specificity of the individual PDZ domains of SITAC. Collectively, these data show that, although for unknown reasons the specificity of the individual PDZ domains of SITAC cannot be assayed by two-hybrid analysis, the full-length molecule has a restricted specificity in position -2, since it binds only a subset of the baits bound by TACIP18, and opens the possibility that SITAC binds proteins ending in C, a novel specificity for PDZ proteins.
Identification of the L6 Antigen as a SITAC Binding Protein
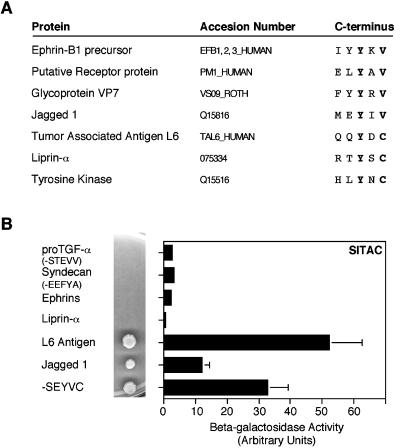
To identify possible binding partners, we searched the databases for human transmembrane molecules containing the sequence preferentially bound by SITAC in vitro Y-X-V/C-COOH. As shown in Figure 4A, several proteins containing this sequence were identified. Analysis of the binding of the C-termini of four of the proteins identified revealed that the L6 antigen (L6A), a cell surface transmembrane protein initially identified because it is overexpressed in lung, breast, ovarian and colon carcinomas (Hellström et al., 1986), showed interaction with SITAC as judged by two-hybrid analysis (Figure 4B). Also the C-terminus of Jagged, one of the ligands of the Notch receptor, showed detectable, albeit quantitative lower, interaction with SITAC (Figure 4B). These results extend the observation that, in vitro, SITAC binds preferentially to proteins that end in C.
Figure 4.
Analysis of the interaction of SITAC with the C-terminus of different transmembrane proteins. (A) C-terminal amino acid sequence of proteins containing the sequence Y-X-V/C-COOH. (B) Yeasts expressing the indicated C-termini fused to the GAL4 DBD were transformed with SITAC fused to the GAL4 AD and assayed for growth in -His/-Ade plates or for β-galactosidase activity. The histograms are the averages of triplicate determinations.
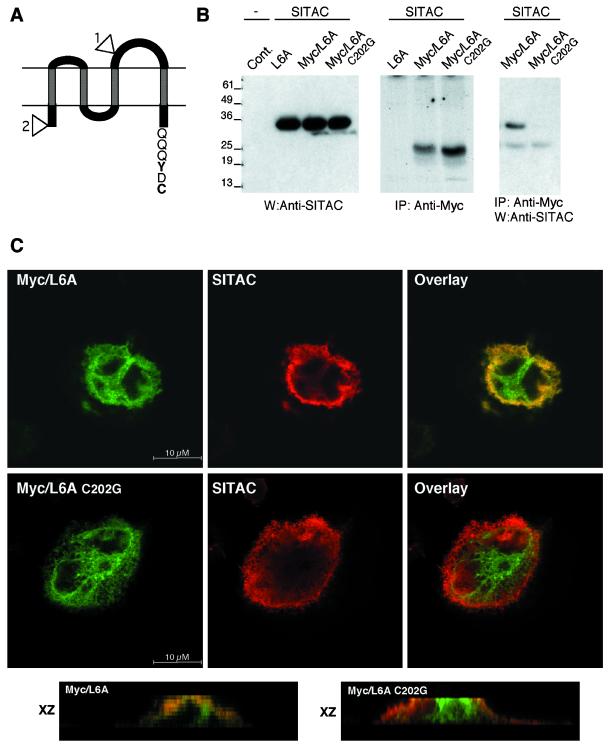
To analyze the possible interaction between SITAC and L6A in cells, we first introduced the Myc epitope into the third loop of L6A, and the resulting construct was named Myc1/L6A (Figure 5A). Then, we analyzed the expression of SITAC and Myc1/L6A in transiently cotransfected CHO cells by western blotting or by immunoprecipitation of metabolically labeled materials with specific antibodies. In agreement with this and a previous report (Marken et al., 1992), the expected 38 or 24 kDa species, corresponding to SITAC or Myc1/L6A, respectively, were specifically detected (Figure 5B, left, middle). Finally, immunoprecipitation with anti-Myc antibodies, followed by western blotting with anti-SITAC antibodies, clearly showed the presence of SITAC in Myc1/L6A immunoprecipitates (Figure 5B, right). This result shows the association between SITAC and Myc1/L6A in cultured cells. To test whether this association depends on the C-terminal C of L6A, we analyzed the binding of the mutant construct Myc1/L6A C202G, to SITAC. As shown in Figure 5B, this C-terminal mutant did not show detectable association with SITAC, confirming the requirement of the C-terminal C of L6A for its interaction with SITAC in a cellular environment.
Figure 5.
Interaction between SITAC and L6 antigen in transfected cells. (A) Schematic showing the topology of L6 antigen. The hatched boxes represent transmembrane regions, and the numbered triangles indicate the location of the Myc epitopes in the Myc1/L6A and Myc2/L6A constructs, respectively. The sequence of the C-terminus is shown. The upper part of the drawing corresponds to the extracellular domain. (B) CHO cells transiently cotransfected with control DNA, SITAC, L6A without the Myc epitope, Myc2/L6A, or Myc2/L6A C202G as indicated were lysed and subjected to western blotting with anti-SITAC antibodies, metabolically labeled, lysed, and immunoprecipitated with anti-Myc antibodies and subjected to SDS-PAGE and fluorography or lysed, subjected to immunoprecipitation, followed by western blotting with anti-SITAC antibodies. (C) CHO cells cotransfected with SITAC and Myc/L6A, or SITAC and Myc/L6A C202G, as indicated were stained with anti-Myc and anti-SITAC antibodies and were analyzed by confocal microscopy. Single confocal planes through the Z axis or several consecutive confocal planes stacked through the XZ axis corresponding to the overlayed images are shown.
To assess the subcellular localization of wild-type L6A and to compare it with that of the C-terminal mutant, we analyzed cells transiently transfected by confocal microscopy. Because the Myc epitope in Myc1/L6A is not accessible to anti-Myc antibodies in intact cells (our unpublished results), we used a L6A construct tagged at the N-terminus (Myc2/L6A, Figure 5A). In cells cotransfected with Myc2/L6A and SITAC, a clear colocalization area of both proteins is observed, particularly in the apical region of the cell (Figure 5C, upper panels and XZ stack). When a similar analysis was performed with the C-terminal mutant Myc2/L6A C202G, little colocalization with SITAC at the apical region of the cell could be detected (Figure 5C, middle panels and XZ stack), indicating a different subcellular localization of Myc2/L6A C202G relative to SITAC. Collectively, the results presented here show that the PDZ domains of SITAC have the ability to interact in vivo with L6A, a transmembrane protein whose C-terminus contains the sequence -Y-D-C-COOH, a novel specificity bound by PDZ domains. In cells, the interaction SITAC/L6A is required for the colocalization of both molecules in the same subcellular compartment.
DISCUSSION
PDZs have been the most recently characterized type of peptide binding domains that mediate protein–protein interactions. Although the structural basis of the interaction between PDZ domains and their targets is reasonably well understood, novel aspects of this interaction are acknowledged as novel PDZ proteins are been identified. In this report, we present the identification and characterization of SITAC, a protein containing two adjacent PDZ domains whose primary sequence and modular organization are similar to those of the previously described protein TACIP18/Syntenin/mda-9.
Several groups have shown the binding of TACIP18, a protein that contains two PDZ domains, to the cytoplasmic domains of Syndecan (Grootjans et al., 1997), Ephrin B (Brückner et al., 1999, Lin et al., 1999, Torres et al., 1998) or proTGF-α (Fernández-Larrea et al., 1999). Although the functional meaning of the interaction between TACIP18 and Syndecans or Ephrins has not been determined yet, it has been proposed that TACIP18 may function as localizing transmembrane ligands for interaction with their receptors or for coupling these ligands to downstream signaling effectors (Brückner et al., 1999, Grootjans et al., 1997, Lin et al., 1999, Torres et al., 1998). Mutations in the cytoplasmic domain of proTGF-α that prevent its interaction with TACIP18 also cause the retention of the former in the early secretory pathway, suggesting a role for TACIP18 in the correct targeting of proTGF-α to the cell surface (Fernández-Larrea et al., 1999). Despite the sequence similarity between SITAC and TACIP18, the binding specificity of this PDZ proteins appears to be different since none of the known ligands of TACIP18 show interaction with SITAC in vitro.
When assayed by two-hybrid, constructs containing only one of the PDZ domains of SITAC scored negatively, preventing the analysis of their individual specificity. Using full-length SITAC and a panel of baits containing point mutations in position 0 and -2, we determined that baits with the C-termini F/Y/W-X-V/C-COOH interact with SITAC. Because SITAC contains two PDZ domains, this consensus sequence possibly represents the combination of the repertoires of C-termini bound by each PDZ domain, if they have different binding specificities. Alternatively, both PDZ domains could be interacting with the same baits, if they have a common specificity. Although several groups, using different assays, have independently observed that the two PDZ domains of TACIP18 are necessary for its interaction with the cytoplasmic tail of Syndecan (Grootjans et al., 1997), B class Ephrins (Lin et al., 1999) or proTGF-α (Fernández-Larrea et al., 1999), the structural reason for this peculiarity is still unknown. A novel characteristic of at least one of the PDZ domains of SITAC is the ability to bind peptides ending in C. Although only proteins with a small hydrophobic amino acid (typically V, L, or I) are normally considered targets of PDZ domains, previous results indicated that C could also be at the C-termini of PDZ-binding proteins. A massive screening of peptides containing random C-terminal sequences performed to define the specificity of the PDZ domain of neuronal nitric oxide synthase (nNOS), showed that the amino acid in position 0 of the 89% of the peptides selected is V. Interestingly, the only amino acids found to also bind to the PDZ of nNOS, albeit at very low percentages (4.2, 3.1, 2.1, and 1%, respectively) were I, L, C, and P (Stricker et al., 1997). Therefore, considering previous evidence and the results presented in this report, we propose that certain PDZ domains bind proteins ending in C and, furthermore, that at least one of the PDZ domains of SITAC binds this type of partner.
Searching the databases for transmembrane proteins possibly bound by SITAC, we identified the L6 antigen (L6A) as a putative partner of SITAC, because it contains the sequence -Y-D-C-COOH. L6A was first identified as a protein overexpressed in several human carcinomas, including that of colon (Hellström et al., 1986) and is classified as a distant member of the transmembrane-4 superfamily (TM4SF), a group of 20 cell surface proteins that have been implicated in the formation and stabilization of functional signaling complexes at the cell surface (Maecker et al., 1997). L6A has been proposed as a target for immunotherapy of different carcinomas (Hellström et al., 1986), and the efficacy of this treatment has been recently shown (DeNardo et al., 1997). Coimmunoprecipitation and colocalization experiments showed that SITAC interacts with L6A in cultured cells. This result and the fact that both L6A and SITAC are expressed in colon carcinomas (Hellström et al., 1986, and Borrell-Pagès and Arribas, unpublished data), suggest that L6A and SITAC could form a complex in colon carcinomas. The mRNA expression of human L6A in normal tissues has not been reported. The murine ortholog is expressed in lung, lymph node, kidney, and skin (Marken et al., 1994). If this pattern of expression is conserved in humans, the interaction of SITAC and L6A could take place in normal lung, where the expression of SITAC is detectable, albeit at low level.
As is the case of many cell surface molecules, immunofluorescence analysis of permeabilized cells shows accumulation of L6A in intracellular compartments. This steady-state distribution of cell surface molecules is frequently interpreted as the accumulation, in different compartments of the secretory pathway, of molecules in transit to, or endocyted from, the cell surface. Colocalization analysis indicates that SITAC interacts with L6A in one of such compartments. The colocalization area is located in the apical region of the cell, as judged by the stack of single planes through the XZ axis obtained by confocal microscopy. In contrast, an L6A C-terminal mutant that shows a lack of binding to SITAC also shows a greatly reduced area of colocalization with SITAC. These observations show that the interactions between the PDZs of SITAC and L6A are critical to precisely colocalize both molecules in the same apical compartment when overexpressed in culture cells and to open the possibility that SITAC could be required for the apical distribution of L6A.This result is in agreement with the current view that many PDZ proteins act as scaffolds in submembranous compartments. However, the possible functional significance of the interaction SITAC/L6A needs further characterization, as alternative possibilities are also plausible. Similar to TACIP18, which seems to mediate the secretion of proTGF-α (Fernández-Larrea et al., 1999), SITAC could also participate in the secretion of L6A. Because the function of tetraspanins is not fully understood, alternatively, SITAC could form part of a scaffold to organize possible signaling complexes containing L6A. The existence of such complexes assembled via PDZ domains, also known as “transducisomes,” has been suggested (Tsunoda et al., 1997) In summary, our results strongly suggest that SITAC exemplifies a new type of PDZ interaction with proteins with a C-terminal C, such as L6A. Although the blockade of this interaction leads to a lack of physical colocalization between SITAC and L6A, future experiments should address in detail the functional meaning of the possible in vivo interaction between SITAC and L6A.
ACKNOWLEDGMENTS
We thank Dr. José G. Castaño and Joaquín Oliva for the anti-SITAC polyclonal antibody, Anna Merlos-Suárez for critical reading of the manuscript, and Dr. Marta Valeri from the Confocal Microscopy facility, Hospitals Vall d'Hebron for superb technical assistance. This work was supported by grants from the Spanish Comisión Interministerial de Ciencia y Tecnología (S.A.F2000–0203), Fundació La Marató de T.V3 (036/97), and Fundació “la Caixa” (98/056–01) to J.A., a predoctoral fellowship from the Fundació “la Caixa”to M. B.-P., and two postdoctoral fellowship from the Fundació per a la Recerca i Docència dels Hospitals Vall d'Hebron to J. F.-L. and A. B.
The accession number for SITAC is AAF80369
REFERENCES
- Brückner K, Labrador JP, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Cho K, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- DeNardo SJ, Kukis DL, Kroger LA, O'donnell RT, Lamborn KR, Miers LA, DeNardo DG, Meares CF, DeNardo GL. Synergy of Taxol and radioimmunotherapy with yttrium-90-labeled chimeric L6 antibody: Efficacy and toxicity in breast cancer xenografts (antibodyybreast carcinoma) Proc Natl Acad Sci USA. 1997;94:4000–4004. doi: 10.1073/pnas.94.8.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–772. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Larrea J, Merlos-Suárez A, Ureña JM, Baselga J, Arribas J. A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-α to the cell surface. Mol Cell. 1999;3:423–433. doi: 10.1016/s1097-2765(00)80470-0. [DOI] [PubMed] [Google Scholar]
- Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, David G. Syntenin, a PDZ. protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström I, Horn D, Linsley P, Brown JP, Brankovan V, Hellström KE. Monoclonol mouse antibodies raised against human lung carcinoma. J Biol Chem. 1986;46:3917–3923. [PubMed] [Google Scholar]
- Hoskins R, Hajinal A, Harp S, Kim SK. The C. elegansvulval induction gene lin-2 encodes a member of the MAGUK family of cell junction proteins. Development. 1995;122:97–111. doi: 10.1242/dev.122.1.97. [DOI] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B-class ephrins constitutes a PDZ domain binding motif. J Biol Chem. 1999;274:3726–3733. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Marken JS, Bajorath J, Edwards CP, Farr AG, Schieven GL, Hellstrom I, Hellstrom KE, Aruffo A. Membrane topology of the L6 antigen and identification of the protein epitope recognized by the L6 monoclonal antibody. J Biol Chem. 1994;269:7397–7401. [PubMed] [Google Scholar]
- Marken JS, Schieven GL, Hellström I, Hellström KE, Aruffo A. Cloning and expression of the tumor-associated antigen L6. Proc Natl Acad Sci USA. 1992;89:3503–3507. doi: 10.1073/pnas.89.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Prasad R, Gu Y, Alder H, Nakamura T, Canaani O, Saito H, Huebner K, Gale RP, Nowell PC, Kuriyama K. Cloning of the ALL-1 fusion partner, the AF-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res. 1993;53:5624–5628. [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog D-H, Hirokawa N. Kinesin Superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chisthi AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxy-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Stricker NL, Christopherson KS, Yi BA, Schatz PJ, Raab RW, Dawes G, Bassett DEJ, Bredt DS, Li M. PDZ domain of neuronal nitric oxide synthase recognizes novel C-terminal peptide sequences. Nat Biotechnol. 1997;15:336–342. doi: 10.1038/nbt0497-336. [DOI] [PubMed] [Google Scholar]
- Tochio H, Zhang Q, Mandal P, Li M, Zhang M. Solution structure of the extended neuronal nitric oxide synthase PDZ domain complexed with an associated peptide. Nat Struct Biol. 1999;6:417–421. doi: 10.1038/8216. [DOI] [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signaling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- Ureña JM, Merlos-Suárez A, Baselga J, Arribas J. The cytoplasmic carboxy-terminal amino acid determines the subcellular localization of proTGF-a and membrane type matrix metalloprotease (MT1-MMP) J Cell Sci. 1999;112:773–784. doi: 10.1242/jcs.112.6.773. [DOI] [PubMed] [Google Scholar]
- Whitfield CW, Bénard C, Barnes T, Hekimi S, Kim SK. Basolateral localization of the Caenorhabditis elegansepidermal growth factor receptor in epithelial cells by the PDZ protein LIN-10. Mol Biol Cell. 1999;10:2087–2100. doi: 10.1091/mbc.10.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]